Highlights
-
•
First report of a secondary somatic glioblastoma arising from MCT-MT in a patient with underlying Li-Fraumeni syndrome.
-
•
The rarity of glioblastoma arising from MCT-MT warrants investigation for underlying genetic predisposition.
-
•
Glioblastomas arising from MCT-MT appear to exhibit wild type IDH gene status.
-
•
Advanced-stage glioblastoma arising from MCT-MT exhibits aggressive behavior and requires adjuvant therapy.
-
•
Optimal adjuvant therapy regimen for glioblastoma arising from MCT-MT remains unknown.
Keywords: Secondary somatic glioblastoma, Mature ovarian cystic teratoma, Li-Fraumeni Syndrome, TP53, IDH, Advanced-stage treatment
1. Introduction
Mature cystic teratomas (MCTs) are the most common type of benign ovarian germ cell tumor (Gershenson et al., 2019). Malignant transformation (MT) of MCT (i.e. secondary somatic malignancy) occurs in approximately 2.4 % of cases, with the majority presenting as squamous cell carcinomas (Sakuma et al., 2010). Though extremely rare, MCT-MTs may also consist of neuroectodermal-type tumors, including glioblastoma (Bjersing et al., 1989, Den Boon et al., 1999, Liang et al., 2016). Data surrounding their presentation, behavior, and response to chemotherapy are very limited. Here, we present a case of aggressive glioblastoma arising from a mature cystic teratoma in an 18-year-old patient with underlying Li-Fraumeni syndrome.
2. Case presentation
An 18-year-old woman, gravida 0, presented to an outpatient gynecology visit with a one-month history of abdominal pain and increasing abdominal girth. Her past medical and surgical histories were unremarkable. Family history was notable for multiple primary malignancies diagnosed before age 40, including a father with lung cancer (non-smoker), a paternal aunt with bilateral breast cancer (BRCA negative) and liver sarcoma, and a paternal great uncle with melanoma. Pelvic ultrasonography demonstrated a 26.2 cm cystic right adnexal mass with peripheral vascularity. Tumor markers including CEA, CA 19–9, AFP, HCG, LDH, inhibin A, inhibin B, and total testosterone were within normal limits. Her CA-125 level was mildly elevated at 69 U/mL with a reference range of < 35 U/mL. Given these findings and her symptoms, she underwent laparoscopic right ovarian cystectomy which was complicated by surgical spill of cyst contents. Intraoperative evaluation of the cyst appeared benign on frozen section.
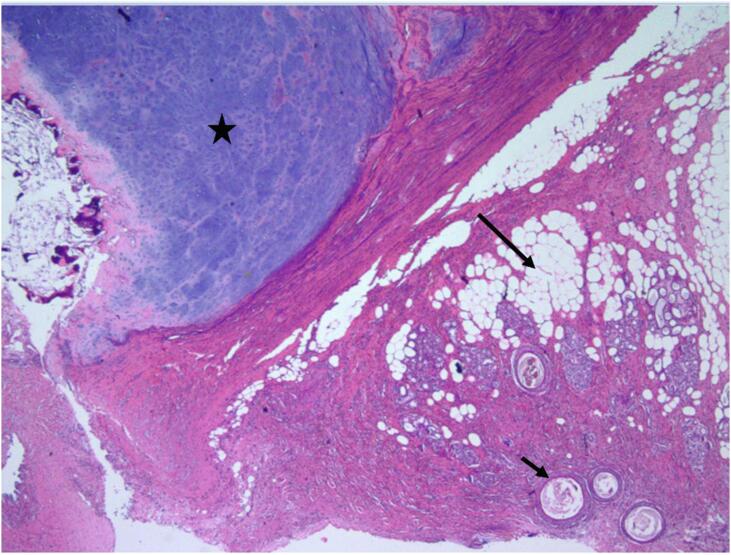
Permanent histologic sectioning of the ovarian cyst wall revealed areas of mature cartilage, adipose tissue and skin adnexa consistent with a mature teratoma (Fig. 1).
Fig. 1.
Mature ovarian teratoma component showing adipose tissue (long arrow), cartilage (star), and skin adnexa (short arrow) (Hematoxylin & eosin, 4x).
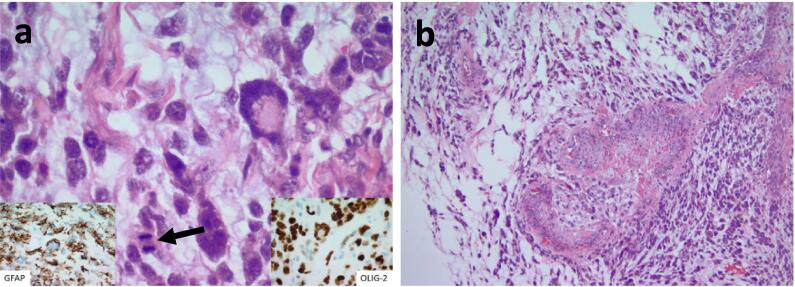
It also demonstrated a high-grade malignant component composed of discohesive, pleomorphic cells with atypical mitosis (Fig. 2a) and microvascular proliferation (Fig. 2b) set within a glial background. Immunohistochemical (IHC) stains of the specimen confirmed positivity of GFAP and OLIG-2, in addition to focal positivity for S-100. IHC was negative for SALL4, desmin, synaptophysin, chromogranin, pankeratin, CK7, CK20, EMA, Melan-A, Glypican-3, and TTF-1. Normal retained nuclear staining was noted for BRG1 (SMARCA4) and INI1. There was no evidence of immature teratoma elements within the specimen. Expert pathologic review performed independently at two high volume centers confirmed a diagnosis of neuroectodermal-type malignancy (glioblastoma) arising in a background of mature ovarian teratoma.
Fig. 2.
A) markedly pleomorphic tumor cells with mitotic figure (arrow) with positivity for gfap (left inlet) and olig-2 (right inlet). b) vascular proliferation with malignant tumor cells in the background.
In the setting of this diagnosis and the patient’s family history of cancer, Medical Genetics was consulted and germline next-generation sequencing (NGS) was performed. Results identified a germline deleterious mutation in the TP53 gene, c.329G > C, confirming a diagnosis of Li-Fraumeni syndrome, as well as a variant of uncertain significance (VUS) in the MLH1 gene, c.1775G > C. The patient was referred to Reproductive Endocrinology and Infertility and decided not to pursue fertility preservation prior to treatment.
Given the patient’s final pathological findings, brain MRI and CT of the chest, abdomen, and pelvis were performed and found no evidence of metastatic disease. The decision was made to proceed with a staging procedure and possible tumor debulking. At time of exploratory laparotomy, approximately one month following her negative imaging, peritoneal entry was notable for moderate ascites and diffuse intraperitoneal plaque-like disease. Frozen pathology was positive for malignancy. Primary cytoreductive surgery was performed requiring hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node debulking, appendectomy, rectosigmoid resection with re-anastomosis, near complete pelvic peritonectomy, and resection of an umbilical nodule at the site of prior laparoscopic entry. No gross residual disease was present at the conclusion of the procedure. Final pathology confirmed stage IVB (due to positive umbilical nodule) secondary neuroectodermal-type malignancy (glioblastoma) arising in a mature teratoma of the ovary. All metastatic elements demonstrated malignant neuroectodermal elements with no teratoma component. The patient recovered well and was discharged on postoperative day six in stable condition.
Based on consensus between gynecologic oncology, neuro-oncology, and interdisciplinary pathology, combination systemic therapy with carboplatin, etoposide, and atezolizumab was initiated. Somatic NGS was also performed to assess for therapeutic targets within the tumor. Results identified low tumor mutational burden (1 mut/Mb), microsatellite stability, high loss of heterozygosity (27 %), ROS-1 pathogenic fusion, positive expression of programmed death-ligand 1 (PD-L1), and negative expression of estrogen receptor (ER). Given this tumor profile, the patient was referred to the TAPUR trial for ectrectinib or crizotinib given her ROS-1 pathogenic fusion, or atezolizumab with talazoparib given her high loss of heterozygosity. (American Society of Clinical Oncology, 2019) The patient’s treatment course (one cycle of carboplatin, etoposide, and atezolizumab) was complicated by multiple readmissions for symptomatic ascites and uncontrolled pain with rapid progression of disease, however, and she elected for comfort care prior to receiving TAPUR trial therapy. She passed away shortly thereafter, with an overall survival of 2 months following initiation of treatment.
3. Discussion
Malignant transformation of a mature ovarian teratoma into a primary neuroectodermal tumor is exceptionally rare. Including cases that do not specify the maturity or immaturity of associated ovarian teratomas, approximately 10 reports of MCT-MT to primary ovarian glioblastoma have been described to date (Bjersing et al., 1989, Yadav et al., 1999, Shirley et al., 1971, Kleinman et al., 1993). These studies suggest the average age at presentation is approximately 19 years and ranges from ages 9 – 41. Most common presenting symptoms include abdominal pain and sensation of an abdominal or pelvic mass. Cancer stage of IA at diagnosis is most common, though few case reports discuss cancer stage. In our case, the patient was 18 years old at diagnosis and presented with abdominal pain and increasing abdominal girth. She was diagnosed with stage IVB disease at the time of her staging laparotomy. However, given no intraoperative findings of peritoneal involvement at the time of ovarian cystectomy, she had at least stage IC1 disease at the time of her primary surgery.
As highlighted by den Boon et al., the rarity of glioblastoma arising in a mature ovarian teratoma warrants investigation for underlying genetic predisposition. (Den Boon et al., 1999) Thorough intake of patient family history serves as an important first-line measure for assessing such inherited risk. Our patient had an extensive family history of cancer, including BRCA negative breast cancer and soft tissue sarcoma. She met Chompret Criteria for Li-Fraumeni Syndrome (LFS) genetic testing and was found to harbor a pathologic germline TP53 mutation. (Tinat et al., 2009) To our knowledge, this is the first case to identify secondary somatic glioblastoma of the ovary in a patient with LFS. Frequency of any germline mutation in this patient population is unknown, as germline testing was not reported in previously published cases. Consultation to Genetics should be considered in these patients and may help identify genes which can be targeted with therapy.
The molecular signature of secondary glioblastoma arising from mature cystic teratoma of the ovary is not well understood. While the morphology of these tumors resembles that of IDH-wild type central nervous system (CNS) tumors, for example, it is uncertain if the lack of an IDH mutation is characteristic of all ovarian glioblastomas. (WHO Classification of Tumours Editorial Board, 2021) In our case, IHC for IDH1(R132H) was negative and NGS did not find evidence of IDH1 or IDH2 mutation. This is consistent with a prior study performed by Liang and colleagues. (Liang et al., 2016) Among four cases of high-grade ovarian gliomas exhibiting vascular proliferation resembling glioblastoma, three were evaluated for IDH1 and/or IDH2 mutations. One of four cases arose from a mature teratoma, while the remainder were associated with immature teratomas. They identified NGS-determined negativity for IDH1 or IDH2 mutations in two samples and immunofluorescence-determined negativity for IDH1 mutations in the third. Though TP53 mutations have not previously been described in these cases, ours is particularly interesting as TP53 mutations are characteristic of primary brain glioblastoma. Regarding MLH1, we detected a VUS and are limited in reporting its presence within the MCT-MT ovarian glioblastoma molecular signature. Sample size remains low and additional cases will be extremely helpful in defining the molecular signature or drivers of this rare gynecologic tumor.
Unlike treatment of glioblastoma in the brain, first-line therapy for ovarian glioblastoma includes cytoreductive surgery. Surgery alone may even prove curative in cases of low-stage cancers. (Shirley et al., 1971) Very little data exist to drive treatment selection in advanced-stage cases. Three cases describe the use of chemotherapy and radiation, for example, but do not clarify drug names, drug doses, or treatment schedules (Yadav et al., 1999, Shirley et al., 1971, Kleinman et al., 1993). The most contemporary study from Liang et al. report treatment and follow up data for 3 patients with high-grade ovarian gliomas, all of whom underwent unilateral salpingo-oophorectomy for early-stage disease. One patient received adjuvant bleomycin, etoposide, and cisplatin and had no evidence of disease at 18 months. The other two patients were observed postoperatively and had no evidence of disease at 5 and 8 months. The decision to use combination carboplatin, etoposide, and atezolizumab in our case was driven by expert opinion that the tumor’s behavior and response to chemotherapy may more closely resemble that of a neuroepithelial tumor (Murdock et al., 2018, Horn et al., 2018). Regardless of adjuvant therapy course, overall survival of advanced-stage ovarian glioblastoma remains poor. This may simply reflect the aggressive nature of these cancers. It may also suggest they demonstrate a response to chemotherapy which is distinct from their cerebral counterparts.
Of the clinicopathologic studies with available treatment and follow up information, most patients had early-stage disease and received no adjuvant therapy (Table 1). In our case report, surgical spill of cyst contents occurred at initial surgery and peritoneal carcinomatosis was noted at the time staging laparotomy. This highlights the aggressive nature of this histology. Furthermore, in the limited patient reports available, long disease-free intervals are observed for patients with ovarian confined disease receiving no additional therapy. This similarly stresses the importance of avoiding intraoperative rupture within the peritoneal cavity, as cases with later stage disease have poorer prognosis.
Table 1.
Treatment and follow up data for case series reporting glioblastoma arising from a mature ovarian teratoma. NA – not reported, NOS – not otherwise specified, RSO – right salpingo-oophorectomy, LSO – left salpingo-oophorectomy, LO – left oophorectomy, TAH-BSO – total abdominal hysterectomy bilateral salpingo-oophorectomy, NED – no evidence of disease, AWD – alive with disease, DOD – dead of disease.
| Year | Author | Age | Stage | Surgery | Adjuvant therapy | Follow-up |
|---|---|---|---|---|---|---|
| 1989 | Bjersing et al (Bjersing et al., 1989) | 34 | NA | Conservative surgery, NOS | None | NED, 3 years |
| 1993 | Kleiman et al (Kleinman et al., 1993) | 6 | IA | RSO | None | DOD, 2 years |
| 17 | IA | LSO | None | NED, 4 years | ||
| 15 | IA | LO | None | NED, 3 years |
||
| 16 | IIA | TAH-BSO | None | DOD, 5 years |
||
| 15 | IIA | RSO, LO | None | AWD, 1 year |
||
| 22 | III | RO | Chemotherapy, Radiotherapy, NOS |
DOD, 4 months | ||
| 2016 | Liang et al (Liang et al., 2016) | 24 | NA | RSO | None | NED, 5 months |
| 2023 | Bussies et al * | 18 | IVB | TAH-BSO, debulking | Carboplatin, etoposide, atezolizumab × 1 cycle | DOD, 2 months |
4. Conclusions
Glioblastoma arising in a mature ovarian teratoma is an exceptionally rare event. It presents most commonly in young women who complain of abdominal pain and mass effect. Though treatment of early-stage cases may be treated with cytoreductive surgery alone, advanced-stage cancers demonstrate aggressive behavior and require additional therapy. Optimal adjuvant treatment regimen remains unknown, and response to treatment may differ from glioblastoma of the brain. Our case provides invaluable molecular data which may facilitate future treatment selection. It is also the first to identify secondary somatic glioblastoma of the ovary in a patient with Li-Fraumeni Syndrome, underscoring the importance of evaluating for an underlying genetic predisposition in this patient population.
5. Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Parker L. Bussies, Email: bussiep@ccf.org.
Lindsey Beffa, Email: beffal2@ccf.org.
References
- American Society of Clinical Oncology. (2019). TAPUR: testing the use of Food and Drug Administration (FDA) approved drugs that target a specific abnormality in a tumor gene in people with advanced stage cancer. NCT02693535.
- Bjersing L., Cajander S., Rogo K., Ottosson U.B., Stendahl U. Glioblastoma multiform in a dermoid cyst of the ovary. Eur. J. Gynaecol. Oncol. 1989;10(6):389–392. [PubMed] [Google Scholar]
- Den Boon J., Van Dijk C.M., Helfferich M., Peterse H.L. Glioblastoma multiforme in a dermoid cyst of the ovary. A case report. Eur. J. Gynaecol. Oncol. 1999;20(3):187–188. [PubMed] [Google Scholar]
- Gershenson, D. M., Pappo, A. S., & Garcia, R. L. (2019). Ovarian germ cell tumors: Pathology, epidemiology, clinical manifestations, and diagnosis. Uptodate R.
- Horn L., Mansfield A.S., Szczęsna A., Havel L., Krzakowski M., Hochmair M.J., Liu S.V. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- Kleinman G.M., Young R.H., Scully R.E. Primary neuroectodermal tumors of the ovary. A report of 25 cases. Am. J. Surg. Pathol. 1993;17(8):764–778. doi: 10.1097/00000478-199308000-00002. [DOI] [PubMed] [Google Scholar]
- Liang L., Olar A., Niu N., Jiang Y., Cheng W., Bian X.W., Liu J. Primary glial and neuronal tumors of the ovary or peritoneum: a clinicopathologic study of 11 cases. Am. J. Surg. Pathol. 2016;40(6):847. doi: 10.1097/PAS.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock T., Orr B., Allen S., Ibrahim J., Sharma R., Ronnett B.M., Rodriguez F.J. Central Nervous System-type Neuroepithelial Tumors and Tumor-like Proliferations Developing in the Gynecologic Tract and Pelvis. Am. J. Surg. Pathol. 2018;42(11):1429–1444. doi: 10.1097/PAS.0000000000001131. [DOI] [PubMed] [Google Scholar]
- Sakuma M., Otsuki T., Yoshinaga K., Utsunomiya H., Nagase S., Takano T., Yaegashi N. Malignant transformation arising from mature cystic teratoma of the ovary: a retrospective study of 20 cases. Int. J. Gynecol. Cancer. 2010;20(5) doi: 10.1111/igc.0b013e3181daaf1d. [DOI] [PubMed] [Google Scholar]
- Shirley R.L., Piro A.J., Crocker D.W. Malignant Neural Elements in a Benign Cystic Teratoma. A Case Report. Obst. Gynecol. 1971;37(3):402–407. [PubMed] [Google Scholar]
- Tinat J., Bougeard G., Baert-Desurmont S., Vasseur S., Martin C., Bouvignies E., Frébourg T. 2009 version of the Chompret criteria for Li Fraumeni syndrome. J. Clin. Oncol. 2009;27(26):e108–e109. doi: 10.1200/JCO.2009.22.7967. [DOI] [PubMed] [Google Scholar]
- WHO Classification of Tumours Editorial Board . 5th ed. International Agency for Research on Cancer; Lyon: 2021. World Health Organization Classification of Tumours of the Central Nervous System. [Google Scholar]
- Yadav A., Lellouch-Tubiana A., Fournet J.C., Quazza J.E., Kalifa C., Sainte-Rose C., Jaubert F. Glioblastoma multiforme in a mature ovarian teratoma with recurring brain tumours. Histopathology. 1999;35(2):170–173. doi: 10.1046/j.1365-2559.1999.00695.x. [DOI] [PubMed] [Google Scholar]