Introduction
Transseptal puncture (TSP) is the main access route during left-sided ablations. Fluoroscopy is still the most common guidance tool [1]. However, for localization of the fossa ovalis (FO), transesophageal or intracardiac echocardiography can be used [2, 3]. Considering the fact that little is known about the topic, we evaluated the usefulness of anatomical landmarks reconstructed on the 3D map of the right atrium for detecting the posterior border for the optimal TSP site.
Methods
Patients and preprocedural management
One hundred patients who underwent an ablation for paroxysmal or persistent atrial fibrillation in our center between June 2020 and November 2021 were prospectively enrolled.
The ablation procedure was performed under deep analgosedation. A 6F decapolar catheter Inquiry™ (Abbott, St. Paul, MN, USA) was inserted into the right atrium (RA), and an accurate three-dimensional electroanatomical map (EAM) using the EnSite Precision system (Abbott, St. Paul, MN, USA) was obtained. TSP was performed under fluoroscopic control. For more precise anatomical orientation, the reconstructed 3D multislice-detector computed tomography images were integrated into the EAM.
Postprocedural analysis
As published before, we have marked the CS (coronary sinus)–SVC (superior vena cava) line from the anterior margin of the CS ostium (mid-point) to the anterior margin of the SVC directly at the highest transition point to the right atrial appendage in LAO projection [4]. The difference is that Eichenlaub et al. used the magnetic field–based 3D mapping system for this purpose. In contrast, in our study, the electrical impedance field–based 3D mapping system was used. After marking the anterior line, RA geometry was then angulated towards LAO/LLat until the CS–SVC line was strictly vertical, and then the map was cranialized to a 30° angle, into the plane view of the FO. In this angulation, we marked two additional lines as follows:
SVC line: from an anterior margin of SVC at the transition into RA/RAA to the posterior margin of IVC at the transition into RA
IVC (inferior vena cava) line: from an anterior margin of IVC at the transition into RA to the posterior margin of SVC at the transition to RA
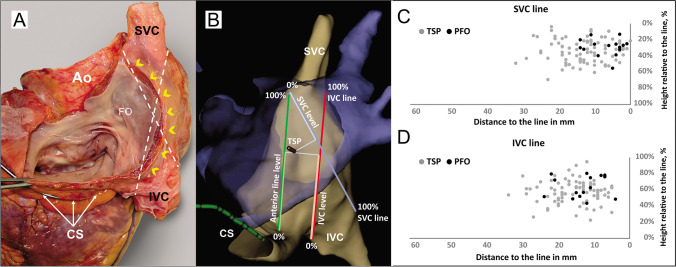
We measured the distance and height of the TSP or persistent foramen ovale (PFO) relative to the abovementioned lines and also to the CS ostium (the most superior part). The height was measured as a percentage relative to the length of the lines. Distances were measured relative to their perpendicular point of intersection with the lines (Fig. 1).
Fig. 1.
A Anatomical specimen showing the structures of interatrial septum from the left atrial side. Dotted lines describes the SVC and IVC lines; the yellow arrowheads along the Waterstones groove. B 3D-electroanatomical map of the right atrium (gray) and the integrated CT scan of the left atrium in blue. Schematical illustration of additional anatomical measurements: Anterior; SVC and IVC lines; distances from the TSP to the lines as well as level of the TSP according to the line (measured in percentage): anterior line level; SVC level and IVC level. C and D Scatter plots demonstrating TSP sites in relation to the lines. SVC, superior vena cava; IVC, inferior vena cava; TSP, transseptal puncture (gray dots); PFO, persistent foramen ovale (black dots). FO, fossa ovalis; Ao, ascending aorta; IVC, inferior vena cava; SVC, superior vena cava; CS, coronary sinus; TSP, transseptal puncture
Statistical analysis
A Student’s test was used for the comparison of continuous variables. A two-tailed p-value < 0.05 was considered statistically significant. All calculations were performed using the IBM SPSS Statistics 27.0, NY, USA.
Results
We have included 100 consecutive patients who underwent ablation due to paroxysmal (n = 34, 34%) or persistent atrial fibrillation (n = 66, 66%). The average age of patients was 66 ± 11 years, 73 patients (73%) were male. Further baseline characteristics are listed in Table 1.
Table 1.
Baseline characteristics and postprocedural analysis
| Measurement | |
|---|---|
| Baseline characteristics | |
| Age, years | 66 ± 11 |
| Sex (males) | 73 (73%) |
| Arterial hypertension | 65 (65%) |
| BMI, kg/m2 | 28 ± 6 |
| BSA, m2 | 2 ± 0.3 |
| DM | 13 (13%) |
| SHD | 33 (33%) |
| LVEF, % | 55 ± 4 |
| LA-diameter in CT, mm | 48 ± 11 |
| AF type | |
| Paroxysmal | 34 (34%) |
| Persistent | 66 (66%) |
| AF duration, years | 2 ± 0.5 |
| Re-do procedure | 34 (34%) |
| Postprocedural analysis | |
| Anterior line | |
| Length anterior line, mm | 50 ± 11 |
| Distance from TSP site to the anterior line, mm | 8 ± 5 |
| Height of TSP site relative to anterior line, % | 55 ± 1 |
| SVC line | |
| Length SVC line, mm | 53 ± 11 |
| Distance from TSP site to SVC line, mm | 12 ± 7 |
| Height of TSP site relative to SVC line, % | 31 ± 1 |
| IVC line | |
| Length IVC line, mm | 58 ± 12 |
| Distance to IVC line, mm | 15 ± 6 |
| Height of TSP site relative to IVC-line, % | 56 ± 1 |
| CS distance, mm | 15 ± 8 |
BMI body mass index, BSA body surface area, DM diabetes mellitus, LVEF left ventricular ejection fraction, SHD structural heart disease, LA left atrium, AF atrial fibrillation, SVC superior vena cava, TSP transseptal puncture, IVC inferior vena cava, CS coronary sinus. Values are given as a median ± standard deviation
All TSP sites were posterior to the anterior line (mean distance—8 ± 5 mm). The TSP site was found to be predominantly on the superior level of the SVC line and was at the mean distance of 12 ± 7 mm of it (Table 1). The TSP site in one patient was on the SVC line. All TSP sites were separated from the IVC line at the mean distance of 15 ± 6 mm and tended to be projected on the middle level of the IVC line. All sites of the transseptal passage were above the CS level, and the mean distance to it was 15 ± 8 mm (Fig. 1).
Discussion
The main findings of the current study are:
The electrical impedance field–based 3D mapping system appears to have the same potential benefits for localization of the anterior border of the transseptal puncture site as the magnetic field–based 3D mapping system.
The proposed anatomical lines provide accurate localization of the posterior border of the TSP area.
In recent publications, colleagues used the magnetic field–based 3D mapping system and proved that the anterior line demarcates the anterior border of FO and can be used for guiding the TSP [4, 5]. However, they did not provide information about landmarks which demarcates the posterior border of the FO. Once TSP is produced too posteriorly, towards the Waterstone groove, a major complication can occur.
In our study, we were able to show that the anterior borderline of FO described before is reproducible also when using a 3D navigation system based on electrical impedance. Moreover, the new additional lines of SVC and IVC corresponded to the posterior boundaries of the FO region. The intersecting character of the SVC and IVC lines reproduces the convex nature of the Waterstone groove. No TSP site was projected on the outer side of the presented lines; in other words, TSP was always inside of the geometrical figure built by the aforementioned lines. The clinical significance of these lines for TSP and their effect on reducing fluoroscopy time will be explored in ongoing studies.
New technical achievements, like visualized sheaths and RF needle as well as the utilization of three-dimensional intracardiac echocardiography, are getting more attention in terms of fluoroscopy time reduction [6–8]. Therefore, geometric localization of the FO using the aforementioned lines could be another step towards minimizing the fluoroscopy. All described methods need to be further evaluated and compared in large, randomized trials. However, fluoroscopy remains the main method of TSP so far.
Study limitation
The presented data was collected from a single center with a relatively small number of patients.
Conclusion
The novel SVC and IVC lines together with the anterior line based on a 3D mapping system are corresponding to the TSP site. Further study is needed to prove safety and/or influence on the fluoroscopy time reduction once using these landmarks.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Data will be made available from the authors on reasonable request.
Declarations
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee.
Competing interests
P. Sommer is an advisory board member for Abbott, Biosense Webster, Boston Scientific, and Medtronic. The other authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matoshvili Z, Bastani H, Bourke T, Braunschweig F, Drca N, Gudmundsson K, et al. Safety of fluoroscopy-guided transseptal approach for ablation of left-sided arrhythmias. Europace. 2017;19:2023–2026. doi: 10.1093/europace/euw432. [DOI] [PubMed] [Google Scholar]
- 2.Zuercher R, Herling A, Schmidt MT, Bachmann M, Winnik S, Duru F, et al. Transesophageal echocardiography-guided transseptal left atrial access to improve safety in patients undergoing pulmonary vein isolation. J Clin Med. 2022;11:2546. doi: 10.3390/jcm11092546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Žižek D, Antolič B, Prolič Kalinšek T, Štublar J, Kajdič N, Jelenc M, et al. Intracardiac echocardiography-guided transseptal puncture for fluoroless catheter ablation of left-sided tachycardias. J Interv Card Electrophysiol. 2021;61:595–602. doi: 10.1007/s10840-020-00858-z. [DOI] [PubMed] [Google Scholar]
- 4.Eichenlaub M, Weber R, Minners J, Allgeier HJ, Jadidi A, Müller-Edenborn B, et al. 3D mapping for the identification of the fossa ovalis in left atrial ablation procedures: a pilot study of a first step towards an electroanatomic-guided transseptal puncture. Europace. 2020;22:732–738. doi: 10.1093/europace/euaa034. [DOI] [PubMed] [Google Scholar]
- 5.Bohnen M, Minners J, Eichenlaub M, Weber R, Allgeier HJ, Jadidi A, et al. Feasibility and safety of a three-dimensional anatomic map-guided transseptal puncture for left-sided catheter ablation procedures. Europace. 2023;25:1126–1134. doi: 10.1093/europace/euac262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imnadze G, Ajaj T, Bante H, Sohns C, Sommer P. Transseptal puncture without fluoroscopy using a radiofrequency needle: a case series. Cardiol J. 2021;28:655–662. doi: 10.5603/CJ.a2020.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalaph M, Sommer P, Lucas P, Guckel D, Fink T, Sciacca V, et al. First clinical experience using a visualized sheath for atrial fibrillation ablation. Pacing Clin Electrophysiol. 2022;45:922–929. doi: 10.1111/pace.14555. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Ze F, Yuan CZ, Zhou X, Wang L, Duan JB, et al. The safety and efficiency of fluoroless site-specific transseptal puncture guided by three-dimensional intracardiac echocardiography. J Interv Card Electrophysiol. 2022;65(3):643–649. doi: 10.1007/s10840-022-01298-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available from the authors on reasonable request.