Abstract
The plasma lipoprotein distribution of free nystatin (Nys) and liposomal nystatin (L-Nys) in human plasma samples with various lipoprotein lipid and protein concentrations and compositions was investigated. To assess the lipoprotein distributions of Nys and L-Nys, human plasma was incubated with Nys and L-Nys (equivalent to 20 μg/ml) for 5 min at 37°C. The plasma was subsequently partitioned into its lipoprotein and lipoprotein-deficient plasma fractions by step-gradient ultracentrifugation, and each fraction was analyzed for Nys content by high-pressure liquid chromatography. The lipid and protein contents and compositions of each fraction were determined with enzymatic kits. Following the incubation of Nys and L-Nys in human plasma the majority of Nys recovered within the lipoprotein fractions was recovered from the high-density lipoprotein (HDL) fraction. Incorporation of Nys into liposomes consisting of dimyristoylphosphatidylcholine and dimyristoylphosphatidylglycerol significantly increased the percentage of drug recovered within the HDL fraction. Furthermore, it was observed that as the amount of HDL protein decreased the amounts of Nys and L-Nys recovered within this fraction decreased. These findings suggest that the preferential distribution of Nys and L-Nys into plasma HDL may be a function of the HDL protein concentration.
It is well known that a number of xenobiotic compounds bind to or associate with plasma proteins such as albumin and alpha-1-glycoprotein (14, 24, 39). However, recent studies have shown that amphiphilic and hydrophobic compounds such as nystatin (Nys) (28), cyclosporine (29), amphotericin B (AmB) (37), and annamycin (33, 35) associate with the plasma lipoproteins. Plasma lipoproteins are a heterogeneous group of proteins responsible for the transport of hydrophobic nutrients throughout the body.
Since disease states significantly influence lipoprotein content and composition resulting in altered therapeutic outcomes, it may be reasonable to suggest that these changes in the lipoprotein profile would affect the degree of drug association and distribution within the lipoprotein subclasses (34). The result of such an effect could lead to alterations in the biopharmaceutical and/or pharmacological action of the drug. It has been demonstrated for a number of hydrophobic drugs that changes in their pharmacokinetic and pharmacodynamic profiles occur following their administration to cancer, human immunodeficiency virus-infected, and bone marrow transplant patients compared to their profiles following their administration to nondiseased individuals (16, 23, 31). These changes may substantially interfere with the clinician’s ability to provide the most effective dose of the drug to his or her patient population while minimizing any untoward effects.
Of interest are the amphiphilic polyene antibiotics Nys and AmB because they exhibit similar characteristics. These include their insolubility in water (1), plasma lipoprotein binding (28, 37), and availability as liposomal formulations (28, 37) and the fact that they can be administered to patients who frequently display abnormal plasma lipoprotein profiles (16, 23, 31). Since Nys and AmB, when incorporated into liposomes, associate with plasma lipoproteins upon incubation in human plasma (28, 37), we have hypothesized that changes in the lipoprotein lipid and protein concentrations may be one factor which alters the pharmacokinetics and pharmacodynamics of these drugs in patients with disease.
Our laboratory has extensively investigated the effects of liposomal encapsulation of AmB on the plasma lipoprotein distribution of AmB (32, 34, 37). Furthermore, the changes in the efficacy and toxicity of liposomal AmB (L-AmB) compared to that of AmB have also been studied (15, 18–20, 22, 31, 32, 38). Preliminary work has reported that the incorporation of AmB into liposomes composed of dimyristoylphosphatidylcholine (DMPC) and dimyristoylphosphatidylglycerol (DMPG) (7:3 [wt/wt]) at a lipid-to-drug ratio of 10:1 (wt/wt) significantly affects the plasma lipoprotein distribution of AmB (32, 34, 37). When AmB is incubated in human plasma, it distributes mainly within the high-density lipoprotein (HDL) fraction. However, when AmB is encapsulated into liposomes composed of negatively and positively charged phospholipids, the amount of AmB associated with the HDL fraction increases, whereas the amount of low-density lipoprotein (LDL)-associated AmB decreases, compared to the amount in the nonliposomal formulation of AmB (37). We have further observed that AmB was significantly less toxic to LLC PK1 renal cells (a cell line derived from the proximal tubular cells of swine) when it was associated with HDL than when it was unassociated with the lipoprotein (36). Conversely, LDL-associated AmB was just as toxic to the cells as unbound AmB. However, when the amounts of the high-affinity surface LDL receptors were decreased, LDL-associated AmB was less toxic to LLC PK1 renal cells than the free, unbound drug. These data suggested that the increased toxicity observed with the LDL-associated AmB was a result of its interaction with the high-affinity LDL receptors located on the surface of the cell (36). Furthermore, an increase in the amount of AmB-associated LDL would suggest an increase in toxicity to the renal cells. Recent studies with transplant patients have demonstrated this link of AmB-induced nephrotoxicity to LDL levels in the body (31).
The purpose of this study was to determine the relationship of the Nys and the liposomal Nys (L-Nys) distribution in plasma to the lipid and protein concentrations and compositions of lipoproteins. Our hypothesis was that the human plasma lipoprotein distribution of Nys and L-Nys is dependent on the composition—specifically, the total cholesterol, esterified cholesterol, free cholesterol, total triglyceride, phospholipid, and total protein contents—of lipoproteins. Furthermore, the incorporation of nystatin into liposomes alters the plasma lipoprotein distribution of the drug compared to the lipoprotein distribution of free Nys.
MATERIALS AND METHODS
Chemicals and reagents.
Aronex Pharmaceuticals Inc. (The Woodlands, Tex.) generously donated Nys powder (lot no. 48515-0853); Lederle Pharmaceuticals) and L-Nys (batch no. 007). Pooled human plasma was obtained from the Vancouver Red Cross (Vancouver, British Columbia, Canada). Methanol, tetrahydrofuran (THF), and other organic solvents were purchased from Fisher Scientific Canada (Toronto, Ontario, Canada). LDL-Direct Plus Cholesterol Ratio System kits were purchased from Isolab Inc. (Akron, Ohio). Sodium bromide, ammonium acetate, and 5,5′-dithio-bis(2-nitrobenzoic acid) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Cholesterol reagent, cholesterol calibrators, triglyceride (int) reagent, triglyceride calibrators, and protein assay kits were purchased from Sigma Diagnostics (St. Louis, Mo.). Free cholesterol analysis kits and phospholipid analysis kits were purchased from Boehringer Mannheim (Laval, Quebec, Canada).
Preparation of analytes and solutions. (i) Nys solution.
An Nys solution (1 mg/ml) was prepared by dissolving 2 mg of accurately weighed Nys powder into 2 ml of methanol. The mixture was covered, protected from light, and stored at 4°C for the duration of the experiment.
(ii) L-Nys suspension.
The method of formation of multilamellar liposomes containing Nys was similar to that for the preparation of L-AmB described previously (17, 21). Briefly, Nys dissolved in methanol was mixed with chloroform solutions of DMPC and DMPG (21). Organic solvents were removed by evaporation under vacuum, and the resultant powder contained a 7:3 (wt/wt) ratio of DMPC:DMPG and a total lipid-to-drug ratio of 10:1 (wt/wt). The mean diameter of the liposomal preparation was 321 ± 192 nm, as determined by quasielectric light scattering with a Nicomp submicron particle sizer (model 270; Pacific Scientific; Santa Barbara, Calif.) (27, 28). The L-Nys suspension was prepared by the addition of 100 mg of accurately weighed L-Nys powder (commercially prepared) to 10 ml of 0.9% sodium chloride. The mixture was dispersed by hand shaking for 1 min, incubated at 37°C for 10 min, and then further agitated for another 1 min. The resulting L-Nys suspension contained a final Nys concentration of 1 mg/ml and was used immediately upon reconstitution.
Heterogeneity of human plasma lipoprotein profiles.
In order to obtain human plasma with variegated lipoprotein lipid compositions, human plasma with dissimilar lipid profiles (we relied on the variability within the human population to obtain plasma with naturally occurring differences in lipoprotein lipid content and composition) was obtained.
Prescreening of human plasma.
Randomly selected, freshly drawn (i.e., removed from the donor within the previous 48 h) human plasma was obtained from the Vancouver Red Cross and frozen. Initial screening of individual patient plasma samples for differences in the lipoprotein lipid composition were performed with the LDL-Direct Plus Cholesterol Ratio System (37). Patient plasma samples were selected on the basis of significant differences in the amounts of either total cholesterol or total triglyceride of the separated lipoprotein fractions.
Lipoprotein separation by step-gradient ultracentrifugation.
For the separation of lipoprotein plasma components by step-gradient ultracentrifugation, sodium bromide density solutions were carefully layered on top of the plasma sample in order of highest to lowest density. The density of the plasma sample was initially altered such that it had the greatest density of all layers in the gradient. The samples were ultracentrifuged overnight, and separation of the lipoprotein fractions was accomplished in a single spin. Each distinct layer was removed and separated for further analysis.
(i) Treatment of plasma with free Nys or L-Nys.
To an ultraclear centrifuge tube (Beckman Instruments, Inc., Palo Alto, Calif.), 2.94 and 3 ml of human plasma was added for sample (n = 8 for Nys and n = 6 for L-Nys) and standard curve (n = 4) purposes, respectively. The contents of all tubes were prewarmed to 37°C. To the sample tubes was added either 60 μl of an Nys solution (1 mg/ml) or 60 μl of an L-Nys suspension (1 mg/ml). The final concentration of Nys in human plasma for both Nys and L-Nys formulations was 20 μg/ml. Immediately after the addition of Nys or L-Nys, the samples were returned to 37°C and incubated for 5 min, whereupon they were removed and cooled on ice for 30 min. The plasma used for standard curve purposes was subjected to identical incubation and cooling times identical to those used for the plasma samples.
(ii) Separation of lipoprotein constituents.
Briefly, solutions of all densities (density [δ] = 1.006, 1.063, and 1.21 g/ml) were stored at 4°C prior to the layering of the gradient. To the previously cooled plasma used for standard curves and the plasma samples was added 1.02 g of accurately weighed sodium bromide (0.34 g of sodium bromide per 1.0 ml of plasma) in order to modify the density of the plasma to approximately 1.25 g/ml (11, 13, 30). Once the sodium bromide had dissolved into the plasma, 2.8 ml of the highest-density sodium bromide solution (δ = 1.21 g/ml) was carefully layered on top of the plasma. By using the same volume of 2.8 ml, the next highest sodium bromide solution (δ = 1.063 g/ml) was layered on top of the sample, followed by 2.8 ml of the lowest-density solution (δ = 1.006 g/ml).
The ultracentrifuge tubes were balanced, placed into individual titanium buckets, and capped. The buckets were placed into their respective positions on an SW 41 Ti swinging bucket rotor (Beckman Instruments, Inc.) and centrifuged at 40,000 rpm for 18 h at a temperature of 15°C in a Beckman L8-80M Ultracentrifuge (Beckman Instruments, Inc.). Upon completion of the run, the ultracentrifuge tubes were carefully removed from the titanium buckets. Each tube showed four visibly distinct regions represented by the very low density lipoprotein (VLDL), LDL, HDL, and lipoprotein-deficient plasma (LPDP) fractions. Subsequently, each of the layers was removed with a Pasteur pipette from the top to the bottom layer, respectively, and the volumes of each of the fractions were measured. Fractions removed for standard curve purposes were pooled together. All sample and pooled standard curve fractions were transferred to clean test tubes, covered, and stored at 4°C until further analysis.
(iii) Controls.
LPDP was used as the control medium (30). The LPDP was acquired by the technique of step-gradient ultracentrifugation as described above. The LPDP was dialyzed (molecular weight cutoff, 1,000) against a 0.9% sodium chloride solution for 24 h at a temperature of 4°C and was then used as the control medium for the incubation of Nys or L-Nys. Preparation of the samples with Nys or L-Nys as well as separation of constituents was carried out by techniques identical to those described above for step-gradient ultracentrifugation. After ultracentrifugation, the removal of each “lipoprotein” fraction was estimated by comparison to previously separated lipoprotein fractions (i.e., removal of the layer from where VLDL would normally reside was based on previous separations of actual plasma in which the layers were visible). The removal of the nonlipoprotein fractions in the control medium was estimated as being close to the placement of their separated lipoprotein counterparts in plasma.
Nys quantification.
Determination of the Nys contents of the fractions separated by both step-gradient and sequential ultracentrifugation was carried out as described above. Furthermore, each individually separated fraction—VLDL, LDL, HDL, and LPDP—was assayed for Nys in a like manner.
(i) Standard curve preparation.
To a series of test tubes labeled 0, 0.625, 1.25, 2.5, 5, 10, and 20 μg/ml was added 0.5 ml of pooled standard curve fraction, and to a test tube labeled 40 μg/ml was added 0.96 ml of the same pooled fraction. All volumes were prewarmed to 37°C. A 40-μl aliquot of either an Nys solution (1 mg/ml) or an L-Nys suspension (1 mg/ml) was then added to the test tube labeled 40 μg/ml, and the test tube was vortexed for 10 s and returned to 37°C. After 5 min, the series of test tubes was removed from 37°C and 0.5 ml from the test tube labeled 40 μg/ml was transferred to the test tube labeled 20 μg/ml. This mixture was vortexed for 10 s, whereupon a 0.5-ml aliquot of this mixture was subsequently transferred to the test tube labeled 10 μg/ml. This procedure of serial dilution was carried out for the remaining tubes used for the standard curve (not including the test tube labeled 0 μg/ml), and once the serial dilution was completed, the test tubes labeled 0, 0.625, 1.25, 2.5, 5, 10, and 20 μg/ml were returned to 37°C for 5 min. Note that 0.5 ml from the test tube labeled 0.625 μg/ml was discarded to provide a final volume of 0.5 ml for all concentrations used for the standard curve. The test tube labeled 40 μg/ml was immediately placed in ice and was cooled to 4°C for 30 min. After 5 min of incubation, the remaining tubes used for the standard curves were removed from 37°C and cooled on ice with the test tube labeled 40 μg/ml (28, 37).
(ii) Determination of Nys content within the separated lipoprotein fraction.
To an appropriately labeled test tube, a 0.5-ml aliquot of sample was added. To these sample tubes as well as the tubes used for the standard curve was added 1.0 ml of dichloromethane. The mixture was vortexed for 10 s, and all samples were dried under a steady stream of nitrogen at ambient temperature. Once the sample was dried, the Nys was extracted from the residue with a series of methanol washes. Extraction efficiency was determined to be >90% (28). Briefly, a 2.0-ml aliquot of methanol was added to the residue, and the mixture was vortexed for 20 s. The mixture was allowed to stand for 5 min and was vortexed again for 20 s. All test tubes were then centrifuged at 1,200 × g for 2 min at 15°C. The supernatant was transferred to a clean test tube, and the procedure was repeated an additional two times. The supernatant from each of the three extraction steps was pooled with the previous supernatant to provide a final volume of approximately 6 ml of methanol. This pooled methanol was then dried to completion under a steady stream of nitrogen at ambient temperature. Immediately prior to analysis, the residue was reconstituted with 0.5 ml of methanol and was injected onto the column.
HPLC apparatus.
The high-pressure liquid chromatography (HPLC) system consisted of a Waters 600 Controller (Waters Corporation, Milford, Mass.) interfaced to a Waters 717plus Autosampler and a Waters 486 Tunable Absorbance Detector. The detector was set at a UV absorbance wavelength of 306 nm and an absorbance sensitivity of 0.05 absorbance units-full scale. All results were recorded on a Waters 746 Data Module integrator. Attenuation was set to 256, and the chart speed was set to 0.5 cm/min. Samples (volume, 100 μl) were injected onto a Zorbax SB-C18 column (4.6 by 150 mm; particle size, 5 μm) prefitted with a Zorbax Reliance SB-C18 guard column (4.6 by 12.5 mm; particle size, 5 μm) (Rockland Technologies, Inc.). Chromatographic separation was carried out at ambient temperature. The mobile phase used a gradient flow and consisted of a 0.0385% ammonium acetate–THF (90:10 [wt/wt]) mixture termed mobile phase A and 100% THF termed mobile phase B (provided by Aronex Pharmaceuticals Inc.) (unpublished data). The flow rate was 1.5 ml/min.
Lipid and protein content analysis of lipoprotein and lipoprotein-deficient plasma fractions.
Total plasma and lipoprotein triglycerides, esterified cholesterol, free cholesterol, phospholipids (phosphatidylcholine), and protein concentrations were determined by enzymatic assays purchased from Sigma Chemical Co. and Boehringer Mannheim (28, 37).
Experimental design.
Nys and L-Nys (20 μg of drug/ml of plasma, a concentration close to peak levels in observed in the plasma of mice after the administration of an intravenous bolus [28]) were incubated in human plasma from three separate patients for 5 min at 37°C. Following incubation the plasma samples were placed on ice to prevent redistribution of drug within the plasma sample. At these temperatures (4°C) the appearance of the lipoprotein changes. It no longer has a fluid-like appearance but rather is more solid or crystallized (2). In effect, by rapidly cooling the plasma, the redistribution of Nys is effectively minimized (data not shown). The plasma was then partitioned into its lipoprotein and lipoprotein-deficient plasma fractions: VLDL, consisting of chylomicrons and VLDLs; LDL, consisting of intermediate-density lipoproteins and LDLs; HDL, consisting of all subclasses of HDL; and LPDP, consisting of all nonlipoprotein constituents of plasma. Separation of the plasma components was accomplished by step-gradient ultracentrifugation. Each lipoprotein fraction was analyzed for cholesterol (total, esterified, and unesterified), triglyceride, phospholipid, and protein concentrations as well as the Nys and L-Nys distributions. A comparison of the Nys and L-Nys distributions to the lipoprotein lipid and protein profiles was then performed in order to determine any relationships which may exist in determining the plasma lipoprotein distributions of Nys and L-Nys.
Statistical analysis.
Differences in the lipid and protein contents of the separated lipoprotein fractions were determined by analysis of variance (InStat; GraphPad Software). Differences between the distribution of Nys and L-Nys in the separated lipoprotein fractions for each patient plasma sample were also determined by analysis of variance. Critical differences were assessed by Tukey post hoc tests (28). Differences were considered significant if P was <0.05. All data are expressed as means ± standard deviations. The linear relationship between Nys or L-Nys recovery and lipid or protein content within the separated lipoprotein and lipoprotein-deficient fractions was determined by using the Pearson correlation coefficient as described previously (29).
RESULTS
Quantification of Nys by HPLC.
In order to determine the concentration of Nys or L-Nys within each separated lipoprotein and lipoprotein-deficient fraction, the resultant peak area from the chromatogram of the sample obtained by HPLC was compared to that from a standard curve for that same fraction. These external calibration curves were prepared and used for every separated fraction from each of the three patient plasma samples. A separate standard curve was prepared for each of the four separated fractions: VLDL, LDL, HDL, and LPDP. These four standard curves were prepared for each of the three different plasma samples.
The standard curves for each plasma lipoprotein and lipoprotein-deficient fraction were linear for Nys and L-Nys over a range of at least 1.25 to 20 μg/ml; the correlation coefficient was greater than 0.993 for each regression line (Table 1). The retention times of Nys and L-Nys following extraction from human plasma lipoprotein and lipoprotein-deficient plasma fractions were approximately 9.06 and 9.38 min, respectively; at these retention times no detectable peaks were observed in the fraction blank (0 μg of Nys or L-Nys per ml) for each standard curve. The area of each peak was determined, and the sum of these peaks was used to generate a concentration point in the creation of the linear standard curve. The intra-assay variability for all of the calibration curves was between 7 and 12%.
TABLE 1.
Representative linear calibration curves for Nys and L-Nys determined in the separated lipoprotein and lipoprotein-deficient plasma fractions for patient plasma sample 2
| Drug formulation and separated fraction for which calibration curve was determined | Equationa | r2 | Concn range (μg/ml) for standard curve |
|---|---|---|---|
| Free Nys | |||
| VLDL | y = 371,342x + 33,780 | 0.9945 | 0.625–20 |
| LDL | y = 474,277x − 10,189 | 0.9984 | 1.250–20 |
| HDL | y = 458,813x + 5,465 | 0.9983 | 1.250–20 |
| LPDP | y = 407,716x + 69,343 | 0.9992 | 1.250–20 |
| L-Nys | |||
| VLDL | y = 391,806x − 101,245 | 0.9956 | 0.625–20 |
| LDL | y = 412,100x + 60,128 | 0.9972 | 1.250–20 |
| HDL | y = 418,018x + 372,403 | 0.9992 | 1.250–20 |
| LPDP | y = 365,657x + 37,080 | 0.9999 | 1.250–20 |
y, peak area (in microvolts per second); x, concentration of Nys (in micrograms per milliliter).
Lipid and protein analyses.
For every separated lipoprotein and lipoprotein-deficient fraction of each patient plasma sample, lipid and protein profiles were compiled. All lipid and protein concentrations were determined with colorimetric enzyme analysis kits and were measured with a UV spectrophotometer. For the determination of the total cholesterol, total triglyceride, and total protein concentrations in the lipoprotein and lipoprotein-deficient plasma fractions, standard curves were created and used. The standard curves for total cholesterol and total triglyceride concentrations were linear over ranges of 12.5 to 200 and 15.625 to 250 mg/dl, respectively. For the standard curve for the total protein concentration, while not linear, a concentration range of 50 to 400 μg/ml was used for total protein concentration determination. The concentrations of each of the separated fraction samples in the samples with unknown concentrations were measured directly from their respective standard curves. For the other lipid components of the lipoprotein and lipoprotein-deficient plasma fractions (i.e., free cholesterol, cholesteryl ester, and phospholipid), measurement of their concentrations was determined directly by using the equation described previously (28, 37).
Lipid and protein compositions of separated fractions. (i) Cholesterol.
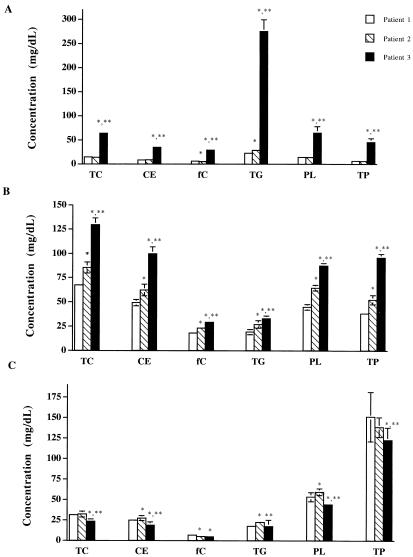
When human plasma was partitioned into its lipoprotein fractions by step-gradient ultracentrifugation, the following differences in the total cholesterol concentrations of three patient samples were observed. While there was no significant difference between the total cholesterol concentration in the VLDL fraction of plasma from patients 1 and 2, a significantly greater total cholesterol concentration was observed in the plasma of patient 3 compared to those observed in the plasma of both patients 1 and 2 (Fig. 1A). In the separated LDL fraction, the total cholesterol concentration significantly increased from patient samples 1 through 3, respectively (Fig. 1B). While there was no difference between the HDL total cholesterol concentration in the HDL fractions of patient samples 1 and 2, there was a significant decrease in HDL total cholesterol concentration in the HDL fraction of the sample from patient 3 (Fig. 1C). The total plasma cholesterol concentration showed a significant increase from patient samples 1 through 3, respectively (data not shown).
FIG. 1.
Lipid and protein contents and compositions within VLDLs (A), LDLs (B), and HDLs (C) of patient plasma samples 1 (□), 2 ( ), and 3 (⧫). TC, total cholesterol; CE, cholesteryl ester; fC, free cholesterol; TG, triglyceride; PL, phospholipid; TP, total protein. Data are represented as means ± standard deviations (n = 14). ∗, P < 0.05 versus patient sample 1; ∗∗, P < 0.05 versus patient sample 2.
(ii) Cholesteryl ester.
The cholesteryl ester concentration in the VLDL fraction showed no difference between patient samples 1 and 2, but there was a significant increase in the esterified cholesterol concentration in the sample from patient 3 compared to that in samples from patients 1 and 2 (Fig. 1A). The esterified cholesterol concentration within the LDL fraction significantly increased from patient samples 1 through 3, respectively (Fig. 1B). The HDL cholesteryl ester concentration in the HDL fraction of patient 2 was significantly greater than that of patient 1, whereas the cholesteryl ester concentration for patient 3 was observed to be significantly less than those for both patient samples 1 and 2 (Fig. 1C). For the total plasma cholesteryl ester concentration there was a significant rise in value from patient samples 1 through 3, respectively (data not shown).
(iii) Free cholesterol.
Within the VLDL fraction there was a significant decrease in the free cholesterol concentration for patient 1 compared to that for patient 2 (Fig. 1A). However, a significant increase in the free cholesterol concentration in the VLDL fraction of patient sample 3 compared to that for patient samples 1 and 2 was observed (Fig. 1A). Within the LDL fraction, the unesterified cholesterol concentration significantly increased from patient samples 1 through 3, respectively (Fig. 1B). The unesterified cholesterol concentration in the HDL fractions of patients 2 and 3 showed no differences when they were compared to each other, but both concentrations were significantly lower than the HDL free cholesterol concentration of patient 1 (Fig. 1C). The total plasma free cholesterol concentration showed a significant increase from patient sample 1 through patient sample 3, respectively (data not shown).
(iv) Triglycerides.
The triglyceride concentration within the VLDL fraction significantly increased from patient sample 1 through patient sample 3, respectively (Fig. 1A). The triglyceride concentration in the LDL fraction also showed a significant increase from patient sample 1 through patient sample 3, respectively (Figure 1B). Within the HDL fraction, however, there was a significantly higher concentration of triglyceride in patient sample 2 than in both patient samples 1 and 3 (Fig. 1C). No difference was observed between the HDL triglyceride concentration of patient samples 1 and 3 (Fig. 1C). The total plasma triglyceride concentration significantly increased from patient sample 1 through patient sample 3, respectively (data not shown).
(v) Phospholipids.
The phospholipid concentration (as a measurement of the concentration of phosphatidylcholine, which is the major phospholipid of lipoproteins [6, 7]) within the VLDL fraction was not different between patient samples 1 and 2. However, a significantly greater phospholipid concentration was observed in patient sample 3 (Fig. 1A). For the phospholipid content in the LDL fraction, a significant increase from patient sample 1 through patient sample 3 was evident (Fig. 1B). The HDL phospholipid concentration in the HDL fraction of patient sample 2 was significantly greater than that of patient sample 1. However, the phospholipid concentration in the HDL layer of patient sample 3 was significantly lower than that of both patient samples 1 and 2 (Fig. 1C). A significant increase in total plasma phospholipid concentration from patient sample 1 through patient sample 3 was observed (data not shown).
(vi) Protein.
The following differences in total protein concentration were observed when the patient plasma samples were separated into their lipoprotein and lipoprotein-deficient fractions. A significant increase in the total protein concentration of VLDL for patient sample 3 compared to that for patient samples 1 and 2 was observed (Fig. 1A). A significant increase in the total protein concentration was observed within the LDL fraction of patient samples 1 through 3, respectively (Fig. 1B). For the HDL fraction there was a significant decrease in total protein concentration from patient samples 1 through 3, respectively (Fig. 1C). In the LPDP fraction, there was a significant decrease in total protein concentration when comparing patient samples 2 and 1 and then a significant increase when comparing patient samples 2 and 3 (data not shown). There was no significant difference between the LPDP protein concentration of patient samples 1 and 3 (data not shown). No difference between the total plasma total protein concentration of patient samples 1 and 3 was observed; however, a significant decrease in total plasma total protein concentration of patient sample 2 compared to those of patient samples 1 and 3 was noted (data not shown).
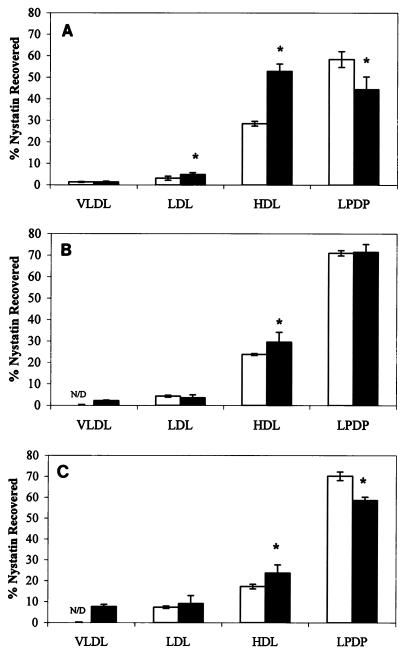
Nys distribution within separated plasma component fractions.
Table 2 describes the human plasma lipoprotein and lipoprotein-deficient fraction distributions of Nys and L-Nys in three different plasma samples. The distribution of Nys and L-Nys in the lipoprotein and lipoprotein-deficient fractions of three different human plasma samples incubated at 37°C for 5 min showed the following differences. For plasma incubated with Nys, only patient sample 1 showed a detectable amount of Nys in the VLDL fraction. Within the assay’s limit of detection, no detectable amount of Nys was recovered from the VLDL fractions of patient samples 2 and 3. A significant increase in the amount of Nys recovered from the LDL fraction of samples 1 through 3, respectively, was observed. For the separated HDL fraction, however, the opposite was true. There was a significant decrease in the amount of Nys recovered from plasma samples 1 through 3, respectively. In the LPDP fraction, there was a significant increase in the amount of Nys recovered in samples 2 and 3 compared to the amount recovered in sample 1. The total recovery of Nys in the three plasma samples incubated with Nys was above 90% for each patient sample.
TABLE 2.
Distribution of free Nys and L-Nys in lipoprotein and lipoprotein-deficient fractions of three different human patient samplesa
| Drug formulation and plasma sample no. | % Drug in
the following fraction:
|
% Recovery | |||
|---|---|---|---|---|---|
| VLDL | LDL | HDL | LPDP | ||
| Free Nys | |||||
| 1 | 1.38 ± 0.20 | 3.15 ± 0.96 | 28.48 ± 1.09 | 58.34 ± 3.68 | 91.26 ± 4.99 |
| 2 | ND | 4.28 ± 0.46b | 23.80 ± 0.46b | 71.06 ± 1.22b | 99.70 ± 1.21 |
| 3 | ND | 7.31 ± 0.56b,c | 17.23 ± 1.10b,c | 70.20 ± 2.06b | 95.37 ± 2.17 |
| L-Nys | |||||
| 1 | 1.42 ± 0.35 | 4.91 ± 0.83 | 52.78 ± 3.44 | 44.41 ± 5.88 | 103.52 ± 4.90 |
| 2 | 2.20 ± 0.27b | 3.54 ± 1.37 | 29.60 ± 4.52b | 71.51 ± 3.67b | 106.85 ± 8.00 |
| 3 | 7.64 ± 1.01b,c | 9.07 ± 3.84b,c | 23.65 ± 3.92b,c | 58.62 ± 1.54b,c | 98.97 ± 7.08 |
The plasma was incubated at 37°C for 5 min, Nys and L-Nys were used at 20 μg/ml of human plasma. Data are expressed as means ± standard deviations (n = 8 for Nys; n = 6 for L-Nys). ND, Nys concentrations were nondetectable within range of the standard curve.
P < 0.05 versus patient sample 1.
P < 0.05 versus patient sample 2.
For plasma incubated with L-Nys, all samples showed detectable amounts of L-Nys in the VLDL fraction. Although the increase in L-Nys recovered from the VLDL fraction of patient sample 2 was small compared to that for patient sample 1, the difference was significant. For patient sample 3 a significant increase in the percentage of L-Nys recovered from the VLDL fraction compared to that for both patient samples 1 and 2 was demonstrated. In the LDL fraction, there was no significant difference between samples 1 and 2; however, a significant increase in the amount of L-Nys recovered from the LDL fraction of patient sample 3 compared to those recovered from patient samples 1 and 2 was observed. In the HDL fraction, a pattern similar to that observed for the distribution of Nys in HDL was seen. There was a significant decrease in the percent recovery of L-Nys within the HDL fraction of patient samples 1 through 3, respectively. The total recovery of Nys in the three plasma samples incubated with L-Nys was above 98% for each patient sample.
Figure 2A describes the distribution of Nys versus L-Nys in the lipoprotein and lipoprotein-deficient fractions of patient plasma sample 1. With the exception of the VLDL fraction, for all other fractions significant differences between the percent recoveries of Nys in the lipoprotein and lipoprotein-deficient fractions were found when the percent recoveries for the two different formulations (Nys and L-Nys) are compared. Within the LDL fraction, there was a significant increase in the percentage of L-Nys recovered compared to the percentage of the Nys formulation recovered. When L-Nys was used, a significantly greater percentage of Nys was recovered within the HDL fraction compared to the percent recovered when the Nys formulation was used. However, within the LPDP fraction, a significant decrease in the percentage of L-Nys recovered compared to the percentage of Nys recovered was observed.
FIG. 2.
Distributions of free Nys (□) and L-Nys ( ) in lipoprotein and lipoprotein-deficient fractions of patient plasma sample 1 (A), patient plasma sample 2 (B), and patient plasma sample 3 (C). Data are represented as means ± standard deviations (n = 8 for Nys; n = 6 for L-Nys). ∗, P < 0.05 versus Nys.
Figure 2B shows the distribution of Nys and L-Nys in patient plasma sample 2. Significant changes in the Nys distribution in the VLDL and HDL fractions were observed between the two different formulations. In both instances, a significant increase in the amount of Nys recovered in these two fractions was observed when the plasma sample was incubated with L-Nys compared to the amount recovered when the sample was incubated with Nys. No difference in the distribution of Nys versus L-Nys was observed in the LDL and LPDP fractions.
Figure 2C shows the distribution of Nys and L-Nys in patient plasma sample 3. The distributions of Nys and L-Nys in the LDL fraction were not significantly different. In both the VLDL fraction and the HDL fraction, there was a significant increase in the percentage of Nys recovered when the sample was incubated with L-Nys. Within the LPDP fraction, a significantly lower percentage of L-Nys was recovered compared to the percent recovery of Nys.
When Nys and L-Nys were incubated in the LPDP fraction, the majority of drug (>85%) was recovered in the density fraction from 1.21 to 1.25 g/ml, suggesting that the distribution of Nys and L-Nys is not a function of formulation density (data not shown).
Effect of lipoprotein composition on Nys and L-Nys distributions.
In order to determine the effect of lipoprotein content and composition on the distributions of Nys and L-Nys within each separated lipoprotein fraction, comparisons of the amounts of Nys or L-Nys recovered and the lipoprotein content and composition of each separated fraction were performed. The values obtained for each of the three patient plasma samples were compared. The following relationships were calculated: Nys or L-Nys to (i) total cholesterol, (ii) cholesteryl ester, (iii) free cholesterol, (iv) triglyceride, (v) phospholipid, (vi) total protein, (vii) core lipid, and (viii) coat lipid concentrations. The relationship between Nys and VLDL lipid and protein content could not be performed for patient plasma samples 2 and 3 due to the nondetectable Nys levels within those samples (Table 3). As a result, comparisons of these ratios within the separated VLDL fractions were not done for Nys. Tables 3 and 4 present the correlation coefficients for the analyses performed with each of the four separated fractions for both the Nys and the L-Nys formulations, respectively.
TABLE 3.
Correlational analysis comparing amount of Nys recovered to lipid and protein contents and compositions of the separated lipoprotein and lipoprotein-deficient plasma fractionsa
| Component or ratio |
r value
|
|||
|---|---|---|---|---|
| VLDL-Nys | LDL-Nys | HDL-Nys | LPDP-Nys | |
| TC | NAb | 0.936c | 0.855c | NA |
| CE | NA | 0.938c | 0.739c | NA |
| fC | NA | 0.874c | 0.230 | NA |
| TG | NA | 0.731c | 0.252 | NA |
| PL | NA | 0.905c | 0.464 | NA |
| TP | NA | 0.926c | 0.595 | 0.312 |
| Core lipid content (CE + TG) | NA | 0.929c | 0.325 | NA |
| Coat lipid content (fC + PL) | NA | 0.903c | 0.539 | NA |
| TC:TP ratio | NA | −0.815c | 0.709c | NA |
| TG:TP ratio | NA | −0.735c | 0.481 | NA |
| PL:TP ratio | NA | −0.731c | 0.018 | NA |
| TC:TG ratio | NA | 0.642c | 0.735c | NA |
Calculations are based on the Pearson correlation coefficient values with significance. TC, total cholesterol; CE, cholesteryl ester; fC, free cholesterol; TG, total triglyceride; PL, phospholipid; TP, total protein.
NA, not applicable; analysis was not performed due to a lack of sufficient data.
P < 0.05.
TABLE 4.
Correlational analysis comparing amount of L-Nys recovered to lipid and protein contents and compositions of the separated lipoprotein and lipoprotein-deficient plasma fractionsa
| Component or and ratio |
r value
|
|||
|---|---|---|---|---|
| VLDL-Nys | LDL-Nys | HDL-Nys | LPDP-Nys | |
| TC | 0.972b | 0.583 | 0.416 | NAc |
| CE | 0.960b | 0.590 | 0.149 | NA |
| fC | 0.979b | 0.521 | 0.626b | NA |
| TG | 0.967b | 0.559 | 0.326 | NA |
| PL | 0.972b | 0.549 | 0.421 | NA |
| TP | 0.982b | 0.642b | 0.879b | 0.037 |
| Core lipid content (CE + TG) | 0.967b | 0.594 | 0.289 | NA |
| Coat lipid content (fC + PL) | 0.975b | 0.544 | 0.511 | NA |
| TC:TP ratio | −0.924b | −0.577 | −0.431 | NA |
| TG:TP ratio | 0.803b | −0.480 | 0.042 | NA |
| PL:TP ratio | −0.910b | −0.692b | −0.341 | NA |
| TC:TG ratio | −0.927b | 0.026 | −0.327 | NA |
Within the LDL fraction of the three plasma samples, a strong positive correlation exists between Nys and almost all lipid and protein components. In assessing the linear relationship between Nys and the individual components of LDL, a correlation coefficient of 0.731 to 0.938 was calculated (Table 3). Therefore, as the amount of lipid or protein increased within the LDL fraction, there was a proportional increase in the amount of Nys recovered from that fraction as well. However, a negative correlation existed between the ratio of lipid to protein compared to the amount of Nys recovered. As the ratio of total cholesterol, triglyceride, or phospholipid to total protein increased within the LDL fraction, a subsequent decrease in the amount of Nys recovered from the LDL fraction was observed (Table 3). However, the relationship between the amount of Nys recovered from the LDL fraction and the total cholesterol:triglyceride ratio of that fraction showed a positive correlation coefficient (r = 0.642) (Table 3).
Few strong linear relationships existed between Nys and the individual lipid components of HDL; only those relationships involving the amount of Nys recovered to the total cholesterol (r = 0.855) and cholesteryl ester (r = 0.739) contents are apparent (Table 3). A positive relationship between the amount of total protein within the HDL fraction and the amount of Nys recovered from the HDL fraction does exist (r = 0.595); however, the association is not as strong as those observed with total cholesterol and cholesteryl ester (Table 3). A positive correlation exists between the amount of Nys within the HDL fraction and the total cholesterol-to total-protein ratio (r = 0.709) and the total cholesterol-to-total triglyceride ratio (r = 0.735) (Table 3). No linear relationship appears to exist between the total protein content and the amount of Nys in the LPDP fraction.
There was a strong positive correlation between the amount of L-Nys recovered and the amounts of all lipid and protein components of the VLDL fraction. The correlation coefficients for these linear relationships ranged from r equal to 0.960 for L-Nys versus cholesteryl ester to r equal to 0.982 for L-Nys versus total protein (Table 4). However, a strong negative relationship (r = −0.924) was evident between the amount of L-Nys and the total cholesterol-to-total protein ratio (Table 4). As the total cholesterol-to-total protein ratio increased, the amount of L-Nys within the VLDL fraction decreased proportionally. The opposite was true for L-Nys versus the triglyceride-to-total protein ratio. As the ratio of triglyceride to total protein increased, so did the amount of L-Nys recovered from the VLDL fraction (r = 0.803) (Table 4). For the amount of L-Nys versus the phospholipid-to-total protein ratio, a strong negative correlation was again observed (r = −0.910) (Table 4). As the ratio of total cholesterol to total triglyceride increased, the amount of L-Nys within the VLDL fraction decreased proportionally (r = −0.927) (Table 4).
Within the LDL fraction, the linear relationships between L-Nys and lipoprotein content and composition are not as strong. The largest correlation coefficients were found to be associated with the relationships of L-Nys versus total cholesterol (r = 0.583), esterified cholesterol (r = 0.590), total protein (r = 0.642), and core lipid (r = 0.594) contents (Table 4). All of these relationships showed positive correlations; as the amount of lipid or protein within the LDL fraction increased, so did the amount of L-Nys recovered from within that fraction. Negative correlations were observed for the ratios of total cholesterol, triglyceride, and phospholipid to total protein compared to the amount of L-Nys. The strongest of these relationships showed that as the ratio of phospholipid to total protein increased, the amount of L-Nys decreased within the LDL fraction in a proportional manner (r = −0.692) (Table 4). Within the HDL fraction, the only correlations of significance were observed in a comparison of the amount of L-Nys recovered within the HDL fraction to the amount of free cholesterol (r = 0.626) and the amount of total protein (r = 0.879) recovered in the fraction (Table 4). These strong positive correlations showed that as the amount of free cholesterol or total protein within the HDL fraction increased, a proportional increase in the amount of L-Nys recovered from within that fraction was observed. No significant relationship existed between the amount of L-Nys associated within the LPDP fraction and the total protein content of the LPDP fraction (Table 4).
DISCUSSION
The objective of this study was to determine the plasma lipoprotein distribution of Nys and L-Nys in different human patient samples and subsequently to ascertain the relationship of the drug distribution to the lipid and protein compositions of these separated lipoprotein fractions. When Nys is incubated in human plasma, the majority of the drug is recovered from the LPDP fraction. However, within the lipoprotein fractions themselves, the majority of Nys is found to be associated with HDL. This same pattern of association is similar for L-Nys; the majority of lipoprotein-associated L-Nys is found in HDL. The incorporation of Nys into liposomes composed of DMPC and DMPG results in an overall increase in the amount of drug recovered in the lipoprotein fractions. In the attempt to determine the relationship between the associated drug and the lipoprotein content and composition, all components of the lipoprotein were considered. Trends in the relationship of the drug distribution to the lipid or protein content and composition of the lipoprotein fractions are summarized in Table 5.
TABLE 5.
Important trends observed in comparison of Nys and L-Nys distributions within plasma lipoproteins and contents and compositions of these lipoprotein fractionsa
| Lipoprotein fraction | Trends observed (patient sample 1 →
patient sample 3)
|
r2 | ||
|---|---|---|---|---|
| Lipoprotein profile | Nys recovery | Ratiob | ||
| Free Nys | ||||
| LDL | ↑TC | ↑Nys | No change | 0.936 |
| ↑CE | ↑Nys | No change | 0.938 | |
| ↑fC | ↑Nys | No change | 0.874 | |
| ↑Core | ↑Nys | No change | 0.929 | |
| HDL | ↓TP | ↓Nys | ↓ | 0.595 |
| L-Nys | ||||
| VLDL | ↑TG | ↑L-Nys | No change | 0.967 |
| HDL | ↓TP | ↓L-Nys | ↓ | 0.879 |
TC, total cholesterol; CE, cholesteryl ester; fC, free cholesterol; core, core lipids (cholesteryl ester plus total triglyceride); TP, total protein; TG, total triglyceride; ↑, increase; ↓, decrease.
Ratio of amount of Nys to amount of lipid or protein in separated fraction.
Within the LDL fraction, a noticeable increase in all lipoprotein components from patient samples 1 through 3 is evident (Fig. 1B). This suggests an increase in LDL particle number and lipid mass. Similarly, the amount of Nys associated with LDL increases (Table 2). Thus, as the amount of lipid or protein within the LDL fraction increases, so does the amount of Nys associated with that fraction (Table 3). One other important factor to be considered is the number of LDL particles within the LDL fraction. It is evident from the data that the concentration of each individual component of LDL is increasing from patient sample 1 to patient sample 3. Therefore, the data suggest that the association of Nys with LDL is not a function of a specific component or amount of component within LDL but, rather, is a function of particle number or total lipid mass of the LDL fraction.
Within the HDL fraction the relationship of the Nys distribution to the lipid or protein content is markedly different from that observed within the LDL fraction. The amount of Nys recovered from the HDL fraction steadily drops from patient samples 1 to 3; however, in almost all situations, the individual lipid components of HDL increase in concentration in patient sample 2 with compared to their concentrations in patient sample 1. A subsequent decrease in concentration then occurs in patient sample 3 (Fig. 1C). One exception to this observation is with free cholesterol. A gradual decrease in free cholesterol concentration is seen from samples 1 to 3. However, the decrease in free cholesterol concentration does not decrease with the amount of Nys recovered within the HDL fraction in a proportional manner (Table 3). Thus, one would expect the ratio of Nys to free cholesterol to remain constant if the decrease in both factors were proportional. However, the ratio of Nys to free cholesterol in HDL is erratic between the three patient samples and shows no distinguishable pattern. These findings suggest that neither free cholesterol nor any other individual lipoprotein lipid constituent plays a major deciding role in the distribution of Nys within the HDL fraction.
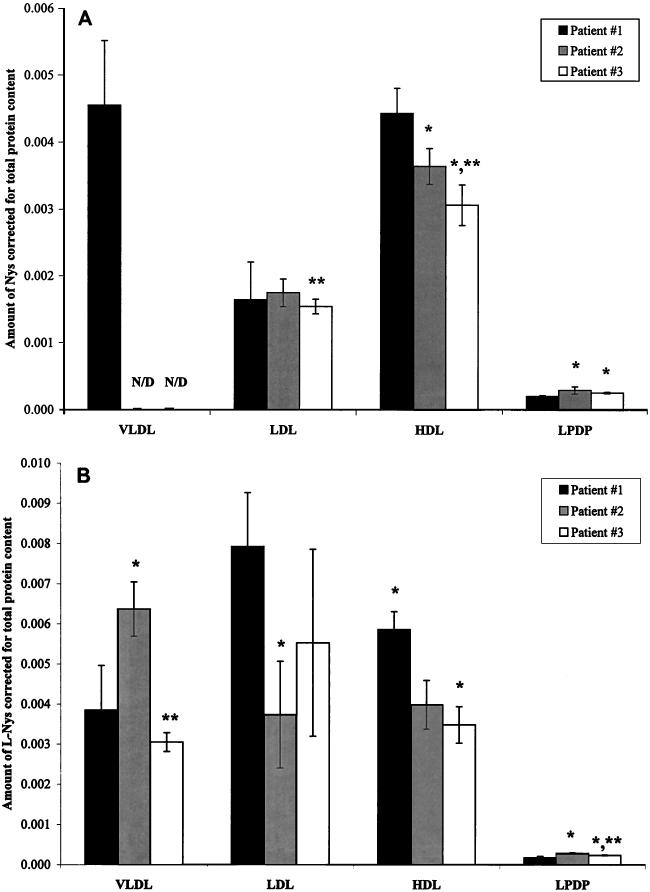
Nevertheless, as with the decrease in the amount of Nys within the HDL fraction, the total protein content of HDL reflects a similar pattern. The total protein concentration decreases from patient samples 1 through 3, respectively, but not in a manner proportional to the amount of Nys, which is redistributed. As the concentration of total protein decreases, the amount of Nys within the HDL fraction also decreases, but in a greater proportion (Fig. 3A). If the amount of Nys were to decrease in the same proportion as the total protein concentration, the ratio of Nys to total protein would remain constant; as observed in Fig. 3A, this is not the case. The ratio of Nys to total protein decreases from patient samples 1 to 3, respectively. These findings suggest that while a relationship between Nys distribution and the total protein content of HDL does exist, it is not simply a function of protein mass.
FIG. 3.
Nys-to-total protein ratio within the four separated plasma fractions of three patient plasma samples following the incubation of Nys (A) or L-Nys (B) in human plasma for 5 min at 37°C. Data are represented as means ± standard deviations (n = 8 for Nys; n = 6 for L-Nys). ∗, P < 0.05 versus patient sample 1; ∗∗, P < 0.05 versus patient sample 2; N/D, nondetectable.
Following the incubation of L-Nys in three different human plasma samples the following relationships were observed. Within the VLDL fraction a detectable concentration of Nys was observed in the plasma of all patients when the plasma was incubated with L-Nys (Table 2). This allowed the establishment of any possible relationships between the percentage of L-Nys recovered within the VLDL fraction and the lipid or protein concentration of that fraction. As the concentration of triglyceride increased from patient sample 1 through patient sample 3 (Fig. 1A), the distribution of L-Nys in the VLDL fraction reflected a similar pattern (Table 2). Subsequently, these increases in triglyceride concentration and L-Nys distribution were observed to be fairly proportional to each other, as demonstrated by the correlation of L-Nys recovery to triglyceride content for the samples from the three patients (Table 4). These data suggest that triglyceride concentration may play a role in the amount of L-Nys that distributes to VLDL. However, the percentage of the initial amount of L-Nys, incubated in plasma, which was recovered within the VLDL fraction was small compared to the majority of lipoprotein-associated L-Nys found in the HDL fraction. This suggests that this association may be due to an increase in particle number or an increase in the amount of triglyceride found within VLDL.
Findings similar to those observed in the distribution of Nys in the HDL fraction were also seen in the distributions of L-Nys in the HDL fractions of the three patient samples. A decrease in the total protein content (Fig. 1C) as well as a decrease in the distribution of L-Nys (Table 2) was observed within the HDL fraction. As with the relationship of percent Nys recovery and the amount of total protein, the decrease in L-Nys recovery and the decrease in protein content is not proportional. This is illustrated by the ratio of L-Nys to total protein content within the HDL fractions of patient samples 1 to 3 (Fig. 3B). A proportional decrease in the percentage of L-Nys recovered within the HDL fraction and the total protein content of the HDL fraction would show no change in the ratio of L-Nys recovered to total protein content for patients samples 1 to 3. Instead, a decrease in this ratio was observed. These data suggest that although protein content does play a role in the distribution of L-Nys in the HDL fraction, it is not simply a total protein mass phenomenon.
Another determining factor that supports the hypothesis that protein content may influence drug distribution lies in the comparison of the distributions of Nys and L-Nys within the individual lipoprotein fractions. The majority of Nys and L-Nys distributed within the lipoprotein fractions is found in HDL (Table 2). If the distribution of Nys or L-Nys within the plasma lipoproteins was dependent on the type or amount of lipid, a greater percentage of drug would be recovered within the other two lipoprotein fractions::VLDL (which is the major triglyceride carrier) and LDL (which is the major cholesterol carrier). However, the majority of the drug recovered from the lipoprotein fractions is found within the HDL fraction. Thus, the data suggest that the association of Nys or L-Nys with plasma lipoproteins is not dependent on the amount or type of lipid.
Additional factors pertaining to the protein content and composition of HDL may also influence drug distribution. One such factor influencing the affinity of Nys for the protein content of HDL could be the type of apoprotein(s) associated with HDL. As discussed above, numerous apoproteins are found with the HDL lipoproteins: apoproteins A, apoproteins C, and apoproteins E (25). Of these apoproteins, apoproteins C and E are also found with other lipoprotein classes as well (25). Apoproteins A, however, are found to be preferentially associated with HDL. Therefore, it is believed that if there is a preference for the association of Nys with an apoprotein in the HDL fraction, it lies with the apoproteins A.
Another element that may play a role in the distribution of Nys within HDL could be the number and type of HDL particles. When separating the plasma lipoproteins by the step-gradient ultracentrifugation method described above, all HDL subclasses were grouped into one general HDL fraction. Separation of these HDL subclasses could not be accomplished by this method of lipoprotein separation. Therefore, in the scope of this work it was impossible to distinguish which types of HDL were present in each of the patient plasma samples. HDL2 and HDL3 are the two major subpopulations of HDL present in plasma, and each of these is composed of different amounts of lipids and apoproteins (6, 8–10, 12). Both subpopulations contain the same number of A2 apoproteins; however, HDL2 contains one more A1 apoprotein than does HDL3 (8, 25). Therefore, apoprotein A1 could specifically play a role in the distribution of Nys within HDL, depending on the subpopulations of the separated HDL fraction.
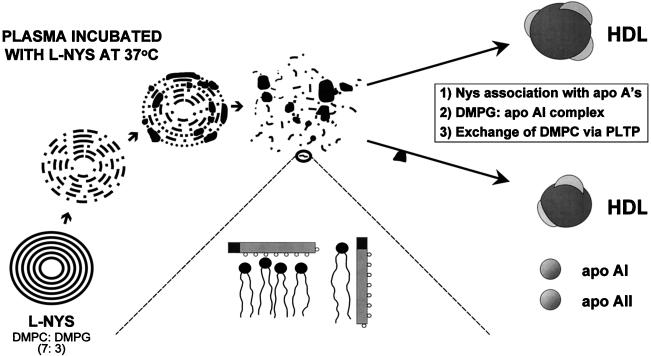
The incorporation of Nys into liposomes consisting of DMPC and DMPG at a ratio of 7:3 (wt/wt) resulted in an even greater percentage of the drug distributing to the HDL fraction of all patient samples compared to that for the nonliposomal formulation (Fig. 2A to C). These findings are supported by the earlier work of several investigators. Damen et al. (3–5) determined that upon the incubation of liposomes containing [14C]DMPC with whole plasma, a transfer of phospholipid between small unilamellar liposomes and HDL was observed. This transfer of 14C-labeled phosphatidylcholine represented an exchange with the phosphatidylcholine of HDL rather than a net transfer (5). Furthermore, Surewicz and coworkers (26) have since demonstrated that this exchange of phosphatidylcholine between small unilamellar liposomes and HDL is one that exclusively involves the outer monolayer of the liposomal membrane. Therefore, the introduction of liposomes into the circulation provides an opportunity for the phosphatidylcholine of the liposome not only to interact with all components of the plasma but also to be incorporated into the membrane bilayer of newly formed nascent HDL (8, 10). Furthermore, if the phosphatidylcholine is complexed with a drug molecule, that molecule may also be incorporated into the structure of the HDL particle. Hence, by the incorporation of Nys into multilamellar liposomes consisting of phosphatidylcholine, an increase in the distribution of Nys into HDL may be facilitated (Fig. 4).
FIG. 4.
Interaction of L-Nys with HDLs. apo AI, apolipoprotein AI; apo AII, apolipoprotein AII, PLTP, phospholipid transfer protein.
Human apolipoprotein AI and phospholipid complexes have also been studied. The association of apolipoprotein AI with multilamellar liposomes consisting of acidic phospholipids, such as DMPG, rapidly formed thermally stable complexes over a wide temperature range (26). Further evidence is provided from our work with L-AmB. When L-AmB composed of the same phospholipids used for L-Nys was incubated for 60 min at 37°C in human serum, more than 90% of AmB’s and DMPG’s liposome concentrations prior to incubation in serum were found with the HDL fraction (37). In addition, the DMPG-to-AmB molar ratio found in the HDL fraction was similar to the initial molar ratio for these liposomes before incubation in serum (37). These findings suggest that liposomes containing DMPG, such as L-Nys, may have the ability to target compounds specifically to HDL due to the ability of DMPG to complex with apolipoprotein AI. Therefore, any DMPG-drug complex introduced into human plasma, initially either as an intact liposome or as a lipid-drug complex, would likely increase the level of distribution of that drug into HDL (Fig. 4). This may be yet another mechanism by which the distribution of Nys into HDL is increased upon the incorporation of Nys into liposomes consisting of DMPC and DMPG. Additional factors such as HDL particle size, charge, and total surface area may also influence the distribution of the drug into HDL. Our laboratory is investigating these factors.
In conclusion, targeting of Nys to HDL by the mechanisms discussed herein may prove to be therapeutically beneficial to the patient. It has been demonstrated that upon the incorporation of AmB into liposomes similar in composition to L-Nys, the incidence of nephrotoxicity was significantly reduced (31, 34, 36). Not only was this effect a result of the increased distribution into HDL, but there was also a subsequent decrease in the association with LDL. As a result, larger doses were able to be delivered with greater efficacy and reduced side effects, thereby improving the therapeutic outcome (14). Therefore, with the incorporation of Nys into liposomes made up of DMPC and DMPG, a similar result may be realized.
ACKNOWLEDGMENTS
This work was funded by Aronex Pharmaceuticals Inc. and the Medical Research Council of Canada (grant MA-14484). S.M.C. is supported by a scholarship provided by the Douglas and Jean Bailey University of British Columbia Endowment Fund. F.W.S. is partially supported by funds provided by the Science Council of British Columbia.
REFERENCES
- 1.Budavari S, editor. The Merck index. 11th ed. Rahway, N.J: Merck and Co. Inc.; 1989. [Google Scholar]
- 2.Cushley R J, Treleaven W D, Parmar Y I, Chana R S, Fenske D B. Surface diffusion in human serum lipoproteins. Biochem Biophys Res Commun. 1987;146:1139–1145. doi: 10.1016/0006-291x(87)90766-2. [DOI] [PubMed] [Google Scholar]
- 3.Damen J, Regts J, Scherphof G. Transfer of [14C]phosphatidylcholine between liposomes and human plasma high density lipoprotein. Partial purification of a transfer-stimulating plasma factor using a rapid transfer assay. Biochim Biophys Acta. 1982;712:444–452. doi: 10.1016/0005-2760(82)90271-5. [DOI] [PubMed] [Google Scholar]
- 4.Damen J, Regts J, Scherphof G. Transfer and exchange of phospholipid between small unilamellar liposomes and rat plasma high density lipoproteins. Dependence on cholesterol content and phospholipid composition. Biochim Biophys Acta. 1981;665:538–545. doi: 10.1016/0005-2760(81)90268-x. [DOI] [PubMed] [Google Scholar]
- 5.Damen J, Waite M, Scherphof G. The in vitro transfer of [14C]dimyristoylphosphatidylcholine from liposomes to subfractions of human plasma high density lipoproteins as resolved by isoelectric focusing. FEBS Lett. 1979;105:115–119. doi: 10.1016/0014-5793(79)80898-4. [DOI] [PubMed] [Google Scholar]
- 6.Davis R A, Vance J E. Structure, assembly and secretion of lipoproteins. In: Vance D E, Vance J E, editors. Biochemistry of lipids, lipoproteins and membranes. New York, N.Y: Elsevier; 1996. pp. 473–493. [Google Scholar]
- 7.Davis R A. Lipoprotein structure and secretion. In: Vance D E, Vance J E, editors. Biochemistry of lipids, lipoproteins and membranes. New York, N.Y: Elsevier; 1991. pp. 403–426. [Google Scholar]
- 8.Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984;25:1017–1058. [PubMed] [Google Scholar]
- 9.Fielding C J, Fielding P E. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 10.Fielding P E, Fielding C J. Dynamics of lipoprotein transport in the human circulatory system. In: Vance D E, Vance J E, editors. Biochemistry of lipids, lipoproteins and membranes. New York, N.Y: Elsevier; 1996. pp. 495–516. [Google Scholar]
- 11.Hatch F T, Lees R S. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- 12.Havel R J, Kane J P. Introduction: structure and metabolism of plasma lipoproteins. In: Scriver C R, Beaudet A L, Sly W S, Valle D, editors. The metabolic and molecular basis of inherited disease. Vol. 2. New York, N.Y: McGraw-Hill Book Co.; 1995. pp. 1129–1138. [Google Scholar]
- 13.Havel R J, Eder H A, Bragdon J H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jusko W J, Gretch M. Plasma and tissue protein binding of drugs in pharmacokinetics. Drug Metab Rev. 1976;5:43–140. doi: 10.3109/03602537608995839. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Berestein G. Liposomes as carriers of antifungal drugs. Ann N Y Acad Sci. 1988;544:590–597. doi: 10.1111/j.1749-6632.1988.tb40459.x. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Berestein G, Fainstein V, Hopfer R L, Mehta K, Sullivan M P, Keating M, Rosenblum M G, Mehta R, Luna M, Hersh E M, Reuben J, Juliano R L, Bodey G P. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary study. J Infect Dis. 1985;151:704–710. doi: 10.1093/infdis/151.4.704. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Berestein G, Rosenblum M, Mehta R. Altered tissue distribution of amphotericin B by liposomal encapsulation: comparison of normal mice to mice infected with Candida albicans. Cancer Drug Delivery. 1984;1:199–205. doi: 10.1089/cdd.1984.1.199. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Berestein G, Hopfer R L, Mehta R T, Mehta K, Hersh E M, Juliano R L. Liposome-encapsulated amphotericin B for treatment of disseminated candidiasis in neutropenic mice. J Infect Dis. 1984;150:278–283. doi: 10.1093/infdis/150.2.278. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Berestein G, Mehta R T, Hopfer R L, Mills K, Kasi L, Mehta K, Fainstein V, Luna M, Hersh E M, Juliano R T. Treatment and prophylaxis of disseminated infection due to Candida albicansin mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983;147:939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- 20.Mehta R T, Lopez-Berestein G. Effect of liposome encapsulation on toxicity and antifungal activity on polyene antibiotics. In: Lopez-Berestein G, Fidler I J, editors. Liposomes in the therapy of infectious diseases and cancer. New York, N.Y: Alan R. Liss, Inc.; 1989. pp. 263–273. [Google Scholar]
- 21.Mehta R T, Hopfer R L, Gunner L A, Juliano R L, Lopez-Berestein G. Formulation, toxicity, and antifungal activity in vitro of liposome-encapsulated nystatin as therapeutic agent for systemic candidiasis. Antimicrob Agents Chemother. 1987;31:1897–1900. doi: 10.1128/aac.31.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta R T, Lopez-Berestein G, Hopfer R L, Mills K, Juliano R L. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochim Biophys Acta. 1984;770:230–234. doi: 10.1016/0005-2736(84)90135-4. [DOI] [PubMed] [Google Scholar]
- 23.Rios A, Rosenblum M, Crofoot G, Lenk R P, Hayman A, Lopez-Berestein G. Pharmacokinetics of liposomal nystatin in patients with human immunodeficiency virus infection. J Infect Dis. 1993;168:153–154. doi: 10.1093/infdis/168.1.253. [DOI] [PubMed] [Google Scholar]
- 24.Rowland M. Plasma protein binding and therapeutic monitoring. Ther Drug Monit. 1980;2:29–37. doi: 10.1097/00007691-198001000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Schneider W J. Removal of lipoproteins from plasma. In: Vance D E, Vance J E, editors. Biochemistry of lipids, lipoproteins and membranes. New York, N.Y: Elsevier; 1996. pp. 517–541. [Google Scholar]
- 26.Surewicz W K, Epand R M, Pownall H J, Hui S W. Human apolipoprotein A-I forms thermally stable complexes with anionic but not with zwitterionic phospholipids. J Biol Chem. 1986;261:16191–16197. [PubMed] [Google Scholar]
- 27.Wasan E K, Reimer D L, Bally M B. Plasmid DNA is protected against ultrasonic cavitation-induced damage when complexed to cationic liposomes. J Pharm Sci. 1996;85:427–433. doi: 10.1021/js9504752. [DOI] [PubMed] [Google Scholar]
- 28.Wasan K M, Ramaswamy M, Cassidy S M, Kazemi M, Strobel F W, Thies R L. Physical characteristics and lipoprotein distribution of liposomal nystatin in human plasma. Antimicrob Agents Chemother. 1997;41:1871–1875. doi: 10.1128/aac.41.9.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasan K M, Pritchard P H, Ramaswamy M, Wong W, Donnachie E M, Brunner L J. Differences in lipoprotein lipid concentration and composition modify the plasma distribution of cyclosporine. Pharm Res. 1997;14:1614–1621. doi: 10.1023/a:1012190620854. [DOI] [PubMed] [Google Scholar]
- 30.Wasan, K. M., S. M. Cassidy, F. W. Strobel, and S. Ng. A comparison of step-gradient and sequential ultracentrifugation in determining the lipoprotein distribution of nystatin incorporated into lipid-based vesicles of heterogeneous sizes. Submitted for publication.
- 31.Wasan K M, Conklin J S. Enhanced amphotericin B nephrotoxicity in intensive care patients with elevated levels of low-density lipoprotein cholesterol. Clin Infect Dis. 1997;24:78–80. doi: 10.1093/clinids/24.1.78. [DOI] [PubMed] [Google Scholar]
- 32.Wasan K M, Lopez-Berestein G. Diversity of lipid-based polyene formulations and their behavior in biological systems. Eur J Clin Microbiol Infect Dis. 1997;16:81–92. doi: 10.1007/BF01575125. [DOI] [PubMed] [Google Scholar]
- 33.Wasan K M, Morton R E. Differences in lipoprotein concentration and composition modify the plasma distribution of free and liposomal annamycin. Pharm Res. 1996;13:462–468. doi: 10.1023/a:1016065114515. [DOI] [PubMed] [Google Scholar]
- 34.Wasan K M. Modifications in plasma lipoprotein concentration and lipid composition regulate the biological activity of hydrophobic drugs. J Pharmacol Toxicol Methods. 1996;36:1–11. doi: 10.1016/1056-8719(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 35.Wasan K M, Perez-Soler R. Distribution of free and liposomal annamycin within human plasma is regulated by plasma triglyceride concentrations but not by lipid transfer protein. J Pharm Sci. 1995;84:1094–1100. doi: 10.1002/jps.2600840912. [DOI] [PubMed] [Google Scholar]
- 36.Wasan K M, Rosenblum M G, Cheung L, Lopez-Berestein G. Influence of lipoproteins on renal cytotoxicity and antifungal activity of amphotericin B. Antimicrob Agents Chemother. 1994;38:223–227. doi: 10.1128/aac.38.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wasan K M, Brazeau G A, Keyhani A, Hayman A C, Lopez-Berestein G. Roles of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993;37:246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wasan K M, Vadiei K, Lopez-Berestein G, Luke D R. Pharmacokinetics, tissue distribution, and toxicity of free and liposomal amphotericin B in diabetic rats. J Infect Dis. 1990;161:562–566. doi: 10.1093/infdis/161.3.562. [DOI] [PubMed] [Google Scholar]
- 39.Zini R, Riant P, Barre J, Tillement J P. Disease-induced variations in plasma protein levels. Implications for drug dosage regimens (parts 1 and 2) Clin Pharmacokinet. 1986;19:147–159. doi: 10.2165/00003088-199019020-00004. , 218–229. [DOI] [PubMed] [Google Scholar]