Abstract
Helicobacter pylori is a major etiological agent in gastroduodenal disorders. The adhesion of H. pylori to human gastric epithelial cells is the initial step of H. pylori infection. Inhibition of H. pylori adhesion is thus a therapeutic target in the prevention of H. pylori infection. Experiments were performed to evaluate the effect of rebamipide, a novel antiulcer agent, on H. pylori adhesion to gastric epithelial cells. MKN-28 and MKN-45 cells, derived from human gastric carcinomas, were used as target cells. Ten H. pylori strains isolated from patients with chronic gastritis and gastric ulcer were used in the study. We evaluated the effect of rebamipide on H. pylori adhesion to MKN-28 and MKN-45 cells quantitatively using our previously established enzyme-linked immunosorbent assay. The adhesion of H. pylori to MKN-28 and MKN-45 cells was significantly inhibited by pretreatment of these cells with 100 μg of rebamipide per ml. However, the adhesion was not affected by the pretreatment of H. pylori with rebamipide. On the other hand, the viabilities of H. pylori, MKN-28 cells, and MKN-45 cells were not affected by rebamipide. Our studies suggest that rebamipide inhibits the adhesion of H. pylori to gastric epithelial cells.
In humans, Helicobacter pylori plays a causal role in histologic gastritis (21) and peptic ulcers (4) and is a cofactor in the occurrence of gastric cancer (3). H. pylori infection occurs in the gastric mucosa (20). The adhesion of H. pylori to human gastric epithelial cells is the initial step of H. pylori infection. Inhibition of the adhesion would be the ideal target for the prevention of H. pylori colonization. Accordingly, we have developed an enzyme-linked immunosorbent assay (ELISA) to quantitatively evaluate H. pylori adhesion to gastric epithelial cells (6).
We investigated the effect of rebamipide, a novel antiulcer agent that has antioxidant and free-radical scavenging activities (5, 12, 18), on H. pylori adhesion to gastric epithelial cells using our established ELISA.
MATERIALS AND METHODS
Target cells.
MKN-28 and MKN-45 cells, derived from human gastric carcinomas, were used for the analysis of H. pylori adhesion (11). The cells were suspended at a concentration of 3 × 105 cells/ml in RPMI 1640 medium (ICN Biomedicals, Costa Mesa, Calif.) containing 10% fetal calf serum, penicillin G (100 U/ml), and streptomycin (0.1 mg/ml). For the assay described here, 100 μl of cell suspension was placed in each well of a flat-bottom 96-well tissue culture plate (Falcon 3072; Becton Dickinson, Lincoln Park, N.J.), and the plate was incubated at 37°C under 8% CO2 for 2 days.
Bacteria.
In this study, 10 H. pylori strains, obtained from five patients with chronic gastritis and five patients with gastric ulcer, were used for the evaluation of rebamipide. Following primary isolation, these strains were passaged one to three times and were frozen at −80°C in brain heart infusion broth (Difco, Detroit, Mich.) supplemented with 15% glycerol. Subsequent analyses were performed with strains derived from the frozen stocks. H. pylori was inoculated onto brain heart infusion agar (Difco) containing 8% horse blood, and the plates were incubated at 37°C under 8% CO2 for 5 days (15). The organisms were washed with 10 mM phosphate-buffered saline (PBS; pH 7.4) and were suspended in RPMI 1640 without fetal calf serum and antibiotics at a concentration of 109 bacteria/ml.
Ten Escherichia coli strains, isolated from the feces of healthy volunteers, were used as controls. They were cultured under the same conditions overnight and were suspended in the same way.
Anti-H. pylori antibody.
Polyclonal antibody against H. pylori was prepared from a male specific-pathogen-free New Zealand White rabbit (weight, 3.5 kg). The rabbit was immunized by the following schedule. Three basal immunizations with a mixture of three different H. pylori clinical isolates (1.6 × 108 bacteria) were given subcutaneously at 7-day intervals. After 1 week, four booster injections of the same immunogen were given intravenously at 7-day intervals. The antibody was purified by using a protein A Cellulofine column (Chisso, Tokyo, Japan). The specificity of this antibody was tested by Western blotting.
Rebamipide and related compounds.
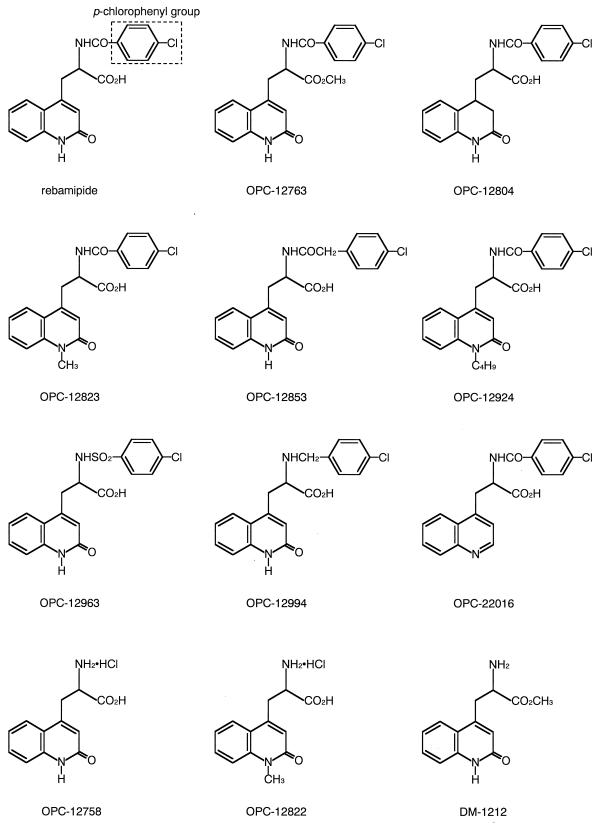
Rebamipide and 11 related compounds, OPC-12763, OPC-12804, OPC-12823, OPC-12853, OPC-12924, OPC-12963, OPC-12994, OPC-22016, OPC-12758, OPC-12822, and DM-1212, were synthesized at Otsuka Pharmaceutical Co. (Tokushima, Japan) (Fig. 1) (19). Bovine serum albumin (BSA) was used as the control agent.
FIG. 1.
Structures of rebamipide and 11 related compounds.
ELISA.
After the MKN-28 or MKN-45 cells had formed confluent monolayers, the medium was decanted from the microplates. The plates were then washed three times with PBS, 100 μl of H. pylori suspension (109 bacteria/ml) was added to each well, and the plates were incubated at 37°C under 8% CO2 for 90 min. The plates were then washed three times to remove the unadhered H. pylori, 100 μl of 8% paraformaldehyde was added to each well, and adherent H. pylori and cells were fixed at 4°C for 60 min. After the plates were washed, 100 μl of 1% H2O2 in methanol was added to each well and the plates were incubated at room temperature for 10 min, inactivating the endogenous peroxidase. After the plates were washed, 100 μl of rabbit anti-H. pylori polyclonal antibody (10 μg/ml) was added to each well and the plates were incubated for 2 h at 37°C. After the plates were washed, 100 μl of peroxidase-conjugated goat anti-rabbit immunoglobulins (Wako Chemicals, Osaka, Japan) diluted 1:1,000 in PBS was added to each well and the plates were incubated for 2 h at 37°C. After the final wash, 100 μl of o-phenylenediamine (0.4 mg/ml) in 100 mM citrate-phosphate buffer (pH 5.0) containing 0.02% H2O2 was added to each well and the plates were incubated at room temperature for 15 min. The reaction was terminated by adding 50 μl of 2 M H2SO4. The optical density (OD) of the reaction was measured at 490 nm with a microplate reader (model 3550 EIA Reader; Bio-Rad, Richmond, Calif.). The OD represents the amount of H. pylori adhering to the target cells (6).
Effect of rebamipide on MKN-28 and MKN-45 cells.
Before the assay of H. pylori adhesion to MKN-28 or MKN-45 cells by ELISA, 100 μl of RPMI 1640 medium containing rebamipide or BSA (25 to 100 μg/ml) was added to each well containing MKN-28 or MKN-45 cells, and the plates were incubated at 37°C under 8% CO2 for 30 to 120 min. After the cells were washed and the rebamipide or BSA was removed, 100 μl of H. pylori suspension (109 bacteria/ml) was added to each well, and the plates were incubated at 37°C under 8% CO2 for 90 min. After the cells were washed, the amount of H. pylori adhering to the target cells was quantified by ELISA.
The viabilities of MKN-28 and MKN-45 cells were assessed by using a Cell Counting Kit (Dojindo, Kumamoto, Japan) (7, 8) before and after the treatment with rebamipide. With this kit, the viability of the cells was represented by the OD at 450 nm.
Effect of rebamipide on H. pylori.
Before the assay of H. pylori adhesion to MKN-28 cells, H. pylori was suspended in RPMI 1640 medium containing rebamipide (25 to 100 μg/ml), and the plates were incubated at 37°C under 8% CO2 for 90 min. The H. pylori treated with rebamipide was washed with PBS and was resuspended in RPMI 1640 medium at a concentration of 109 bacteria/ml. Subsequently, 100 μl of this H. pylori suspension was added to each well containing MKN-28 cells, and the plates were incubated at 37°C under 8% CO2 for 90 min. After the cells were washed, the amount of adherent H. pylori was quantified by ELISA.
The viability of H. pylori was assessed by measuring the numbers of CFU of the H. pylori suspension before and after the treatment with rebamipide. The MICs of rebamipide for the H. pylori were determined by the agar dilution method. The H. pylori strains were inoculated onto brain heart infusion blood agar plates containing rebamipide (25 to 1,600 μg/ml), and the plates were incubated at 37°C under 8% CO2 for 3 days. The MICs were the lowest concentrations of rebamipide that visibly inhibited bacterial growth.
Effect of rebamipide on E. coli adhesion to MKN-28 cells.
Before the assay of E. coli adhesion to MKN-28 cells, the cells were pretreated with rebamipide or BSA (25 to 100 μg/ml) for 90 min. After washing for the removal of rebamipide or BSA, 100 μl of the E. coli suspension (109 bacteria/ml) was added to each well, and the plates were incubated at 37°C under 8% CO2 for 60 min. After the cells were washed, the amount of E. coli adhering to MKN-28 cells was quantified by ELISA with rabbit anti-E. coli polyclonal antibody (Biogenesis, Poole, United Kingdom) in place of anti-H. pylori antibody.
Assay of rebamipide binding to MKN-28 cells.
For the assay of rebamipide binding to MKN-28 cells, 5 × 105 cells were added to each well of a flat-bottom, 24-well tissue culture plate (Falcon 3047; Becton Dickinson) and the plates were incubated at 37°C under 8% CO2 for 24 h. After the MKN-28 cells had formed confluent monolayers, 500 μl of RPMI 1640 medium containing 25 to 100 μg of rebamipide per ml, which consisted of a 1:100 mixture of 14C-labeled (114 mCi/mmol) and nonlabeled rebamipide, was added to each well and the plates were incubated at 37°C under 8% CO2 for 60 min. After the MKN-28 cells were washed twice, the cells were lysed at 0°C with 1 M NaOH for 10 min and neutralized with 1 M HCl. After a scintillator (Aquasol-2; Packard, Meriden, Conn.) was added to the cell lysate, the radioactivity was measured with a liquid scintillation counter (Liquid Scintillation System LS5000CE; Beckman, Fullerton, Calif.) and the amount of rebamipide binding to MKN-28 cells was calculated.
Effects of related compounds.
Before the adhesion assay, 100 μl of RPMI 1640 medium containing one of the rebamipide-related compounds (100 μg/ml) was added to each well containing MKN-28 cells and the plates were incubated at 37°C under 8% CO2 for 90 min. After the cells were washed, 100 μl of the H. pylori suspension (109 bacteria/ml) was added to each well and the plates were incubated at 37°C under 8% CO2 for 90 min. After the cells were washed, the amount of adherent H. pylori was quantified by ELISA.
Statistics.
Data are presented as means ± standard deviations (SDs). The difference between rebamipide and the control was evaluated by paired Student’s t test. The correlation between the effect of rebamipide and the concentration of rebamipide was evaluated by Spearman’s rank correlation. A two-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
Effect of rebamipide on MKN-28 and MKN-45 cells.
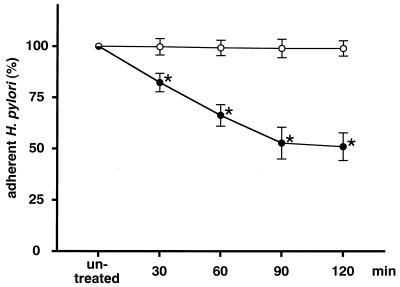
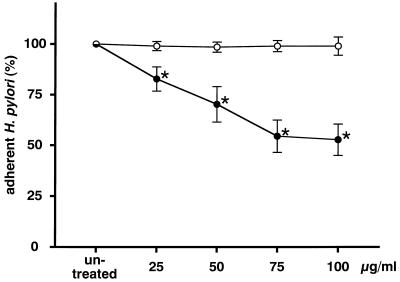
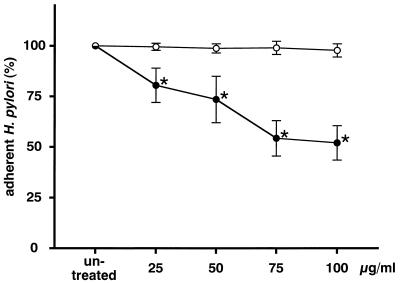
The amount of H. pylori adhering to MKN-28 cells was reduced by pretreating MKN-28 with rebamipide and was dependent on the incubation time (Fig. 2). The inhibitory activity reached a plateau after 90 min of incubation, at which point the experiments were carried out. The amount of H. pylori adhering to MKN-28 cells decreased in a dose-dependent manner (r = −0.963; P < 0.05 by Spearman’s rank correlation) with the concentration of rebamipide (Fig. 3). Furthermore, the amount of H. pylori adhering to MKN-45 cells also decreased in a dose-dependent manner (r = −0.974; P < 0.05 by Spearman’s rank correlation; Fig. 4). However, there was a difference in the reproducibility of the results between MKN-28 and MKN-45 cells. MKN-28 cells showed more consistent results than MKN-45 cells, which indicates that MKN-28 cells are more suitable for the analysis of H. pylori adhesion than MKN-45 cells.
FIG. 2.
Effect of rebamipide on MKN-28 cells. MKN-28 cells were treated with 100 μg of rebamipide per ml (•) or BSA (○) for 30 to 120 min. The amount of adherent H. pylori is expressed as the percentage of the amount of H. pylori adhering to untreated target cells. Each value represents the mean ± SD for 10 strains tested in this study. The difference between rebamipide and BSA was evaluated by two-tailed paired Student’s t test. ∗, P < 0.01.
FIG. 3.
Effect of rebamipide on MKN-28 cells. MKN-28 cells were treated with 25 to 100 μg of rebamipide per ml (•) or BSA (○) for 90 min. The amount of adherent H. pylori is expressed as the percentage of the amount of H. pylori adhering to untreated target cells. Each value represents the mean ± SD for 10 strains tested in this study. The difference between rebamipide and BSA was evaluated by two-tailed paired Student’s t test. ∗, P < 0.01.
FIG. 4.
Effect of rebamipide on MKN-45 cells. MKN-45 cells were treated with 25 to 100 μg of rebamipide per ml (•) or BSA (○) for 90 min. The amount of adherent H. pylori is expressed as the percentage of the amount of H. pylori adhering to untreated target cells. Each value represents the mean ± SD for 10 strains tested in this study. The difference between rebamipide and BSA was evaluated by two-tailed paired Student’s t test. ∗, P < 0.01.
On the other hand, BSA at the same concentrations did not inhibit H. pylori adhesion. The viabilities of MKN-28 and MKN-45 cells were not affected by the treatment with rebamipide (Table 1).
TABLE 1.
Effect of rebamipide on MKN-28 and MKN-45 cells and H. pyloria
| Rebamipide concn (μg/ml) | Viability of MKN-28 cells (OD450) | Viability of MKN-45 cells (OD450) | Adhesion activity of H. pylori (% adherent H. pylori) | Viability of H. pylori (CFU/ml [109]) |
|---|---|---|---|---|
| 0 | 1.59 ± 0.03 | 1.51 ± 0.08 | 100.00 ± 0.00 | 1.00 ± 0.03 |
| 25 | 1.60 ± 0.04 | 1.51 ± 0.07 | 93.95 ± 6.57 | 1.01 ± 0.04 |
| 50 | 1.60 ± 0.06 | 1.52 ± 0.06 | 93.75 ± 6.81 | 1.02 ± 0.05 |
| 75 | 1.61 ± 0.06 | 1.53 ± 0.06 | 92.45 ± 6.62 | 1.03 ± 0.07 |
| 100 | 1.61 ± 0.08 | 1.53 ± 0.07 | 91.96 ± 6.06 | 1.04 ± 0.08 |
MKN-28 and MKN-45 cells and H. pylori were treated with 25 to 100 μg of rebamipide per ml for 90 min. The viabilities of MKN-28 and MKN-45 cells are expressed as the OD at 450 nm (OD450). The viability of H. pylori is expressed as CFU. The adhesion activity of H. pylori is expressed as the amount of adherent H. pylori. Each value represents the mean ± SD.
Effect of rebamipide on H. pylori.
The adhesion activity of H. pylori to MKN-28 cells was not affected by the pretreatment of H. pylori with rebamipide (Table 1). The viability of H. pylori was not affected by the treatment with rebamipide (Table 1). The MICs of rebamipide for all H. pylori strains tested in this study were >1,600 μg/ml, indicating that rebamipide has no activity against H. pylori in vitro.
Effect of rebamipide on E. coli adhesion to MKN-28 cells.
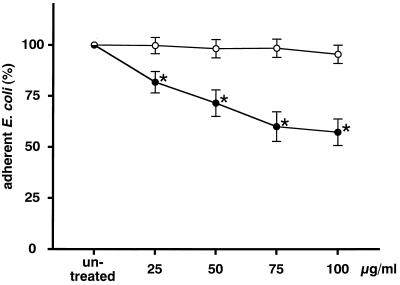
The results for E. coli were similar to those for H. pylori. The amount of E. coli adhering to MKN-28 cells was reduced by the pretreatment of MKN-28 cells with rebamipide (Fig. 5) and was dependent on the dose of rebamipide (r = −0.971; P < 0.05 by Spearman’s rank correlation).
FIG. 5.
Effect of rebamipide on E. coli adhesion to MKN-28 cells. MKN-28 cells were treated with 25 to 100 μg of rebamipide per ml (•) or BSA (○) for 90 min. The amount of adherent E. coli is expressed as the percentage of the amount of E. coli adhering to untreated target cells. Each value represents the mean ± SD for 10 strains tested in this study. The difference between rebamipide and BSA was evaluated by two-tailed paired Student’s t test. ∗, P < 0.01.
Binding of rebamipide to MKN-28 cells.
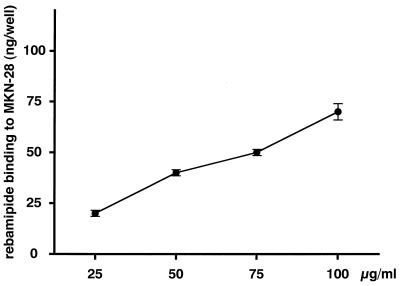
In order to examine the direct binding of rebamipide to target cells, a binding assay was carried out. The amount of rebamipide that bound to MKN-28 cells increased in a dose-dependent manner (r = 0.992; P < 0.01 by Spearman’s rank correlation) (Fig. 6).
FIG. 6.
Binding of rebamipide to MKN-28 cells. MKN-28 cells were treated with 25 to 100 μg of 14C-labeled rebamipide per ml for 60 min. Values are expressed as the amount of rebamipide bound to MKN-28 cells per well. Each value represents the mean ± SD.
Effects of related compounds.
The effects of rebamipide-related compounds on H. pylori adhesion to MKN-28 cells are indicated in Table 2. The compounds, each of which has a p-chlorophenyl group, reduced the level of adhesion. On the other hand, the compounds which do not have this group did not.
TABLE 2.
Effects of related compoundsa
| Compound | % Adherent H. pylori | Difference (P) between each compound and BSA | Presence of p-chlorophenyl group |
|---|---|---|---|
| OPC-12763 | 52.56 ± 8.45 | <0.01 | + |
| OPC-12804 | 51.46 ± 7.90 | <0.01 | + |
| OPC-12823 | 54.11 ± 5.98 | <0.01 | + |
| OPC-12853 | 53.87 ± 8.11 | <0.01 | + |
| OPC-12924 | 53.78 ± 6.79 | <0.01 | + |
| OPC-12963 | 55.22 ± 6.93 | <0.01 | + |
| OPC-12994 | 54.16 ± 7.13 | <0.01 | + |
| OPC-22016 | 54.96 ± 8.04 | <0.01 | + |
| OPC-12758 | 90.57 ± 13.70 | NS | − |
| OPC-12822 | 88.31 ± 14.88 | NS | − |
| DM-1212 | 91.09 ± 15.02 | NS | − |
| Rebamipide | 52.81 ± 7.80 | <0.01 | + |
MKN-28 cells were treated with 100 μg of one of the rebamipide-related compounds per ml for 90 min. The amount of adherent H. pylori is expressed as the percentage of the amount of H. pylori adhering to untreated target cells. Each value represents the mean ± SD for the 10 strains tested in this study. The difference between each compound and BSA was evaluated by two-tailed paired Student’s t-test. NS, not significant.
DISCUSSION
In this study, MKN-28 and MKN-45 cells, derived from human gastric carcinomas (11), were used as target cells. The manners of adhesion may differ in these cells and normal human gastric mucosal cells. However, normal human gastric mucosal cells are not available for laboratory adhesion assays and such cells, obtained from biopsied or surgical specimens, may show heterogeneous characteristics. Therefore, we adopted these cell lines as target cells to obtain reproducible results.
The adhesion of H. pylori to MKN-28 and MKN-45 cells was significantly inhibited by the pretreatment of these cells with 100 μg of rebamipide per ml for 90 min compared with the level of adhesion inhibition for the controls. This concentration can be achieved in the gastric mucous layer with the recommended clinical dose of rebamipide (13). Furthermore, rebamipide did not affect the viabilities of MKN-28 and MKN-45 cells at this concentration. These results suggest that rebamipide can inhibit H. pylori adhesion to gastric epithelial cells without affecting the viability of the cells. On the other hand, H. pylori adhesion to MKN-28 cells was not affected by the pretreatment of H. pylori with the same concentration of rebamipide. This indicates that rebamipide directly affects the gastric epithelial cells and does not act on H. pylori.
Rebamipide bound to MKN-28 cells and reduced the level of H. pylori adhesion in a dose-dependent manner. These results suggest that target molecules of rebamipide exist on or within MKN-28 cells. These target molecules could be responsible for the adhesion of H. pylori to MKN-28 cells. Several adhesins of H. pylori have been identified (1, 2, 9, 10). One possible mechanism is that rebamipide has some structural similarity to those adhesins and competitively inhibits H. pylori adhesion. To investigate the precise antiadhesion mechanism of rebamipide, the target molecules must be identified. On the other hand, rebamipide and related compounds which showed antiadhesion activities had the p-chlorophenyl group, without exception. Thus, this group may play an important role in the antiadhesion mechanism of rebamipide.
However, rebamipide did not completely inhibit H. pylori adhesion. The adhesion of H. pylori to gastric epithelial cells will be due to some combinations of H. pylori adhesins and their receptors. Rebamipide may only partially inhibit the combinations. On the other hand, rebamipide also inhibited E. coli adhesion to MKN-28 cells, which indicates that the antiadhesion effect of rebamipide is not specific for H. pylori. The target molecules of rebamipide may be common receptors for bacterial adhesion to alimentary tract epithelial cells.
Rebamipide itself did not directly affect the viability of H. pylori in vitro. However, our studies suggest that rebamipide has potential as an agent for the prevention of H. pylori adhesion. Furthermore, from preliminary data, triple therapy with omeprazole, amoxicillin, and rebamipide combined showed a strong eradication effect in a human clinical trial (14). In the gastric mucosa, H. pylori localizes on the surface of the epithelial cells as well as in the mucous layer (16). H. pylori colonized on epithelial cells can induce mucosal injury by direct and indirect mechanisms (17). On the other hand, H. pylori in the mucous layer can survive after insufficient eradication therapy, and the organisms adhere again to gastric epithelial cells and recolonize. Thus, the antiadhesion effect of rebamipide can contribute to the prevention of H. pylori recolonization, provided that the clinical dose of rebamipide completely overlays the surface of the gastric epithelial cells. As a result, prolonged use of rebamipide combined with antibiotics may enhance the eradication rate (14).
In conclusion, rebamipide may have potential as a new therapeutic agent against H. pylori infection.
ACKNOWLEDGMENTS
This study was supported in part by a Grant-in-Aid for Scientific Research (grant 09770188) from the Japanese Ministry of Education, Science, Sports and Culture, a grant (grant 8-14) from the Japanese Ministry of Health and Welfare, and a grant from the Sapporo Medical University Foundation.
REFERENCES
- 1.Evans D G, Evans D J, Jr, Moulds J J, Graham D Y. N-Acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988;56:2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans D G, Karjalainen T K, Evans D J, Jr, Graham D Y, Lee C H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175:674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman D the Eurogast Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 4.Graham D Y. Helicobacter pylori: its epidemiology and its role in duodenal ulcer disease. J Gastroenterol Hepatol. 1991;6:105–113. doi: 10.1111/j.1440-1746.1991.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 5.Han B G, Kim H S, Rhee K H, Han H S, Chung M H. Effects of rebamipide on gastric cell damage by Helicobacter pylori-stimulated human neutrophils. Pharmacol Res. 1995;32:201–207. doi: 10.1016/s1043-6618(05)80023-4. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi S, Sugiyama T, Yachi A, Yokota K, Hirai Y, Oguma K, Fujii N. A rapid and simple method to quantify Helicobacter pylori adhesion to human gastric MKN-28 cells. J Gastroenterol Hepatol. 1997;12:373–375. doi: 10.1111/j.1440-1746.1997.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 7.Ishiyama M, Shiga M, Sasamoto K, Mizoguchi M, He P. A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem Pharm Bull. 1993;41:1118–1122. [Google Scholar]
- 8.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K, Watanabe M. Novel cell proliferation and cytotoxicity assays using a tetrazolium salt that produces a water-soluble formazan dye. In Vitro Toxicol. 1995;8:187–190. [Google Scholar]
- 9.Lingwood C A, Huesca M, Kuksis A. The glycerolipid receptor for Helicobacter pylori (and exoenzyme S) is phosphatidylethanolamine. Infect Immun. 1992;60:2470–2474. doi: 10.1128/iai.60.6.2470-2474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lingwood C A, Wasfy G, Han H, Huesca M. Receptor affinity purification of a lipid-binding adhesin from Helicobacter pylori. Infect Immun. 1993;61:2474–2478. doi: 10.1128/iai.61.6.2474-2478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motoyama T, Hojo H, Watanabe H. Comparison of seven cell lines derived from human gastric carcinomas. Acta Pathol Jpn. 1986;36:65–83. doi: 10.1111/j.1440-1827.1986.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 12.Naito Y, Yoshikawa T, Tanigawa T, Sakurai K, Yamasaki K, Uchida M, Kondo M. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study. Free Radic Biol Med. 1995;18:117–123. doi: 10.1016/0891-5849(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 13.Naito Y, Yoshikawa T, Iinuma S, Miyazaki R, Yagi N, Yoshida N, Osumi T, Hirao Y, Kondo M. Local gastric and serum concentrations of rebamipide following oral administration to patients with chronic gastritis. Arzneimittelforschung. 1996;46:698–700. [PubMed] [Google Scholar]
- 14.Nebiki H, Arakawa T, Kioka K, So K, Okawa K, Oka H, Yamada H, Harihara S, Ando K, Uchida T, Ito H, Higuchi K, Kobayashi K. Increase in the rate of cure of Helicobacter pylori infection by addition of rebamipide to omeprazole plus amoxicillin. Gastroenterology. 1997;112:A232. [Google Scholar]
- 15.Queiroz D M M, Mendes E N, Rocha G A. Indicator medium for isolation of Campylobacter pylori. J Clin Microbiol. 1987;25:2378–2379. doi: 10.1128/jcm.25.12.2378-2379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu T, Akamatsu T, Ota H, Katsuyama T. Immunohistochemical detection of Helicobacter pylori in the surface mucous gel layer and its clinicopathological significance. Helicobacter. 1996;1:197–206. doi: 10.1111/j.1523-5378.1996.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 17.Smoot D T, Resau J H, Earlington M H, Simpson M, Cover T L. Effects of Helicobacter pylori vacuolating cytotoxin on primary cultures of human gastric epithelial cells. Gut. 1996;39:795–799. doi: 10.1136/gut.39.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki M, Miura S, Mori M, Kai A, Suzuki H, Fukumura D, Suematsu M, Tsuchiya M. Rebamipide, a novel antiulcer agent, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994;35:1375–1378. doi: 10.1136/gut.35.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida M, Tabusa F, Komatsu M, Morita S, Kanbe T, Nakagawa K. Studies on 2(1H)-quinolinone derivatives as gastric antiulcer active agents. 2-(4-Chlorobenzoylamino)-3-[2(1H)-quinolinon-4-yl]propionic acid and related compounds. Chem Pharm Bull. 1985;33:3775–3786. doi: 10.1248/cpb.33.3775. [DOI] [PubMed] [Google Scholar]
- 20.Warren J R, Marshall B J. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 21.Wyatt J I, Dixon M F. Chronic gastritis—a pathogenic approach. J Pathol. 1988;154:113–124. doi: 10.1002/path.1711540203. [DOI] [PubMed] [Google Scholar]