Abstract
Y-688 is a new fluoroquinolone with increased activity against ciprofloxacin-resistant staphylococci. The MICs of Y-688 and other quinolones were determined for 58 isolates of ciprofloxacin-resistant and methicillin-resistant Staphylococcus aureus (MRSA). The MICs at which 50% and 90% of bacteria were inhibited were ≥128 and ≥128 mg/liter, respectively, for ciprofloxacin, 16 and 32 mg/liter, respectively, for sparfloxacin, and 0.25 and 1 mg/liter, respectively, for Y-688. This new quinolone was further tested in rats with experimental endocarditis due to either of two isolates of ciprofloxacin-resistant MRSA (namely, P8/128 and CR1). Infected animals were treated for 3 days with ciprofloxacin, vancomycin, or Y-688. Antibiotics were administered through a computerized pump to simulate human-like pharmacokinetics in the serum of rats. The anticipated peak and trough levels of Y-688 were 4 and 1 mg/liter at 0.5 and 12 h, respectively. Treatment with ciprofloxacin was ineffective. Vancomycin significantly decreased vegetation bacterial counts for both organisms (P ≲ 0.05). In contrast, Y-688 only marginally decreased vegetation bacterial counts (P ≳ 0.05). Moreover, several vegetation that failed Y-688 treatment grew staphylococci for which the MICs of the test antibiotic were increased two to eight times. Y-688 also selected for resistance in vitro, and isolates for which the MICs were increased eight times emerged at a frequency of ca. 10−8. Thus, in spite of its low MIC for ciprofloxacin-resistant MRSA, Y-688 failed in vivo and its use carried the risk of resistance selection. The fact that ciprofloxacin-resistant staphylococci became rapidly resistant to this potent new drug suggests that the treatment of ciprofloxacin-resistant MRSA with new quinolones might be more problematic than expected.
Ever since their introduction into the armamentarium of antimicrobial agents, fluorinated quinolones have emerged as major antibacterial compounds against gram-negative microorganisms. In the late 1980s, relatively new drugs such as ciprofloxacin also emerged, and it was hoped that these drugs could solve the increasing problem posed by multidrug-resistant gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) in hospitals. However, extensive use of such quinolones very rapidly selected for quinolone-resistant MRSA (14, 21, 22). More than 90% of these organisms are now resistant to ciprofloxacin in certain places (21).
Quinolone-resistant staphylococci can readily be selected for in vitro by exposure to stepwise increasing concentrations of these agents (9, 21). In vivo, this phenomenon may have been accentuated by the relatively low therapeutic margin of the earlier quinolones against these bacteria. For example, the MIC of ciprofloxacin for susceptible S. aureus ranges between 0.25 and 1 mg/liter (3, 25). In comparison, therapeutic doses of this drug produce peak and trough concentrations in the serum of humans of 2.5 and 0.5 mg/liter, respectively (2, 18), i.e., not much greater than the ciprofloxacin MIC for the most susceptible staphylococci. This is an ideal situation for resistance selection in test tubes (9, 21) and thus may have provided the perfect conditions for the emergence of quinolone-resistant MRSA in the clinical environment.
The resistance of S. aureus to fluoroquinolones involves at least three different mechanisms, which are often combined in highly resistant organisms. One mechanism is the active efflux of the drugs by the NorA transporter (27). The two other mechanisms result from modifications of the quinolone molecular targets of the bacterium, i.e., the DNA gyrase (gyrA mutants) (13) and/or the topoisomerase IV (grlA mutants) (10). Newer quinolones, including sparfloxacin and others, have lower levels of susceptibility to NorA-mediated efflux and may have higher affinities for bacterial gyrases and topoisomerases (16, 20, 27). Therefore, they are more potent, in a weight-to-weight ratio, than older quinolones against staphylococci and other gram-positive pathogens. Some of these molecules are active even against MRSA strains showing low-level resistance to ciprofloxacin. However, these compounds may fail against high-level ciprofloxacin-resistant MRSA encountered in the clinical environment (5, 12, 23). Therefore, quinolones with increased activity against ciprofloxacin-resistant MRSA are still needed.
Y-688 (17) is a novel molecule of this family demonstrating in vitro activity against both ciprofloxacin-susceptible and ciprofloxacin-resistant S. aureus (26). If this activity is preserved in vivo, the drug could become extremely important owing to its effectiveness against multidrug-resistant MRSA. To investigate this question, the therapeutic efficacy of Y-688 was tested in rats with experimental aortic endocarditis due to ciprofloxacin-resistant MRSA. The new compound was administered to mimic the anticipated pharmacokinetics in the serum of humans. Control drugs included vancomycin, which is the sole drug uniformly proposed for the treatment of severe MRSA infections, and ciprofloxacin, which was used as a negative control.
MATERIALS AND METHODS
Microorganisms and growth conditions.
Two ciprofloxacin-resistant MRSA strains were used for the experiments with animals. The first, called MRSA P8/128, was generated in the laboratory by serial exposure of the ciprofloxacin-susceptible parent strain MRSA P8 to the drug (5). The second, called MRSA CR1, was recovered from a patient with an MRSA infection. Both isolates were class 2 to 3 heterogeneous MRSA according to Tomasz et al. (24). Parent strain MRSA P8 was also used as a control for ciprofloxacin susceptibility in in vitro tests. In addition, a panel of 58 ciprofloxacin-resistant MRSA isolates originating from various geographical areas (20 isolates from our own strain collection and 38 isolates kindly provided by P. Hohl, Roche, Basel, Switzerland) were tested for their in vitro susceptibility to Y-688, and some of them were also tested for their in vitro susceptibility to sparfloxacin. Unless otherwise stated, the bacteria were grown at 35°C either in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) with aeration in a shaking incubator at 120 rpm or on Columbia agar plates (Becton Dickinson Microbiology Systems, Cockeysville, Md.). The media were supplemented with 2% NaCl. Bacterial stocks were kept at −70°C in tryptic soy broth supplemented with 10% (vol/vol) glycerol.
Antibiotics.
Y-688 was provided by Yoshitomi Pharmaceutical Industries Ltd. (Fukuota, Japan); ciprofloxacin was purchased from Bayer AG (Wuppertal, Germany); sparfloxacin was provided by Rhône-Poulenc Rorer (Antony, France); and vancomycin was purchased from Eli Lilly (Geneva, Switzerland). Before being diluted to the desired concentrations, Y-688 was solubilized in 100% pure glacial acetic acid, sparfloxacin was solubilized in 0.1 N sodium hydroxide, and vancomycin was solubilized in sterile water.
Susceptibility testing and time-kill curve experiments.
The MICs were determined by a previously described broth macrodilution method (19), with a final inoculum of 105 to 106 CFU/ml. The MIC was defined as the lowest antibiotic concentrations which inhibited visible bacterial growth after 24 h of incubation at 35°C.
For time-kill curve experiments, series of flasks containing fresh prewarmed medium were inoculated with ca. 106 CFU/ml (final concentration) from an overnight culture of bacteria and the bacteria were further incubated at 35°C with aeration. Immediately after inoculation, the antibiotics were added to the flasks at final concentrations approximating the peak and trough levels of antibiotics produced in the serum of humans after the administration of therapeutic doses of the drugs (see Results section). These concentrations were (i) 4 and 1 mg/liter, respectively, for Y-688 and (ii) 40 and 5 mg/liter, respectively, vancomycin (1). Ciprofloxacin (4 mg/liter) was also tested in certain experiments. Viable counts were determined just before and at various times after the addition of the antibiotics by plating adequate dilutions of the cultures on agar plates. To avoid antibiotic carryover, 0.5-ml samples of the cultures were transferred from the flasks into microcentrifuge tubes, and the bacteria were spun and resuspended twice in antibiotic-free medium to remove residual drugs. Then the bacterial suspensions were serially diluted and plated on Columbia agar.
Production of endocarditis and infusion pump installation.
Sterile aortic valve vegetations were produced in rats as described previously (15). An intravenous (i.v.) line was inserted via the jugular vein into the superior vena cava and was connected to a programmable infusion pump (Pump 44; Harvard Apparatus, Inc., South Natick, Mass.) to deliver the antibiotics (11). The pump was set to deliver a volume of 0.2 ml of saline per h to keep the catheter open until the onset of therapy. No. i.v. lines were placed in the control animals.
Bacterial endocarditis was induced 24 h after catheterization by i.v. challenge of the animals with 0.5 ml of saline containing 105 CFU of either MRSA strain. This inoculum was 10 times larger than the minimum inoculum producing endocarditis in 90% of the untreated rats.
Antibiotic treatment of experimental endocarditis.
Treatment was started 12 h after bacterial challenge and lasted for 3 days. The antibiotics were delivered at changing flow rates via the infusion pump described above in order to stimulate the drug kinetics in the serum of humans produced by therapeutic doses of the test antibiotics. For Y-688, the anticipated kinetics in the serum of humans was 4 mg/liter at 0.5 h after administration and 1 mg/liter at 12 h, considering a terminal plasma elimination half-life in humans of 3 h. Vancomycin was given to simulate the administration of 1 g of the drug given i.v. every 12 h to humans (1), and ciprofloxacin was given to simulate the administration of 750 mg of the drug given orally every 12 h (2, 18). This required a total amount of antibiotic (in milligrams per kilogram of body weight per 12 h) of 24 mg of Y-688, 23.2 mg of vancomycin, and 37.4 mg of ciprofloxacin.
Antibiotic concentrations.
The concentrations of antibiotic in serum were determined on day 2 of therapy for groups of three to six uninfected or infected rats. For determination of the levels in the serum of infected animals we used the internal controls of therapeutic experiments, in which adequate drug delivery was tested routinely. Blood was drawn by puncturing the periorbital sinuses and the animals at several time points during and after antibiotic administration. Antibiotic concentrations were determined by an agar diffusion bioassay with antibiotic medium 1 (Difco Laboratories, Detroit, Mich.) and with Bacillus subtilis ATCC 6633 as the indicator organism. The diluent was pooled rat serum. The limits of detection of the assays were 0.06 mg/liter for Y-688, 0.6 mg/liter for vancomycin, and 0.12 mg/liter for ciprofloxacin. The linearity of the standard curves was assessed by a regression coefficient of ≥0.995, and intraplate and interplate variations were ≤10%.
Evaluation of infection.
The control rats were killed at the onset of treatment (i.e., 12 h after inoculation) in order to measure both the frequency and the severity of valvular infection at the start of therapy. Treated rats were killed 6 h after the trough level of the last antibiotic dose of either test drug was reached. At that time, no residual antibiotic could be detected in the blood. The valvular vegetations were dissected by using sterile precautions, weighed, homogenized in 1 ml of saline, and serially diluted before being plated for colony counts. Quantitative blood cultures and spleen cultures were performed in parallel. Some animals died before the end of treatment due to either complications of the operation itself (such as possible catheter-induced arrythmia) or the infection process, or both. Among these animals, data only for rats which had received at least two-thirds of the treatment were taken into account for vegetation bacterial counts. Blood and spleen cultures were not performed for these animals. The number of colonies growing on the plates were determined after 48 h of incubation at 35°C. The bacterial densities in the vegetations were expressed as log10 CFU per gram of tissue. The minimum detection level was ≥2 log10 CFU/g of vegetation. For statistical comparisons of differences between the vegetation bacterial densities for the various treatment groups, culture-negative vegetations were considered to contain 2 log10 CFU/g.
Selection for antibiotic resistance.
The propensity of Y-688 to select for drug-resistant derivatives was tested in vitro by spreading large (1010 CFU) as well as smaller (105 CFU) bacterial numbers onto agar plates containing increasing concentrations of the test antibiotic. The results were expressed as a population analysis profile by plotting the number of colonies growing on the plates against the concentrations of Y-688 present in the plates.
The emergence of resistant derivatives was also detected during therapy for endocarditis. In each case of Y-688 treatment failure, 1 of the approximately 100 colonies growing from the infected vegetations was picked at random from the plates, grown in an independent liquid culture, and retested for the MIC of the drug for the colony. Because the investigational compound Y-688 was available in limited quantities, this screening was not performed for bacteria recovered from the spleens. The screening was also not performed for vancomycin-treated animals.
Statistical analysis.
The median vegetation bacterial densities for the various treatment groups were compared by the nonparametric Mann-Whitney rank sum test. Bonferroni’s correction was used for multiple group comparisons. Differences between groups were considered significant when P was ≤0.05.
RESULTS
Antibiotic susceptibility and time-kill curves experiments.
Table 1 presents the MICs of the drugs studied in rats and of the recently introduced compound sparfloxacin for the ciprofloxacin-susceptible control strain MRSA P8 and the two ciprofloxacin-resistant MRSA strains used in the studies with animals (strain P8/128 and strain CR1). As a control, we also tested the drug susceptibilities of an additional panel of 58 unrelated clinical isolates of ciprofloxacin-resistant MRSA. The MIC at which 50% and 90% of this group of organisms were inhibited by the test quinolones were ≥128 and ≥128 mg/liter, respectively, for ciprofloxacin, 16 and 32 mg/liter, respectively, for sparfloxacin (only 20 strains were tested), and 0.25 and 1 mg/liter, respectively, for Y-688. Although sparfloxacin was more effective than ciprofloxacin, its MICs were beyond therapeutic levels since the peak concentrations of this drug in serum are below 4 mg/liter in humans (5, 6). In contrast, the new compound, Y-688, was uniformly active against all the ciprofloxacin-resistant isolates tested and was ≥32 times more effective than sparfloxacin and the other newer quinolones tested against these isolates (data not presented). Vancomycin was effective against both organisms tested in rats.
TABLE 1.
Comparative activities of the test antibiotics against the ciprofloxacin-susceptible control strain MRSA P8 and two ciprofloxacin-resistant MRSA strains used in experiments with animals
| Drug | MIC (mg/liter)
|
||
|---|---|---|---|
| MRSA P8 | MRSA P8/128a | MRSA CR1b | |
| Ciprofloxacin | 0.25 | >128 | 128 |
| Y-688 | 0.006 | 0.25 | 0.5 |
| Sparfloxacin | 0.06 | 2 | 16 |
| Vancomycin | 2 | 2 | 2 |
Strain P8/128 is a ciprofloxacin-resistant derivative generated by serial exposures of the ciprofloxacin-susceptible parent MRSA P8 to the drug.
Strain CR1 is a ciprofloxacin-resistant clinical isolate of MRSA.
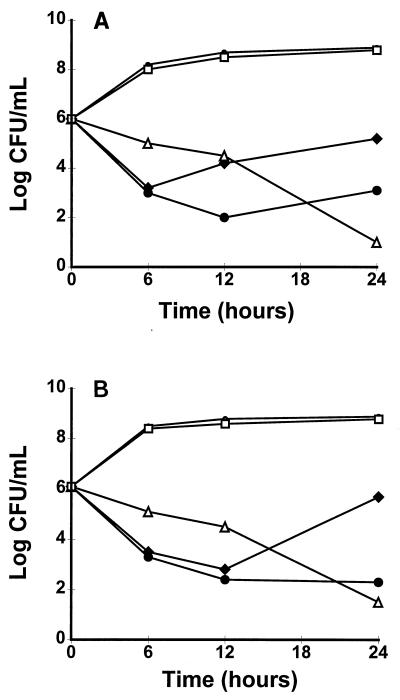
Figure 1 indicates that Y-688 was bacterial against the bacteria that were used to infect the animals. It can be seen that Y-688 induced a rapid decrease in viable counts (≥3 log10 CFU) during the 6 h of in vitro exposure to the drug. This decline was observed whether the drug concentrations were adjusted to mimic the peak (4 mg/liter) or the trough (1 mg/liter) levels of antibiotic achieved in the serum of rats during therapy. At the lower concentration, however, the initial decrease in viable counts was followed by a progressive regrowth of the bacteria after 12 to 24 h of incubation. This phenomenon was not due to antibiotic degradation but was due to the emergence of Y-688-resistant derivatives for which the MICs of the drug were increased 8 to 16 times (MIC, 4 to 8 mg/liter); these derivatives had permeated the culture by 24 h and were stable upon regrowth for up to 15 generations on antibiotic-free agar plates. As expected, vancomycin used at the peak level achievable in human serum (40 mg/liter) was slowly bactericidal, whereas ciprofloxacin (4 mg/liter) was ineffective (Fig. 1).
FIG. 1.
Results of in vitro time-kill experiments performed with test compounds against the ciprofloxacin-resistant strains MRSA P8/128 (laboratory mutant) (A) and MRSA CR1 (clinical isolate) (B). Y-668 was added to the cultures at final concentrations which approximated the peak (4 mg/liter) and trough (1 mg/liter) antibiotic concentrations in the serum of rats. Vancomycin and ciprofloxacin concentrations (40 and 4 mg/liter, respectively) simulated the peak serum levels obtained in both the serum of humans and rats during antibiotic therapy (see Materials and Methods). •, control; •, Y-688 (4.0 mg/liter); ⧫, Y-688 (1.0 mg/liter); ▵, vancomycin (40 mg/liter); □, ciprofloxacin (4 mg/liter).
Antibiotic concentration in the serum of rats.
The levels of Y-688 in the serum of rats were measured at 0.5 h (peak concentration) and 12 h (trough concentration) after the start of drug administration. The antibiotic levels at these respective time points were (mean ± standard deviation of 6 to 12 determinations for individual animals pooled from more than one experiment) 3.98 ± 0.63 mg/liter for the time of the peak concentration and 0.61 ± 0.27 mg/liter for the time of trough concentration. These values were adjusted to approximate in serum of the animals the pharmacokinetics of the compound anticipated in the serum of humans. Ciprofloxacin was administered to mimic the kinetics produced by 750 mg of the drug given orally every 12 h in the serum of humans (2, 18), and vancomycin was administered to stimulate the kinetics of 1 g of the drug given intravenously every 12 h in the serum of humans (1), as recently described (9).
Therapy for experimental endocarditis.
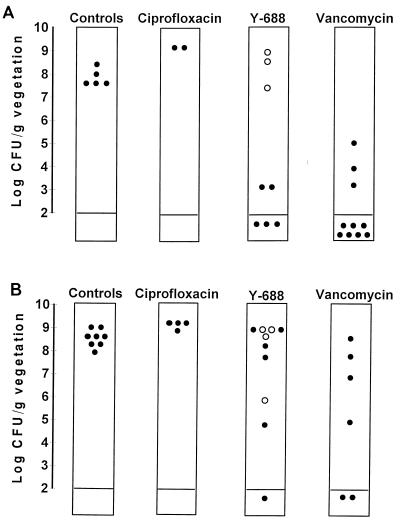
Figure 2 depicts the therapeutic results. A few animals were treated with ciprofloxacin to establish the inefficacy of this compound against the specific bacteria used in these experiments. All these rats were heavily infected after 3 days of therapy, and the bacteria continued to grow in the vegetations, despite drug treatment. Vancomycin treatment, used as a positive control, decreased significantly the vegetation bacterial densities of both test MRSA strains compared to the densities in animals killed at the start of antibiotic administration (P < 0.05). In comparison, Y-688 failed to decrease significantly the vegetation infections caused by either of the two organisms tested (P > 0.05 compared to the controls). Moreover, in three of five animals with treatment failures due to the ciprofloxacin-resistant strain P18/128, the MIC of the test drug for 1 colony picked randomly, from among 100 colonies growing on the plates was increased twofold. This suggested that Y-688 might have selected for variants with decreased drug susceptibility during in vivo therapy. This finding was confirmed in subsequent experiments performed with clinical isolate MRSA CR1. For four of nine animals that were treatment failures in this group, the MIC of the test quinolone for 1 colony picked randomly from among 100 colonies had increased four- to eightfold.
FIG. 2.
Results of Y-688 therapy for experimental endocarditis due to ciprofloxacin-resistant isolates MRSA P8/128 (laboratory mutant) (A) and MRSA CR1 (clinical isolate) (B). Each dot in the columns represents the vegetation bacterial density (log10 CFU per gram of vegetation) in a single rat. Open dots indicate the vegetations which grew staphylococcal derivatives for which the Y-688 MICs had increased two- to eightfold after 3 days of therapy. Vancomycin treatment significantly (P < 0.05) decreased the vegetation bacterial titers of both test organisms compared to those for untreated controls. In contrast, Y-688 did not significantly affect the vegetation infection caused by either of these bacteria (P > 0.05). Ciprofloxacin-treated animals, which were used as negative controls, were not considered in the statistical evaluation.
A more precise assessment of the frequency of resistant colonies in vegetation cultures was not performed. However, the fact that at least 1% of the colonies from about one-half of the animals that were treatment failures were less susceptible to the test drug indicated that resistance selection in vivo was a genuine problem. Of note, for these Y-688-resistant derivatives selected in vivo, the MICS of sparfloxacin were also increased, increasing from 2 to 16 mg/liter for the parent bacteria (Table 1) to 8 to 64 mg/liter for the resistant derivatives. This indicated that the newly acquired mechanism of resistance was not specific for the selecting drug but also extended to other quinolones.
Thus, although Y-688 showed promising activity in standard in vitro MIC tests, it clearly engendered the risk of resistance selection both in vivo and in vitro, as indicated by the time-kill curve experiments.
Frequency of resistance selection in vitro.
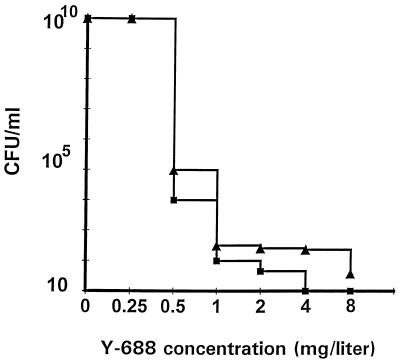
To assess more accurately the frequency of selection of resistance to Y-688, large and medium-sized inocula (1010 and 105 CFU, respectively) were spread onto agar plates containing increasing concentrations of the drug. Figure 3 presents the population analysis profile of both MRSA P8/128 and MRSA CR1 in such an experiment. It can be seen that subpopulations with various degrees of resistance preexisted in the original inoculum. Both test bacteria harbored subpopulations with low levels of resistance (two times the MIC) at a high frequency (ca. 10−5). The laboratory derivative P8/128 did not grow colonies on Y-688 at concentrations of greater than 2 mg/liter (four to eight times the MIC). In contrast, clinical isolate CR1 grew colonies resistant to at least 8 mg/liter (i.e., 16 times the MIC) at a frequency of ca. 10−8. Thus, since the rats were challenged with precisely 10−5 CFU, it is likely that both organisms already contained subpopulations with some degree of resistance when they were injected into the animals.
FIG. 3.
Population analysis profile of the ciprofloxacin-resistant laboratory mutant P8/128 (closed squares) and the ciprofloxacin-resistant clinical isolate CR1 (closed triangles) grown on agar plates containing increasing concentrations of Y-688. Large bacteria numbers (up to 1010 CFU) were spread on plates containing twofold increasing concentrations of the drug. Resistant variants able to grow in the presence of increased concentrations of Y-688 were counted after 48 h of incubation at 35°C.
Spleen and blood cultures.
Whenever assessable, the spleen and blood cultures were also examined. However, due to the scarcity of the investigational compound, Y-688-resistant derivatives were not searched for in these organs.
Spleen cultures were positive for all (13 of 13) of the control rats at the start of therapy, with a mean ± standard deviation of 4.12 ± 0.77 CFU/g of tissue. Blood cultures were also positive for most (10 of 13) of the control animals and ranged between 2 and >1,000 CFU/ml. For treated animals, in contrast, the general rule was that only rats harboring ≥6 log10 CFU/g of vegetation had positive spleen and/or blood cultures.
In the case of Y-688 treatment, for 1 of four assessable rats infected with MRSA P8/128 bacteria could be grown from both the spleen (4.5 CFU/g) and the blood (>1,000 CFU/ml). The vegetation bacterial titer in this rat was 9.15 CFU/g. In contrast, the vegetation bacterial titers in the three rats with sterile organs were ≤2.0 CFU/g. In rats infected with clinical isolate CR1, for six of seven animals spleen cultures were positive (4.9 ± 0.37 CFU/g) and for four of them blood cultures were also positive (>1,000 CFU/ml). The vegetation bacterial titers in these animals ranged between 6.28 and 9.36 CFU/g. Finally, the majority (13 of 16) of vancomycin-treated rats had vegetation bacterial densities below the critical limit of 6 log10 CFU/g at the time of autopsy. The three rats with higher vegetation bacterial densities were not assessable for spleen and blood cultures, because they died before the time of killing. Note that the dissociation between positive vegetation cultures and negative spleen and blood cultures in treated animals is a common phenomenon. It is most likely due to the “helper” effect of professional macrophages present in the spleen.
DISCUSSION
The present experiments show that the new quinolone Y-688 is effective in vitro in standard MIC tests against highly ciprofloxacin-resistant isolates of MRSA, irrespective of whether the organisms had acquired the quinolone resistance in vitro or from the clinical environment.
When tested in vivo, on the other hand, use of the new quinolone against experimental endocarditis due to the ciprofloxacin-resistant laboratory mutant MRSA P8/128 appeared to be only partially successful. Although it decreased the bacterial density in the vegetations of treated rats compared to the density in the vegetations of untreated control rats, this decrease was not statistically significant (P = 0.12). Moreover, for three of the treatment failures the MIC of the test drug for the bacteria grown from infected vegetations had increased twofold. This observation suggested that Y-688 might have selected for staphylococcal variants with altered drug susceptibility during in vivo therapy. This hypothesis was further confirmed when Y-688 therapy was administered to rats infected with clinical isolate MRSA CR1. Indeed, four of the treatment failures due to this bacterium harbored in their vegetations organisms for which the MICs of Y-688 had increased four- to eightfold. Therefore, even though Y-688 had good in vitro activity against the two test isolates, the experiments with the rats showed that the novel compound was prone to select for fluoroquinolone resistance in S. aureus under the in vivo conditions used in this trial.
The reasons for resistance selection in strains MRSA P8/128 and MRSA CR1 may be multiple. One potentially important factor might be related to the pharmacodynamics of the drug. Some compounds diffuse poorly into cardiac vegetations and thus provide suboptimal antibiotic levels at the infection foci (4). Quinolones such as perfloxacin, temafloxacin, and sparfloxacin were shown to diffuse homogeneously inside rabbit vegetations (4a). On the other hand, we recently observed that ciprofloxacin diffused into rat vegetations at a level about two times less than that for sparfloxacin (unpublished observation), thus indicating that there might be differences in the diffusion capabilities between certain quinolones. Poor intravegetation drug levels could provide ideal conditions for the selection of resistance in vivo and would be compatible with the fact that at low concentrations of Y-688, the drug selected for Y-688 resistance in in vitro time-kill curve experiments. It is also noteworthy that the new drug was less effective against clinical isolate CR1 than against laboratory strain P8/128. Strain CR1 was somewhat less susceptible than P8/128 in vitro and more easily yielded resistant derivatives (see below). Therefore, the therapeutic margin of the new compound was less optimal against the clinical isolate than against the laboratory strain.
Alternatively, the rapid emergence of resistance could be due to intrinsic properties of the bacteria. In the present experiments, in vitro tests indicated that in the original inocula of both MRSA P8/128 and MRSA CR1 subpopulations for which MICs were increased were present at a relatively high frequency (ca. 10−8). For MRSA CR1, these subpopulations were resistant to at least 8 mg/liter (i.e., 16 times the MIC), indicating that the drug concentrations in the serum (peak concentrations, 4 mg/liter) were already insufficient to prevent the emergence of resistance.
The resistance phenotype selected by Y-688 conferred cross-resistance to other quinolones, such as sparfloxacin, which was tested in the present study. Therefore, this increased level of resistance appeared to come on top of other quinolone resistance mechanisms already present in the test staphylococci. The nature of the additional change(s) was not determined in the present experiments. However, the observation is not trivial. It indicates that staphylococci have not attained their acme in terms of quinolone resistance. It seems that once resistance to ciprofloxacin is acquired, staphylococci can further increase their level of resistance to more potent quinolones quite easily. It is possible that MRSA P8/128 and CR1, which were already resistant to ciprofloxacin, had some advantage for the development of further resistance to the newer quinolone Y-688. If true, this might challenge the whole concept of developing newer quinolones active against ciprofloxacin-resistant staphylococci. The answer to this question needs further investigation.
Regarding vancomycin, although antibiotic therapy was considered effective by statistical analysis, a few rats in the treated groups remained heavily infected. This is a common observation in the animal model of endocarditis, and a definitive explanation for this phenomenon has yet to be found (8). In the present experiments, these failures were not due to inadequate drug administration, because we routinely tested for this possibility in each experiment by the use of internal controls. It is possible that the nonresponding rats had greater vegetation bacterial titers than the responding animals at the start of therapy. Indeed, we recently showed that under such conditions, cell wall-active drugs may fail to sterilize the vegetations due to the effect of the so-called phenotypic tolerance (7).
In conclusion, the present experiments highlight the dichotomy between the good efficacy of Y-688 against ciprofloxacin-resistant MRSA in vitro and its tendency to fail as a treatment and to select for drug-resistant derivatives in vivo. Regarding the experiments with animals, it is possible that the administration of higher doses of the drug might restore the therapeutic efficacy. Indeed, the therapeutic margin of Y-688 might have been too low to afford effective therapy. More worrisome, however, is the fact that ciprofloxacin-resistant staphylococci very rapidly became resistant to the new test quinolone. While the mechanism of this resistance has yet to be elucidated, it might be a primary indication that the treatment of infections caused by ciprofloxacin-resistant MRSA with new quinolones will be more problematic than expected.
ACKNOWLEDGMENTS
We thank Yoshitomi Pharmaceutical Industries Ltd., Fukuota, Japan, for compound Y-668.
We also thank F. Hoffmann-La Roche AG, Basel, Switzerland, for supporting the study.
REFERENCES
- 1.Blouin R A, Bauer L A, Miller D D, Record K E, Griffen W O. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21:575–580. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borner K, Höffken G, Lode H, Koeppe P, Prinzing C, Glatzel P, Wiley R, Olschewski P, Sievers B, Reinitz D. Pharmacokinetics of ciprofloxacin in healthy volunteers after oral and intravenous administration. Eur J Clin Microbiol. 1986;5:179–186. doi: 10.1007/BF02013983. [DOI] [PubMed] [Google Scholar]
- 3.Chin N X, Neu H C. Ciprofloxacin, a quinolone carboxylic acid compound active against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984;25:319–326. doi: 10.1128/aac.25.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cremieux A C, Maziere B, Vallois J M, Ottaviani M, Azancot A, Raffoul H, Bouvet A, Pocidalo J J, Carbon C. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J Infect Dis. 1989;159:938–944. doi: 10.1093/infdis/159.5.938. [DOI] [PubMed] [Google Scholar]
- 4a.Crémieux A C, Saleh-Mghir A, Vallois J M, Ottaviani M, Pocidalo J J, Carbon C. Program and abstracts of the 31st Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1991. Pattern and diffusion of three quinolones throughout infected cardiac vegetations studied by autoradiography, abstr. 357; p. 158. [Google Scholar]
- 5.Entenza J M, Blatter M, Francioli P, Glauser M P, Moreillon P. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Sparfloxacin treatment of experimental endocarditis due to ciprofloxacin-susceptible or laboratory-selected resistant methicillin-resistant Staphylococcus aureus, abstr. B-9; p. 23. [Google Scholar]
- 6.Entenza J M, Blatter M, Francioli P, Glauser M P, Moreillon P. Parenteral sparfloxacin compare with ceftriaxone in treatment of experimental endocarditis due to penicillin-susceptible and -resistant streptococci. Antimicrob Agents Chemother. 1994;38:2683–2688. doi: 10.1128/aac.38.12.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Entenza J M, Caldelari I, Glauser M P, Francioli P, Moreillon P. Importance of genotypic and phenotypic tolerance in the treatment of experimental endocarditis due to Streptococcus gordonii. J Infect Dis. 1997;175:70–76. doi: 10.1093/infdis/175.1.70. [DOI] [PubMed] [Google Scholar]
- 8.Entenza J M, Fluckiger U, Glauser M P, Moreillon P. Antibiotic treatment of experimental endocarditis due to methicillin-resistant Staphylococcus epidermidis. J Infect Dis. 1994;170:100–109. doi: 10.1093/infdis/170.1.100. [DOI] [PubMed] [Google Scholar]
- 9.Entenza J M, Vouillamoz J, Giddey M, Glauser M P, Moreillon P. Levofloxacin versus ciprofloxacin, flucloxacillin, or vancomycin for treatment of experimental endocarditis due to methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1662–1667. doi: 10.1128/aac.41.8.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 11.Flückiger U, Francioli P, Blaser J, Glauser M P, Moreillon P. Role of amoxicillin serum level for successful prophylaxis of experimental endocarditis due to tolerant streptococci. J Infect Dis. 1994;169:1397–1400. doi: 10.1093/infdis/169.6.1397. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rodriguez J A, Garcia J E, Garcia Garcia M I, Fresnadillo M J, Trujillano I, Garcia Sanchez E. In vitro activity of four new fluoroquinolones. J Antimicrob Chemother. 1994;34:53–64. doi: 10.1093/jac/34.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Goswitz J J, Willard K E, Fasching C E, Peterson L R. Detection of gyrA mutations associated with ciprofloxacin resistance in methicillin-resistant Staphylococcus aureus: analysis by polymerase chain reaction and automated direct DNA sequencing. Antimicrob Agents Chemother. 1992;36:1166–1169. doi: 10.1128/aac.36.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harnett N, Brown S, Krishnan C. Emergence of quinolone resistance among clinical isolates of methicillin-resistant Staphylococcus aureus in Ontario. Antimicrob Agents Chemother. 1991;3:1911–1913. doi: 10.1128/aac.35.9.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heraief E, Glauser M P, Freedmann L. Natural history of aortic valve endocarditis in rats. Infect Immun. 1982;37:127–131. doi: 10.1128/iai.37.1.127-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitani H, Kuroda T, Moriguchi A, Hikida K, Ao H, Yokoyama Y, Hirayama F, Ikeda Y. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Novel 7-substituted-fluoroquinolones as potent antibacterial agents: synthesis and structure-activity relationships, abstr. F-190; p. 146. [Google Scholar]
- 18.Lettieri J T, Rogge M C, Kaiser L, Echols R M, Heller A H. Pharmacokinetic profiles of ciprofloxacin after single intravenous and oral doses. Antimicrob Agents Chemother. 1992;36:993–996. doi: 10.1128/aac.36.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 20.Piddock L J. New quinolones and gram-positive bacteria. Antimicrob Agents Chemother. 1994;38:163–169. doi: 10.1128/aac.38.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders, C. C., W. E. Sanders, Jr., and K. S. Thomson. 1995. Fluoroquinolone resistance in staphylococci: new challenges. Eur. J. Clin. Microbiol. Infect. Dis. 14(Suppl. 1):6–11. [PubMed]
- 22.Shalit L, Beerger S A, Gorea A, Frierman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates on a general hospital. Antimicrob Agents Chemother. 1989;33:593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Zhang Y X, Ishida H, Sato K, Hayakawa I. Mechanisms of 4-quinolone resistance in quinolone-resistant and methicillin-resistant Staphylococcus aureus isolates from Japan and China. J Med Microbiol. 1995;42:214–219. doi: 10.1099/00222615-42-3-214. [DOI] [PubMed] [Google Scholar]
- 24.Tomasz A, Nachman S, Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991;35:124–129. doi: 10.1128/aac.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfson J S, Hooper D C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989;2:378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama Y, Morimoto M, Iwao E, Yamamoto K, Honjo K, Hyrayama F, Ikeda Y. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC: American Society for Microbiology; 1995. Y-688, a new fluoroquinolone with high activity against quinolone-resistant gram-positive bacteria, abstr. F-191; p. 146. [Google Scholar]
- 27.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]