Abstract
Soybean is a leguminous crop known for its efficient nitrogen utilization and ease of cultivation. However, its intercropping with maize may lead to severe reduction in its growth and yield due to shading effect of maize. This issue can be resolved by the appropriate application of essential plant nutrient such as molybdenum (Mo). Aim of this study was to assess the effect of Mo application on the morphological and physiological characteristics of soybean intercropped with maize. A two-year field experiment was conducted for this purpose, and Mo was applied in the form of sodium molybdate (Na2MoO4), and four different levels were maintained i.e., 0, 60, 120 and 180 g ha-1. Soybean exhibited varying responses to different levels of molybdenum (Mo) application. Notably, in both sole and intercropped cropping systems, the application of Mo at a rate of 120 g ha-1 demonstrated the highest level of promise compared to other application levels. However, most significant outcomes were pragmatic in soybean-maize intercropping, as application of Mo @ 120 g ha-1 significantly improved soybean growth and yield attributes, including leaf area index (LAI; 434 and 441%), total plant biomass (430 and 461%), transpiration rate (15 and 18%), stomatal conductance (9 and 11%), and yield (15 and 20%) during year 2020 and 2021 respectively, as compared to control treatment. Similarly, Mo @ 120 g ha-1 application resulted in highest total grain yield (626.0 and 725.3 kg ha-1) during 2020 and 2021 respectively, which exceeded the grain yields of other Mo levels under intercropping. Moreover, under Mo application level (120 g ha-1), grain NPK and Mo contents during years 2020 and 2021 were found to be 1.15, 0.22, 0.83 and 68.94 mg kg-1, and 1.27, 0.25, 0.90 and 72.18 mg kg−1 under intercropping system increased the value as compared to control treatment. Findings of current study highlighted the significance of Mo in enhancing soybean growth, yield, and nutrient uptake efficiency in maize-soybean intercropping systems.
Keywords: molybdenum, soybean, intercropping, physiological, nutrient-use-efficiency
1. Introduction
Worldwide, agriculture is currently facing numerous challenges, including growing populations, land use change, urban sprawl, industrial development and climate change, all of which contribute to food security issues (Kalugina, 2014; Hu et al., 2016). One of the leading global challenges is to conserve natural resources, agricultural productivity, and biodiversity while addressing food security (Brown, 2002; Jiren et al., 2021). Soybean (Glycine max L.), a vital leguminous crop, is valued for its high-quality oil and protein, encompassing essential amino acids crucial for human health (Hou et al., 2015; Zeeshan et al., 2023). In 2019, global soybean production exceeded 333 million tons (Faostat, 2019; Jahan et al., 2023). Intercropping has been identified as a potential solution to promote sustainable agricultural development by improving land utilization rates and ensuring higher and sustainable crop yields (Bedoussac et al., 2015; Iqbal et al., 2019). Intercropping involves growing two or more crop species together for their specific cropping periods. Recent studies have shown that cassava intercropping is prevalent in America and Africa (Zhang et al., 2015). Legume-cereal intercropping, particularly maize-bean intercropping, is a common practice in East and Southern Africa (Mucheru-Muna et al., 2010; Fischer et al., 2020). Intercropping is also widely practice in Asian countries like China, such as wheat-maize (Gao et al., 2014), maize-soybean (Liu et al., 2018), sunflower-soybean (de la Fuente et al., 2014), and maize-pea (Teng et al., 2016). Despite vast potential and applicability of intercropping, certain challenges may arise, including nutrient competition, imbalances, and resource acquisition between co-existing crops (Huss et al., 2022). Intercropping also enables the adjacent crops to ensure greater yields owing to complimentary utilization of limited resources (Wang et al., 2023).
One of the leading concern in maize-soybean intercropping is the shading effect of maize, and soybean plants are highly responsive to these shading conditions caused by maize plants (Raza et al., 2020a). Reduced light exposure results in morphological and physiological changes in soybean, such as increased plant height and higher susceptibility to lodging (Li et al., 2014). Lodging of soybean is a major issue in maize-soybean intercropping systems, which inhibits the transportation of photo-assimilates, nutrients and water ultimately, leading to decreased crop yield (Liu et al., 2015; Wu et al., 2017). However, appropriate supply of essential plant nutrients such as iron (Fe), molybdenum (Mo) etc. can alleviate the shading induced adverse impacts on soybean growth, yield and physiology under maize-soybean intercropping. Despite the ongoing researches in improving the shade tolerance, and associated lodging losses in soybean, limited data is available in literature regarding the beneficial impacts of essential plant nutrients such as Mo in improving the shade tolerance in soybean under maize-soybean intercropping system (Oliveira et al., 2022).
Molybdenum (Mo) is an essential micronutrient that serves a crucial role in nitrogen (N) metabolism as a cofactor for nitrate reductase and nitrogenase. (Mendel, 2013; Cakmak et al., 2023). In legumes, Mo is particularly important for N-fixation, as it directly affects nitrogenase functioning in root nodules (Yang et al., 2020). Numerous studies have highlighted the significance of Mo in soybean production (Laltlanmawia et al., 2004; Raut et al., 2004; Kadlag et al., 2006) as well as effects of its combined application with Rhizobium (Sunita et al., 2000; Adkine et al., 2011). Improved crop growth and development in terms of branch number, root nodules, leaf chlorophyll content, grain and straw yield, seed protein, and oil contents have been observed through the use of zinc, iron, and seed priming with Mo (Tiwari et al., 2018). High soybean yields necessitate substantial nitrogen (N) input, with the most cost-effective source of N for soybean being biological nitrogen fixation (BNF) by the symbiotic association between plant and bacteria, mainly belonging to genus Bradyrhizobium (Htwe et al., 2019). Interspecific competition between maize-soybean intercropping leads to more efficient utilization of applied N (Zhang et al., 2023). Adding Bradyrhizobium with these three micronutrients zinc, iron, and molybdenum have resulted in a remarkable yield response in soybean as reported earlier by Tiwari et al. (2018) and Joglekar et al. (2023). Furthermore, Mo can influence the interaction between maize and soybean in intercropping systems, potentially impacting overall crop productivity and quality (Abendroth et al., 2017; Oliveira et al., 2022). Thus, understanding the role of Mo in maize-soybean intercropping systems is crucial for optimizing crop management practices and maximizing productivity in order to address the existing knowledge gap. Present study aimed at to assess the impact of Mo application on morphological and physiological attributes of soybean intercropped with maize. Hypothesized adequate Mo application and subsequent availability to soybean promotes plant growth, N-fixation, and biomass accumulation, enhancing overall productivity and resource utilization in such systems. These findings are expected to be of practical importance for farmers, agronomists, and researchers in the fields of plant nutrition, crop physiology, and agroecosystems, providing valuable insights to optimize crop management strategies.
2. Materials and methods
Current field study was carried out at experimental area of Sindh Agriculture University Tandojam, Hyderabad, Pakistan located at (26.1° N, 68.5° E). Meteorological data of experimental site are presented in Table 1 . Experimental units were arranged by following randomized complete block design (RCBD) with three replications. Foliar applications of Mo in the form of sodium molybdate (Na2MoO4) was done at various rates 0, 60, 120 and 180 g ha-1), and it was applied during the R1 growth stage of soybean crop by dissolving in 200 L of water.
Table 1.
Two years month-wise average meteorological (2020–2021) in Tandojam.
| Month | Temperature (°C) | Relative Humidity (%) |
Sunshine (hour) |
Wind Speed (km day−1) |
||
|---|---|---|---|---|---|---|
| Maximum | Minimum | Mean | ||||
| January | 24.0 | 9.10 | 16.55 | 68 | 8.40 | 21.82 |
| February | 27.4 | 11.4 | 19.40 | 64 | 8.80 | 18.71 |
| March | 33.2 | 16.0 | 24.60 | 58 | 9.20 | 21.38 |
| April | 38.3 | 21.2 | 29.75 | 54 | 9.70 | 35.18 |
| May | 40.4 | 25.4 | 32.90 | 61 | 10.2 | 50.77 |
| June | 44.9 | 27.2 | 33.05 | 67 | 8.60 | 64.13 |
| July | 46.3 | 26.9 | 31.60 | 74 | 7.20 | 52.55 |
| August | 38.1 | 26.2 | 30.65 | 77 | 8.10 | 32.96 |
| September | 35.2 | 24.4 | 29.80 | 75 | 9.30 | 48.54 |
| October | 35.5 | 19.8 | 27.65 | 69 | 9.50 | 13.36 |
| November | 31.1 | 14.9 | 23.00 | 64 | 9.00 | 16.03 |
| December | 25.5 | 10.7 | 18.10 | 67 | 8.20 | 18.71 |
Source: Meteorological station from Agriculture Research Institute Tandojam, Sindh Pakistan.
Physicochemical properties of experimental soils before planting are presented in Table 2 . Soil texture was determined using Hydrometer method (Bouyoucos, 1962; Qasim et al., 2023). Five grams of soil were mixed with 10 ml 1 N HCl and 50 ml distilled water. Boiled 2 mins, cooled, and 3 drops phenolphthalein added. Titration against 1 N NaOH followed. Calcium carbonate calculated using (Horváth et al., 2005) respectively. Soil EC was determined by soil water extraction (1:2) method by using conductivity meter. Soil pH was measured by soil-water suspension method (U.S. Salinity Laboratory Lab Staff, 1954). Organic carbon was determined by modified Walkley-Black titration and ammonium saturation methods, respectively (Walkley and Black, 1934). Extractable phosphorus was determined using the Olsen method (Olsen and Sommers, 1982). Extractable potassium was determined by mixing 5 g of soil with 1 N ammonium acetate solution, followed by flame photometry analysis (Rhoades, 1983), and available nitrogen was determined by alkaline potassium permanganate method (Wang et al., 2013).
Table 2.
Physico-chemical properties of experimental soils.
| Property | Soil |
|---|---|
| Texture | Silt clay loam |
| pH (1:5 soil water extract) | 7.42 |
| EC | 0.93 dS m−1 |
| Calcium carbonate | 13.37% |
| Organic matter | 0.85% |
| Total N | 0.07% |
| Extractable phosphorus | 3.62 mg kg−1 |
| Extractable Potassium | 146.0 mg kg−1 |
2.1. Seed procurement and agronomic practices
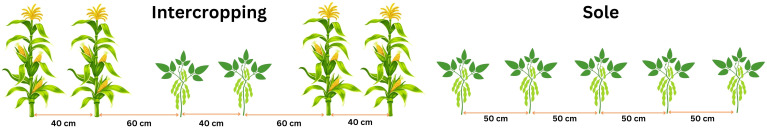
Present study utilized two crop varieties, determinate soybean ‘NARC−16’ and semi-compact maize ‘DK-6317’. Seeds were procured from National Agricultural Research Centre (NARC), Pakistan. Two rows of soybean per strip were planted at row-to-row spacing of 40 × 60 cm. Sole soybean plots had row spacing of 50 cm ( Figure 1 ). All the agronomic practices were performed manually. Basal fertilization was applied before sowing, with a dose of N (120 kg ha-1 urea), P (180 kg ha-1 diammonium phosphate), and K (150 kg ha-1 sulfate of potash) for maize and N (75 kg ha-1 urea), P (150 kg ha-1 diammonium phosphate), and K (100 kg ha-1 sulfate of potash) for soybean. Two additional N doses were applied at 60 and 100 kg ha-1 for maize at the V6 and tasseling stages (Abendroth et al., 2017). Experimental plots were irrigated with 550 ± 100 mm water throughout the experiment using the furrow irrigation method.
Figure 1.
The arrangement of Maize and Soybean crops in strip intercropping and sole cropping systems was varied, with different planting patterns used in each.
2.2. Sampling and measurements
2.2.1. Observation of plant morphological characteristics
Root fresh and dry weights were recorded using an analytical balance and were expressed in g plant−1. Whereas, stem internodal length, shoot height and root length were measured using a tape. Stem and internode diameter and lodging were also measured using the vernier caliper. Leaf area and number of plants were assessed five times (at 45, 65, 85, 105, and 125 days) after sowing (DAS) in two consecutive years. Five soybean plants were destructively sampled from each plot at each time point. The leaf area was determined by multiplying the maximum width and length of the leaves by a crop-specific coefficient factor of 0.75 for soybean (Gao et al., 2010). Leaf area index (LAI) was then calculated as followed (Raza et al., 2019).
| (1) |
2.2.2. Observation of yield and yield components
Phenological plant stages were observed by collecting five soybean plants from each plot during the study ( Table 3 ) that indicated the growth stages of the plants at specific DAS (45, 65, 85, 105, and 125 DAS). Ten soybean plants from each planting pattern were collected for total dry matter accumulation analysis (Raza et al., 2019). Plant parts were separated into root, straw, and grain, and sun-dried for 7-10 days to reach a constant weight. Total dry matter accumulation was determined by summing the dry matter of each part. To measure seed yield, 40 soybean plants were collected and sun-dried, then threshed and weighed to determine yield (kg ha-1) (Raven, 1988; Mao et al., 2012).
Table 3.
Phenological stages observed for soybean in the intercropping system at specific DAS.
| DAS | Soybean Growth Stages |
|---|---|
| 45 | V2-V3 |
| 65 | V5 |
| 85 | R2-R3 |
| 105 | R5-R6 |
| 125 | R7 |
2.3. Physiological and biochemical indexes
2.3.1. Photosynthetic indices
Leaf photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr) were measured on sunny days between 09:00-12:00 (local time) from fully expanded top leaves (3 leaves per experimental units from 3 different plants) with a Li-Cor 6400 photosynthesis measuring system. Measurements were performed on the 4th and 5th fully expanded leaves at 100 DAS (R6).
2.3.2. Chlorophyll content analysis
Chlorophyll content was determined during reproductive stages by taking three replication from each leaf of a plant using a portable chlorophyll meter SPAD-502 (Minolta, Japan) (Hong et al., 2005). The value of chlorophyll (Chl) content (mg m−2) was estimated from corresponding SPAD values by using the following equation:
| (2) |
2.3.3. Antioxidant enzymes
Analyses involving antioxidant enzyme activities were performed in triplicates. Activities of superoxide dismutase (SOD) was measured by using a photochemical reduction of nitro blue tetrazolium (NBT) inhibition method (Kalimuthu et al., 2022), where reaction mixture consisted of a potassium-phosphate buffer (125 mm, pH 7.8), 3 mM MgSO4, 3.1 mM EDTA, 2% polyvinyl polypyrrolidone (PVPP), methionine (130 mM), riboflavin (600 µM), NBT (22.5 mM), and plant extract. Reaction was illuminated for 15 min and absorbance was measured at 560 nm. One unit of SOD activity was defined as 50% reduction of A560 control. Catalase (CAT) activity was determined by measuring the decrease in absorbance of reaction mixture containing H2O2 (100 mM), plant extract, and a potassium-phosphate buffer (125 mm, pH 7.0) at 240 nm (Kalimuthu et al., 2022). Peroxidase (POD) activity was determined by measuring the increase in absorbance of reaction mixture consisting of ascorbate solution (5 mM), H2O2 (100 mM), EDTA solution (1 mM), plant extract, and a potassium-phosphate buffer (125 mm, pH 7.0) at 290 nm (Probst et al., 2021). In addition, malondialdehyde contents (MDA) in soybean leaves were determined by following the method of Sheng and Zhu (2019), which involved the homogenization of soybean leaves in the presence of trichloroacetic acid (TCA; 10%) followed by their centrifugation at 9000g for 20 minutes. Reaction mixture for this assay comprised of 2 mL of aliquot, 2 mL of 0.6% thiobarbituric acid, and heated for 20 minutes at 100°C followed by their abrupt cooling in an ice bath. Finally, absorbance of the mixture was checked at 532 and 450 nm for MDA determination.
2.4. Determination of N, P, K, and Mo contents in different parts of the plant
At physiological maturity, 30 soybean and 15 maize plants were randomly selected from each experimental unit, and were separated into grain, straw and roots, and dried at 70°C for 72 h to determine N, P, K, and Mo content. The N concentration was determined using the Kjeldahl Apparatus, P concentration was estimated using the Vanadomolybdate method (Xia et al., 2013), and K concentration was measured using the FAAS method (Varian 250 plus). The NPK uptake was calculated by multiplying the biomass of each organ by the N, P, and K concentrations, and presented as kilogram per hectare (Mao et al., 2012). Similarly, for Mo analysis, approximately 0.2 g of dried tissue was microwave-digested in 5 mL HNO3 (4/1 v/v) at 160°C for 45 min and diluted, and Mo content was quantified using inductively coupled plasma optical emission spectroscopy (ICP-OES) (Perkin Elmer Optima 8300, Massachusetts, USA).
2.5. Statistical analysis
Data was analyzed for normal distribution and homogeneity of variance. Log transformations were applied where necessary to correct deviations from these assumptions. Analysis was performed using Microsoft Excel 2013 (Microsoft Corp. Washington, USA), IBM SPSS Statistics V22.0 (SPSS Inc., Chicago, New Mexico, USA). One-way analysis of variance (ANOVA) was used to statistically analyze the obtained data. Later, data was arranged in MS Excel 2013, and their relevant means and standard errors were also computed by MS Excel 2013.
3. Results
3.1. Growth and yield attributes
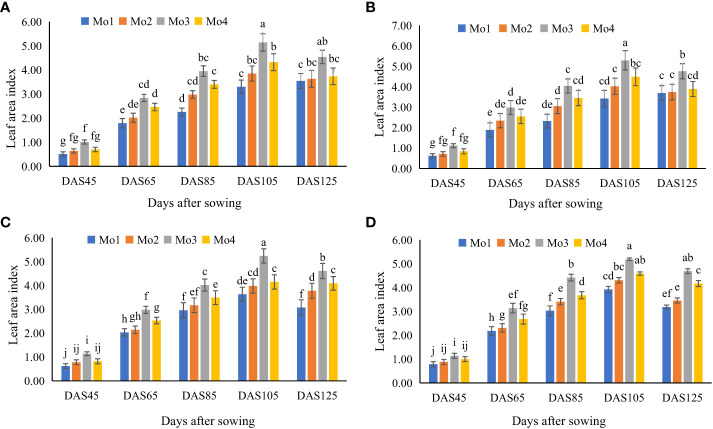
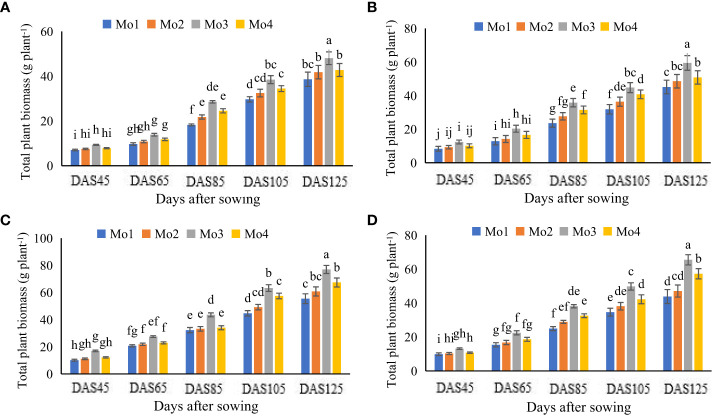
Results regarding growth attributes revealed a significant impact of Mo application in both years under both cropping systems ( Figures 2 , 3 ). However, Mo application @ 120 g ha-1 proved to be the most significant in this regard. It was observed that in sole soybean plantation, maximum increment in growth attributes such as leaf area index (LAI; 436 and 393%), plant height (16 and 12%), root length (107 and 102%), nodules per plant (18 and 17%), stem diameter (15 and 16%), total plant biomass (TPB; 454 and 364%), yield (19 and 18%) were observed as compared to control treatment after 105 DAS during year 2020 and 2021. Similarly, in soybean-maize intercropping system, Mo application @ 120 g ha-1 enhanced the LAI (434 and 441%), TPB (430 and 461%), plant height (12 and 13%), root length (94 and 86%), nodules per plant (14 and 15%), stem diameter (12 and 14%), and yield (15 and 20%) after 125 DAS during year 2020 and 2021, respectively ( Tables 4 , 5 ). On average, more improvement in all the plant growth and yield attributes was evident after Mo application during 2021 as compared to the preceding year (2020).
Figure 2.
Effect of different levels of Mo on leaf area index (m2) of soybean at different time intervals, (A) sole soybean during 2020, (B) sole soybean during 2021, (C) soybean intercropped during 2020 and (D) soybean intercropped during 2021. The columns sharing same letters are statistically non-significant (Tukey’s HSD test) at p< 0.05. The error bars indicate standard deviation.
Figure 3.
Effect of different levels of Mo on total plant biomass (g) at different time intervals, (A) sole soybean during 2020, (B) sole soybean during 2021, (C) soybean intercropped during 2020 and (D) soybean intercropped during 2021. The columns sharing same letters are statistically non-significant (Tukey’s HSD test) at p< 0.05. Error bars indicate standard deviation.
Table 4.
Soybean yield in maize-soybean intercropping system.
| Treatments | 2020 | 2021 | ||
|---|---|---|---|---|
| Sole Soybean | Soybean Intercropped | Sole Soybean | Soybean Intercropped | |
| Mo1 | 906.7 c | 521.6 c | 1014.8 d | 631.1 c |
| Mo2 | 1008.2 bc | 558.5 bc | 1079.7 c | 653.1 bc |
| Mo3 | 1122.4 a | 626.0 a | 1192.9 a | 725.3 a |
| Mo4 | 1065.1 ab | 592.4 ab | 1136.2 b | 688.6 b |
| P value (0.05) | 0 | 0 | 0.0001 | 0 |
Different lowercase letters, in a single column, show significant differences among concentration means at p< 0.05.
Table 5.
Morphological properties of soybean under sole and intercropping with maize in different (plant−1) during 2020 and 2021.
| Sole Soybean | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | |||||||||
| T | PH | RL | NoP | SDIM | IL | PH | RL | NoP | SDIM | IL |
| Mo1 | 55.5 ± 0.81 d | 81.4 ± 2.18 d | 23.9 ± 0.46 d | 5.3 ± 0.22 c | 5.4 ± 0.10 b | 57.6 ± 1.71 c | 88.2 ± 1.32 d | 24.7 ± 0.42 d | 5.5 ± 0.14 c | 5.6 ± 0.21 b |
| Mo2 | 58.4 ± 0.83 c | 104.3 ± 1.36 c | 25.3 ± 0.57 c | 5.6 ± 0.28 bc | 5.3 ± 0.09 ab | 60.7 ± 1.98 b | 112.4 ± 1.41 c | 26.2 ± 0.52 c | 5.8 ± 0.19 bc | 5.5 ± 0.26 ab |
| Mo3 | 64.4 ± 0.94 a | 168.5 ± 2.35 a | 27.9 ± 0.78 a | 6.1 ± 0.41 a | 5.0 ± 0.12 a | 65.9 ± 1.14 a | 178.3 ± 8.23 a | 29.0 ± 0.71 a | 6.4 ± 0.33 a | 5.2 ± 0.34 a |
| Mo4 | 61.6 ± 1.19 b | 136.2 ± 1.14 b | 26.7 ± 0.66 b | 5.9 ± 0.29 ab | 5.1 ± 0.07 ab | 65.1 ± 1.65 a | 139.7 ± 1.22 b | 27.7 ± 0.62 b | 6.1 ± 0.20 ab | 5.3 ± 0.30 a |
| P value (0.05) | 0 | 0.0345 | 0 | 0.0037 | 0.1015 | 0.0001 | 0.0135 | 0 | 0.0024 | 0.0476 |
| Soybean intercropping | ||||||||||
| Mo1 | 77.1 ± 2.20 d | 96.2 ± 1.17 | 34.7 ± 1.03 d | 7.4 ± 0.49 c | 4.1 ± 0.18 d | 80.3 ± 2.17 d | 105.03 ± 2.34 d | 36.0 ± 0.61 d | 7.6 ± 0.43 c | 4.3 ± 0.23 c |
| Mo2 | 80.9 ± 2.32 c | 129 ± 1.62 | 36.4 ± 1.09 c | 7.8 ± 0.39 bc | 4.2 ± 0.14 c | 84.3 ± 2.29 c | 138.23 ± 1.42 c | 37.8 ± 0.64 c | 8.0 ± 0.31 bc | 4.4 ± 0.28 b |
| Mo3 | 86.6 ± 2.66 a | 186.2 ± 1.24 | 39.5 ± 1.15 a | 8.3 ± 0.34 a | 4.5 ± 0.16 a | 90.4 ± 2.13 a | 195.11 ± 2.32 a | 41.2 ± 0.70 a | 8.7 ± 0.25 a | 4.6 ± 0.29 a |
| Mo4 | 83.8 ± 3.03 b | 155.6 ± 1.01 | 38.1 ± 1.15 b | 8.1 ± 0.53 ab | 4.4 ± 0.14 b | 87.4 ± 1.44 b | 164.45 ± 1.41 b | 39.7 ± 0.68 b | 8.4 ± 0.46 ab | 4.5 ± 0.30 ab |
| P value (0.05) | 0 | 0.0125 | 0 | 0.015 | 0 | 0.0001 | 0.015 | 0 | 0.012 | 0.0015 |
T, Treatments; PH, Plant height; RL, Root length; NoP, number of nodules plant−1; SDIM, steam diameter (mm); IL, internode length (inch). Different lowercase letters, in a single column, show significant differences among concentration means at p< 0.05.
3.2. Physiological attributes
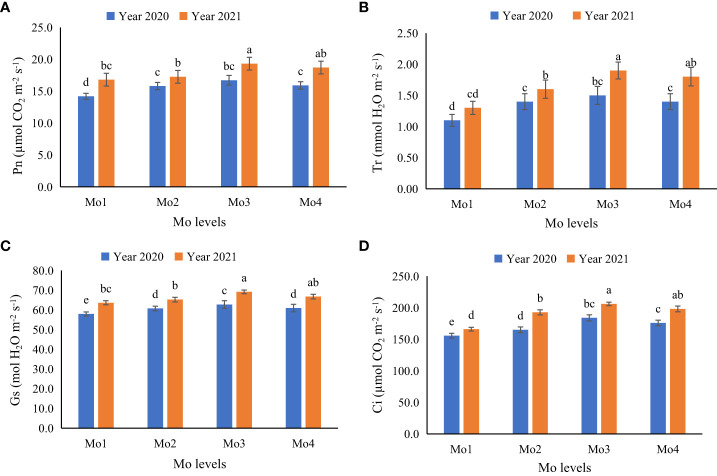
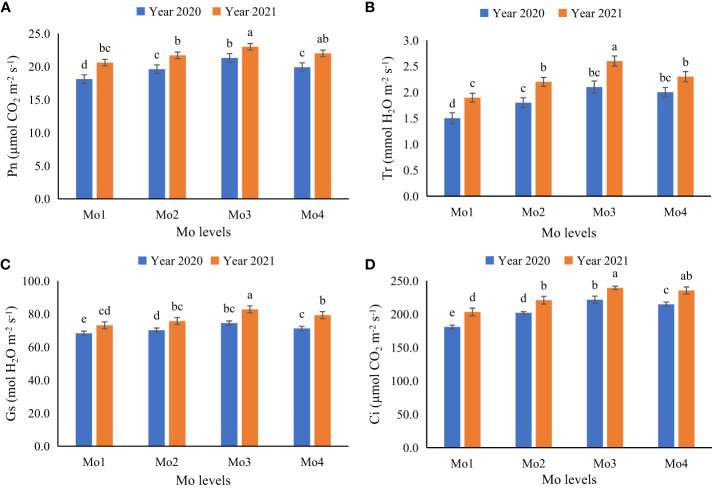
In terms of plant physiological attributes, it was observed that exogenous application of Mo led to a significant improvement in all the measured physiological attributes i.e., transpiration rate, stomatal conductance, inter-cellular CO2 concentration, and photosynthetic rate, as well as chlorophyll content (SPAD value). However, application of Mo @ 120 g ha-1 yielded significantly better results as compared to other Mo levels. It was observed that during year 2020, under sole soybean plantation and soybean-maize intercropping, application of Mo @ 120 g ha-1 improved transpiration rate (12 and 15%), stomatal conductance (9 and 10%), inter-cellular CO2 concentration (13 and 11%), and photosynthetic rate (11 and 11%) respectively. However, during 2021, more promising outcomes were pragmatic in terms of physiological attributes i.e., transpiration rate, stomatal conductance, inter-cellular CO2 concentration, and photosynthetic rate. Under both sole and intercropped soybean, application of Mo @ 120 g ha-1 significantly enhanced the physiological attributes i.e., transpiration rate (18 and 19%), stomatal conductance (11 and 11%), inter-cellular CO2 concentration (13 and 12%), and photosynthetic rate (12 and 11%) respectively as compared to control treatment ( Figures 4 – 6 ). It was obvious from the results of physiological attributes that more promising outcomes of Mo application @ 120 g ha-1 were observed under soybean-maize intercropping during both the years.
Figure 4.
Effect of different levels of Mo on gaseous exchange attributes of soybean leaves at different Mo application levels for sole spybean, (A) Photosynthetic rate (Pn; µmol CO2 m−2 s−1), (B) Transpiration rate (Tr; mmol H2O m−2 s−1), (C) Stomatal conductance (Gs; mol H2O m−2 s−1) and (D) inter-cellular CO2 concentration (Ci; µmol CO2 m−2 s−1). The columns sharing same letters are statistically non-significant (Tukey’s HSD test) at p< 0.05. Error bars indicate standard deviation.
Figure 6.
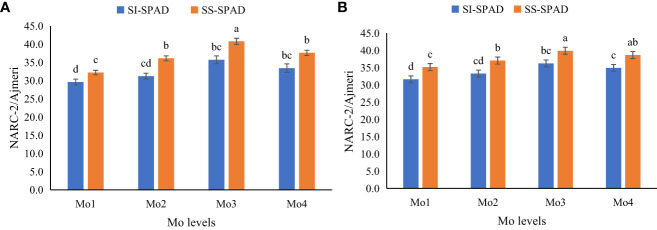
Effect of different levels of Mo on SPAD value of soybean leaves grown solely and intercropped with maize, (A) SPAD value in 2020 and (B) SPAD value in 2021. The columns sharing different letters are statistically non-significant (Tukey’s HSD test) at p< 0.05. Error bars indicate standard deviation. SI, Soybean intercropped; SS, Sole Soybean.
Figure 5.
Effect of different levels of Mo on gaseous exchange attributes of soybean leaves at different Mo application levels for intercropping soybean, (A) Photosynthetic rate (Pn; µmol CO2 m−2 s−1), (B) Transpiration rate (Tr; mmol H2O m−2 s−1), (C) Stomatal conductance (Gs; mol H2O m−2 s−1) and (D) inter-cellular CO2 concentration (Ci; µmol CO2 m−2 s−1). The columns sharing same letters are statistically non-significant (Tukey’s HSD test) at p< 0.05. Error bars indicate standard deviation.
3.3. Antioxidant activities
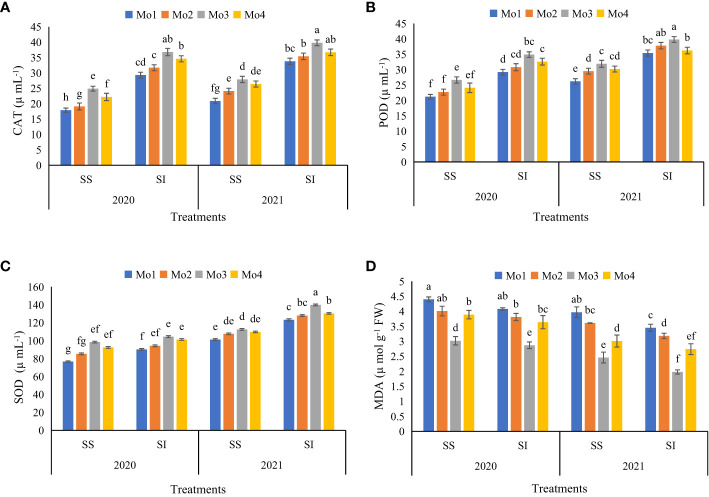
Application of Mo also showed significantly positive effects on various antioxidant activities in soybean leaves such as catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) during sole and intercropping with maize over two consecutive years (2020–2021). During sole plantation in 2020-21, Mo application at a rate of 120 g ha-1 led to significant increment in antioxidant enzyme activities CAT (14 and 15%), SOD (13 and 12%), and POD (7 and 5%) respectively, as compared to control treatment. However, in soybean maize intercropping, Mo application @ 120 g ha-1 yielded more significant outcomes in all the measured antioxidant activities, including CAT (11 and 12%), POD (5 and 6%), and SOD (11 and 11%) as compared to control treatment ( Figure 7 ). Furthermore, lipid oxidation was evaluated in terms of malondialdehyde (MDA) contents in soybean leaves. Results revealed that Mo application at a rate of 120 g ha-1 significantly reduced MDA contents in soybean leaves, indicating a significant decrease in lipid peroxidation. In the sole and intercropped soybean cropping systems, Mo application at a rate of 120 g ha -1 during 2020 led to significant reduction in MDA contents by 30 and 31%, respectively as compared to control treatment. However, during the subsequent year (2021), MDA contents were further decreased by 38 and 43% respectively, in the sole and intercropped soybean cropping systems with the application of Mo @ 120 g ha-1 ( Figure 7 ).
Figure 7.
Effect of different levels of Mo on antioxidant activities (A) Catalase, (B) Peroxidase, (C) Superoxide dismutase and (D) malondialdehyde contents in soybean leaves during year 2020–2021. The columns sharing different letters are statistically non-significant (Tukey’s HSD test) at p< 0.05. Error bars indicate standard deviation.
3.4. Nutritional attributes
Results in terms of nutritional attributes of soybean such as nitrogen (N), phosphorous (P), potassium (K), and Mo contents were significantly affected by Mo treatments. However, among different application rates, Mo application @ 120 g ha−1 was found to be the most efficient in both cropping systems. During year 2020, Mo application at 120 g ha-1 significantly increased the N, P, K and Mo contents in roots (10, 13, 13 and 15%), straw (7, 13, 10 and 17%), and grains (10, 10, 10 and 20%) of soybean grown solely ( Table 6 ). Whereas, in the same year during intercropping with maize, NPK and Mo contents in roots (13, 13, 17 and 16%), straw (9, 10, 17 and 16%) and grains (11, 12, 26 and 18%) of soybean were also significantly increased under Mo application rate of 120 g ha-1 ( Table 6 ). Furthermore, during year 2021, relatively more significant outcomes in terms of nutrient uptake were pragmatic, where nutrient uptake in different parts of sole and intercropped soybean were significantly increased after the application of Mo @ 120 g ha-1 i.e., NPK and Mo contents in roots (15, 13, 12 and 15% as well as 19, 17, 24 and 17%), straw (12, 13, 14 and 18% as well as 17, 12, 18 and 17%) and grains (12, 14, 14 and 21% as well as 15, 14, 18 and 19%) were increased respectively in sole grown and intercropped soybean crop as compared to control treatment during year 2021 ( Table 6 ).
Table 6.
Total nitrogen, phosphorus, potassium, and molybdenum uptake under soybean grown as sole crop in different parts of the soybean (plant−1) during 2020 and 2021.
| 2020 | 2021 | ||||||
|---|---|---|---|---|---|---|---|
| Nutrients | Treatments | Root | Straw | Grain | Root | Straw | Grain |
| Nitrogen | Mo1 | 0.31 ± 0.03 b | 0.48 ± 0.03 b | 0.85 ± 0.05 bc | 0.34 ± 0.03 c | 0.58 ± 0.04 c | 0.89 ± 0.04 c |
| Mo2 | 0.35 ± 0.03 ab | 0.51 ± 0.03 b | 0.88 ± 0.06 b | 0.40 ± 0.03 b | 0.62 ± 0.04 b | 0.93 ± 0.06 b | |
| Mo3 | 0.41 ± 0.02 a | 0.62 ± 0.03 a | 0.96 ± 0.05 a | 0.47 ± 0.03 a | 0.68 ± 0.03 a | 1.01 ± 0.05 a | |
| Mo4 | 0.38 ± 0.03 ab | 0.57 ± 0.03 ab | 0.91 ± 0.05 ab | 0.42 ± 0.03 ab | 0.65 ± 0.03 ab | 0.97 ± 0.05 ab | |
| P value (0.05) | 0.009 | 0.0025 | 0.0017 | 0.0055 | 0.0025 | 0.0006 | |
| Phosphorus | Mo1 | 0.03062 ± 0.00 b | 0.0447 ± 0.00 c | 0.0967 ± 0.02 bc | 0.0322 ± 0.00 b | 0.0560 ± 0.00 b | 0.1443 ± 0.02 c |
| Mo2 | 0.03412 ± 0.00 ab | 0.0561 ± 0.00 b | 0.1112 ± 0.02 b | 0.0369 ± 0.00 ab | 0.0589 ± 0.00 b | 0.1504 ± 0.02 b | |
| Mo3 | 0.03811 ± 0.00 a | 0.0618 ± 0.00 a | 0.1521 ± 0.02 a | 0.0399 ± 0.00 a | 0.0632 ± 0.00 a | 0.1633 ± 0.02 a | |
| Mo4 | 0.03510 ± 0.00 ab | 0.0582 ± 0.00 ab | 0.1361 ± 0.02 ab | 0.0384 ± 0.00 ab | 0.0611 ± 0.00 ab | 0.1568 ± 0.02 ab | |
| P value (0.05) | 0.4547 | 0.0701 | 0.0079 | 0.4547 | 0.0701 | 0.0029 | |
| Potassium | Mo1 | 0.0420 ± 0.00 ab | 0.5441 ± 0.07 b | 0.2891 ± 0.02 b | 0.0438 ± 0.00 b | 0.5558 ± 0.06 c | 0.2997 ± 0.01 c |
| Mo2 | 0.0432 ± 0.00 ab | 0.5585 ± 0.07 ab | 0.2969 ± 0.02 b | 0.0459 ± 0.00 ab | 0.5865 ± 0.07 b | 0.3156 ± 0.02 b | |
| Mo3 | 0.0479 ± 0.00 a | 0.5994 ± 0.07 a | 0.3177 ± 0.02 a | 0.0492 ± 0.00 a | 0.6362 ± 0.08 a | 0.3419 ± 0.03 a | |
| Mo4 | 0.0450 ± 0.00 ab | 0.5753 ± 0.07 ab | 0.3054 ± 0.02 ab | 0.0477 ± 0.00 ab | 0.6112 ± 0.08 ab | 0.3292 ± 0.02 ab | |
| P value (0.05) | 0.4547 | 0.0003 | 0.0219 | 0 | 0.0049 | 0.0219 | |
| Molybdenum | Mo1 | 16.40 ± 0.26 d | 22.47 ± 0.18 d | 46.84 ± 0.36 d | 18.09 ± 0.27 d | 23.30 ± 0.13 d | 48.96 ± 0.49 d |
| Mo2 | 17.35 ± 0.20 c | 23.14 ± 0.18 c | 49.93 ± 0.40 c | 19.14 ± 0.22 c | 24.69 ± 0.10 c | 52.36 ± 0.55 c | |
| Mo3 | 20.62 ± 0.03 a | 26.19 ± 0.24 a | 56.19 ± 0.43 a | 22.16 ± 0.04 a | 28.41 ± 0.14 a | 60.10 ± 0.61 a | |
| Mo4 | 19.32 ± 0.14 b | 24.06 ± 0.18 b | 53.22 ± 0.42 b | 20.28 ± 0.15 b | 26.17 ± 0.10 b | 55.92 ± 0.58 b | |
| P value (0.05) | 0 | 0 | 0 | 0 | 0 | 0 | |
Different lowercase letters, in a single column, show significant differences among concentration means at p< 0.05.
4. Discussion
Effect of Mo application on soybean growth and yield characteristics has been studied extensively in recent years (Bittner, 2014; Qin et al., 2017; Mahilane and Singh, 2018; Quddus et al., 2020; Rana et al., 2020; Ali et al., 2021; Oliveira et al., 2022; Nasar et al., 2022a). In present study, application of Mo at different levels were tested in sole and intercropping with maize. Observations revealed a positive correlation between Mo application and LAI, consistent with the findings of (Nasar et al., 2022a; Oliveira et al., 2022), and may be attributed to Mo mediated improvement of photosynthetic efficiency (Gao et al., 2010). Increased total plant biomass as observed in current study is also consistent with the findings of previous researchers (Raza et al., 2020b; Hussain et al., 2021), which might be attributed to the role of Mo in N metabolism, enhancing N-use efficiency and promoting plant growth (Probst et al., 2021). Additionally, higher biomass values in intercropping compared to sole cropping systems may be due to the efficient utilization of resources, such as light, water, and nutrients as suggested by (Gao et al., 2010). Similarly, positive correlation between Mo application and various morphological plant attributes are in agreement with the previously reported studies (Qin et al., 2017; Heshmat et al., 2021; Nasar et al., 2022a), which might be correlated with the enhanced application and subsequent role of Mo in various enzymatic processes related to plant growth and development (Bittner, 2014; Probst et al., 2021). Moreover, improvement in these traits in intercropping system suggests that Mo application can enhance the compatibility of soybean in intercropping systems, ensuring better growth and yield (Ren et al., 2017).
In current study, Mo application also improved soybean yield in both sole and intercropping system, which is also consistent with the outcomes of previous researchers (Qin et al., 2017; Mahilane and Singh, 2018; Rana et al., 2020; Ali et al., 2021; Nasar et al., 2022b). However, lower yields were observed during intercropping than in sole soybean cultivation, which might be related to provision of competition between maize and soybean for resources (Ren et al., 2017; Raza et al., 2020b). However, increased grain yield with increasing Mo levels in intercropping treatments suggests that Mo can mitigate the adverse effects of intercropping on soybean yield (Oliveira et al., 2022). Additionally, higher aggregate outputs observed in intercropping treatments with higher Mo levels align with previous studies, indicating the potential for increased yield in intercropping systems (Ren et al., 2017; Raza et al., 2019; Raza et al., 2020b). In addition to that, improved soybean physiology after Mo application in both years under sole and intercropping systems was due to the role of Mo as an essential component of the enzyme nitrogenase which is involved in N-fixation (Zuber and Kang, 1978), which contributed to the improvement in the efficiency of photosynthetic apparatus as well as stomatal conductance (Wu et al., 2017). Similar results were previously reported by (Kaiser et al., 2005; Kadlag et al., 2006). Furthermore, increase in chlorophyll content as observed in this study could be linked to the role of Mo in the formation of Mo-cofactor, which is involved in the synthesis of chlorophyll and other essential biomolecules (Mendel, 2013; Probst et al., 2021), as suggested by Li et al. (2018). Moreover, positive influence of Mo on antioxidant activities in soybean was also pragmatic in current study, which has been earlier reported by (Tiwari et al., 2018; Oliveira et al., 2022). In contrast, while the application of Mo led to a decline in lipid peroxidation through potential enhancement of antioxidant enzyme activities in soybean plants, facilitating ROS scavenging and protection against oxidative stress, plants can also mobilize intrinsic defense mechanisms to counteract ROS, including the activation of antioxidant enzymes and the synthesis of osmotic adjustment compounds, as highlighted by (Lv et al., 2019; Jia et al., 2021; Wang et al., 2023) in their respective studies.
Positive interaction between Mo application and nutritional uptake by plants was observed, as previously reported by (Raza et al., 2019). Mo is an essential micronutrient for plants and plays a crucial role in N metabolism (Walkley and Black, 1934). It has been reported that Mo application enhances N accumulation, seed yield, and seed protein content in soybean (Campo et al., 2009) as was observed in current study. Previous research are supported by our results, which show an increase in plant nutrient profile quality under both the solo and intercropping systems. (Raut et al., 2004; Weisany et al., 2015; Zhao et al., 2022). Moreover, Mo has been reported to exert positive effects on photosynthesis (Probst et al., 2021), which may further contribute to enhanced nutrient uptake as observed in this study. These improvements in the soybean growth, yield, morphology, physiology, and antioxidant activities after Mo application have significant implications for agricultural productivity, particularly in maize-soybean intercropping systems. Improved photosynthetic performance and stomatal conductance can contribute to increased biomass and grain yields (Wolff and Coltman, 1990; Raza et al., 2021; Mirriam et al., 2022). Moreover, enhanced antioxidant activities can help improve the stress tolerance of soybean plants under the shaded conditions commonly encountered in intercropping systems (Zuber and Kang, 1978; Li et al., 2014; Wu et al., 2017). As a result, Mo application can potentially enhance the overall productivity and sustainability of maize-soybean intercropping systems (Gao et al., 2010; Liu et al., 2015; Raza et al., 2020b).
5. Conclusions
Intercropping of soybean and maize has numerous benefits but shading stress on soybean due to maize canopy can negatively impact its growth and yield. Exogenous application of Mo has shown considerable potential in mitigating the negative effects of shading stress and improving soybean growth and yield. Molybdenum (Mo) is directly involved in photosynthetic process of plants and it also stimulates the assimilatory enzyme activities under stressed conditions, and in this way, help the plant to sustain shading stress. In a field trial, different levels of Mo were applied and evaluated for their effects on growth and yield parameters. Results showed that all the tested levels of Mo significantly improved soybean growth and yield, with the most efficient level being 120 g ha-1. These findings have significant implications for the development of sustainable intercropping practices in agriculture, which can promote optimal land utilization and enhance crop productivity, contributing to food security and environmental sustainability. Application of Mo proved to be a suitable alternative in improving the shade tolerance in soybean under soybean maize intercropping system. Following schematic diagram shows the mechanism of induced shade tolerance in soybean after Mo application.
Soybean-maize intercropping improved photosynthesis and enzyme activities
improved photosynthesis and enzyme activities shade tolerance
shade tolerance improved plant growth and physiology.
improved plant growth and physiology.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, ZJ and SC. Methodology, SA. Software, MQ. Validation, ZJ, SA and MQ. Formal analysis, SA. Investigation, ZJ. Resources, SA. Data curation, MA. Writing—original draft preparation, ZJ. Writing—review and editing, MQ, JD and YW. Visualization, SA. Supervision, SC. Project administration, SC. Funding acquisition, SC. All authors have read and agreed to the published version of the manuscript.
Funding Statement
The work was funded by National Natural Science Foundation of China (32172121), Sichuan Science and Technology Program (2022YFH0051) and Sichuan Innovation Team Project of National Modern Agricultural Industry Technology System, China (SCCXTD-2023-20).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Any opinions, finding, and conclusions or recommendations expressed in this material are those of authors and do not necessarily reflect the views of National Science Foundation of China.
References
- Abendroth L. J., Woli K. P., Myers A. J. W., Elmore R. W. (2017). Yield-based corn planting date recommendation windows for Iowa. Crop Forage Turfgrass Manage. 3, 1–7. doi: 10.2134/cftm2017.02.0015 [DOI] [Google Scholar]
- Adkine P. M., Mankar D. D., Khandait V. M., Bhandare V. L., Nawlakhe S. M. (2011). Effect of boron, molybdenum and potassium nitrate on growth, yield and economics of soybean. J. Soils Crop 21, 116–123. [Google Scholar]
- Ali M. M., Li B., Zhi C., Yousef A. F., Chen F. (2021). Foliar-supplied molybdenum improves phyto-nutritional composition of leaves and fruits of loquat (Eriobotrya japonica Lindl.). Agronomy 11, 892. doi: 10.3390/agronomy11050892 [DOI] [Google Scholar]
- Bedoussac L., Journet E.-P., Hauggaard-Nielsen H., Naudin C., Corre-Hellou G., Jensen E. S., et al. (2015). Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 35, 911–935. doi: 10.1007/s13593-014-0277-7 [DOI] [Google Scholar]
- Bittner F. (2014). Molybdenum metabolism in plants and crosstalk to iron. Front. Plant Sci. 5, 28. doi: 10.3389/fpls.2014.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyoucos G. J. (1962). Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 54, 464–465. doi: 10.2134/agronj1962.00021962005400050028x [DOI] [Google Scholar]
- Brown J. K. M. (2002). Yield penalties of disease resistance in crops. Curr. Opin. Plant Biol. 5, 339–344. doi: 10.1016/S1369-5266(02)00270-4 [DOI] [PubMed] [Google Scholar]
- Cakmak I., Brown P., Colmenero-Flores J. M., Husted S., Kutman B. Y., Nikolic M., et al. (2023). Chapter 7-Micronutrients. In In Marschner’s Mineral Nutrition of Plants (4th Edition) (Academic Press; ), 283–385. doi: 10.1016/B978-0-12-819773-8.00017-4 [DOI] [Google Scholar]
- Campo R. J., Araujo R. S., Hungria M. (2009). Molybdenum-enriched soybean seeds enhance N accumulation, seed yield, and seed protein content in Brazil. F. Crop Res. 110, 219–224. doi: 10.1016/j.fcr.2008.09.001 [DOI] [Google Scholar]
- de la Fuente E. B., Suárez S. A., Lenardis A. E., Poggio S. L. (2014). Intercropping sunflower and soybean in intensive farming systems: evaluating yield advantage and effect on weed and insect assemblages. NJAS-Wageningen J. Life Sci. 70, 47–52. doi: 10.1016/j.njas.2014.05.002 [DOI] [Google Scholar]
- Faostat F. (2019) Food and agriculture organization of the united nations-statistic division. Available at: https://www.fao.org/faostat/en/#data.
- Fischer J., Böhm H., Heβ J. (2020). Maize-bean intercropping yields in Northern Germany are comparable to those of pure silage maize. Eur. J. Agron. 112, 125947. doi: 10.1016/j.eja.2019.125947 [DOI] [Google Scholar]
- Gao Y., Duan A., Qiu X., Sun J., Zhang J., Liu H., et al. (2010). Distribution and use efficiency of photosynthetically active radiation in strip intercropping of maize and soybean. Agron. J. 102, 1149–1157. doi: 10.2134/agronj2009.0409 [DOI] [Google Scholar]
- Gao Y., Wu P., Zhao X., Wang Z. (2014). Growth, yield, and nitrogen use in the wheat/maize intercropping system in an arid region of northwestern China. F. Crop Res. 167, 19–30. doi: 10.1016/j.fcr.2014.07.003 [DOI] [Google Scholar]
- Heshmat K., Asgari Lajayer B., Shakiba M. R., Astatkie T. (2021). Assessment of physiological traits of common bean cultivars in response to water stress and molybdenum levels. J. Plant Nutr. 44, 366–372. doi: 10.1080/01904167.2020.1822395 [DOI] [Google Scholar]
- Hong F., Zhou J., Liu C., Yang F., Wu C., Zheng L., et al. (2005). Effect of nano-TiO 2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 105, 269–279. doi: 10.1385/BTER:105:1-3:269 [DOI] [PubMed] [Google Scholar]
- Horváth B., Opara-Nadi O., Beese F. (2005). A simple method for measuring the carbonate content of soils. Soil Sci. Soc Am. J. 69, 1066–1068. doi: 10.2136/sssaj2004.0010 [DOI] [Google Scholar]
- Hou J., Stacey G., Cheng J. (2015). Exploring soybean metabolic pathways based on probabilistic graphical model and knowledge-based methods. EURASIP J. Bioinforma. Syst. Biol. 2015, 1–13. doi: 10.1186/s13637-015-0026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htwe A. Z., Moh S. M., Soe K. M., Moe K., Yamakawa T. (2019). Effects of biofertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of mung bean, cowpea, and soybean. Agronomy 9, 77. doi: 10.3390/agronomy9020077 [DOI] [Google Scholar]
- Hu F., Gan Y., Chai Q., Feng F., Zhao C., Yu A., et al. (2016). Boosting system productivity through the improved coordination of interspecific competition in maize/pea strip intercropping. F. Crop Res. 198, 50–60. doi: 10.1016/j.fcr.2016.08.022 [DOI] [Google Scholar]
- Huss C. P., Holmes K. D., Blubaugh C. K. (2022). Benefits and risks of intercropping for crop resilience and pest management. J. Econ. Entomol. 115, 1350–1362. doi: 10.1093/jee/toac045 [DOI] [PubMed] [Google Scholar]
- Hussain S., Shafiq I., Chattha M. S., Mumtaz M., Brestic M., Rastogi A., et al. (2021). Effect of Ti treatments on growth, photosynthesis, phosphorus uptake and yield of soybean (Glycine max L.) in maize-soybean relay strip intercropping. Environ. Exp. Bot. 187, 104476. doi: 10.1016/j.envexpbot.2021.104476 [DOI] [Google Scholar]
- Iqbal N., Hussain S., Ahmed Z., Yang F., Wang X., Liu W., et al. (2019). Comparative analysis of maize–soybean strip intercropping systems: A review. Plant Prod. Sci. 22, 131–142. doi: 10.1080/1343943X.2018.1541137 [DOI] [Google Scholar]
- Jahan M. S., Zhao C. J., Shi L. B., Liang X. R., Jabborova D., Nasar J., et al. (2023). Physiological mechanism of melatonin attenuating to osmotic stress tolerance in soybean seedlings. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1193666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Ma M., Chen J., Wu S. (2021). Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 22 (3), 1088. doi: 10.3390/ijms22031088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiren T. S., Leventon J., Jager N. W., Dorresteijn I., Schultner J., Senbeta F., et al. (2021). Governance challenges at the interface of food security and biodiversity conservation: a multi-level case study from Ethiopia. Environ. Manage. 67, 717–730. doi: 10.1007/s00267-021-01432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar P., Ferrell B. D., Jarvis T., Haramoto K., Place N., Dums J. T., et al. (2023). Spontaneously produced lysogenic phages are an important component of the soybean Bradyrhizobium mobilome. MBio 14, e00295–e00223. doi: 10.1128/mbio.00295-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlag A. D., Patil J. C., Kasture M. C., Jagdhani A. D. (2006). Effect of molybdenum on yield and quality of soybean. Ann. Plant Physiol. 20, 281. [Google Scholar]
- Kaiser B. N., Gridley K. L., Ngaire Brady J., Phillips T., Tyerman S. D. (2005). The role of molybdenum in agricultural plant production. Ann. Bot. 96, 745–754. doi: 10.1093/aob/mci226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimuthu P., Harmer J. R., Baldauf M., Hassan A. H., Kruse T., Bernhardt P. V. (2022). Electrochemically driven catalysis of the bacterial molybdenum enzyme YiiM. Biochim. Biophys. Acta (BBA)-Bioenergetics 1863, 148523. doi: 10.1016/j.bbabio.2021.148523 [DOI] [PubMed] [Google Scholar]
- Kalugina Z. I. (2014). Agricultural policy in Russia: Global challenges and the viability of rural communities. Int. J. Sociol. Agric. Food 21, 115–131. doi: 10.48416/ijsaf.v21i1.158 [DOI] [Google Scholar]
- Laltlanmawia L., Singh A. K., Sharma S. K. (2004). Effect of phosphorus and molybdenum on yield, protein content and nutrient uptake by soybean on acid soils of Nagaland. J. Indian Soc Soil Sci. 52, 199–202. [Google Scholar]
- Li Y., Jin Q., Yang D., Cui J. (2018). Molybdenum sulfide induce growth enhancement effect of rice (Oryza sativa L.) through regulating the synthesis of chlorophyll and the expression of aquaporin gene. J. Agric. Food Chem. 66 (16), 4013–4021. doi: 10.1021/acs.jafc.7b05940 [DOI] [PubMed] [Google Scholar]
- Li T., Liu L.-N., Jiang C.-D., Liu Y.-J., Shi L. (2014). Effects of mutual shading on the regulation of photosynthesis in field-grown sorghum. J. Photochem. Photobiol. B Biol. 137, 31–38. doi: 10.1016/j.jphotobiol.2014.04.022 [DOI] [PubMed] [Google Scholar]
- Liu X., Rahman T., Song C., Yang F., Su B., Cui L., et al. (2018). Relationships among light distribution, radiation use efficiency and land equivalent ratio in maize-soybean strip intercropping. F. Crop Res. 224, 91–101. doi: 10.1016/j.fcr.2018.05.010 [DOI] [Google Scholar]
- Liu W., Zou J., Zhang J., Yang F., Wan Y., Yang W. (2015). Evaluation of soybean (Glycine max) stem vining in maize-soybean relay strip intercropping system. Plant Prod. Sci. 18, 69–75. doi: 10.1626/pps.18.69 [DOI] [Google Scholar]
- Lv J., Christie P., Zhang S. (2019). Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environ. Sci. Nano 6, 41–59. doi: 10.1039/C8EN00645H [DOI] [Google Scholar]
- Mahilane C., Singh V. (2018). Effect of zinc and molybdenum on growth, yield attributes, yield and protein in grain on summer blackgram (Vigna mungo L.). Int. J. Curr. Microbiol. Appl. Sci. 7, 1156–1162. doi: 10.20546/ijcmas.2018.701.140 [DOI] [Google Scholar]
- Mao L., Zhang L., Li W., van der Werf W., Sun J., Spiertz H., et al. (2012). Yield advantage and water saving in maize/pea intercrop. F. Crop Res. 138, 11–20. doi: 10.1016/j.fcr.2012.09.019 [DOI] [Google Scholar]
- Mendel R. R. (2013). The molybdenum cofactor. J. Biol. Chem. 288, 13165–13172. doi: 10.1074/jbc.R113.455311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirriam A., Mugwe J., Raza M. A., Seleiman M. F., Maitra S., Gitari H. H. (2022). Aggrandizing soybean yield, phosphorus use efficiency and economic returns under phosphatic fertilizer application and inoculation with Bradyrhizobium. J. Soil Sci. Plant Nutr. 22 (4), 5086–5098. doi: 10.1007/s42729-022-00985-8 [DOI] [Google Scholar]
- Mucheru-Muna M., Pypers P., Mugendi D., Kung’u J., Mugwe J., Merckx R., et al. (2010). A staggered maize–legume intercrop arrangement robustly increases crop yields and economic returns in the highlands of Central Kenya. F. Crop Res. 115, 132–139. doi: 10.1016/j.fcr.2009.10.013 [DOI] [Google Scholar]
- Nasar J., Wang G.-Y., Ahmad S., Muhammad I., Zeeshan M., Gitari H., et al. (2022. a). Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 13, 988055. doi: 10.3389/fpls.2022.988055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasar J., Wang G. Y., Zhou F. J., Gitari H., Zhou X. B., Tabl K. M., et al. (2022. b). Nitrogen fertilization coupled with foliar application of iron and molybdenum improves shade tolerance of soybean under maize-soybean intercropping. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1014640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira S. L., Crusciol C. A. C., Rodrigues V. A., Galeriani T. M., Portugal J. R., Bossolani J. W., et al. (2022). Molybdenum foliar fertilization improves photosynthetic metabolism and grain yields of field-grown soybean and maize. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.887682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S. R., Sommers L. E. (1982). “Phosphorus,” in Methods of soil analysis, 2nd edn. Eds. Page A. L., Miller R. H., Keeney D. R. (Soil Sci. Soc. Am. Madison, WI, USA; ), 403–430. [Google Scholar]
- Probst C., Yang J., Krausze J., Hercher T. W., Richers C. P., Spatzal T., et al. (2021). Mechanism of molybdate insertion into pterin-based molybdenum cofactors. Nat. Chem. 13, 758–765. doi: 10.1038/s41557-021-00714-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim M., Ju J., Zhao H., Bhatti S. M., Saleem G., Memon S. P., et al. (2023). Morphological and physiological response of tomato to sole and combined application of vermicompost and chemical fertilizers. Agronomy 13, 1508. doi: 10.3390/agronomy13061508 [DOI] [Google Scholar]
- Qin S., Hu C., Tan Q., Sun X. (2017). Effect of molybdenum levels on photosynthetic characteristics, yield and seed quality of two oilseed rape (Brassica napus L.) cultivars. Soil Sci. Plant Nutr. 63, 137–144. doi: 10.1080/00380768.2017.1286232 [DOI] [Google Scholar]
- Quddus M. A., Anwar M. B., Naser H. M., Siddiky M. A., Hussain M. J., Aktar S., et al. (2020). Impact of zinc, boron and molybdenum addition in soil on mungbean productivity, nutrient uptake and economics. J. Agric. Sci. 12, 115–129. doi: 10.5539/jas.v12n9p115 [DOI] [Google Scholar]
- Rana M., Bhantana P., Sun X. C., Imran M., Shaaban M., Moussa M., et al. (2020). Molybdenum as an essential element for crops: an overview. Int. J. Sci. Res. Growth 24, 18535. [Google Scholar]
- Raut S. S., Chore C. N., Deotale R. D., Waghmare H. U., Hatmode C. N., Yenprediwar M. D. (2004). Response of seed dressing with biofertilizers and nutrient on chemical, biochemical, yield and yield contributing parameters of soybean. J. Soils Crop 14, 66–70. [Google Scholar]
- Raven J. A. (1988). The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytol. 109, 279–287. doi: 10.1111/j.1469-8137.1988.tb04196.x [DOI] [Google Scholar]
- Raza A., Asghar M. A., Ahmad B., Bin C., Iftikhar Hussain M., Li W., et al. (2020. a). Agro-techniques for lodging stress management in maize-soybean intercropping system—a review. Plants 9, 1592. doi: 10.3390/plants9111592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza M. A., Cui L., Qin R., Yang F., Yang W. (2020. b). Strip-width determines competitive strengths and grain yields of intercrop species in relay intercropping system. Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-78719-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza M. A., Feng L. Y., van der Werf W., Iqbal N., Khan I., Hassan M. J., et al. (2019). Optimum leaf defoliation: A new agronomic approach for increasing nutrient uptake and land equivalent ratio of maize soybean relay intercropping system. F. Crop Res. 244, 107647. doi: 10.1016/j.fcr.2019.107647 [DOI] [Google Scholar]
- Raza M. A., Gul H., Wang J., Yasin H. S., Qin R., Bin Khalid M. H., et al. (2021). Land productivity and water use efficiency of maize-soybean strip intercropping systems in semi-arid areas: A case study in Punjab Province, Pakistan. J. Clean. Prod. 308, 127282. doi: 10.1016/j.jclepro.2021.127282 [DOI] [Google Scholar]
- Ren Y. Y., Wang X. L., Zhang S. Q., Palta J. A., Chen Y. L. (2017). Influence of spatial arrangement in maize-soybean intercropping on root growth and water use efficiency. Plant Soil 415, 131–144. doi: 10.1007/s11104-016-3143-3 [DOI] [Google Scholar]
- Rhoades J. D. (1983). Soluble salts. Methods soil anal. Part 2 Chem. Microbiol. Prop. 9, 167–179. doi: 10.2134/agronmonogr9.2.2ed.c10 [DOI] [Google Scholar]
- Sunita S., Sontakey P. Y., Beena N., Prema M., Deotale R. D. (2000). Influence of seed inoculation with Rhizobium and molybdenum on soyabean roots. J. Soils Crop 10, 128–130. [Google Scholar]
- Teng Y., Zhao C., Chai Q., Hu F., Feng F. (2016). Effects of postponing nitrogen topdressing on water use characteristics of maize-pea intercropping system. Acta Agron. Sin. 42, 446–455. doi: 10.3724/SP.J.1006.2016.00446 [DOI] [Google Scholar]
- Tiwari A. K., Prakash V., Ahmad A., Singh R. P. (2018). Effect of biofertilizers and micronutrients on nutrient uptake, growth, yield and yield attributes of lentil (Lens culinaris L.). Int. J. Curr. Microbiol. Appl. Sci. 7, 3269–3275. doi: 10.20546/ijcmas.2018.702.392 [DOI] [Google Scholar]
- U.S. Salinity Laboratory Staff . (1954). Diagnosis and improvement of saline and alkali soils. USDA Hdbk 60, 160. [Google Scholar]
- Walkley A., Black I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003 [DOI] [Google Scholar]
- Wang G. Y., Ahmad S., Wang Y., Wang B. W., Huang J. H., Jahan M. S., et al. (2023). Multivariate analysis compares and evaluates drought and flooding tolerances of maize germplasm. Plant Physiol. 193 (1), 339–355. doi: 10.1093/plphys/kiad317 [DOI] [PubMed] [Google Scholar]
- Wang Q., Ebbs S. D., Chen Y., Ma X. (2013). Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics 5, 753–759. doi: 10.1039/c3mt00033h [DOI] [PubMed] [Google Scholar]
- Weisany W., Raei Y., Pertot I. (2015). Changes in the essential oil yield and composition of dill (Anethum graveolens L.) as response to arbuscular mycorrhiza colonization and cropping system. Ind. Crops Prod. 77, 295–306. doi: 10.1016/j.indcrop.2015.09.003 [DOI] [Google Scholar]
- Wolff X. Y., Coltman R. R. (1990). Productivity under shade in Hawaii of five crops grown as vegetables in the tropics. J. Am. Soc Hortic. Sci. 115, 175–181. doi: 10.21273/JASHS.115.1.175 [DOI] [Google Scholar]
- Wu Y., Gong W., Yang W. (2017). Shade inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 7, 9259. doi: 10.1038/s41598-017-10026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H.-Y., Wang Z.-G., Zhao J.-H., Sun J.-H., Bao X.-G., Christie P., et al. (2013). Contribution of interspecific interactions and phosphorus application to sustainable and productive intercropping systems. F. Crop Res. 154, 53–64. doi: 10.1016/j.fcr.2013.07.011 [DOI] [Google Scholar]
- Yang J., Song Z., Ma J., Han H. (2020). Toxicity of molybdenum-based nanomaterials on the soybean–rhizobia symbiotic system: implications for nutrition. ACS Appl. Nano Mater. 3, 5773–5782. doi: 10.1021/acsanm.0c00943 [DOI] [Google Scholar]
- Zeeshan M., Hu Y. X., Guo X. H., Sun C. Y., Salam A., Ahmad S., et al. (2023). Physiological and transcriptomic study reveal SeNPs-mediated AsIII stress detoxification mechanisms involved modulation of antioxidants, metal transporters, and transcription factors in Glycine max L.(Merr.) roots. Environ. pollut. 317, 120637. doi: 10.1016/j.envpol.2022.120637 [DOI] [PubMed] [Google Scholar]
- Zhang W.-P., Liu G.-C., Sun J.-H., Zhang L.-Z., Weiner J., Li L. (2015). Growth trajectories and interspecific competitive dynamics in wheat/maize and barley/maize intercropping. Plant Soil 397, 227–238. doi: 10.1007/s11104-015-2619-x [DOI] [Google Scholar]
- Zhang G., Yang H., Zhang W., Bezemer T. M., Liang W., Li Q., et al. (2023). Interspecific interactions between crops influence soil functional groups and networks in a maize/soybean intercropping system. Agr. Ecosyst. Environ. 355, 108595. doi: 10.1016/j.agee.2023.108595 [DOI] [Google Scholar]
- Zhao X., Dong Q., Han Y., Zhang K., Shi X., Yang X., et al. (2022). Maize/peanut intercropping improves nutrient uptake of side-row maize and system microbial community diversity. BMC Microbiol. 22, 14. doi: 10.1186/s12866-021-02425-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M. S., Kang M. S. (1978). Corn lodging slowed by sturdier stalks. Crop Soils. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.