Abstract
We show that the coumeromycin antibiotic novobiocin, a potent inhibitor of ADP ribosylation, prevents lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-6, and IL-10 secretion in human peripheral blood mononuclear cells. It shares these cytokine-suppressing properties with other inhibitors of ADP ribosylation. We found that novobiocin prevents TNF-α production by inhibiting translation of the TNF-α mRNA. Elevated TNF-α levels in mice treated with d-galactosamine (GalN)-LPS or GalN-TNF were not reduced by novobiocin; however, the drug exhibited hepatoprotective properties. Novobiocin causes downregulation of the surface molecules on monocytes, among which CD14 was the most affected. The diminished expression of surface molecules was not observed on T and B lymphocytes. Similar to other inhibitors of ADP ribosylation, novobiocin prevents LPS-induced phosphate labelling of γ-actins.
Antibiotics are widely used as bacteriostatic or bactericidal drugs in the therapy of bacterial infections. Whereas the interaction between antibiotics and bacteria and between bacteria and the immune system has been well studied, little is known about the effects of antibiotics on the immune system. In the study described in the present paper we studied the effect of the antibiotic novobiocin on the immune functions of human monocytes. The coumarin novobiocin is produced by Streptomyces (S. niveaus and S. spheroides), and its main targets are gram-positive bacteria. It acts by inhibiting the bacterial gyrase activity. Novobiocin interferes with metabolic processes not only in bacteria but also in eukaryotic cells. It has been shown to function as a potent inhibitor of ADP ribosylation (1), i.e., the covalent attachment of multiple or single residues of the ADP ribose moiety of NAD to various proteins. ADP ribosylation, like phosphorylation, constitutes an important mechanism in posttranslational modifications of cellular proteins (10). By using the inhibitor novobiocin we tried to assess how far this form of modification might be involved in the regulation of certain monocyte functions. In the study described here we showed that novobiocin effectively suppresses the production of proinflammatory cytokines as well as the anti-inflammatory cytokine interleukin-10 (IL-10). It induces shedding of CD14 and modulates the expression of other surface antigens. When administered to mice receiving GalN-lipopolysaccharide (LPS) novobiocin prevents an increase in serum transaminase activity. We found that novobiocin inhibits protein biosynthesis and alters the phosphorylation state of cytosolic proteins, indicating that it may exert its immunomodulatory properties by interference with these intracellular events probably via ADP ribosylation-dependent processes.
MATERIALS AND METHODS
Reagents.
LPS (Escherichia coli O55:B5), novobiocin, and nicotinamide were from Sigma (Deisenhofen, Germany). l-[4,5-3H]leucine (specific activity, 58 Ci/mmol) and [γ-32P]ATP (specific activity, 5,000 Ci/mmol) were purchased from Amersham Buchler (Braunschweig, Germany).
Cell preparation.
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by centrifugation over a Ficoll-Paque (Pharmacia, Freiburg, Germany) density gradient (23). After repeated washing in phosphate-buffered saline (PBS) the PBMCs were further separated by counterflow centrifugation with the J6-MC elutriator system (Beckman Instruments, Palo Alto, Calif.). The monocyte fraction consisted of >92% monocytes, as determined by morphology and immunofluorescence staining with a monoclonal antibody (MAb) against CD14 (BL-M/G14). Peritoneal macrophages were collected from adult BALB/c mice that immediately after killing had been injected with 3 ml of RPMI 1640 containing 10% fetal calf serum (FCS).
Stimulation of cells.
PBMCs or monocytes (4 × 106/ml) were suspended in RPMI 1640 supplemented with 10% (vol/vol) FCS and antibiotics in 24-well culture plates (662160; Greiner Frickenhausen, Germany) and incubated with novobiocin or nicotinamide for 10 min prior to the addition of LPS for 6 h. The supernatants were collected, centrifuged, and analyzed for cytokine concentrations.
Preparation of cytosolic supernatant and in vitro phosphorylation.
After monocytes (4 × 106/ml) suspended in Falcon tubes (Becton Dickinson, Heidelberg, Germany) had been stimulated as described above, they were washed twice in ice-cold PBS. To disrupt the cells, the cell pellet was resuspended in ice-cold permeabilizing buffer containing 10 mM Tris-HCl (pH 7.8), 1 mM EDTA, 4 mM MgCl2, 30 mM 2-mercaptoethanol, and 1 mM vanadate, left on ice for 10 min, and sonicated (12 strokes; intensity, 1.1; duty cycle, 80%; Branson Sonifier 250; Danbury, Branson, Conn.). The cytosol was obtained by ultracentrifugation at 100,000 × g for 1 h.
Aliquots of the cytosolic supernatant containing about 30 μg of protein in a volume of 50 μl of buffer were added to 25 μl of the phosphorylation reaction mixture. The mixture contained 100 mM Tris-HCl (pH 7.8), 120 mM MgCl2, 0.01% leupeptin, 0.1 mM phenylmethylsulfonyl fluoride, and [γ-32P]ATP (5 μCi/aliquot). The reaction mixtures were incubated for 10 min at 37°C. The reactions were terminated by precipitating the proteins with methanol as described by Wessel and Flügge (27). The dried pellets were solubilized in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (14) and boiled for 3 min. The proteins were separated by SDS-PAGE (12% polyacrylamide gel) (14). The gels were stained with Coomassie blue (Serva Blue G; Serva, Heidelberg, Germany) (18) and dried, and autoradiography was performed with DuPont Cronex film 4 (DuPont, Bad Homburg, Germany) with Kodak X-OMAT intensifying screens (Eastman Kodak Co., New Haven, Conn.) at −70°C for 3 to 5 days.
Soluble CD14 quantitation.
The amount of soluble CD14 in the supernatants was determined by enzyme-linked immunosorbent assay (ELISA) as described previously (7). The ELISA kit was kindly provided by C. Krüger (Immuno Biological Laboratories, Hamburg, Germany).
Flow cytometry analysis.
The cells (2 × 105/50 μl of PBS) were incubated in the presence of 50 μl of MAb for 45 min at 4°C. The cells were then washed in ice-cold PBS containing 10% Haemacel and 0.1% sodium azide and were incubated for 30 min at 4°C with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse antibody (SIFIN, Berlin, Germany). After washing and fixation in 1% formaldehyde, the cells were analyzed in a FACScan analyzer (Becton Dickinson, San Jose, Calif.). The mean fluorescence intensities of labelled cells were recorded after gating of the cells by using their forward and side scatter properties. Ten thousand events were analyzed per sample and are presented as histograms with a four-decade logarithmic scale.
MAbs.
Fluorescein (FITC)-conjugated anti-CD14 MAbs were from Biometec (Greifswald, Germany); anti-HLA DR/DQ (MAb BL-Ia1), anti-CD2 (MAb BL-TP2a), anti-CD3 (MAb BL-TP3b), anti-CD4 (MAb BL-TH4), anti-CD5 (MAb BL-TP5), anti-CD6 (MAb BL-TP6a), anti-CD8 (MAb BL-TS8/2), anti-CD11b (MAb BL-M/G1), anti-CD11c (MAb BL-M11c), anti-CD14 (MAb BL-M/G14), anti-CD21 (MAb BL-B21), anti-CD22 (MAb BL-B22), anti-CD31 (MAb BL-M/G31), anti-CD40 (MAb BL-B40), anti-CD43 (MAb BL-TP43), anti-CD45 (MAb BL-Leuk45), anti-CD72 (BL-B72), and anti-CD76 (MAb BL-B76) MAbs were from DiaMAk (Leipzig, Germany); isotype control antibodies (immunoglobulin G1-FITC and immunoglobulin G2b-FITC) were from Sigma (Deisenhofen, Germany).
Protein synthesis.
Monocytes suspended at 2 × 105/ml in leucine-deficient RPMI 1640 supplemented with 10% dialyzed FCS were incubated with novobiocin in the presence of L-[4,5-3H]leucine (4 μCi/ml; specific activity, 58 Ci/mmol). After 3 h the cells were diluted with 2 ml of ice-cold 0.9% NaCl and were centrifuged (at 1,100 × g for 10 min). The washed cells were precipitated with 2 ml of 25% (wt/vol) trichloroacetic acid after the addition of 100 μl of 0.1% (wt/vol) bovine serum albumin. After 30 min at 4°C the resulting precipitates were recovered by centrifugation (3,000 × g for 10 min). The pellets were resuspended in 250 μl of 1 N KOH, precipitated again, collected on Whatman GF/C glass fiber filters, and washed with 25% trichloroacetic acid. The radioactivity on the filters was counted by liquid scintillation spectrometry.
Reverse transcription-PCR of TNF mRNA.
Monocytes (106/ml) were incubated in RPMI 1640 supplemented with 10% (vol/vol) FCS and antibiotics in Eppendorf vials (Eppendorf, Hamburg, Germany) in the presence and absence of LPS and novobiocin or nicotinamide. After 2 h the cells were washed in PBS and RNA was isolated with the RNeasy Total RNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Subsequent reverse transcription was performed as described previously (22). Genomic DNA was prepared by standard protocols (16). PCR was carried out in an automatic DNA thermal cycler (Crocodile III; Appligene, Reutlingen, Germany). To amplify cytokine-specific cDNA fragments the following gene-specific, intron-spanning primers were used: tumor necrosis factor alpha (TNF-α) sense primer (5′-GAG TGA CAA GCC TGT AGC-3′), TNF-α antisense primer (5′-CCC TTC TCC AGC TGG AAG-3′), β-actin sense primer (5′-AGC GGG AAA TCG TGC GTG-3′), and β-actin antisense primer (5′-CAG GGT ACA TGG TGG TGG TGC-3′). The reaction mixture containing 2 μl of cDNA, 1 μM sense and antisense primers, 200 μM deoxynucleoside triphosphates (MWG Biotech, Ebersberg, Germany), and 1.3 U of Taq polymerase (Gibco-BRL, Eggenstein, Germany) in a final volume of 35 μl. The cycle program was set for denaturation at 95°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1.5 min for a total of 25 cycles (β-actin) or 30 cycles (TNF-α). Electrophoresis of the PCR products was performed on 1.5% agarose gels (Gibco-BRL) containing 1 μg of ethidium bromide per ml. The DNA molecular weight marker VI (Boehringer, Mannheim, Germany) was used.
Detection of cytokines in culture supernatants.
Supernatants were collected for measurement of IL-1, IL-6, IL-10, and TNF-α concentrations. Determination of IL-1 concentration was based on the proliferation of IL-1-dependent human fibroblasts (15). IL-10 concentration was measured by a commercially available ELISA (Biosource, Ratingen, Germany) according to the manufacturer’s instructions. The concentrations of TNF-α and IL-6 were determined by ELISA. The primary anti-IL-6 and anti-TNF-α antibodies as well as the secondary purified rabbit polyclonal anti-IL-6 and TNF-α antibodies were kindly provided by W. Buurman (Maastricht University, Maastricht, The Netherlands). The peroxidase-labelled goat anti-rabbit antibody was from SIFIN. Mouse TNF-α concentration was measured by a commercially available ELISA (Genzyme, Cambridge, Mass.).
Shedding of CD14.
The cells (4 × 106/ml) were incubated with a FITC-labelled anti-CD14 MAb for 20 min at 4°C. The cells were washed with PBS and incubated in RPMI 1640 containing 10% FCS with LPS (100 ng/ml) or without LPS in the presence or absence of novobiocin. After washing of the cells, the cells were analyzed by fluorescence-activated cell sorter (FACS) analysis. A decrease in fluorescence indicates shedding of CD14; it is not the result of internalization since internalized CD14 is also detected by this method. No change in fluorescence could mean either no modulation or internalization.
GalN-LPS-TNF-α challenge in vivo.
BALB/c mice (age, 6 to 8 weeks) were obtained from the internal animal breeding house of the Institute of Experimental and Clinical Pharmacology and Toxicology (University of Erlangen-Nürnberg, Erlangen, Germany). The animals received humane care according to the guidelines of the National Institutes of Health as well as the legal requirements in Germany and were maintained under controlled conditions (22°C, 55% humidity, 12-h day and 12-h night rhythm) and were fed a standard laboratory chow (Altromin 1313) ad libitum. All reagents were injected in a total volume of 250 μl per 25-g mouse. The mice were pretreated intraperitoneally with 100 mg of novobiocin (Sigma, Deisenhofen, Germany) per kg of body weight; control animals received saline instead of novobiocin. Sixty minutes later the mice were challenged intraperitoneally either with a combination of 700 mg of d-galactosamine (GalN; Carl Roth GmbH + Co., Karlsruhe, Germany) plus 10 μg of LPS per kg from Salmonella abortus equi, S form (Metalon GmbH, Ragow, Germany), or with 700 mg of GalN per kg intraperitoneally plus 7.5 μg TNF-α (kindly provided by G. R. Adolf, Bender & Co., Vienna, Austria) intravenously. For determination of circulating TNF-α, blood samples were taken from the tail vein 60 min after GalN-LPS challenge. Eight hours after challenge blood was withdrawn by cardiac puncture and placed into heparinized syringes while the mice were under lethal pentobarbital sodium (Nembutal 150 mg/kg given intravenously; Sanofi-Ceva, Wirtschaftsgenossenschaft Deutscher Tierärzte eG, Hannover, Germany) anesthesia for assessment of liver injury. Hepatocyte damage in vivo was assessed by measuring the activity of the liver-specific enzyme alanine aminotransferase (ALT) in plasma as described by Bergmeyer (3) by an automated procedure. TNF-α concentration was determined by ELISA (Pharmingen, Hamburg, Germany). A polyclonal sheep anti-mouse TNF-α capture antibody, purified on protein G columns (Pharmacia, Freiburg, Germany), was used to replace the Pharmingen capture MAb (13).
RESULTS
Effect of novobiocin on cytokine production.
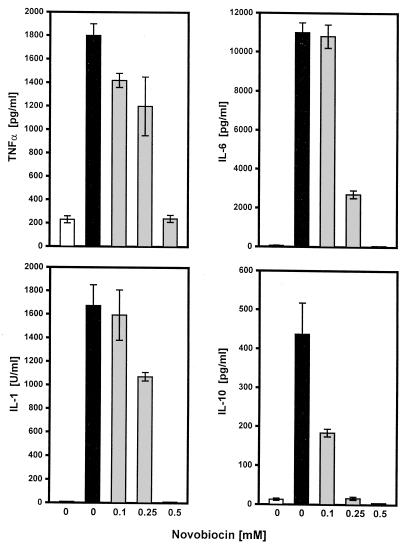
As indicated in Fig. 1, treatment of PBMCs with novobiocin results in a dose-dependent decrease in TNF-α, IL-1, IL-6, and IL-10 concentrations. At 0.5 mM, cytokine production was nearly completely abolished. Toxic effects can be excluded because cell viability as judged by trypan blue exclusion and by FACS analysis (staining with fluorescein diacetate and propidium iodide) was not altered by 0.5 mM novobiocin. FACS analysis was used to determine the percentage of necrotic cells. The cells were incubated with fluorescein diacetate (green fluorescence indicates living cells) and propidium iodide (red fluorescence indicates dead cells), and the percentage of necrotic cells was calculated according to the fluorescence intensities. Whereas in the absence of novobiocin 1 to 2% of the cells were necrotic, the percentage of necrotic cells hardly increased (4 to 5%) in the presence of 0.5 mM novobiocin.
FIG. 1.
Effect of novobiocin on LPS-induced cytokine release. PBMCs (4 × 106/ml) were incubated in the absence or presence of different concentrations of novobiocin for 10 min before the addition of LPS (100 ng/ml) for 6 h. Supernatants were harvested and analyzed for cytokine content as described in Materials and Methods. Values are means ± standard deviations of one of four experiments. ■, with LPS; □, without LPS; , preincubation with novobiocin.
To assess the effects of novobiocin at different stages of LPS-induced TNF-α production we performed a time course study (Table 1). PBMCs were incubated in the presence of LPS for 6 h, and novobiocin was added either before the addition of LPS, at the same time as the addition of LPS, or after the addition of LPS.
TABLE 1.
Time course of suppression of TNF-α formation by novobiocina
| Time of addition of novobiocin (min) | TNF-α concn (pg/ml) | Inhibition (% of that of control) |
|---|---|---|
| −10 | 602 ± 23 | 93.2 |
| 0 | 610 ± 21 | 93.1 |
| 15 | 672 ± 42 | 92.4 |
| 60 | 596 ± 21 | 93.3 |
| 120 | 1,192 ± 42 | 86.5 |
| 180 | 1,914 ± 80 | 78.3 |
| 240 | 4,262 ± 135 | 51.7 |
| 300 | 5,361 ± 75 | 39.9 |
| Control (no novobiocin) | 8,824 ± 242 |
PBMCs (4 × 106/ml) were incubated in the presence of LPS (100 ng/ml) for 6 h. Novobiocin (0.5 mM) was added at different times, and after 6 h the TNF-α level in the supernatant was measured. Values are means ± standard deviations of one of three experiments performed in duplicate. Values in the absence of novobiocin were 8,824 ± 242 pg/ml.
TNF-α production was almost completely suppressed when novobiocin was added 10 min before the addition of LPS, simultaneously with the addition of LPS, and 1 h after stimulation with LPS. The inhibition gradually declined with increasing intervals between the time of stimulation with LPS and the subsequent addition of novobiocin. After 4 h novobiocin still affected TNF-α production, presumably by inhibiting protein synthesis.
Novobiocin inhibits protein synthesis.
The inhibition of LPS-stimulated cytokine secretion by novobiocin occurs in a setting in which many proteins are being synthesized by both constitutive and induced mechanisms. Thus, we assumed that novobiocin should affect protein biosynthesis. Table 2 indicates that the incubation of monocytes for 3 h with novobiocin leads to a concentration-dependent inhibition of [3H]leucine incorporation into proteins. Seventy percent inhibition was observed in the presence of 0.5 mM novobiocin.
TABLE 2.
Effect of novobiocin on leucine uptakea
| Addition | Level of 3H incorporation (cpm [103]) | Inhibition (% of that of control) |
|---|---|---|
| None | 4.46 ± 0.63 | |
| Novobiocin | ||
| 0.125 mM | 2.89 ± 0.14 | 35.5 |
| 0.25 mM | 2.14 ± 0.13 | 52.1 |
| 0.5 mM | 1.46 ± 0.05 | 67.3 |
Monocytes (2 × 105/ml) were incubated with l-[4,5-3H]leucine (4 μCi/ml) in the absence or presence of different concentrations of novobiocin. After 3 h, the levels of incorporation of l-[4,5-3H]leucine into proteins was measured. The results are means ± standard deviations of one of two experiments performed in duplicate.
Novobiocin does not inhibit TNF-α mRNA expression.
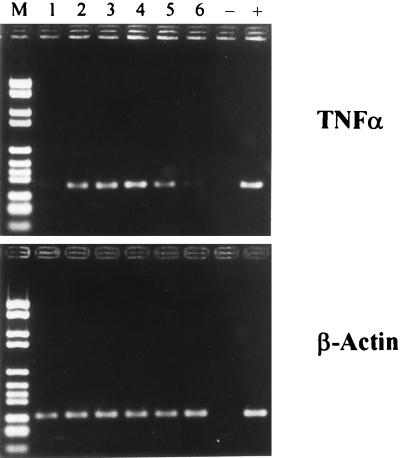
In previous studies we have shown that inhibitors of ADP ribosylation, namely, nicotinamide, 3-aminobenzamide, 3-methoxybenzamide, and meta-iodobenzylguanidine, not only suppress protein synthesis but also suppress mRNA synthesis (8, 11). To test whether novobiocin also acts at the transcriptional level, we measured its effects on TNF-α mRNA expression. Monocytes were stimulated with LPS in the presence or in the absence of novobiocin or nicotinamide. Two hours after incubation mRNA expression of TNF-α was determined by reverse transcription-PCR. As seen in Fig. 2, LPS induces TNF-α mRNA expression (lane 2). In contrast to nicotinamide (lane 6), novobiocin (lanes 3 to 5) does not suppress mRNA levels at any novobiocin concentration tested.
FIG. 2.
Effect of novobiocin on the expression of TNF-α mRNA. Monocytes (2 × 106/ml) were incubated for 10 min with various concentrations of novobiocin or nicotinamide before 100 ng of LPS per ml was added for 2 h. mRNA was isolated and reverse transcription-PCR was then performed with primers specific for TNF-α and β-actin. Data represent the results of one of four representative experiments. Lanes: 1, unstimulated monocytes; 2, LPS-stimulated monocytes; 3 to 5, LPS-stimulated monocytes in the presence of 0.1, 0.25, and 0.5 mM novobiocin, respectively; 6, LPS-stimulated monocytes in the presence of 25 mM nicotinamide. The controls were no cDNA (lane −) and defined cDNA of the concerned protein (lane +). Lane M, marker (DNA molecular weight marker VI).
Novobiocin prevents an increase in transaminase activity in mice.
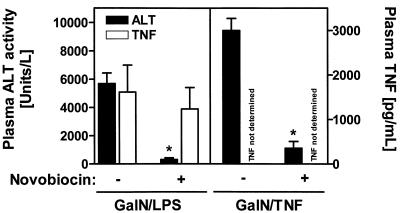
Having shown that novobiocin inhibits LPS-induced TNF-α production in human monocytes we next assessed whether the drug had similar effects in mice treated with GalN-LPS or GalN-TNF.
Treatment with these compounds has been described to result in increased serum TNF-α levels and transaminase activities (6). As seen in Fig. 3 novobiocin effectively prevents an increase in transaminase activity when it is given prior to the administration of GalN-LPS. It hardly affects TNF-α concentrations. The effective suppression of transaminase activity by novobiocin was also observed when LPS was exchanged for TNF-α in the animal model. The inability of novobiocin to reduce the TNF-α concentrations in this model made us measure the effect of novobiocin on the TNF-α concentrations in the supernatants of mouse peritoneal macrophages stimulated with LPS. Treatment with novobiocin does not result in a dose-dependent decrease in TNF-α concentrations in mouse macrophages, as observed in human monocytes. For determination of TNF-α concentrations, the supernatants of mouse macrophages (106/ml) that were incubated in the absence or presence of different concentrations of novobiocin for 10 min before the addition of LPS (100 ng/ml) for 6 h were harvested and analyzed for TNF-α content. For mouse macrophages receiving no treatment and treatments with LPS alone, LPS–0.1 mM novobiocin, LPS–0.25 mM novobiocin, and LPS–0.5 mM novobiocin, the TNF-α concentrations (means ± standard deviations of one of two experiments) were 0, 200 ± 14, 338 ± 14, 796 ± 4, and 168 ± 9 pg/ml, respectively. The values increased with increasing concentrations of novobiocin, and only at a novobiocin concentration of 0.5 mM was a decrease which was not as drastic as that in human monocytes observed. This species-dependent response to novobiocin may in part account for the failure of novobiocin to suppress TNF-α concentrations in the animal model.
FIG. 3.
In vivo protection of mice from GalN-LPS- and GalN–TNF-α-induced liver injury due to novobiocin pretreatment. BALB/c mice received 700 mg of GalN per kg plus 10 μg of LPS per kg or 700 mg of GalN per kg plus 7.5 μg of TNF-α per kg 1 h after pretreatment with 100 mg of novobiocin per kg. Control animals were pretreated with saline instead of novobiocin. At 8 h after challenge blood was withdrawn for an alanine aminotransferase level determination. The plasma TNF-α level was determined 60 min following LPS treatment. Data are expressed as means ± standard errors of the means (n = 6 for GalN-LPS or n = 3 for GalN–TNF-α). ∗, P < 0.05 versus saline-pretreated mice.
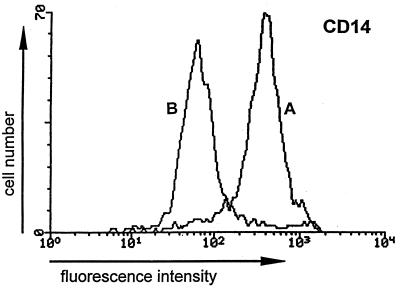
Decreased expression of CD14 is due to shedding.
As shown in Fig. 4, CD14 expression seems be extremely susceptible to novobiocin. To verify that the loss of CD14 was due to shedding and not to internalization, the cells were stained with an FITC-labelled anti-CD14 MAb and were incubated in the presence or absence of 0.5 mM novobiocin (Table 3). After various times, the fluorescence was measured. Compared to the fluorescence analyzed at time zero, there was a gradual decrease starting after 4 h of incubation. After 6 h the fluorescence was reduced to 22% of the original level. This means that CD14 had been shed; if it had been internalized the fluorescence would not have changed. The shedding is mainly caused by novobiocin; it is observed only to a minor extent in the absence of novobiocin (Table 3). To confirm that CD14 has been released, the amount of soluble CD14 was determined in the supernatants of cells that had been stimulated with LPS in the presence or absence of novobiocin. As seen in Table 4, treatment with novobiocin results in an increase in the soluble CD14 level, and after 6 h the soluble CD14 level is twice as high as that measured in the absence of novobiocin.
FIG. 4.
Effect of novobiocin on CD14 expression. PBMCs (4 × 106/ml) were incubated in the presence or absence of novobiocin (0.5 mM) for 10 min before LPS (100 ng/ml) was added for 6 h. The cells were analyzed by flow cytometry with MAbs against CD14. The fluorescence of labelled monocytes and lymphocytes was recorded after gating of the cells by using their forward and side scatter properties. Curves represent isotype-matched controls for cells treated with LPS in the presence (B) or absence (A) of novobiocin. Data represent the results of one of three experiments.
TABLE 3.
Effect of novobiocin on antibody-induced modulation of CD14 expression on human monocytesa
| Time (min) | Mean fluorescence (% of that of control) in the
presence of the following:
|
|
|---|---|---|
| LPS at 100 ng/ml | Novobiocin at 0.5 mM + LPS at 100 ng/ml | |
| 0 | 100 | 100 |
| 120 | 100 | 100 |
| 240 | 97 | 90 |
| 360 | 88 | 22 |
Monocytes (4 × 106/ml) were stimulated with LPS (100 ng/ml) in the absence or presence of 0.5 mM novobiocin. After 10 min the cells were incubated with FITC-labelled anti-CD14 MAb at 4°C for 20 min. Then cells were washed and incubated in culture medium at 37°C. After the indicated times cells were analyzed by FACS analysis. The mean fluorescence of the cells at time zero was taken as 100% (control). Data from one of three experiments are shown.
TABLE 4.
Effect of novobiocin on shedding of CD14a
| Time (min) | Soluble CD14 concn
(ng/ml) in the presence of the following:
|
|
|---|---|---|
| LPS at 100 ng/ml | Novobiocin at 0.5 mM + LPS at 100 ng/ml | |
| 0 | 0 | 0 |
| 120 | 0 | 0 |
| 240 | 5.5 ± 0.1 | 9.0 ± 1.1 |
| 360 | 25.0 ± 3.0 | 50.5 ± 8.2 |
PBMCs (4 × 106/ml) were incubated in the presence or absence of LPS (100 ng/ml) for 6 h. Novobiocin was added 10 min before addition of LPS for 6 h. Supernatants were harvested and analyzed for soluble CD14 by ELISA. Values are means ± standard deviations of one of two experiments.
Novobiocin changes the phosphorylation state of cytosolic proteins.
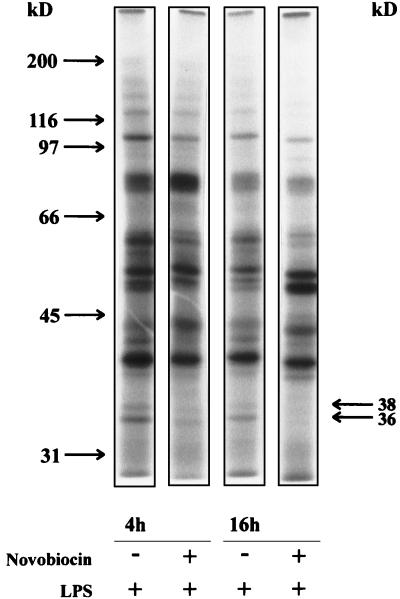
To investigate whether the phosphorylation pattern of activated cells was affected by novobiocin we stimulated monocytes with LPS for different times in the presence and absence of novobiocin and separated the cytosolic proteins by SDS-PAGE.
As illustrated in Fig. 5 preincubation with 0.5 mM novobiocin for 10 min resulted in changes in the phosphorylation states of several proteins. The changes were more pronounced after a 16-h incubation time than after a 4-h incubation time.
FIG. 5.
Effect of novobiocin on phosphorylation of cytosolic proteins. Monocytes (4 × 106/ml) were incubated in the presence or absence of 0.5 mM novobiocin for 10 min before stimulation with LPS (100 ng/ml). After 4 h, cytosolic supernatants were prepared and incubated with [γ-32P]ATP for 10 min. The proteins were separated by SDS-PAGE as described in Materials and Methods. The autoradiograph is representative of three experiments.
Novobiocin led to a diminished phosphorylation of cytosolic proteins of 36 and 38 kDa. This effect is also seen when other inhibitors of ADP ribosylation are used (11).
DISCUSSION
Novobiocin prevents LPS-induced TNF-α and IL-6 synthesis. This effect is shared by two other inhibitors of ADP ribosylation, namely, meta-iodobenzylguanidine and nicotinamide (11). In contrast to these two compounds, novobiocin does not reduce the TNF-α activity at the transcriptional level but reduces it at the translational level. By comparison, the TNF-α-suppressive drugs pentoxifylline and thalidomide exert their effects by inhibiting transcription of the TNF-α gene (4) or by enhancing the degradation of TNF-α mRNA (17), respectively. Thus, a combination of novobiocin with either drug may have synergistic effects.
Novobiocin prevents not only the production of proinflammatory cytokines but also that of the anti-inflammatory cytokine IL-10. IL-10 production has been shown to be under the control of TNF-α (24) so that the absence of TNF-α may be related to the missing IL-10 synthesis.
The ability of novobiocin to prevent TNF-α production in vitro made us examine its protective effects against an increase in TNF-α production and transaminase activity in GalN-sensitized LPS- or TNF-α-challenged mice. Novobiocin led to a drastic decrease in transaminase activity but did not reduce elevated TNF-α levels. The failure of novobiocin to suppress TNF-α production might be species-dependent since the TNF-α production of mouse macrophages seems to be less sensitive to novobiocin than the TNF-α production of human monocytes. These findings indicate that novobiocin does not protect mice from the deleterious effects of GalN-LPS by inhibiting endogenous TNF-α production. The beneficial effect of novobiocin becomes evident at the level of apoptosis induction by TNF-α, the central mediator of GalN-LPS-induced liver injury. TNF-α destroys liver cells only in GalN-sensitized mice. The mechanism by which novobiocin may inhibit the apoptotic pathway is not known.
Novobiocin not only possesses cytokine-suppressive activity but it also inhibits expression of MHC class II antigens (HLA-DR/DQ) and molecules that are involved in the leukocyte-endothelial cell interaction and in leukocyte migration (CD11b/11c; CD31) (data not shown). Given the role of MHC II molecules in antigen presentation, one might speculate that these cells are less effective accessory cells and that they are reduced in their ability to adhere to the endothelium and to migrate into tissues. Thus, these cells may be less efficient in encountering a bacterial challenge.
One major initiator in innate immune responses to bacterial infections is LPS, the major component of the outer membrane of gram-negative bacteria (20). LPS complexed with LPS-binding protein interacts with CD14 receptors on the cell membrane (mCD14) leading to subsequent cell activation (26, 28). CD14 in its soluble form (2) also binds to LPS and transmits its action to cells which are devoid of mCD14, such as endothelial or epithelial cell (5), and at higher concentrations it blocks the binding of LPS to monocytes (21). The level of CD14 expression was found to be drastically reduced in response to novobiocin (Fig. 4). Because the diminished level of expression was due to shedding an increase in the level of soluble CD14 was observed (Table 4).
The consequences of this effect are difficult to predict. Monocytes may be prevented from responding to LPS or to other stimuli like soluble peptidoglycan which also interact with mCD14 (25), or on the other hand, endothelial cells may be converted to an activation phenotype expressing both proinflammatory and procoagulant properties (19). When analyzing the expression of surface molecules on T and B lymphocytes, neither T-cell-specific (CD2, CD3, CD4, CD5, CD6, and CD8) nor B-cell-specific (CD21, CD22, CD40, CD72, and CD76) proteins were downregulated, indicating that the selective suppressive effects of the drug on monocytes are not secondary to a generalized mechanism.
The fact that novobiocin inhibits LPS-induced cytokine production implies that it may interfere with signal transduction events that have been shown to be involved in cytokine synthesis such as phosphorylation-dephosphorylation. Treatment of monocytes with novobiocin results in changes in the phosphorylation pattern. It inhibits phosphorylation of several proteins including γ-actins (9). The fact that two other inhibitors of ADP ribosylation were also shown to inhibit phosphorylation of the proteins (11) points to a role of ADP ribosylation in processes resulting in phosphorylation of β- and γ-actins. The extent of a correlation between inhibition of cytokine production and inhibition of phosphorylation of β- and γ-actins remains to be established.
The novobiocin concentrations that we used in the in vitro and in vivo studies are within the range of concentrations achievable in plasma (0.16 mM and more), which are reached when novobiocin is used as an anticancer drug at 4 g/day (12). Thus, application of novobiocin for the treatment of bacterial infections or in clinical trials as an anticancer drug may be accompanied by modulated immune responses such as cytokine production and the expression of surface molecules on monocytes. It may block these reactions by virtue of its ability to inhibit endogenous ADP ribosylation.
ACKNOWLEDGMENT
This work was in part supported by the Deutsche Forschungsgemeinschaft (grant Ha 2484/1-1).
REFERENCES
- 1.Banasik M, Komura H, Shimoyama M, Ueda K. Specific inhibitors of poly (ADP-ribose) synthetase and mono (ADP-ribosyl) transferase. J Biol Chem. 1992;267:1569–1575. [PubMed] [Google Scholar]
- 2.Bazil V, Strominger J L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. [PubMed] [Google Scholar]
- 3.Bergmeyer H U. Methods of enzymatic analysis. 3rd ed. Vol. 82. Weinheim, Germany: Verlag Chemie; 1984. [Google Scholar]
- 4.Doherty G M, Jensen J C, Alexander H R, Buresh C M, Norton J A. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery (St Louis) 1991;110:192–198. [PubMed] [Google Scholar]
- 5.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5948. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunwald U, Krüger C, Westemann J, Leikowsky A, Ehlers M, Schütt C. An enzyme-linked immunosorbent assay for the quantification of solubilized CD14 in biological fluids. J Immunol Methods. 1992;155:225–232. doi: 10.1016/0022-1759(92)90289-6. [DOI] [PubMed] [Google Scholar]
- 8.Hauschildt S, Scheipers P, Bessler W G. Inhibitors of poly (ADP-ribose) polymerase suppress lipopolysaccharide-induced nitrite formation in macrophages. Biochem Biophys Res Commun. 1991;179:865–871. doi: 10.1016/0006-291x(91)91898-m. [DOI] [PubMed] [Google Scholar]
- 9.Hauschildt S, Schwarz C, Heine H, Ulmer A J, Flad H D, Rietschel E T, Jensen O N, Mann M. Actin: a target of lipopolysaccharide-induced phosphorylation in human monocytes. Biochem Biophys Res Commun. 1997;241:670–674. doi: 10.1006/bbrc.1997.7887. [DOI] [PubMed] [Google Scholar]
- 10.Hayaishi O, Ueda K, editors. ADP-ribosylation reactions: biology and medicine. New York, N.Y: Academic Press, Inc.; 1982. [Google Scholar]
- 11.Heine H, Ulmer A J, Flad H-D, Hauschildt S. Lipopolysaccharide-induced change of phosphorylation of two cytosolic proteins in human monocytes is prevented by inhibitors of ADP-ribosylation. J Immunol. 1995;155:4899–4908. [PubMed] [Google Scholar]
- 12.Kennedy M J, Armstrong D K, Huelskamp A M, Ohly K, Clarke B V, Colvin O M, Grochow L B, Chen T L, Davidson N E. Phase I and pharmacologic study of the alkylating agent modulator novobiocin in combination with high-dose chemotherapy for the treatment of metastatic breast cancer. J Clin Oncol. 1995;13:1136–1143. doi: 10.1200/JCO.1995.13.5.1136. [DOI] [PubMed] [Google Scholar]
- 13.Küsters S, Gantner F, Künstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Loppnow H, Flad H-D, Dürrbaum I, Musehold J, Fetting R, Ulmer A J, Herzbeck H, Brandt E. Detection of interleukin-1 with human dermal fibroblasts. Immunobiology. 1989;179:283–291. doi: 10.1016/S0171-2985(89)80023-3. [DOI] [PubMed] [Google Scholar]
- 16.Maniatis T, Fritsch E F, Sambrook J. Extraction, purification and analysis of messenger RNA from eukaryotic cells. In: Nolan C, editor. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. p. 71. [Google Scholar]
- 17.Moreira A L, Sampaio E P, Zmuidzinas A, Frindt P, Smith K A, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuhoff V, Stamm R, Eibl H. Clear background and highly sensitive protein staining with Coomassie blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis. 1985;6:427. [Google Scholar]
- 19.Noel R F, Jr, Sato T T, Mendez C, Johnson M, Pohlmann T H. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria require soluble CD14. Infect Immun. 1995;63:4046–4053. doi: 10.1128/iai.63.10.4046-4053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raetz C R H. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 21.Schütt C, Schilling T, Grunwald U, Schönfeld W, Krüger C. Endotoxin neutralising capacity of soluble CD14. Res Immunol. 1992;143:71–78. doi: 10.1016/0923-2494(92)80082-v. [DOI] [PubMed] [Google Scholar]
- 22.Thanhäuser A, Reiling N, Böhle A, Toellner K M, Duchrow M, Scheel D, Schluter C, Ernst M, Flad H-D, Ulmer A J. Pentoxifylline: a potent inhibitor of IL-2 and IFN-gamma biosynthesis and BCG-induced cytotoxicity. Immunology. 1993;80:151–156. [PMC free article] [PubMed] [Google Scholar]
- 23.Ulmer A J, Scholz W, Ernst M, Brandt E, Flad H-D. Isolation and subfractionation of human peripheral blood mononuclear cells (PMNC) by density gradient centrifugation on Percoll. Immunobiology. 1984;116:238–250. doi: 10.1016/S0171-2985(84)80042-X. [DOI] [PubMed] [Google Scholar]
- 24.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-α in human monocytes IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- 25.Weidemann B, Brade H, Rietschel E T, Dziarki R, Bazil V, Kusumoto S, Flad H-D, Ulmer A J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein S L, June C H, Franco A L. Lipopolysaccharide-induced protein tyrosine phosphorylation in human macrophages is mediated by CD14. J Immunol. 1993;151:3829–3838. [PubMed] [Google Scholar]
- 27.Wessel D, Flügge U I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 28.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]