Abstract
A 75-year-old woman on hemodialysis for end-stage renal failure due to polycystic kidney disease developed dark spots on her limbs. She had been treated for extended spectrum beta-lactamase–producing Escherichia coli bacteremia by a rectovaginal fistula and was on long-term oral minocycline (cumulative dose 45 g). Physical examination revealed dark patches on her forearms and lower legs but no trunk hyperpigmentation or visual impairment. Blood tests were normal. Skin biopsy confirmed minocycline-induced hyperpigmentation. Minocycline-induced pigmentation is categorized into types I–IV, each with unique clinical and histopathological features. Types I and II are reversible upon discontinuing minocycline, whereas types III and IV are permanent. The patient was diagnosed with type II pigmentation, generally occurring with a cumulative dose exceeding 70–100 g; however, her lower dose (45 g) led to pigmentation, possibly influenced by her vitamin D deficiency. Clinicians should evaluate the antimicrobial indication and treatment period, considering not only the benefits but also the side effects and antimicrobial resistance. If minocycline is used, attention should be paid to minocycline-induced hyperpigmentation, and this possibility should be communicated to patients to enable early detection.
Keywords: Minocycline-induced hyperpigmentation, Hemodialysis, Extended spectrum betalactamase-producing Escherichia coli, Vitamin D deficiency
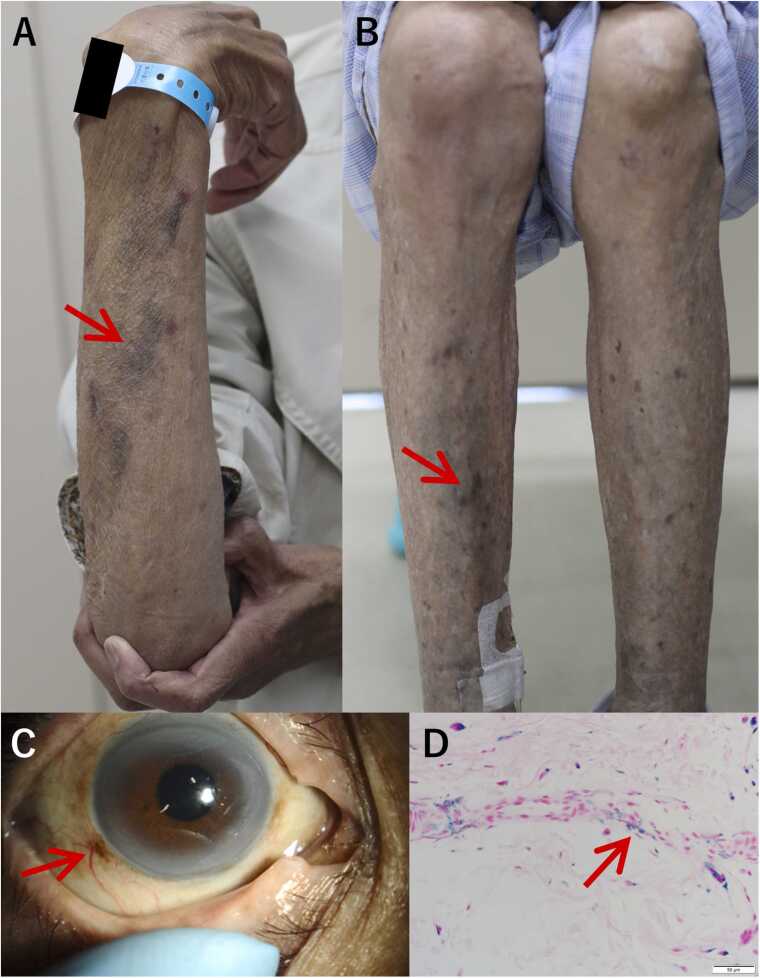
A 75-year-old woman who had been undergoing hemodialysis for end-stage renal failure for 24 years due to polycystic kidney disease had dark spots on her extremities for 2 months. She was taking oral active vitamin D preparations for dialysis-associated vitamin deficiency. She had been hospitalized and treated for recurrent extensive bacteraemia due to extended spectrum beta-lactamase–producing Escherichia coli associated with a rectovaginal fistula 7 months after surgery for rectal cancer and receiving oral minocycline as a long-term suppressive therapy since (cumulative dose, 45 g). Physical examination revealed multiple dark patches but no pain or itching on both forearms and lower legs (Fig. A and B). No trunk hyperpigmentation was observed. Scleral hyperpigmentation was observed (Fig. 1C); however, no visual impairment was noted. Blood tests revealed that serum iron, thyroid-stimulating hormone, cortisol, and adrenocorticotropic hormone levels and antinuclear antibody titers were within normal limits. A skin biopsy was performed on the dark-gray pigmented area on the right lower leg; histopathological testing with Berlin blue staining indicated minocycline-induced hyperpigmentation (Fig. 1D). The forearm and lower leg pigmentation resolved 4 months after minocycline discontinuation.
Fig. 1.
Dark pigmentation (arrow) on the forearm. (B) Dark pigmentation (arrow) on the leg. (C) Pigmentation (arrow) on the sclera around the corneal ring of the right eye. (D) Histopathological findings of the specimen with Berlin-blue staining. All layers of the dermis show deposition of blue granules that are positive for iron (arrow) and oedema with Berlin-blue staining.
Minocycline-induced pigmentation is classified into types I–IV, which have different clinical and histopathological characteristics and prognoses [1], [2]. Type I is characterized by blue-black pigmentation on scarred or inflamed facial areas, whereas type II is characterized by blue-grey pigmentation on the lower legs and forearms. Therefore, for an accurate diagnosis, it is important to exclude other hyperpigmentation disorders. Based on histopathological testing, types I and II are caused by the phagocytosis of haemosiderin and chelation of minocycline by macrophages, and differentiating between the two types can be challenging. Discontinuing minocycline can reverse type I and II pigmentation; however, types III and IV are permanent. In this patient, hyperpigmentation observed on the anterior aspect of the upper and lower extremities was clinically considered type II pigmentation. Type II and III pigmentation can occur with a cumulative minocycline dose exceeding 70–100 g [3]. However, in this case, accumulation with a lower dose of 45 g led to pigmentation, which may have been influenced by vitamin D deficiency associated with minocycline-induced hyperpigmentation [4]. Clinicians should evaluate the antimicrobial indication and treatment period, considering not only the benefits but also the side effects and antimicrobial resistance. If minocycline is used, attention should be paid to minocycline-induced hyperpigmentation, and this possibility should be communicated to patients to enable early detection.
Funding
There was no funding source for this publication.
Ethical approval
Not applicable.
Consent
Informed consent has been obtained for the publication of this clinical image.
Author contributions
TO was involved in literature review and drafted the manuscript. All authors revised the manuscript for critical intellectual content and approved the submitted version.
Declaration of Competing Interest
None.
Acknowledgments
The authors thank the physicians, nurses, and clinical staff of Aso Iizuka Hospital for their excellent work.
Contributor Information
Tomohide Okinaka, Email: tokinakah1@aih-net.com.
Kento Fukumitsu, Email: kfukumitsu@aih-net.com.
Nozomi Okamura, Email: nokamurah2@aih-net.com.
Liya Wang, Email: liyawang.pek0@gmail.com.
Yoshihiro Ohishi, Email: yohishih3@aih-net.com.
Yoshiko Miyazaki, Email: ymiyazakih5@aih-net.com.
Takashi Matono, Email: tmatonoh1@aih-net.com.
References
- 1.Basler R.S. Minocycline-related hyperpigmentation. Arch Dermatol. 1985;121(5):606–608. doi: 10.1001/archderm.1985.01660050058015. [DOI] [PubMed] [Google Scholar]
- 2.Mouton R.W., Jordaan H.F., Schneider J.W. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29(1):8–14. doi: 10.1111/j.1365-2230.2004.01421.x. [DOI] [PubMed] [Google Scholar]
- 3.Eisen D., Hakim M.D. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431–440. doi: 10.2165/00002018-199818060-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hanada Y., Berbari E.F., Steckelberg J.M. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3(1):ofv107. doi: 10.1093/ofid/ofv107. [DOI] [PMC free article] [PubMed] [Google Scholar]