Abstract
Background and Objective
Since 2016, new therapies have transformed the standard of care for lung cancer, creating a need for up-to-date evidence for health economic modelling. We developed a discrete event simulation of advanced lung cancer treatment to provide estimates of survival outcomes and healthcare costs in the Australian setting that can be updated as new therapies are introduced.
Methods
Treatment for advanced lung cancer was modelled under a clinician-specified treatment algorithm for Australia in 2022. Prevalence of lung cancer subpopulations was extracted from cBioPortal and the Sax Institute’s 45 and Up Study, a large prospective cohort linked to cancer registrations. All costs were from the health system perspective for the year 2020. Pharmaceutical and molecular diagnostic costs were obtained from public reimbursement fees, while other healthcare costs were obtained from health system costs in the 45 and Up Study. Treatment efficacy was obtained from clinical trials and observational study data. Costs and survival were modelled over a 10-year horizon. Uncertainty intervals were generated with probabilistic sensitivity analyses. Overall survival predictions were validated against real-world studies.
Results
Under the 2022 treatment algorithm, estimated mean survival and costs for advanced lung cancer 10 years post-diagnosis were 16.4 months (95% uncertainty interval [UI]: 14.7–18.1) and AU$116,069 (95% UI: $107,378–$124,933). Survival and costs were higher assuming optimal treatment utilisation rates (20.5 months, 95% UI: 19.1–22.5; $154,299, 95% UI: $146,499–$161,591). The model performed well in validation, with good agreement between predicted and observed survival in real-world studies.
Conclusions
Survival improvements for advanced lung cancer have been accompanied by growing treatment costs. The estimates reported here can be used for budget planning and economic evaluations of interventions across the spectrum of cancer control.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-023-01291-6.
Key Points for Decision Makers
| In recent years, lung cancer treatment has been transformed with the arrival of novel systemic therapies. As a result, existing data on healthcare costs and survival outcomes may no longer be relevant, creating difficulties for health economic evaluations. |
| We developed and validated a discrete event simulation of lung cancer treatment; and found that new treatments have led to improvements in short-term overall survival with accompanying increases in healthcare costs. |
| Simulation modelling can be used to address data gaps created by evolving standards in oncology, enabling timely decision making on health policy. |
Introduction
Health economic evaluations used to inform public health investments are often limited by the availability of data inputs. Clinical data on the benefits of new interventions are typically available at the time that evaluations are undertaken. However, cost data are generally scarce, largely because they are a function of clinical practices, resource utilisation and health system funding schemes that are highly idiosyncratic. Consequently, estimates of absolute costs are rarely transferrable across countries, clinical populations or time periods.
For lung cancer, up-to-date costs data are especially scarce owing to recent developments in treatment. Since 2016, the therapeutic landscape for advanced lung cancer has changed dramatically with the arrival of immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) targeting tumours with alterations in the epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) genes, among others. These offer improved survival outcomes over chemotherapy and are widely subsidised in many health systems. In Australia, all existing studies reporting on the costs associated with advanced lung cancer predate the arrival of these treatments and do not reflect current practice [1–4]. Moreover, even studies that report outcomes in recently diagnosed cohorts may be of limited use in economic evaluations, which often require assumptions to be made about costs over the spectrum of potential survival times. Thus, data that are necessarily truncated due to limited follow-up can pose challenges.
The absence of data relating to treatment outcomes is a limiting factor when modelling preventive interventions for which the rationale is often to avert the poorer survival and steeper costs associated with advanced disease. For example, a national lung cancer screening programme was recently recommended in Australia [5]. However, because of the scale of this investment, the final implementation of the programme will depend heavily on its budgetary consequences, which can only be determined with up-to-date healthcare costs data.
As lung cancer is the leading cause of cancer death [6], it is imperative that researchers can reliably address questions of budget impact and cost effectiveness for new interventions even in the absence of mature data. To this end, simulation modelling plays an important role. Contemporary treatment algorithms can be combined with clinical trial data and drug prices to provide up-to-date estimates of survival and costs. For example, the iTEN model simulated non-small cell lung cancer (NSCLC) treatment under the Canadian treatment algorithm for 2018 [7].
The aim of this study was to develop and validate a discrete event simulation of advanced lung cancer treatment and survival for the Australian setting, ‘txSim’. Modelled outputs include per-patient survival time and costs for advanced NSCLC, extensive-stage small cell lung cancer (ES-SCLC), and other-specified or not-otherwise-specified (Other/NOS) lung cancer with Australian standards of care in 2022.
Materials and Methods
Model Structure
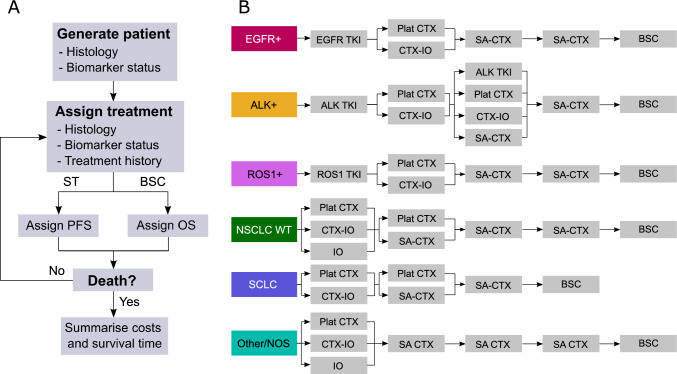
The txSim model is a discrete event simulation of advanced lung cancer treatment and survival. Each individual in the model was generated with an indication detailing their tumour histology and biomarker status (Fig. 1A) and treatment was simulated according to treatment patterns specifying the probability of each line of therapy and the distribution of drugs prescribed in those lines of therapy. Individuals who do not receive treatment are assigned best supportive care (BSC) only. The model estimated costs from a health system perspective, capturing treatments listed on the Schedule of Pharmaceutical Benefits or recommended for public subsidisation by the Pharmaceutical Benefits Advisory Committee. Individuals in the model were not prescribed multiple treatments of the same class (e.g. ICIs after prior immunotherapy) unless explicitly allowed (e.g. rechallenge).
Fig. 1.
Simplified structure of the txSim model (A) and simplified treatment algorithm for advanced lung cancer in 2022 (B). ALK anaplastic lymphoma kinase, EGFR epidermal growth factor receptor, BSC best supportive care, CTX-IO chemo-immunotherapy, IO immunotherapy, NSCLC WT wild-type non-small cell lung cancer, OS overall survival, Other/NOS other-specified and not-otherwise-specified lung cancer, PFS progression-free survival, Plat CTX platinum chemotherapy, ROS1 c-ros oncogene 1, SA-CTX single-agent chemotherapy, SCLC small cell lung cancer, ST systemic therapy, TKI tyrosine kinase inhibitor
We assumed that overall survival (OS) was equal to the sum of the progression-free survival (PFS) time for every drug received and the OS time associated with BSC, which were drawn from parametric distributions estimated from relevant data in clinical trials or observational studies. As PFS includes both tumour progression events and death, we assumed that 10% of progression events were deaths, roughly matching observations reported by Kocher et al. [8]. All other deaths occurred at the end of BSC. Treatment costs within each line of therapy were counted up to disease progression or the end of the treatment course. The model produced OS and cost estimates for 50,000 individuals, which were averaged. The model was programmed in R version 4.2.0 [9].
Datasets
Data on the distribution of lung cancer histological subtypes and healthcare costs were derived from the Sax Institute’s 45 and Up Study. The 45 and Up Study is a longitudinal cohort study of 267,357 residents in New South Wales (NSW) Australia aged ≥ 45 years (recruited 2005–2009) [10]. Participants were recruited from the Services Australia Medicare enrolment database by a postal questionnaire with a response rate of ~ 19% (~ 11% of the NSW population aged ≥ 45 years). Individuals aged ≥ 80 years were oversampled by a factor of two and all individuals living in regional and remote areas were invited. Participants gave consent for their questionnaire data to be linked to health records from the NSW Cancer Registry (January 1994–December 2013), Admitted Patient Data Collection (July 2004–June 2016), Emergency Department Data Collection (January 2005–June 2017), Registry of Births Deaths and Marriages (January 2006–June 2016), Pharmaceutical Benefits Scheme (June 2004–June 2016) and Medicare Benefits Schedule (June 2004–June 2016). Probabilistic linkage with the NSW Cancer Registry, Admitted Patient Data Collection, Emergency Department Data Collection and Registry of Births Deaths and Marriages records was performed by the Centre for Health Record Linkage [11]. The Centre for Health Record Linkage used a best-practice approach in privacy-preserving record linkage [12] along with the open-source probabilistic record linkage software Choice Maker [13]. The probabilistic matching process is highly accurate (false-positive and false-negative rates < 0.4%); a detailed explanation of the process has been published elsewhere [14]. Medicare Benefits Schedule and Pharmaceutical Benefits Scheme records were provided by Services Australia, with deterministic linkage performed using a unique identifier supplied to the Sax Institute. Ethics approval for the 45 and Up Study as a whole was provided by the University of NSW Human Research Ethics Committee and for this analysis by the NSW Population and Health Services Research Ethics Committee.
Data on the prevalence of molecular biomarkers for lung adenocarcinoma were derived from cBioPortal [15, 16], an open access repository of cancer biology studies for which participating patients provided informed consent. Data from four study populations in cBioPortal were pooled: the Memorial Sloan Kettering Cancer Centre’s MSK-IMPACT cohort [17], the Cancer Genome Atlas [18, 19], TRAcking Non-small Cell Lung Cancer Evolution Through Therapy (TRACERx) study [20], and the Clinical Proteomic Tumour Analysis Consortium [21]. Additional details are available in the Electronic Supplementary Material (ESM).
Model Inputs
Lung Cancer Subpopulations
Individuals with advanced lung cancer were assigned histological subtypes (non-squamous cell carcinoma [NSCC], squamous cell carcinoma [SCC], ES-SCLC, and Other/NOS disease) based on proportions estimated in the 45 and Up Study (see ESM).
Non-squamous cell carcinoma was further subdivided into patients with actionable EGFR mutations, ALK fusions and ROS1 fusions. Patients with SCC and patients with NSCC with EGFR-, ALK- or ROS1-wild type (WT) tumours could have high or low programmed death ligand-1 (PD-L1) expression (defined as a PD-L1 tumour proportion score ≥ 50% or < 50%). The prevalence of high PD-L1 expression for patients with SCC and EGFR-, ALK-, and ROS1-WT NSCC were sourced from the global EXPRESS study [22]. The prevalence of EGFR mutations, ALK fusions and ROS1 fusions in NSCC was derived from cBioPortal [15, 16].
Survival Inputs
The simulation scheduled progression events and death over multiple lines of therapy. Event times were randomly drawn from parametric distributions estimated from published Kaplan–Meier curves from relevant clinical trials and observational studies [23–53]. While inputs were selected to be indication specific (reflecting the appropriate population, drug and line of therapy), substitutes were used where data were limited. We assumed that single-agent chemotherapy regimens used for second-line NSCLC treatment had similar efficacy to one another and were represented by PFS outcomes in patients receiving docetaxel and pemetrexed [41]. We also assumed that PFS with second-line chemotherapy for ES-SCLC (other than platinum-etoposide rechallenge) was equivalent to PFS observed with topotecan [40].
Individual-level time-to-event data were reconstructed from Kaplan–Meier curves using the algorithm by Guyot et al. [54] as implemented in the IPDfromKM package for R [55]. Common parametric distributions (exponential, lognormal, Weibull) were fitted to individual patient data with the fitdistrplus package [56]. Distributions were selected by visual inspection and the Akaike Information Criterion. Selected distributions are provided in Table 4 of the ESM.
Time-to-event outcomes for selected classes of treatment were correlated across lines of therapy. This was implemented by means of a copula where PFS times fell at the same percentile for all (correlated) treatments received. Thus, an individual who achieved the median (50th percentile) PFS of their first-line therapy would achieve the median PFS for their second-line therapy. See ESM for further details.
Treatment Patterns
The 2022 treatment algorithm was obtained from three practicing medical oncologists from metropolitan sites in Australia (Brisbane/Sydney) and is provided in simplified form in Fig. 1B. Briefly, the algorithm assumed that patients with WT NSCLC received combined chemo-immunotherapy, immunotherapy or chemotherapy alone. Patients with ES-SCLC received chemo-immunotherapy or chemotherapy alone. Patients with actionable genetic alterations (EGFR, ALK, ROS1) received targeted therapies followed by platinum-based chemotherapy (with or without atezolizumab and bevacizumab), then single-agent chemotherapy. Other/NOS, a heterogeneous category, could receive chemo-immunotherapy, immunotherapy or chemotherapy alone. See ESM for details.
We applied systemic therapy utilisation rates from the 45 and Up Study reported in a previous publication [57]. For patients with NSCLC, the probabilities of first-, second- and third-line therapy were 51%, 58% and 40% conditional on receiving the preceding line of therapy. The corresponding probabilities for patients with ES-SCLC were 79%, 40% and 25%. In patients with Other/NOS disease, first- and second-line treatment probabilities were 31% and 28%, while the proportion receiving third-line therapy was assumed to be 50%. The proportion receiving fourth-line therapy across all groups was also assumed to be 50%. Given the significant attrition occurring after second-line therapy, assumptions regarding the uptake of treatment in later lines of therapy had little impact on model outputs.
Costs
Molecular testing costs (obtained from the Medicare Benefits Schedule) were only applied to patients with NSCLC who received treatment on the grounds that molecular diagnostics are only relevant when treatment is intended. We assumed that all patients with NSCC received EGFR mutation tests, ALK immunohistochemistry (IHC), ROS1 IHC and PD-L1 IHC; while patients with SCC received PD-L1 IHC. Further, patients who test positive with ALK or ROS1 IHC were assumed to receive fluorescent in situ hybridisation tests to confirm gene rearrangement (ESM).
Monthly treatment costs were calculated based on protocols described on eviQ, an online resource for Australian treatment protocols in oncology [58]. Drug costs were obtained from reimbursement amounts in the June 2022 Schedule of Pharmaceutical Benefits. In the main analysis, immunotherapy was administered for a maximum of 24 months. See ESM for details.
We estimated healthcare expenditure not related to systemic therapy (henceforth “background” costs) from participants in the 45 and Up Study diagnosed with incident lung cancer using our previously published methods [4]. Specifically, we quantified excess healthcare costs for participants diagnosed with lung cancer over a 3-year period or until death, compared to matched controls, with all costs directly relating to anti-cancer systemic therapy excluded. Costs were divided into an initial phase, continuing care phase and terminal phase, where expenses in the initial and continuing care phases were summarised as monthly costs while expenses in the terminal phase were modelled as a function of time to death. See ESM for details.
Model Validation
The model was co-developed with a medical oncologist (DK) and, in this way, underwent both conceptual validation and data validation per AdViSHE recommendations [59]. Computerised model validation was performed through model verification, the results of which are provided in the ESM.
For the operational validation of txSim, we simulated treatment patterns reported in published observational studies and compared predicted and observed survival outcomes. We reproduced studies of the overall NSCLC population [8, 60–63], patients with ES-SCLC [64, 65], patients with EGFR-mutated NSCLC receiving TKIs [66, 67], patients with ALK-rearranged NSCLC receiving TKIs [68, 69] and patients with NSCLC receiving immunotherapy [70]. For each of the studies selected, treatment was simulated to the last reported line of therapy, after which observations were censored. Survival predictions for the validation scenarios were then calculated using the Kaplan–Meier method. To adequately reproduce the treatment patterns observed in each cohort, observational studies were restricted to those that had a minimum follow-up of 18 months or reported the number of patients alive and continuing on each line of therapy at the end of the follow-up. See ESM for details.
We assessed model accuracy by comparing simulated and observed survival probabilities and restricted mean survival times (RMSTs) for each observational study available. Observed survival probabilities and RMSTs were estimated using either reconstructed individual patient data or, where individual patient data could not be accurately approximated [8, 65], the area under the survival curve (if available). Agreement between predicted and observed RMSTs was then assessed using the Bland–Altman analysis. Additionally, we estimated mean absolute error and mean absolute percentage error (MAPE) of survival probabilities at 6-month intervals within subgroups of interest.
Model Outputs and Sensitivity Analyses
The main outputs of this model were mean costs (expressed in 2020 Australian dollars) and mean survival time (in months) at 10 years post-diagnosis. Survival probabilities at 1, 2, 5 and 10 years were also reported.
A probabilistic sensitivity analysis was performed to capture the effect of parameter uncertainty. Model parameters were randomly drawn from their respective distributions (Table 1) over 500 simulations, each containing 20,000 patients. We estimated uncertainty intervals (representing the 2.5th and 97.5th percentiles) for mean per-patient costs and survival time at 10 years across these simulations. Beta distributions were specified for binary probabilities while Dirichlet distributions were used for parameters with multiple mutually exclusive categories (histology, biomarker status, treatment options). Parameters for time-to-event distributions were drawn from multivariate normal distributions. Uncertainty regarding monthly healthcare costs in the initial and continuing care phases were represented with gamma distributions, while parameters for the terminal phase cost function were drawn from a multivariate normal distribution. The model’s predictions and corresponding uncertainty intervals were plotted against the number of probabilistic sensitivity analysis iterations (from 1 to 500) to assess the plausibility of convergence (ESM).
Table 1.
Summary of parameter inputs for the txSim model and their respective data sources
| Model inputs | PSA distribution | Data sources |
|---|---|---|
| Histological subtype | Dirichlet | 45 and Up Study (See Electronic Supplementary Material [ESM] Section 1.1) |
| Biomarker prevalence | ||
| NSCC biomarkers | Dirichlet | TCGA [18, 19], TRACERx [20], MSK-IMPACT cohort [17], CPTAC [21], and EXPRESS [22]. See ESM Section 1.2. |
| PD-L1 expression for SCC | Beta | EXPRESS [22] |
| Survival | ||
| Progression-free survival | Multivariate normal | Clinical trial and observational data [25–53] reconstructed from Kaplan–Meier curves. See ESM Section 2.1. |
| Overall survival for best supportive care | Multivariate normal | Observational study data [23, 24], reconstructed from Kaplan-Meier curves. See ESM Section 2.1. |
| Death on progression | None | Assumed to be 10%, matching observations by Kocher et al. 2015 [8] |
| Treatment patterns | ||
| Real-world systemic therapy rates | Beta | 45 and Up Study [57]. See ESM Section 3.2. |
| Optimal systemic therapy ratesb | None | Jacob et al. 2010 [71] |
| Drug-specific probabilities | None | Elicited from three clinicians. See ESM Section 3.1. |
| Costs | ||
| Tumour diagnostic tests | None | Medicare Benefits Schedule reimbursement fee (ESM Section 4.1) |
| Drugs | None | Drug reimbursement amount from Pharmaceutical Benefits Scheme, regimen information on eviQ [58], drug administration fee in the Medicare Benefits Schedule. See ESM Section 4.2. |
| Background costsa | Gamma and multivariate normal | 45 and Up Study; modified analysis to [4] (ESM Section 4.3) |
CPTAC Clinical Proteomic Tumour Analysis Consortium, NSCC non-squamous cell carcinoma, PD-L1 programmed death ligand-1, PSA probabilistic sensitivity analysis, SCC squamous cell carcinoma, TCGA The Cancer Genome Atlas, TRACERx TRAcking Non-small Cell Lung Cancer Evolution Through Therapy
aHealth system costs not related to systemic therapy
bUsed in "optimal utilisation" sensitivity analyses
We conducted a “low-price” analysis to capture the potential influence of special pricing arrangements, in which the prices of targeted and immunotherapeutic agents were discounted by 50%. Further, we performed an “optimal utilisation” analysis in which first-line therapy rates expected under optimal care were applied [71]. In this sensitivity analysis, 79% of patients with NSCLC and 90% of patients with ES-SCLC received systemic therapy. Additionally, a “treat-to-progression” sensitivity analysis was conducted in which ICIs were received until disease progression or death.
Results
Validation
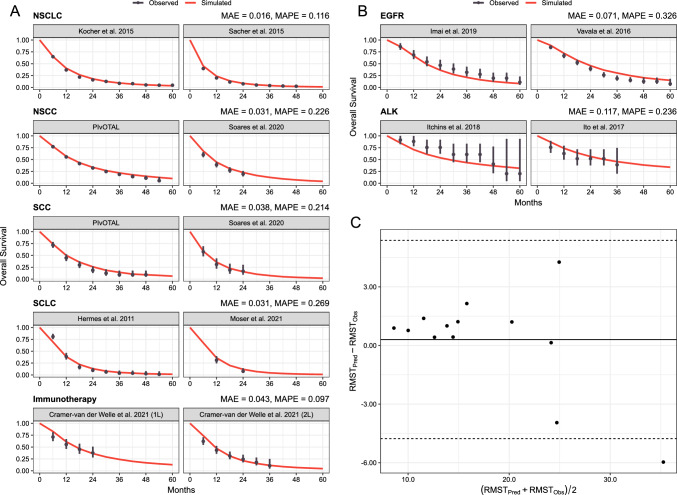
Overall, survival probabilities predicted by txSim matched observations from real-world studies, though the degree of accuracy varied by subpopulation (Fig. 2A, B). The model performed best for individuals with ES-SCLC and unselected NSCLC (Fig. 2A). The largest error was observed for EGFR-positive NSCLC and ALK-rearranged NSCLC (Fig. 2B). However, in both populations, mean absolute error was below 12%. Discrepancies between predicted and observed RMSTs ranged from − 6.0 to 4.3 months, but agreement was acceptable overall with an average bias of only 0.3 months (Fig. 2C). Restricted mean survival times could not be estimated for one study [65] but this study was retained for the comparison of survival probabilities.
Fig. 2.
Comparison of txSim predictions against observed survival outcomes in real-world studies for patients with non-small cell lung cancer (NSCLC), unselected non-squamous cell carcinoma (NSCC), squamous cell carcinoma (SCC), small-cell lung cancer (SCLC) and NSCLC receiving immunotherapy (A); patients with NSCLC with epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations (B); and Bland–Altman plot showing agreement in simulated and observed restricted mean survival times [RMST] (C). Estimates of mean absolute percentage error (MAPE) and mean absolute error (MAE) are within each subgroup
Model Outputs
At 10 years, mean survival time and cost for advanced lung cancer were 16.4 months (95% uncertainty interval: 14.7–18.1) and $116,069 (95% uncertainty interval: $107,378–124,933) [Table 2]. The “optimal utilisation” analysis found that 10-year OS and treatment costs increased across all scenarios, with survival ranging from 19.1 to 22.5 months and costs that ranged from $146,499 to $161,591 in 95% of simulations in the probabilistic sensitivity analysis. Costs were also higher in the “treat-to-progression” sensitivity analysis (in which immunotherapy was allowed to be administered beyond 24 months), with 10-year per-patient costs ranging from $121,252 to $144,720. Cumulative costs are reported in Fig. 24 of the ESM.
Table 2.
Model-estimated survival outcomes and per-patient costs for advanced lung cancer in Australia.
| Base case | Sensitivity analyses | |||
|---|---|---|---|---|
| Low price | Optimal utilisation | Treat to progression | ||
| Per-patient cost (2020 AUD) | ||||
| Meana (95% UI) | $116,069 (107,378–124,933) | $82,845 (77,422–87,800) | $154,299 (146,499–161,591) | $133,854 (121,252–144,720) |
| Overall survival | ||||
| 1 year (95% UI) | 40.5% (36.6–44.9) | b | 51.9% (49.1–55.2) | b |
| 2 year (95% UI) | 20.0% (17.3–23.1) | b | 27.1% (24.7–30.1) | b |
| 5 year (95% UI) | 4.8% (3.7–5.9) | b | 6.7% (5.5–8.2) | b |
| 10 year (95% UI) | 1.2% (0.9–1.7) | b | 1.8% (1.3–2.4) | b |
| Meana (95% UI) | 16.4 months (14.7–18.1) | b | 20.5 months (19.1–22.5) | b |
Note: 95% UIs give the 2.5th and 97.5th percentiles across 500 simulations (of 20,000 patients each) in the probabilistic sensitivity analysis
AUD Australian dollars, UI uncertainty interval
aEstimated up to 10 years after diagnosis
bSurvival outcomes identical to the base-case analysis
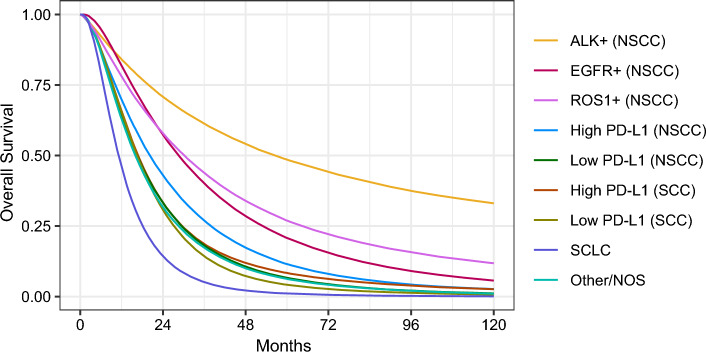
Overall survival for histological and molecular subpopulations receiving treatment under a 2022 Australian treatment algorithm is presented in Fig. 3 and associated treatment costs in Table 3. Among those who were treated, mean survival and costs 10-years post-diagnosis were highest for individuals with ALK rearrangement (65.0 months; $529,005), ROS1 rearrangement (43.8 months; $323,826) and EGFR mutations (39.0 months; $330,341). Mean costs were similar between patients with WT NSCC and patients with SCC, which ranged from $198,669 (NSCC with high PD-L1 expression) and $169,108 (SCC with low PD-L1 expression). Despite the arrival of ICIs for ES-SCLC, prognosis for ES-SCLC was predicted to remain low with an estimated mean OS of 14.4 months and an associated cost of $122,867 after 10 years. The mean cost for patients with Other/NOS disease was $179,418, although the expert panel expressed uncertainty regarding treatment patterns within this population.
Fig. 3.
Model-estimated overall survival (OS) probability in advanced lung cancer subpopulations receiving systemic therapy. ALK anaplastic lymphoma kinase, EGFR epidermal growth factor receptor, NSCC non-squamous cell carcinoma, Other/NOS other-specified or not-otherwise-specified; PD-L1 programmed death ligand 1, ROS1 c-ros oncogene 1, SCC squamous cell carcinoma, SCLC small cell lung cancer
Table 3.
Model-estimated overall survival and mean costs for advanced lung cancer subpopulations. Outcomes represent patients who receive at least one line of therapy
| Overall survival | Mean costa (2020 AUD) | |||||
|---|---|---|---|---|---|---|
| Mean (months)a | 1 year (%) | 2 years (%) | 5 years (%) | 10 years (%) | ||
| ALK+ (NSCC) | 65.0 | 84 | 71 | 49 | 33 | $529,005 |
| EGFR+ (NSCC) | 39.0 | 82 | 57 | 21 | 6 | $330,341 |
| High PD-L1 (NSCC) | 28.8 | 70 | 43 | 12 | 3 | $198,669 |
| High PD-L1 (SCC) | 24.8 | 66 | 33 | 8 | 3 | $172,129 |
| Low PD-L1 (NSCC) | 23.3 | 64 | 33 | 7 | 1 | $177,607 |
| Low PD-L1 (SCC) | 21.4 | 65 | 31 | 4 | 1 | $169,108 |
| Other/NOS | 22.7 | 63 | 32 | 6 | 1 | $179,418 |
| ROS1+ (NSCC) | 43.8 | 78 | 58 | 27 | 12 | $323,826 |
| SCLC | 14.4 | 46 | 14 | 1 | 0 | $122,867 |
ALK anaplastic lymphoma kinase, AUD Australian dollars, EGFR epidermal growth factor receptor, NSCC non-squamous cell carcinoma, Other/NOS other-specified or not-otherwise-specified, PD-L1 programmed death ligand 1, ROS1 c-ros oncogene 1, SCC squamous cell carcinoma, SCLC small-cell lung cancer
aEstimated up to 10 years after diagnosis
Discussion
We developed a discrete event simulation platform (txSim) to estimate contemporary survival outcomes and costs for Australians with advanced lung cancer. The model performed well when validated against survival outcomes in observational studies, with acceptable agreement and accuracy across a variety of molecular and histological subpopulations. Under the 2022 treatment algorithm for advanced lung cancer, we predicted an average per-patient cost of $116,069, with 5-year survival estimates that ranged from 49% for individuals with an ALK rearrangement to 1% for ES-SCLC. Costs were proportional to the length of treatment, with targeted therapies accruing the most expenditure per patient. The model was sensitive to assumptions relating to drug pricing, the duration of immunotherapy and systemic therapy utilisation rates, but provides a plausible range of values that can be applied in health economic evaluations and updated over time.
Validation
The model performed well in external validation, closely predicting survival outcomes in real-world observational cohorts. The MAPE estimates ranged from 11.6 to 32.6%, indicating similar accuracy to the Canadian iTEN model that achieved MAPE estimates of 11.6–49.3% [7]. However, MAPE may be a suboptimal measure of accuracy for quantities close to zero. Predictions for ES-SCLC cohorts, which had the poorest survival, had a MAPE of 26.9% but only a mean absolute error of 1.3%, indicating only a minor discrepancy on the scale of survival probabilities.
Model accuracy varied between subpopulations with larger discrepancies observed among patients with driver oncogenes. Overall survival was both overestimated in an Italian sample [67] and underestimated in a Japanese sample [66] of EGFR-positive patients, suggesting that there is heterogeneity in survival outcomes for this group not explained by treatment patterns alone. Furthermore, model-predicted outcomes for ALK-positive NSCLC may be underestimated. In the validation, the model significantly underestimated OS for ALK-rearranged NSCLC reported by Itchins et al. [69]. Consistent with this, we found that 5-year survival for patients with ALK-rearranged NSCLC receiving treatment was estimated to be 49% whereas 62.5% of patients receiving alectinib in the ALEX trial survived to 5 years [25]. However, it is likely that real-world survival will be poorer than observed in clinical trials, which select the healthiest subset of patients. Cramer-van der Welle et al. [70] showed that OS among Dutch patients with NSCLC receiving immunotherapy was poorer than reported in clinical trials. Encouragingly, these observations were reproduced with our model (see Fig. 2A).
The Clinical and Economic Impact of New Therapies
Lung cancer survival statistics in Australia will likely continue to improve. For stage IV lung cancer cases diagnosed in 2011–2016, 1-, 2- and 5-year relative survival rates were 19.2%, 8.3% and 3.2% [72]. Our base-case analysis predicted appreciable increases in OS to 40.5%, 20.0% and 4.8%, under a 2022 treatment algorithm, with mean survival of 16.4 months. The “optimal utilisation” analysis, where we applied treatment rates expected if all patients with a performance status of 0–1 received systemic therapy [71], yielded improved outcomes over the base case (21.6 vs 16.4 months), highlighting the importance of optimising access to, and minimising inequities in care, particularly as more effective therapies become available.
Novel therapies are expected to substantially increase healthcare costs. The mean cost of advanced lung cancer 10 years post-diagnosis was predicted to be $116,069, representing a 90% increase over an “average case” cost of $61,183 (in 2020 AUD) for metastatic lung cancers diagnosed between 2006 and 2013 [4]. This growth has been driven by the availability of novel TKIs and ICIs, as well as the improving efficacy of treatment that results in longer treatment courses. Unsurprisingly, we found that per-patient costs were highest for patients with oncogene-driven NSCLC receiving TKIs. Consistent with this, allowing immunotherapy to be administered beyond 24 months resulted in a 15% increase in per-patient costs. It should be noted that considerable uncertainty remains regarding the prices of new medications in Australia, which may differ from public reimbursement amounts. The results of our “low-price” analysis indicate only a 36% increase since 2013 [4], which should be taken as a plausible minimum for the recent growth in costs.
Evolving treatment standards have implications for the budgetary consequences of preventive policies. Early detection through screening has recently been recommended by the Medical Services Advisory Committee, which advises on decisions to publicly fund medical interventions in Australia, with funding for the programme announced in May 2023 [5]. However, the total economic consequences of the programme beyond the direct cost of implementation and CT imaging will be determined in part by the costs of treatment, which are subject to change because of innovation. Thus, in planning the programme, costs will need to be re-evaluated both at implementation and over the life of the programme.
Limitations
While efforts were made to select appropriate inputs for the simulation, simplifying assumptions were necessary. First, we applied systemic therapy utilisation rates observed between 2006 and 2016 [57], which may since have increased with the introduction of more effective and tolerable treatment options. These were applied uniformly across all patients with NSCLC, ignoring the potential heterogeneity between molecular subpopulations. Notably, ALK- and ROS1-positive NSCLC are characterised by earlier age at onset [73], which is a predictor of treatment uptake [57]. Hence, survival and cost predictions for these subpopulations may be underestimated. Second, we assumed that all individuals treated received molecular testing. Third, we did not account for all sources of uncertainty, particularly in relation to the treatment algorithm specified. Methods to elicit subjective uncertainties relating to model parameters may be an avenue of future development. Fourth, we assumed that drug costs would remain static over the modelled 10-year horizon when they, in fact, may change because of statutory price reductions or the introduction of generics.
It should be noted that, although population-based, the 45 and Up Study is not a representative sample [10], meaning that parameters drawn from these data may not be fully representative of the broader lung cancer population. In particular, the average age at lung cancer diagnosis in the cohort was slightly older than the national average [57], suggesting that treatment rates in the model are underestimated. Additionally, smoking prevalence in the 45 and Up Study cohort as a whole was lower [10], resulting in potential differences in the distribution of histological subtypes.
Treatment-related adverse events were not explicitly costed in this analysis. Extreme toxicities can lead to hospitalisation and may be very costly. However, the background costs applied in the model were derived from a sample of 45 and Up Study participants with lung cancer, many of whom received systemic therapy. Therefore, healthcare costs associated with adverse events occurring in this sample were likely to be captured (i.e. excess costs of general practitioner visits, medications, hospitalisations). Further, our approach offers the advantage of capturing the incidence of adverse events in real-world patients (noting the generalisability limitations in the 45 and Up Study) who may be more frail than clinical trial populations [74]. Nevertheless, the implementation of background costs here is imperfect, particularly when applied to patients receiving TKIs, which have a more favourable toxicity profile compared with chemotherapy.
Finally, the generalisability of the results is limited by the evolving nature of treatment. Since conducting this analysis, tepotinib for NSCLC harbouring MET exon 14 skipping mutations has been listed on the Pharmaceutical Benefits Scheme and will likely alter the costs presented. However, this limitation also highlights the advantage of the txSim platform, which can be flexibly adapted to changing practices and deliver timely estimates at a fraction of the resource cost of an observational study.
Conclusions
Survival expectations and health expenditure for advanced lung cancer have increased in recent years with the emergence of novel systemic therapies. During periods of innovation, real-world observational data will not always be available to meet the needs of policy decision making. Such needs must be addressed through other means such as modelling. The txSim platform allows for early estimates of the clinical and economic impact of novel treatments in the absence of mature observational data.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was completed using data collected through the 45 and Up Study (http://www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and partners: the Heart Foundation; NSW Ministry of Health; NSW Department of Communities and Justice; and Australian Red Cross Lifeblood. We thank the many thousands of people participating in the 45 and Up Study, the Centre for Health Record Linkage, Services Australia, NSW Ministry of Health, and the Cancer Institute NSW for use of their data. We also thank the Secure Unified Research Environment (SURE) for providing secure access to the data in this study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Funding
This work was funded by a Medical Research Future Fund Preventive and Public Health Research Initiative: 2019 Target Health System and Community Organisation Research Grant Opportunity, No. MRF1200535.
Conflict of Interest
Karen Canfell is a co-principal investigator of an investigator-initiated trial of cervical screening, “Compass”, run by the Australian Centre for Prevention of Cervical Cancer (ACPCC), which is a government-funded not-for-profit charity. Compass receives infrastructure support from the Australian government and the ACPCC has received equipment and a funding contribution from Roche Molecular Diagnostics, USA. Karen Canfell is also a co-principal investigator on a major implementation program Elimination of Cervical Cancer in the Western Pacific, which has received support from the Minderoo Foundation and the Frazer Family Foundation and equipment donations from Cepheid Inc. Zarnie Lwin declares participation on advisory boards/scientific advisory committees for, travel support from, and/or honoraria from Pfizer, AstraZeneca, Bristol Myers Squibb and Merck. Kwun M. Fong, David Goldsbury, Brett G.M. Hughes, Deme Karikios, Preston Ngo, Marianne Weber and Stephen Wade have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Data Availability
Data from the 45 and Up Study used in this study cannot be made available by the authors on grounds of confidentiality and privacy. The data are available from the data custodians (Sax Institute, Services Australia, NSW Ministry of Health) for approved research projects. Enquiries can be made to the Sax Institute (see https://www.saxinstitute.org.au/solutions/45-and-up-study/use-the-45-and-up-study). Molecular tumour datasets used in this study are available on cBioPortal (see https://www.cbioportal.org).
Code Availability
Not applicable.
Author Contributions
Conceptualisation: PN; methodology: PN, DK, SW, ZL, BH; software: PN; validation: PN; analysis: PN, DG; original draft: PN, MW; review and editing: DK, DG, SW, ZL, BH, KF, KC; supervision: KC, MW.
References
- 1.Rosenthal MA, Webster PJ, Stuart-Harris RC, Langlands AO, Boyages J, Gebski VJ. The cost of treating small cell lung cancer. Med J Aust. 1992;156:605–611. doi: 10.5694/j.1326-5377.1992.tb121456.x. [DOI] [PubMed] [Google Scholar]
- 2.Access Economics. Cost of cancer in NSW: a report by Access Economics Pty Limited for The Cancer Council NSW (2007). Cancer Council NSW. https://www.cancercouncil.com.au/wp-content/uploads/2010/11/costofcancer_summary.pdf.
- 3.Kang S, Koh E-S, Vinod SK, Jalaludin B. Cost analysis of lung cancer management in South Western Sydney. J Med Imaging Radiat Oncol. 2012;56:235–241. doi: 10.1111/j.1754-9485.2012.02354.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldsbury DE, Weber MF, Yap S, Rankin NM, Ngo P, Veerman L, et al. Health services costs for lung cancer care in Australia: estimates from the 45 and Up Study. PLoS One. 2020;15:e0238018. doi: 10.1371/journal.pone.0238018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medical Services Advisory Committee. Application No. 1699 National Lung Cancer Screening Program; 2022. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1699-public [Accessed 8 Jun 2023].
- 6.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 7.Moldaver D, Hurry M, Evans WK, Cheema PK, Sangha R, Burkes R, et al. Development, validation and results from the impact of treatment evolution in non-small cell lung cancer (iTEN) model. Lung Cancer. 2020;139:185–194. doi: 10.1016/j.lungcan.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Kocher F, Hilbe W, Seeber A, Pircher A, Schmid T, Greil R, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87:193–200. doi: 10.1016/j.lungcan.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 9.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2022. https://www.r-project.org/ [Accessed 8 Jun 2023].
- 10.Bleicher K, Summerhayes R, Baynes S, Swarbrick M, Navin Cristina T, Luc H, et al. Cohort profile update: the 45 and Up Study. Int J Epidemiol. 2023;52:e92-101. doi: 10.1093/ije/dyac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centre for Health Record Linkage (CHeReL); 2022. https://www.cherel.org.au/ [Accessed 15 Mar 2022].
- 12.Kelman CW, Bass AJ, Holman CDJ. Research use of linked health data: a best practice protocol. Aust N Z J Public Health. 2002;26:251–255. doi: 10.1111/j.1467-842X.2002.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 13.ChoiceMaker. Open Source ChoiceMaker technology; 2022. https://oscmt.sourceforge.io/ [Accessed 13 Oct 2022].
- 14.Bentley JP, Ford JB, Taylor LK, Irvine KA, Roberts CL. Investigating linkage rates among probabilistically linked birth and hospitalization records. BMC Med Res Methodol. 2012;12:149. doi: 10.1186/1471-2288-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017;7:596–609. doi: 10.1158/2159-8290.CD-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173:291–304.e6. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 21.Gillette MA, Satpathy S, Cao S, Dhanasekaran SM, Vasaikar SV, Krug K, et al. Proteogenomic characterization reveals therapeutic vulnerabilities in lung adenocarcinoma. Cell. 2020;182:200–5.e35. doi: 10.1016/j.cell.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174–179. doi: 10.1016/j.lungcan.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Yu JL, Simmons C, Victor JC, Han D, Hogeveen S, Leighl N, et al. Impact of new chemotherapeutic and targeted agents on survival in stage IV non-small cell lung cancer. Oncologist. 2011;16:1307–1315. doi: 10.1634/theoncologist.2011-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien MER, Ciuleanu T-E, Tsekov H, Shparyk Y, Cuceviá B, Juhasz G, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 25.Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim D-W, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31:1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 26.Novello S, Mazières J, Oh I-J, de Castro J, Migliorino MR, Helland Å, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29:1409–1416. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camidge DR, Kim HR, Ahn M-J, Yang JCH, Han J-Y, Hochmair MJ, et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L trial. J Thorac Oncol. 2021;16:2091–2108. doi: 10.1016/j.jtho.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Soria J-C, Tan DSW, Chiari R, Wu Y-L, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 29.Kim D-W, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–463. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 31.Shaw AT, Riely GJ, Bang Y-J, Kim D-W, Camidge DR, Solomon BJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30:1121–1126. doi: 10.1093/annonc/mdz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 33.Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 34.Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi T, Ando M, Asami K, Okano Y, Fukuda M, Nakagawa H, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA) J Clin Oncol. 2014;32:1902–1908. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- 36.Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Zhang Q, Liang L, Li J, Liu Z, Li W, et al. Crizotinib vs platinum-based chemotherapy as first-line treatment for advanced non-small cell lung cancer with different ROS1 fusion variants. Cancer Med. 2020;9:3328–3336. doi: 10.1002/cam4.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi A, Chiodini P, Sun J-M, O’Brien MER, von Plessen C, Barata F, et al. Six versus fewer planned cycles of first-line platinum-based chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2014;15:1254–1262. doi: 10.1016/S1470-2045(14)70402-4. [DOI] [PubMed] [Google Scholar]
- 39.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 40.Baize N, Monnet I, Greillier L, Geier M, Lena H, Janicot H, et al. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2020;21:1224–1233. doi: 10.1016/S1470-2045(20)30461-7. [DOI] [PubMed] [Google Scholar]
- 41.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 42.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 43.Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 44.Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:1156–1170. doi: 10.1016/j.jtho.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 45.Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro CJ, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39:723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 48.Kim D-W, Tiseo M, Ahn M-J, Reckamp KL, Hansen KH, Kim S-W, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–2498. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 49.Dziadziuszko R, Krebs MG, De Braud F, Siena S, Drilon A, Doebele RC, et al. Updated integrated analysis of the efficacy and safety of entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small-cell lung cancer. J Clin Oncol. 2021;39:1253–1263. doi: 10.1200/JCO.20.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32:881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 53.Leighl NB, Hellmann MD, Hui R, Carcereny E, Felip E, Ahn M-J, et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med. 2019;7:347–357. doi: 10.1016/S2213-2600(18)30500-9. [DOI] [PubMed] [Google Scholar]
- 54.Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2021;21:111. doi: 10.1186/s12874-021-01308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delignette-Muller ML, Dutang C. fitdistrplus: an R package for fitting distributions. J Stat Softw. 2015;64:1–34. doi: 10.18637/jss.v064.i04. [DOI] [Google Scholar]
- 57.Ngo P, Goldsbury DE, Karikios D, Yap S, Yap ML, Egger S, et al. Lung cancer treatment patterns and factors relating to systemic therapy use in Australia. Asia Pac J Clin Oncol. 2022;18:e235–e246. doi: 10.1111/ajco.13637. [DOI] [PubMed] [Google Scholar]
- 58.eviQ. Cancer treatments online; 2022. https://www.eviq.org.au/ [Accessed 15 Mar 2022].
- 59.Vemer P, Corro Ramos I, van Voorn GAK, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34:349–361. doi: 10.1007/s40273-015-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sacher AG, Le LW, Lau A, Earle CC, Leighl NB. Real-world chemotherapy treatment patterns in metastatic non-small cell lung cancer: are patients undertreated? Cancer. 2015;121:2562–2569. doi: 10.1002/cncr.29386. [DOI] [PubMed] [Google Scholar]
- 61.Soares M, Antunes L, Redondo P, Borges M, Hermans R, Patel D, et al. Real-world treatment patterns and survival outcomes for advanced non-small cell lung cancer in the pre-immunotherapy era in Portugal: a retrospective analysis from the I-O Optimise initiative. BMC Pulm Med. 2020;20:240. doi: 10.1186/s12890-020-01270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee DH, Isobe H, Wirtz H, Aleixo SB, Parente P, de Marinis F, et al. Health care resource use among patients with advanced non-small cell lung cancer: the PIvOTAL retrospective observational study. BMC Health Serv Res. 2018;18:147. doi: 10.1186/s12913-018-2946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Castro J, Tagliaferri P, de Lima VCCCC, Ng S, Thomas M, Arunachalam A, et al. Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care (Engl) 2017;26:e12734. doi: 10.1111/ecc.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermes A, Waschki B, Gatzemeier U, Reck M. Vergleich von Behandlungsergebnissen in zwei Patientengruppen (2004–2005 und 2007–2008) mit einem kleinzelligen Bronchialkarzinom im begrenzten und metastasierten Krankheitsstadium. Pneumologie. 2011;65:203–207. doi: 10.1055/s-0030-1256122. [DOI] [PubMed] [Google Scholar]
- 65.Moser SS, Bar J, Kan I, Ofek K, Cohen R, Khandelwal N, et al. Real world analysis of small cell lung cancer patients: prognostic factors and treatment outcomes. Curr Oncol. 2021;28:317–331. doi: 10.3390/curroncol28010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imai H, Kaira K, Mori K, Kotake M, Mitani M, Kawashima N, et al. Post-progression survival is highly linked to overall survival in patients with non-small-cell lung cancer harboring sensitive EGFR mutations treated with first-line epidermal growth factor receptor-tyrosine kinase inhibitors. Thorac Cancer. 2019;10:2200–2208. doi: 10.1111/1759-7714.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vavalà T, Follador A, Tiseo M, Galetta D, Morabito A, Di Maio M, et al. BE-POSITIVE: beyond progression after tyrosine kinase inhibitor in EGFR-positive non small cell lung cancer patients. Lung Cancer. 2016;95:73–81. doi: 10.1016/j.lungcan.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Ito K, Hataji O, Kobayashi H, Fujiwara A, Yoshida M, D’Alessandro-Gabazza CN, et al. Sequential therapy with crizotinib and alectinib in ALK-rearranged non-small cell lung cancer: a multicenter retrospective study. J Thorac Oncol. 2017;12:390–396. doi: 10.1016/j.jtho.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 69.Itchins M, Hayes SA, Gill AJ, Cooper W, O’Connell R, Howell VM, et al. Pattern of care and survival of anaplastic lymphoma kinase rearranged non-small cell lung cancer (ALK+ NSCLC) in an Australian Metropolitan Tertiary Referral Centre: a retrospective cohort analysis. Asia Pac J Clin Oncol. 2018;14:e275–e282. doi: 10.1111/ajco.12877. [DOI] [PubMed] [Google Scholar]
- 70.Cramer-van der Welle CM, Verschueren MV, Tonn M, Peters BJM, Schramel FMNH, Klungel OH, et al. Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep. 2021;11:6306. doi: 10.1038/s41598-021-85696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacob S, Hovey E, Ng W, Vinod S, Delaney GP, Barton MB. Estimation of an optimal chemotherapy utilisation rate for lung cancer: an evidence-based benchmark for cancer care. Lung Cancer Amst Neth. 2010;69:307–314. doi: 10.1016/j.lungcan.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 72.Australian Institute of Health and Welfare. Cancer data in Australia; 2022. https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/about [Accessed 17 Nov 2022].
- 73.Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA. 2019;321:1391–1399. doi: 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prince RM, Atenafu EG, Krzyzanowska MK. Hospitalizations during systemic therapy for metastatic lung cancer: a systematic review of real world vs clinical trial outcomes. JAMA Oncol. 2015;1:1333–1339. doi: 10.1001/jamaoncol.2015.3440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the 45 and Up Study used in this study cannot be made available by the authors on grounds of confidentiality and privacy. The data are available from the data custodians (Sax Institute, Services Australia, NSW Ministry of Health) for approved research projects. Enquiries can be made to the Sax Institute (see https://www.saxinstitute.org.au/solutions/45-and-up-study/use-the-45-and-up-study). Molecular tumour datasets used in this study are available on cBioPortal (see https://www.cbioportal.org).