Abstract
Purpose
Limited knowledge is available on the incidence of febrile neutropenia (FN) in intermediate-risk patients and the rationale for use of granulocyte colony-stimulating factor (G-CSF) in these patients. We aimed to estimate the rate at which patients associated with intermediate risk (10–20%) of FN would develop ≥ 1 episode of FN with a commonly used chemotherapy regimen in clinical practice.
Methods
This prospective, real-world, observational, multinational, multicenter study (December 2016–October 2019) recruited patients with solid tumors or Hodgkin’s/non-Hodgkin’s lymphoma. Patients receiving chemotherapy with intermediate risk of FN, but not G-CSF as primary prophylaxis were included and observed for the duration of the chemotherapy (≤ 6 cycles and ≤ 30 days after the last chemotherapy administration).
Results
In total, 364 patients (median age, 56 years) with 1601 cycles of chemotherapy were included in the analysis. The incidence of FN was 5% in cycle 1, 3% in cycles 2–3, and 1% in cycles 4–6. The rate of patients with ≥ 1 episode of FN was 9%, and 59% of FN events were reported during cycle 1. The rate of grade 4 neutropenia in cycle 1 was 11%, and 15% of patients experienced ≥ 1 episode of grade 4 neutropenia.
Conclusions
Overall, the incidence of FN was low, with a high incidence in cycle 1 and a decrease in the subsequent cycles. These results provide the real FN risk for common chemotherapy regimens in patients generally excluded from clinical trials. Prophylactic G-CSF in intermediate-risk patients could be considered as per clinician’s judgement.
Keywords: Chemotherapy, Neutropenia, Febrile Neutropenia, Risk
Introduction
Despite the advances in oncology and anti-cancer therapies such as immuno-oncology and targeted therapies, chemotherapeutic agents still play a pivotal role in the management of certain solid tumors and hematological malignancies [1]. Chemotherapy-related adverse events are high and are associated with substantial morbidity and mortality [2]. One of the most frequent side effects of cytotoxic agents is neutropenia, which increases the risk of infection [1]. Febrile neutropenia (FN) is the most serious manifestation of neutropenia and a key driver of chemotherapy dose delays and/or reductions. This modification in dose intensity may impact treatment efficacy, frequently leading to hospital admissions with significant mortality due to infection-related complications [1, 3]. Furthermore, FN is accompanied by high costs attributable to the increased use of antibiotics and unplanned hospitalizations [4]. During the COVID-19 pandemic, prevention and management of severe neutropenia and FN has become even more challenging due to oncology patients’ limited or remote access to health care. Major concerns during the pandemic include delayed or interrupted standard cancer care, risk of COVID-19 infection spread in healthcare facilities and among healthcare workers, a need to keep the number of face-to-face visits to the minimum possible, and implementation of optimal FN prevention strategies [5].
The incidence of neutropenia varies between 2 and 50% and is dependent on various factors such as patient-related risk factors, type of cancer, chemotherapy, and genetic susceptibility [3, 6, 7]. FN occurs in 13–21% of patients receiving standard myelosuppressive chemotherapy regimens for metastatic solid tumors, most frequently during the first cycle (23–36%) [8].
The therapeutic action of granulocyte colony-stimulating factors (G-CSFs) results from the production and activation of neutrophils, increasing their numbers and migration in the blood. They can be used as either primary or secondary prophylaxis to reduce the risk, severity, and duration of FN, or as an adjunct to support the delivery of dose-dense (increased frequency) or dose-intense (increased dose) myelosuppressive regimens [1, 3, 7]. The use of G-CSFs as primary prophylaxis for the prevention of FN has been shown to reduce the relative risk of FN by 46% on average across 15 studies analyzed in a systematic review, with infection-related mortality and early deaths decreasing by 45% and 40%, respectively [9].
The latest updates of the leading international guidelines formulated by the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), the Infectious Diseases Society of America (IDSA), the National Comprehensive Cancer Network (NCCN, consensus-based guidelines), the European Organization for Research and Treatment in Cancer (EORTC), and the Multinational Association for Supportive Care in Cancer (MASCC) recommend prophylactic treatment with G-CSF in patients receiving chemotherapy associated with a high risk of FN development (≥ 20%), as well as in patients receiving chemotherapy with an intermediate risk of FN development (10–20%) in the presence of defined risk factors, including age ≥ 65 years, poor performance status, and prior FN [3, 6, 7, 10–12].
Data on FN risk are usually derived from phase III trials with inclusion criteria that limit the participation of patients with a potentially higher risk of FN. Therefore, analysis of FN incidence based on data from stringently designed prospective clinical trials encompassing patients receiving intermediate-risk chemotherapy in the real-world oncology practice is a priority.
A previous publication has described the study design and methodology of this type of prospective international multicenter observational real-world study [13]. The present report discusses the incidence of chemotherapy-induced FN in patients with an intermediate risk of FN development.
Methods
Study endpoints
The study's primary objective was to assess the incidence of FN (≥ 1) in patients treated with available chemotherapy regimens, which, according to published guidelines, are expected to be associated with a 10–20% risk of FN. The primary endpoint was FN incidence, defined as an absolute neutrophil count of less than 0.5 IU or 500 cells/µL of blood and temperature ≥ 38.5 °C after the first cycle of a chemotherapy regimen that is expected to be associated with intermediate risk of FN and without G-CSF prophylaxis. Secondary outcome measures included the overall incidence of FN after all chemotherapy cycles, the incidence of complicated FN after each chemotherapy cycle, and rates of morbidities (diarrhea or oral mucositis) associated with each cycle that might increase the risk of infectious complications. Other secondary endpoints included: time to the first occurrence of FN, distribution of cycle number for the first episode of FN, and impact of risk of neutropenia.
Study design
This real-world, prospective (December 2016 to October 2019), observational, and multinational study recruited patients through a secure website. Patients were observed for the duration of the chemotherapy (≤ 6 cycles and ≤ 30 days after the last administration of chemotherapy) after they signed the informed consent forms. A total of 364 patients was enrolled from the following countries: Pakistan (n = 60), Lebanon (n = 13), Switzerland (n = 12), Belgium (n = 33), South Africa (n = 86), and Spain (n = 160).
The study initially intended to develop a risk model using the incidence of FN as a binary outcome, stratifying data by age, comorbidities, and other risk factors; however, the study was powered to have 1000 patients, but only 364 eligible patients were recruited [13].
Patients
Adult patients diagnosed with solid tumors or Hodgkin’s/non-Hodgkin’s lymphoma, with planned administration of an accepted and available chemotherapy regimen in adjuvant, neo-adjuvant, or metastatic settings during the accrual period, were included in the study. Accepted chemotherapy regimens had an expected intermediate risk of FN in the range of 10–20% as per published guidelines [14] and study protocol [13]. Patients included in the study did not have any planned administration of G-CSF as primary prophylaxis and had not previously received another chemotherapy line. Patients who had received FN prophylaxis (with antibiotics or available G-CSF), prior high-dose chemotherapy, or stem cell transplantation were excluded. Patients scheduled to receive other chemotherapy regimens than those listed as having a FN risk of 10–20% or patients with abnormal kidney (creatinine > 1.5 × upper limit of normal) and liver function (aspartate transaminase and alanine transaminase > 2 × upper limit of normal) were also excluded.
Patients were required to provide independent, ethics committee-approved informed consent as per national laws for participation in the study. Patients were also required to comply with blood sampling. On day 8 of the first chemotherapy cycle, a blood sample for hematological counts was taken at selected sites (optional), and on day 1 of subsequent chemotherapy cycles, routine blood samples for hematological counts were taken.
Statistical analysis
Baseline demographic variables including age, gender, and comorbidities were collected. Additional variables related to blood test parameters (neutrophils, lymphocytes, etc.) at baseline and during follow-up were also recorded/documented in a website specifically developed for the study. Comorbidities were grouped according to the Charlson comorbidity index (CCI) [15, 16] available online (https://www.mdcalc.com/charlson-comorbidity-index-cci). FN development was considered a binary outcome (ignoring the severity or risk). Descriptive statistics were used to tabulate patient characteristics. The incidence of neutropenia and FN were determined as proportions of the population.
Analyses of the data involved stratification by age (younger or older than 65 years) and examination of interactions between age, comorbidities, and other risk factors identified during the development of the logistic regression model. We then performed univariate (Chi2/Fisher’s exact) analyses to examine associations between patient risk factors and the prevalence of neutropenia and/or FN. Two-sided P-values of < 0.05 were considered statistically significant. Multivariate models included only variables that exhibited a univariate association with the dependent variable, that is, the prevalence of neutropenia (P < 0.05). The time to first event was estimated using the Kaplan–Meier plot and compared using the log-rank test. NCSS 2021 software for Windows (USA) was used for statistical analyses.
Results
Patient characteristics
A total of 364 patients was evaluable at seven centers in six countries; the median age of the patients was 56 years, and two-thirds were female (67%). Among the underlying cancers, breast cancer (53%) and colon cancer (32%) accounted for more than 80% of the cases. Notably, most of the patients received chemotherapy as part of neoadjuvant or adjuvant schemes; only about 29% of the patients had metastatic disease at baseline (Table 1).
Table 1.
Baseline characteristics
| Characteristics | N = 364 |
|---|---|
| Age (years), median (range) | 56 (17–81) |
| Female, n (%) | 246 (67%) |
| Underlying cancer, n (%) | |
| Breast cancer | 192 (52.8%) |
| Colorectal cancer | 117 (32.1%) |
| Non-Hodgkin’s lymphoma | 17 (4.7%) |
| Prostate cancer | 18 (5.0%) |
| Germ cell tumor | 9 (2.5%) |
| Non-small cell lung cancer | 6 (1.7%) |
| Gastric cancer | 3 (0.8%) |
| Esophageal cancer | 2 (0.5%) |
| Metastatic disease | 104 (28.6%) |
| Charlson score, n (%) | |
| Mild (1–2) | 200 (54.9%) |
| Moderate (3–4) | 64 (17.6%) |
| Severe (≥ 5) | 100 (27.5%) |
| Weight loss ≥ 10% | |
| Yes | 24 (6.7%) |
| No | 311 (85.4%) |
| Unknown | 29 (8%) |
Chemotherapy regimen modifications
The various chemotherapy regimens received by patients with a particular cancer have been previously described [13]. A total of 1601 chemotherapy cycles was administered to 364 patients. The median number of planned chemotherapy cycles was four, administered to 157 patients (43%), whereas 129 patients (36%) received six cycles of chemotherapy (Table 2). The majority of the patients (92%) completed their planned cycles of chemotherapy. Chemotherapy dose reductions and delays were observed in 78 (22%) and 148 (41%) patients, respectively. Of the total 1601 cycles, dose reductions and delays were observed in 173 (11%) and 222 (14%) cycles, respectively. The most common reasons underpinning the dose reductions and dose delays were hematological toxicity (29% and 35%), non-hematological toxicity (42% and 26%), and logistical reasons (0.02% and 27%, respectively).
Table 2.
Administration of chemotherapy data
| Number of cycles received | |
| Median | 4 cycles |
| Number of patients with 4 cycles, n (%) | 157 (43%) |
| Number of patients with 6 cycles, n (%) | 129 (36%) |
| No chemotherapy given, n | 1 patient |
| Reason for stopping follow-up, n (%) | |
| Chemotherapy complete | 336 (92%) |
| Lost to follow-up | 8 (2%) |
| Patient died | 6 (2%) |
| Other reason | 13 (4%) |
| Number of cycles with | |
| -Dose reduction | 173 (11%) |
| -Delay in chemotherapy administration | 222 (14%) |
| Number of patients with | |
| -Dose reduction | 78 (22%) |
| -Delay in chemotherapy administration | 148 (41%) |
In total, 104 cases of diarrhea and 105 mucositis incidents were observed, of which 14% and 6%, respectively, were ≥ grade 3.
Incidence rates of neutropenia and FN
Rates of FN were 5% in cycle 1, reduced by almost half in cycle 2 and 3 (3%, each), and 1% in cycles 4–6. The rate of patients with ≥ 1 episode of FN was 9% (95% CI: 6–12%). Site-wise incidence of FN was as follows: Aga Khan University (n = 22), Barcelona (n = 17), Bordet (n = 1), Rosebank (n = 1), and Orense (n = 1).
In total, 272 neutropenia episodes was observed. As shown in Table 3 and Fig. 1, the rates of all-grade neutropenia were similar: around 20% in the first three cycles (22%, 20%, and 19%, respectively); in cycles 4 and 5, the rates decreased to 12% and 15%, respectively; and a further decrease was observed in cycle 6 (7%). Except for cycle 5, an overall decrease was determined in the rates of all-grade neutropenia.
Table 3.
Rates of neutropenia and FN
| Neutropenia: | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 |
|---|---|---|---|---|---|---|
| N = 363 | N = 342 | N = 328 | N = 299 | N = 142 | N = 129 | |
| Grade 1 | 11 | 19 | 24 | 14 | 8 | 0 |
| Grade 2 | 12 | 19 | 14 | 10 | 7 | 4 |
| Grade 3 | 16 | 15 | 11 | 8 | 6 | 1 |
| Grade 4 | 38 | 15 | 12 | 3 | 1 | 4 |
| Rate of grade 4 neutropenia, 95% CI | 0.11 (0.08–0.14) | 0.04 (0.02–0.07) | 0.04 (0.02–0.06) | 0.01 (0.002–0.03) | 0.01 (< 0.01–0.04) | 0.03 (< 0.01–0.08) |
| Rate of all grades of neutropenia, 95% CI | 0.22 (0.17–0.26) | 0.20 (0.16–0.25) | 0.19 (0.15–0.24) | 0.12 (0.09–0.16) | 0.22 (0.10–0.23) | 0.07 (0.03–0.13) |
| Febrile neutropenia: | 19 | 10 | 9 | 2 | 1 | 1 |
| Rate of FN, 95% CI | 0.05 (0.03–0.08) | 0.03 (0.01–0.05) | 0.03 (0.01–0.05) | < 0.01 (< 0.01–0.02) | < 0.01 (< 0.01–0.04) | < 0.01 (< 0.01–0.04) |
CI confidence interval, FN febrile neutropenia
Fig. 1.
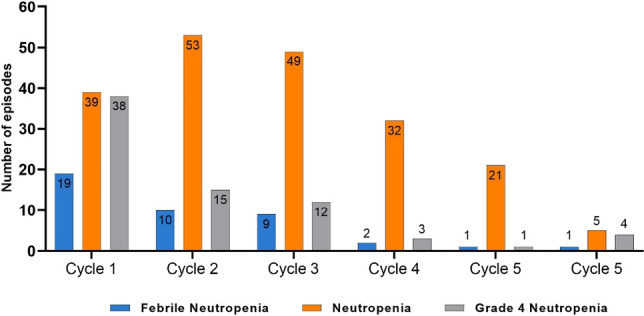
Total neutropenia and FN events per cycle
Rates of grade 1–4 neutropenia are presented in Table 3. Patients in cycle 1 experienced a relatively higher rate of grade 4 neutropenia (11%), which decreased to 4% in cycles 2 and 3, and 1% each in cycles 4 and 5, followed by an increase in cycle 6 (3%). Overall, the rates of neutropenia of the respective grades did not show considerable consistency (Fig. 1). In 56 patients, ≥ 1 grade 4 neutropenia occurred during follow-up. The rate of patients with ≥ 1 episode of grade 4 neutropenia was 15% (95% CI: 1–20%).
Of the 32 patients who developed FN, more than half of the reported episodes (59%) occurred during cycle 1. Similar findings were observed for time to neutropenia; 48% of episodes occurred during cycle 1 among the 158 patients who developed neutropenia. Percentages of development of FN and grade 4 neutropenia by cycle are presented in Table 4.
Table 4.
Percentages of grade 4 neutropenia and FN by cycle
| FN events (N = 32) | Grade 4 Neutropenia events | |
|---|---|---|
| Cycle 1 | 59% | 48% |
| Cycle 2 | 19% | 28% |
| Cycle 3 | 16% | 14% |
| Cycle 4 | 0% | 6% |
| Cycle 5 | 3% | 2% |
| Cycle 6 | 3% | 1% |
Subgroup analysis
The overall incidence of FN was 9.5%. The age group of ≥ 65 years showed 16% incidence of grade 4 neutropenia and 8% incidence of FN. In gender‑wise distribution, the occurrence of grade 4 neutropenia and FN was similar in males (17% and 10%) and females (15% and 8%, respectively). The patients diagnosed at metastatic stage showed a greater incidence of grade 4 neutropenia (14%) and FN (7%). Other subgroup analysis outcomes are presented in Table 5.
Table 5.
Frequency of grade 4 neutropenia and FN by patient characteristic
| Patient characteristic | Grade 4 neutropenia | Febrile neutropenia |
|---|---|---|
| Age ≥ 65 years | 14 (16%) | 7 (8%) |
| Weight loss ≥ 10% | 2 (8%) | 1 (4%) |
| Metastatic disease | 15 (14%) | 7 (7%) |
| Charlson score – mild & moderate | 42 (16%) | 22 (8%) |
| Charlson score – severe | 15 (15%) | 10 (10%) |
| Gender – male | 20 (17%) | 12 (10%) |
| Gender – female | 37 (15%) | 20 (8%) |
| Creatinine (mg/dL) | 0.5 | 0.79 |
Discussion
Although current chemotherapy regimens for solid tumors are well tolerated in most tumor types, they are sometimes associated with severe and life-threatening events such as FN [4]. To our knowledge, our study is the first prospective, real-world, observational multinational study that evaluated the incidence of FN in different cycles of chemotherapy in patients with intermediate-risk FN and analyzed the associated dose reduction and treatment delays in chemotherapy in different types of cancers. The study excluded patients receiving G-CSF as primary prophylaxis.
Investigator-reported incidence of FN varies considerably for the same chemotherapy regimen. The results of this study demonstrated different outcomes from different sites/countries, with The Aga Khan University and Barcelona representing 58% of the cohort and reporting 93% of the FN incidents, whereas Rosebank and Bordet representing 33% of the cohort and reporting only 5% of the FN incidents. This difference could be attributed to patient selection, different patient characteristics, different chemotherapy regimens, or differential use of G-CSF for prophylaxis after the second chemotherapy cycle.
Evidence suggests that FN rates in the real-world setting may be higher than those reported in randomized controlled trials (RCTs), a possible reason being the stringent eligibility criteria for entry into RCTs. In a systematic review and meta-analysis of 65 observational (n = 7812 patients) and 110 RCT (n = 42,257 patients) cohorts involving 29 breast cancer chemotherapy regimens, the unadjusted FN rate was 11.7% and 7.9% in the observational and RCT cohorts, respectively [17]. The present study reflected this number, with an FN rate of 11.6%. Our data suggested that FN incidence is similar to the FN rates of real-world data and other RCTs; however, our study might have been biased due to the exclusion of patients receiving G-CSF as primary prophylaxis.
This is a real-world study, as opposed to G-CSF Phase III clinical trials, in which clinical and laboratory parameters are recorded frequently. In real-world studies, these parameters are recorded as frequently. The main aim of this research was to establish real-world incidence encompassing the world's various regions. The study design and the frequency of the investigations were done to resemble clinical practice as closely as possible in many settings worldwide. Frequent testing might indeed overestimate the incidence of FN, resulting in overtreatment. Additionally, this approach might decrease quality of life and increase costs.
The rate of patients with ≥ 1 episode of FN was 9% in the present study. This rate was higher than results observed in a previously published RCT [18] and a real-world study [19]. In the RCT, the overall proportion of patients having ≥ 1 occurrence of FN was 5.8% for the 7/10-day group [18]. Similar results were observed in the real-world study in which the rate of patients who developed ≥ 1 episode of FN was 6.1% [19]. The higher number of chemotherapy cycles administered in the present study (n = 1601), compared to the RCT (n = 1302) and the real-word study (n = 912), might be a possible reason for this difference. Other reasons could be related to patient heterogeneity, type of regimen used, different inclusion criteria, different tumor types, or lack of control for dose reductions and dose delays.
In the present analysis, the rate of episodes of FN in each cycle varied; 5% were encountered in cycle 1, followed by 3% and < 1% in cycles 2 and 6, respectively. A similar pattern, but a higher rate of FN, was noted in an observational study; of 19 patients, the first (36.36%) and second (22.72%) cycles of chemotherapy exhibited a higher incidence of FN cases than other chemotherapy cycles [20]. Culakova et al. reported 9.7% incidence of FN in cycle 1, followed by 5.7% and 3.8% in cycles 2 and 3, respectively [21]. A decrease in neutropenic events was observed in subsequent cycles because of clinical interventions such as reductions in chemotherapy dose intensity and/or additional use of supportive care measures (G-CSF prophylaxis or antibiotics) [21]. Reduced relative dose intensity was more common for older patients, those with poor performance status, and obese patients [21]. The present study results correlate with those of previous studies in that the incidence was higher in cycles 1 and 2, but varied in the cycles that followed [22].
Currently, chemotherapy-induced myelosuppression, which reduces chemotherapy dose intensity and potentially limits therapeutic efficacy, is managed with chemotherapy dose delays/reductions and lineage-specific supportive care interventions [23]. The present analysis observed dose reductions and delays in 78 (22%) and 148 (41%) patients, respectively. These results were in line with previously published data. Results from one RCT [18] showed that the overall proportion of patients having ≥ 1 occurrence of either chemotherapy dose delay or reduction or discontinuation was 37.64% and 39.42% for the 5- and 7/10-day groups, respectively [18]. In a retrospective cohort study, considerable variability in dose delay of ≥ 7 days was seen among cancer types and among chemotherapy regimens, which varied from 9.3% to 16.3% for different cancer types [24].
In this study, a high incidence of treatment dose reductions and dose delays, including patients receiving adjuvant treatment, were recorded. It should be noted that this observational study did not have specific recommendations regarding dose modifications; therefore, these reductions and delays reflect clinicians' and patients' preferences.
In the setting of the COVID-19 pandemic, where clinicians aim to minimize patients’ risk of potential infection and need for hospital visits, the prophylaxis and management of FN is of particular importance. As FN can result in the need for hospitalization, ESMO recommends that physicians consider using regimens with a lower risk of FN in patients who are not being treated with curative intent [25]. ESMO [25] and NCCN [26] also suggest expanding the indication for G-CSF after chemotherapy to lower the risk of FN but acknowledge that this may require additional outpatient visits. Similar to the NCCN and ESMO recommendations, the American Society of Clinical Oncology (ASCO) [27] recognizes that during the COVID-19 pandemic, it may be reasonable for patients at lower risk of FN to be prescribed growth factors [27]. Furthermore, with wider availability of biosimilars for G-CSF, the cost-effectiveness of prophylactic treatment in intermediate-risk patients may be a rationale for consideration.
In the current study, we were unable to develop a risk model for FN in this set of chemotherapy regimens with an expected FN rate of 10–19% or identify a particular set of patients at higher risk of FN owing to slow recruitment and low patient numbers. The primary aim of the current study was to develop a model for predicting FN in oncology patients receiving chemotherapy with intermediate risk (10–20% risk) involving 1000 patients in 20 centers worldwide. This recruitment was not achieved; however, we report a secondary endpoint of the study describing the incidence of FN in 364 patients recruited.
The heterogeneity of the patient population in terms of tumor type and the wide range of chemotherapy agents made the interpretation of risk factors difficult. Further research on patient populations with similar tumor types and chemotherapy schedules may help stratify the patients by age and comorbidities. With wider use of mixed anticancer regimens combining chemoimmunotherapy with targeted therapies for many cancer types, the incidence of FN may be further reduced. Prophylactic G-CSF in intermediate-risk patients may be considered according to the clinician’s judgement. A quality-of-life component was also recorded and will be reported separately.
The study limitations are mainly the lack of data about anticancer treatments including concomitant chemo-immuno therapies (current regimens for non-small cell lung cancer and other cancers) and COVID-19 infection because of the study period. Other limitations include lack of data related to financial status or healthcare system, G-CSF coverage, and unavailability of data about patient preferences and specific education of patients regarding FN at each participating center.
In conclusion, in patients receiving chemotherapy regimens with an intermediate risk of FN, the rate of FN was on par with other RCTs and real-world studies. The rates were high in cycles 1 and 2 but varied in the subsequent cycles. This large observational, real-world study found that cancer patients with intermediate-risk of FN (10–20%) can be treated prophylactically with G-CSF in alignment with the current guidelines. Overall, this prospective study provides insights and real-world evidence to support the implementation of the current guidelines in daily practice.
Acknowledgements
We thank patients and their families, investigators, and staff from all the participating sites. We thank Saurabh Agarwal, PhD, and Kavita Garg, PhD, CMPP™, of Novartis Healthcare Pvt. Ltd. for providing medical writing assistance with this manuscript.
Author’s contribution
TS, LH, DM, JL, NF, MO, MI, and KT have contributed to the collection and compilation of data. BLR DM and MO have contributed to conception and design. BLR, TS, DM, JD, MO, MI, and BLR, TS, RA, KT have contributed to data analysis and interpretation, and DM, NF, JD, JL, MO, MI, and KT have contributed to manuscript writing. All authors have critically reviewed and approved final manuscript for submission.
Funding
Open access funding provided by University of Pretoria. This work was supported by research funding from Novartis Pharma AG, Basel, Switzerland.
Data availability
Novartis supports the publication of scientifically rigorous analysis that is relevant to patient care, regardless of a positive or negative outcome. Qualified external researchers can request access to anonymized patient-level data, respecting patient-informed consent, through www.clinicalstudydatarequest.com, according to requirements noted on the web portal.
Declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol and all amendments were reviewed by the independent ethics committee or institutional review board for each center. The ethics approval was obtained from the Ethics Review Committee of The Aga Khan University (Pakistan; Reference number: 2019–0827-2451), Hotel-Dieu de France Ethics Committee of the Université Saint-Joseph de Beyrouth (Lebanon; Reference number: CEHDF 1038), Ethics Committee Northwest and Central Switzerland (EKNZ, Switzerland; Reference number: 2018–01603), Ethics Committee of Institute Jules Bordet (Belgium, Reference number: B079201628399), Pharma-Ethics, an Independent Research Ethics Committee (South Africa, Reference number: 161115075), and Clinical Research Ethics Committee of the Hospital Clinic de Barcelona (Spain; Reference number: HCB/2017/0661). Informed consent was obtained from each patient in writing before enrolment in the study.
Consent for publication
All authors give consent for the publication of the manuscript in the Journal, “Supportive Care in Cancer”.
Competing interests
BLR reports a research grant, personal fees, advisory boards, speaker engagements from Novartis and Sandoz, and personal fees and speaker engagements from Amgen South Africa, during the conduct of the study. MGM, CF, ZS, AAJ, HRK, FE, RAP, JG, LH, TS, TC, RA and JK have no relevant financial or non-financial interests to disclose. AK was a part of a study that received research grant from Sanofi. AK and NM are current or previous employees of Sandoz/Hexal AG.
Footnotes
Andriy Krendyukov is a former employee of Sandoz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tralongo AC, Antonuzzo A, Pronzato P, Sbrana A, Turrini M, Zoratto F, Danova M. Management of chemotherapy-induced neutropenia in patients with cancer: 2019 guidelines of the Italian Medical Oncology Association (AIOM) Tumori Journal. 2020;106(4):273–280. doi: 10.1177/0300891620927093. [DOI] [PubMed] [Google Scholar]
- 2.Kuderer NM, Desai A, Lustberg MB, Lyman GH (2022) Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat Rev Clin Oncol 19(11):681-697. 10.1038/s41571-022-00685-3 [DOI] [PubMed]
- 3.Klastersky J, De Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, Herrstedt J. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol. 2016;27:v111–v118. doi: 10.1093/annonc/mdw325. [DOI] [PubMed] [Google Scholar]
- 4.Kasi PM, Grothey A. Chemotherapy-induced neutropenia as a prognostic and predictive marker of outcomes in solid-tumor patients. Drugs. 2018;78(7):737–745. doi: 10.1007/s40265-018-0909-3. [DOI] [PubMed] [Google Scholar]
- 5.Cooksley T, Font C, Scotte F, Escalante C, Johnson L, Anderson R, Rapoport B. Emerging challenges in the evaluation of fever in cancer patients at risk of febrile neutropenia in the era of COVID-19: a MASCC position paper. Support Care Cancer. 2021;29(2):1129–1138. doi: 10.1007/s00520-020-05906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BL R Febrile Neutropenia - Guideline Update and Approach to Management of Both High and Intermediate-Risk Patients. https://mascc.org/wp-content/uploads/2022/04/1114_Rapoport_Strauss-1-2_Sat.pdf. Accessed November 23 2022
- 7.NCCN Clinical Practice Guidelines in Oncology (2022) Prevention and Treatment of Cancer-Related Infections, version 1. 2022. National Cancer Comprehensive Network website. https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf. Accessed November 23 2022 [DOI] [PubMed]
- 8.Weycker D, Li X, Edelsberg J, Barron R, Kartashov A, Xu H, Lyman GH. Risk and consequences of chemotherapy-induced febrile neutropenia in patients with metastatic solid tumors. J Oncol Pract. 2015;11(1):47–54. doi: 10.1200/JOP.2014.001492. [DOI] [PubMed] [Google Scholar]
- 9.Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25(21):3158–67. 10.1200/JCO.2006.08.8823 [DOI] [PubMed]
- 10.Aapro M, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman G, Pettengell R, Tjan-Heijnen V, Walewski J. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young J-AH, Wingard JR. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 12.Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 13.Rapoport BL, Aapro M, Paesmans M, Van Eeden R, Smit T, Krendyukov A, Klastersky J (2018) Febrile neutropenia (FN) occurrence outside of clinical trials: occurrence and predictive factors in adult patients treated with chemotherapy and an expected moderate FN risk. Rationale and design of a real-world prospective, observational, multinational study. BMC Cancer 18 (1):1–8 [DOI] [PMC free article] [PubMed]
- 14.Li Y, Family L, Yang S-J, Klippel Z, Page JH, Chao C. Risk of febrile neutropenia associated with select myelosuppressive chemotherapy regimens in a large community-based oncology practice. J Natl Compr Canc Netw. 2017;15(9):1122–1130. doi: 10.6004/jnccn.2017.7010. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J-M, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 17.Truong J, Lee EK, Trudeau ME, Chan KK. Interpreting febrile neutropenia rates from randomized, controlled trials for consideration of primary prophylaxis in the real world: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):608–618. doi: 10.1093/annonc/mdv619. [DOI] [PubMed] [Google Scholar]
- 18.Clemons M, Fergusson D, Simos D, Mates M, Robinson A, Califaretti N, Zibdawi L, Bahl M, Raphael J, Ibrahim MFK, Fernandes R, Pitre L, Aseyev O, Stober C, Vandermeer L, Saunders D, Hutton B, Mallick R, Pond GR, Awan A, Hilton J. A multicentre, randomised trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy-induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951–957. doi: 10.1016/j.annonc.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Palukuri NR, Yedla RP, Bala SC, Kuruva SP, Chennamaneni R, Konatam ML, Gundeti S. Incidence of febrile neutropenia with commonly used chemotherapy regimen in localized breast cancer. South Asian J Cancer. 2020;9(1):4–6. doi: 10.4103/sajc.sajc_439_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philip MLSN, Sebastian MA, Mateti UV, et al. Assessment of chemotherapy-induced febrile neutropenia in cancer patients. IJMPO. 2019;40(2):249–256. doi: 10.4103/ijmpo.ijmpo_31_18. [DOI] [Google Scholar]
- 21.Culakova E, Poniewierski MS, Wolff DA, Dale DC, Crawford J, Lyman GH. The impact of chemotherapy dose intensity and supportive care on the risk of febrile neutropenia in patients with early stage breast cancer: a prospective cohort study. Springerplus. 2015;4:396. doi: 10.1186/s40064-015-1165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosmer W, Malin J, Wong M. Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support Care Cancer. 2011;19(3):333–341. doi: 10.1007/s00520-010-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyman GH, Kuderer NM, Aapro M. Improving outcomes of chemotherapy: established and novel options for myeloprotection in the COVID-19 era. Front Oncol. 2021;11:697908. doi: 10.3389/fonc.2021.697908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawatkar AA, Farias AJ, Chao C, Chen W, Barron R, Vogl FD, Chandler DB. Hospitalizations, outcomes, and management costs of febrile neutropenia in patients from a managed care population. Support Care Cancer. 2017;25(9):2787–2795. doi: 10.1007/s00520-017-3692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Society for Medical Oncology (2020) Supportive Care Strategies During the COVID-19 Pandemic. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/supportivecare-in-the-covid-19-era. Accessed November 23 2022
- 26.Griffiths EA, Alwan LM, Bachiashvili K, Brown A, Cool R, Curtin P, Geyer MB, Gojo I, Kallam A, Kidwai WZ, Kloth DD, Kraut EH, Lyman GH, Mukherjee S, Perez LE, Rosovsky RP, Roy V, Rugo HS, Vasu S, Wadleigh M, Westervelt P, Becker PS (2020) Considerations for use of hematopoietic growth factors in patients with cancer related to the COVID-19 pandemic. J Natl Compr Canc Netw 1–4. 10.6004/jnccn.2020.7610 [DOI] [PMC free article] [PubMed]
- 27.American Society of Clinical Oncology (2020) COVID-19 Patient Care Information. Cancer Treatment & Supportive Care. https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during-covid-19/cancer-treatment-supportive-care. Accessed November 23 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Novartis supports the publication of scientifically rigorous analysis that is relevant to patient care, regardless of a positive or negative outcome. Qualified external researchers can request access to anonymized patient-level data, respecting patient-informed consent, through www.clinicalstudydatarequest.com, according to requirements noted on the web portal.


