Abstract
Background
We performed a systematic overview of the cost-effectiveness analyses (CEAs) comparing Non-insulin antidiabetic drugs (NIADs) with other NIADs for the treatment of type 2 diabetes mellitus (T2DM), using decision-analytical modelling (DAM), focusing on both the economic results and the underlying methodological choices.
Methods
Eligible studies were CEAs using DAM to compare NIADs within the glucagon-like peptide-1 (GLP1) receptor agonists, sodium-glucose cotransporter-2 (SGLT2) inhibitors, or dipeptidyl peptidase-4 (DPP4) inhibitor classes with other NIADs within those classes for the treatment of T2DM. The PubMed, Embase and Econlit databases were searched from 1 January 2018 to 15 November 2022. Two reviewers screened the studies for relevance by titles and abstracts and then for eligibility via full-text screening, extracted the data from the full texts and appendices, and then stored the data in a spreadsheet.
Results
The search yielded 890 records and 50 studies were eligible for inclusion. The studies were mainly based on a European setting (60%). Industry sponsorship was found in 82% of studies. The CORE diabetes model was used in 48% of the studies. GLP1 and SGLT2 products were the main comparators in 31 and 16 studies, respectively, while one study had DPP4 and two had no easily discernible main comparator. Direct comparison between SGLT2 and GLP1 occurred in 19 studies. At a class level, SGLT2 dominated GLP1 in six studies and was cost effective against GLP1 once as part of a treatment pathway. GLP1 was cost effective in nine studies and not cost effective against SGLT2 in three studies. At a product level, oral and injectable semaglutide, and empagliflozin, were cost effective against other within-class products. Injectable and oral semaglutide were more frequently found cost effective in these comparisons, with some conflicting results. Most of the modelled cohorts and treatment effects were sourced from randomised controlled trials. The following model assumptions varied depending on the class of the main comparator: choice of and reasoning behind risk equations, the time until the treatment switch, and how often the comparators were discontinued. Diabetes-related complications were emphasised on par with quality-adjusted life-years as model outputs. The main quality issues were regarding the description of alternatives, the perspective of analysis, the measurement of costs and consequences, and patient subgroups.
Conclusion
The included CEAs using DAMs have limitations that hinder their ability to inform decision makers on the cost-effective choice: lack of updated reasoning behind the choice of key model assumptions, over-reliance on risk equations based on older treatment practices, and sponsorship bias. The question of which NIAD is cost effective for the treatment of which T2DM patient is a pressing one and the answer remains unclear.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-023-01268-5.
Key Points for Decision Makers
| Compared with their older counterparts, newer Non-insulin antidiabetic drugs are cost effective for treating type 2 diabetes mellitus and show great promise in treating diabetes and its complications through indirect and direct effects. An overview of the recent findings in the cost-effectiveness literature and the underlying methodological choices in the decision-analytical models could aid decision makers in prioritisation. |
| This review found that as comparators, glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors were the favoured classes. Within these classes, semaglutide (injectable or oral) and empagliflozin were the favoured products, respectively. It is challenging to provide conclusions on the cost-effective option among these products due to different underlying methodological choices, sponsorship bias, and outdated information populating the model. |
| Decision makers face several difficulties when prioritising between the newer Non-insulin antidiabetic drug. The field of cost-effectiveness analyses in type 2 diabetes mellitus could benefit from using modelling practices, mainly treatment switch assumptions and risk equations, that better align with real-world practice and contemporary follow-up data for modelling treatment effects over time. |
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic progressive condition that poses a growing public health concern worldwide [1]. The estimated global total diabetes-related health expenditure for adults with diabetes will reach US dollars (US$) 1.03 trillion in 2030 and US$1.05 trillion in 2045 [1]. A UK model estimates that around 90% of the total healthcare costs for treating diabetes can be attributed to T2DM [2]. Furthermore, the costliest aspect of diabetes is the diabetes-related complications (DRCs), which carry great direct and indirect economic costs and a massive impact on health-related quality of life [3–5].
Several Non-insulin antidiabetic drugs (NIADs) are used for the pharmacological treatment of T2DM, where the management of hyperglycaemia is central. The main NIAD classes are commonly divided into older NIADs (oNIADs; e.g. metformin [MET], sulphonylurea [SU], and thiazolidinediones), and newer NIADs (nNIADs; e.g. dipeptidyl peptidase-4 [DPP4] inhibitors, glucagon-like peptide-1 [GLP1] receptor agonists, sodium-glucose cotransporter-2 [SGLT2] inhibitors) [6].
In 2018, a paradigm shift occurred where treatment guidelines in the consensus reports from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [7–16] increasingly emphasise using products from the GLP1 and SGLT2 classes of pharmaceuticals. This emphasis was based on cardiovascular outcome trials, which were shown to have a protective effect against cardiorenal DRCs while treating hyperglycaemia and promoting weight loss [10]. Later updates have led to the recommendation of even earlier use of GLP1 or SGLT2 products, independent of HbA1c or first-line medication, if the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD), chronic kidney disease (CKD), or heart failure (HF) [14].
Prevention or reduction of the occurrence of DRCs could lead to substantial improvements in the quality of life for patients with T2DM and a reduction in the increasing economic burden of the T2DM pandemic [3]. It thus becomes increasingly important to evaluate the value for money of the nNIADs [17]. One of the most commonly used methods for evaluating the cost effectiveness of diabetes treatment is the decision analytical model (DAM). Many factors complicate predicting how diabetes progresses over time, and DAMs can incorporate multiple sources of evidence to estimate how interventions differ over a long time horizon. Using many sources also allows DAMs to compensate for the short durations of clinical trials [18].
The ADA issued guidelines for diabetes modelling in 2004, highlighting seven different factors that complicate diabetes modelling: the long time horizon of disease progression, the involvement of multiple organ systems, the use of several types of medications that affect different outcomes, the vast array of complications that differ in terms of costs and how they affect quality of life, and the difficulties related to diagnosis [19]. These guidelines suggest that confidence in the models and their reliability can increase if transparency, validation, and the inclusion of different types of uncertainty are considered when constructing them. Adherence to these guidelines was investigated in a review from 2015, where it was found to be lacking but improving [20]. Methodological aspects of the models have also been reviewed [21–23], and Asche et al. have commented that clinically inconsequential changes in clinical parameters are given too much weight in DAMs and that time horizons in the models are too long [24].
One significant barrier to using nNIADs more than oNIADs is that the price has consistently been much higher [6, 10]. Despite the acquisition cost being much higher [11, 13–15], the literature on cost effectiveness has favoured the nNIADs [25–31] for second-line treatment. However, it remains unclear which of the nNIADs is the cost-effective choice, for which patients, and under which model assumptions [25]. An overview of the underlying methodological choices in the DAMs used to compare these nNIADs can provide decision makers with a better understanding of the basis of the economic results on which they base their decisions on choosing the appropriate nNIAD. We therefore aimed to provide an overview of the economic outcomes and differences in methodological choices, trends, and model assumptions by conducting a systematic review of the literature on cost-effectiveness analyses (CEAs) using DAM to compare nNIADs against other nNIADs, for the treatment of patients with T2DM.
Methods
This systematic review was conducted in 2021, updated in 2022, and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [32].
Eligibility Criteria
Types of Studies
Studies were eligible to be included if they were CEA or cost-utility analyses that used any DAM to compare one or several nNIADs as interventions for the treatment of T2DM. The studies must have compared at least one nNIAD with another, either comparing different classes or products or the same classes and products within those classes (e.g., GLP1 class vs. SGLT2 class, or GLP1 product vs. SGLT2 product, such as semaglutide vs. empagliflozin).
Editorials, comments, conference abstracts, protocols, reviews, and studies that were not original research articles published in English were excluded.
Types of Intervention
The primary aim of the interventions in the CEAs must be managing the hyperglycaemic aspect of T2DM. The therapy in the intervention can be nNIADs monotherapy, combination therapy of nNIADs with oNIADs, nNIADs, or insulin (INS).
Types of Comparators
Eligible comparators include nNIADs of the SGLT2, GLP1, or DPP4 classes on the product level or aggregated class-level representations based on the pooling of data from the treatment effect of several products.
Information Sources and Literature Search Strategy
Data were collected by searching the PubMed, EMBASE, and EconLit databases. The initial search period was from 1 January 2018 to 8 October 2020. The search was updated twice and the final search date was 15 November 2022. The search was limited to start from 2018 to include studies from the year the 2018 ADA/EASD consensus report was published [10]. The literature search was first performed in PubMed using a combination of Medical Subject Heading (MeSH) terms and free-text keywords, and subsequently adapted to EMBASE and EconLit. In addition, reference lists in eligible studies were scanned to identify additional relevant articles. The entire search strategy is available in Online Resource Appendix A1.
Selection Process
Screening Process
First, titles and abstracts were screened for relevance by two authors independently (HVBL and EPJ), erring to the side of inclusion and blinding the decision process using the browser application Rayyan [33]. Second, HVBL and EPJ accessed the full text and reviewed the studies in-depth for final inclusion based on the predefined eligibility criteria.
Data Collected and the Collection Process
A spreadsheet for collecting data from the studies was developed and tested on all studies to ensure the validity of the extraction sheet. After an iterative process, the testing resulted in a standardised sheet for data collection. Data were extracted under three main categories: (1) basic study characteristics, including country of study, funding source, analysis perspective, time horizon, model type and name, subgroups analysed, and background medication and combination therapy of the modelled cohort; (2) main outcomes of the economic evaluation and sensitivity analysis (SA), such as cost, effect measure, incremental cost-effectiveness ratio (ICER) and currency, the key drivers of results, and types and results of SA; and (3) modelling parameters and assumptions, including the source of baseline characteristics of the modelled cohort, patient subgroup, treatment effect, adverse events (AEs), DRCs, risk equations, treatment switch, and treatment switch type. The term ‘main comparator’ was used when a comparator in the study was easily discernible as the one against which all other drugs were compared.
Quality Assessment of the Included Studies
The overall quality of the included studies was assessed using Drummond’s 10-point checklist for assessing economic evaluations [17], which consists of 10 essential questions with supporting sub-questions. HVBL assessed all studies and EPJ assessed a random sample of 10 studies. Any disagreement was resolved through consensus, while major disagreement resulted in EPJ assessing more studies until consensus was achieved. The answers to the 10 main questions are presented in Table 4, along with a summary of the main results. Each question was answered using one of the following four responses: ‘Yes’ (adequate), ‘Partial yes’ (partially adequate), ‘Unclear’ (cannot tell), ‘No’ (not adequate), and ‘Not applicable’. For the checklist questions that were subjective in nature, the ADA treatment guidelines [10, 11, 13–15] were used as a reference regarding the adequacy of the answers.
Table 4.
Quality assessment via Drummonds 10-point checklist
| Study, year | Research aim | Alternatives described | Effectiveness established | Identification of costs and consequences | Measurement of costs and consequences | Valuation of costs and consequences | Extrapolation and discounting | Incremental analysis | Sensitivity analysis presentation | Discussion of study results |
|---|---|---|---|---|---|---|---|---|---|---|
| Barnett, 2018 [37] | Yes | Partial yes | Yes | Partial yes | Unclear | Unclear | Yes | Yes | Partial yes | Partial yes |
| Basson, 2018 [36] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Ericsson, 2018 [35] | Yes | Partial yes | Yes | Yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Tzanetakos, 2018 [34] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Ericsson, 2019 [44] | Yes | Partial yes | Yes | Yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Gæde, 2019 [52] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Hunt, 2019 [43] | Yes | Partial yes | Yes | Yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Johansen, 2019 [68] | Yes | Partial yes | Yes | Yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Malkin, 2019a [41] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Yes | Partial yes |
| Malkin, 2019b[42] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Yes | Partial yes |
| Raya, 2019 [39] | Yes | Partial yes | Partial yes | Partial yes | Unclear | Yes | Yes | Yes | Yes | Partial yes |
| Viljoen, 2019 [38] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Yes | Partial yes |
| Bain, 2020 [51] | Yes | Yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Capel, 2020 [50] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Gorgojo-MartíNez, 2020 [49] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Johansen, 2020 [48] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Martín, 2020 [47] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Capehorn, 2021 [57] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Guzauskas, 2021 [64] | Yes | Yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Malkin, 2021 [53] | Yes | Yes | Yes | Partial yes | Unclear | Partial yes | Yes | Yes | Yes | Yes |
| Risebrough, 2021 [70] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Ehlers, 2022a [58] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Ehlers, 2022b [59] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Ekhlasi, 2022 [83] | Partial yes | Partial yes | Yes | Partial yes | Partial yes | Partial yes | Yes | Yes | Partial yes | Yes |
| Eliasson, 2022 [60] | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Partial yes | Unclear |
| Franch-Nadal, 2022 [63] | Yes | Yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Hu, 2022 [79] | Yes | Partial yes | Yes | Partial yes | Unclear | Unclear | Yes | Yes | Partial yes | Partial yes |
| Malkin, 2022 [61] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Ruan, 2022 [81] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Stafford, 2022 [73] | Yes | Partial yes | Yes | Yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Viljoen, 2022 [62] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Chien, 2020 [75] | Yes | Yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Yes | Partial yes |
| Hu, 2021 [76] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Lin, 2021 [78] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Zupa, 2021 [71] | Yes | Yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Yes | Partial yes |
| Chakravarty, 2018 [65] | Yes | Yes | Yes | No | No | Partial yes | NA | Yes | Partial yes | Partial yes |
| Neslusan, 2018 [66] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Hou, 2019 [74] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Ramos, 2019 [40] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Ramos, 2020a [45] | Yes | Yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | No | Partial yes |
| Ramos, 2020b [46] | Yes | Yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Reifsnider, 2020 [69] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Yes | Partial yes |
| Van der Linden, 2020 [54] | Yes | Partial yes | Yes | Yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
| Ehlers, 2021 [56] | Yes | Yes | Yes | Partial yes | Partial yes | Yes | Yes | Yes | Partial yes | Yes |
| Gourzoulidis, 2021 [55] | Yes | Partial yes | Yes | Partial yes | Partial yes | Yes | Yes | Yes | Partial yes | Yes |
| Ramos, 2021 [77] | Yes | Yes | Yes | Partial yes | Partial yes | Yes | No | Yes | Partial yes | Partial yes |
| Reifsnider, 2021 [67] | Yes | Yes | Yes | Partial yes | Partial yes | Yes | Yes | Yes | Partial yes | Yes |
| Lasalvia, 2022 [82] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Partial yes |
| Peng, 2022 [80] | Yes | Partial yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Partial yes |
| Reifsnider, 2022 [72] | Yes | Partial yes | Yes | Partial yes | Unclear | Yes | Yes | Yes | Partial yes | Yes |
NA not applicable, Partial yes only a partial answer to the question
Results
Identified Studies
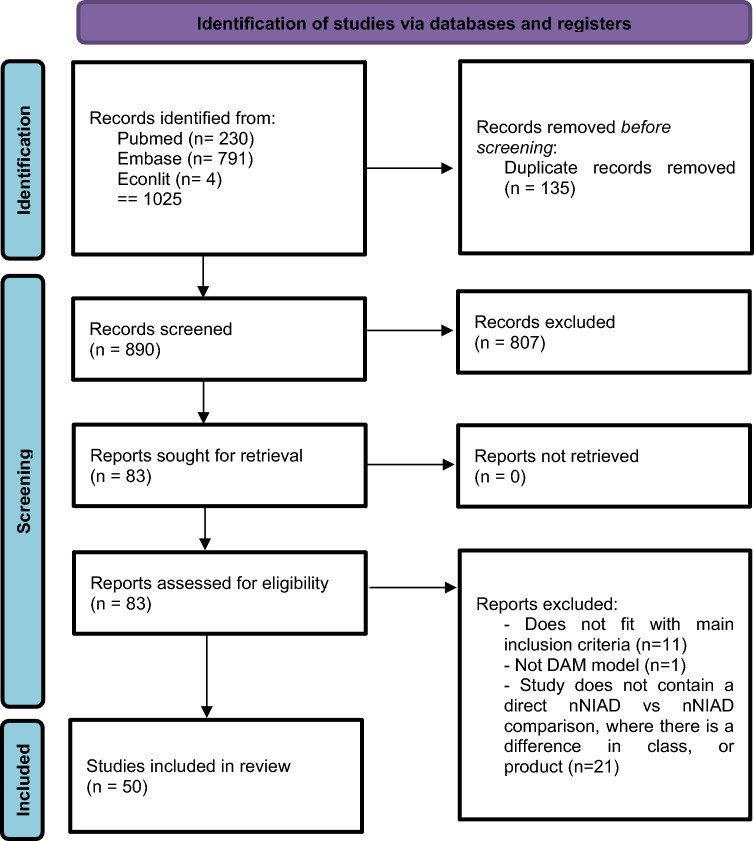
From the initial search, 890 unique studies were identified, of which 807 were removed after title and abstract screening, leaving 83 full-text articles. After reviewing the full texts, 50 studies met the inclusion criteria and were included for data extraction (see Fig. 1).
Fig. 1.
Flow-chart for study selection, adapted from the PRISMA-guidelines for the reporting systematic reviews [32]. DAM decision-analytical model, nNIAD Non-insulin antidiabetic drugs
Basic Characteristics
The results of extracted data from included studies can be found in Table 1, which summarises the general characteristics of the studies. The majority of studies (n = 30) were conducted in Europe [34–63], of which many were conducted in the UK (n = 9) [37, 38, 40, 45, 46, 48, 51, 57, 62], and one-third of the studies were conducted in North America (n = 10) [64–73] and Asia (n = 8) [74–81]. Two studies were conducted outside these regions: one each from Colombia [82] and Iran [83].
Table 1.
General characteristics of the studies
| Study, year | Country | Comparison | Funding | Perspective | Time horizon, years | Model type | Model name | Subgroups analysed | Background medication | Combination therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| Barnett, 2018 [37] | UK | Switch from sitagliptin 100 mg 1d to liraglutide 1.8 mg 1w within the first year of analysis vs. no switch | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 8.5 | None stated | Dual therapy | MET |
| Basson, 2018 [36] | France | Dulaglutide 1.5 mg 1w vs. exenatide QW ?? mg 1w | Eli Lilly | Statutory health insurance | 40 | Markov with Monte Carlo simulation | QuintilesIMS CORE Diabetes Model | None stated | Triple therapy or above | MET+SU |
| Ericsson, 2018 [35] | Sweden | Liraglutide 1.8 mg 1d + basal vs. lixisenatide 20 mg 1d + basal and IDegLira vs. lixisenatide 20 mg + basal | Novo Nordisk | Societal | 40 | Markov chains | IHECM-T2D | None stated | Dual therapy | MET |
| Tzanetakos, 2018 [34] | Greece | Exenatide QW2 mg 1w vs. INS glargine, liraglutide 1.2 mg 1d | AstraZeneca | Third-party payer | 40 | Discrete event stochastic simulation | Cardiff | None stated | Dual and triple therapy | MET, MET+SU |
| Ericsson, 2019 [44] | Sweden | Semaglutide-I 1 mg 1w vs. dulaglutide 1.5 mg 1w, Lixisenatide ?? mg 1d | Novo Nordisk | Societal | 40 | Markov cohort with risk equations | IHE-DCM | None stated | Dual and triple therapy | MET, MET+INS |
| Gæde, 2019 [52] | Denmark | Semaglutide-I 0.5 and 1 mg 1w vs. dulaglutide 1.5 mg 1w and semaglutide-I 0.5 and 1 mg 1w vs. exenatide QW ?? mg 1w, liraglutide 1.2 and 1.8 mg 1d, Lixisenatide ?? mg 1d | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual therapy | MET |
| Hunt, 2019 [43] | Netherlands | Semaglutide-I 0.5 and 1 mg 1w vs. INS glargine U100 1d and semaglutide-I 0.5 and 1 mg 1w vs. dulaglutide 0.75 and 1.5 mg 1w | Novo Nordisk | Societal | Lifetime | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | A subgroup analysis on BMI 30+, 35+ | Dual and triple therapy | MET, MET+SU |
| Johansen, 2019 [68] | Canada | Semaglutide-I 0.5 and 1 mg 1w vs. dulaglutide 0.75 and 1.5 mg 1w | Novo Nordisk | Societal | 40 | Markov cohort with risk equations | IHECM-T2D | None stated | Dual and triple therapy | MET |
| Malkin, 2019a [41] | Estonia | Semaglutide-I 1 mg 1w vs. liraglutide 1.2 mg 1d | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | Whole analysis on BMI >35 | Dual and triple therapy | MET, SU, TZD |
| Malkin, 2019b [42] | Slovakia | Semaglutide-I 0.5 and 1 mg 1w vs. dulaglutide 1.5 mg 1w | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | Whole analysis on BMI >35 | Dual therapy | MET |
| Raya, 2019 [39] | Spain | IDegLira vs. GLP1, INS + GLP1, basal INS, MDI | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 8.5 | Four subgroups: MDI, GLP1+INS, basal INS, GLP1 | Dual and triple therapy | MET, GLP1, INS |
| Viljoen, 2019 [38] | UK | Semaglutide-I 0.5 and 1 mg 1w vs. dulaglutide 1.5 mg 1w | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | THIN cohort used in SA | Dual therapy | MET |
| Bain, 2020 [51] | UK | Semaglutide-O 14 mg 1d vs. empagliflozin 25 mg 1d, liraglutide 1.8 mg 1d, sitagliptin 100 mg 1d | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual and triple therapy | MET, SU, SGLT2 |
| Capel, 2020 [50] | Spain | Exenatide QW 2 mg 1w vs. dulaglutide 1.5 mg 1w, liraglutide 1.2 and 1.8 mg 1d, lixisenatide 20 mg 1d | AstraZeneca | Healthcare payer | 40 | Discrete event stochastic simulation | Cardiff | None stated | Dual therapy | MET |
| Gorgojo-MartíNez, 2020 [49] | Spain | Semaglutide-I 0.5 and 1 mg 1w vs. empagliflozin 10 and 25 mg | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual and triple therapy | MET, TZD, MET+TZD |
| Johansen, 2020 [48] | UK | Semaglutide-I 1 mg 1w vs. liraglutide 1.2 mg 1d | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual and triple therapy | MET, SU, S, COMBOS |
| Martín, 2020 [47] | Spain | Semaglutide-I 0.5 and 1 mg 1w vs. dulaglutide 1.5 mg 1w, sitagliptin 100 mg 1d | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual and triple therapy | Vs. dulaglutide: MET vs. sitagliptin: MET, SU, TZD |
| Capehorn, 2021 [57] | UK | Semaglutide-I 1 mg 1w vs. empagliflozin 25 mg 1d | Novo Nordisk | Healthcare payer | Lifetime | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual therapy | MET |
| Guzauskas, 2021 [64] | USA | Semaglutide-O 14 mg 1d vs. empagliflozin 10 and 25 mg 1d, liraglutide 1.8 mg 1d, sitagliptin 100 mg 1d, background (MET + SU) | Not industry | Healthcare payer | Lifetime | Individual patient-level Monte Carlo microsimulation | UKPDS OM2 | None stated | Dual and triple therapy | MET+SU |
| Malkin, 2021 [53] | Netherlands | Semaglutide-O 14 mg 1d vs. empagliflozin 25 mg 1d, sitagliptin 100 mg 1d, liraglutide 1.8 mg 1d | Novo Nordisk | Societal | Lifetime | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | A subgroup analysis on BMI 30+ | Dual and triple therapy | MET, SU, SGLT2 |
| Risebrough, 2021 [70] | USA | Semaglutide-O 14 mg 1d vs. dulaglutide 1.5 mg 1w, liraglutide 1.8 mg 1d, Semaglutide-I 1 mg 1w | Novo Nordisk | Payer perspective | Lifetime | State transition cohort model with competing risk approach | Not stated | None stated | Dual and triple therapy | 1–2 OADs |
| Ehlers, 2022a [58] | Denmark | Semaglutide-O ?? mg 1d vs. empagliflozin ?? mg 1d | Boehringer Ingelheim | Health sector | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9.5 | None stated | Dual therapy | MET |
| Ehlers, 2022b [59] | Denmark | Semaglutide-I 1 mg 1w vs. empagliflozin 25 mg 1d | Boehringer Ingelheim | Payers’ perspective | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9.5 | None stated | Triple therapy or above | MET |
| Ekhlasi, 2022 [83] | Iran | Dulaglutide 1.5 mg 1w vs. liraglutide 1.8 mg 1d | Not industry | Health system | 10 | Markov state transition model | Not stated | None stated | Triple therapy or above | ≥2 OADs |
| Eliasson, 2022 [60] | Sweden | Semaglutide-O 14 mg 1d vs. empagliflozin 25 mg 1d, sitagliptin 100 mg 1d | Novo Nordisk | Societal and payers | 40 | Markov state transition model | IHE-DCM | None stated | Dual and triple therapy | MET, MET+SU |
| Franch-Nadal, 2022 [63] | Spain | Semaglutide-O 14 or 7 mg 1d vs. empagliflozin 25 mg 1d, sitagliptin 100 mg 1d, liraglutide 1.8 mg 1w | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9.0 | None stated | Dual and triple therapy | MET, SU, SGLT2 |
| Hu, 2022 [79] | China | Semaglutide-I 1 mg 1w vs. dulaglutide 1.5 mg 1w | Not industry | Healthcare providers | 40 | Individual patient-level Monte Carlo microsimulation | UKPDS OM2 | None stated | Dual therapy | MET |
| Malkin, 2022 [61] | Portugal | Semaglutide-O 14 mg 1d vs. empagliflozin 25 mg 1d, dulaglutide 1.5 mg 1w | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9.0 | None stated | Dual and triple therapy | 1–2 OADs |
| Ruan, 2022 [81] | China | Semaglutide-I 0.5 or 1 mg 1w vs. dulaglutide 1.5 mg 1w | Novo Nordisk | Healthcare system | 40 | Markov state transition model | IHE-DCM | None stated | Dual therapy | MET |
| Stafford, 2022 [73] | Canada | Semaglutide-I 1 mg 1w vs. canagliflozin 300 mg 1d | Novo Nordisk | Healthcare payer and societal | 40 | Markov state transition model and individual patient simulation | IHE-DCM and ECHO-T2DM | None stated | Dual therapy | MET |
| Viljoen, 2022 [62] | UK | Semaglutide-I 1 mg vs. dulaglutide 3 mg 1w, dulaglutide 4.5 mg 1w | Novo Nordisk | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9.0 | None stated | Dual therapy | MET |
| Chien, 2020 [75] | Taiwan | Comparison of 8 different strategies with 5 second-line classes (SGLT2, DPP4, GLP1, SU, ins) | Not industry | National health insurance | 40 | Patient-level fixed-time increment, Monte Carlo microsimulation model | Cardiff | Scenario analysis had Taiwanese T2DM individuals | Triple therapy or above | MET |
| Hu, 2021 [76] | China | Dapagliflozin 10 mg 1d + saxagliptin 5 mg 1d vs. dapagliflozin 10 mg 1d vs. saxagliptin 5 mg 1d | Not industry | Healthcare service providers | 50 | Individual patient-level Monte Carlo microsimulation | UKPDS OM2 | None stated | Triple therapy or above | MET |
| Lin, 2021 [78] | China | Five different strategies for DPP4 inhibitors were compared: linagliptin 5 mg, saxagliptin 5 mg, alogliptin 25 mg, sitagliptin 100 mg, and vildagliptin 50 mg | Not industry | Healthcare service providers | Lifetime | Risk equation model/submodels/unclear terms | COMT | None stated | Dual therapy | MET |
| Zupa, 2021 [71] | USA | Empagliflozin 25 mg 1d vs. semaglutide-I 1 mg 1w | Not industry | Healthcare system | 3 | Markov state transition model | Not stated | Alternate case where patients started with complications consistent with EMPA-REG OUTCOME and SUSTAIN 6 populations | Dual and triple therapy | SoC (none stated) |
| Chakravarty, 2018 [65] | USA | Dapagliflozin vs. liraglutide, SU, DPP4, pioglitazone | AstraZeneca | Third-party payer | 1 | Decision tree | Not stated | None stated | Dual therapy | MET |
| Neslusan, 2018 [66] | USA | Canagliflozin 300 mg 1d vs. dapagliflozin 10 mg 1d | Janssen Scientific Affairs | Third-party payer | 30 | Markov with microsimulation at the patient level | ECHO-T2DM | None stated | Dual therapy | MET |
| Hou, 2019 [74] | China | Canagliflozin 100 mg 1d vs. dapagliflozin 10 mg 1d | Not industry | Healthcare service providers | Lifetime | Risk equation model/submodels/unclear terms | COMT | None stated | Dual therapy | MET |
| Ramos, 2019 [40] | UK | Empagliflozin ?? mg 1d + SoC vs. saxagliptin ?? mg 1d + SoC, sitagliptin ?? mg 1d + SoC | Boehringer Ingelheim | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual and triple therapy | MET, SU, GLP1, TZD, MEG, INS |
| Ramos, 2020a [45] | UK | Empagliflozin ?? mg 1d + SoC vs. SoC, liraglutide ?? mg 1d + SoC | Boehringer Ingelheim | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual and triple therapy | MET, SU, TZD, MEG, INS added as per SoC |
| Ramos, 2020b [46] | UK | Empagliflozin 25 mg 1d vs. semaglutide-O 14 mg 1d | Boehringer Ingelheim | Healthcare payer | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual therapy | MET |
| Reifsnider, 2020 [69] | USA | Comparison of two different second-line strategies, empagliflozin or sitagliptin | Boehringer Ingelheim | Healthcare payer | Lifetime | Individual patient-level Monte Carlo microsimulation | DICE platform | No subgroup, but analysis divided into T2DM individuals with or without CVD | Triple therapy or above | MET |
| Van der Linden, 2020 [54] | Netherlands | Dapagliflozin ?? mg 1d vs. DPP4 (represented by sitagliptin mainly) | AstraZeneca | Societal | 40 | Fixed-time increment stochastic simulation model | Cardiff | None stated | Triple therapy or above | MET+SU |
| Ehlers, 2021 [56] | Denmark | Empagliflozin ?? mg 1d vs. liraglutide 1.8 mg 1d | Boehringer Ingelheim | Healthcare sector | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual therapy | MET |
| Gourzoulidis, 2021 [55] | Greece | Empagliflozin ?? mg 1d vs. dapagliflozin ?? mg 1d | Boehringer Ingelheim | Public payer | Lifetime | Individual patient-level discrete-event simulation | Not stated | None stated | Dual and triple therapy | SoC (MET, SU, INS) |
| Ramos, 2021 [77] | China | Empagliflozin ?? 1d vs. liraglutide ?? 1w, sitagliptin ?? mg1d | Boehringer Ingelheim | Healthcare payer providers | 50 | Markov with Monte Carlo simulation | IQVIA CORE Diabetes Model v. 9 | None stated | Dual and triple therapy | SoC (MET, SU, INS) |
| Reifsnider, 2021 [67] | USA | Empagliflozin ?? 1d vs. dapagliflozin ?? mg 1d, canagliflozin ?? mg 1d | Boehringer Ingelheim | Third-party payer | Lifetime | Individual patient-level discrete-event simulation | Not stated | None stated | Dual and triple therapy | SoC (MET, SU, INS) |
| Lasalvia, 2022 [82] | Colombia | Dapagliflozin ?? mg 1d vs. DPP4 | AstraZeneca | Unclear, third-party payer or healthcare system | 5 | Patient-level fixed-time increment, Monte Carlo microsimulation model | Cardiff | None stated | Dual therapy | MET |
| Peng, 2022 [80] | Taiwan | SGLT2 vs. DPP4 | Not industry | Healthcare sector | 10 | Markov state transition model | Not stated | With and without CVD history | Unclear | Unclear |
| Reifsnider, 2022 [72] | USA | Empagliflozin ?? mg 1d vs. liraglutide ?? mg 1d | Boehringer Ingelheim | Payers’ perspective | Lifetime | Individual patient simulation | Not stated | With and without CVD history | Triple therapy or above | MET |
When no dosage or ‘??’ is written, the dosage was unclear. Comparisons list the main comparators and then the alternatives separated by a comma, while ‘and’ is written to show that several different comparisons were made. When one medication is combined with another in the comparison, it is explicitly stated or symbolised with a ‘+’ symbol
1d once per day, 1w once per week, BMI body mass index, CVD cardiovascular disease, COMT Chinese Outcomes Model for T2DM, CORE Centre for Outcomes Research, Cardiff Cardiff Diabetes Model, DICE discretely integrated condition event, DPP4 dipeptidylpeptidase-4 inhibitors, ECHO-T2DM Health Outcomes Model of Type 2 Diabetes Mellitus, GLP1 glucagon-like peptide-1 receptor agonist, IDegLira combination of liraglutide and INS degludec, IHECM-T2D Swedish Institute for Health Economics Cohort Model for T2DM, INS insulin, MDI multiple daily injections, MEG meglitinide, MET metformin, SA sensitivity analysis, SGLT2 sodium-glucose cotransporter-2 inhibitors, SU sulfonylurea, semaglutide-I or O semaglutide injection or oral, SoC standard of care, T2DM type 2 diabetes mellitus, TZD thiazolidinedione, UKPDS UK Prospective Diabetes Study, UKPDS OM UK Prospective Diabetes Study outcomes model
Only 9 of 50 studies were not sponsored by the industry [64, 71, 74–76, 78–80, 83]. Novo Nordisk funded the most studies (n = 23) [35, 37–39, 41–44, 47–49, 51–53, 57, 60–63, 68, 70, 73, 81], followed by Boehringer Ingelheim (n = 11) [40, 45, 46, 55, 56, 58, 59, 67, 69, 72, 77]. The remaining five studies were funded by AstraZeneca [34, 50, 54, 65, 82] and one each by Eli Lilly [36] and Janssen Scientific Affairs [66].
The vast majority of modelling studies (n = 24) [36–43, 45–49, 51–53, 56–59, 61–63, 77] used IQVIA's CORE Diabetes Model (CDM) [84, 85], followed by the Cardiff Diabetes Model [86] (n = 5) [34, 50, 54, 75, 82], the Swedish Institute for Health Economics Cohort Model for T2DM (IHE-DCM) [87] (n = 5) [35, 44, 60, 68, 81], the UK Prospective Diabetes Study (UKPDS) Outcomes Model 2 (UKPDS-OM2) [88] (n = 3) [64, 76, 79], the Chinese Outcomes Model for T2DM (COMT) [89] (n = 2) [74, 78], and one study each with the Economic and Health Outcomes Model of T2DM (ECHO-T2DM) [90] and the Discretely Integrated Condition Event platform [91]. One study used both the IHE-DCM and the ECHO-T2DM to produce and compare their results [73]. Eight studies did not state the model name, of which three studies [71, 80, 83] used Markov state transition models, three used individual patient simulations [55, 67, 72], and one each used a state transition cohort model with a competing risk approach [70] and a decision tree [65].
The majority of studies conducted analyses using the payer’s perspective (n = 31) [34, 36–42, 45–52, 55, 57, 59, 61–67, 69, 70, 72, 75, 77]. Less frequently, a healthcare sector perspective (n = 10) [56, 58, 59, 71, 74, 76, 78–81] and a societal perspective were used in six studies [35, 43, 44, 53, 54, 68]. Two studies used both a payer’s perspective and a societal perspective [60, 73], while the perspective employed by one study was unclear [82].
Few studies (n = 3) had a time horizon of 5 years or lower [65, 71, 82]. The majority had a time horizon of either 40 years (n = 12) [34–36, 44, 50, 54, 60, 68, 73, 75, 79, 81] or 50 years (n = 21) [37–42, 45–49, 51, 52, 56, 58, 59, 61–63, 76, 77], while some studies (n = 11) reported a lifetime horizon [43, 53, 55, 57, 64, 67, 69, 70, 72, 74, 78]. The remainder had a time horizon of 30 years [66] or 10 years [80, 83].
The most frequently used background medication for the modelled cohorts at baseline was MET (n = 25) [35, 37, 38, 42, 46, 50, 52, 56–59, 62, 65, 66, 68, 69, 72–76, 78, 79, 81, 82], while most of the remaining studies (n = 23) reported MET combined with a range of other classes of medications [34, 36, 39–41, 43–45, 47, 48, 51, 53–55, 60, 61, 63, 64, 67, 70, 71, 77, 80, 83]. Sometimes the background medication was described as ‘Standard of Care’ (SoC) or ‘1–2 oral antidiabetic drugs’. Two studies had unclear reporting on the background medication [71, 80]. With regard to combination therapy, most studies (n = 22) involved dual or triple therapy, while some studies (n = 19) had dual therapy and the remaining eight studies had triple therapy or above [36, 54, 59, 69, 72, 75, 76, 83]. In one study, the background medication was unclear [80].
Cost-Effectiveness Results and Uncertainty
This section first describes how the comparisons between the classes are presented and then the methodological choices regarding the CEAs. An overview can be found in Table 2.
Table 2.
Cost-effectiveness and uncertainty results
| Study, year | Comparison | Cost, year | Effect measure | Which is cost effective | ICER (currency) | Threshold | Quadrant (location in source) | Key driver | Sensitivity analysis | First sensitive factor | Second sensitive factor | Third sensitive factor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barnett, 2018 [37] | Liraglutide vs. sitagliptin | Direct, 2016 | QALY, LY, incidence of DRC, onset of DRC | Liraglutide | 15,423/QALY, (GBP) | GBP20,000/QALY | QNE (Fig. 2) | Lower rates and delayed onset of DRCs offset the higher treatment cost | DSA generally confirmed findings. Except lipid or hypoglycaemia difference only led to the same effect but more cost, and BMI or SBP difference only led to 150k+ and 279k+ ICER; PSA likewise | Lipid difference only | Hypoglycaemia difference only | BMI difference only |
| Basson, 2018 [36] | Dulaglutide vs. exenatide QW | Direct, 2014 | QALY, LY, incidence of DRC, onset of DRC | Dulaglutide | Dominant (EUR) | EUR30,000/QALY | QSE (Fig. 2) | Lower rates and delayed onset of DRCs offset the higher treatment cost | DSA generally confirmed findings; PSA likewise | Variation in treatment efficacy | Time on treatment | Shorter time horizon (10 years) |
| Ericsson, 2018 [35] | Liraglutide + basal INS vs. lixisenatide + basal INS, IDegLira | Direct, 2016; indirect, 2015 | QALY, LY, incidence of DRC | Liraglutide and IDegLira |
Vs. lixisenatide: 30.802/QALY Scenarios: IDegLira vs. lixisenatide: 34.800/QALY | 23.984/QALY | dominant (SEK) |
SEK100,000–1,000,000/QALY | QNE for liraglutide vs. lixisenatide (Fig. 1, Table 4); QNE for IDegLira vs. lixisenatide = QNE or QSE dependent on dosage (Table 5) | More complications avoided | DSA generally confirmed findings. Except liraglutide was not CE when HbA1c changes were assumed equal; PSA likewise | Hba1c reduction of liraglutide critical in achieving CE | Liraglutide 1.8 mg replaced with 1.2 mg led to liraglutide being dominant | Shorter time horizon (10 years) |
| Tzanetakos, 2018 [34] | Exenatide QW vs. INS glargine, liraglutide | Direct, 2016 | QALY, LY, incidence of DRC | Exenatide QW | Vs. INS glargine: 4499/QALY vs. liraglutide: 2827/QALY (EUR) | EUR36,000/QALY | QNE for exenatide once-weekly vs. IG, QNE for exenatide once-weekly vs. liraglutide (Table 3) | Vs. INS glargine: lower cumulative incidence of DRCs (IHD, MI, hypoglycaemia) offset higher acquisition cost vs. liraglutide: lower cumulative incidence of DRCs (CHF, stroke) and fatality offset higher acquisition cost | DSA generally confirmed findings; PSA likewise | Lower utility to BMI weights | Switching HbA1c threshold | Hba1c effect of exenatide QW 1w set to upper limit |
| Ericsson, 2019 [44] | Semaglutide-I vs. dulaglutide, lixisenatide | Direct, 2018; indirect, 2016 | QALY, LY, incidence of DRC | Semaglutide-I | Vs. dulaglutide: dominant, vs. lixisenatide: dominant (SEK) | SEK500,000/QALY | QSE for semaglutide-I vs. dulaglutide, QSE for semaglutide-I vs. lixisenatide (Fig. 1) | For both comparisons: longer time to onset of DRCs, reduction of DRC incidence (particularly retinopathy) led to reduced costs and higher quality of life with semaglutide-I | DSA confirmed findings. Except with intensification at lower HbA1c threshold for semaglutide-I vs. dulaglutide; PSA confirmed base case | No drift in last intensification | Intensification at HbA1c 7.5% | Shorter time horizon (20 years) |
| Gæde, 2019 [52] | Semaglutide-I vs. dulaglutide, exenatide QW, liraglutide, lixisenatide | Direct, 2017 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | Primary analysis: dominant Secondary analysis: semaglutide-I 0.5 mg dominant, semaglutide-I 1 mg dominant (DKK) | DKK250,000/QALY (based on UK threshold) | QSE for semaglutide-I 0.5 mg vs. dulaglutide, QSE for semaglutide-I 1 mg vs. dulaglutide (Table 2) | Delay to treatment intensification, lower rates, and delayed onset of DRCs | DSA generally confirmed findings. Including only statistically significant differences made semaglutide-I 0.5 mg non-CE. With different treatment intensification, semaglutide-I was non-dominant but still CE; PSA confirmed base case | Only statistically significant differences | Treatment switch at 3 or 5 years made semaglutide-I 1 and 0.5 mg not dominant | Shorter time horizon (10 years) |
| Hunt, 2019 [43] | Semaglutide-I vs. INS glargine, dulaglutide | Direct + indirect, 2017 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I |
Semaglutide-I 0.5 mg vs. dulaglutide – direct: 4671/QALY direct + indirect: dominant Semaglutide-I 1 mg vs. dulaglutide – direct: 2861/QALY direct + indirect: dominant Semaglutide-I 0.5 mg vs. INS glargine – direct: 11,310/QALY direct + indirect: 4988/QALY Semaglutide-I 1 mg vs. INS glargine – direct: 7515/QALY direct + indirect: 495/QALY (EUR) |
EUR12,900/QALY | Direct costs: QNE for semaglutide-I 0.5 mg vs. glargine, QNE for semaglutide-I 1 mg vs. glargine, QNE for semaglutide-I 0.5 mg vs. dulaglutide, QNE for semaglutide-I 1 mg vs. dulaglutide with indirect costs: QNE, QNE, QSE, and QSE respectively (Table 4) | Semaglutide-I vs. INS glargine: reduction in cumulative incidence of DRCs and increased time to their onset. Largest cost saving from avoided CVD complications. This offset higher pharmacy costs of semaglutide-I. Combining direct + indirect reduced ICER further. Semaglutide-I vs. dulaglutide: same, but avoided ophthalmological complications most notable. Combining direct + indirect led to semaglutide-I dominating | DSA confirmed findings; PSA likewise | Hypoglycaemia difference only | SBP difference only | Lipid difference only |
| Johansen, 2019 [68] | Semaglutide-I vs. dulaglutide | Direct + indirect, 2017 | QALY, LY, incidence of DRC | Semaglutide-I | Semaglutide-I 0.5 mg vs. dulaglutide 0.75 mg: dominant, semaglutide-I 1 mg vs. dulaglutide 1.5 mg: dominant (CAD) | CAD50,000/QALY | QSE for semaglutide-I 0.5 mg vs. dulaglutide 0.5 mg, QSE for semaglutide-I 1 mg vs. dulaglutide 1.5 mg (Fig. 3, Table 3) | Higher cost of semaglutide-I due to longer treatment period (than dulaglutide) before switch to INS, offset by reduced complications, and longer onset until them. Same concept led to higher QALY for semaglutide-I | DSA confirmed findings; PSA: low dose, 66% probability of being CE at 50k/QALY; high dose 73% for PSA scenario analyses: 98% CE for both dose comparisons | Scenario: HbA1c drift at 0.14%, discontinuing GLP1 at 8% HbA1c threshold, and replacing it with INS | Scenario: No INS effect, HbA1c remain at 8% when reached | Shorter time horizon (10 years) |
| Malkin, 2019 [41] | Semaglutide-I vs. liraglutide | Direct, drug, unknown year, DRC 2017 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | 523/QALY (EUR) | EUR52,390/QALY (3 times Estonian GDP/capita) | QNE (Table 4) | Reduced incidence and delayed time to onset of DRC and avoidance of ulcer, amputation, neuropathy, hypoglycaemia, CVD | DSA confirmed findings. In some cases, semaglutide-I dominated; PSA likewise | Shorter time horizon (10 years) | Use of UKPDS 82 | 0% discount rate |
| Malkin, 2019 [42] | Semaglutide-I vs. dulaglutide | Direct, year unknown | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | Semaglutide-I 0.5 vs. dulaglutide 1.5: dominant, semaglutide-I 1 vs. dulaglutide 1.5: dominant (EUR) | EUR25,536/QALY (28 times average monthly wage in Slovakia) | QSE for semaglutide-I 0.5 mg vs. dulaglutide 1.5 mg, QSE for semaglutide-I 1 mg vs. dulaglutide 1.5 mg (Table 5) | Reduced incidence and delayed time to onset of DRC and avoidance of ulcer, amputation, neuropathy, hypoglycaemia, CVD | DSA confirmed findings. In DSA it is dominant all the time; PSA is 57–72% likely to be CE | Treatment switch at 7.5% HbA1c | Statistically significant differences only | 0% discount rate |
| Raya, 2019 [39] | IDegLira vs. GLP1, INS mix + GLP1, basal INS, MDI | Direct, 2016 | QALY, LY, incidence of DRC, onset of DRC | IDegLira | Vs. MDI: 3013/QALY, vs. basal: 6890/QALY, vs. GLP1: dominant, vs. GLP1 + INS: dominant (EUR) | EUR30,000/QALY | QNE for IDegLira vs. MDI, QNE for IDegLira vs. basal, QSE for IDegLira vs. INS+GLP1, QSE for IDegLira vs. GLP1 (Table 4, Fig. 2) | Vs. MDI: improved glycemic control led to fewer DRCs, higher cost offset by reduced cost of DRC vs. GLP1 + INS: cost saving because of fewer DRCs vs. basal: higher cost offset by reduced DRCs and delayed onset vs. GLP1: cost saving in general, fewer DRCs | DSA generally confirmed findings. Highly sensitive to (leads to non-CE) abolishment of HbA1c difference though (vs. MDI); PSA likewise | HbA1c difference abolishment vs. MDI OR basal INS | NPH INS cost applied | Statistically significant differences only |
| Viljoen, 2019 [38] | Semaglutide-I vs. dulaglutide | Direct, 2016 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | Semaglutide-I 0.5 vs. dulaglutide 1.5: dominant, semaglutide-I 1 vs. Dulaglutide 1.5: dominant (GBP) | GBP20,000/QALY | QSE for semaglutide-I 0.5 mg vs. dulaglutide 1.5 mg, QSE for semaglutide-I 1 mg vs. dulaglutide 1.5 mg (Tables 2 and 4, ESM Fig. 2) | Reduced incidence and delayed time to onset of DRC, higher cost due to increased survival offset by fewer DRCs | DSA generally confirmed findings; PSA likewise | Only including statistically significant differences between semaglutide-I 0.5 mg and dulaglutide 1.5 mg | Treatment switch at 7.5% | Dominant in all other scenarios |
| Bain 2020, [51] | Semaglutide-O vs. empagliflozin, sitagliptin, liraglutide | Direct (2019 drug; 2018 DRC) | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-O 14 mg | Vs. empagliflozin: 11,006/QALY vs. sitagliptin: 4930/QALY vs. liraglutide: dominant (GBP) | GBP20,000–30,000/QALY | QNE for semaglutide-O 14 mg vs. empagliflozin 25 mg, QNE for semaglutide-O 14 mg vs. sitaglipin100 mg, QSE for semaglutide-O 14 mg vs. liraglutide 1.8 mg (Table 3, Fig. 2) | Benefit from reduced incidence of DRC and longer mean time to onset of any DRC with semaglutide-O in all comparisons. Increased cost of semaglutide-O from higher acquisition price and longer time to treatment intensification | DSA confirmed findings. Dominance over liraglutide and CE against others unchanged; PSA confirmed base case | Shorter time horizon (10 years) | Treatment switch at 8.0% HbA1c | 26-week treatment effects applied |
| Capel, 2020 [50] | Exenatide QW vs. dulaglutide, liraglutide, lixisenatide | Direct, 2018 | QALY | Exenatide QW 2 mg 1w | Vs. dulaglutide: dominant, vs. liraglutide 1.2 mg: dominant, vs. liraglutide 1.8 mg: dominant, vs. lixisenatide: dominant (EUR) | EUR20,000/QALY gained | QSE for exenatide vs. dulaglutide, QSE for exenatide vs. liraglutide 1.2 mg, QSE for exenatide vs. liraglutide 1.8 mg, QSE for exenatide vs. lixisenatide, (Table 3, Fig. 1) | None stated | DSA completely confirmed findings in base case; PSA likewise | Dominant conclusion not changed | Dominant conclusion not changed | Dominant conclusion not changed |
| Gorgojo-MartíNez, 2020 [49] | Semaglutide-I vs. empagliflozin | Direct, 2018 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I |
Semaglutide-I 1 mg vs. empagliflozin 10 mg: 161/QALY Semaglutide-I 1 mg vs. empagliflozin 25 mg: 625/QALY (EUR) |
EUR30,000/QALY | QNE for semaglutide-I 0.5 mg vs. empagliflozin 10 mg, QNE for semaglutide-I 1 mg vs. empagliflozin 25 mg (Table 3) | Lower rates and delayed onset of DRCs led to higher clinical benefit for semaglutide-I. Higher acquisition cost and longer survival led to higher cost for semaglutide-I. Greater Hba1c reduction biggest contributor to superiority over empagliflozin | DSA generally confirmed findings, except some changes made semaglutide-I CE instead of dominant; PSA likewise | Shorter time horizon (10 years) | Lower 95% CI of HbA1c treatment difference | 5% discount rates |
| Johansen, 2020 [48] | Semaglutide-I vs. liraglutide | Direct, 2018 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | Semaglutide-I dominant (GBP) | GBP20,000/QALY | QSE (Table 2, Fig. 4) | Longer time to onset of DRCs, time to intensification, higher survival, greater ‘avoidance’ of DRCs | DSA completely confirmed findings, except under a statistically deterministic scenario, where it was still CE; PSA likewise | Shorter time horizon (10 years) | SGLT2 and SU discontinued at treatment intensification | Only statistically significant differences |
| Martín, 2020 [47] | Semaglutide-I vs. dulaglutide, sitagliptin | Direct (2019 drug; 2018 DRC) | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | 0.5 mg semaglutide-I vs. dulaglutide: dominant, 1 mg semaglutide-I vs. dulaglutide: dominant, 0.5 mg semaglutide-I vs. sitagliptin: dominant, 1 mg semaglutide-I vs. sitagliptin: dominant (EUR) | EUR30,000/QALY | QSE for semaglutide-I 1 mg vs. dulaglutide, QSE for semaglutide-I 1 mg vs. sitagliptin, QSE for semaglutide-I 0.5 mg vs. dulaglutide, QSE for semaglutide-I 0.5 mg vs. sitagliptin (Table 2) | Longer time to onset of DRC, time to intensification, higher survival, greater ‘avoidance’ of DRCs | DSA completely confirmed findings; PSA likewise | Dominant conclusion not changed | Dominant conclusion not changed | Dominant conclusion not changed |
| Capehorn, 2021 [57] | Semaglutide-I vs. empagliflozin | Direct, 2019 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | 4439/QALY (GBP) | GBP20,000/QALY | QNE (Table 2, Fig. 4) | Reduced incidence of DRCs, delayed onset of DRCs, delayed treatment intensification, driven by greater HbA1c reduction | DSA confirmed findings; PSA likewise | Fixed HbA1c over time and treatment intensification at 3 years | Shorter time horizon (10 years) | Different hypoglycaemia disutilities |
| Guzauskas, 2021 [64] | Semaglutide-O vs. empagliflozin, liraglutide, sitagliptin, MET + SU | Direct, 2020 | QALY, LY, incidence of DRC | Semaglutide-O, except vs. empagliflozin | Vs. empagliflozin: 458.400/QALY vs. liraglutide: 40.100/QALY vs. sitagliptin: 145.200/QALY vs. background (MET+SU): 117.500/QALY (US$) | US$100,000–250,000/QALY | QNE for semaglutide-I vs. liraglutide, QNE for semaglutide-I vs. empagliflozin, QNE for semaglutide-I vs. sitagliptin, QNE for semaglutide-I vs. background medication (Table 2) | Semaglutide-O had fewer MACE and cardiovascular deaths | No mention of robustness to change; but costs/QALYs very sensitive to changes in treatment effect | Change in MACE reduction of semaglutide-O | Changes in Hba1c reduction of semaglutide-O | Change in heart failure and nephropathy HR of semaglutide-O |
| Malkin, 2021 [53] | Semaglutide-O vs. empagliflozin, sitagliptin, liraglutide | Direct + indirect, 2019 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-O | Semaglutide-O vs. empagliflozin: 13,770/QALY, w indirect = 7061/QALY Semaglutide-O vs. sitagliptin: 5938/QALY, w indirect = 516/QALY Semaglutide-O vs. liraglutide: dominate, w indirect cost = dominate (EUR) | EUR20,000/QALY | QNE for semaglutide-O vs. empagliflozin, QNE for semaglutide-O vs. sitagliptin, QSE for semaglutide-O vs. liraglutide. with indirect cost: QNE, QNE, and QSE, respectively (Table 3, Fig. 1) | Greater Hba1c reductions were the key driver of clinical benefits in all comparisons. BMI made smaller contributions. Other RF made small or no contributions | DSA generally confirmed findings; PSA showed 52.7, 70.8%, 68.3% chance of semaglutide-O being CE vs. empagliflozin, sitagliptin, liraglutide, respectively | Treatment switch at 8.0% HbA1c | Use of UKPDS 82 | Shorter time horizon (30 years) |
| Risebrough, 2021 [70] | Semaglutide-O vs. dulaglutide, liraglutide, semaglutide-I | Direct, 2019 | QALY, LY, incidence of DRC, event-free survival | Semaglutide-O | Vs. semaglutide-I: 163,737/QALY, vs. dulaglutide: dominate, vs. liraglutide: dominate (US$) | US$20,000/QALY | QNE for semaglutide-O vs. injectable semaglutide, QSE for semaglutide-O vs. dulaglutide, QSE for semaglutide-O vs. liraglutide (Table 5) | Small differences in AE estimates, HbA1c benefits and event-free survival led to cost savings vs. dulaglutide and liraglutide | DSA confirmed findings; PSA focuses on semaglutide-O vs. semaglutide-I and shows that with increasing WTP, semaglutide-I is more likely to be CE | Daily cost of semaglutide-O | Weight treatment effect of semaglutide-O | Daily cost of semaglutide-O |
| Ehlers, 2022 [58] | Semaglutide-O vs. empagliflozin | Direct, 2020 | QALY, LY, incidence of DRC | Semaglutide-O not cost effective | Semaglutide-O vs. empagliflozin: 1,930,548/QAL (DKK) | DKK357,100/QALY (1 time GDP/capita) | QNE (Fig. 1) | The cost-effectiveness result was driven by a major difference in treatment costs, reflecting the large unit cost difference of Semaglutide-O vs. empagliflozin | DSA confirmed findings; PSA likewise, semaglutide-O was CE in 16% of simulations at set WTP | Discontinuation of semaglutide-O and empagliflozin, and switch to higher-dose, long-acting INS alogliptin in third-line | Shorter time horizon (5 years) | Using trial product estimand |
| Ehlers, 2022 [59] | Semaglutide-I vs. empagliflozin | Direct, 2020 | QALY, LY | Semaglutide-I not cost effective | Semaglutide-I vs. empagliflozin: 745,561/QALY, (DKK and EUR) | DKK357,100/QALY (1 time GDP/capita) | QNE (Fig. 1) | None stated | DSA confirmed findings; PSA likewise | Third-line treatment assumption (comparators replaced with INS) | Third-line treatment assumption (comparators replaced with INS) + third-line occurring at 8% HbA1c instead of 7.5% | Shorter time horizon (5 years) |
| Ekhlasi, 2022 [83] | Dulaglutide vs. liraglutide | Direct, 2018 | QALY | Dulaglutide | Dominant (US$) | US$3598.483/QALY (1 time GDP/capita in Iran 2018) | QSE (Fig. 2) | None stated | DSA confirmed findings; PSA likewise | Cost of liraglutide | Cost of dulaglutide | Hba1c reduction of liraglutide |
| Eliasson, 2022 [60] | Semaglutide-O vs. empagliflozin, sitagliptin | Direct + indirect, 2019 | QALY, incidence of DRC | Semaglutide-O | Direct: vs. empagliflozin = 239,001/QALY, vs. sitagliptin = 120,848/QALY, indirect: vs. empagliflozin = 191,721/QALY, vs. sitagliptin 95,234/QALY (SEK) | SEK500,000/QALY | Direct costs: QNE for semaglutide-O vs. empagliflozin, QNE for semaglutide-O vs. sitagliptin, with indirect costs: QNE and QNE (Fig. 2, Table 1) | Greater reductions in HbA1c led to fewer DRCs, longer time to INS initiation, fewer hypo events, and, lastly, better projected survival | DSA confirmed findings; PSA likewise | 3-year fixed duration until INS initiation | No QOL impact of BMI change | 0% discount rate |
| Franch-Nadal, 2022 [63] | Semaglutide-O vs. empagliflozin, sitagliptin, liraglutide | Direct, 2020 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-O | Vs. empagliflozin: 1339/QALY, vs. sitagliptin: dominant, vs. liraglutide: dominant, 7 mg semaglutide-O vs. sitagliptin: 2011/QALY (EUR) | EUR30,000/QALY | QNE for semaglutide-O vs. empagliflozin, QSE for semaglutide-O vs. sitagliptin, QSE for semaglutide-O vs. liraglutide, QNE for 7 mg semaglutide-O vs. sitagliptin (Table 1) | Extra clinical benefit from using semaglutide-O was due to reduced cumulative incidence and later onset of DRCs. Higher treatment cost of semaglutide-O was offset by the lower DRCs | DSA confirmed findings; PSA likewise | Shorter time horizon (10 years) | UKPDS HbA1c progression with no changes in treatment intensification | Lower 95% CI of HbA1c estimated treatment difference applied |
| Hu, 2022 [76] | Semaglutide-I vs. dulaglutide | Direct, 2021 | QALY, LY, incidence of DRC | Semaglutide-I | 26,957.44/QALY(US$) | US$12,551.5–37,654.50/QALY (1–3 times GDP/capita) | QNE (Fig. 2) | None stated | DSA showed high sensitivity to time-related factors, reversing conclusions of base-case; PSA showed 30.2%, 48.2% and 2.8% chance of being CE at above 3 times GDP/capita, between 1 and 3 times GDP/capita, and below 1 times GDP/capita, respectively | Discounting factor | Shorter time horizon | MI disutility score |
| Malkin, 2022 [61] | Semaglutide-O vs. empagliflozin, dulaglutide | Direct, 2021 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-O | Vs. empagliflozin: 23,571/QALY, vs. dulaglutide: 23,927/QALY, (EUR) | EUR30,000/QALY | QNE for semaglutide-O vs. empagliflozin, QNE for semaglutide-O vs. dulaglutide (Fig. 2) | Reduced incidence and time to onset of DRCs, the higher costs of semaglutide-O were offset by this. Higher HbA1c and weight reduction were the biggest drivers when comparing with empagliflozin and dulaglutide, respectively | DSA confirmed findings; PSA likewise | Shorter time horizon (10 years) | Discount factor of 0% | Only statistically significant differences from NMA |
| Ruan, 2022 [81] | Semaglutide-I vs. dulaglutide | Direct, 2021 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | Semaglutide-I dominant (CNY) | CNY80,976/QALY (1 times GDP/capita) | QSE (Fig. 2) | Semaglutide-I reduced and delayed the occurrence of DRCs, and reduced mortality. This offset the increase treatment cost | DSA confirmed findings; PSA likewise | Shorter time horizon (5 years) | Shorter time horizon (10 years) | HbA1c threshold at 7.0% |
| Stafford, 2022 [73] | Semaglutide-I vs. canagliflozin | Direct + indirect, 2019 | QALY, LY, incidence of DRC | Semaglutide-I | Using the IHE-DCM model: 14,127/QALY; using the ECHO-T2DM model: 13,188/QALY (CAD) | CAD50,000/QALY | QNE and QNE using both models (Fig. 1) | QALY gains for semaglutide-I were mainly driven by later use of INS caused by higher HbA1c reduction, and greater initial weight loss. Fewer DRCs offset some of the higher treatment costs of semaglutide-I | DSA confirmed findings; PSA likewise | 10% worse HbA1c effect for semaglutide-I | HbA1c threshold set to 7.5% | Discount rate at 3.5% |
| Viljoen, 2022 [62] | Semaglutide-I vs. dulaglutide | Direct, 2020 | QALY, LY, incidence of DRC, onset of DRC | Semaglutide-I | Vs. dulaglutide 3 mg: dominant, vs. dulaglutide 4.5 mg: 228/QALY (GBP) | GBP20,000/QALY | QSE for semaglutide-I vs. dulaglutide 3 mg, QNE for semaglutide-I vs. Dulaglutide 4.5 mg (Fig. 3) | Extra clinical benefit from using semaglutide-I was due to reduced cumulative incidence and later onset of DRCs. Higher treatment cost of semaglutide-I was offset by the lower DRCs | DSA confirmed findings; PSA likewise | HbA1c threshold set to 7.5% | Lower 95% CI of HbA1c treatment difference | Addition of basal INS, then basal bolus |
| Chien, 2020 [75] | No main vs. classes: MET, SU, DPP4, SGLT2, GLP1, unspecified INS | Direct, 2019 | QALY, LY, incidence of DRC | Arm7 (SGLT2) |
Arm 6: – Arm 7 dominates Arm 3 and Arm 8 is cost effective against Arm 1 and Arm 2 Arm 4 and Arm 5 extended dominated Arm 1: MET+SU -> +DPP4 Arm 2: MET +SGLT2 -> +DPP4 Arm 3: MET+DPP4 -> +SU Arm 4: MET+DPP4 -> +SGLT2 Arm 5: MET+GLP1 -> +SU Arm 6: MET+SU -> +DPP4 Arm 7: MET+SU -> +SGLT2 Arm 8: MET+INS -> +SU (NT) |
NT770,770/QALY (forecasted GDP/capita in Taiwan in 2019) |
Arm 7 vs. Arm 3: QSE Arm 7 vs. Arm 8: QSE Arm 7 vs. Arm 1: QNE Arm 7 vs. Arm 2: QNE Arm 4 and Arm 5: extended dominated (Table 3, text) |
None stated | DSA confirmed findings; PSA likewise | Baseline HbA1c | Baseline age | Hba1c threshold |
| Hu, 2021 [76] | No main vs. dapagliflozin + saxagliptin, dapagliflozin, saxagliptin | Direct, 2019 | QALY, LY | Dapagliflozin | Dapagliflozin + saxagliptin vs. saxagliptin: 217,530/QALY dapagliflozin vs. dapagliflozin + saxagliptin: dominate dapagliflozin vs. saxagliptin: 12,191/QALY (US$) | US$10,425.29–31,275.88/QALY (1–3 times GDP/capita in 2019) | QSE for dapagliflozin + MET vs. dapagliflozin + saxagliptin + MET, QNE for dapagliflozin + MET vs. saxagliptin + MET, QNE for dapagliflozin + saxagliptin + MET vs. saxagliptin (Table 4) | None stated | DSA confirmed findings; PSA likewise | Discount rate | Saxagliptin acquisition cost | Dapagliflozin acquisition cost |
| Lin, 2021 [78] | No main vs. linagliptin 5 mg, saxagliptin 5 mg, alogliptin 25 mg, sitagliptin 100 mg, and vildagliptin 50 g | Direct, 2019 | QALY, LY, incidence of DRC | Alogliptin | Alogliptin vs. linagliptin: extended dominate, vs. saxagliptin: dominate, vs. vildagliptin: dominate (US$) | US$10,276/QALY (China capita per GDP in 2019) | QSE for alogliptin vs. linagliptin, QSE for alogliptin vs. saxagliptin: QSE for alogliptin vs. vildagliptin | Reduced cumulative incidence of DRCs | DSA confirmed findings; PSA likewise | Cost of alogliptin and sitagliptin | Reduction of HbA1c for sitagliptin and alogliptin | Discount rate |
| Zupa, 2021 [71] | Empagliflozin vs. semaglutide-I | Direct, 2020 | QALY, LY | Semaglutide-I | 19,964/QALY (US$) | US$50,000–100,000/QALY | QNE (text) | None stated | DSA confirmed findings; PSA likewise | Daily cost of semaglutide-I | Heart failure risk of semaglutide-I | Stroke risk of semaglutide-I |
| Chakravarty, 2018 [65] | Dapagliflozin vs. liraglutide, SU, DPP4, pioglitazone | Direct, 2016 | QALY | Dapagliflozin | Vs. liraglutide: dominant, vs. DPP4: dominant, vs. TZD: 25.835/QALY, vs. SU: 19.005/QALY (US$) | US$50,000/QALY | QSE for dapagliflozin vs. GLP1, QNE for dapagliflozin vs. SU, QSE for dapagliflozin vs. DPP4, QNE for dapagliflozin vs. TZD (Fig. 4, Table 5) | Change in body weight | DSA generally confirmed findings; PSA likewise | Change in weight impact of treatment | Change in Hba1c | Change in SBP |
| Neslusan, 2018 [66] | Canagliflozin vs. dapagliflozin | Direct, 2016 | QALY, LY, incidence of DRC, onset of DRC | Canagliflozin | Dominant (US$) | US$100,000/QALY | QSE (Fig. 2) | Cost offsets from higher acquisition cost, and QALY gains were driven by better HbA1c lowering, which also led to lower event rates from complications, longer time to INS, less INS use | DSA completely confirmed findings; PSA likewise | Shorter time horizon (5 years) | Later treatment intensification | Real-world patient characteristics |
| Hou, 2019 [74] | Canagliflozin vs. dapagliflozin | Direct 2017 | QALY, LY, incidence of DRC | Canagliflozin | Canagliflozin100mg dominant (US$) | US$9117/QALY (GDP/capita of China in 2017) | QSE (Table 3) | Driven by the reduced cumulative incidence of macrovascular and microvascular complications | DSA big impact from cost of drugs, moderate/small impact of disutility/costs of complications; PSA confirmed base case | Cost of canagliflozin and dapagliflozin | Disutility | Cost of complications |
| Ramos, 2019 [40] | Empagliflozin vs. sitagliptin, saxagliptin | Direct, 2018 | QALY, LY, incidence of DRC | Empagliflozin | Vs. sitagliptin: 6464/QALY, vs. saxagliptin: 3878/QALY (GBP) | GBP20,000/QALY | QNE for empagliflozin vs. sitagliptin, QNE for empagliflozin vs. saxagliptin, (Table 5, Fig. 3) | Higher initial cost of SGLT2 offset by higher QALYs and LYs. Higher cost amd more DRCs for empagliflozin because of increased survival. Lower renal complication costs | DSA confirmed findings; PSA likewise | Shorter time horizon (5 years) | Hba1c threshold for treatment switch at 9% | Cardiovascular outcomes up to 3 years |
| Ramos, 2020 [45] | Empagliflozin vs. SoC and liraglutide | Direct, 2018 | QALY, LY, incidence of DRC | Empagliflozin | Empagliflozin + SoC dominant vs. liraglutide + SoC, 6428/QALY vs. SoC alone (GBP) | GBP20,000–30,000/QALY | QSE for empagliflozin vs. liraglutide, QNE for empagliflozin vs. SoC (Table 5, Fig. 3) | Treatment costs, survival, lower CV mortality | DSA confirmed findings; PSA likewise | Shorter time horizon (5 years) | Treatment switch threshold at 9% | CVOT outcome benefits applied for full treatment duration |
| Ramos, 2020 [46] | Empagliflozin vs. Semaglutide-O | Direct, year unknown | QALY, LY, heart failure | Empagliflozin | With hHF: empagliflozin dominant, without hHF: ICER = 186,690/QALY (GBP) | GBP20,000–30,000/QALY | QSE for empagliflozin (with hHF effect) vs. oral semaglutide, QSW for empagliflozin (without hHF effect) vs. oral semaglutide (Table 4, Fig. 1) | Inclusion of hHF effect of empagliflozin. Lower cost of empagliflozin | DSA confirmed findings; but some scenarios very sensitive; PSA likewise | Excluding the treatment effect on hHF | Treatment intensification at different HbA1c thresholds | BMI polynomial utility approach |
| Reifsnider, 2020 [69] | Empagliflozin vs. sitagliptin | Direct, 2018 | QALY, LY, CVD-free LY, incidence of DRC | Empagliflozin | Base case: 6967/QALY in CVD: 3589/QALY in non-CVD: 12,577/QALY (US$) | US$50,000–150,000/QALY | QNE for base case, QNE in CVD population, QNE in non-CVD population (Table 1, ESM Fig. SA3) | Base case: least complications with empagliflozin in the CVD pop: longer CVD-free survival and less cardiovascular death, fewer rates of DRCs in general in the non-CVD pop: lower or similar rates of DRCs | DSA confirmed findings, and showed empagliflozin to be either CE or dominant, depending on parameter. More likely to be dominant in the CVD pop.; PSA confirmed findings | Rebate percentage applied to the wholesale acquisition cost | Shorter time horizon (1 year) | Adherence to empagliflozin (80%*) or commercial perspective |
| Van der Linden, 2020 [54] | Dapagliflozin vs. DPP4 | Direct + indirect, 2018 | QALY, LY, incidence of DRC | Dapagliflozin | Vs. DPP4 class: dominant (EUR) | EUR20,000/QALY | QSE (Table 7, Fig. 2) | Dapagliflozin reduced the incidence of micro- and macrovascular complications, in exchange for more urinary tract infections and gastrointestinal infections, which increased quality of life. Dapagliflozin was cost saving due to lower treatment costs and reduced DRCs | DSA confirmed findings. No change from being dominant; PSA confirmed base case | Dominant conclusion not changed | Dominant conclusion not changed | Dominant conclusion not changed |
| Ehlers, 2021 [56] | Empagliflozin vs. liraglutide | Direct, 2019 | QALY, LY, incidence of DRC | Empagliflozin | Empagliflozin dominant (DKK) | DKK357,100/QALY (1 times the GDP/capita) | QSE (Fig. 2, Table 4) | Longer survival of empagliflozin, higher total cost of liraglutide | DSA confirmed findings. In scenario where liraglutide effects were extended to 13 years (Hba1c 8.5%), liraglutide would have extreme ICER (>3 mill/QALY); PSA likewise | Dominant conclusion not changed | Dominant conclusion not changed | Dominant conclusion not changed |
| Gourzoulidis, 2021 [55] | Empagliflozin vs. dapagliflozin | Direct, 2020 | QALY, LY, incidence of DRC | Empagliflozin | Vs. dapagliflozin: 965/QALY (EUR) | EUR36,000/QALY | QNE (Table 3, ESM Fig. 1) | Empagliflozin had reduced many DRCs, with longer life as a result | DSA confirmed findings, but empagliflozin showed dominance in 3-year model, not shown in tornado diagram; PSA confirmed findings | Discount rate of costs | HR: dapagliflozin vs. empagliflozin | Discount rate health |
| Ramos, 2021 [77] | Empagliflozin vs. liraglutide, sitagliptin | Direct, 2019 | QALY, LY, incidence of DRC | Empagliflozin | Vs. liraglutide: dominant vs. sitagliptin: 75,349/QALY (RMB) | RMB212,676/QALY (3 times GDP/capita) | QSE for empagliflozin vs. liraglutide, QNE for empagliflozin vs. sitagliptin, (Table 4, Fig. 3) | Longer survival of empagliflozin, lower cost from less HF and renal complications, despite higher costs from longer survival | DSA confirmed findings, except when changing treatment switch for liraglutide to 13 years, at HbA1c 8.5%, which puts empagliflozin in QSW; PSA confirmed base case | Effects of liraglutide extended to 13 years /HbA1c threshold of 8.5% | Shorter time horizon (5 years) | CV outcomes only used for 3 years |
| Reifsnider, 2021 [67] | Empagliflozin vs. dapagliflozin, canagliflozin | Direct, 2020 | QALY, LY, incidence of DRC | Empagliflozin | Empagliflozin vs. canagliflozin: dominate empagliflozin vs. dapagliflozin: 3054/QALY Empagliflozin vs. SoC: 32,848/QALY (US$) | US$50,000–150,000/QALY | QSE for empagliflozin vs. canagliflozin, QNE for empagliflozin vs. dapagliflozin, (Table 1, ESM Fig. OS1) | Longer overall survival and reduced rates of clinical events | DSA generally confirmed findings. Using treatment effects that favoured the comparators, made comparators dominant; PSA confirmed base case | Reducing HR for comparator vs. empagliflozin | Shorter time horizon (1, 3, 5, and 10 years) | Commercial perspective |
| Lasalvia, 2022 [82] | Dapagliflozin vs. DPP4 | Direct, 2020 | QALY, LY, incidence of DRC | Dapagliflozin | 1964.80/QALY (US$) | US$5710–17,129.9/QALY (1–3 times GDP/capita) | QNE (Table 4) | None stated | DSA confirmed findings; PSA likewise | Change of time horizon | Hba1c threshold for treatment switch at 9% | Weight reduction effect maintenance |
| Peng, 2022 [80] | SGLT2 vs. DPP4 | Direct, 2020 | QALY, incidence of DRC | SGLT2 | With CVD history: 3244.07/QALY; without CVD history: 4185.64/QALY (US$) | US$30,038–90,114/QALY (1–3 times GDP/capita) | QNE and QNE for comparisons with and without CVD history (Table 1) | None stated | DSA confirmed findings; PSA likewise | Cost of DPP4 | HR of SGLT2 vs. DPP4 on all-cause death | HR of SGLT2 vs. DPP4 on stroke |
| Reifsnider, 2022 [72] | Empagliflozin vs. liraglutide | Direct, 2019 | QALY, LY, incidence of DRC | Empagliflozin | Empagliflozin dominant (US$) | None stated | QSE (Table 1) | Fewer DRCs over time and longer survival due to empagliflozin's effect on patients with CVD | DSA completely confirmed findings; PSA likewise | Disutility of injectable treatment | Drug acquisition cost | Treatment effect for patients with CVD (HR of empagliflozin vs. liraglutide) |
Comparisons list the main comparators and then the alternatives separated by a comma, while ‘and’ is written to show that several different comparisons were made. When one medication is combined with another in the comparison, it is explicitly stated or symbolised with a ‘+’ symbol
1w once per week, AE adverse event, BMI body mass index, CAD Canadian dollars, CE cost-effective, CHF chronic heart failure, CI confidence interval, CNY Chinese Yuan, CV cardiovascular, CVD cardiovascular disease, CVOT Cardiovascular Outcome Trial, DKK Danish kroner, DPP4 dipeptidylpeptidase-4 inhibitors, DRCs diabetes-related complications, DSA deterministic sensitivity analysis, ECHO-T2DM Health Outcomes Model of Type 2 Diabetes Mellitus, ESM electronic supplementary, material, EUR Euro, GBP British pound sterling, GDP gross domestic product, GLP1 glucagon-like peptide-1 recepter agonist, HR hazard ratio, hHF hospitalisation for heart failure, ICER incremental cost-effectiveness ratio, IDegLira combination of liraglutide and INS degludec, IHD ischaemic heart disease, IHE-DCM Institute for Health Economics Cohort Model for T2DM, INS insulin, LY life years, MACE major adverse cardiac events, MET metformin, MDI multiple daily injections, MI myocardial infarction, NMA network meta-analysis, NT Taiwan new dollar, pop population, PSA probabilistic sensitivity analysis, QALY quality-adjusted life-years, QOL quality of life, QSE south-east quadrant, QNE north-east quadrant, QSW south-west quadrant, QW every week, RMB Renminbi, SBP systolic blood pressure, SEK Swedish kroner, SoC standard of care, US$ United States dollar, SGLT2 sodium-glucose cotransporter-2 inhibitors, semaglutide-I/O semaglutide injectable/oral, SU sulfonylurea, TZD thiazolidinedione, UKPDS UK Prospective Diabetes Study, WTP willingness to pay
Overview of Comparisons between the Newer Non-insulin Antidiabetic Drugs
In the included studies, the GLP1 and SGLT2 classes were the main comparators in 31 and 16 studies, respectively, while one study used DPP4 exclusively [78]. The remaining three studies compared alternatives where it was difficult to discern which was the main comparator [75, 76]. No studies evaluated the cost effectiveness of combining the two most frequently compared classes—SGLT2 and GLP1. The dosage of one or more comparators was mentioned in 35 of 50 studies. When a single product was represented as two different comparators with different dosages, the comparator with the higher dosage was always favoured. The results of the comparisons are presented in three sections based on the most frequent comparisons between classes of nNIADs: (1) GLP1 compared with GLP1 or another non-SGLT2; (2) SGLT2 compared with SGLT2 or another non-GLP1; and (3) GLP1 compared with SGLT2. The study, which compared different products from the DPP4 class, found alogliptin to be cost effective [78]. To simplify the descriptions of the comparators in Sects. 3.3.2–3.3.4, the nNIAD mentioned first is considered the main comparator unless otherwise stated. The results of the comparisons are briefly summarised below. Table 2 provides further details on the comparisons and the economic results in general.
Glucagon-Like Peptide-1 (GLP1) Compared with GLP1 or Another Non-Sodium-Glucose Cotransporter-2 (SGLT2)
Comparisons of products within the GLP1 class occurred in 14 studies, and comparisons of the GLP1 class of products against non-SGLT2 products occurred in six studies.
In 10 studies, injectable semaglutide was found to be primarily dominant but cost effective against other GLP1 products [39, 42, 43, 45, 49, 53, 63, 69, 80, 82]. In the one study where oral semaglutide was compared with injectable semaglutide, oral semaglutide was dominant against it and all other comparators [70]. The three remaining comparisons were between other GLP1 products and gave conflicting results [36, 50, 83]. For the six comparisons with GLP1 and other classes as comparators, injectable semaglutide was dominant compared with dulaglutide and sitagliptin [47], and cost-effective compared with INS glargine [43]. Exenatide was found to be cost effective against INS glargine and liraglutide [34], while liraglutide was cost effective against sitagliptin [37]. IDegLira, a combination of liraglutide and INS degludec, was categorised as an nNIAD of the GLP1 class if compared with nNIADs or similar combinations of nNIADs and INS. IDegLira and liraglutide, combined with INS, were found to be cost effective or dominant compared with other GLP1 products combined with INS, GLP1 as a class, and multiple forms of INS treatment [35, 39].
SGLT2 Compared with SGLT2 or Another Non-GLP1
Comparisons of products within the SGLT2 class occurred in four studies, and in six studies, SGLT2 products were compared with SGLT2 or non-GLP1 products.
When only SGLT2 products were compared, empagliflozin was found to be cost effective against dapagliflozin [55, 67] and dominant against canagliflozin [67], while canagliflozin was dominant against dapagliflozin in two studies [66, 74]. For the six comparisons with SGLT2 and other classes, empagliflozin was found to be cost effective against sitagliptin [69] and saxagliptin [40], while dapagliflozin was found to be dominant in one study against the DPP4 class and cost effective in another [54, 82]. Finally, dapagliflozin in combination with MET was the dominant strategy in a study comparing combinations of dapagliflozin and saxagliptin [76], and one class-level comparison found SGLT2 to be cost effective against DPP4 [80].
GLP1 Compared with SGLT2
Direct comparison of GLP1 and SGLT2 occurred in 19 studies. Aggregating the results to a class-level perspective, GLP1 was cost effective against SGLT2 in nine comparisons and not cost effective in three, while SGLT2 dominated GLP1 in six comparisons. Additionally, one study found SGLT2 as a third-line choice to be the cost-effective option in a set of comparisons where introducing GLP1 as a third-line choice was dominated.
From the product-level perspective, semaglutide (oral or injectable) and empagliflozin were the most frequently used main comparators. Oral semaglutide was found to be cost effective in five of seven comparisons with empagliflozin [51, 53, 58, 60, 61, 63, 64] but was not cost effective in two comparisons [58, 64]. Injectable semaglutide was found to be cost effective in all comparisons with empagliflozin [49, 57, 59] except one [59]. Injectable semaglutide was also found to be cost effective against canagliflozin [73]. Empagliflozin dominated liraglutide in all their comparisons [45, 56, 72, 77]. When empagliflozin was compared with injectable semaglutide and oral semaglutide, it dominated the latter [71] but was not cost effective against the former [46]. Dapagliflozin was compared with liraglutide once and dominated [65]. One study compared injectable semaglutide and empagliflozin [71] with no easily discernible main comparator and found the former to be cost effective.
Willingness to Pay and Incremental Cost-Effectiveness Ratio (ICER) Estimates
In the majority of studies (n = 21), a willingness-to-pay (WTP) threshold similar to that used in the UK (GBP20–30,000/quality-adjusted life-years [QALYs] or equivalent value in Euros or local currency) was utilised [34, 36–40, 45–55, 57, 61–63], followed by the World Health Organisation (WHO)-recommended WTP threshold of one to three times the gross domestic product per capita (n = 14) [41, 56, 58, 59, 74–83]. The remaining studies used a North American WTP threshold (n = 9) [64–71, 73] or a Swedish threshold (n = 3) [35, 60], and one study each used thresholds specific to The Netherlands [43] and Slovakia [42], while the last study did not state a threshold [72].
A total of 116 ICER estimates for base cases and reported scenarios were extracted from the included studies. Most of these ICERs were in the northeast quadrant of an incremental cost-effectiveness plane (n = 65; higher costs and effects), while the remaining were located in the southeast quadrant (n = 54; lower costs, higher effects), except one that was found in the southwest quadrant (lower costs, lower effects). When comparing the base case and the scatterplot resulting from the probabilistic sensitivity analysis (PSA), there was a clear consensus about the cost effectiveness and location of the estimates, indicating ICER estimates that were robust to changes in parameters chosen in the respective analyses.
Effect Measures and Key Drivers
All studies used QALYs and life-years (LYs) as effect measures, except for five studies [50, 60, 65, 80, 83] that only used QALYs. The majority of the included studies emphasised presenting the DRC output of the models and how this output affected the results, while six studies [50, 65, 76] did not report DRC output. Among the studies that reported DRC output, 19 reported both DRC incidence and the time until onset [36–39, 41–43, 47–49, 51–53, 57, 61–63, 66, 81], while 24 only reported incidence [34, 35, 40, 44, 45, 54–56, 58, 60, 64, 67–70, 72–75, 77–80, 82]. Two studies focused explicitly on the effect of the treatments on CVD-related DRCs, with one including hospitalisation from HF [46] and included CVD-free LYs [69]. One study included event-free survival as an effect measure [70].
The reporting of key drivers was based on an explicit description of the key drivers of the results of the economic analysis, taken from a dedicated section. If not described explicitly, the authors interpreted the key drivers as the results reported as most important for their results. The authors observed a general tendency to emphasise the effect of the nNIADs on time until the onset of DRCs and reduction of the cumulative incidence of DRCs, or both, as being key in achieving cost effectiveness over the comparators. It was not possible to discern whether key drivers of the results were reported in nine studies [50, 59, 71, 75, 76, 79, 80, 82, 83].
Sensitivity Analysis
All included studies conducted both deterministic sensitivity analysis (DSA) and PSA. The included studies either directly reported parameters that had the greatest impact on the ICER or supplied graphs or tables from which it was possible to extract the information. We extracted information on the parameters that affected the results most, second most, and third most, according to the degree to which the change in the parameter affected the ICER. These parameters were further grouped into broad categories. Among these categories, the most frequently occurring first-, second-, and third-most sensitive parameters were changes to the time horizon of the analysis, changes to the treatment effect of the comparators, and differing assumptions regarding the cost of the comparators, respectively. Four studies [47, 50, 54, 56] reported that the conclusion of dominance remained unchanged (see Table 2 for more details).
Model Parameters and Assumptions
This section presents the key model parameters and assumptions from the included studies, along with notable differences between the studies. A detailed presentation can be found in Table 3.
Table 3.
Model parameters
| Study, year | Comparison | Baseline table | Treatment effect | Adverse events | Diabetes-related complications | Risk equations | Treatment switch | Treatment switch type |
|---|---|---|---|---|---|---|---|---|
| Barnett, 2018 [37] | Liraglutide vs. sitagliptin | Yes | HbA1c, systolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, severe hypoglycaemic event, non-severe hypoglycaemic event | Severe hypoglycaemic event, non-severe hypoglycaemic event | Ophthalmic, diabetic foot, renal complications, CVD |
Base case: UKPDS 68; SA: UKPDS 82 |
After 3 years = comparators discontinued, all progress to basal INS NPH | Comparators discontinued |
| Basson, 2018 [36] | Dulaglutide vs. exenatide QW | Yes | HbA1c, systolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, nausea, severe hypoglycaemic event, non-severe hypoglycaemic event, injection site reaction | Severe hypoglycaemic event, non-severe hypoglycaemic event, nausea, injection site reaction | Myocardial infarction, ischaemic heart disease, heart failure, stroke, severe vision loss, amputation, peripheral vascular disease, gross proteinuria, neuropathy, ulcer, hemodialysis, peritoneal dialysis, renal transplant, cataract, retinopathy, macular edema, vision-threatening retinopathy |
Base case: No specific UKPDS RE mentioned; SA: none stated |
After 2 years = comparators discontinued, basal INS glargine IU40 | Comparators discontinued |
| Ericsson, 2018 [35] | Liraglutide + basal INS vs. lixisenatide + basal INS, IDegLira | Yes | HbA1c, BMI, systolic blood pressure, total cholesterol, LDL, HDL, triglycerides, severe hypoglycaemic event, non-severe hypoglycaemic event | Severe hypoglycaemic event, non-severe hypoglycaemic event | Proliferative retinopathy, retinopathy, macular edema, severe vision loss, neuropathy, peripheral vascular disease, amputation, microalbuminuria, gross proteinuria, end-stage renal disease, ischaemic heart disease, myocardial infarction, stroke, heart failure |
Base case: SweNDR, UKPDS OM2; SA: none stated |
After 8.8% HbA1c threshold = comparators discontinued, patients switched to basal-bolus | Comparators discontinued |
| Tzanetakos, 2018 [34] | Exenatide QW vs. INS glargine, liraglutide | Yes | HbA1c, weight, total cholesterol, HDL, systolic blood pressure, discontinuation in general, non-severe hypoglycaemic event, severe hypoglycaemic event, nausea | Discontinuation in general, non-severe hypoglycaemic event, severe hypoglycaemic event, nausea | Myocardial infarction, congestive heart failure, stroke, amputation, ischaemic heart disease, blindness, end-stage renal disease, non-severe hypoglycaemic event, severe hypoglycaemic event |
Base case: UKPDS 68; SA: none stated |
After 8% HbA1c threshold = comparators discontinued, replace with basal INS rescue regimen | Comparators discontinued |
| Ericsson, 2019 [44] | Semaglutide-I vs. dulaglutide, lixisenatide | Yes | HbA1c, BMI, systolic blood pressure, total cholesterol, LDL, HDL, triglycerides, severe hypoglycaemic event, non-severe hypoglycaemic event | Severe hypoglycaemic event, non-severe hypoglycaemic event | Proliferative retinopathy, non-proliferative retinopathy, macular edema, severe vision loss, neuropathy, peripheral vascular disease, amputation, microalbuminuria, macroalbuminuria, end-stage renal disease, ischaemic heart disease, myocardial infarction, stroke, heart failure |
Base case: SweNDR; SA: none stated |
Uncontrolled on MET: HbA1c 8.22% exceeded = basal INS added, GLP1 stopped. Next step not described Uncontrolled on INS: HbA1c 8.31% exceeded = bolus added, GLP1 stopped | Comparators discontinued |
| Gæde, 2019 [52] | Semaglutide-I vs. dulaglutide, exenatide QW, liraglutide, lixisenatide | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, microalbuminuria, gross proteinuria, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, proliferative retinopathy, macular edema, cataract, blindness, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe hypoglycaemic event, severe nocturnal hypoglycaemic event, diabetic ketoacidosis, lactic acid event, laser treatment, neuropathy, amputation, gangrene, ulcer |
Base case: UKPDS 68; SA: none stated |
After HbA1c 7.5% exceeded = add basal INS, 8.0 exceeded = add basal-bolus + stop GLP1 | Comparators continued, then discontinued |
| Hunt, 2019 [43] | Semaglutide-I vs. INS glargine, dulaglutide | Yes | HbA1c, systolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Severe hypoglycaemic event, non-severe hypoglycaemic event, severe nocturnal hypoglycaemic event, non-severe nocturnal hypoglycaemic event | myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, hemodialysis, peritoneal dialysis, renal transplant, non-severe hypoglycaemic event, severe hypoglycaemic event, laser treatment, cataract, blindness, neuropathy, amputation, gangrene, ulcer |
Base case: none stated; SA: UKPDS 68 and 82 |
After 3 years = comparators discontinued and all get same dosage of basal INS glargine U100 | Comparators discontinued |
| Johansen, 2019 [68] | Semaglutide-I vs. dulaglutide | Yes | HbA1c, systolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event | Background retinopathy, macular edema, proliferative retinopathy, severe vision loss, neuropathy, amputation, microalbuminuria, macroalbuminuria, end-stage renal disease, ischaemic heart disease, myocardial infarction, stroke, congestive heart failure, non-severe hypoglycaemic event, severe hypoglycaemic event | Base case: UKPDS 82 and 68, CDC, WESDR, REP; SA: UKPDS 68 | After 3 years = comparators discontinued, basal INS Hagedorn for all | Comparators discontinued |
| Malkin, 2019 [41] | Semaglutide-I vs. liraglutide | Yes | HbA1c, systolic blood pressure, BMI | None stated | Any, stroke, myocardial infarction, angina, congestive heart failure, peripheral vascular disease, neuropathy, amputation, ulcer, end-stage renal disease, gross proteinuria, microalbuminuria, macular edema, cataract, proliferative retinopathy, background retinopathy, severe vision loss |
Base case: UKPDS 68; SA: UKPDS 82 |
After 5 years = comparators discontinued, all get same dosage of basal INS glargine U100 | Comparators discontinued |
| Malkin, 2019 [42] | Semaglutide-I vs. dulaglutide | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Any, stroke, myocardial infarction, angina, congestive heart failure, peripheral vascular disease, neuropathy, amputation, ulcer, end-stage renal disease, gross proteinuria, microalbuminuria, macular edema, cataract, proliferative retinopathy, background retinopathy, severe vision loss |
Base case: UKPDS 68; SA: none stated |
After 3 years = comparators discontinued, all get same dosage of basal INS glargine U100 | Comparators discontinued |
| Raya, 2019 [39] | IDegLira vs. GLP1, INS mix + GLP1, basal INS, MDI | No | HbA1c, systolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI | Hypoglycaemia only in SA | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, microalbuminuria, gross proteinuria, haemodialysis, peritoneal dialysis, renal transplant, background retinopathy, proliferative retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, non-severe hypoglycaemic event, severe hypoglycaemic event, gangrene, laser treatment |
Base case: none stated; SA: UKPDS OM2, UKPDS OM |
After 5 years = comparators discontinued, all progress to basal bolus | Comparators discontinued |
| Viljoen, 2019 [38] | Semaglutide-I vs. dulaglutide | No | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, severe hypoglycaemic event, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Severe hypoglycaemic event, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Myocardial infarction, angina, peripheral vascular disease, stroke, congestive heart failure, microalbuminuria, gross proteinuria, end-stage renal disease, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, proliferative retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, severe hypoglycaemic event, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event |
Base case: UKPDS 68; SA: UKPDS 82, UKPDS HbA1c |
After 3 years = comparators discontinued, all progress to basal INS | Comparators discontinued |
| Bain, 2020 [51] | Semaglutide-O vs. empagliflozin, sitagliptin, liraglutide | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, BMI, severe hypoglycaemic event, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, microalbuminuria, gross proteinuria, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event, laser treatment, blindness, gangrene |
Base case: UKPDS 68; SA: UKPDS 82 |
After HbA1c 7.5% exceeded = stop semaglutide, basal INS started | Comparators discontinued |
| Capel, 2020 [50] | Exenatide QW vs. dulaglutide, liraglutide, lixisenatide | No | HbA1c, weight, discontinuation associated with AEs, nausea, non-severe hypoglycaemic event, severe hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, nausea | Ischaemic heart disease, congestive heart failure, myocardial infarction, stroke, amputation, blindness, end-stage renal disease, severe hypoglycaemic event, non-severe hypoglycaemic event |
Base case: UKPDS 68; SA: none stated |
After HbA1c 7.5% exceeded = comparators discontinued, switch to basal INS, 8.0 exceeded = switch to basal-bolus + stop GLP1 | Comparators discontinued |
| Gorgojo-Martínez, 2020 [49] | Semaglutide-I vs. empagliflozin | No | HbA1c, systolic blood pressure, BMI | None stated | Any, stroke, myocardial infarction, angina, congestive heart failure, peripheral vascular disease, neuropathy, amputation, ulcer, end-stage renal disease, gross proteinuria, microalbuminuria, macular edema, cataract, proliferative retinopathy, background retinopathy, severe vision loss, blindness, hemodialysis, non-severe hypoglycaemic event, severe hypoglycaemic event, gangrene |
Base case: none stated; SA: UKPDS 68 and 82 |
Both treatments switched to basal INS glargine U100, after 3 years | Comparators discontinued |
| Johansen, 2020 [48] | Semaglutide-I vs. liraglutide | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, microalbuminuria, gross proteinuria, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, proliferative retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe hypoglycaemic event, severe nocturnal hypoglycaemic event |
Base case: UKPDS 68; SA: UKPDS 82 |
After HbA1c 7.5% exceeded = stop semaglutide, basal INS started | Comparators discontinued |
| Martín, 2020 [47] | Semaglutide-I vs. dulaglutide, sitagliptin | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event | Hypoglycaemia | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, microalbuminuria, gross proteinuria, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event, laser treatment, blindness, gangrene, severe vision loss, end-stage renal disease |
Base case: UKPDS 68; SA: UKPDS 82 |
After 3 years = basal INS glargine U100 | Comparators discontinued |
| Capehorn, 2021 [57] | Semaglutide-I vs. empagliflozin | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate | Non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe hypoglycaemic event, severe nocturnal hypoglycaemic event | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, microalbuminuria, gross proteinuria, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, proliferative retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe hypoglycaemic event, severe nocturnal hypoglycaemic event, laser treatment, blindness |
Base case: UKPDS 68; SA: UKPDS 82 |
After exceeding threshold: HbA1c 7.5% = replace therapy with basal INS | Comparators discontinued |
| Guzauskas, 2021 [64] | Semaglutide-O vs. empagliflozin, liraglutide, sitagliptin, MET+SU | Yes | HbA1c, weight, severe hypoglycaemic event, discontinuation associated with AEs, MACE, heart failure, nephropathy | Severe hypoglycaemic event, discontinuation associated with AEs | Heart failure, ischaemic heart disease, myocardial infarction, stroke, blindness, ulcer, amputation, nephropathy, severe hypoglycaemic event, cardiovascular death, MACE |
Base case: UKPDS OM2; SA: none stated |
After HbA1c 8.5% exceeded = add INS therapy to comparators, except for sitagliptin, which is also discontinued | Comparators continued |
| Malkin, 2021 [53] | Semaglutide-O vs. empagliflozin, sitagliptin, liraglutide | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, BMI, non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | None stated |
Base case: UKPDS 68; SA: UKPDS 82 |
After HbA1c 7.5% exceeded = discontinue drug/comparator, replace with basal INS | Comparators discontinued |
| Risebrough, 2021 [70] | Semaglutide-O vs. dulaglutide,liraglutide,semaglutide-I | Yes | HbA1c, weight, systolic blood pressure, discontinuation associated with AEs, nausea, vomiting, hypoglycaemia, diarrhoea, ischaemic heart disease, myocardial infarction, congestive heart failure, stroke, revascularisation, proteinuria, serum creatinine, neuropathy, ulcer, amputation | Discontinuation associated with AEs, non-severe hypoglycaemic event, severe hypoglycaemic event, nausea, vomiting, diarrhoea | Non-severe hypoglycaemic event, severe hypoglycaemic event, ischaemic heart disease, myocardial infarction, congestive heart failure, stroke, amputation, blindness, renal failure |
Base case: UKPDS OM1; SA: none stated |
After 3 years = switch to INS | Comparators discontinued |
| Ehlers, 2022 [58] | Semaglutide-O vs. empagliflozin | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, BMI | Non-severe hypoglycaemic event, severe hypoglycaemic event, urinary tract infection, gastrointestinal | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, hemodialysis, peritoneal dialysis, renal transplant, non-severe hypoglycaemic event, severe hypoglycaemic event, laser treatment, cataract, blindness, neuropathy, ulcer, amputation, macular edema, urinary tract infection, proliferative retinopathy, background retinopathy |
Base case: UKPDS 82 and 68; SA: none stated |
After HbA1c 7.5% exceeded = add basal INS | Comparators continued |
| Ehlers, 2022 [59] | Semaglutide-I vs. empagliflozin | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, BMI, non-severe hypoglycaemic event, severe hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, urinary tract infection, gastrointestinal | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, hemodialysis, peritoneal dialysis, renal transplant, non-severe hypoglycaemic event, severe hypoglycaemic event, laser treatment, cataract, blindness, neuropathy, ulcer, amputation, macular edema, urinary tract infection, proliferative retinopathy, background retinopathy |
Base case: UKPDS 68 and 82; SA: none stated |
After HbA1c 7.5% exceeded = add basal INS | Comparators continued |
| Ekhlasi, 2022 [83] | Dulaglutide vs. liraglutide | No | HbA1c, hypoglycaemia | Hypoglycaemia | Hypoglycaemia, nephropathy, retinopathy, myocardial infarction, stroke | None stated | No switch | No switch/NA |
| Eliasson, 2022 [60] | Semaglutide-O vs. empagliflozin, sitagliptin | Yes | HbA1c, systolic blood pressure, total cholesterol, LDL, HDL, triglycerides, BMI, heart rate, white blood cell count, estimated glomerular filtration rate, cardiovascular death, myocardial infarction, stroke, heart failure | Non-severe hypoglycaemic event, severe hypoglycaemic event | Background retinopathy, proliferative retinopathy, macular edema, severe vision loss, neuropathy, peripheral vascular disease, amputation, microalbuminuria, macroalbuminuria, end-stage renal disease, ischaemic heart disease, myocardial infarction, stroke, heart failure, non-severe hypoglycaemic event, severe hypoglycaemic event |
Base case: UKPDS 82, Eastman [99], Bagust [100]; SA: UKPDS 68 |
After HbA1c 8.0% exceeded = stop comparators and initiate basal, after HbA1c 8.0% exceeded again bolus INS was added | Comparators discontinued |
| Franch-Nadal, 2022 [63] | Semaglutide-O vs. empagliflozin, sitagliptin, liraglutide | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, BMI, severe hypoglycaemic event, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Severe hypoglycaemic event, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Myocardial infarction, angina, heart failure, stroke, peripheral vascular disease, hemodialysis, peritoneal dialysis, renal transplant, non-severe hypoglycaemic event, severe hypoglycaemic event, cataract, blindness, neuropathy, amputation, gangrene, ulcer |
Base case: UKPDS 68; SA: UKPDS 82 |
After HbA1c 7.5% exceeded = replace with basal INS | Comparators discontinued |
| Hu, 2022 [79] | Semaglutide-I vs. dulaglutide | Yes | HbA1c, weight, BMI, systolic blood pressure, heart rate | None stated | Ischaemic heart disease, myocardial infarction, heart failure, stroke, amputation, blindness, renal failure, ulcer, end-stage renal disease |
Base case: UKPDS OM2; SA: none stated |
After 5 years = substitute with basal INS | Comparators discontinued |
| Malkin, 2022 [61] | Semaglutide-O vs. empagliflozin, dulaglutide | Yes | HbA1c, systolic blood pressure, total cholesterol, HDL, BMI, non-severe hypoglycaemic event, severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, severe nocturnal hypoglycaemic event, non-severe nocturnal hypoglycaemic event | Myocardial infarction, angina, heart failure, stroke, peripheral vascular disease, microalbuminuria, gross proteinuria, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, proliferative retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, non-severe hypoglycaemic event, severe hypoglycaemic event, severe nocturnal hypoglycaemic event, non-severe nocturnal hypoglycaemic event |
Base case: UKPDS 68; SA: UKPDS 82 |
After HbA1c 7.5% exceeded = replace with basal INS | Comparators discontinued |
| Ruan, 2022 [81] | Semaglutide-I vs. dulaglutide | Yes | HbA1c, systolic blood pressure, total cholesterol, LDL, HDL, triglycerides, BMI, heart rate, white blood cell count, estimated glomerular filtration rate, ischaemic heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, non-severe hypoglycaemic event, severe hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event | Background retinopathy, proliferative retinopathy, macular edema, severe vision loss, neuropathy, peripheral vascular disease, amputation, microalbuminuria, macroalbuminuria, end-stage renal disease, ischaemic heart disease, myocardial infarction, stroke, heart failure, non-severe hypoglycaemic event, severe hypoglycaemic event | Base case: UKPDS 68 and 82 and SweNDR, Fremantle | After 1 years = replace with basal INS | Comparators discontinued |
| Stafford, 2022 [73] | Semaglutide-I vs. canagliflozin | Yes | HbA1c, systolic blood pressure, total cholesterol, LDL, HDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event | Background retinopathy, proliferative retinopathy, macular edema, blindness, neuropathy, ulcer, amputation, microalbuminuria, macroalbuminuria, chronic kidney disease, end-stage renal disease, myocardial infarction, ischaemic heart disease, heart failure, stroke, peripheral vascular disease, non-severe hypoglycaemic event, severe hypoglycaemic event |
Base case: UKPDS 82; SA: UKPDS 68 |
After HbA1c 8% reached = replace with basal INS, and after HbA1c 8% exceeded again, basal-bolus | Comparators discontinued |
| Viljoen, 2022 [62] | Semaglutide-I vs. dulaglutide | Yes | HbA1c, BMI | Set to 0 | Myocardial infarction, angina, heart failure, stroke, peripheral vascular disease, hemodialysis, peritoneal dialysis, renal transplant, non-severe hypoglycaemic event, severe hypoglycemic event, cataract, blindness, neuropathy, amputation, gangrene, ulcer, microalbuminuria, gross proteinuria, background retinopathy, proliferative retinopathy, macular edema |
Base case: UKPDS 68; SA: UKPDS 82 |
After 3 years = replace with basal INS | Comparators discontinued |
| Chien, 2020 [75] | No main vs. classes: MET, SU, DPP4, SGLT2, GLP1, Unspecified INS | Yes | HbA1c, weight, systolic blood pressure, total cholesterol, HDL, non-severe hypoglycaemic event, severe hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, urinary tract infection, gastrointestinal | Ischaemic heart disease, myocardial infarction, congestive heart failure, stroke, amputation, blindness, end-stage renal disease, severe hypoglycaemic event |
Base case: UKPDS 68; SA: none stated |
Allocation to dual therapy after HbA1c 8.01% reached. Unclear when what threshold led to allocation to triple therapy or INS+MET | Comparators continued, then discontinued |
| Hu, 2021 [76] | Dapagliflozin + saxagliptin combination therapy vs. dapagliflozin monotherapy vs. saxagliptin monotherapy | Yes | HbA1c, systolic blood pressure, fasting plasma glucose, HDL, weight | Severe hypoglycaemic event, non-severe hypoglycaemic event, discontinuation associated with AEs | Ischaemic heart disease, myocardial infarction, congestive heart failure, stroke, blindness, end-stage renal disease, amputation, ulcer, hypoglycaemia, genitourinary infection, urinary tract infection |
Base case: UKPDS OM2; SA: none stated |
Not stated | No switch/NA |
| Lin, 2021 [78] | No main vs. linagliptin 5 mg, saxagliptin 5 mg, alogliptin 25 mg, sitagliptin 100 mg, and vildagliptin 50 mg | No | HbA1c | None stated | Myocardial infarction, stroke, congestive heart failure, atherosclerotic cardiovascular disease, cardiovascular death, end-stage renal disease, blindness, neuropathy, amputation | None stated | No switch | No switch/NA |
| Zupa, 2021 [71] | Empagliflozin vs. semaglutide-I | No | Heart failure, myocardial infarction, stroke, nephropathy, end-stage renal disease, all-cause mortality | Genitourinary infection | Heart failure, stroke, myocardial infarction, nephropathy, end-stage renal disease | None stated | No switch | No switch/NA |
| Chakravarty, 2018 [65] | Dapagliflozin vs. liraglutide, SU, DPP4, pioglitazone | Yes | HbA1c, weight, systolic blood pressure, hypoglycaemia | Hypoglycaemia | None stated | None stated | NA | No switch/NA |
| Neslusan, 2018 [66] | Canagliflozin vs. dapagliflozin | Yes | HbA1c, systolic blood pressure, BMI, total cholesterol, LDL, HDL, triglycerides, non-severe hypoglycaemic event, severe hypoglycaemic event, male genital mycotic infection, female genital mycotic infection, lower urinary tract infection, upper urinary tract infection, volume depletion-related AEs, osmotic diuresis-related AEs, discontinuation associated with AEs | Non-severe hypoglycaemic event, severe hypoglycaemic event, male genital mycotic infection, female genital mycotic infection, lower urinary tract infection, upper urinary tract infection, volume depletion-related AEs, osmotic diuresis-related AEs, discontinuation associated with AEs | Myocardial infarction, ischaemic heart disease, congestive heart failure, stroke, peripheral vascular disease, CVD, proliferative retinopathy, background retinopathy, macular edema, blind one eye, blind two eyes, neuropathy, ulcer, amputation, macroalbuminuria, microalbuminuria, chronic kidney disease in stages3a–5, end-stage renal disease |
Base case: UKPDS 82 and 68, CDC, WESDR, REP; SA: none stated |
INTENSIFY after: >7% HbA1c/>140 SBP/>2.6 LDL (comparators discontinued) | Comparators discontinued |
| Hou, 2019 [74] | Canagliflozin vs. dapagliflozin | No | HbA1c, systolic blood pressure, total cholesterol, HDL | Urinary tract infection, gastrointestinal | Myocardial infarction, stroke, congestive heart failure, end-stage renal disease, blindness, neuropathy, amputation | Base case: COMT model | No switch | No switch/NA |
| Ramos, 2019 [40] | Empagliflozin vs. sitagliptin, saxagliptin | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event, genitourinary infection | Non-severe hypoglycaemic event, severe hypoglycaemic event, genitourinary infection | Myocardial infarction, angina, peripheral vascular disease, stroke, congestive heart failure, microalbuminuria, gross proteinuria, end-stage renal disease, background retinopathy, proliferative retinopathy, macular edema, severe vision loss, cataract, ulcer, amputation, neuropathy, non-severe hypoglycaemic event, severe hypoglycaemic event, hemodialysis, peritoneal dialysis, laser treatment, renal transplant, genitourinary infection, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event, gangrene |
Base case: UKPDS 82; SA: none stated |
All switch to basal bolus when 8.5% HbA1c reached | Comparators discontinued |
| Ramos, 2020 [45] | Empagliflozin vs. SoC and liraglutide | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event, severe hypoglycaemic event | Non-severe hypoglycaemic event, severe hypoglycaemic event, severe hypoglycaemic event, genitourinary infection | Myocardial infarction, angina, heart failure, stroke, peripheral vascular disease, microalbuminuria, gross proteinuria, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, proliferative retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, severe nocturnal hypoglycaemic event, severe hypoglyceemic event, severe nocturnal hypoglycaemic event, severe hypoglycaemic event, laser treatment, blindness, gangrene |
Base case: UKPDS 82 and 68, ADVANCE, SweNDR, ARIC, Fremantle, PROCAM; SA: none stated |
After HbA1c 8.5% exceeded = meal INS (57 IU) and INS glargine (94 IU) started, uncertain if liraglutide and empagliflozin and MET still on | Comparators discontinued |
| Ramos, 2020 [46] | Empagliflozin vs. semaglutide-O | Yes |
(1) HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, BMI; (2) HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, BMI, heart failure |
Severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event, severe hypoglycaemic event, severe hypoglycaemic event | Myocardial infarction, angina, heart failure, stroke, peripheral vascular disease, microalbuminuria, macroalbuminuria, hemodialysis, peritoneal dialysis, renal transplant, background retinopathy, proliferative retinopathy, macular edema, severe vision loss, cataract, neuropathy, ulcer, amputation, non-severe nocturnal hypoglycaemic event, severe nocturnal hypoglycaemic event, non-severe hypoglycaemic event, severe hypoglycaemic event, laser treatment, blindness |
Base case: UKPDS 68, UKPDS 82; SA: none stated |
After HbA1c 7.5% exceeded = basal INS started, semaglutide-I and empagliflozin and MET still on | Comparators continued |
| Reifsnider, 2020 [69] | Empagliflozin vs. sitagliptin | Yes |
(1) Without CVD: HbA1c, weight, systolic blood pressure (2) With CVD: Risk equations from EMPA-REG OUTCOME |
Urinary tract infection, genital mycotic infection, upper respiratory tract infection, nasopharyngitis, headache | Myocardial infarction, stroke, heart failure, ischaemic heart disease, blindness, amputation, renal failure, cardiovascular death, transient ischaemic attack, revascularisation, macroalbuminuria, renal injury, angina, ulcer |
Base case: UKPDS OM2; SA: none stated |
Addition of either sitagliptin or empagliflozin as third-line, and INS as third-line. Rates from Montvida [101] | Comparators continued |
| Van der Linden, 2020 [54] | Dapagliflozin vs. DPP4 | Yes | HbA1c, systolic blood pressure, total cholesterol, weight, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, non-severe hypoglycaemic event, urinary tract infection, gastrointestinal, discontinuation in general | Non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, non-severe hypoglycaemic event, urinary tract infection, gastrointestinal, discontinuation in general | Ischaemic heart disease, myocardial infarction, congestive heart failure, stroke, amputation, blindness, end-stage renal disease, non-severe hypoglycaemic event, non-severe nocturnal hypoglycaemic event, severe hypoglycaemic event, urinary tract infection, gastrointestinal |
Base case: UKPDS68; SA: none stated |
After HbA1c 8% exceeded = replace comparators with basal INS; when that fails, add bolus minus SU | Comparators discontinued |
| Ehlers, 2021 [56] | Empagliflozin vs. liraglutide | Yes | HbA1c et al. not reported, cardiovascular death, all-cause mortality, composite endpoint, heart failure, stroke, myocardial infarction | Non-severe hypoglycaemic event, severe hypoglycaemic event, severe nocturnal hypoglycaemic event, non-severe nocturnal hypoglycaemic event, genitourinary infection | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, hemodialysis, peritoneal dialysis, renal transplant, non-severe hypoglycaemic event, severe hypoglycaemic event, laser treatment, cataract, blindness, neuropathy, amputation, gangrene, ulcer, microalbuminuria, end-stage renal disease, background retinopathy |
Base case: UKPDS 82; SA: none stated |
After 9 years (HbA1c = 8.5%), both arms switched to basal-bolus therapy | Comparators discontinued |
| Gourzoulidis, 2021 [55] | Empagliflozin vs. dapagliflozin | No | Cardiovascular death, myocardial infarction, stroke, composite renal outcome, genital mycotic infection, acute kidney injury, severe hypoglycaemic event | Severe hypoglycaemic event, genital mycotic infection, acute kidney injury | Myocardial infarction, stroke, heart failure, cardiovascular death, composite renal outcome, severe hypoglycaemic event | None stated | No switch | No switch/NA |
| Ramos, 2021 [77] | Empagliflozin vs. liraglutide, sitagliptin | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, BMI, estimated glomerular filtration rate, non-severe hypoglycaemic event, severe hypoglycaemic event, genitourinary infection | Non-severe hypoglycaemic event, severe hypoglycaemic event, genitourinary infection | Myocardial infarction, angina, congestive heart failure, stroke, peripheral vascular disease, hemodialysis, peritoneal dialysis, renal transplant, non-severe hypoglycaemic event, severe hypoglycaemic event, laser treatment, cataract, blindness, neuropathy, amputation, gangrene, ulcer |
Base case: UKPDS 82; SA: none stated |
After exceeding threshold: HbA1c 8.5% = replace therapy with basal-bolus INS | Comparators discontinued |
| Reifsnider, 2021 [67] | Empagliflozin vs. dapagliflozin, canagliflozin | No | Cardiovascular death, myocardial infarction, stroke, heart failure, progression of albuminuria, composite renal outcome, genital mycotic infection, acute kidney injury, amputation, bone fracture, severe hypoglycaemic event | Severe hypoglycaemic event, genital mycotic infection, acute kidney injury, amputation, bone fracture | Cardiovascular death, myocardial infarction, stroke, heart failure, angina, transient ischaemic attack, revascularisation, progression of albuminuria, composite renal outcome, genital mycotic infection, acute kidney injury, amputation, bone fracture, severe hypoglycaemic event | Inhouse, based on EMPA-REG OUTCOME | No switch | No switch/NA |
| Lasalvia, 2022 [82] | Dapagliflozin vs. DPP4 | Yes | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, haematocrit,weight | Non-severe hypoglycaemic event, severe hypoglycemic event, genitourinary infection | Ischaemic heart disease, myocardial infarction, congestive heart failure, stroke, amputation, blindness, end-stage renal disease, ulcer, non-severe hypoglycaemic event, severe hypoglycaemic event, genitourinary infection |
Base case: UKPDS 68; SA: UKPDS 82 |
After HbA1c 8.3% or 8.1% = ‘receive rescue therapy with basal INS’ | Comparators discontinued |
| Peng, 2022 [80] | SGLT2 vs. DPP4 | No | HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, hematocrit, weight | Unclear | Myocardial infarction, heart failure, stroke, all-cause mortality | None stated | No switch | No switch/NA |
| Reifsnider, 2022 [72] | Empagliflozin vs. liraglutide | Yes | HbA1c, weight, systolic blood pressure, cardiovascular death, myocardial infarction, stroke, heart failure, macroalbuminuria, renal injury, renal failure | Genital mycotic infection, urinary tract infection, nausea, hypoglycaemia, injection site reaction | Myocardial infarction, stroke, heart failure, ischaemic heart disease, blindness, ulcer, amputation, renal failure, cardiovascular death, angina, revascularisation, macroalbuminuria, renal injury, renal failure, hypoglycaemia |
Base case: UKPDS 68 and 82; SA: none stated |
Addition of either liraglutide or empagliflozin as third-line, and INS as fourth-line. Rates from Montvida [101] | Comparators continued |
AE adverse events, ARIC Atherosclerosis Risk in Communities, ADVANCE Action in Diabetes and Vascular Disease: preterax and diamicron-MR controlled evaluation, BMI body mass index, CDC Centers for Disease Control and Prevention, COMT Chinese Outcomes Model for T2DM, CVD cardiovascular disease, DPP4 dipeptidylpeptidase-4 inhibitors, Fremantle The Fremantle Diabetes Study, GLP1 glucagon-like peptide-1 receptor agonist, HDL high-density lipoprotein, IDegLira combination of liraglutide and INS degludec, INS insulin, LDL low-density lipoprotein, MACE major adverse cardiac events, MDI multiple daily injections, MET metformin, NA not available, PROCAM Prospective Cardiovascular Münster, QW every week, REP Rochester Epidemiology Project, SA sensitivity analysis, SoC standard of care, SU sulfonylurea, SGLT2 sodium-glucose cotransporter-2 inhibitors, SweNDR Swedish National Diabetes Register, UKPDS UK Prospective Diabetes Study, UKPDS OM UK Prospective Diabetes Study outcomes model, WESDR Wisconsin Epidemiologic Study of Diabetic Retinopathy
Source of Baseline Characteristics of the Modelled Cohorts
The baseline characteristics of the modelled cohorts were sourced from randomised controlled trials (RCTs) in most studies (42/50), some of which focused on cardiovascular outcomes. Real-world data from observational studies or data from other CEAs were only used in eight studies [39, 50, 64, 65, 69, 72, 80, 83]. Baseline tables describing the characteristics of the modelled cohort were unavailable in one study [38, 39, 49, 50, 67, 71, 74, 78, 80, 83]. The characteristics of the cohorts in the studies were most frequently sourced from the SUSTAIN 7 [92] (n = 10) and PIONEER 2 (n = 10) trials [93], followed by EMPA-REG OUTCOME [94] (n = 7), and PIONEER 3 (n = 5) [95]. These trials tested the efficacy of injectable semaglutide, oral semaglutide, and empagliflozin.
However, there was considerable variation in the patient eligibility criteria across these trials. For example, SUSTAIN 7 excluded patients with HF, CKD, and retinopathy, while PIONEER 2 excluded patients with renal impairment, retinopathy, and pancreatitis, and EMPA-REG OUTCOME only included patients with established CVD (see Table 3 for more details on which trials were used as the basis for the modelled cohorts).
Subgroup Analysis
Explicit subgroup analysis was performed in 11 studies. Four studies analysed a subgroup with body mass index (BMI) ≥30 [41–43, 53]; two of these studies performed their base-case analysis on a cohort with BMI ≥35 [41, 42], while the other two performed a separate subgroup analysis on individuals with BMI ≥30 [43, 53]. A subgroup analysis on individuals with and without a history of DRCs, primarily CVD, was performed in four studies [69, 71, 72, 80]. One study used four different subgroups from the EXTRA study, and the two remaining studies used a cohort with different characteristics than the base-case cohort to test their results [38, 75].
Model Inputs: Treatment Effect and Adverse Events
The treatment effect and AEs were sourced from multiple sources, with RCTs being the most common (n = 36), followed by network meta-analyses (n = 16), indirect treatment comparisons (n = 8), or other CEAs (n = 6). The studies frequently used treatment effects from the SUSTAIN 7 (n = 9), PIONEER 2 (n = 9), EMPA-REG OUTCOME (n = 8), and PIONEER 3 (n = 6) trials.
The most frequently used treatment effects were changes in HbA1c (n = 47), systolic blood pressure (n = 41), total cholesterol (n = 33), and high-density lipoprotein (n = 33). Similar treatment effects were used between the studies, although those with SGLT2 as the main comparator had a broader range of treatment effects than those with GLP1 as the main comparator. Five studies with empagliflozin as the main comparator [46, 55, 56, 67, 72] and four studies with oral semaglutide [60, 64, 70, 81] used treatment effects specific to the reduction of CVD as an additional separate part of the treatment effect parameters. One study comparing the DPP4 class of products also included CVD-related effects [71]. Studies with GLP1 as the main comparator focused more on hyperglycaemia than those with SGLT2.
Most studies included drug-related AEs, and the most frequently used were variations of hypoglycaemic events: severe (n = 35), non-severe (n = 33), severe nocturnal (n = 13), and non-severe nocturnal (n = 12). Only one study, with GLP1 as the main comparator, included diabetic ketoacidosis [52]. For more information on treatment and adverse effects, see Table 3; for the sources of model input, see Online Resource Appendix A2.
Key Model Assumptions
Two key model assumptions used in the included studies are reported in this section: (1) the risk equations used to predict the development of DRCs over time; and (2) the time until, and conditions leading to, the treatment switch. There were notable differences in how these assumptions were applied in the studies (see Table 3 for details).
The UKPDS 68 [96] and 82 [88] risk equations were the most frequently used. However, there were differences in what risk equations were used in the base-case analysis, which depended on the class of the main comparator. Among the 27 studies using UKPDS 68 in the base-case scenario, 20 studies had GLP1 as the main comparator [34, 37–39, 41, 42, 47, 48, 50–53, 57–59, 61–63, 68, 81] and six studies had SGLT2 as the main comparator [45, 46, 54, 66, 72, 82], while one study [75] had no main comparator. Of the 20 times UKPDS 82 was used in the base-case, eight studies had SGLT2 as the main comparator [40, 45, 46, 56, 66, 67, 69, 72, 77], 10 studies had GLP1 as the main comparator [35, 39, 58–60, 64, 68, 73, 79, 81], and one study [76] had no main comparator. In eight of the studies mentioned above, both risk equations were used for the base-case; half of these studies had GLP1 as the main comparator [58, 59, 68, 81] and the other half had SGLT2 as the main comparator [45, 46, 66, 72]. GLP1 was the main comparator in all of the 18 studies that used these risk equations in the SA, with three using UKPDS 68 [60, 68, 73], 13 using UKPDS 82 [37–39, 41, 47, 48, 51, 53, 57, 61–63, 82], and two using both [43, 49]. Only one study with SGLT2 as the main comparator used UKPDS 82 in the SA [82].
Explicit reasoning for the choice of risk equation was only present in a few cases. Three studies with SGLT2 as the main comparator cited model fit as the reason [40, 45, 56], while eight GLP1 studies cited advice from model proprietors as the reason [37, 38, 47, 49, 57, 61–63]. Different aspects of the risk equations were sometimes used simultaneously. For example, in the study by Neslusan et al. [66], the change in HbA1c, systolic blood pressure (SBP), and lipids was modelled using UKPDS 68, macrovascular complications were modelled using UKPDS 82, and microvascular complications were modelled using a combination of four different risk equations [97–100]. Six studies either did not use risk equations or had unclear reporting of their use [55, 65, 71, 78, 80, 83]. For details on the risk equations used, see Table 3.
Regarding treatment switch, it occurred either at a predefined period after treatment initiation of the main comparators [36–39, 41–43, 47, 49, 62, 68–70, 72, 79, 81], when the HbA1c levels of the cohort reached a certain threshold [34, 35, 40, 46, 48, 50–52, 54, 57–60, 62–64, 66, 73, 75, 82], or a combination of the two [44, 45, 53, 56, 77]. These variations in treatment switch occurred in 16, 20, and 5 studies, respectively. The time to treatment switch was shorter for studies with GLP1 as the main comparator (n = 20, mean = 3.95 years, standard deviation = 2.7 years) than for the SGLT2 studies (n = 3, mean = 10 years, standard deviation = 2.6 years). At the point of treatment switch, the comparators were either discontinued and replaced with a different medication, often INS [34–45, 47–51, 53, 54, 56, 57, 60–63, 66, 68, 70, 73, 76, 77, 81, 82], continued while different medications could be added [46, 58, 59, 64, 69, 72], or continued for one step and then discontinued at a later step [52, 75], as reported in 33, 6, and 2 studies, respectively. Studies with GLP1 as the main comparator had the comparators discontinued 84% of the time, while those with SGLT2 had the comparators discontinued 47% of the time. Nine did not report a treatment switch [55, 65, 67, 74, 76].
Model Outputs—Diabetes-Related Complications
In the conducted CEAs, the most common output related to DRC was a summary of incidence or onset, most frequently both. Only two studies did not include DRCs as the output [53, 65], while the majority had similar items within the category. Overall, the difference in DRC outputs between the studies was small when present. SGLT2 studies reported HF and cardiovascular death more often and had more varied outputs relating to nephropathy, while GLP1 studies had more varied outputs regarding hypoglycaemia and retinopathy (see Table 3 for all extracted data).
The following categories represented the DRCs: CVD, nephropathy, neuropathy, retinopathy, AEs, and others. The most-reported DRC category was CVD, represented by stroke, myocardial infarction, HF or congestive HF, peripheral vascular disease, angina, ischaemic heart disease, CV death, composite major adverse cardiac events, and transient ischaemic attack. The nephropathic complications were represented by microalbuminuria, end-stage renal disease, haemodialysis, renal transplant, peritoneal dialysis, gross proteinuria, macroalbuminuria, composite renal outcome, renal injury, renal failure, progression of albuminuria, nephropathy, and acute kidney injury. Similar to the treatment effects, AEs as the model output were almost exclusively hypoglycaemia, divided into subcategories of severe and non-severe, and sometimes further divided into non-nocturnal and nocturnal. AEs associated with certain classes were also present, such as genitourinary infection for the SGLT2 class and gastrointestinal issues for GLP1. The neuropathy group consisted of amputation, ulcer, and neuropathy. Macular oedema, blindness, cataract, vision loss, background and proliferative retinopathy, and non-proliferative retinopathy were included in the retinopathy group, whereas laser treatment, gangrene, and revascularisation were included in the ‘others’ category.
Quality of the Included Studies
The answers to the essential questions of the quality assessment checklist are provided in Table 4. Answers to questions 1, 3, 6, 7, and 8 were adequate in almost all studies; however, answers to questions 2, 4, 5, 9, and 10 had some inadequacies. The description of comparators (question 2) was frequently partially adequately described due to the omission of relevant comparators to the research question (e.g., GLP1 products were compared only with GLP1 products and no SGLT2 products). Additionally, most studies lacked a societal perspective in their analyses (question 4). The accuracy of cost and consequence measurement (question 5) was often unclear due to the use of data that may not represent current treatment practice (i.e., the UKPDS risk equations) and the frequent use of short times to treatment switch, which may not reflect modern treatment practice. The heterogeneity of patient groups was rarely explored when the studies addressed uncertainty (question 9). Finally, the discussion sections (question 10) had inadequacies, mainly regarding comparison with other studies and generalisability.
Discussion
Main Findings
This systematic review aimed to provide an overview of the cost-effectiveness outcomes and the underlying methodological choices of the most recent studies published. In the included studies, SGLT2 and GLP1 products were the comparators of choice among the nNIADs. Empagliflozin and semaglutide (oral and injectable) were the most frequently used main comparators and were found to be cost effective compared with other products within their respective classes. This aligns with the recent ADA reports recommending using products from the SGLT2 and GLP1 classes based on the patient’s risk level and treatment goals [15, 16]. However, only 38% of studies compared SGLT2 and GLP1 directly. The lack of direct comparisons could be due to a lack of direct comparison data, although several of the included studies have performed indirect treatment comparisons or network meta-analyses, or used existing comparisons from the literature.
The cost effectiveness findings can be seen from both a class and a product level. From the class-level perspective, one class was not favoured over the other, but GLP1 never dominated SGLT2. On the other hand, SGLT2 dominated GLP1 in six comparisons. The dominance of SGLT2 over GLP1 seems limited to the older GLP1 products, as only one of the dominant results was between empagliflozin and the newer line of GLP1 products, oral semaglutide [46]. From the product perspective, semaglutide (oral or injectable) was more frequently the main comparator against empagliflozin (n = 11) than vice versa (n = 2) and was cost effective in the majority of these comparisons.
Sponsorship bias may be inferred from the fact that all industry-funded studies found their product to be cost effective or dominating, except for two studies that compared their competitors’ product against their own (i.e. injectable and oral semaglutide were compared with empagliflozin), and found the competitor to be not cost effective [58, 59]. In the non-industry-sponsored studies where SGLT2 and GLP1 were compared, oral semaglutide was found to be cost effective against all comparators except empagliflozin [64]. In contrast, the other study found injectable semaglutide to be cost effective against empagliflozin [71].
There were considerable regional differences in the choice of main comparators, with Europe favouring GLP1, the Asian region favouring SGLT2 slightly more, and the North American region seemed to favour both equally. Most included studies were conducted in Europe, possibly explained by its increased focus on prioritising healthcare resources. However, Asia and North America may benefit more from increased prioritisation of healthcare resources, as the current and projected incidence of T2DM is much higher than in the European region [1].
It can be seen as a shortcoming that only 11 studies explicitly defined the subgroups they modelled. Presenting results for different subgroups might help differentiate SGLT2 and GLP1 products more. Emphasising the differences in their respective effects might be especially important since the Danish Medicines Counsel has adopted the simplified assumption that their effects are equivalent [102]. The modelled cohorts were almost exclusively in a broad category described as patients with T2DM uncontrolled on one or several first-line medications. Most studies with empagliflozin as the main comparator based their cohort on the EMPA-REG OUTCOME trial, which only included patients with established CVD and focused more on explicitly incorporating the reduction of cardiorenal DRCs as separate treatment-effect parameters. Including the effect as separate parameters could lead to more accurate models since GLP1 and SGLT2 have demonstrated a reduction of DRCs that occur independent of the antihyperglycaemic effect [15]. However, one of the included studies argued that including both the direct and indirect effects carries the risk of double-counting, which could overestimate the effect [61]. The majority of the included studies focused on translating the differences between the comparators in terms of how they changed common physiological markers (HbA1c, SBP, cholesterol, etc.) into differences in DRCs over time. Some have critiqued the approach of translating these frequently minor differences into large effects over a time horizon that might be too long [25].
Regarding differences in the inclusion of AEs, this review highlighted the omission of diabetic ketoacidosis as an AE when SGLT2 was a comparator. It is a rare but well-established and potentially deadly AE, especially for INS-dependent T2DM [15, 94, 103–107], that included only one study [52] with GLP1 as the main comparator included. These differences between the models, which in some cases seem to be dependent on the class of the main comparator, are problematic, and decision makers need to pay special attention to this, as the results of the economic analysis could be greatly affected by them.
Most studies (48%) of DAMs used in the health economic evaluations were based on the CDM from the IQVIA™ [84, 85]. Still, the high representation of this model does not necessarily reflect its superiority compared with other models available. Studies with GLP1 as the main comparator use this model 61% of the time, while those with SGLT2 use it 33% of the time. The Mount Hood meetings exemplify that there are several complex simulation models specific to diabetes, each with own its strengths and weaknesses [108–111].
Development of the physiological parameters over time in the context of the disease is primarily governed by risk equations that represent assumptions about the progression of the disease. The present review focused on which risk equations were used and whether it was used for the base-case analysis or the SA. The most commonly used risk equations were UKPDS 68 and 82, which are based on the UKPDS study [96] from 1977 to 1997, while UKPDS 82 uses 10 years of follow-up beyond that [88]. While these risk equations are currently widely used, contemporary studies may be able to produce more accurate risk equations that better reflect modern treatment practices. The choice of risk equation seemed connected to the class of the main comparator, and the reasoning behind the choice was rarely explicit in the studies. The GLP1 studies that were explicit in their reasoning cited recommendations from the model proprietors (i.e. of the CDM). The authors of this paper could not find any such recommendations in the model validation paper, where the UKPDS 82 has been incorporated since 2014 [88]. The three studies with SGLT2 cited model fit, and reference to model proprietor recommendations was absent, even though they also used the CDM. We recommend that the reasoning behind the choice of risk equations is always stated explicitly, as it may be difficult to explain why using UKPDS 68 was the better choice since the UKPDS 82 risk engine is an updated and improved version based on a larger dataset over an extended period, with more significant predictors and event types, and, in general, predicted fewer DRC events [88].
Another key assumption relates to the time from initiation of the comparators in the study until they are either switched out, additional medication is added, or are replaced by rescue therapy. Studies with GLP1 as the main comparator and predefined time to switch often cited a report that states that the mean treatment duration of GLP1 was 29.35 months [112]. However, treatment guidelines known to the authors of this paper recommend lifelong treatment with the nNIADs, and only stopping if their use was contraindicated. Models that use a short preset time-to-treatment switch where the comparator was replaced with INS might not be representative of real-world practice, and the assumption might lead to misinforming decision makers about the long-term consequences of utilising that comparator.
The quality of the included studies was, in large part, adequate. The inadequate answers were about the omission of relevant comparators, lack of societal perspective, and the accuracy of measuring costs and consequences. These quality issues may however be a limitation of available data and not a lack of consideration from the authors of the included studies. However, the methods used in other studies were rarely discussed, except for some recent studies [58, 59, 61–63, 72, 73], where the authors directly commented on the advantages and disadvantages of their own and others’ methods.
Connecting Main Findings to Other Studies
Other reviews in this area find that SGLT2 and GLP1 appear to be cost effective for patients with T2DM uncontrolled on MET and other background oNIADs. Ruan et al. [30] focused on DPP4 and found it to be cost effective as a second-line treatment compared with sulfonylurea (SU) and INS, but not SGLT2 and GLP1. Bagepally et al. [28] found GLP1 to be cost effective compared with SU, DPP4, and TZD in high-income countries, while Bagepally et al. [29] found SGLT2 to be cost effective against SU, but not DPP4, in high-income countries. Zozaya et al. [31], Rahman et al. [26], and Yoshida et al. [27] focused on SGLT2 and found the class to be cost effective against SU, TZD and DPP4. Additionally, Yoshida et al. found SGLT2 to be cost effective against α-glucosidase inhibitors, INS, and SoC. However, Yoshida et al. and Zozaya et al. cautioned that the heterogeneity of the studies included in their studies made it challenging to determine in which treatment scenarios nNIADs were most cost effective.
The lack of CEAs or systematic reviews focusing on DPP4 seemingly conflicts with a global report regarding NIAD market shares that found DPP4s to be the most commonly used nNIAD, followed by GLP1 and SGLT2 [113].
Limitations of the Study
The present review has several limitations. The literature search was restricted to a brief period, and it could be argued that not including non-DAM methodologies is a limitation as valuable information was lost, even if DAMs are considered the best tool to inform decision makers. It could also be argued that systematic reviews should always strive to provide a unified conclusion based on the literature instead of a descriptive overview and broad qualitative synthesis of the results. However, the diversity of the methodological choices, settings, and the high risk of sponsorship bias studies, makes attempting to provide a conclusion on which nNIADs are cost effective challenging to interpret for decision makers. Only including studies that compare nNIADs with other nNIADs was also a limitation. Previous reviews conclude that nNIADs were cost effective compared with oNIADs; however, given the high cost of nNIADs, this is probably limited to second-line treatment. One study [114] has estimated that SGLT2 and GLP1 need a price reduction of 70% to be cost effective compared with MET. The choice of quality assessment checklist in the present study could be considered a limitation as it was not model-specific; however, model specifics are reported as part of the main data extraction. An additional limitation is excluding non-English-language papers since this could likely have excluded many papers from the Asian region. Finally, it is a limitation of this review that we included all published papers as separate studies without discussing whether some papers should be interpreted as adaptations of the same modelling study to different settings. This distinction is not trivial however but underlines the same conclusion that simply counting the number of published papers in favour of a certain conclusion about cost effectiveness does not necessarily count as ‘more evidence’ of this finding.
Strengths of the Study
The present review has some key strengths. Extracting a broad range of information about the assumptions and input and output parameters of DAMs can provide valuable insight into the underlying methodological choices. In a field as complicated as modelling the cost effectiveness of T2DM treatment, it might be beneficial to go beyond only reporting a combination of the economic outcomes and using a checklist that mainly evaluates the included paper’s quality on an ordinal scale of high to low quality. More information for decision makers might provide a better foundation for making decisions.
Suggestions for Future Research
More studies should be conducted directly comparing the products of the SGLT2 and GLP1 classes. Additionally, the combination of SGLT2 and GLP1 as a single treatment intervention was not examined in any of the included studies, but combining these two highly effective drugs using different biological pathways [15] may be worth exploring. However, the most benefit could be gained from conducting more studies for middle- to low-income countries due to their higher prevalence and incidence of T2DM. If the Danish Medicines Counsel is right in their assumption that the SGLT2 and GLP1 can be seen as equivalent in effect [102], SGLT2 is likely to be a good choice for these countries.
However, concluding the cost effectiveness of nNIADs for treating T2DM must be cautiously approached due to the differences between the studies, lack of transparency, and the sponsorship bias outlined in the present and previous reviews. We advise future reviews aiming to conclude on this to take this into account and to specify in what context their conclusion applies. For systematic reviews to be better able to derive conclusions, we suggest establishing better practices and consensus for conducting CEAs evaluating the cost effectiveness of T2DM treatments with a DAM. The central clinically relevant model assumptions, parameters, and comparators must be included, and the explicit reasoning for choices must be provided and presented as clearly and transparently as possible. Implementing a base-case cohort in DAMs, based on a representative sample of the population large enough to examine the effect on different subgroups, could greatly benefit the ability of these studies to reflect the consequences of more usage of the nNIADs in a real-world context in contrast to the highly selected populations of the RCTs used in the included studies. Finally, more observational research is needed into the different aspects of the treatment switch assumption regarding the time until INS is added to, or replaces, the nNIADs and the proportion of addition compared with substitution. Involving clinicians could aid in addressing many of these issues, especially in clarifying and validating the underlying methodological choices in terms of how they reflect and can be transferred to a real-world context and understood by decision makers.
Conclusions
There are several challenges associated with drawing conclusions about which of the nNIADs is the cost-effective option for the second-line treatment of T2DM. First, the data used to model disease progression may not reflect modern treatment practice. Second, the lack of reasoning behind key methodological choices, some of which may conflict with treatment guidelines. Third, the assumptions regarding the time until changes to the comparators may not be representative of current practice. Finally, some methodological differences seem to depend on the class of the main comparator in the analysis, and sponsorship bias may also affect results.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Declarations
Compliance with ethics guidelines
This article is based on previous studies and does not involve any new studies of human or animal subjects performed by the authors.
Funding
This study was supported by Boehringer Ingelheim, who had no role in the design, analysis, or interpretation of the results in this study. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to Boehringer Ingelheim’s substances and intellectual property considerations.
Conflicts of Interest
Peter Vestergaard is the head of research at Steno Diabetes Center North Denmark, funded by the Novo Nordisk Foundation. Lars Holger Ehlers has received funding for research and compensation for lecture activities from Boehringer Ingelheim. Henrik Vitus Bering Laursen has received partial funding for his PhD from Steno Diabetes Center North Denmark, which in turn is funded by the Novo Nordisk Foundation, and additional funding from Boehringer Ingelheim. Emmelie Ploug Jørgensen declares that she has no conflicts of interest.
Author contributions
HVBL was responsible for the conception of the study question and the search strategy, along with collecting, storing, and analysing data, writing the manuscript, and screening the included studies. EPJ contributed to the screening of studies, collecting data, writing and commenting on the manuscript, and providing interpretations of the results. LHE and PV provided ideas for the conception of the study question, generated ideas regarding focus areas of the analysis, provided comments and corrections to the manuscript, and provided feedback throughout the writing period. All authors read and approved the final manuscript.
Data availability statements
The data collected and generated from the included studies can be sent upon request to the corresponding author.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 2021, Tenth edition. 2021
- 2.Hex N, Bartlett C, Wright D, et al. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs: estimating current and future costs of Type 1 and Type 2 diabetes in the UK. Diabet Med. 2012;29(7):855–862. doi: 10.1111/j.1464-5491.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 3.Cannon A, Handelsman Y, Heile M, et al. Burden of illness in type 2 diabetes mellitus. JMCP 2018; 24 (9): S5–S13. 10.18553/jmcp.2018.24.9-a.s5 [DOI] [PMC free article] [PubMed]
- 4.Janssen LMM, Hiligsmann MJC, Elissen AMJ, et al. Burden of disease of type 2 diabetes mellitus: cost of illness and quality of life estimated using the Maastricht Study. Diabet Med. 2020 doi: 10.1111/dme.14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman WH, Braffett BH, Kuo S, et al. What are the clinical, quality-of-life, and cost consequences of 30 years of excellent vs poor glycemic control in type 1 diabetes? J Diabetes Complicat. 2018;32(10):911–915. doi: 10.1016/j.jdiacomp.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyay J, Polyzos SA, Perakakis N, et al. Pharmacotherapy of type 2 diabetes: an update. Metabolism. 2018;78:13–42. doi: 10.1016/j.metabol.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American diabetes association and the European Association for the study of diabetes. Diabetes Care. 2006;29(8):1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in Type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2008;31(1):173–175. doi: 10.2337/dc08-9016. [DOI] [PubMed] [Google Scholar]
- 9.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012 Jun; 55 (6): 1577–1596. 10.1007/s00125-012-2534-0 [DOI] [PubMed]
- 10.Davies MJ, Dalessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018; 61 (12): 2461–2498. 10.1007/s00125-018-4729-5 [DOI] [PubMed]
- 11.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020; 63 (2): 221–228. 10.1007/s00125-019-05039-w [DOI] [PubMed]
- 12.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2020. Dia Care 2020 Jan; 43 (Supplement 1): S98–S110. 10.2337/dc20-S009 [DOI] [PubMed]
- 14.American Diabetes Association AD. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021 Jan; 44 (Supplement 1): S111–S124. 10.2337/dc21-S009 [DOI] [PubMed]
- 15.American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022; 40 (Supplement_1): S64–S74. 10.2337/dc17-S011 [DOI] [PubMed]
- 16.ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2023. Diabetes Care. 2022;46(1):S140–S157. doi: 10.2337/dc23-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael F, Drummond MJ, Sculpher KC, Greg LS, George WT. Methods for the economic evaluation of health care programmes 4th edition. 2015
- 18.Charokopou M, Sabater FJ, Townsend R, et al. Methods applied in cost-effectiveness models for treatment strategies in type 2 diabetes mellitus and their use in Health Technology Assessments: a systematic review of the literature from 2008 to 2013. Curr Med Res Opin. 2016;32(2):207–218. doi: 10.1185/03007995.2015.1102722. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Consensus Panel Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–2265. doi: 10.2337/diacare.27.9.2262. [DOI] [PubMed] [Google Scholar]
- 20.Govan L, Wu O, Lindsay R, et al. How do diabetes models measure up? A review of diabetes economic models and ADA guidelines. JHEOR 2015;3(2):132–152. 10.36469/9831 [DOI] [PMC free article] [PubMed]
- 21.Tarride J-E, Hopkins R, Blackhouse G, et al. A review of methods used in long-term cost-effectiveness models of diabetes mellitus treatment. Pharmacoeconomics. 2010;28(4):255–277. doi: 10.2165/11531590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Yi Y, Philips Z, Bergman G, et al. Economic models in type 2 diabetes. Curr Med Res Opin. 2010;26(9):2105–2118. doi: 10.1185/03007995.2010.494451. [DOI] [PubMed] [Google Scholar]
- 23.Kengne AP, Colagiuri S, Heller S, et al. The Framingham and UK prospective diabetes study (UKPDS) risk equations do not reliably estimate the probability of cardiovascular events in a large ethnically diverse sample of patients with diabetes: the action in diabetes and vascular disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) study. Diabetologia. 2010;53(5):821–831. doi: 10.1007/s00125-010-1681-4. [DOI] [PubMed] [Google Scholar]
- 24.Asche CV, Hippler SE, Eurich DT. Review of models used in economic analyses of new oral treatments for type 2 diabetes mellitus. Pharmacoeconomics. 2014;32(1):15–27. doi: 10.1007/s40273-013-0117-7. [DOI] [PubMed] [Google Scholar]
- 25.Hong D, Si L, Jiang M, et al. Cost effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and dipeptidyl peptidase-4 (DPP-4) inhibitors: a systematic review. Pharmacoeconomics. 2019;37(6):777–818. doi: 10.1007/s40273-019-00774-9. [DOI] [PubMed] [Google Scholar]
- 26.Rahman W, Solinsky PJ, Munir KM, et al. Pharmacoeconomic evaluation of sodium-glucose transporter-2 (SGLT2) inhibitors for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2019;20(2):151–161. doi: 10.1080/14656566.2018.1543408. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida Y, Cheng X, Shao H, et al. A systematic review of cost-effectiveness of sodium-glucose cotransporter inhibitors for type 2 diabetes. Curr DiabRep. 2020;20(4):1–19. doi: 10.1007/s11892-020-1292-5. [DOI] [PubMed] [Google Scholar]
- 28.Bagepally BS, Chaikledkaew U, Gurav YK, et al. Glucagon-like peptide 1 agonists for treatment of patients with type 2 diabetes who fail metformin monotherapy: systematic review and meta-analysis of economic evaluation studies. BMJ Open Diabet Res Care. 2020;8(1):2. doi: 10.1136/bmjdrc-2019-001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagepally BS, Gurav YK, Anothaisintawee T, et al. Cost utility of sodium-glucose cotransporter 2 inhibitors in the treatment of metformin monotherapy failed type 2 diabetes patients: a systematic review and meta-analysis. Value Health. 2019;22(12):1458–1469. doi: 10.1016/j.jval.2019.09.2750. [DOI] [PubMed] [Google Scholar]
- 30.Ruan Z, Zou H, Lei Q, et al. Pharmacoeconomic evaluation of dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus: a systematic literature review. Exp Rev Pharmacoecon Outcomes Res. 2022 doi: 10.1080/14737167.2022.2042255. [DOI] [PubMed] [Google Scholar]
- 31.Zozaya N, Capel M, Simón S, et al. A systematic review of economic evaluations in Non-insulin antidiabetic treatments for patients with type 2 diabetes mellitus. Glob Region Health Technol Assess. 2019 doi: 10.1177/2284240319876574. [DOI] [Google Scholar]
- 32.Page MJ, McKenzie JE, Bossuyt PM, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020 doi: 10.1136/bmj.n71. [DOI] [PubMed] [Google Scholar]
- 33.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzanetakos C, Bargiota A, Kourlaba G, et al. Cost effectiveness of exenatide once weekly versus INS glargine and liraglutide for the treatment of type 2 diabetes mellitus in Greece. Clin Drug Investig. 2018;38(1):67–77. doi: 10.1007/s40261-017-0586-0. [DOI] [PubMed] [Google Scholar]
- 35.Ericsson Å, Glah D, Lorenzi M, et al. Cost-effectiveness of liraglutide versus lixisenatide as add-on therapies to basal INS in type 2 diabetes. PLoS ONE. 2018;13(2):e0191953. doi: 10.1371/journal.pone.0191953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basson M, Ntais D, Ayyub R, et al. The cost-effectiveness of dulaglutide 1.5mg versus exenatide QW for the treatment of patients with type 2 diabetes mellitus in France. Diabetes Ther. 2018;9(1):13–25. doi: 10.1007/s13300-017-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnett AH, Arnoldini S, Hunt B, et al. Switching from sitagliptin to liraglutide to manage patients with type 2 diabetes in the UK: a long-term cost-effectiveness analysis. Diabetes Obes Metab. 2018;20(8):1921–1927. doi: 10.1111/dom.13318. [DOI] [PubMed] [Google Scholar]
- 38.Viljoen A, Hoxer CS, Johansen P, et al. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611–621. doi: 10.1111/dom.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raya PM, Blasco FJA, Hunt B, et al. Evaluating the long-term cost-effectiveness of fixed-ratio combination INS degludec/liraglutide (IDegLira) for type 2 diabetes in Spain based on real-world clinical evidence. Diabetes Obes Metab. 2019;21(6):1349–1356. doi: 10.1111/dom.13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos M, Foos V, Ustyugova A, et al. Cost-effectiveness analysis of empagliflozin in comparison to sitagliptin and saxagliptin based on cardiovascular outcome trials in patients with type 2 diabetes and established cardiovascular disease. Diabetes Ther. 2019;10(6):2153–2167. doi: 10.1007/s13300-019-00701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malkin SJP, Russel-Szymczyk M, Liidemann G, et al. Once-weekly semaglutide versus once-daily liraglutide for the treatment of type 2 diabetes: a long-term cost-effectiveness analysis in Estonia. Diabetes Ther. 2019;10(1):159–176. doi: 10.1007/s13300-018-0542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malkin SJP, Russel-Szymczyk M, Psota M, et al. The management of type 2 diabetes with once-weekly semaglutide versus dulaglutide: a long-term cost-effectiveness analysis in Slovakia. Adv Ther. 2019;36(8):2034–2051. doi: 10.1007/s12325-019-00965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt B, Malkin SJP, Moes RGJ, et al. Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands. BMJ Open Diab Res Care. 2019;7(1):e000705. doi: 10.1136/bmjdrc-2019-000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ericsson Å, Fridhammar A. Cost-effectiveness of once-weekly semaglutide versus dulaglutide and lixisenatide in patients with type 2 diabetes with inadequate glycemic control in Sweden. J Med Econ. 2019;22(10):997–1005. doi: 10.1080/13696998.2019.1614009. [DOI] [PubMed] [Google Scholar]
- 45.Ramos M, Ustyugova A, Hau N, et al. Cost–effectiveness of empagliflozin compared with liraglutide based on cardiovascular outcome trials in Type II diabetes. J Compar Effectiv Res. 2020 doi: 10.2217/cer-2020-0071. [DOI] [PubMed] [Google Scholar]
- 46.Ramos M, Cummings MH, Ustyugova A, et al. Long-term cost-effectiveness analyses of empagliflozin versus oral semaglutide, in addition to metformin, for the treatment of type 2 diabetes in the UK. Diabetes Ther. 2020;11(9):2041–2055. doi: 10.1007/s13300-020-00883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martín V, Vidal J, Malkin SJP, et al. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide and sitagliptin in the spanish setting. Adv Ther. 2020;37(10):4427–4445. doi: 10.1007/s12325-020-01464-1. [DOI] [PubMed] [Google Scholar]
- 48.Johansen P, Chubb B, Hunt B, et al. Evaluating the long-term cost-effectiveness of once-weekly semaglutide versus once-daily liraglutide for the treatment of type 2 diabetes in the UK. Adv Ther. 2020;37(5):2427–2441. doi: 10.1007/s12325-020-01337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorgojo-Martínez JJ, Malkin SJP, Martín V, et al. Assessing the cost-effectiveness of a once-weekly GLP-1 analogue versus an SGLT-2 inhibitor in the Spanish setting: Once-weekly semaglutide versus empagliflozin. J Med Econ. 2020;23(2):193–203. doi: 10.1080/13696998.2019.1681436. [DOI] [PubMed] [Google Scholar]
- 50.Capel M, Ciudin A, Mareque M, et al. Cost-effectiveness analysis of exenatide versus glp-1 receptor agonists in patients with type 2 diabetes mellitus. PharmacoEconomics Open. 2020;4(2):277–286. doi: 10.1007/s41669-019-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bain SC, Hansen BB, Malkin SJP, et al. Oral semaglutide versus empagliflozin, sitagliptin and liraglutide in the UK: long-term cost-effectiveness analyses based on the PIONEER clinical trial programme. Diabetes Ther. 2020;11(1):259–277. doi: 10.1007/s13300-019-00736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gæde P, Johansen P, Tikkanen CK, et al. Management of patients with type 2 diabetes with once-weekly Semaglutide versus dulaglutide, exenatide ER, liraglutide and Lixisenatide: a cost-effectiveness analysis in the Danish setting. Diabetes Ther. 2019;10(4):1297–1317. doi: 10.1007/s13300-019-0630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malkin. The long-term cost-effectiveness of oral semaglutide in the Netherlands based on the PIONEER 2, 3 and 4 randomized controlled trials | Elsevier Enhanced Reader. Diabetes Research and Clinical Practice 2021. 10.1016/j.diabres.2021.108759 [DOI] [PubMed]
- 54.Van der Linden N, Van Olst S, Nekeman S, et al. The cost-effectiveness of dapagliflozin compared to DPP-4 inhibitors in the treatment of type 2 diabetes mellitus in the Netherlands. Diabet Med. 2020;38(4):2. doi: 10.1111/dme.14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gourzoulidis G, Tzanetakos C, Ioannidis I, et al. Cost-effectiveness of empagliflozin compared with dapagliflozin for the treatment of patients with type 2 diabetes mellitus and established cardiovascular disease in Greece. Clin Drug Investig. 2021;41(4):371–380. doi: 10.1007/s40261-021-01013-w. [DOI] [PubMed] [Google Scholar]
- 56.Ehlers LH, Lamotte M, Monteiro S, et al. The cost-effectiveness of empagliflozin versus liraglutide treatment in people with type 2 diabetes and established cardiovascular disease. Diabetes Ther. 2021;12(5):1523–1534. doi: 10.1007/s13300-021-01040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Capehorn M, Hallén N, Baker-Knight J, et al. Evaluating the cost-effectiveness of once-weekly semaglutide 1 mg versus empagliflozin 25 mg for treatment of patients with type 2 diabetes in the UK setting. Diabetes Ther. 2021;12(2):537–555. doi: 10.1007/s13300-020-00989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehlers LH, Lamotte M, Ramos MC, et al. The cost–effectiveness of oral semaglutide versus empagliflozin in Type 2 diabetes in Denmark. J Compar Effectiv Res. 2022;11(1):29–37. doi: 10.2217/cer-2021-0169. [DOI] [PubMed] [Google Scholar]
- 59.Ehlers LH, Lamotte M, Ramos MC, et al. The cost-effectiveness of subcutaneous semaglutide versus empagliflozin in type 2 diabetes uncontrolled on metformin alone in Denmark. Diabetes Ther. 2022;13(3):489–503. doi: 10.1007/s13300-022-01221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eliasson B, Ericsson Å, Fridhammar A, et al. Long-term cost effectiveness of oral semaglutide versus empagliflozin and sitagliptin for the treatment of type 2 diabetes in the Swedish setting. PharmacoEconomics Open. 2022;6(3):343–354. doi: 10.1007/s41669-021-00317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malkin SJP, Carvalho D, Costa C, et al. The long-term cost-effectiveness of oral semaglutide versus empagliflozin and dulaglutide in Portugal. Diabetol Metab Syndr. 2022;14(1):32. doi: 10.1186/s13098-022-00801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viljoen A, Chubb B, Malkin SJP, et al. The long-term cost-effectiveness of once-weekly semaglutide 1 mg vs dulaglutide 3 mg and 45 mg in the UK. Eur J Health Econ. 2022 doi: 10.1007/s10198-022-01514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franch-Nadal J, Malkin SJP, Hunt B, et al. The cost-effectiveness of oral semaglutide in Spain: a long-term health economic analysis based on the PIONEER clinical trials. Adv Ther. 2022;39(7):3180–3198. doi: 10.1007/s12325-022-02156-8. [DOI] [PubMed] [Google Scholar]
- 64.Guzauskas GF, Rind DM, Fazioli K, et al. Cost-effectiveness of oral semaglutide added to current antihyperglycemic treatment for type 2 diabetes. JMCP. 2021;27(4):455–468. doi: 10.18553/jmcp.2021.27.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakravarty A, Rastogi M, Dhankhar P, et al. Comparison of costs and outcomes of dapagliflozin with other glucose-lowering therapy classes added to metformin using a short-term cost-effectiveness model in the US setting. J Med Econ. 2018;21(5):497–509. doi: 10.1080/13696998.2018.1434182. [DOI] [PubMed] [Google Scholar]
- 66.Neslusan C, Teschemaker A, Willis M, et al. Cost-effectiveness analysis of canagliflozin 300 mg versus dapagliflozin 10 mg added to metformin in patients with type 2 diabetes in the United States. Diabetes Ther. 2018;9(2):565–581. doi: 10.1007/s13300-018-0371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reifsnider OS, Kansal AR, Gandhi PK, et al. Cost-effectiveness of empagliflozin versus canagliflozin, dapagliflozin, or standard of care in patients with type 2 diabetes and established cardiovascular disease. BMJ Open Diabetes Res Care. 2021;9(1):e001313. doi: 10.1136/bmjdrc-2020-001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johansen P, Håkan-Bloch J, Liu AR, et al. Cost effectiveness of once-weekly semaglutide versus once-weekly dulaglutide in the treatment of type 2 diabetes in Canada. PharmacoEcon Open. 2019;3(4):537–550. doi: 10.1007/s41669-019-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reifsnider O, Kansal A, Pimple P, et al. Cost-effectiveness analysis of empagliflozin versus sitagliptin as second-line therapy for treatment in patients with type 2 diabetes in the United States. Diabetes Obes Metab. 2020;23(3):791–799. doi: 10.1111/dom.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Risebrough NA, Baker TM, Zhang L, et al. Lifetime cost-effectiveness of oral semaglutide versus dulaglutide and liraglutide in patients with type 2 diabetes inadequately controlled with oral antidiabetics. Clin Ther. 2021;43(11):1812–1826.e7. doi: 10.1016/j.clinthera.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 71.Zupa MF, Codario RA, Smith KJ. Cost–effectiveness of empagliflozin versus weekly semaglutide as add-on therapy for Type 2 diabetes. J Compar Effectiv Res. 2021;10(15):1133–1141. doi: 10.2217/cer-2021-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reifsnider OS, Pimple P, Brand S, et al. Cost-effectiveness empagliflozin versus liraglutide for type 2 diabetes in the United States. Diabet Obes Metab. 2022;24(4):652–661. doi: 10.1111/dom.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stafford S, Bech PG, Fridhammar A, et al. Cost-effectiveness of once-weekly semaglutide 1 mg versus canagliflozin 300 mg in patients with type 2 diabetes mellitus in a Canadian setting. Appl Health Econ Health Policy. 2022;20(4):543–555. doi: 10.1007/s40258-022-00726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou X, Wan X, Wu B. Cost-effectiveness of canagliflozin versus dapagliflozin added to metformin in patients with type 2 diabetes in China. Front Pharmacol. 2019;10:480. doi: 10.3389/fphar.2019.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chien C-L, Chen Y-C, Malone DC, et al. Cost-utility analysis of second-line anti-diabetic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. Curr Med Res Opin. 2020;36(10):1619–1626. doi: 10.1080/03007995.2020.1815686. [DOI] [PubMed] [Google Scholar]
- 76.Hu S, Deng X, Ma Y, et al. Cost-utility analysis of dapagliflozin versus saxagliptin treatment as monotherapy or combination therapy as add-on to metformin for treating type 2 diabetes mellitus. Appl Health Econ Health Policy. 2021;19(1):69–79. doi: 10.1007/s40258-020-00603-7. [DOI] [PubMed] [Google Scholar]
- 77.Ramos M, Men P, Wang X, et al. Cost-effectiveness of empagliflozin in patients with type 2 diabetes and established cardiovascular disease in China. Cost Eff Resour Alloc. 2021;19(1):46. doi: 10.1186/s12962-021-00299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin W-Q, Cai Z, Chen T, et al. Cost-effectiveness of dipeptidylpeptidase-4 inhibitors added to metformin in patients with type 2 diabetes in China. Front Endocrinol. 2021;12:684960. doi: 10.3389/fendo.2021.684960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu S, Gu S, Qi C, et al. Cost-utility analysis of semaglutide for type 2 diabetes after its addition to the National Medical Insurance System in China. Diabet Obes Metab. 2022 doi: 10.1111/dom.14881. [DOI] [PubMed] [Google Scholar]
- 80.Peng Z, Yang C, Ou H, et al. Cost-effectiveness of sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors among patients with type 2 diabetes with and without established cardiovascular diseases: a model-based simulation analysis using 10-year real-world data and targeted literature review. Diabetes Obesity Metabolism. 2022;24(7):1328–1337. doi: 10.1111/dom.14708. [DOI] [PubMed] [Google Scholar]
- 81.Ruan Z, Ung COL, Shen Y, et al. Long-term cost-effectiveness analysis of once-weekly semaglutide versus dulaglutide in patients with type 2 diabetes with inadequate glycemic control in China. Diabetes Ther. 2022;13(10):1737–1753. doi: 10.1007/s13300-022-01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lasalvia P, Gil-Rojas Y, García Á. Cost-effectiveness of dapagliflozin compared to DPP-4 inhibitors as combination therapy with metformin in the treatment of type 2 diabetes mellitus without established cardiovascular disease in Colombia. Expert Rev Pharmacoecon Outcomes Res. 2022;22(6):955–964. doi: 10.1080/14737167.2022.2044310. [DOI] [PubMed] [Google Scholar]
- 83.Ekhlasi M, Taheri S, Yousefi N. Cost-effectiveness of dulaglutide versus liraglutide for management of type 2 diabetes mellitus in Iran. Value Health Region Issues. 2022;32:54–61. doi: 10.1016/j.vhri.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 84.McEwan P, Foos V, Palmer JL, et al. Validation of the IMS CORE diabetes model. Value Health. 2014;17(6):714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and costeffectiveness of interventions in diabetes mellitus (Types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(sup1):S5–S26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 86.McEwan P, Evans M, Bergenheim K. (CARDIFF) A population model evaluating the costs and benefits associated with different oral treatment strategies in people with type 2 diabetes. Diabetes Obes Metab. 2010;12(7):623–630. doi: 10.1111/j.1463-1326.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 87.Lundqvist A, Carlsson KS, Johansen P, et al. Validation of the IHE cohort model of type 2 diabetes and the impact of choice of macrovascular risk equations. PLoS ONE. 2014;9(10):e110235. doi: 10.1371/journal.pone.0110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayes AJ, Leal J, Gray AM, et al. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–1933. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 89.Wu B, Ma J, Zhang S, et al. (COMT, Wu 2018a) development and validation of a health policy model of type 2 diabetes in Chinese setting. J Comparat Effect Res. 2018;7(8):749–763. doi: 10.2217/cer-2018-0001. [DOI] [PubMed] [Google Scholar]
- 90.Willis M, Johansen P, Nilsson A, et al. Validation of the economic and health outcomes model of type 2 diabetes mellitus (ECHO-T2DM) Pharmacoeconomics. 2017;35(3):375–396. doi: 10.1007/s40273-016-0471-3. [DOI] [PubMed] [Google Scholar]
- 91.Caro JJ. Discretely integrated condition event (DICE) simulation for pharmacoeconomics. Pharmacoeconomics. 2016;34(7):665–672. doi: 10.1007/s40273-016-0394-z. [DOI] [PubMed] [Google Scholar]
- 92.Pratley RE, Aroda VR, Lingvay I, et al. (SUSTAIN 7) Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 93.Rodbard HW, Rosenstock J, Canani LH, et al. (PIONEER 2) oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–2281. doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 94.Zinman B, Wanner C, Lachin JM, et al. (EMPA-REG OUTCOME - CVD) empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 95.Rosenstock J, Allison D, Birkenfeld AL, et al. PIONEER 3) Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with Type 2 diabetes uncontrolled with metformin alone or with sulfonylurea (PIONEER 3. JAMA. 2019;321(15):1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: the United Kingdom prospective diabetes study (UKPDS) outcomes model (UKPDS no. 68) Diabetologia. 2004;47(10):1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 97.Hoerger TJ, Wittenborn JS, Segel JE, et al. A health policy model of CKD: 1. Model construction, assumptions, and validation of health consequences. Am J Kidney Dis. 2010;55(3):452–462. doi: 10.1053/j.ajkd.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 98.Hoerger TJ, Wittenborn JS, Segel JE, et al. A health policy model of CKD: 2 the cost-effectiveness of microalbuminuria screening. Am J Kidney Dis. 2010;55(3):463–473. doi: 10.1053/j.ajkd.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 99.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM: I Model construction and assumptions. Diabetes Care. 1997;20(5):725–734. doi: 10.2337/diacare.20.5.725. [DOI] [PubMed] [Google Scholar]
- 100.Bagust A, Hopkinson PK, Maier W, et al. An economic model of the long-term health care burden of Type II diabetes. Diabetologia. 2001;44(12):2140–2155. doi: 10.1007/s001250100023. [DOI] [PubMed] [Google Scholar]
- 101.Montvida O, Shaw J, Atherton JJ, et al. Long-term trends in antidiabetes drug usage in the US: real-world evidence in patients newly diagnosed with type 2 diabetes. Dia Care. 2018;41(1):69–78. doi: 10.2337/dc17-1414. [DOI] [PubMed] [Google Scholar]
- 102.Medicinrådet. Medicinrådets behandlingsvejledning T2DM. 2021. Available at: https://medicinraadet.dk/anbefalinger-og-vejledninger/behandlingsvejledninger/type-2-diabetes
- 103.European Medicines Agency. EMA confirms recommendations to minimise ketoacidosis risk with SGLT2 inhibitors for diabetes. European Medicines Agency. 2018 Sep. Available at: https://www.ema.europa.eu/en/news/ema-confirms-recommendations-minimise-ketoacidosis-risk-sglt2-inhibitors-diabetes. Accessed 6 Mar 2020
- 104.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 105.Neal B, Perkovic V, Mahaffey KW, et al. (CANVAS) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 106.Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:4365. doi: 10.1136/bmj.k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hamblin PS, Wong R, Ekinci EI, et al. SGLT2 inhibitors increase the risk of diabetic ketoacidosis developing in the community and during hospital admission. J Clin Endocrinol Metab. 2019;104(8):3077–3087. doi: 10.1210/jc.2019-00139. [DOI] [PubMed] [Google Scholar]
- 108.The Mount Hood 4 Modeling Group Computer modeling of diabetes and its complications: a report on the fourth mount hood challenge meeting. Diabetes Care. 2007;30(6):1638–1646. doi: 10.2337/dc07-9919. [DOI] [PubMed] [Google Scholar]
- 109.Palmer AJ. Computer modeling of diabetes and its complications: a report on the fifth mount hood challenge meeting. Value in Health. 2013;16(4):670–685. doi: 10.1016/j.jval.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Palmer AJ, Si L, Tew M, et al. Computer modeling of diabetes and its transparency: a report on the eighth mount hood challenge. Value in Health. 2018;21(6):724–731. doi: 10.1016/j.jval.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Si L, Willis MS, Asseburg C, et al. Evaluating the ability of economic models of diabetes to simulate new cardiovascular outcomes trials: a report on the ninth mount hood diabetes challenge. Value Health. 2020;23(9):1163–1170. doi: 10.1016/j.jval.2020.04.1832. [DOI] [PubMed] [Google Scholar]
- 112.Clarivate. Type 2 Diabetes | Current Treatment | EU5 | 2017. Available at: https://clarivate.com/products/research-reports/report/cutrmd0016-2017-biopharma-type-2-diabetes-current-treatment-eu5-2017/
- 113.Fortune Business Insights. Diabetes drugs market size, share and industry analysis by drug class (INS, DPP-4 inhibitors, GLP-1 receptor agonists, SGLT2 inhibitors), diabetes type (type 1, type 2), route of administration (oral, subcutaneous, intravenous), distribution channel (online pharmacies, hospital pharmacies, retail pharmacies) & regional forecast 2019–2026. 2018. Available at: https://www.fortunebusinessinsights.com/industry-reports/diabetes-drugs-market-100570.
- 114.Choi JG, Winn AN, Skandari MR, et al. First-line therapy for type 2 diabetes with sodium–glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists. Ann Intern Med. 2022 doi: 10.7326/M21-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected and generated from the included studies can be sent upon request to the corresponding author.