Abstract
Introduction
NF-YB transcription factor is an important regulatory factor in plant embryonic development.
Results
In this study, 15 longan NF-YB (DlNF-YB) family genes were systematically identified in the whole genome of longan, and a comprehensive bioinformatics analysis of DlNF-YB family was performed. Comparative transcriptome analysis of DlNF-YBs expression in different tissues, early somatic embryogenesis (SE), and under different light and temperature treatments revealed its specific expression profiles and potential biological functions in longan SE. The qRT-PCR results implied that the expression patterns of DlNF-YBs were different during SE and the zygotic embryo development of longan. Supplementary 2,4-D, NPA, and PP333 in longan EC notably inhibited the expression of DlNF-YBs; ABA, IAA, and GA3 suppressed the expressions of DlNF-YB6 and DlNF-YB9, but IAA and GA3 induced the other DlNF-YBs. Subcellular localization indicated that DlNF-YB6 and DlNF-YB9 were located in the nucleus. Furthermore, verification by the modified 5'RNA Ligase Mediated Rapid Amplification of cDNA Ends (5' RLM-RACE) method demonstrated that DlNF-YB6 was targeted by dlo-miR2118e, and dlo-miR2118e regulated longan somatic embryogenesis (SE) by targeting DlNF-YB6. Compared with CaMV35S- actuated GUS expression, DlNF-YB6 and DlNF-YB9 promoters significantly drove GUS expression. Meanwhile, promoter activities were induced to the highest by GA3 but suppressed by IAA. ABA induced the activities of the promoter of DlNF-YB9, whereas it inhibited the promoter of DlNF-YB6.
Discussion
Hence, DlNF-YB might play a prominent role in longan somatic and zygotic embryo development, and it is involved in complex plant hormones signaling pathways.
Keywords: Dimocarpus longan Lour., somatic embryogenesis, NF-YB family, nuclear transcription factor, expression profiles
1. Introduction
Nuclear factor-Y (NF-Y), a transcription factor ubiquitous in eukaryotes, is also known as CAATT binding factor or heme activator protein (HAP) (Edwards et al., 1998; Calvenzani et al., 2012). NF-Y was first identified in yeast and subsequently found to be widespread in animals and plants (McNabb et al., 1997); the NF-Y subunit usually has only one or two coding genes in animals and yeast, whereas it has multiple coding genes in plants. NF-Y is not only involved in plant growth development but also plays a key role in the interaction between plants and microorganisms and the environment (Petroni et al., 2012). NF-Y is composed of three distinct subunits, namely, NF-YA (or CBF-B, HAP2), NF-YB (or CBF-A, HAP3), and NF-YC (or CBF-C, HAP5) (Nakshatri et al., 1996). They interact with each other to jointly regulate the cis-acting original of CCAAT in the promoter region of downstream target genes in the nucleus, thereby activating or inhibiting the expression of downstream target genes (Baudin et al., 2015).
In Arabidopsis thaliana, NF-YA, NF-YB, and NF-YC have 10, 13, and 13 family members, respectively (Siefers et al., 2009). NF-YB transcription factors play an important role in plant growth development and stress resistance. At present, NF-YB gene family has been cloned and identified in many plants, including 13 in Oryza sativa L. (Zhou et al., 2018), 21 in Zea mays (Xu et al., 2019), and 28 in Glycine max (Zheng et al., 2012). The NF-YB11 in Triticum aestivum responded to drought stress by regulating downstream gene expression (Zhao et al., 2022), overexpression of NF-YB10 inhibited hypocotyl elongation and upregulated the expression of heat response genes under high temperature stress of Arabidopsis thaliana (Shao, 2022). The PbNF-YB21 promoted root growth with highly lignified and enlarged xylem vessels and enhanced drought resistance by abscisic acid-mediated indoleacetic acid transport (Zhou et al., 2020). The study of Zhang et al. also showed that overexpression of PdNF-YB21 promoted root growth and increased the biomass of poplar (Zhang Y. et al., 2021).
The NF-YB family was first reported in A. thaliana. LEC1 gene was first isolated from A. thaliana by Lotan et al. (1998). It was found to be an essential gene in embryogenesis. The overexpression of AtLEC1 could fully trigger embryogenetic potential and induce somatic embryos on the plant leaf surface. Then, the LEC1 gene was cloned in corn (Zhang et al., 2002), alfalfa (Orlowska et al., 2017), cotton (Yazawa et al., 2004), and other plants. AtNF-YB9 (leafy cotyledon1, LEC1) and AtNF-YB6 (LEC1-like, L1L) played key roles in early embryogenesis (Lotan et al., 1998; Kwong et al., 2003). They were also important regulatory factors of plant growth development, which were involved in seed development and maturation (Tang et al., 2018). LEC1 protein interacted with gibberellin signaling inhibitor DELLA protein to regulate auxin accumulation and promote embryogenesis (Elahi et al., 2016; Hu et al., 2018; Xu J. et al., 2022), and LEC1 could convert somatic cells into embryonic cells (Lotan et al., 1998; Orlowska et al., 2017). NF-YB9, NF-YB2, and NF-YB3 were involved in the regulation of the flowering time of A. thaliana (Cai et al., 2007; Shen et al., 2020; Xu G. et al., 2022). The sequence of L1L was similar to that of LEC1 with high homology (Yazawa et al., 2004). AtLEC1 and AtL1L could activate the CRC and SUCROSE SYNTHASE 2 (SUS2) promoters with the NF-YC subunit by interacting with the bZIP67 that binds to the ABA response elements (Yamamoto et al., 2009). Overexpression of BnL1L in A. thaliana promoted the expression of its fatty acid synthesis genes, indicating that L1L gene was involved in fatty acid synthesis. In rice, OsNF-YB9 (L1L) interacted with SPK and played a key role in seed development (Niu et al., 2021). Paul et al. found that L1L gene was highly expressed in the early stage of SE of grape, and the development of grape embryos would be affected under 2,4-D treatment (Schellenbaum et al., 2008). Wei found that L1L gene was highly expressed in young petioles and embryogenic callus of Liriodendron hybrids (Wei, 2009). The cells labeled with LhL1L in young petioles may be residual pre-embryonic cells after seed germination, and their embryogenic properties will be lost with the maturation of petioles. L1L gene was involved in somatic embryogenesis by regulating the content of IAA in Helianthus annuus × H. tuberosus (Chiappetta et al., 2009).
Longan (Dimocarpus longan Lour.) belongs to the tropical and subtropical characteristic woody fruit tree; its embryo growth and development situation are closely related to fruit quality, setting rate, and yield. Therefore, it is of great significance to study the embryonic development of longan (Chen Y. et al., 2020). However, the zygote embryo of longan is wrapped in its pulp, the early embryonic development state cannot be observed, and it is difficult to obtain materials. In addition, there are genetic differences among different zygote embryos, so it is difficult to study related molecular biology in longan zygote embryos. Longan somatic embryogenesis (SE) is an excellent system for studying the embryonic development of woody plants; it has the advantages of high-frequency occurrence (Lin and Lai, 2010), high synchronization, strong regeneration ability, easy sampling, and consistent genetic background. Recently, the first whole-genome database of Sapindaceae was established by second-generation sequencing of longan (Lin et al., 2017). Then, the third-generation sequencing of the longan genome was available, which provided complete and comprehensive genomic information. The three-dimensional structure of chromatin during early SE by Hi-C technique revealed the dynamic change of genome during longan SE (Chen et al., 2023), and the improvement of longan genetic transformation system further laid the foundation for studying the molecular mechanism during longan SE (Xu et al., 2023; Zhang et al., 2023). Although the NF-YB family has been characterized in several plants, however, the comprehensive data regarding the evolution, expression patterns, and functions of the NF-YB family in longan are still unavailable. The release of longan genomic data and transcriptomics of SE provides the basis for the identification and functional analysis of longan genes. Therefore, DlNF-YB family were identified based on longan genomic database, their expression patterns under different exogenous hormone treatments were analyzed, and the functions of DlNF-YB6 and DlNF-YB9 promoter were studied, which laid a foundation for studying the regulatory mechanism of NF-YB family during longan SE.
2. Materials and methods
2.1. Plant materials and treatments
The synchronized embryogenic cultures at early developmental stages, including friable embryogenic callus (EC), incomplete compact pro-embryogenic cultures (ICpEC), and globular embryos (GE) were obtained by the method of Lai et al. (1997). Different development stages of zygotic embryo were collected in June to isolate the total RNA. When the young fruits emerged, cotyledon embryo stage was marked as S1; then, the zygotic embryo were collected every 5 days, marked as S2, S3, S4, S5, S6, S7, and S8, ordinal. For EC suspension culture, 2 g of 18-day subculture longan EC was transferred to MS liquid basal medium (2% sucrose) supplemented with 2,4-D (1.0 mg/L), inositol (100 mg/L) with agitation at 110 rpm at 25°C under dark conditions for 5 days (Chen et al., 2018a). Then, the suspension cell was transferred to MS liquid basal medium (2% sucrose) supplemented with 2,4-D (1.0 mg/L), KT (0.5 mg/L), and AgNO3 (5 mg/L) with agitation at 110 rpm at 25°C under dark conditions for 5 days. Two grams of 18-day subculture longan EC was transferred to MS liquid basal medium (2% sucrose) supplemented with 2,4-D (0.5, 1.0, 1.5, and 2.0 mg/L), IAA (0.5, 1.0, 1.5, and 2.0 mg/L), GA3 (3, 6, 9, and 12 mg/L), ABA (3, 6, 9, and 12 mg/L), N-1- naphthylphthalamic acid (NPA: 5, 10, 20,30, 40, and 50 mg/L), and Paclobutrazol (PP333: 0.05, 0.1, 0.3, 1, 2, and 3 mg/L) with agitation at 120 rpm at 25°C under dark conditions for 24 h, with three replicates. EC culture in MS liquid basal medium (2% sucrose) was used as control. All samples were frozen in liquid nitrogen immediately for 5 min after collecting and then stored at −80°C for total RNA extraction.
2.2. Identification and characterization of the NF-YB family in longan
The second generation of longan genome sequencing data (SRA050205) (Lin et al., 2017) and the third generation of longan genome data were provided by our research team (BioProject accession: PRJNA792504; genome sequence of D. longan: SRR17675476) (Chen et al., 2023). Arabidopsis thaliana protein sequence was downloaded from https://www.arabidopsis.org/. The latest Hidden Markov Model (HMM) for NF-YB transcription factor (PF00808) (http://pfam.xfam.org/) was used to perform HMM searches against the annotated entire protein datasets of longan with an E-value cutoff of 1e−5 using HMMER 3.0. The amino acid sequence of the A. thaliana NF-YB was used as the seed sequence. TBtools software (Chen C. et al., 2020) was used for sequence bidirectional alignment, combined with the results of NCBI analysis of the protein structure domain of the candidate sequence. The members without the domain were removed. The DlNF-YB family was named according to the A. thaliana naming method. The amino acid number (aa), isoelectric point (pI), molecular weight (MW), instability coefficient, and average hydrophilicity were predicted by Expasy Protparam (https://web.expasy.org/protparam/). The subcellular localization of DlNF-YBs were predicted by WOLF PSORT (https://wolfpsort.hgc.jp/). The gene structure of DlNF-YB family was analyzed by TBtools using the GFF file of ‘Honghezi ‘ longan.
2.3. Phylogenetic evolution and synteny analysis of DlNF-YB
Arabidopsis thaliana protein sequence data were downloaded from https://www.arabidopsis.org/, Gossypium spp. protein sequence data were downloaded from https://www.cottongen.org/, Oryza sativa protein sequence data were downloaded from https://rapdb.dna.affrc.go.jp/, Litchi chinensis Sonn (Hu et al., 2022) genome data were downloaded from https://data.mendeley.com/datasets/kggzfwpdr9/1, Medicago sativa L. (Du et al., 2022) genome data were downloaded from https://figshare.com/articles/dataset/Medicago_sativa_genome_and_annotation_files/12623960, Larix kaempferi (Li et al., 2021; Li et al., 2023) genome data were downloaded from NCBI (NCBI BioProject number: PRJNA648500), and ‘Jidanben’ longan (CRA004281) (Wang et al., 2022) genome data were downloaded from https://ngdc.cncb.ac.cn/. MEGA5.05 software was used to construct the phylogenetic tree, and the neighbor-joining method (NJ) was used to set the bootstrap value to 1,000 for repetition. The phylogenetic tree was perfected using the online tool Chiplot (https://www.chiplot.online/), and TBtools was used to predict and visualize the collinearity between longan and litchi.
2.4. Analysis of conserved motifs, cis-acting regulatory elements, and protein interaction analysis of DlNF-YB
Analysis of conserved motif of DlNF-YB protein sequence by MEME (http://meme-suite.org/tools/meme). The 2,000- bp sequence upstream of the transcription start site of genes in the DlNF-YB family was extracted from the longan genome file, and the cis-acting elements of the promoter were analyzed by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html). The results were visualized with Tbtools. Protein–protein interactions (PPI) of DlNF-YBs were analyzed using STRING (https://string-db.org/), A. thaliana was selected as the model plant, and the confidence level was set to 0.400 to analyze the protein interaction of DlNF-YB family members.
2.5. Expression analysis of DlNF-YB family at the early stage of SE, different tissues, different light quality, and different temperatures
The FPKM (fragments per kilo-base of exon per million fragments mapped) values of DlNF-YB family members were extracted from the transcriptomes of early SE (Lin et al., 2017) (NEC [non-embryogenic callus], EC [embryogenic callus], ICpEC [incomplete compact pro-embryogenic cultures], GE [globular embryos]), different tissues (NCBI BioProject number: PRJNA326792) (young fruit, seed, flower, flower bud, leaf, pulp, root, and stem), different light qualities (Li et al., 2019) (blue, white, and dark as the control), and different temperatures (15°C, 25°C, and 35°C) (NCBI BioProject number PRJNA889670), normalized by log2 FPKM. TBtoosl software was used to visualize and analyze the expression level of each member.
2.6. qRT-PCR analysis of DlNF-YB family
The total RNA of longan EC, ICpEC, GE, and EC treated with different hormones were extracted by TransZolUp kit (TransGen Biotech), and the total RNA of different development stages of the zygotic embryo was extracted by BioTeke kit (Cat. No. RP3301). The cDNA was synthesized with a Hifair®III 1st Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus) (Yeasen). Primer design was performed using Primer3 online software (https://primer3.ut.ee/cgi-bin/primer3/primer3web_results.cgi) ( Supplementary Table 1 ). DlACTB, DlEF-la, and DlUBQ were used as internal control genes (Lin and Lai, 2010). The qPCR system was 20 μL, including HRbio™ qPCR SYBR®Green Master Mix (No Rox) (Heruibio), 8.2 μL ddH2O, 1 µL of 10-fold diluted cDNA, and 0.4 μL specific primer pairs, with three replicates. The reaction procedure was 95°C for 30 s, followed by 40 cycles of 95°C for 10 s and 58°C for 30 s, and 2−ΔCT was used to calculate genes expression (Livak and Schmittgen, 2002; Vandesompele et al., 2002). SPSS software was used for significant analysis of gene differences, and GraphPad 8.0.2 was used for the draft.
2.7. Subcellular localization of DlNF-YB
The CDS sequences of DlNF-YB6 and DlNF-YB9 were selected to design subcellular localization primers by DNAMAN6 ( Supplementary Table 1 ) and synthesized by Beijing Tsingke Biology Co., Ltd. According to Zhang X. et al., 2021, the pCAMBIA1302-GFP vector was constructed and transiently expressed in Allium cepa epidermal cells by Agrobacterium-mediated transformation. The pCAMBIA1302-GFP vector was co-transformed into A. cepa as a positive control. 4',6-Diamidino-2-phenylindol (DAPI) was used as a maker of nuclear localization. After 3 days of co-culture under 28°C, analysis was performed by a laser scanning confocal microscope (OLYMPUS, FV1200, Tokyo, Japan; GFP wavelength, 475 nm; DAPI wavelength, 450 nm).
2.8. miRNAs prediction of DlNF-YB family and cleavage site verification by modified 5'RLM-RACE method
The target miRNAs of DlNF-YB family were predicted using an online software psRNAtarget, and the expected value was set to 5. The modified 5'RLM-RACE method was used to verify the cleavage site of miRNA, and the extracted RNA of EC, ICpEC, and GE of longan was mixed with 1: 1 for reverse transcription, referenced to the FirstChoice® RLM-RACE Kit instructions for the synthesis of cDNA template. The CDS sequences between 90 and 160 bp near the predicted cleavage site were selected for primer design ( Supplementary Table 1 ). Nested PCR was used for amplification, the amplified products were verified by electrophoresis, and the glue was recovered and connected for transformation. After PCR verification, the bacterial liquid was tested.
2.9. DlNF-YB promoter cloning and functional analysis
The core promoter region sequences of DlNF-YB6 and DlNF-YB9 were selected by DNAMAN6 software to design primers ( Supplementary Table 1 ); the 1,244 bp and 760 bp promoter sequences were selected to construct pCAMBIA1301-DlNF-YB6pro::GUS and pCAMBIA1301-DlNF-YB9pro::GUS fusion vector. The pCAMBIA1301::GUS vector containing the 35S promoter was used as a positive control. The bacteria liquid was transiently expressed in Nicotiana benthamiana epidermal cells by Agrobacterium-mediated transformation. After 3 days of co-culture, the injection leaves were sampled and placed in a refrigerator at −80°C to detect the expression level of NbGUS.
To examine the effects of hormones on the transcriptional level of the DlNF-YB6 and DlNF-YB9 promoter, 100 μmol/L IAA, ABA, and GA3 were sprayed on tobacco leaves 48 h after Agrobacterium solution injection and cultured at 25°C, 16/8 h (light/dark) for 24 h. Spraying water was used as a control to compare the expression levels of NbGUS in each group under different hormone treatments.
3. Results
3.1. Identification, physicochemical properties, and gene structure analysis of DlNF-YB family
The potential members of the NF-YB family were obtained by using the Hidden Markov Model of the NF-YB family and compared with the amino acid sequence of NF-YB family of A. thaliana. A total of 14 DlNF-YB genes were identified from the second generation of longan genome database, and 16 DlNF-YB genes were identified from the third generation of longan genome database. The conserved domains of the 16 candidate sequences were analyzed using SMART and Pfam databases. One sequence that did not contain CBFD_NFYB_HMF (Dlo003663) was removed. The second-generation results were correlated with the third-generation results. Finally, 15 DlNF-YB genes were identified, according to the similarity of AtNF-YBs sequences, and combined with evolutionary tree classification, the 15 genes were named as DlNF-YB1, DlNF-YB2, DlNF-YB3, DlNF-YB3-like, DlNF-YB4, DlNF-YB4-like, DlNF-YB5, DlNF-YB6, DlNF-YB7, DlNF-YB7-like, DlNF-YB8, DlNF-YB9, DlNF-YB9-like, DlNF-YB10, and DlNF-YB11 ( Supplementary Table 2 ).
The physicochemical properties of the DlNF-YBs showed that the number of aa in DlNF-YBs protein sequences ranged from 141 to 320aa. The pI ranged from 4.98 to 8.11, DlNF-YB8 and DlNF-YB10 were basic proteins, and the other 13 were acidic proteins ( Supplementary Table 2 ). The hydrophilicity coefficient of DlNF-YBs were less than zero, indicating that all of them were hydrophilic proteins with different hydrophilicity gradients. The instability coefficients ranged from 32.88 to 65.85; all were unstable proteins. Subcellular localization prediction showed that DlNF-YB9-like and DlNF-YB11 were located in the cytoplasm, DlNF-YB4 and DlNF-YB6 were located in the mitochondrial matrix, and other members were located in the nucleus, indicating that DlNF-YBs may mainly play a regulatory role in the nucleus.
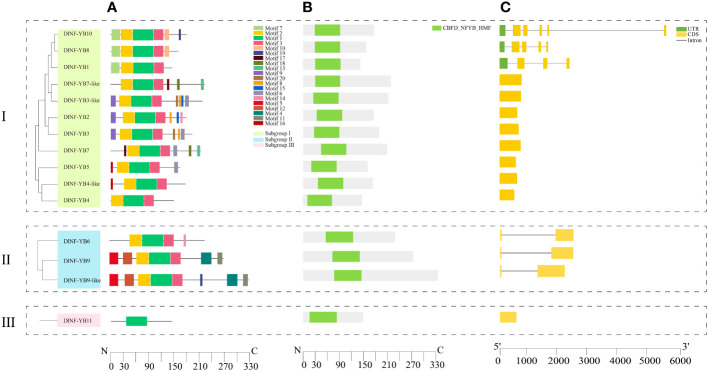
TBtools software was used to analyze and visualize the gene structure and domain of DlNF-YB family, and the results showed that ( Figures 1B, C ) the number of introns in each member of the DlNF-YB family ranges from 0 to 5, including DlNF-YB2, DlNF-YB3, DlNF-YB3-like, DlNF-YB4, DlNF-YB4-like, DlNF-YB5, DlNF-YB7, DlNF-YB7-like, and DlNF-YB11 had no introns, DlNF-YB6, DlNF-YB9 and DlNF-YB9-like contained 1 intron, DlNF-YB1 contained 3 introns, DlNF-YB8 contained 4 introns, only DlNF-YB10 contained 5 introns. All members contain CBFD_NFYB_HMF.
Figure 1.
Conserved motif, domain, and gene structure of DlNF-YB family. (A) The motif composition of DlNF-YB proteins. The motifs, numbers 1–20, were displayed in different colored boxes; (B) domain of DlNF-YB proteins. Green boxes indicate CBFD_NFYB_HMF; (C) exon–intron structure of DlNF-YB genes. Yellow boxes indicate exons; green boxes indicate UTR; black lines indicate introns.
3.2. Phylogenetic analysis and synteny analysis of DlNF-YB
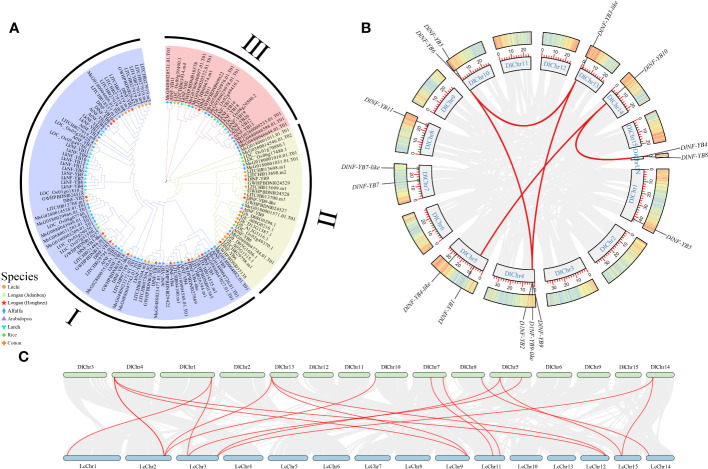
In the phylogenetic tree of the NF-YB family from eight species, namely, ‘Honghezi’ longan, ‘Jidanben’ longan, A. thaliana, Litchi chinensis Sonn, Gossypium spp., Oryza sativa, Medicago sativa L., and Larix kaempferi, MEGA 5.05 was constructed for multiple sequence analysis according to the classification of NF-YB in A. thaliana, combined with the homology clustering analysis of DlNF-YB family. By constructing phylogenetic evolutionary tree to explore its evolutionary characteristics, the results suggested that the NF-YB could be categorized into three subgroups ( Figure 2A ). DlNF-YB6, DlNF-YB9, and DlNF-YB9-like were distributed in subgroup II; only DlNF-YB11 was distributed in subgroup III. A total of 13 members with ‘Jidanben’ longan had higher homology; DlNF-YB8 and DlNF-YB10 had high homology with litchi, indicating that different varieties of longan changed during the evolutionary process. It is speculated that there may be diversity or species-specific biological functions of DlNF-YBs due to the grouping differences and genetic relationships between species during the evolutionary process.
Figure 2.
Phylogenetic tree and collinear analysis of NF-YB family. (A) Phylogenetic tree of NF-YB in longan, Arabidopsis, litchi, rice, and cotton; (B) chromosome localization and collinearity analysis of NF-YB family members in longan (gray line represents collinearity block in longan genome; red line represents linear gene pairs related to DlNF-YB family genes); (C) interspecific collinearity analysis of longan and litchi (gray line represents collinearity blocks in longan and litchi; red line represents line gene pairs related to NF-YB family).
TBtools software was used to obtain collinearity gene pairs. The collinearity analysis of DlNF-YB family revealed that five tandem duplication events in seven members ( Figure 2B ); they were DlNF-YB6 and DlNF-YB3-like, DlNF-YB6 and DlNF-YB9, DlNF-YB3-like and DlNF-YB2, DlNF-YB10 and DlNF-YB1, and DlNF-YB9-like and DlNF-YB8. The 13 DlNF-YB genes were unevenly distributed on 8 of the 15 chromosomes of longan, and DlNF-YB4 and DlNF-YB8 were located on the unknown chromosome.
To study the evolutionary relationships between longan and litchi NF-YB family, the interspecies collinearity analysis was conducted using their genomes. The results represented that the collinearity relationship between litchi and longan was quite close ( Figure 2C ); NF-YB family of litchi was also distributed on eight chromosomes, and 11 members of longan had collinearity relationship with litchi, displaying high correlation, thereby indicating that the genetic similarity between them is high in the evolutionary process.
3.3. Analysis of conserved motif, promoter cis-acting element, and protein interaction of DlNF-YB
In order to understand the distribution of protein conserved motifs of DlNF-YB family, the online software MEME was used for motif prediction analysis. As shown in Figure 1A , we found that motif 2 and motif 3 were only missing in DlNF-YB11; motif 1 was distributed in all members, indicating that motif 1 was highly conserved in the DlNF-YB family. In contrast, motif 17 was only detected in DlNF-YB4-like and DlNF-YB5.
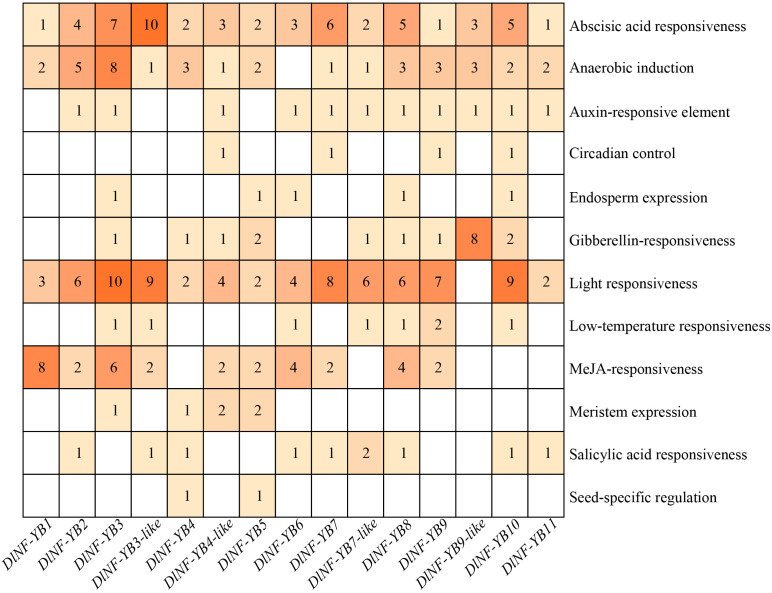
To further explore the functions of DlNF-YBs in longan, the cis-acting elements localized upstream of the translation initiation site were predicted using the PlantCARE online software. The results indicated that the DlNF-YB family all contains core promoter elements that ensure normal transcription and abscisic acid response elements ( Figure 3 ), suggesting that they all respond to abscisic acid (ABA). In addition, there were 14 DlNF-YBs that could to respond to light (except DlNF-YB9-like), 11 DlNF-YBs contained auxin response elements, and 9 DlNF-YBs contained methyl jasmonic acid (MeJA) response elements. Seven DlNF-YBs (DlNF-YB3, DlNF-YB6, DlNF-YB7-like, DlNF-YB8, DlNF-YB9, DlNF-YB10, and DlNF-YB12) contained low temperature response elements. DlNF-YB family also contained a variety of elements related to plant growth and development, such as circadian control, endosperm expression elements, seed-specific development, and protein binding site.
Figure 3.
Distribution of cis-acting elements of DlNF-YB genes in longan. Different colors and numbers in the figure indicate the number of components, and the blank spaces indicate no components.
DlNF-YB6 and DlNF-YB9 contained multiple core regulatory elements ( Supplementary Figure S1 ). Further analysis with PlantCare found that DlNF-YB9 contained several hormone response elements, among which only one ABRE responded to ABA, one TGA element responded to auxin, and CGTCA motif and TGACG motif responded to MeJA. Similarly, DlNF-YB6 contained AuxRR core for auxin responsiveness, two TGACG motifs and two CGTCA motifs for MeJA responsiveness, one TCA element for salicylic acid (SA) responsiveness, and three ABRE for ABA responsiveness. It could be seen that DlNF-YB6 and DlNF-YB9 all responded to hormone regulation. In addition, there were also elements involved in seed-specific regulation (GCN4_motif), a large number of light-responsive elements (G-Box, GT1-motif), and low-temperature responsive elements (LTR).
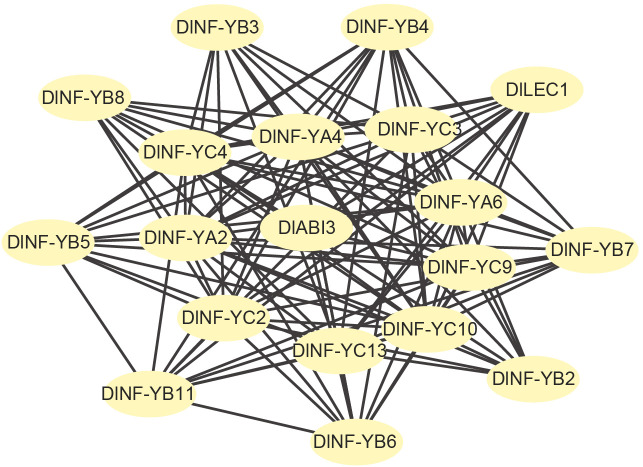
To better understand the interactions between DlNF-YB family and other family members. STRING software was used to analyze PPI. The results ( Figure 4 ) showed that there was a strong interaction between NF-YB family members and also with subgroups A and B. DlNF-YB family not only interacts with members within the family but also interacts with ABI3.
Figure 4.
Protein–protein interactions (PPI) of DlNF-YB family members.
3.4. Expression pattern analysis of DlNF-YB family
3.4.1. FPKM analysis of DlNF-YB family during early SE
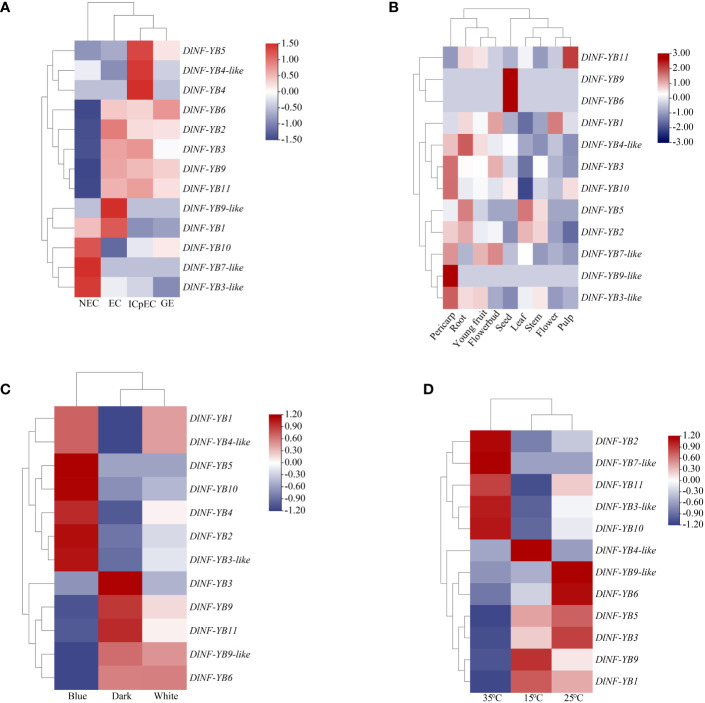
The FPKM values of DlNF-YB were analyzed based on the transcriptome of longan early SE (NEC, EC, ICpEC, and GE); DlNF-YB2, DlNF-YB3, DlNF-YB6, DlNF-YB9, and DlNF-YB11 were mainly expressed at the early stage of SE, but barely expressed at the NEC stage ( Figure 5 ). The expression level of DlNF-YB9 was extremely high at the EC stage and gradually decreased with SE, indicating that DlNF-YB9 played an essential role in the induction and maintenance of embryogenic callus in longan. The expression level of DlNF-YB6 was maintained at a high level from the EC stage to the GE stage, indicating that DlNF-YB6 had a positive regulatory effect on longan SE. DlNF-YB3-like, DlNF-YB7-like, and DlNF-YB10 were specifically expressed at the NEC stage. DlNF-YB7-like was barely expressed at the early stage of longan SE. The expression level of DlNF-YB3-like was extremely low at the early stage of SE, and the expression level gradually decreased with SE, which was also much lower than that at the NEC stage, indicating that the function of the NF-YB family may not be limited to the early stage of SE.
Figure 5.
Expression patterns of DlNF-YB family members based on FPKM values. (A) FPKM value of DlNF-YB family during longan SE. (B) FPKM value of DlNF-YB family in different tissues. (C) FPKM value of DlNF-YB family under different light quality treatments. (D) FPKM value of DlNF-YB family under different temperature treatments. All plant materials were ‘Honghezi’ longan; different colors on the scale bar represent different transcript levels.
3.4.2. FPKM analysis of DlNF-YB family in different tissues
FPKM value analysis of DlNF-YB family based on transcriptomes of longan different tissues (young fruit, seed, flower, flower bud, leaf, pulp, root, and stem) showed that ( Figure 5 ) DlNF-YB6 and DlNF-YB9 were highly expressed in seeds but not in other tissues, indicating that they were seed-specific genes and may play a key role in seed dormancy and embryonic development. The higher expression of DlNF-YB5 in roots and leaves suggested that DlNF-YB5 was involved in the growth and development of longan. DlNF-YB1 was highly expressed in flower buds and flowers; however, it was minimal or undetected in other tissues, indicating that it was mainly involved in the development of floral organs and flowering induction. DlNF-YB3, DlNF-YB3-like, and DlNF-YB10 were highly expressed in the pulp, indicating that the above genes may be involved in fruit development. In general, DlNF-YB family showed different expression patterns in different tissues, indicating that DlNF-YB family played an important role in the growth and development of longan.
3.4.3. Analysis of FPKM values of DlNF-YB family under different light treatments
The FPKM values of DlNF-YB were analyzed based on longan EC in transcripts treated with different light qualities (white, black, and blue); the DlNF-YB family had different expression trends under different light quality treatments ( Figure 5 ). With dark treatment compared to controls, DlNF-YB1, DlNF-YB2, DlNF-YB3-like, DlNF-YB4, DlNF-YB4-like, DlNF-YB5, and DlNF-YB10 presented high expression in blue light treatment. It explained that blue light treatment could promote the expression of the above genes in longan EC. However, the expression levels of DlNF-YB3, DlNF-YB6, DlNF-YB9, DlNF-YB9-like, and DlNF-YB11 under blue light treatment were lower than those under dark treatment. It can be seen that the DlNF-YB family also plays a critical role in light response.
3.4.4. FPKM analysis of DlNF-YB family under different temperature treatments
FPKM value analysis of DlNF-YB based on the longan EC- treated transcriptome under different temperatures (15°C, 25°C, and 35°C) was performed. Compared with 25°C treatment ( Figure 5 ), DlNF-YB2, DlNF-YB3-like, DlNF-YB7-like, DlNF-YB10, and DlNF-YB11 were specifically expressed at 35°C. DlNF-YB1, DlNF-YB3, DlNF-YB5, and DlNF-YB9 were significantly expressed at 15 °C; and only DlNF-YB4-like showed an inhibitory expression trend at 15°C and 35°C. Therefore, most of the DlNF-YB family may play an essential role in the self-repair process under temperature stress.
3.5. The qRT-PCR analysis of DlNF-YB family
3.5.1. Expression analysis of DlNF-YB family during early SE
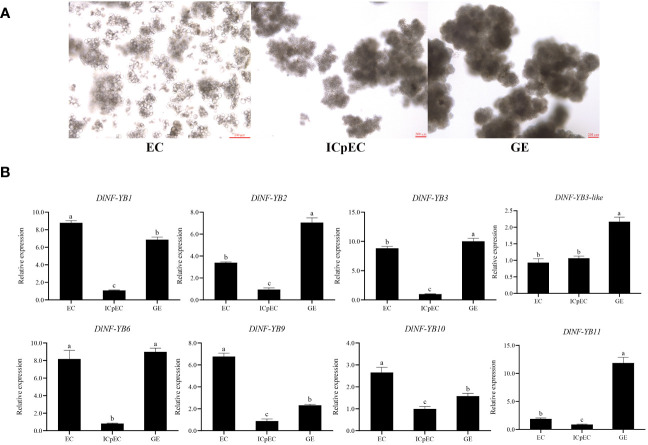
Based on FPKM analysis, DlNF-YB1, DlNF-YB2, DlNF-YB3, DlNF-YB3-like, DlNF-YB6, DlNF-YB9, DlNF-YB10, and DlNF-YB11 had significant differential expression during early SE of longan. The different expression patterns of these DlNF-YBs were analyzed by qRT-PCR. The expression trends of DlNF-YB1, DlNF-YB2, DlNF-YB6, DlNF-YB9, and DlNF-YB11 were similar to the transcriptome data, while the qRT-PCR results of the other members were different from the RNA-seq date. It can be seen that the expression of DlNF-YBs in longan had a certain degree of spatial and temporal specificity during somatic embryogenesis, and the specific function needs to be further verified. The qRT-PCR result of DlNF-YB family at EC, ICpEC, and GE stages indicated that most of the DlNF-YB were highly expressed in the EC stage, except DlNF-YB11 ( Figure 6 ). The expression of DlNF-YB1, DlNF-YB2, DlNF-YB3, and DlNF-YB6 at EC and GE stages were significantly higher than those at ICpEC stage. The expression of DlNF-YB6 during longan early SE was similar to that of FPKM, which remained at a high level at the EC and GE stages, suggesting that DlNF-YB6 had a positive regulatory effect on longan SE. DlNF-YB9 was highly expressed at the EC stage, and DlNF-YB11 was highly expressed at the GE stage, which showed a contradictory trend, indicating that these two genes played a key role at different stages of SE, while DlNF-YB3-like and DlNF-YB10 were expressed throughout the SE stage. The above results indicated that DlNF-YB family was involved in longan early SE and played different roles.
Figure 6.
D. longan early stage of somatic embryogenesis and qRT-PCR analysis of longan DlNF-YB family during early somatic embryogenesis. Note: (A) Longan early stage of somatic embryogenesis. EC, embryogenic callus; ICpEC, incomplete compact pro-embryogenic cultures; GE, globular embryos. (B) The qRT-PCR analysis of DlNF-YB members the early stage of SE. The internal reference genes were DlACTB, DlEF-la, and DlUBQ, with three biological replicates, and significant differences with lowercase letters abc, p < 0.05.
3.5.2. Expression analysis of DlNF-YB family at different development stages of zygotic embryos
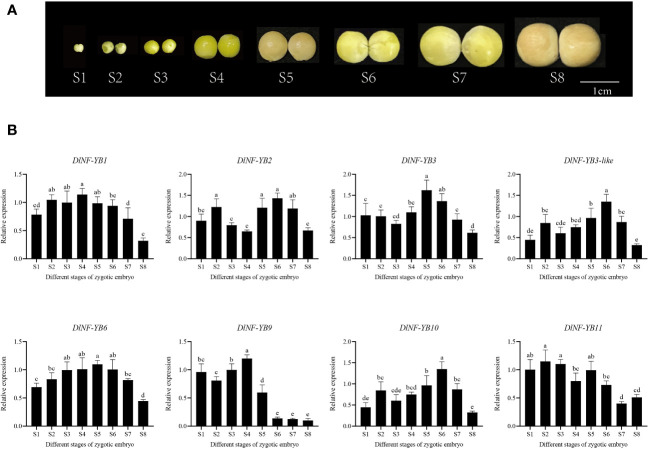
The qRT-PCR results showed that DlNF-YBs were detected at all development stages of zygotic embryos ( Figure 7 ), and their expression patterns were different. Among them, DlNF-YB9 was highly expressed at S1–S5 stage of zygotic embryos, and its expression was extremely low at S6–S8 stage. DlNF-YB1 and DlNF-YB6 showed an overall high level of expression throughout the development stage of zygotic embryos, with an initial increasing trend and a subsequent decrease, with the lowest expression at S8 stage. The expression patterns of DlNF-YB2, DlNF-YB3, DlNF-YB3-like, DlNF-YB10, and DlNF-YB11 were similar during the development of zygotic embryos, showing a similar double-peaked “M “ trend, except that the expression of DlNF-YB11 was the lowest at S7 stage; the others decreased to the lowest at S8 stage. Therefore, the DlNF-YB family may play different regulatory roles during the development of longan zygotic embryos.
Figure 7.
qRT-PCR analysis of longan DlNF-YB family at different development stages of zygotic embryos. (A) The different developmental stages of zygotic embryos. (B) The relative expression of different stages of zygotic embryos. The internal reference genes were DlACTB, DlEF-la, and DlUBQ, with three biological replicates, and significant differences in different lowercase letters, p < 0.05.
3.5.3. Expression analysis of DlNF-YB family under different exogenous hormone treatments
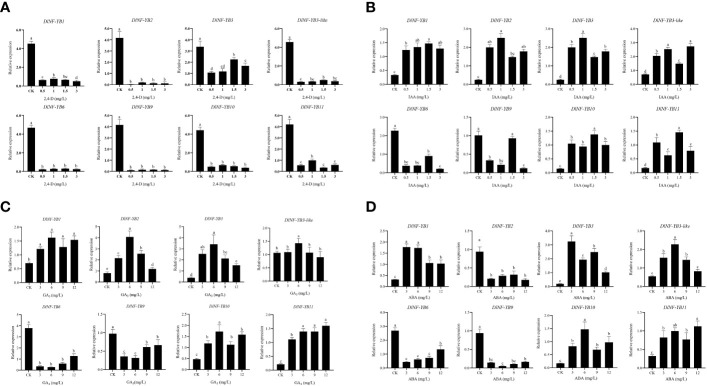
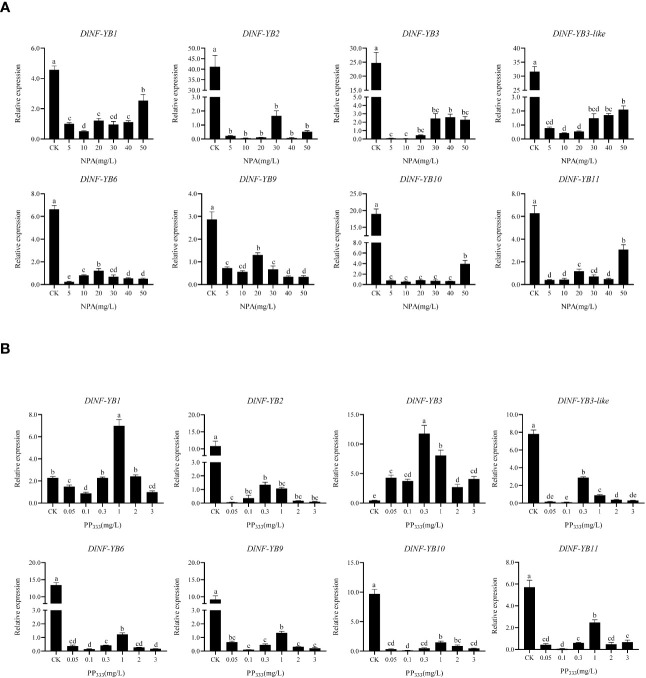
The qRT-PCR result showed that the expression of DlNF-YB was significantly lower under different concentrations of 2,4-D treatment than that of the control, indicating that exogenous 2,4-D could significantly inhibit the expression of DlNF-YBs ( Figure 8A ). Under different concentrations of exogenous IAA treatment, the expression of DlNF-YB1, DlNF-YB2, DlNF-YB3, DlNF-YB3-like, DlNF-YB10, and DlNF-YB11 were significantly higher than those of the control, indicating that exogenous IAA could promote their transcription levels. The expressions of DlNF-YB6 and DlNF-YB9 were significantly lower than that of the control under exogenous IAA treatment ( Figure 8B ), except that DlNF-YB9 was not significantly different under the 1.5 mg/L IAA treatment and control. Under the treatment of 5, 10, 20, 30, 40, and 50 mg/L NPA, the transcription levels of DlNF-YBs were significantly lower than those of the control; especially under the treatment of 5, 10, and 20 mg/L, the expression of DlNF-YBs were extremely low or barely expression, indicating that exogenous NPA could significantly inhibit the expression of DlNF-YBs ( Figure 9A ). Meanwhile, the expression of DlNF-YB1, DlNF-YB2, DlNF-YB3, DlNF-YB3-like, DlNF-YB10, and DlNF-YB11 under NPA treatment showed the opposite trend with that under exogenous IAA treatment.
Figure 8.
qRT-PCR analysis of DlNF-YB family under the 2,4-D, IAA, ABA, GA3 treatments. (A) qRT-PCR analysis of DlNF-YB family under the 2,4-D treatment; (B) qRT-PCR analysis of DlNF-YB family under the IAA treatment; (C) qRT-PCR analysis of DlNF-YB family under the ABA treatment; (D) qRT-PCR analysis of DlNF-YB family under the GA3 treatment. The internal reference genes were DlACTB, DlEF-la, and DlUBQ, with three biological replicates, and significant differences in different lowercase letters, P < 0.05.
Figure 9.
qRT-PCR analysis of DlNF-YB family under the NPA and PP333 treatment. (A) qRT-PCR analysis of DlNF-YB family under the NPA treatment; (B) qRT-PCR analysis of DlNF-YB family under the PP333 treatment. The internal reference genes were DlACTB, DlEF-la, and DlUBQ, with three biological replicates, and significant differences in different lowercase letters, P < 0.05.
The expression of DlNF-YB family under different concentrations of GA3 treatment showed that exogenous GA3 could significantly promote the expression of DlNF-YB1, DlNF-YB2, DlNF-YB3, and DlNF-YB10, and the expression level was the most significant under 6 mg/L GA3 treatment. The expression level of DlNF-YB11 gradually increased with the increase in concentration. Among those treatment, only 6 mg/L GA3 can promote the expression of DlNF-YB3-like. However, exogenous GA3 significantly inhibited the transcription levels of DlNF-YB6 and DlNF-YB9, which was almost the same as the expression pattern under exogenous IAA treatment ( Figure 8C ). The qRT-PCR result of DlNF-YB family under different concentrations of PP333 treatment suggested that the transcription level of DlNF-YB1 decreased first, then increased and then decreased with the increase in PP333 concentration ( Figure 9B ). The expression of DlNF-YB1 was highest under 1 mg/L PP333 treatment, which was significantly higher than that of the control. Treatment with different concentrations of PP333 significantly promoted the expression of DlNF-YB3 but significantly inhibited the expression of DlNF-YB2, DlNF-YB3-like, DlNF-YB6, DlNF-YB9, DlNF-YB10, and DlNF-YB11.
Under different concentrations of ABA treatment, the expression levels of DlNF-YB2, DlNF-YB6, and DlNF-YB9 were significantly lower than those of the control, indicating that exogenous ABA inhibited the transcription of DlNF-YB2, DlNF-YB6, and DlNF-YB9 ( Figure 8D ). At the same time, the expression levels of DlNF-YB1, DlNF-YB3, DlNF-YB3-like, DlNF-YB10, and DlNF-YB11 were significantly higher than those of the control, indicating that exogenous ABA could promote the transcription of DlNF-YB1, DlNF-YB3, DlNF-YB3-like, DlNF-YB10, and DlNF-YB11. In summary, DlNF-YB can actively respond to the treatment of exogenous hormones, and there are differences in the response mechanism to exogenous hormones. DlNF-YB may regulate the somatic embryo process of longan through the hormone signal transduction pathway.
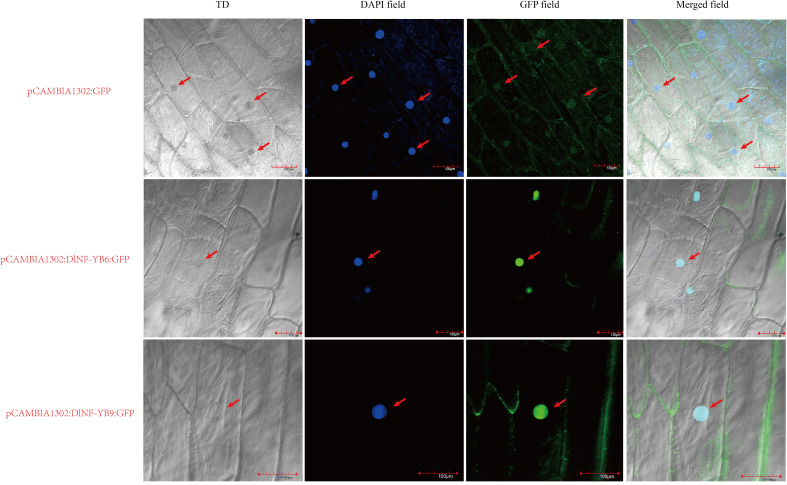
3.6. Subcellular localization of DlNF-YB6 and DlNF-YB9
According to the prediction results of WOLF PSORT software, DlNF-YB9 (LEC1) and DlNF-YB6 (L1L) were selected for verification. The prediction results showed that DlNF-YB9 was located in the nucleus, and DlNF-YB6 was located in the mitochondrial matrix. The results showed that ( Figure 10 ) all the cells of the onion inner epidermis injected with bacterial liquid had GFP fluorescence signals. The GFP fluorescence signals were distributed in the nucleus, indicating that both DlNF-YB6 and DlNF-YB9 were located in the nucleus and participated in the regulation of longan SE as nuclear transcription factors.
Figure 10.
Subcellular localization of DlNF-YB6 and DlNF-YB9. TD is a transmission light channel, the scale is 100 μM, and the arrow represents the localizations of GFP and DAPI fluorescence signal in cells.
3.7. miRNAs prediction of DlNF-YB family and verification of cleavage site of DlNF-YB10
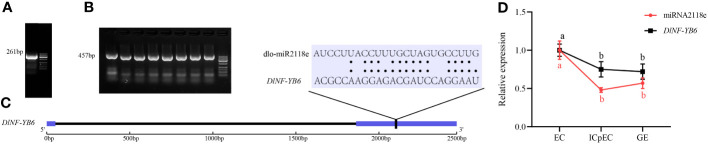
The miRNA- targeted regulation of DlNF-YB members was predicted by psRNATarget. As shown in Supplementary Table 3 , DlNF-YB1, DlNF-YB4-like, DlNF-YB5, DlNF-YB7, DlNF-YB7-like, DlNF-YB9, DlNF-YB9-like, and DlNF-YB11 were not regulated by miRNA. The expression of DlNF-YB2 and DlNF-YB3-like was regulated by dlo-miR156a in a cleavage inhibition manner, with a minimum expected value of 4. Multiple miRNAs simultaneously targeted DlNF-YB2, DlNF-YB3, and DlNF-YB3-like. Based on different expectations, the cleavage site of the DlNF-YB6 (L1L) target gene was verified by the improved RLM-RACE method. The results indicated that DlNF-YB6 was targeted by dlo-miR2118e, where the cleavage site was the classic 10th CCU/UUG ( Figure 11 ). The expression analysis of miRNA and its target genes in the three stages of longan SE showed that both of them were downregulated in the early stage of longan SE from EC to ICpEC, while at the stage of ICpEC to GE, dlo-miR2118e was upregulated and its target gene DlNF-YB6 was downregulated, indicating that there was a negative regulatory relationship between the ICpEC to GE. In conclusion, dlo-miR2118e could regulate longan SE by targeting DlNF-YB6.
Figure 11.
Verification of cleavage site between dlo-miR2118e and its target gene DlNF-YB6. (A) The amplified gel map. (B) The bacterial liquid PCR verification gel map. (C) The cleavage site diagram. The black line part indicates the cleavage site. (D) the quantitative expression of dlo-miR2118e and DlNF-YB6 in the early stages of longan SE. U6 snRNA was used as a miRNA to quantitatively express a single internal reference gene, and DlUBQ was used as a target gene to quantitatively express a single internal reference gene, with three biological replicates. Different lowercase letters showed significant differences, p < 0.05.
3.8. Functional verification of DlNF-YB6 and DlNF-YB9 promoter
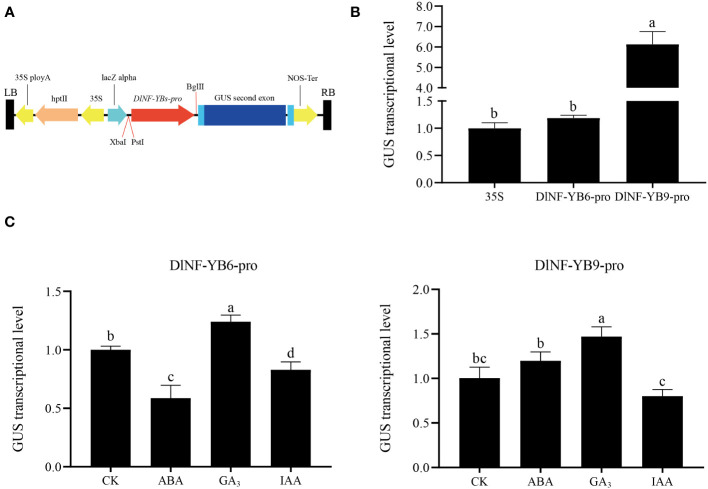
The promoter of DlNF-YB6 and DlNF-YB9, which contained transcription start sites and important cis-acting element regions, were cloned from longan gDNA for pCAMBIA1301-DlNF-YB6pro::GUS and pCAMBIA1301-DlNF-YB9pro::GUS vector construction. The transient transformation in N. benthamiana showed that the promoters of DlNF-YB6 and DlNF-YB9 could promote the expression of GUS, among which DlNF-YB9 had the strongest ability to actuate the expression of GUS, and the activation of DlNF-YB9 promoter was approximately 6.14 times that of 35S promoter ( Figure 12 ). The expression of GUS gene under different hormone treatments was compared by using N. benthamiana leaves infected with pCAMBIA1301:GUS bacterial solution and sprayed with water as control. The highest GUS transcription level was detected in the GA3-treated samples, and the DlNF-YB6 and DlNF-YB9 promoter- driven GUS transcription levels under GA3 treatment were approximately 1.5-fold compared with the control.
Figure 12.
GUS transcriptional level generated by 35S promoter and DlNF-YB6/9 promoter in N. benthamiana and GUS transcriptional level generated by 35S promoter and DlNF-YB6/9 promoter in N. benthamiana under different hormone treatments. (A) The schematic diagram of DlNF-YB6/9-pro vector fragment; (B) the expression level of transiently transformed tobacco GUS gene; (C) the expression level of GUS gene in transformed tobacco leaves under different hormone treatments. Nb18S was used as an internal reference gene for N. benthamiana GUS gene expression, with three biological replicates, and significant differences with lowercase letters abc, p < 0.05.
Under ABA treatment, DlNF-YB6 promoter-driven GUS gene was inhibited, which was approximately 50% of the control; on the contrary, the DlNF-YB9 promoter-driven GUS gene expression, which was approximately 1.2 times of the control. Meanwhile, IAA-treated samples presented a repressed effect, both of which were approximately 80% –83% of the control.
4. Discussion
4.1. DlNF-YB may be involved in longan somatic and zygotic embryogenesis
AtNF-YB9 (LEC1) and AtNF-YB6 (L1L) play important roles in early embryogenesis (Lotan et al., 1998; Kwong et al., 2003). In our study, based on the genome-wide identification of the DlNF-YB family and its expression analysis during early SE and zygotic embryo, the results indicated that a large number of longan NF-YB genes were highly expressed at EC to GE stage and the development of the zygotic embryo. DlNF-YB6 and DlNF-YB9 were expressed specifically at EC stage and changed with the process of SE, suggesting that DlNF-YB6 and DlNF-YB9 were specific to the early stage of longan SE and played a crucial role in the development stage of EC. Chen et al. (2018b) found that ectopic expression of GhNF-YB22 promoted the formation of EC and promoted the embryonic development of cotton. RNA-seq shows the change trend of gene expression in the whole sample, but it cannot guarantee that the change trend of every gene is consistent with qRT-PCR (Everaert et al., 2017). In our study, the qRT-PCR trends of some members were different from RNA-seq date, presumably due to samples not being from the same batch and related to primer selection. The cleavage site of DlNF-YB6 was verified by the modified RLM- RACE method, and the result suggested that DlNF-YB6 was targeted by dlo-miR2118e. Meanwhile, both showed a negative regulatory relationship from ICpEC to GE. These results clearly show that DlNF-YBs played a prominent role in longan SE and zygotic embryo development. Niu et al. (2021) found that OsNF-YB7 and OsNF-YB9 played a crucial role in the formation of rice seeds, and the absence of OsNF-YB7 and OsNF-YB9 would lead to abnormal seed development and death. According to the FPKM values of different tissues of longan, DlNF-YB6 and DlNF-YB9 were highly expressed in the seed, suggesting that they might be involved in longan seed development. AtNF-YB2 played a key role in the flowering processes (Cai et al., 2007), and Arabidopsis HAP3b controlled flowering time by regulating the flowering gene AtFT (Kumimoto et al., 2008). Furthermore, DlNF-YB1 was highly expressed in flower buds and flower stages, indicating that it played an important role in the flowering process of longan.
4.2. DlNF-YB involved in longan SE through hormones and stress response
Hormones are involved in the growth and development of most plants. Gao (2001) found that low concentration of ABA could promote SE and further regulated SE in carrot. Moreover, ABA can also improve the tolerance of many plant embryos and inhibit premature germination of embryos, thereby improving the quality of SE (Vahdati et al., 2008; Rai et al., 2011). The expression of AsNF-YB3 was promoted by exogenous ABA during tobacco seed germination (Sun et al., 2016). In Jatropha curcas seedlings, JcNF-YB3 and JcNF-YB10 genes were upregulated to varying degrees under the action of exogenous ABA (Huang et al., 2017). From our study, exogenous ABA could significantly upregulate the expression of most members of the DlNF-YB family, but inhibit DlNF-YB2, DlNF-YB6, and DlNF-YB9 expression. Therefore, DlNF-YB might be involved in ABA signal transduction and also participate in longan SE. It was found that plants treated with NPA had a phenotype similar to PIN family protein mutants, and NPA may play a role by inhibiting PIN family (Abas et al., 2021). Subsequently, Yang et al. (2022) found that IAA and NPA had certain similarities in binding mode, but NPA molecules were larger and bound to PIN1 in a higher affinity way, which verified the efficient inhibition of NPA. Meanwhile, NPA could directly target PIN proteins. Supplementary 2,4-D, NPA in longan EC notably inhibited the expression of DlNF-YBs. IAA suppressed the expression of DlNF-YB6 and DlNF-YB9, but the other DlNF-YBs were induced by IAA. Moreover, under the addition of NPA, the expression trends of DlNF-YB1, DlNF-YB2, DlNF-YB3, DlNF-YBlike, DlNF-YB10, and DlNF-YB11 showed an opposite trend compared with that under IAA treatment. It is speculated that DlNF-YB family may participate in the process of NPA inhibiting the effect of PIN family. Meanwhile, GA3 inhibited the expression of DlNF-YB6 and DlNF-YB9, while it promoted the expression of other members. Promoters of DlNF-YB6 and DlNF-YB9 driven GUS gene expression were different. The results of spraying exogenous hormones showed that GA3 promoted the expression of GUS gene driven by DlNF-YB6 and DlNF-YB9 promoter, while IAA inhibited it, consistent with the study that GA3 significantly induced DlRan3A promoter activity (Tian et al., 2015). GA3 is involved in somatic embryogenesis and regulates the expression of transcription factors related to somatic embryogenesis. By supplementing exogenous GA3 to change endogenous GA levels, it is also related to the acceleration of starch hydrolysis by GA3 by increasing α-amylase activity (Lai and Chen, 2002; Rudus et al., 2002). Therefore, DlNF-YBs may also play a role in the energy metabolism signal transduction pathway during longan SE. However, ABA induced the activities of DlNF-YB9 promoter, whereas it inhibited the DlNF-YB6 promoter activities. ABA plays an essential role in promoting the maturation of plant somatic embryos; meanwhile, the content of ABA was different during longan SE (Lai and Chen, 2002; Langhansová et al., 2004). It was found that DlNF-YB6 and DlNF-YB9 played a role in different stages of longan SE. Although the opposite effects of ABA on transcription of DlNF-YB6 and DlNF-YB9 promoters suggest that they may be involved in ABA signaling pathways, their main functions and action time were different during longan SE. The result now proves that the DlNF-YB family was involved in various hormone signal transduction pathways and affected longan SE.
Previous studies have shown that for longan in the embryonic development period, if the temperature continues at 34°C–38°C, it will lead to embryonic development stagnation and the formation of small fruit (Nong et al., 2006). In addition, high temperature stress was carried out during the proliferation of longan EC, and it was found that the proliferation rate of longan EC increased at 35°C, but the growth of longan EC stagnated at 40°C (Wang, 2019). The expression of DlERF6 increased at 35°C in the early three stages of longan embryo development. Overexpression of DlERF6 increased TAG (triacylglycerol) content at high temperature and inhibited longan SE (Zhang, 2022). The expression of DlNF-YB family under different temperature treatments showed that, except for DlNF-YB2 and DlNF-YB10, the expression of others were promoted at high temperature (35°C) or low temperature (15°C). In A. thaliana, AtNF-YB3 forms NF-Y complex with AtNF-YA2 and AtNF-YC10 (Sato et al., 2014), which was involved in plant heat stress response and improved plant survival rate, and overexpression of AtNF-YB3 plants enhanced heat resistance (Sato et al., 2019). AtNF-YB10 inhibited hypocotyl elongation induced by mild and heat stresses under light, suggesting that AtNF-YB10 may be a negative regulator of thermomorphogenesis (Shao, 2022). Analysis of the cis-acting elements of the promoter revealed that the DlNF-YB family contained many CCAAT boxes. The study found that this response element could confer plant resistance to cold stress (Shi and Chan, 2014). In Phaseolus vulgaris, miR2118 was upregulated under ABA and drought stress (Valdés-López et al., 2010). In summary, the DlNF-YB family can participate in the process of longan SE by responding to abiotic stress.
5. Conclusions
Based on the genome data of D. longan, we conducted an in-depth analysis of the DlNF-YB family, and 15 DlNF-YB genes were identified in the D. longan genome. A comprehensive bioinformatics analysis of DlNF-YB family was performed, under different light and temperature treatments, revealing its specific expression profiles and potential biological functions in longan SE. In addition, DlNF-YB6 was targeted by dlo-miR2118e, and dlo-miR2118e could regulate longan SE by targeting DlNF-YB6. Through the study of promoters, DlNF-YB6 and DlNF-YB9 promoters could generate the expression of GUS in different degrees. Gene expression analysis showed that DlNF-YBs were expressed in different degrees during longan SE, suggesting their specific functions, and DlNF-YBs were regulated by ABA, GA3, and IAA, revealing the DlNF-YBs response to hormone signal pathways. This study explained that DlNF-YBs play a prominent role during longan SE, which provided a reference for better research on the functions of DlNF-YBs.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material .
Author contributions
MT: Data curation, Formal Analysis, Writing – original draft. XG: Writing – original draft, Investigation, Methodology, Project administration. WM: Resources, Writing – original draft, Methodology. JL: Data curation, Methodology, Writing – original draft. GZ: Project administration, Software, Writing – original draft. ZL: Writing – review & editing, Funding acquisition. YL: Writing – review & editing, Funding acquisition. YC: Funding acquisition, Writing – review & editing.
Acknowledgments
Thanks to the Institute of Horticultural Biotechnology of Fujian Agriculture and Forestry University for providing conditions for this study. Thanks to the workers who contributed to our research.
Funding Statement
The research was funded by The Natural Science Foundation of Fujian Provincial (2021J01086), the Natural Science Foundation of Fujian Province (2020J01543), and the College of Horticulture, Fujian Agriculture and Forestry University Youth Academic Backbone Training Fund (102/722022011).
Abbreviations
Dl, Dimocarpus longan Lour.; aa, amino acids; NEC, non-embryogenic callus; EC, embryogenic callus; ICpEC, incomplete compact pro-embryogenic cultures; GE, globular embryos; SE, somatic embryogenesis; DNA, deoxyribonucleic acid; gDNA, genomic DNA; RNA, ribonucleic acid; miRNA, microRNA; cDNA, complementary DNA; RACE, rapid amplification of cDNA ends; PCR, polymerase chain reaction; qPCR, quantitative real-time PCR; CDS, coding sequence; UTR, untranslated regions; bp, base pairs; μL, microliter; mL, milliliter; g, gram; d, day; h, hour; min, minute; s, second; rpm, revolutions per minute; FPKM, Fragments Per Kilo-base of exon per Million fragments mapped; 2,4-D, 2,4-Dichlorophenoxyacetic acid; IAA, Indole-3-acetic acid; ABA, abscisic acid; GA, gibberellin; SA, salicylic acid; MeJa, methyl jasmonate; NPA, N-1-naphthylphthalamic acid; PP333, paclobutrazol; DAPI 4′,6-diamidino-2-phenylindol, 4′,6-diamidino-2-phenylindol; GFP, green fluorescent protein.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1255436/full#supplementary-material
The nucleotide sequence of DlNF-YB6 and DlNF-YB9 promoter. Note: The putative regulatory elements and hormone-responsive elements on both strands are shown in bold and underlined, which was performed by the PlantCARE database.
References
- Abas L., Kolb M., Stadlmann J., Janacek D. P., Lukic K., Schwechheimer C., et al. (2021). Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proc. Natl. Acad. Sci. U. S. A. 118, e2020857118. doi: 10.1073/pnas.2020857118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin M., Laloum T., Lepage A., Rípodas C., Ariel F., Frances L., et al. (2015). A phylogenetically conserved group of Nuclear factor-Y transcription factors interact to control nodulation in legumes. Plant Physiol. 169, 2761–2773. doi: 10.1104/pp.15.01144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Ballif J., Endo S., Davis E., Liang M., Chen D., et al. (2007). A putative ccaat-binding transcription factor is a regulator of flowering timing in arabidopsis. Plant Physiol. 145, 98–105. doi: 10.1104/pp.107.102079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvenzani V., Testoni B., Gusmaroli G., Lorenzo M., Gnesutta N., Petroni K., et al. (2012). Interactions and ccaat-binding of Arabidopsis thaliana NF-Y subunits. PloS One 7, e42902. doi: 10.1371/journal.pone.0042902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Chen H., Zhang Y., Thomas H. R., Frank M. H., He Y., et al. (2020). Tbtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. doi: 10.1016/j.molp.2020.06.009 [DOI] [PubMed] [Google Scholar]
- Chen Y., Lin X., Lai Z. (2020). Advances in somatic embryogenesis of Dimocarpus Longan Lour. Chin. J. Trop. Crops 41, 1990–2002. doi: 10.3969/j.issn.1000-2561.2020.10.006 [DOI] [Google Scholar]
- Chen Y., Xie D., Ma X., Xue X., Liu M., Xiao X., et al. (2023). Genome-wide Hi-C analysis reveals hierarchical chromatin interactions during early somatic embryogenesis. Plant Physiol. 193 (1), 555–577. doi: 10.1093/plphys/kiad348 [DOI] [PubMed] [Google Scholar]
- Chen Y., Xu X., Chen X., Chen Y., Zhang Z., Xu X., et al. (2018. a). Seed-specific gene MOTHER of FT and TFL1(MFT) involved in embryogenesis, hormones and stress responses in Dimocarpus longan Lour. Int. J. Mol. Sci. 19, 2403. doi: 10.3390/ijms19082403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yang Z., Xiao Y., Wang P., Wang Y., Ge X., et al. (2018. b). Genome-wide analysis of the NF-YB gene family in Gossypium hirsutum L. And characterization of the role of GhdNF-YB22 in embryogenesis. Int. J. Mol. Sci. 19, 483. doi: 10.3390/ijms19020483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappetta A., Fambrini M., Petrarulo M., Rapparini F., Michelotti V., Bruno L., et al. (2009). Ectopic expression of Leafy cotyledon1-like gene and localized auxin accumulation mark embryogenic competence in epiphyllous plants of Helianthus annuus x H. Tuberosus . Ann. Bot. 103, 735–747. doi: 10.1093/aob/mcn266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Junfeng Y., Li Q., He C., Pang Y. (2022). Identification and characterization of abiotic stress-responsive NF-YB family genes in medicago. Int. J. Mol. Sci. 2 (23), 6906. doi: 10.3390/ijms23136906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D., Murray J. A., Smith A. G. (1998). Multiple genes encoding the conserved ccaat-box transcription factor complex are expressed in arabidopsis. Plant Physiol. 117, 1015–1022. doi: 10.1104/pp.117.3.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi N., Duncan R. W., Stasolla C. (2016). Modification of oil and glucosinolate content in canola seeds with altered expression of brassica napus Leafy cotyledon 1 . Plant Physiol. Biochem. PPB 100, 52–63. doi: 10.1016/j.plaphy.2015.12.022 [DOI] [PubMed] [Google Scholar]
- Everaert C., Luypaert M., Maag J. L. V., Cheng Q. X., Dinger M. E., Hellemans J., et al. (2017). Benchmarking of RNA-sequencing analysis workflows using whole-transcriptome RT-qPCR expression data. Sci. Rep. 7 (1), 1559. doi: 10.1038/s41598-017-01617-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S. (2001). Effects of ABA and PEG on the embryogenesis and regulation of carrot (Daucus carota L.) Somatic embryos. J. Northwest A & F Univer. (Nat. Sci. Ed.) 2, 13–16. doi: 10.13207/j.cnki.jnwafu.2001.02.003 [DOI] [Google Scholar]
- Hu G., Feng J., Xiang X., Wang J., Salojärvi J., Liu C., et al. (2022). Two divergent haplotypes from a highly heterozygous lychee genome suggest independent domestication events for early and late-maturing cultivars. Nat. Genet. 54, 73–83. doi: 10.1038/s41588-021-00971-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhou L., Huang M., He X., Yang Y., Liu X., et al. (2018). Gibberellins play an essential role in late embryogenesis of arabidopsis. Nat. Plants 4, 289–298. doi: 10.1038/s41477-018-0143-8 [DOI] [PubMed] [Google Scholar]
- Huang Y. Y., Deng M. H., Shi W. S., Gong M., Chen H. W., Wen J. F., et al. (2017). Cloning and expression analysis of JcNF-YB3 and JcNF-YB10 from jatropha curcas. Zhiwu Shengli Xuebao/Plant Physiol. J. 53, 1059–1066. doi: 10.13592/j.cnki.ppj.2016.0504 [DOI] [Google Scholar]
- Kumimoto R. W., Adam L., Hymus G. J., Repetti P. P., Reuber T. L., Marion C. M., et al. (2008). The nuclear factor y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta 228, 709–723. doi: 10.1007/s00425-008-0773-6 [DOI] [PubMed] [Google Scholar]
- Kwong R., Bui A., Lee H., Kwong L., Fischer R., Goldberg R., et al. (2003). Leafy cotyledon1-like defines a class of regulators essential for embryo development. Plant Cell 15, 5–18. doi: 10.1105/tpc.006973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z., Chen C. (2002). Changes of endogenous phytohormones in the process of somatic embryogenesis in longan. Chin. J. Trop. Crops (02), 41–47. doi: 10.3969/j.issn.1000-2561.2002.02.008 [DOI] [Google Scholar]
- Lai Z., Pan L., Chen Z. (1997). Establishment and maintenance of longan embryogenic cell lines. J. Fujian Agric. Forestry University(Natural Sci. Edition) (02), 33–40. doi: CNKI:SUN:FJND.0.1997-02-006 [Google Scholar]
- Langhansová L., Konrádová H., Vanêk T. (2004). Polyethylene glycol and abscisic acid improve maturation and regeneration of Panax ginseng somatic embryos. Plant Cell Rep. 22 (10), 725–730. doi: 10.1007/s00299-003-0750-2 [DOI] [PubMed] [Google Scholar]
- Li W., Lee J., Yu S., Wang F., Lv W., Zhang X., et al. (2021). Characterization and analysis of the transcriptome response to drought in Larix kaempferi using PacBio full-length cDNA sequencing integrated with de novo RNA-seq reads. Planta 253 (2), 28. doi: 10.1007/s00425-020-03555-3 [DOI] [PubMed] [Google Scholar]
- Li H., Lyu Y., Chen X., Wang C., Yao D., Ni S., et al. (2019). Exploration of the effect of blue light on functional metabolite accumulation in longan embryonic calli via rna sequencing. Int. J. Mol. Sci. 20, 441. doi: 10.3390/ijms20020441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ren X., Shao L., Huang X., Zhang C., Wang X., et al. (2023). Comprehensive Analysis of the NF-YB Gene Family and Expression under Abiotic Stress and Hormone Treatment in Larix kaempferi. Int. J. Mol. Sci. 24 (10), 8910. doi: 10.3390/ijms24108910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Lai Z. (2010). Reference gene selection for qpcr analysis during somatic embryogenesis in longan tree. Plant Sci. 178, 359–365. doi: 10.1016/j.plantsci.2010.02.005 [DOI] [Google Scholar]
- Lin Y., Min J., Lai R., Wu Z., Chen Y., Yu L., et al. (2017). Genome-wide sequencing of longan (Dimocarpus longan Lour.) Provides insights into molecular basis of its polyphenol-rich characteristics. Gigascience 6, 1–14. doi: 10.1093/gigascience/gix023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K., Schmittgen T. (2002). Analysis of relative gene expression data using real-time quantitative PCR. Methods (San Diego Calif.) 25, 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lotan T., Ohto M., Yee K. M., West M. A., Lo R., Kwong R. W., et al. (1998). Arabidopsis leafy cotyledon1 is sufficient to induce embryo development in vegetative cells. Cell 93, 1195–1205. doi: 10.1016/s0092-8674(00)81463-4 [DOI] [PubMed] [Google Scholar]
- McNabb D. S., Tseng K. A., Guarente L. (1997). The saccharomyces cerevisiae HAP5P homolog from fission yeast reveals two conserved domains that are essential for assembly of heterotetrameric ccaat-binding factor. Mol. Cell. Biol. 17, 7008–7018. doi: 10.1128/mcb.17.12.7008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakshatri H., Bhat-Nakshatri P., Currie R. A. (1996). Subunit association and dna binding activity of the heterotrimeric transcription factor NF-Y is regulated by cellular redox. J. Biol. Chem. 271, 28784–28791. doi: 10.1074/jbc.271.46.28784 [DOI] [PubMed] [Google Scholar]
- Niu B., Zhang Z., Zhang J., Zhou Y., Chen C. (2021). The rice LEC1-like transcription factor OsNF-YB9 interacts with SPK, an endosperm-specific sucrose synthase protein kinase, and functions in seed development. Plant J. For Cell Mol. Biol. 106, 1233–1246. doi: 10.1111/tpj.15230 [DOI] [PubMed] [Google Scholar]
- Nong W., Huang J., Li G., Li G. (2006). Research on the reason of severely small size fruit phenomenon in longan. J. South. Agric. 3, 314–316. doi: 10.3969/j.issn.2095-1191.2006.03.031 [DOI] [Google Scholar]
- Orlowska A., Igielski R., Łagowska K., Kępczyńska E. (2017). Identification of LEC1, L1L and polycomb repressive complex 2 genes and their expression during the induction phase of medicago truncatula gaertn. Somatic embryogenesis. Plant Cell Tissue Organ Culture (Pctoc.) 129, 119–132. doi: 10.1007/s11240-016-1161-8 [DOI] [Google Scholar]
- Petroni K., Kumimoto R. W., Gnesutta N., Calvenzani V., Fornari M., Tonelli C., et al. (2012). The promiscuous life of plant nuclear factor y transcription factors. Plant Cell 24, 4777–4792. doi: 10.1105/tpc.112.105734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M., Shekhawat N., Harish, Gupta A., Phulwaria M., Ram K., et al. (2011). The role of abscisic acid in plant tissue culture – a review of recent progress. Plant Cell Tissue Organ Culture 106, 179–190. doi: 10.1007/s11240-011-9923-9 [DOI] [Google Scholar]
- Rudus I., Kępczyńska E., Kępczyński J. (2002). Regulation of Medicago sativa L. somatic embryogenesis by gibberellins. Plant Growth Regul. 36, 91–95. doi: 10.1023/A:1014751125297 [DOI] [Google Scholar]
- Sato H., Mizoi J., Tanaka H., Maruyama K., Qin F., Osakabe Y., et al. (2014). Arabidopsis dpb3-1, a dreb2a interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with nf-y subunits. Plant Cell 26, 4954–4973. doi: 10.1105/tpc.114.132928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Suzuki T., Takahashi F., Shinozaki K., Yamaguchi-Shinozaki K. (2019). NF-YB2 and NF-YB3 have functionally diverged and differentially induce drought and heat stress-specific genes. Plant Physiol. 180, 1677–1690. doi: 10.1104/pp.19.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenbaum P., Jacques A., Maillot P., Bertsch C., Mazet F., Farine S., et al. (2008). Characterization of vvserk1, vvserk2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.). Plant Cell Rep. 27, 1799–1809. doi: 10.1007/s00299-008-0588-8 [DOI] [PubMed] [Google Scholar]
- Shao Y. (2022). Proteomic analysis of thermomorphogenic responses in Arabidopsis (Zhejiang University: PhD dissertation; ). [Google Scholar]
- Shen C., Liu H., Guan Z., Yan J., Zheng T., Yan W., et al. (2020). Structural insight into dna recognition by CCT/NF-YB/YC complexes in plant photoperiodic flowering. Plant Cell 32, 3469–3484. doi: 10.1105/tpc.20.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Chan Z. (2014). Athap5a modulates freezing stress resistance in Arabidopsis independent of the cbf pathway. Plant Signaling Behav. 9, e29109. doi: 10.4161/psb.29109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefers N., Dang K. K., Kumimoto R. W., Bynum W. E., Tayrose G., Holt B. F., et al. (2009). Tissue-specific expression patterns of arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 149, 625–641. doi: 10.1104/pp.108.130591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Ren A., Zhang X., Lian H., Zhou S., Liu S., et al. (2016). Overexpression of a garlic Nuclear factor Y (NF-Y) B gene, AsNF-YB3, affects seed germination and plant growth in transgenic tobacco. Plant Cell Tissue Organ Culture (Pctoc.) 127, 513–523. doi: 10.1007/s11240-016-1076-4 [DOI] [Google Scholar]
- Tang G., Xu P., Ma W., Wang F., Liu Z., Wan S., et al. (2018). Seed-specific expression of atlec1 increased oil content and altered fatty acid composition in seeds of peanut (Arachis hypogaea L.). Front. Plant Sci. 9, 260. doi: 10.3389/fpls.2018.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q., Lin Y., Yang M., Zhang D., Lai R., Lai Z. (2015). DlRan3A is involved in hormone, light, and abiotic stress responses in embryogenic callus of Dimocarpus longan Lour. Gene 569 (2), 267–275. doi: 10.1016/j.gene.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Vahdati K., Bayat S., Ebrahimzadeh H., Jariteh M., Mirmasoumi M. (2008). Effect of exogenous ABA on somatic embryo maturation and germination in persian walnut (Juglans regia L.). Plant Cell Tissue Organ Culture 93, 163–171. doi: 10.1007/s11240-008-9355-3 [DOI] [Google Scholar]
- Valdés-López O., Yang S., Aparicio-Fabre R., Graham P., Reyes J., Vance C., et al. (2010). Microrna expression profile in common bean (Phaseolus vulgaris) under nutrient deficiency stresses and manganese toxicity. New Phytol. 187, 805–818. doi: 10.1111/j.1469-8137.2010.03320.x [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3 (7), research0034.0031. doi: 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. (2019). Genome-wide identification of HSF family in longan (Dimocarpus longan lour.) and expression analysis in response to heat stress (Master, Fuzhou: Fujian Agriculture and Forestry University; ). [Google Scholar]
- Wang J., Li J., Li Z., Liu B., Zhang L., Guo D., et al. (2022). Genomic insights into longan evolution from a chromosome-level genome assembly and population genomics of longan accessions. Horticult. Res. 9, uhac21. doi: 10.1093/hr/uhac021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P. (2009). Isolation and expression analysis of the marker genes related to somatic embreogenesis of liriodendron hybrids (l. Chinense (hemsl.)Sarg.xl. Tulipifera l.) PhD dissertation. (Nanjing: Nanjing Forestry University; ). [Google Scholar]
- Xu Z., Liu Y., Xu L., An D. (2019). Bioinformatics analysis of nf-yb gene family of transcription factor in Zea mays . Mol. Plant Breed. 17, 3807–3816. doi: 10.13271/j.mpb.017.003807 [DOI] [Google Scholar]
- Xu J., Niu B., Chen C. (2022). Advances on the function of nf-y transcription factors in regulation of plant growth and development. Zhiwu Shengli Xuebao/Plant Physiol. J. 58, 1191–1200. doi: 10.13592/j.cnki.ppj.300045 [DOI] [Google Scholar]
- Xu G., Tao Z., He Y. (2022). Embryonic reactivation of flowering locus c by abscisic acid-insensitive 3 establishes the vernalization requirement in each arabidopsis generation. Plant Cell 34, 2205–2221. doi: 10.1093/plcell/koac077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang C., Xu X., Cai R., Guan Q., Chen X., et al. (2023). Riboflavin mediates m6a modification targeted by mir408, promoting early somatic embryogenesis in longan. Plant Physiol. 192 (3), 1799–1820. doi: 10.1093/plphys/kiad139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Kagaya Y., Toyoshima R., Kagaya M., Takeda S., Hattori T., et al. (2009). Arabidopsis NF-YB subunits LEC1 and LEC1-LIKE activate transcription by interacting with seed-specific ABRE-binding factors. Plant J. 58, 843–856. doi: 10.1111/j.1365-313X.2009.03817.x [DOI] [PubMed] [Google Scholar]
- Yang Z., Xia J., Hong J., Zhang C., Wei H., Ying W., et al. (2022). Structural insights into auxin recognition and efflux by Arabidopsis PIN1. Nature 609, 611–615. doi: 10.1038/s41586-022-05143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K., Takahata K., Kamada H. (2004). Isolation of the gene encoding carrot Leafy cotyledon1 and expression analysis during somatic and zygotic embryogenesis. Plant Physiol. Biochem. PPB Société Française Physiol. Végétale 42, 215–223. doi: 10.1016/j.plaphy.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Zhang S. (2022). Functional study of the ERF6-GPAT regulatory network based on single-cell transcriptome during the early somatic embryogenesis of longan. PhD dissertation. (Fujian Agriculture and Forestry University; ). [Google Scholar]
- Zhang X., Liang Z., Chen X., Zhang S., Lai Z., Lin Y., et al. (2021). Subcellular localization analysis and responses to exogenous ABA and SA in longan callus of DCL4 . Chin. J. Trop. Crops 42, 1514–1519. doi: 10.3969/j.issn.1000-2561.2021.06.002 [DOI] [Google Scholar]
- Zhang Y., Lin S., Zhou Y., Wen J., Kang X., Han X., et al. (2021). PdNF-YB21 positively regulated root lignin structure in poplar. Ind. Crops Prod. 168, 113609. doi: 10.1016/j.indcrop.2021.113609 [DOI] [Google Scholar]
- Zhang S., Wong L., Meng L., Lemaux P. (2002). Similarity of expression patterns of knotted1 and ZmLEC1 during somatic and zygotic embryogenesis in maize (Zea mays L.). Planta 215, 191–194. doi: 10.1007/s00425-002-0735-3 [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhu C., Zhang X., Liu M., Xue X., et al. (2023). Single-cell rna sequencing analysis of the embryogenic callus clarifies the spatiotemporal developmental trajectories of the early somatic embryo in Dimocarpus longan . Plant J. For Cell Mol. Biol. 115 (5), 1277–1297. doi: 10.1111/tpj.16319 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang Y., Bai X., Lin R., Shi G., Du P., et al. (2022). Tanf-yb11, a gene of NF-Y transcription factor family in t. Aestivum, confers plant drought tolerance via modulating osmolyte accumulation and ros homeostasis. J. Integr. Agric. 21, 3114–3130. doi: 10.1016/j.jia.2022.07.058 [DOI] [Google Scholar]
- Zheng W., Xu Z., Feng Z., Li L., Chen M., Chai S., et al. (2012). Genome-wide identification, classification, and expression of NF-YB gene family in soybean. Acta Agronomica Sin. 38, 1570–1582. doi: 10.3724/SP.J.1006.2012.01570 [DOI] [Google Scholar]
- Zhou T., Yu D., Sun Z., Sun X., Tan Y., Sheng X., et al. (2018). Bioinformatics analysis of the NF-YB gene family in rice. Mol. Plant Breed. 16, 5849–5861. doi: 10.13271/j.mpb.016.005849 [DOI] [Google Scholar]
- Zhou Y., Zhang Y., Wang X., Han X., An Y., Lin S., et al. (2020). Root-specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in populus. New Phytol. 227, 407–426. doi: 10.1111/nph.16524 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The nucleotide sequence of DlNF-YB6 and DlNF-YB9 promoter. Note: The putative regulatory elements and hormone-responsive elements on both strands are shown in bold and underlined, which was performed by the PlantCARE database.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material .