Abstract
Background
Sweet Syndrome (SS) is a rare inflammatory skin condition characterized by the sudden appearance of tender, erythematous or violaceous papules, plaques, and nodules typically found on the face, neck, shoulder, upper extremities, and trunk. Often, SS is difficult to diagnose because of its various non-specific manifestations, including fever, arthralgia, myalgia and ocular involvement. In most cases described in literature, cutaneous and pulmonary symptoms of SS present in a concomitant manner. Several reported cases of pulmonary SS have shown that if left untreated, acute respiratory distress syndrome can ensue and progress to fatal respiratory failure.
Case report
A 58-year-old female with acute myeloid leukemia (AML) secondary to chronic lymphocytic leukemia (CLL) presented with new nodular lesions, dyspnea, and fevers. Chest X-ray revealed pulmonary infiltrates. The patient developed new facial lesions and worsening hypoxic respiratory failure. Further infectious workup was negative. She was found to have SS with pulmonary involvement and initiated on high-dose intravenous (IV) steroids with marked clinical improvement.
Conclusions
Major and minor criteria for the diagnosis of lung-associated SS should be carefully evaluated, especially when a biopsy is unavailable. The following case report describes the clinical course and outcomes from treatment for this patient.
Keywords: Pulmonary sweet syndrome, Neutrophilic dermatosis, Acute Myeloid Leukemia
1. Introduction
Sweet Syndrome (SS) is a rare inflammatory skin condition characterized by the sudden appearance of tender, erythematous or violaceous papules, plaques, and nodules typically found on the face, neck, shoulder, upper extremities, and trunk [[1], [2], [3]]. Papule eruption is often distributed asymmetrically and may present as one or multiple lesions [[1], [2], [3]]. SS can be distinguished histologically from other inflammatory skin diseases by a diffuse neutrophilic infiltrate in the upper dermis [[1], [2], [3]]. There are three clinical contexts in which SS occurs: classical (or idiopathic), malignancy-associated, and drug-induced.
Classical Sweet Syndrome (CSS) typically presents in patients between the ages of 30–60 years at a 4-to-1 female-to-male ratio [1,4]. Despite being considered idiopathic, it has been reported in association with infections (particularly upper respiratory tract infections), pregnancy, inflammatory and autoimmune disorders [1]. Recurrence of the dermatosis is seen in approximately one-third of patients with CSS [1].
The original criteria for diagnosing CSS were proposed in 1986 by Su and Liu, but has since been revised [[5], [6], [7]]. Two major criteria required for establishing a diagnosis include (1) sudden onset eruption of tender, painful plaques, or nodules, and (2) neutrophilic infiltrate in the dermis without vasculitis [4]. To confirm diagnosis, a minimum of two of the four minor criteria must also be present: (1) fever >38 °C, (2) illness preceded by an upper respiratory or gastrointestinal infection, or associated with an underlying inflammatory disorder, malignancy, or pregnancy, (3) elevated white cell count with neutrophil predominance and elevated inflammatory markers, and (4) positive response to corticosteroids [4].
Unlike CSS, Malignancy-associated Sweet Syndrome (MASS) occurs with equal frequency in men and women [1]. MASS was first described by Costello in the 1950s and later revisited by Robert Sweet in his seminal article on SS in 1964 [1,8]. Diagnostic criteria for MASS are identical to that of classic SS, but also include the presence of underlying malignancy as a minor criterion and forego inflammatory disease, pregnancy, vaccination or infection [5,6]. MASS can present as a paraneoplastic syndrome in patients with an established cancer diagnosis and has been temporally associated with diagnosis or relapse of cancer [1]. In 1993, Cohen and Kurzrock reported that 21% of patients (96 of 448 individuals spanning 15 studies) with SS co-presented with either a hematologic or solid tumor malignancy [9]. It has also been noted in a more recent retrospective study that 39% of patients with acute myeloid leukemia (AML) and SS were detected to have FLT-3 mutations, suggesting FLT-3 inhibitors may contribute to triggering SS [8].
Lastly, Drug-induced Sweet Syndrome (DISS) has been most frequently associated with patients receiving granulocyte-colony stimulating factor (G-CSF) therapy, though several other medications have been linked to this condition [1]. DISS was first described by Su and Liu in the 1980s in relation to sulfamethoxazole/trimethoprim use, and Walker and Cohen later established its diagnostic criteria [10].
Often, SS is difficult to diagnose due to its various, non-specific manifestations, including fever, arthralgia, myalgia and ocular involvement [1,2]. Pulmonary involvement is a rare presentation of SS, but has been reported in at least 34 cases [11]. Clinical signs and symptoms of pulmonary involvement include fever, dry cough, progressive dyspnea, and diminishing respiratory status [[11], [12], [13]]. Lung histology is characteristic of a distinct, dense neutrophilic infiltrate that is similarly present in other manifestations of SS [[11], [12], [13]]. Chest X-ray findings include unilateral or bilateral interstitial infiltrates and pulmonary opacities, with or without pleural effusions [[11], [12], [13]]. In most cases described in literature, the presentation of cutaneous and pulmonary symptoms in SS often overlap [[11], [12], [13]]. Pulmonary SS could progress to acute respiratory distress syndrome and worsen to fatal respiratory failure if untreated [12].
We present a patient who completed induction chemotherapy for newly diagnosed AML and subsequently presented with new nodular lesions, dyspnea, and fevers.
2. Case report
A 58-year-old female with chronic lymphocytic leukemia (CLL) diagnosed 4 years prior, status post five cycles of FCR (fludarabine, cyclophosphamide, and rituximab), was admitted to USC Norris Comprehensive Cancer Center in May 2021 for induction chemotherapy with D+V (decitabine 20 mg/m2 (day 1–7) plus venetoclax ramp-up (50 mg on day 1, 100 mg on day 2, then 200 mg daily) for 21 days) for newly diagnosed therapy-related AML with complex cytogenetics (FLT3 not detected). Prophylactic levofloxacin, isavuconazonium, acyclovir, and allopurinol were also initiated due to pancytopenia and risk of tumor lysis syndrome based on admission labs. Prior to initiation of chemotherapy, the patient developed a fever in the setting of neutropenia, requiring escalation of empiric antibiotics from cefepime and vancomycin to meropenem on day 3 of chemotherapy cycle 1 (C1D3) per infectious diseases (ID) team. The same day meropenem was started, a pulmonary embolism (PE) was detected on computerized tomography (CT) scan with small bilateral pleural effusions and basilar atelectasis. The hematology team decided to hold D+V on C1D4 due to persistent tachycardia and increasing oxygen requirements in the setting of neutropenic fever.
A repeat chest CT scan was performed the next day and revealed a worsening patchy pneumonia in both upper and right middle lobes as well as worsening left lower lobe atelectasis versus consolidation. The patient's pleural effusions temporarily improved with diuresis without a change in her antibiotic regimen. However, she became tachycardic and tachypneic and was subsequently started on intravenous (IV) sulfamethoxazole/trimethoprim double-strength (DS) to cover Pneumocystis pneumonia and propranolol for heart rate control. Though her tachypnea persisted in the 30s, she demonstrated clinical improvement. Her arterial blood gas (ABG) showed respiratory alkalosis with a pH 7.55, pCO2 30 mmHg and pO2 69 mmHg with an arterial-alveolar (A-a) gradient >100 mmHg. The patient reported acute back pain but remained afebrile overnight and subsequently discharged after resolution of fever.
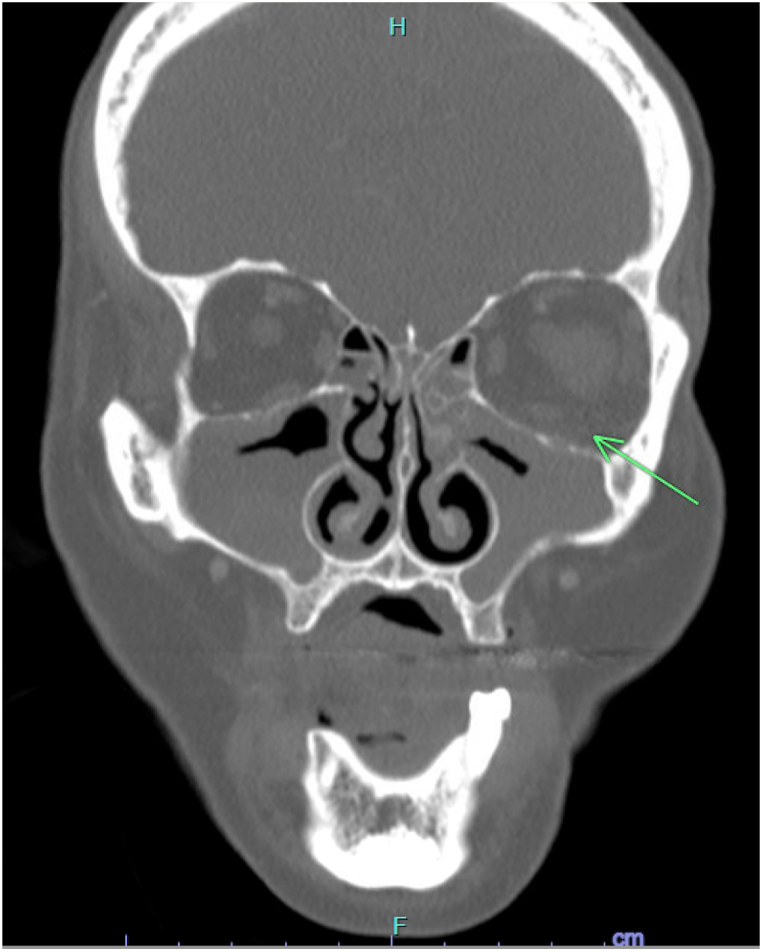
12 days after D+V chemotherapy was held, the patient presented to the clinic with new left-sided facial swelling. Upon evaluation, the dermatology team described a left-sided solitary nodule extending from the dermal to subcutaneous regions, with little epidermal change and minor follicular accentuation (Fig. 1). Infection was the primary concern, as the rapid onset of left-sided facial swelling and dyspnea was in the setting of neutropenia. Other differential diagnoses included neutrophilic dermatosis, such as SS, pseudolymphoma secondary to sulfamethoxazole/trimethoprim, panniculitis, and leukemia cutis. Biopsy of the lesion was non-specific and revealed only mild inflammation with no evidence of SS, leukemia cutis, or frank infection.
Fig. 1.
Patient's maxillofacial CT scan without contrast, where a green arrow indicates a left sided solitary nodule extending from the dermal to subcutaneous regions, with little epidermal change and minor follicular accentuation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Two days after biopsy of the lesion, the patient developed similar plaques along her right eyelid and eyebrow (Fig. 2). These new cutaneous lesions, in the context of ongoing AML and thrombocytopenia, were suggestive of SS. Given the progression with skin involvement, the team decided to re-biopsy the lesion for repeat tissue cultures. Results of the repeat biopsy were consistent with SS; bacterial and fungal stains were negative. The patient was scheduled to restart C1D1 of D+V induction chemotherapy the following day after bacterial and fungal stains returned negative. On C1D2, she was started on prednisone (60 mg by mouth daily). The prednisone was held on C1D3 due to recurrence of fever overnight. Despite holding the steroid, the patient's facial lesions significantly improved by C1D3. The patient was afebrile overnight and resumed prednisone on C1D4. The prednisone was tapered over five weeks, with continued response. The hematology team did not resume chemotherapy after the patient completed 7 days of decitabine and 21 days of venetoclax. Despite slow neutrophil recovery in the setting of AML secondary to MDS, she received filgrastim for one week prior to discharge after resolution of fever, and strict precautions were given to return to the emergency department had she developed another fever.
Fig. 2.
Photograph of the nodular lesions seen on the patient's face and extremities.
Approximately four months later in October 2021, she presented to the clinic with worsening facial pain and fevers (Tmax 38.1 °C) in the setting of neutropenia (absolute neutrophil count (ANC) 390 cells/μL). She was noted to have bilateral purpuric lesions along her lower extremities as well as tenderness along her nasal bridge and below her left eye. At this time, she was on C1D35 of A+V chemotherapy (azacitidine plus venetoclax), with prolonged count recovery. Her bone marrow biopsy showed 2% blasts, which was indicative of residual AML. Cefepime and doxycycline were started empirically for neutropenic fever in the setting of recent IV broad-spectrum antibiotic use, along with prophylactic acyclovir and isavuconazonium. Due to concern for recurrence of cutaneous SS, the patient was also started on prednisone (30 mg by mouth daily) and topical hydrocortisone cream.
Further infectious workup with a maxillofacial CT scan raised concern for invasive fungal disease based on new complete opacification of the maxillary sinuses with hyperdense material on the left side and fat stranding along the left orbit (Fig. 3). The ID team subsequently added amphotericin B and switched doxycycline to daptomycin. Otolaryngologists performed a rigid nasal endoscopy, which was more concerning for acute bacterial sinusitis but with negative nasal biopsy cultures. The patient's fever persisted despite her optimized antimicrobial regimen and negative cultures. A CT scan of the chest revealed new ground glass opacities of the bilateral upper and middle lobes with consolidative opacities of the lower lobes, raising concerns for an infectious versus inflammatory process (Fig. 4). In addition to fever and tachycardia, the patient reported symptoms of dyspnea and productive cough. She underwent bronchoscopy with brushings, but cytology and cultures were unrevealing. Her primary care team elected to rapidly taper the prednisone given concern for worsening infection.
Fig. 3.
Patient's coronal maxillofacial CT scan with contrast, where a green arrow indicates complete opacification of the maxillary sinuses with hyperdense material on the left side and fat stranding along the left orbit. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Patient's axial chest CT scan with contrast, where two green arrows reveal ground glass opacities of the bilateral upper and middle lobes with consolidative opacities of the lower lobes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
More extensive serologic workup and cultures were sent to investigate fungal, mycobacterial, and atypical organisms. She developed new oxygen requirements over the next few days, eventually requiring transfer to the medical intensive care unit (MICU) for high flow nasal cannula (HFNC). Cefepime was broadened to meropenem and both daptomycin and amphotericin B were changed to linezolid and micafungin, respectively. Karius testing, which is designed to detect microbial cell-free DNA in blood, was unrevealing. In November 2021, the patient was initiated on salvage chemotherapy LDAC (low-dose cytarabine, 20 mg administered subcutaneously twice daily day 1–10) plus glasdegib (100 mg by mouth daily) for a 28-day cycle while she remained in the MICU given concern for fevers in the setting of possible residual AML. A repeat chest CT scan demonstrated worsening ground glass opacities and intralobular septal thickening in both lungs as well as worsening consolidative opacities of bilateral lobes and bilateral pleural effusions, for which IV diuretics were initiated.
As the patient remained in the MICU on HFNC, the primary team continued to rule out other possible diagnoses contributing to her presentation. AML could have explained the worsening fevers; however, the lung findings raised concern for pneumonia. Pulmonary edema was also proposed, which was addressed with IV diuretics. Ultimately, the decision was made to consider pulmonary SS and initiate immediate treatment with methylprednisolone (1 mg/kg IV every 12 hours), though this decision would have meant potentially exacerbating any underlying fungal pathology. The following day, the patient defervesced and had rapid improvement in oxygen requirements, which prompted dose reduction of methylprednisolone from 1 mg/kg IV to every 24 hours. On day 3 of steroid therapy, the patient transitioned from methylprednisolone (1 mg/kg IV) to prednisone (60 mg by mouth daily) with continued response. Pan-cultures and Karius testing remained negative; thus, antibiotics were de-escalated. The patient then transferred out of the MICU to continue a 6-week steroid taper and complete salvage chemotherapy without barriers to discharge.
Approximately 3 weeks later in December 2021, the patient presented to the clinic with fever in the setting of neutropenia. She was admitted and started on empiric IV antibiotics, including cefepime, piperacillin/tazobactam, and doxycycline. An extensive infectious work-up was performed including blood cultures, urine analysis, and chest X-ray, all of which were negative. Her treatment course was complicated by a right upper extremity cutaneous papular lesion. Due to concern for cutaneous SS, the dermatology team recommended re-initiation of prednisone (60 mg by mouth daily). Repeat chest imaging was obtained with concern for relapsing pulmonary SS in the setting of persistent fever and oxygen desaturation into the low 90s; however, the imaging did not indicate an invasive pulmonary fungal infection. She began to complain of right submandibular pain, right anterior neck pain, and a new left upper extremity skin lesions; thus, a CT of maxillofacial and neck were obtained, yet unrevealing (Fig. 5). She then transitioned from oral prednisone (60 mg daily) to IV methylprednisolone (1000 mg daily) for 3 days, with improvement. She was discharged on prednisone (60 mg by mouth daily) until scheduled readmission the following week to start a chemotherapy clinical trial. However, the patient contracted COVID-19 in the days leading up to the start of the clinical trial and grew Cladosporium fungemia in a fungal blood culture, thereby preventing eligibility for the clinical trial. During this hospital course, dexamethasone and remdesivir was for 5 days for severe COVID-19 pneumonia, with improvement. Prednisone was dose-reduced (from 60 mg to 30 mg by mouth daily) in the setting of fungemia and amphotericin B was initiated for 15 days prior to switching to voriconazole.
Fig. 5.
Patient's maxillofacial CT with contrast, where a green arrow indicates chronic polypoid mucosal thickening in the maxillary sinuses, but no evidence of acute fungal sinus disease is present. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
After recovering from COVID-19 pneumonia and fungemia, the patient began C2 of LDAC plus glasdegib while completing a 5-week prednisone taper for cutaneous SS in January 2022. Despite completing salvage chemotherapy, the patient had a poor prognosis given her poor performance status, on-going infections, and new onset of gastrointestinal and cardiovascular symptoms. A shared clinical decision was made to transition the patient to home hospice in February 2022, 12 days after completing C2 of LDAC + glasdegib.
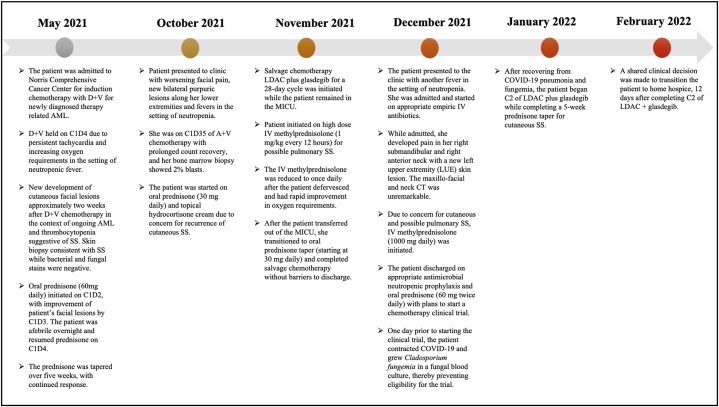
A timeline summarizing the key events of the patient's clinical course may be referenced below in Fig. 6.
Fig. 6.
Summary of the patient's clinical course.
3. Discussion
Diagnosing SS can be challenging due to its potential for multi-organ involvement [11]. Pulmonary manifestations are a rare subset of SS and can present in the acute setting as infiltrates, pleural effusions, or bronchitis obliterans-organizing pneumonia (BOOP) [11]. Higher rates of SS have been noted in patients with coexisting myeloproliferative disorders either before, at the time of, or after the diagnosis of malignancy [4]. In MASS, SS tends to present in older patients and in the presence of ongoing cytopenias [4]. Although this patient had a history of MASS, the diagnosis of pulmonary SS was difficult without biopsy. Additionally, the patient's severely immunocompromised state prompted immediate action to diagnose and treat potentially fatal causes, namely infection. Yet, the patient's cultures and lab tests were repeatedly negative, and despite various regimens of broad-spectrum antimicrobials, there was no evidence of clinical improvement.
Although corticosteroids are an effective, commonly used treatment for SS, there is no standard protocol for the treatment of SS. Patients should be carefully evaluated for treatment based on their clinical presentation and medical history [14]. Systemic corticosteroids have been considered a “gold standard” for both systemic and cutaneous symptoms associated with SS [9]. Clinical improvement is generally seen within 1–2 days, including resolution of skin lesions, with clearance occurring within 7–10 days. The dose and administration of prednisone may range between 0.5 and 1.5 mg/kg/day, with typical doses of 30–60 mg given daily, followed by a taper to 10 mg within 4–6 weeks [9]. Similarly, topical or intralesional corticosteroids have been used to effectively mitigate isolated cutaneous lesions [14]. Alternatives to corticosteroids include colchicine, dapsone, potassium iodide, cyclosporine, indomethacin, and clofazimine [9,14].
A remarkable feature of this case report was the connection between SS to AML, and how treating the patient's AML seemed to play a significant role in alleviating the episodes of SS she experienced. This became evident upon her first induction of D+V after being newly diagnosed with therapy-related AML. Here, the patient received treatment for AML for only 3 days before it was placed on hold. Then, 12 days after holding chemotherapy, the patient presented to the clinic with dermatological lesions consistent with SS on her right eyelid and eyebrow. Improvement of the lesions was seen by C1D3 of restarting D+V, with continued response on C1D4 after prednisone was resumed and tapered for a total of 5 weeks. Several months after on C1D35 of A+V, she presented to the clinic with purpuric lesions in her lower extremities. Notably, her blast count at the time of this presentation was indicative of residual AML, despite her A+V regimen. An additional concern for pulmonary SS developed thereafter, which required treatment with IV methylprednisolone before tapering on prednisone. To address the patient's refractory AML, salvage chemotherapy with LDAC plus glasdegib was initiated while she was tapering steroids. She was admitted to the hospital three weeks later for cutaneous manifestations of SS. After 9 months from her initial presentation, she was ultimately discharged on home hospice due to progressive disease and poor prognosis.
A few studies have investigated the relationship between hematological malignancies, such as AML, and SS. In 2014, Kazmi et al. reported 1% of 2178 patients with AML developed SS upon retrospective review [15]. Further analysis of these patients revealed that the −5/del(5q) karyotype and FMS-related tyrosine kinase-3 (FLT3) mutations were more prevalent in comparison to AML patients who did not develop SS [15]. In another retrospective review in 2016, Varadarajan et al. reported 2.6% patients (2 of 77) with FLT3 internal tandem duplication (ITD)-positive AML developed symptoms of differentiation syndrome along with SS [16]. These patients were treated with a FLT3 inhibitor as part of two concurrent institutional review board–approved clinical trials [16]. The most common side effects of FLT3 inhibitor agents, including midostaurin, are cytopenia, neutropenic fever, gastrointestinal and pulmonary issues [17]. Despite the lack of reported SS in clinical trials with midostaurin, there were recently four case reports of patients with FLT3-positive AML who developed SS while receiving midostaurin [[17], [18], [19], [20], [21]]. Though the mechanism behind this temporal relationship cannot be explained, midostaurin may have contributed to the development of SS in these case reports. Although our patient was diagnosed with therapy-related AML with complex cytogenetics, they did not have FLT3 mutations detected. Our patient's underlying AML is the most likely causative inducer of SS given that FLT3 mutations nor inhibitors were present. However, the pathogenesis of SS in neutropenic AML patients remains uncertain. It is important for clinicians to recognize the implication of the FLT3 mutation not only in the increased relapse rates of AML patients, but also in its possible association with SS.
4. Conclusion
Treatment of this patient's underlying AML contributed to alleviating the recurring episodes of SS. Furthermore, the patient's rapid clinical improvement after initiation of high-dose steroids made evident that pulmonary SS was a probable driving force in her clinical presentation. Rare systemic manifestations of SS should be taken into consideration for patients not responding to antibiotic therapy with clinical compromise. Pulmonary SS should be treated with systemic corticosteroids if there is a high degree of suspicion, even without biopsy. Additionally, multi-specialty collaboration was integral to optimizing the management of this patient's case.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgements
There are no acknowledgements for this research.
References
- 1.Cohen P.R. Sweet's syndrome – a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J. Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casarin Costa J.R., Virgens A.R., de Oliveira Mestre L., et al. Sweet syndrome: clinical features, histopathology, and associations of 83 cases. J. Cutan. Med. Surg. 2017;21(3):211–216. doi: 10.1177/1203475417690719. [DOI] [PubMed] [Google Scholar]
- 3.Yaghmour G., Wiedower E., Yaghmour B., Nunnery S., Duncavage E., Martin M.G. Sweet's syndrome associated with clonal hematopoiesis of indeterminate potential responsive to 5-azacitidine. Ther. Adv. Hematol. 2017;8(2):91–95. doi: 10.1177/2040620716680330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vashisht P., Bansal P., Goyal A., et al. StatPearls Publishing; 2021. Sweet Syndrome. [Updated 2021 Sep 14]. in: StatPearls [Internet]. Treasure Island (FL)https://www.ncbi.nlm.nih.gov/books/NBK431050/ Jan-. Available from: [Google Scholar]
- 5.Su W.P., Liu H.N. Diagnostic criteria for Sweet's syndrome. Cutis. 1986;37(3):167–174. [PubMed] [Google Scholar]
- 6.von den Driesch P. Sweet's syndrome (acute febrile neutrophilic dermatosis) J. Am. Acad. Dermatol. 1994;31(4):535–560. doi: 10.1016/s0190-9622(94)70215-2. [DOI] [PubMed] [Google Scholar]
- 7.Villarreal-Villarreal C.D., Ocampo-Candiani J., Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369–378. doi: 10.1016/j.ad.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Heath M.S., Ortega-Loayza A.G. Insights into the pathogenesis of sweet's syndrome. Front. Immunol. 2019;10:414. doi: 10.3389/fimmu.2019.00414. Published 2019 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen P.R., Kurzrock R. Sweet's syndrome: a review of current treatment options. Am. J. Clin. Dermatol. 2002;3(2):117–131. doi: 10.2165/00128071-200203020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Walker D.C., Cohen P.R. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet's syndrome. J. Am. Acad. Dermatol. 1996;34(5 Pt 2):918–923. doi: 10.1016/s0190-9622(96)90080-8. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Bussy S., Labarca G., Cabello F., Cabello H., Folch E., Majid A. Sweet's syndrome with pulmonary involvement: case report and literature review. Respir Med. Case Rep. 2012;6:16–19. doi: 10.1016/j.rmcr.2012.08.004. Published 2012 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrtens S.H., Hasan Z.U., Halpern S.M., et al. Sweet's syndrome with pulmonary involvement. BMJ Case Rep. CP. 2019;12 doi: 10.1136/bcr-2019-229997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astudillo L., Sailler L., Launay F., et al. Pulmonary involvement in Sweet's syndrome: a case report and review of the literature. Int. J. Dermatol. 2006;45(6):677–680. doi: 10.1111/j.1365-4632.2006.02585. [DOI] [PubMed] [Google Scholar]
- 14.Sweet Syndrome. NORD (National Organization for Rare Disorders) 2015. https://rarediseases.org/rare-diseases/sweet-syndrome/ October 15). Retrieved March 13, 2022, from. [Google Scholar]
- 15.Kazmi S.M., Pemmaraju N., Patel K.P., et al. Characteristics of Sweet Syndrome in patients with acute myeloid leukemia. Clin. Lymphoma, Myeloma & Leukemia. 2015;15(6):358–363. doi: 10.1016/j.clml.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varadarajan N., Boni A., Elder D.E., et al. FLT3 inhibitor-associated neutrophilic dermatoses. JAMA Dermatol. 2016;152(4):480–482. doi: 10.1001/jamadermatol.2015.6121. [DOI] [PubMed] [Google Scholar]
- 17.Stone R.M., Mandrekar S.J., Sanford B.L., et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur A., Jacobs M.S., Raza S. Characteristics of sweet's syndrome associated with novel acute myeloid leukemia targeted drugs-midostaurin and enasidenib. Adv. Cell Gene Ther. 2019;2(4) doi: 10.1002/acg2.61. [DOI] [Google Scholar]
- 19.Alkassis S., Rizwan A., Daoud L., Chi J. Midostaurin-induced Sweet syndrome in a patient with FLT3-ITD-positive AML. BMJ Case Rep. 2021;14(8) doi: 10.1136/bcr-2021-243615. Published 2021 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiewchanvit S., Jamjanya S., Rattanathammethee T., Mahanupab P., Tovanabutra N., Chuamanochan M. Bullous Sweet syndrome in a patient with acute myeloid leukemia treated with midostaurin: rapid response to acitretin and colchicine-A case report. Dermatol. Ther. 2021;34(6) doi: 10.1111/dth.15171. [DOI] [PubMed] [Google Scholar]
- 21.Yasin H., Laytem T., Sutamtewagul G., Ayyappan S. A rare case of midostaurin-associated sweet's syndrome. Case Rep. Hematol. 2022;2022 doi: 10.1155/2022/1099005. Published 2022 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]