Abstract
Purpose
Atopic dermatitis (AD) is a chronic inflammatory skin disorder associated with various comorbidities. However, inconsistent results on the risk of myocardial infarction (MI) and mortality have been reported in patients with AD. This study was aimed to evaluate the risk of MI and all-cause mortality in patients with AD.
Methods
This nationwide population-based retrospective cohort study enrolled 56,205 adults ≥ 20 years of age with AD and 3,825,609 controls without AD from the Korean National Health Service (NHIS) database from 2009 to 2016.
Results
The risk of MI (adjusted hazard ratio [aHR], 1.111, 95% confidence interval [CI], 1.050–1.176) was increased in patients with AD. By AD severity, patients with moderate-to-severe AD had a higher risk of MI (aHR, 1.163, 95% CI, 1.080–1.251) than individuals without AD. The risk of all-cause mortality was only increased for patients with moderate-to-severe AD (aHR, 1.096, 95% CI, 1.040–1.155) compared to individuals without AD. In subgroup analysis, an increased risk of MI was observed in female, non-obese, non-smoking, non-diabetic, and non-dyslipidemic patients with moderate-to-severe AD compared to individuals without AD. An increased risk of all-cause mortality was observed in patients with moderate-to-severe AD compared to non-AD controls among individuals ≥60 years of age and non-smokers.
Conclusions
The risk of MI and all-cause death was increased in patients with moderate-to-severe AD. Even without well-known risk factors for MI and mortality, patients with AD require the proper management and screening for comorbidities to prevent MI and decrease all-cause mortality.
Keywords: Atopic dermatitis, death, mortality, myocardial infarction, adults, female, risk
INTRODUCTION
Atopic dermatitis (AD) is a common chronic relapsing inflammatory dermatosis with an estimated prevalence of up to 10%.1,2 The age distribution of AD shows a bimodal peak, with the first one in early childhood and the second one in middle-aged and older individuals.2 Recently, the systemic nature of AD has attracted interest, focusing on the comorbidities of patients with AD.
Evidence has proven an association between AD and a variety of comorbidities, including asthma, hay fever, food allergies, anxiety, depression, suicidality, obesity, and cardiovascular disease.3 Recently, chronic inflammation in several skin diseases was associated with cardiovascular events.4,5 Therefore, it is necessary to determine the association between AD and cardiovascular disease. Among various cardiovascular disorders, myocardial infarction (MI) is a common cardiogenic emergency characterized by the necrosis of the myocardium due to the cessation of blood supply and is closely associated with significant mortality.6 A recent study by Thyssen et al. 7 reported an increased risk of mortality in patients with AD due to cardiovascular disease compared to controls. Therefore, identifying risk factors and preventing MI and mortality is very important in patients with AD to reduce the disease burden.
Previous studies on the association between AD and MI reported conflicting results. Some studies did not find a significant association between AD and MI.8,9 A meta-analysis using studies from North America and Europe found no significant association between AD and MI.10 However, a study by Silverberg et al. 11 found significantly increased odds of cardiovascular disease, heart attack, and stroke in adult patients with AD. A study by Jung et al. 12 also found an increased risk of MI in patients with AD. We hypothesized that systemic inflammation in AD, like psoriasis, may increase cardiovascular risk, including MI and all-cause mortality. We also hypothesized that patients with more severe AD may have a higher risk of MI or all-cause mortality because of the intense inflammatory responses in severe AD.
To date, data on the impact of AD on MI are sparse and inconsistent. Therefore, we aimed to identify the incidence and impact of AD on the risk of MI using a large-nationwide Korean registry. Furthermore, as MI is associated with subsequent mortality, this study intended to identify the incidence and risk of all-cause mortality in patients with AD.
MATERIALS AND METHODS
Study design, setting, and participants
A nationwide population-based cohort study using data from the National Health Insurance Service (NHIS), a government-operated mandatory social health insurance program containing health information on approximately 50 million South Koreans, was conducted.13,14
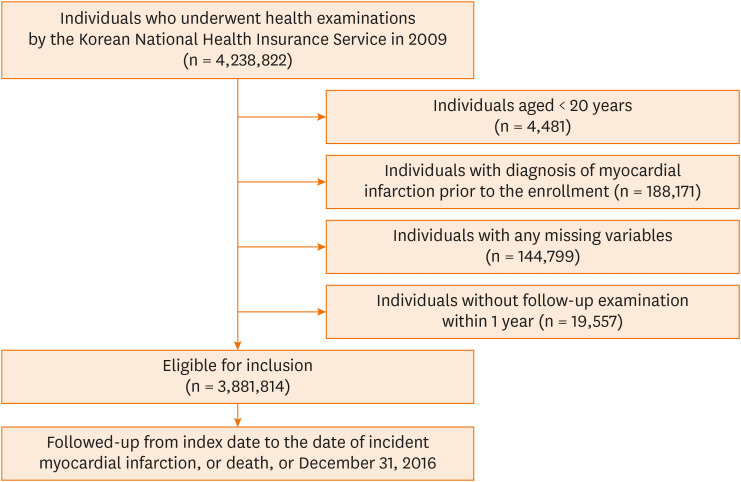
A total of 4,238,822 individuals who underwent health examinations by the Korean NHIS between January 1 and December 31 of 2009 were enrolled. Individuals < 20 years of age (n = 4,481), those diagnosed with MI before enrollment (n = 188,171), and those with missing data (n = 144,799) were excluded. The index date was defined as the date of the initial health check-up. The immortal bias was reduced by considering the lag period in this study. Individuals without a follow-up examination within one year were also excluded (n = 19,557). Finally, a total of 3,881,814 individuals were followed up for newly diagnosed MI or all-cause mortality until December 31, 2016 (Fig. 1). The study protocol was approved by the Institutional Review Board (IRB) of The Catholic University of Korea (IRB approval number: KC21ZISI0965).
Fig. 1. Flowchart of the study.
Definition of AD
We defined AD using the International Classification of Diseases 10th revision (ICD-10) code (L209) for AD with more than 3 claims during the same year and with more than 3 prescription records for AD therapies, including topical or systemic medications or phototherapies for AD during the same year. Mild AD was defined by the ICD-10 code (L209) for AD and the prescription of topical corticosteroids or calcineurin inhibitors or ICD-10 code L209 with more than 3 claims during the same year. Moderate-to-severe AD was defined by ICD-10 code L209 and the prescription of systemic oral medications for AD or phototherapies with more than 12 claims during the same year.
Definition of MI and mortality
MI was defined by ICD-10 code I21 or I22 during hospitalizations or the presence of ICD-10 code I21 or I22 at least twice during the same year. The study population was followed up from baseline to the date of mortality or cardiovascular event or until December 31, 2015, whichever came first.
Definition of variables
Obesity was defined as a body mass index (BMI) of ≥25, which was calculated by dividing weight (kg) by height squared (m2). Smoking status, alcohol consumption, and physical activity were assessed using standardized self-reported questionnaires. Regular exercise was defined as a physical activity performed at least 5 times per week. Baseline comorbidities were assessed based on the combination of past medical history and ICD-10 codes with pharmacy and/or clinical values for each comorbidity. For example, the presence of hypertension was defined as a systolic/diastolic blood pressure of >140/90 mm Hg or at least one claim per year for an antihypertensive prescription under ICD-10 codes I10–I13 or I15. Dyslipidemia was defined as a total cholesterol level of >240 mg/dL or at least one claim per year for a prescription for a lipid-lowering agent under ICD-10 code E78. The presence of diabetes mellitus was defined as treatment with antidiabetic medication under ICD-10 code E10–E14 or a fasting glucose level of ≥126 mg/dL.
Statistical analysis
Data are presented as means ± standard deviation, geometric means (95% confidence interval [CI]), or percentages. The Student’s t-test for continuous variables or χ2 test for categorical variables was used to evaluate differences between the groups.15 The incidence of MI and mortality was calculated by dividing the total number of incident cases by the follow-up period (person-years) and is presented as the number of cases per 1,000 person-years. The difference in the cumulative incidence of MI and mortality based on the severity of AD was calculated by the log-rank test. The Cox proportional hazards regression analysis was performed to examine the association between risk factors and MI and mortality. To control for confounding factors, we used 2 models, according to the adjustment for age, gender, BMI, smoking, drinking, regular exercise, hypertension, diabetes mellitus, and dyslipidemia. A subgroup analysis and interaction testing by the likelihood ratio test were conducted to analyze the differences in the risk of MI and mortality. Individuals with missing data for a specific mandatory parameter were excluded. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and a 2-sided P value of <0.05 indicated statistical significance.
RESULTS
Baseline characteristics
The demographic characteristics of the individuals are summarized in Table 1. Among 3,881,814 individuals, 56,205 had AD, and 3,825,609 did not. The mean age of the AD group was 46.5 years, and the mean age of the non-AD controls was 46.4 years. The patients with AD were more likely to have cardiovascular risk factors, such as hypertension, diabetes mellitus, and dyslipidemia, than the non-AD controls. In contrast, individuals without AD were more likely to be current smokers or heavy drinkers. When the patients with AD were stratified according to severity, 22,183 (39.5%) AD patients were classified as having mild AD, and 34,022 (60.5%) AD patients were classified as having moderate-to-severe AD.
Table 1. Baseline characteristics of the study population.
| Characteristic | AD (n = 56,205) | Non-AD (n = 3,825,609) | P value | ||
|---|---|---|---|---|---|
| Mild (n = 22,183) | Moderate-to-severe (n = 34,022) | ||||
| Age (yr) | 46.4 ± 13.8 | 47.6 ± 15.0 | 45.8 ± 14.9 | < 0.0001 | |
| Sex | < 0.0001 | ||||
| Male | 10,020 (45.2) | 15,713 (46.2) | 2,119,850 (55.4) | ||
| Female | 12,163 (54.8) | 18,309 (53.8) | 1,705,759 (44.6) | ||
| Smoking | < 0.0001 | ||||
| Non-smoker | 14,825 (66.8) | 21,988 (64.6) | 2,252,363 (58.9) | ||
| Ex-smoker | 3,165 (14.3) | 4,532 (13.3) | 543,629 (14.21) | ||
| Current smoker | 4,193 (18.9) | 7,502 (22.1) | 1,029,617 (26.9) | ||
| Drinking status | < 0.0001 | ||||
| None | 12,831 (57.8) | 18,628 (54.8) | 1,931,797 (50.5) | ||
| Mild drinker | 8,051 (36.3) | 13,194 (38.78) | 1,583,332 (41.4) | ||
| Heavy drinker | 1,301 (5.8) | 2,200 (6.5) | 310,480 (8.1) | ||
| Regular exercise, yes | 4,149 (18.7) | 6,026 (17.7) | 690,327 (18.04) | 0.010 | |
| BMI ≥ 25 kg/m2, yes | 7,078 (31.9) | 10,256 (30.1) | 1,236,830 (32.3) | < 0.0001 | |
| Diabetes mellitus, yes | 2,284 (10.3) | 2,472 (7.3) | 309,041 (8.1) | < 0.0001 | |
| Hypertension, yes | 6,507 (29.3) | 8,344 (24.5) | 958,333 (25.1) | < 0.0001 | |
| Dyslipidemia, yes | 4,408 (19.9) | 6,232 (18.3) | 651,821 (17.0) | < 0.0001 | |
| SBP (mmHg) | 121.2 ± 14.9 | 120.9 ± 14.7 | 122.2 ± 15.0 | < 0.0001 | |
| DBP (mmHg) | 75.5 ± 10.0 | 75.4 ± 9.9 | 76.3 ± 10.0 | < 0.0001 | |
| Glucose (mg/dL) | 97.0 ± 24.3 | 95.2 ± 21.3 | 96.9 ± 23.6 | < 0.0001 | |
| Total cholesterol (mg/dL) | 194.0 ± 44.7 | 195.2 ± 41.3 | 195.4 ± 41.3 | < 0.0001 | |
| HDL cholesterol (mg/dL) | 57.7 ± 37.5 | 58.0 ± 33.5 | 56.6 ± 32.3 | < 0.0001 | |
| LDL cholesterol (mg/dL) | 112.8 ± 40.6 | 113.0 ± 37.1 | 113.6 ± 38.6 | 0.0003 | |
| TG (mg/dL) | 108.5 (107.7–109.4) | 108.6 (107.9–109.2) | 112.2 (112.2–112.3) | < 0.0001 | |
Values are means ± standard deviations, numbers (%) or geometric mean (95% confidence interval). The P-values were determined by the Student’s t-test for continuous variables or χ2 test for categorical variables to evaluate differences between the groups.
AD, atopic dermatitis; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Incidence and risk of MI in patients with AD
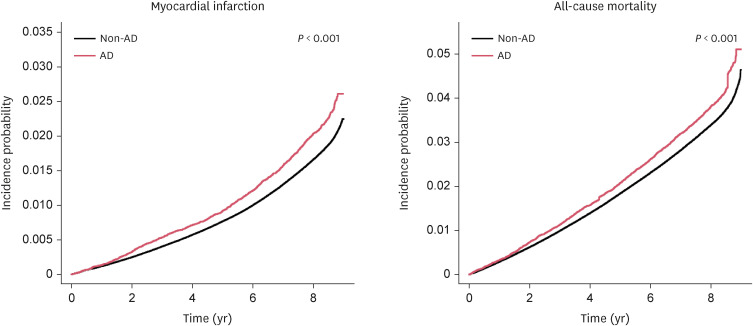
During a mean follow-up of 8.19 years, 69,696 individuals in the entire cohort experienced MI (1.8%). The cumulative incidence of MI was significantly higher in patients with AD than in non-AD controls (Fig. 2, P < 0.001), and the incidence of MI was higher in patients with AD than the non-AD controls (Table 2). The patients with AD showed an increased risk of MI compared to the non-AD controls (adjusted hazard ratio [aHR], 1.111, 95% CI, 1.050–1.176; Table 3). According to AD severity, both the patients with mild AD and moderate-to-severe AD had an increased risk of MI in the non-adjusted model (mild AD: hazard ratio [HR], 1.259, 95% CI, 1.153–1.375; moderate-to-severe AD: HR, 1.186, 95% CI, 1.102–1.277). When adjusted for age, sex, BMI, smoking, drinking, regular exercise, hypertension, diabetes mellitus, and dyslipidemia, only patients with moderate-to-severe AD had an increased risk of MI (aHR, 1.163, 95% CI, 1.080–1.251, Table 3) compared to non-AD controls.
Fig. 2. Cumulative incidence of myocardial infarction and all-cause mortality in patients with AD and non-AD control.
AD, atopic dermatitis.
Table 2. Incidence of myocardial infarction and all-cause mortality in patients with AD compared with non-AD control.
| Variable | Myocardial infarction | All-cause mortality | ||
|---|---|---|---|---|
| AD | Non-AD | AD | Non-AD | |
| Mean follow-up time (yr) | 8.16 | 8.19 | 8.22 | 8.23 |
| No. of person-years | 458,850.8 | 31,337,028.8 | 462,199.8 | 31,520,363.6 |
| No. of new cases (%) | 1,217 (2.1%) | 68,479 (1.7%) | 2,356 (4.1%) | 140,243 (3.6%) |
| Incidence per 1,000 person-years | 2.65 | 2.19 | 5.10 | 4.45 |
AD, atopic dermatitis.
Table 3. Risk of myocardial infarction and all-cause mortality in patients with AD.
| Variable | No. of events | IR | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Non-adjusted | Model 1 | Model 2 | |||||
| Myocardial infarction | |||||||
| Non-AD | 68,479 | 2.185 | 1.000 (Ref.) | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Total AD | 1,217 | 2.652 | 1.215 (1.148–1.286) | 1.130 (1.068–1.196) | 1.111 (1.050–1.176) | ||
| Mild AD | 498 | 2.748 | 1.259 (1.153–1.375) | 1.076 (0.985–1.175) | 1.044 (0.956–1.140) | ||
| Moderate-to-severe AD | 719 | 2.589 | 1.186 (1.102–1.277) | 1.171 (1.088–1.260) | 1.163 (1.080–1.251) | ||
| All-cause mortality | |||||||
| Non-AD | 140,243 | 4.449 | 1.000 (Ref.) | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Total AD | 2,356 | 5.097 | 1.146 (1.100–1.194) | 1.008 (0.967–1.049) | 1.020 (0.980–1.063) | ||
| Mild AD | 935 | 5.512 | 1.152 (1.080–1.228) | 0.912 (0.855–0.973) | 0.923 (0.866–0.985) | ||
| Moderate-to-severe AD | 1,421 | 5.079 | 1.142 (1.084–1.203) | 1.082 (1.027–1.140) | 1.096 (1.040–1.155) | ||
The Cox regression models were used to assess the risks of myocardial infarction and all-cause mortality in patients with mild and moderate-to-severe AD. The incidence rate is per 1,000 person-years. Model 1 was adjusted for age and gender. Model 2 was adjusted for age, gender, body mass index, smoking, drinking, regular exercise, hypertension, diabetes mellitus, and dyslipidemia.
AD, atopic dermatitis; CI, confidence interval; HR, hazard ratio; IR, incidence rate.
Incidence and risk of all-cause mortality in patients with AD
During a mean follow-up of 8.24 years, there were 142,599 cases of all-cause mortality (3.7%) in the entire cohort. A significantly higher cumulative incidence probability of all-cause mortality was observed in patients with AD than in non-AD controls (P < 0.001, Fig. 2). An increased risk of all-cause mortality among AD patients was observed compared to non-AD controls in the non-adjusted model (HR, 1.146, 95% CI, 1.100–1.194, Table 3). However, this no longer remained significant in the fully adjusted model (HR, 1.020, 95% CI, 0.980–1.063). According to AD severity, patients with moderate-to-severe AD showed an increased risk of all-cause mortality in the fully adjusted model (HR, 1.096, 95% CI, 1.040–1.155).
Subgroup analysis of MI and all-cause mortality risk in patients with AD
We conducted stratified analysis by age, sex, obesity, smoking status, drinking habits, regular exercise, diabetes mellitus, hypertension, and dyslipidemia. An increased incidence of MI was observed in AD patients with cardiometabolic disorders, including diabetes mellitus, hypertension, and dyslipidemia, and negative lifestyle factors, including obesity and current smoking, compared to non-AD controls (Supplementary Table S1). In addition, an increased incidence of all-cause mortality was observed in AD patients with cardiometabolic disorders, including hypertension, diabetes mellitus, and dyslipidemia, and negative lifestyle factors, including heavy drinking, non-regular exercising, current smoking, and obesity, compared to non-AD controls (Supplementary Table S1).
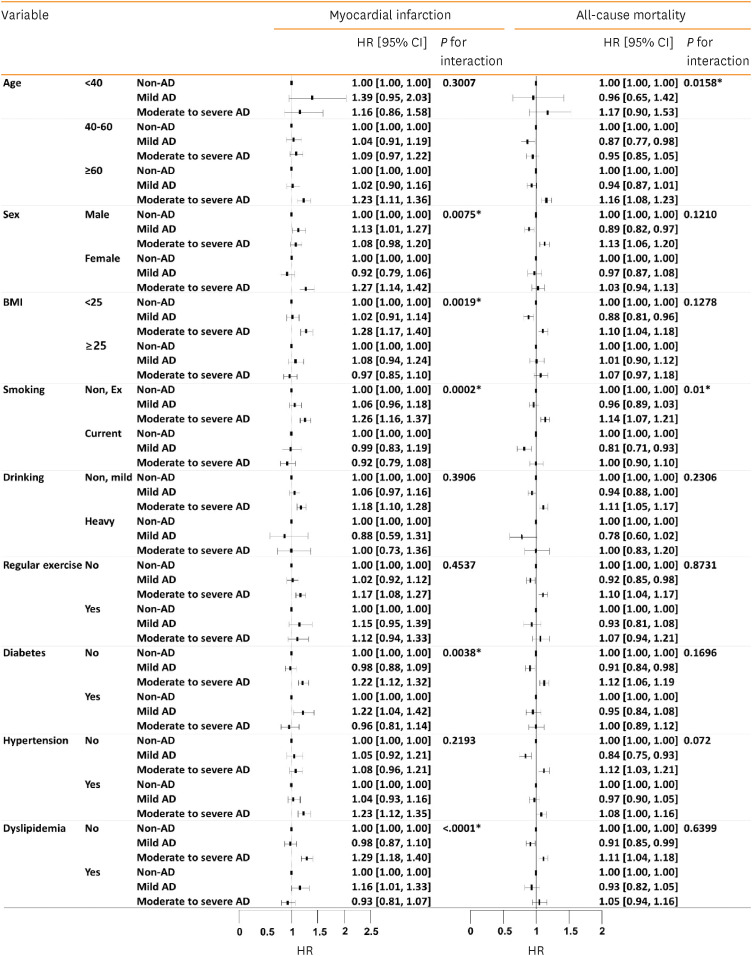
The effect of AD severity on the risk of MI differed significantly according to sex (P for interaction, 0.0075), BMI (P for interaction, 0.0019), and smoking (P for interaction, 0.002; Fig. 3). The risk of MI was significantly increased in females (HR, 1.271, 95% CI, 1.141–1.416), non-obese individuals (HR, 1.281, 95% CI, 1.172–1.399), and non-smokers (HR, 1.260, 95% CI, 1.159–1.369) with moderate-to-severe AD than in those without AD.
Fig. 3. Subgroup analysis of the risks of myocardial infarction and all-cause mortality in patients with AD according to AD severity.
AD, atopic dermatitis; BMI, body mass index; CI, confidence interval; HR, hazard ratio.
*P value is less than 0.05.
The effect of AD severity on the risk of MI differed significantly according to the presence of diabetes (P for interaction, 0.0038) and dyslipidemia (P for interaction, < 0.0001; Fig. 3). Among patients without diabetes mellitus, there was a significantly higher risk of MI in those with moderate-to-severe AD than in those without AD (HR, 1.219, 95% CI, 1.124–1.322). In addition, the risk of MI in non-dyslipidemic individuals was higher in those with moderate-to-severe AD than in those without AD (HR, 1.288, 95% CI, 1.181–1.405).
The effect of AD severity on all-cause mortality significantly differed according to age (P for interaction, 0.0158) and smoking (P for interaction, 0.01). The risk of all-cause mortality was higher among individuals ≥60 years of age with moderate-to-severe AD than those without AD (HR, 1.16, 95% CI, 1.08–1.23). Among non-smokers, the risk of all-cause mortality was also increased in patients with moderate-to-severe AD compared to those without AD (HR, 1.14, 95% CI, 1.07–1.21).
Sensitivity analysis
The sensitivity analysis results were similar to those in the primary analysis. We used varying lag times of up to 5 years in the sensitivity analysis to account for uncertainty regarding the latency period for MI and all-cause mortality. With the lag time set at 3 years, the adjusted HR for MI was 1.112 (95% CI, 1.045–1.183), and for all-cause mortality was 1.139 (95% CI, 1.062–1.221) in patients with AD compared to non-AD-controls (Supplementary Table S2).
DISCUSSION
In the present study, we found that the cumulative incidence of MI and all-cause mortality was significantly higher in individuals with AD compared to non-AD controls using nationwide Korean registry data. After adjusting for possible confounding factors, an increased risk of MI was observed in patients with AD compared to non-AD controls. According to AD severity, a significant increase in the risk of MI and all-cause mortality was found in patients with moderate-to-severe AD compared to non-AD controls.
To date, the risk of MI in AD patients remains unclear due to prior inconsistent results. A recent systematic review and meta-analysis found no significant pooled association between AD and MI in cross-sectional studies.16 However, the authors reported that AD was associated with an increased risk of MI (relative risk, 1.12, 95% CI, 1.00–1.25) based on longitudinal cohort studies,16 consistent with the findings of the present study. The previous cohort studies reporting an increased risk of MI in patients with AD were mostly conducted in European populations, including Denmark,17 Germany,8 and the United Kingdom.18 The significance of this study is that it identified an increased risk of MI in Korean patients with AD, a different ethnicity from previous reports. It is also a strength of this study that we identified an increased risk of MI and all-cause mortality in AD according to the severity of AD.
An increase in all-cause mortality in patients with AD was reported in previous studies.7,19 A study by Silverwood et al. 19 reported that after adjusting for several risk factors, all-cause mortality was increased in patients with severe AD compared to those with non-eczema AD, which is consistent with the findings in this study. Although we could not determine the cause of death in each case due to the dataset used in this study, infectious, urogenital, and cardiovascular events were reported to be the significant risk factors that greatly affected mortality in patients with AD.7 In this study, the effect of AD severity on the risk of all-cause mortality varied significantly according to age and smoking status.
The mechanisms underlying MI and all-cause mortality in AD remain unclear. We hypothesize that chronic inflammation in AD promotes atherosclerosis and the occurrence of cardiovascular events, which also affect mortality. The concept of epidermal interleukin (IL)-1 march could explain the role of AD in the development of cardiovascular events.5 A study by Yamanaka and Mizutani5 suggested that the skin of patients with AD damaged by continuous scratching behavior and skin inflammation promoted the release of several pro-inflammatory cytokines into the systemic circulation. Among various cytokines, they suggested that IL-1 acts as a major inflammatory cytokine involved in both AD and cardiovascular events.5 Several recent experimental studies identified shared inflammatory and cardiovascular markers. A study by Brunner et al. 20 found that inflammatory markers involved in Th2 (IL-13, CCL17, eotaxin-1/CCL11, CCL13, CCL4, IL-10) and Th1 (CXCL10, CXCL11), Th1/Th17/Th22 (IL-12/IL-23p40) pathways were increased in the sera of patients with AD. They also found that serum levels of atherosclerosis-associated proteins, including fractalkine/CX3CL1, CCL8, M-CSF, and HGF, were significantly increased in patients with AD compared to healthy controls.18 A positive correlation between several serum markers associated with atherosclerosis, including E-selectin, PI3/elafin, CCL7, and IL-16, and the severity of atopic dermatitis (AD) was identified.21 The study also reported an increased expression of atherosclerosis-associated proteins, including CCL2, CCL19, SELE, PGF, LOX-1/OLR1, FABP4, MPO, MMPs, RETN, CASP3, TGF-b1, and VEGFA, in the lesional and non-lesional skin of patients with AD compared to healthy controls.21
A recent study using cardiac computed tomography angiography found that the prevalence of coronary artery calcium scores >0 was significantly increased in patients with AD compared to controls,22 showing the increased risk of coronary heart disease in patients with AD.
The subgroup analysis in this study found that patients older than 60 with AD had a significantly increased risk of MI and all-cause mortality compared to healthy controls. A study by He et al. 23 also found that elderly patients with AD (>60 years old) showed an increased expression of atherosclerosis markers (CCL4, CCL7, and SORT) and markers of cardiovascular risk (GDF15, MPO, and ST2) compared to age-matched healthy controls and younger patients with AD. This observation could also be explained by the inflammation present in AD. The chronic low-grade inflammation of senescent cells in elderly patients with AD induces further imbalances in immune cells, including the increased release of Th1 and Th17-associated cytokines and the decreased release of Th2-associated cytokines in the lesional and non-lesioned skin of patients with AD,24,25 which could be associated with an increased risk of MI.26
To further elucidate the impact of metabolic disorders and lifestyle factors on the risk of MI and all-cause mortality in patients with AD, this study stratified patients with AD by the presence of cardiometabolic disorders and lifestyle factors. Consistent with previous observations,27 we found an increased incidence of MI and all-cause mortality in patients with metabolic disorders and negative lifestyle factors. However, after adjusting for possible confounding factors, the risk of MI was significantly increased in patients with moderate-to-severe AD among individuals without metabolic disorders, including diabetes mellitus and dyslipidemia. This finding is consistent with the findings of Wu et al. 27, who also reported an increased risk of cardiovascular disease (odds ratio [OR], 1.25, 95% CI, 1.13–1.39) and major adverse cardiovascular events (OR, 1.22, 95% CI, 1.01–1.47) in patients with AD and without metabolic disorders. In our subgroup analysis, we found an increased risk of MI in AD patients without known lifestyle risk factors for MI. Based on this finding, we suggest that the inflammation present in AD rather than a lifestyle factor could be an independent risk factor for MI. Therefore, in patients with moderate-to-severe AD, even if there are no known risk factors for MI, careful attention should be paid to the possible development of MI.
This study had some limitations. As we utilized the NHIS database, there was the potential for the misdiagnosis or misclassification of AD and MI. Although a combination of diagnostic codes and prescription codes for AD was used in this study to increase the reliability of the diagnosis of AD, the definition of AD used in this study has not been validated. Therefore, there is a potential for misdiagnosis or misclassification of AD. In addition, including patients who participated in the annual health check-ups could have resulted in a selection bias. Moreover, there might be a possibility of unmeasured confounders, such as medications, as we did not consider the effect of various drug exposures on the development of MI and all-cause mortality in patients with AD due to the study design. Thus, further studies about the effect of drug exposure on MI and all-cause death in patients with AD should be conducted in the future.
In conclusion, the findings from this large-scale population-based cohort study could provide insight into the link between AD and MI and all-cause mortality. The study findings suggest a considerable risk of MI and all-cause mortality in patients with moderate-to-severe AD. Thus, in patients with moderate-to-severe AD, even if there are no known risk factors for MI, appropriate management of AD and proper screening for MI should be conducted to prevent the occurrence of MI and decrease all-cause mortality. Future longitudinal prospective studies with different populations are necessary to clarify the association between MI and all-cause mortality in patients with AD.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Subgroup analysis of the incidence rates and risks of myocardial infarction and all-cause mortality in patients with AD
Sensitivity analysis for risk of myocardial infarction and all-cause mortality
References
- 1.Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 2.Laughter MR, Maymone MB, Mashayekhi S, Arents BW, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990-2017. Br J Dermatol. 2021;184:304–309. doi: 10.1111/bjd.19580. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144–151. doi: 10.1016/j.anai.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Furue M, Kadono T. “Inflammatory skin march” in atopic dermatitis and psoriasis. Inflamm Res. 2017;66:833–842. doi: 10.1007/s00011-017-1065-z. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka K, Mizutani H. “Inflammatory skin march”: IL-1-mediated skin inflammation, atopic dermatitis, and psoriasis to cardiovascular events. J Allergy Clin Immunol. 2015;136:823–824. doi: 10.1016/j.jaci.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376:2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 7.Thyssen JP, Skov L, Egeberg A. Cause-specific mortality in adults with atopic dermatitis. J Am Acad Dermatol. 2018;78:506–510. doi: 10.1016/j.jaad.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Standl M, Tesch F, Baurecht H, Rodríguez E, Müller-Nurasyid M, Gieger C, et al. Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074–1081. doi: 10.1016/j.jid.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Drucker AM, Li WQ, Cho E, Li T, Sun Q, Camargo CA, Jr, et al. Atopic dermatitis is not independently associated with nonfatal myocardial infarction or stroke among US women. Allergy. 2016;71:1496–1500. doi: 10.1111/all.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thyssen JP, Halling-Overgaard AS, Andersen YMF, Gislason G, Skov L, Egeberg A. The association with cardiovascular disease and type 2 diabetes in adults with atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2018;178:1272–1279. doi: 10.1111/bjd.16215. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy. 2015;70:1300–1308. doi: 10.1111/all.12685. [DOI] [PubMed] [Google Scholar]
- 12.Jung HJ, Lee DH, Park MY, Ahn J. Cardiovascular comorbidities of atopic dermatitis: using National Health Insurance data in Korea. Allergy Asthma Clin Immunol. 2021;17:94. doi: 10.1186/s13223-021-00590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam DJ, Kwon HW, Lee H, Ahn EK. National healthcare service and its big data analytics. Healthc Inform Res. 2018;24:247–249. doi: 10.4258/hir.2018.24.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn E. Introducing big data analysis using data from National Health Insurance Service. Korean J Anesthesiol. 2020;73:205–211. doi: 10.4097/kja.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SW. Methods for testing statistical differences between groups in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e1 [Google Scholar]
- 16.Ascott A, Mulick A, Yu AM, Prieto-Merino D, Schmidt M, Abuabara K, et al. Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol. 2019;143:1821–1829. doi: 10.1016/j.jaci.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riis JL, Vestergaard C, Hjuler KF, Iversen L, Jakobsen L, Deleuran MS, et al. Hospital-diagnosed atopic dermatitis and long-term risk of myocardial infarction: a population-based follow-up study. BMJ Open. 2016;6:e011870. doi: 10.1136/bmjopen-2016-011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverwood RJ, Forbes HJ, Abuabara K, Ascott A, Schmidt M, Schmidt SAJ, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018;361:k1786. doi: 10.1136/bmj.k1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverwood RJ, Mansfield KE, Mulick A, Wong AYS, Schmidt SAJ, Roberts A, et al. Atopic eczema in adulthood and mortality: UK population-based cohort study, 1998-2016. J Allergy Clin Immunol. 2021;147:1753–1763. doi: 10.1016/j.jaci.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunner PM, Suárez-Fariñas M, He H, Malik K, Wen HC, Gonzalez J, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep. 2017;7:8707. doi: 10.1038/s41598-017-09207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavel AB, Zhou L, Diaz A, Ungar B, Dan J, He H, et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol. 2020;82:690–699. doi: 10.1016/j.jaad.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Hjuler KF, Böttcher M, Vestergaard C, Deleuran M, Raaby L, Bøtker HE, et al. Increased prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med. 2015;128:1325–1334.e2. doi: 10.1016/j.amjmed.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 23.He H, Li R, Choi S, Zhou L, Pavel A, Estrada YD, et al. Increased cardiovascular and atherosclerosis markers in blood of older patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2020;124:70–78. doi: 10.1016/j.anai.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Bocheva GS, Slominski RM, Slominski AT. Immunological aspects of skin aging in atopic dermatitis. Int J Mol Sci. 2021;22:5729. doi: 10.3390/ijms22115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen B, Yang J, Song Y, Zhang D, Hao F. Skin immunosenescence and type 2 inflammation: a mini-review with an inflammaging perspective. Front Cell Dev Biol. 2022;10:835675. doi: 10.3389/fcell.2022.835675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelbertsen D, Andersson L, Ljungcrantz I, Wigren M, Hedblad B, Nilsson J, et al. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol. 2013;33:637–644. doi: 10.1161/ATVBAHA.112.300871. [DOI] [PubMed] [Google Scholar]
- 27.Wu JJ, Amand C, No DJ, Mahajan P, Gadkari A, Ghorayeb E, et al. The use of real-world data to evaluate the association between atopic dermatitis and cardiovascular disease: a retrospective claims analysis. Dermatol Ther (Heidelb) 2021;11:1707–1715. doi: 10.1007/s13555-021-00587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis of the incidence rates and risks of myocardial infarction and all-cause mortality in patients with AD
Sensitivity analysis for risk of myocardial infarction and all-cause mortality