Key Points
Question
Does the addition of resuscitative endovascular balloon occlusion of the aorta (REBOA) to standard care reduce mortality in trauma patients with exsanguinating hemorrhage?
Findings
In this bayesian randomized clinical trial that included 89 patients at 90 days, all-cause mortality was 54% in the REBOA and standard care group vs 42% in the standard care alone group (odds ratio, 1.58; posterior probability of increased odds of death with REBOA, 86.9%).
Meaning
In trauma patients with exsanguinating hemorrhage, a strategy of REBOA and standard care in the emergency department does not reduce, and may increase mortality compared with standard care alone.
Abstract
Importance
Bleeding is the most common cause of preventable death after trauma.
Objective
To determine the effectiveness of resuscitative endovascular balloon occlusion of the aorta (REBOA) when used in the emergency department along with standard care vs standard care alone on mortality in trauma patients with exsanguinating hemorrhage.
Design, Setting, and Participants
Pragmatic, bayesian, randomized clinical trial conducted at 16 major trauma centers in the UK. Patients aged 16 years or older with exsanguinating hemorrhage were enrolled between October 2017 and March 2022 and followed up for 90 days.
Intervention
Patients were randomly assigned (1:1 allocation) to a strategy that included REBOA and standard care (n = 46) or standard care alone (n = 44).
Main Outcomes and Measures
The primary outcome was all-cause mortality at 90 days. Ten secondary outcomes included mortality at 6 months, while in the hospital, and within 24 hours, 6 hours, or 3 hours; the need for definitive hemorrhage control procedures; time to commencement of definitive hemorrhage control procedures; complications; length of stay; blood product use; and cause of death.
Results
Of the 90 patients (median age, 41 years [IQR, 31-59 years]; 62 [69%] were male; and the median Injury Severity Score was 41 [IQR, 29-50]) randomized, 89 were included in the primary outcome analysis because 1 patient in the standard care alone group declined to provide consent for continued participation and data collection 4 days after enrollment. At 90 days, 25 of 46 patients (54%) had experienced all-cause mortality in the REBOA and standard care group vs 18 of 43 patients (42%) in the standard care alone group (odds ratio [OR], 1.58 [95% credible interval, 0.72-3.52]; posterior probability of an OR >1 [indicating increased odds of death with REBOA], 86.9%). Among the 10 secondary outcomes, the ORs for mortality and the posterior probabilities of an OR greater than 1 for 6-month, in-hospital, and 24-, 6-, or 3-hour mortality were all increased in the REBOA and standard care group, and the ORs were increased with earlier mortality end points. There were more deaths due to bleeding in the REBOA and standard care group (8 of 25 patients [32%]) than in standard care alone group (3 of 18 patients [17%]), and most occurred within 24 hours.
Conclusions and Relevance
In trauma patients with exsanguinating hemorrhage, a strategy of REBOA and standard care in the emergency department does not reduce, and may increase, mortality compared with standard care alone.
Trial Registration
isrctn.org Identifier: ISRCTN16184981
This randomized clinical trial compares the effectiveness of resuscitative endovascular balloon occlusion of the aorta and standard care in the emergency department vs standard care alone on mortality in trauma patients with exsanguinating hemorrhage.
Introduction
Hemorrhage is the most common cause of preventable death after trauma.1 The natural history of uncontrolled bleeding is decreasing cardiac output and hypotension, and ultimately failure of compensatory mechanisms with consequent cerebral and myocardial hypoperfusion leading to death.2 In contrast, when hemorrhage is controlled expeditiously, patients often recover.3 Bleeding originating from within the torso is particularly challenging because it cannot be controlled without surgery,4,5,6 and many patients die before they can be taken to an operating room. Temporary aortic occlusion, which is used to limit hemorrhage and maintain cerebral and myocardial perfusion until definitive control of hemorrhage can be obtained, is conceptually attractive.7,8,9
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a novel technique whereby a percutaneously inserted balloon is inflated in the aorta. Large animal studies have shown REBOA to be highly effective,10,11,12,13 but the current evidence for REBOA in injured humans is limited and conflicting. There are case series,14,15,16 cohort studies (retrospective and prospective),17,18,19,20,21 and scoping reviews, systematic reviews, and meta-analyses22,23,24,25,26 with divergent results. There are also military clinical practice guidelines27 that recommend REBOA for profound shock (defined as a systolic blood pressure <90 mm Hg) and for some cases of traumatic cardiac arrest (blunt and penetrating).
A position statement from the American College of Emergency Physicians and the American College of Surgeons28 recommends REBOA for traumatic life-threatening hemorrhage below the diaphragm in patients with hemorrhagic shock who are unresponsive or transiently responsive to resuscitation, and for patients arriving at the hospital in cardiac arrest from trauma due to presumed life-threatening hemorrhage below the diaphragm. However, there are no randomized clinical trials.
The aim of the UK Resuscitative Endovascular Balloon Occlusion of the Aorta (UK-REBOA) trial was to examine the effectiveness of REBOA and standard care compared with standard care alone for the management of uncontrolled torso hemorrhage at specialist major trauma centers in the UK.
Methods
Study Design and Eligibility Criteria
This multicenter, open label, bayesian, group-sequential, registry-enabled, randomized clinical trial was conducted at 16 major trauma centers in the UK (eTable 1 in Supplement 1). The patients were enrolled under the provisions for adults not able to consent for themselves.
The trial protocol was published in advance29 and appears in Supplement 2. The statistical analysis plan was completed before data analysis commenced and appears in Supplement 3. The trial was approved by the Greater Manchester research ethics committee.
Trauma patients aged (or believed to be) 16 years or older presenting to major trauma centers in the UK were eligible for inclusion. Patients had confirmed or suspected life-threatening torso hemorrhage, which was deemed to be amenable to adjunctive treatment with REBOA. Patients were excluded if they were known or thought to be pregnant or had injuries that were clearly not survivable (eTable 2 in Supplement 1). Consent for continued participation in the trial was sought once patients were no longer in a life-threatening condition.
Randomization
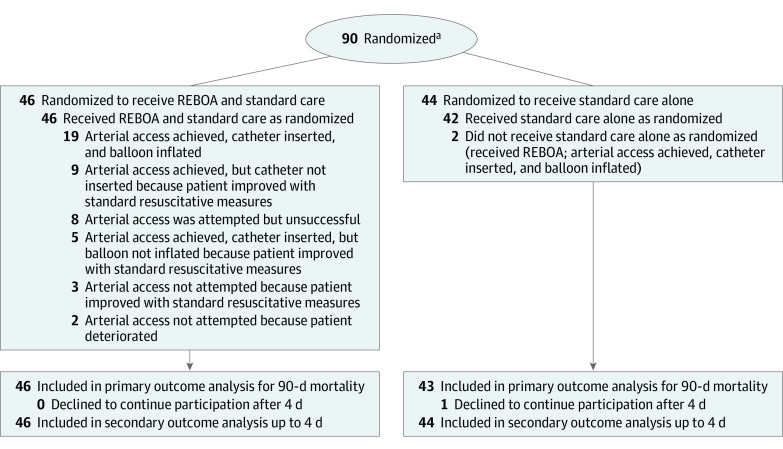
The patients were randomly assigned in a 1:1 ratio to either a strategy of REBOA and standard care or standard care alone (Figure 1). Randomization was performed using a web-based system in random permuted block sizes of 2 or 4; clinicians could access the system using their cell phones. It was not possible for physicians at the trial sites to be blinded to treatment assignments.
Figure 1. Recruitment, Randomization, and Patient Flow in the UK-REBOA Trial.
REBOA indicates resuscitative endovascular balloon occlusion of the aorta; UK-REBOA, UK Resuscitative Endovascular Balloon Occlusion of the Aorta.
aTrauma patients aged 16 years or older were eligible for inclusion in this trial. Patients deemed ineligible for the REBOA intervention were excluded prior to randomization.
Study Interventions
The intervention was the technique of REBOA for the purpose of resuscitation as part of an overall resuscitation strategy. The trial sought to evaluate the technique of REBOA rather than a specific brand of device and therefore did not prescribe or mandate a particular product.
Clinicians using REBOA were required to complete the trial’s training package (eMethods and eFigure 1 in Supplement 1), subsequent in-house training conducted as part of the establishment of each center’s REBOA service, or both. The level of occlusion (zone I, descending thoracic aorta; or zone III, above aortic bifurcation) was left to the judgment of the attending physicians and their assessment of the likely source of hemorrhage.
Patients in the standard care alone group received the expected care that is provided at a major trauma center. Such treatment typically included intubation, balanced blood product transfusion, interventions such as tourniquet application, and early operative or endovascular hemorrhage control. Treatment could also include open aortic occlusion of the thoracic or abdominal aorta.
Primary and Secondary Outcomes
The primary outcome was all-cause mortality at 90 days. Prespecified secondary outcomes included mortality at 6 months, while in the hospital, and within 24 hours, 6 hours, or 3 hours; the need for definitive hemorrhage control procedures (defined as an operation that involved resection of a bleeding organ, ligation of a named vessel, interposition grafting, shunt insertion, packing of a cavity, or angiographic embolization); time to commencement of definitive hemorrhage control procedures; complications; length of stay (hospital-free and intensive care unit–free days); blood product use; and cause of death.
Baseline data were obtained through linkage to the UK Trauma Audit and Research Network registry. Primary outcome and most secondary outcome data were collected directly from assessments of the patients or from NHS Digital, which is the National Health Service’s data repository (eTable 3 in Supplement 1).
Statistical Analysis
The output from a bayesian trial provides a probability for a defined range of treatment effects given the observed data. The trial was designed around the available number of patients based on a retrospective study of national registry data.30 We estimated that 10 high-volume major trauma centers would admit approximately 80 patients who might benefit from REBOA per year, and that approximately half of whom would be enrolled in the trial over a period of 3 years, providing a target recruitment of 120 participants.
The trial was designed to evaluate the clinical effectiveness of REBOA in real-world clinical settings and to answer the question of whether a treatment strategy that includes REBOA reduces mortality in patients with exsanguinating hemorrhage, irrespective of intercurrent events.31 The primary analysis was thus based on the intention-to-treat principle.
The initial design parameters contained an error in the formulation of the variance in the calculations, resulting in an overestimation of the operating characteristics. After consultation with the National Institute for Health Research (the study’s funder) and external reviewers, we relaxed the success threshold and added informative priors, resulting in acceptable probabilities for declaring success if REBOA and standard care were beneficial. Given the direction and size of the observed effect size, these changes did not influence the interpretation of the findings.
The primary outcome was analyzed using bayesian logistic regression with a minimally informative prior on the natural log odds ratio (OR)δ of N(0, 1.282), which rules out extreme effects, and a noninformative prior on the intercept (ie, the log odds of survival with standard care) as N(0, 102). Secondary outcomes were analyzed in the same way using generalized linear models suitable for the outcome distribution.
An adjusted analysis (the covariates for which had been selected a priori without knowledge of the results) was conducted to account for potential between-group imbalances. The treatment effects were summarized as ORs with 95% credible intervals (CrIs) and posterior probability estimates of the OR being less than 1 (would indicate REBOA and standard care were beneficial) or the OR being greater than 1 (would indicate REBOA and standard care were harmful). Two additional principal stratum analyses were conducted to account for intercurrent events and for possible learning curve effects. Any missing data at baseline are reported as missing.
An independent data and safety monitoring committee monitored emerging data (which included a preplanned interim analysis when 40 patients were enrolled and then when 80 patients were enrolled). An independent trial steering committee oversaw the conduct of the trial. Data were collected to assess the number of complications and serious adverse device events. All analyses were performed using Stata version 17 (StataCorp).32
Results
Enrollment, Interim Analyses, and Study Termination
Between October 30, 2017, and March 16, 2022, a total of 90 patients were enrolled and 89 patients were followed up for 90 days. We had originally planned to enroll 120 patients, but the trial was stopped after the second interim analysis, which included 80 patients, because the prespecified stopping rule for harm was met (90.1% posterior probability of OR >1 for mortality at 90 days; stopping criterion was >90%). Given the time required to collect the primary outcome data, a further 10 patients had been enrolled by the time of the interim analysis, resulting in a total of 90 patients.
Of these 90 patients, 46 were randomly assigned to REBOA and standard care vs 44 to standard care alone (Figure 1). One patient in the standard care alone group declined to provide consent for continued participation and data collection 4 days after enrollment, and was therefore excluded from the analyses from that point forward.
Baseline Characteristics
Most patients (69%) were male and most (97%) had experienced blunt trauma (median Injury Severity Score, 41 [IQR, 29-50]; score range, 0-75; a score >15 indicates severe injury) (Table 1). The median age was 41 years (IQR, 31-59 years). The patients had hypotension and tachycardia in the prehospital setting and 23% required cardiopulmonary resuscitation upon arrival at the emergency department.
Table 1. Patient Characteristics.
| REBOA and standard care (n = 46) | Standard care alone (n = 44) | |
|---|---|---|
| Demographics | ||
| Age, median (IQR), y | 46 (33-62) | 39 (30-56) |
| Sex, No. (%) | ||
| Female | 18 (39) | 10 (23) |
| Male | 28 (61) | 34 (77) |
| Mechanism of injury, No. (%) | ||
| Blunt | 44 (96) | 43 (98) |
| Penetrating | 2 (4) | 1 (2) |
| Patient prehospital characteristics | ||
| Systolic blood pressure, mm Hg | ||
| Median (IQR) | 85 (66-120) [n = 34] | 97 (71-128) [n = 37] |
| ≤70, No./total (%) | 11/34 (32) | 9/37 (24) |
| ≤90, No./total (%) | 18/34 (53) | 17/37 (46) |
| Heart rate, median (IQR), beats/min | 113 (94-133) [n = 42] | 109 (76-133) [n = 40] |
| Oxygen saturation, median (IQR), % | 88 (80-95) [n = 32] | 92 (81-98) [n = 43] |
| Glasgow Coma Scale score, median (IQR)a | 10 (3-14) [n = 42] | 10 (3-14) [n = 42] |
| CPR performed, No./total (%) | 10/43 (22) | 11/44 (25) |
| Method of transport, No./total (%) | ||
| Ambulance | 22/45 (49) | 19/43 (43) |
| Helicopter | 17/45 (38) | 21/43 (49) |
| Ambulance and helicopter | 6/45 (13) | 3/43 (7) |
| Patient characteristics in ED | ||
| Time from injury to ED arrival, median (IQR), min | 90 (70-125) [n = 39] | 97 (78-119) [n = 41] |
| Time from ED arrival to randomization, median (IQR), minb | 13 (4-21) [n = 39] | 13 (4-19) [n = 41] |
| Systolic blood pressure, mm Hg | ||
| Median (IQR) | 84 (58-115) [n = 44] | 99 (72-115) [n = 42] |
| ≤70, No./total (%) | 18/44 (41) | 9/42 (21) |
| ≤90, No./total (%) | 26/44 (59) | 19/42 (45) |
| Heart rate, median (IQR), beats/min | 105 (88-123) [n = 45] | 120 (87-135) [n = 43] |
| Oxygen saturation, median (IQR), % | 99 (90-100) [n = 39] | 99 (95-100) [n = 40] |
| Glasgow Coma Scale score, median (IQR) | 3 (3-11) [n = 39] | 3 (3-15) [n = 39] |
| CPR performed, No./total (%) | 4/40 (9) | 4/43 (9) |
| Injury Severity Scorec | ||
| Median (IQR) | 41 (29-50) | 41 (29-50) |
| >25 (very severe), No. (%) | 38 (83) | 38 (86) |
| 16-25 (severe), No. (%) | 7 (15) | 4 (9) |
| 9-15 (moderate), No. (%) | 1 (2) | 1 (2) |
| 1-8 (mild), No. (%) | 0 | 1 (2) |
| Abbreviated Injury Scale score, median (IQR)d | ||
| Head | 3 (0-4) | 0 (0-5) |
| Thorax | 4 (3-4) | 4 (1-4) |
| Abdomen | 2 (0-3) | 2 (0-4) |
| Pelvis | 2 (0-5) | 2 (0-5) |
| Limbs | 2 (2-3) | 3 (2-3) |
Abbreviations: CPR, cardiopulmonary resuscitation; ED, emergency department; REBOA, resuscitative endovascular balloon occlusion of the aorta.
Score range is 3 (patient does not respond to any stimuli, including pain) to 15 (normal level of consciousness).
Three patients in the REBOA and standard care group and 5 patients in the standard care alone group were randomized before ED arrival and were given a time of 0 minutes.
A global anatomical scoring system that provides a measure of trauma severity with a score range from 0 (no injury) to 75 (maximal injury). This score is calculated by summing the squares of the 3 worst-injured body regions (indicated by the highest Abbreviated Injury Scale score). An Abbreviated Injury Scale score of 6 for any body region automatically results in an Injury Severity Score of 75.
An anatomically based, consensus-derived severity scoring system that classifies an individual injury by body region according to severity with a score range from 0 (no injury) to 6 (maximal injury). A score of 1 reflects a minor injury.
Overall, the groups were well matched; however, there were more cases of hypotension among patients in the REBOA and standard care group upon arrival at the emergency department (median systolic blood pressure, 84 mm Hg [IQR, 58-115 mm Hg]) than among patients in the standard care alone group (median systolic blood pressure, 99 mm Hg [IQR, 72-115 mm Hg]). Patients in the REBOA and standard care group also had higher median Abbreviated Injury Scale scores for the head region (eFigures 2-3 in Supplement 1). The distribution of the Abbreviated Injury Scale scores in other body regions was similar across both groups (eFigures 4-7 in Supplement 1).
Treatment Pathways for REBOA
Patients in the REBOA and standard care group had a number of treatment pathways due to intercurrent events (Figure 1). Of the 46 patients in the REBOA and standard care group, 19 (41%) had a device inserted and inflated. A further 17 patients (37%) responded to other resuscitative measures while REBOA insertion was being prepared or performed, and progression to full aortic occlusion was no longer deemed necessary. Two patients (4%) deteriorated before arterial access could be established. Arterial access was attempted but could not be established in 8 patients (17%).
The balloon was inflated in zone I (descending thoracic aorta) in 10 patients (53%) and in zone III (above aortic bifurcation) in 9 patients (47%). The median time from emergency department arrival to balloon inflation was 32 minutes (IQR, 20-47 minutes), and the median duration of inflation was 29 minutes (IQR, 19-64 minutes). Partial REBOA (titrated deflation of the balloon to allow some distal perfusion) was used in 8 patients (42%).
Primary Outcome
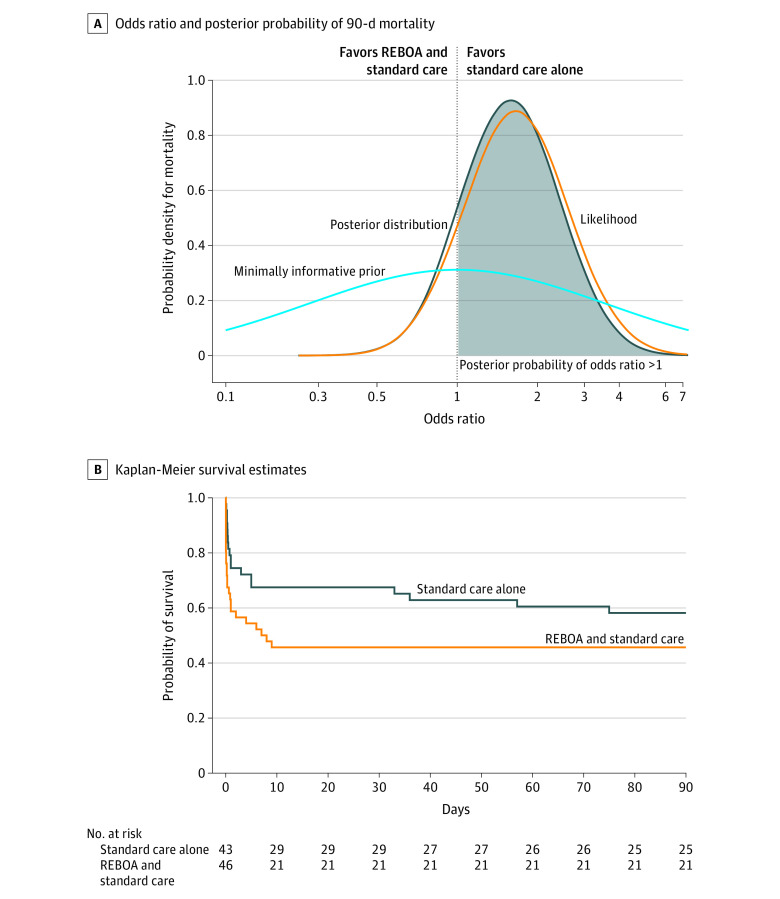
Among the 89 patients who provided consent for data analysis, there were 25 deaths (54%) in the REBOA and standard care group and 18 deaths (42%) in the standard care alone group at 90 days after randomization (Table 2). The prespecified primary unadjusted analysis using the minimally informative prior showed that the OR for mortality at 90 days was 1.58 (95% CrI, 0.72-3.52) for patients in the REBOA and standard care group and the posterior probability of an OR greater than 1 (indicating increased odds of death with REBOA and standard care) was 86.9% (Figure 2A).
Table 2. Primary and Secondary Outcomes.
| REBOA and standard care (n = 46) | Standard care alone (n = 44) | Absolute difference (95% CrI), % |
Effect estimate (95% CrI) | Posterior probability of OR >1, %a | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| All-cause mortality at 90 d, No./total (%) | 25/46 (54) | 18/43 (42)b | 11.3 (−8.1 to 30.1) | OR, 1.58 (0.72 to 3.52) | 86.9 |
| Secondary outcomes | |||||
| Mortality at different time points, No./total (%) | |||||
| Death within 6 mo | 25/46 (54) | 18/43 (42)b | 11.3 (−8.1 to 30.1) | OR, 1.58 (0.72 to 3.52) | 86.9 |
| Death while in the hospital | 25/46 (54) | 18/43 (42)b | 11.3 (−8.1 to 30.1) | OR, 1.58 (0.72 to 3.52) | 86.9 |
| Death within 24 h | 17/46 (37) | 10/44 (23) | 12.5 (−5.0 to 29.6) | OR, 1.85 (0.79 to 4.46) | 91.8 |
| Death within 6 h | 13/46 (28) | 4/44 (9) | 15.8 (1.8 to 30.4) | OR, 3.14 (1.13 to 9.76) | 98.6 |
| Death within 3 h | 11/46 (24) | 2 /44 (5) | 15.1 (3.3 to 28.4) | OR, 4.25 (1.33 to 15.99) | 99.3 |
| Underwent a definitive hemorrhage control procedure, No. (%) | 14 (30) | 19 (43) | −11.5 (−29.6 to 7.1) | OR, 0.60 (0.26 to 1.37) | |
| Time from randomization to definitive hemorrhage control procedure, median (IQR), min | 83 (56 to 156) [n = 12] | 64 (34 to 83) | |||
| Type of definitive hemorrhage control procedure, No. (%)c | |||||
| Hemorrhage control laparotomy | 7 (50) | 12 (63) | |||
| Extremity vascular ligation, shunting, or repair | 2 (14) | 4 (21) | |||
| Pelvic packing | 4 (29) | 1 (5) | |||
| Angioembolization | 2 (14) | 2 (11) | |||
| Hemorrhage control thoracotomy | 1 (7) | 0 | |||
| Intensive care unit–free days at 90 dd | |||||
| Mean (SD) | 35 (40) | 40 (37) | MD, −4.79 (−20.75 to 11.31) | ||
| Median (IQR) | 0 (0 to 80) | 45 (0 to 78) | |||
| Hospital-free days at 90 dd | |||||
| Mean (SD) | 22 (30) | 41 (39) | MD, −18.58 (−32.86 to −3.93) | ||
| Median (IQR) | 0 (0 to 49) | 41 (0 to 82) | |||
| Transfusion requirements | |||||
| Red blood cells, units | |||||
| Mean (SD) | 10 (9) | 11 (9) | IRR, 0.92 (0.66 to 1.29) | ||
| Median (IQR) | 7 (4 to 12) | 9 (4 to 17) | |||
| Plasma, units | |||||
| Mean (SD) | 8 (8) | 11 (10) | IRR, 0.73 (0.49 to 1.08) | ||
| Median (IQR) | 6 (3 to 10) | 7 (4 to 18) | |||
| Platelets, pools | |||||
| Mean (SD) | 1 (3) | 2 (2) | IRR, 0.87 (0.50 to 1.52) | ||
| Median (IQR) | 1 (0 to 2) | 1 (0 to 2) | |||
| Cryoprecipitate, units | |||||
| Mean (SD) | 2 (3) | 2 (3) | IRR, 0.79 (0.41 to 1.53) | ||
| Median (IQR) | 0 (0 to 2) | 2 (0 to 3) | |||
| Tranexamic acid, mg | |||||
| Mean (SD) | 1413 (580) | 1568 (695) | IRR, 0.90 (0.70 to 1.16) | ||
| Median (IQR) | 1000 (1000 to 2000) | 2000 (1000 to 2000) | |||
Abbreviations: CrI, credible interval; MD, mean difference; IRR, incident rate ratio; OR, odds ratio; REBOA, resuscitative endovascular balloon occlusion of the aorta.
An OR greater than 1 indicates REBOA and standard care were harmful.
One patient withdrew on day 4.
Some patients underwent more than 1 procedure.
Patients who died within 90-day follow-up were assigned 0 days.
Figure 2. Primary Outcome and Kaplan-Meier Survival Estimates.
REBOA indicates resuscitative endovascular balloon occlusion of the aorta.
The probability was 3.7% that REBOA and standard care reduces death per the prespecified OR of 0.77 (or lower, corresponding to a greater reduction in mortality). When multivariable regression was used to adjust for differences in the baseline characteristics, the odds of 90-day mortality in the REBOA and standard care group also remained higher than in the standard care alone group (OR, 1.80 [95% CrI, 0.59-5.59]). The posterior probability of an OR greater than 1 was 84.9%. The post hoc analyses of the individual covariates showed only a minimal effect on the results (eTable 4 in Supplement 1). The results of the principal stratum analyses (to account for intercurrent events) and the learning curve effects analysis did not change the overall findings (eAppendices 1-2 in Supplement 1).
Secondary Outcomes
The ORs for mortality and the posterior probabilities of an OR greater than 1 were all increased in the REBOA and standard care group for 6-month, in-hospital, 24-, 6-, and 3-hour mortality and the ORs were increased with earlier mortality end points (Table 2). The survival curves show an early separation of the 2 groups, but also more deaths in the REBOA and standard care group to day 10 (Figure 2B). The causes of death (as determined by the site investigators) appear in eTable 5 in Supplement 1 for each time point. There were more deaths due to bleeding in the REBOA and standard care group (8 of 25 patients [32%]) than in the standard care alone group (3 of 18 patients [17%]) and most of these deaths occurred within 24 hours.
Fourteen patients (30%) in the REBOA and standard care group had a definitive hemorrhage control procedure compared with 19 patients (43%) in the standard care alone group. The median time from randomization to definitive hemorrhage control was 19 minutes longer in the REBOA and standard care group than in the standard care alone group. Blood transfusion requirements were similar in the 2 groups (Table 2). Patients in the standard care alone group had more intensive care unit–free and hospital-free days (Table 2). There were no between-group differences in the number of complications (eTable 6 in Supplement). There were no serious adverse device events.
Discussion
This is the first randomized clinical trial, to our knowledge, to examine the potential clinical effectiveness of REBOA for the management of exsanguinating hemorrhage. There are no other randomized clinical trials of REBOA for trauma patients registered on ClinicalTrials.gov.
Among the 90 patients who were enrolled in the current trial, the REBOA and standard care group was observed to have a high probability (86.9%) of higher mortality at 90 days (the primary outcome) compared with the standard care alone group. It is also noteworthy that the ORs and posterior probabilities for increased mortality increased with earlier time points, which are more specific for deaths due to hemorrhage. The findings were not altered in an adjusted analysis conducted to account for the baseline imbalances. The probability was 3.7% that REBOA and standard care reduces mortality by a worthwhile margin at 90 days, and less so at time points within 24 hours.
The findings from the current study are consistent with a number of previously published observational studies. A retrospective, propensity score–matched study21 from the US, which used data from a national registry, reported a possible detrimental effect of REBOA. Similarly, a retrospective, propensity score–matched study from Japan,20 which was also based on national registry data, showed that treatment with REBOA was associated with higher mortality. The findings from these 2 studies20,21 had been attributed to unmeasured confounders, but are worthy of reevaluation in light of the current study. However, there are also a number of other observational studies that have reported positive benefits with REBOA.23,25
The survival curves demonstrate the probable harmful early effects of REBOA (Figure 2B). The early (within the first few hours) decline in survival likely represents a delay or failure to definitively control hemorrhage as a result of REBOA insertion or during attempts at REBOA insertion in the emergency department. There were fewer patients who underwent a definitive hemorrhage control procedure in the REBOA and standard care group, likely due to the competing risk of early death. For those patients who did undergo such a procedure, it took, on average, an additional 19 minutes to commence these procedures.
Although a small delay to definitive hemorrhage control is to be expected with REBOA, the purported benefit in longer survival was not observed. This can be seen in the increased proportion of early deaths due to uncontrolled hemorrhage. Death due to hemorrhage was more common in the REBOA and standard care group, and all of these deaths occurred within 24 hours (and most of them within 3 hours) of randomization. The excess early deaths also explain why patients in the REBOA and standard care group had fewer hospital-free and intensive care unit–free days than those in the standard care alone group.
Patients in the current trial had higher mortality than trauma patients in other studies of hemorrhage control interventions (eg, the Pragmatic, Randomized Optimal Platelet and Plasma Ratios [PROPPR] trial).33 Most likely this is a result of the inclusion criteria chosen in the current trial, which selected a more seriously injured group of patients. The median Injury Severity Score in the PROPPR trial33 was only 26 compared with a median Injury Severity Score of 41 in the current trial.
There were a number of treatment pathways experienced by patients in the REBOA and standard care group due to intercurrent events. These findings reflect the challenges in obtaining arterial access in patients with severe shock and in distinguishing between patients who are experiencing continuing hemorrhage from those in whom bleeding has stopped. These experiences reflect real life and highlight the complexity of trauma care, and the challenges inherent in evaluating it.
This trial has several strengths. The trial included a comprehensive training program that recognized the challenges of evaluating a technology such as REBOA. The trial was pragmatic in design, with simple inclusion criteria that were based on the clinical judgment of experienced clinicians, allowing them to quickly evaluate suitability for the trial in a pressured clinical setting, and used routinely collected data, minimizing burden on staff.
Limitations
This trial has several limitations. First, the trial’s small size reflects the relative infrequency and etiology of exsanguinating hemorrhage in the UK where blunt trauma predominates, and most penetrating trauma is the result of stabbings rather than gunshot wounds. The results may therefore not be translatable to other settings.
Second, the reorganization of in-hospital trauma care in England has markedly improved mortality from trauma over the past decade34; however, institutional case volume (and operative case volume for hemorrhage control) is lower than in other countries, reflecting high road safety standards and low levels of interpersonal violence.
Third, the responsibility for the control of torso hemorrhage rests with surgeons who provide other care in addition to trauma care. The initial care of trauma patients is the responsibility of senior emergency medicine physicians, but surgeons are called early (even before the arrival of a patient). Nevertheless, these organizational differences may have affected the speed with which trauma patients were treated and operated on, if needed.
Fourth, the relatively low proportion (37%) of patients who underwent a definitive hemorrhage control procedure may be a reflection of the rigorous classification applied. Operations were only counted as definitive hemorrhage control procedures when a bleeding organ had to be resected; a named vessel was ligated, repaired, or shunted; or a cavity was packed. A limitation of this approach is that a bowel resection for mesenteric bleeding, for example, would not have been coded as a definitive hemorrhage control procedure.
Fifth, there were some baseline imbalances between the groups, but the adjusted analyses showed these had little effect on the results, and the proportion of deaths attributed to traumatic brain injury, in particular, were similar in the 2 groups.
Conclusions
In trauma patients with exsanguinating hemorrhage, a strategy of REBOA and standard care in the emergency department does not reduce, and may increase, mortality compared with standard care alone.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
eTable 1. Participating major trauma centers and enrollment by site
eTable 2. Inclusion and exclusion criteria in list form
eTable 3. Definitions of outcomes and sources of data
eTable 4. Adjusted intention to treat analysis for 90 day mortality (primary outcome)
eTable 5. Causes of death
eTable 6. Complications
eMethods. Training information
eFigure 1. Two-day course, sample program
eFigure 2. Distribution of ED systolic blood pressure (mmgHG) on arrival, by group
eFigure 3. Distribution of Head Abbreviated Injury Scales (AIS), by group
eFigure 4. Distribution of Thorax Abbreviated Injury Scales (AIS), by group
eFigure 5. Distribution of Abdomen Abbreviated Injury Scales (AIS), by group
eFigure 6. Distribution of Pelvis Abbreviated Injury Scales (AIS), by group
eFigure 7. Distribution of Limbs Abbreviated Injury Scales (AIS), by group
eAppendix 1. Supplementary analysis: principal stratum/complier average causal effect (PS/CACE) analyses
eAppendix 2. Supplementary analysis: learning curve analysis
eReference
Trial protocol
Statistical analysis plan
Data sharing statement
References
- 1.Berwick D, Downey A, Cornett E, eds. A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury. National Academies of Sciences, Engineering, and Medicine; 2016. doi: 10.17226/23511 [DOI] [PubMed] [Google Scholar]
- 2.Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg. 1995;32(11):925-1002. doi: 10.1016/S0011-3840(05)80008-5 [DOI] [PubMed] [Google Scholar]
- 3.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6)(suppl):S3-S11. doi: 10.1097/01.ta.0000199961.02677.19 [DOI] [PubMed] [Google Scholar]
- 4.Morrison JJ, Rasmussen TE. Noncompressible torso hemorrhage: a review with contemporary definitions and management strategies. Surg Clin North Am. 2012;92(4):843-858, vii. doi: 10.1016/j.suc.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 5.Morrison JJ, Stannard A, Rasmussen TE, Jansen JO, Tai NRM, Midwinter MJ. Injury pattern and mortality of noncompressible torso hemorrhage in UK combat casualties. J Trauma Acute Care Surg. 2013;75(2)(suppl 2):S263-S268. doi: 10.1097/TA.0b013e318299da0a [DOI] [PubMed] [Google Scholar]
- 6.Kisat M, Morrison JJ, Hashmi ZG, Efron DT, Rasmussen TE, Haider AH. Epidemiology and outcomes of non-compressible torso hemorrhage. J Surg Res. 2013;184(1):414-421. doi: 10.1016/j.jss.2013.05.099 [DOI] [PubMed] [Google Scholar]
- 7.Ledgerwood AM, Kazmers M, Lucas CE. The role of thoracic aortic occlusion for massive hemoperitoneum. J Trauma. 1976;16(08):610-615. doi: 10.1097/00005373-197608000-00004 [DOI] [PubMed] [Google Scholar]
- 8.Millikan JS, Moore EE. Outcome of resuscitative thoracotomy and descending aortic occlusion performed in the operating room. J Trauma. 1984;24(5):387-392. doi: 10.1097/00005373-198405000-00003 [DOI] [PubMed] [Google Scholar]
- 9.Sankaran S, Lucas C, Walt AJ. Thoracic aortic clamping for prophylaxis against sudden cardiac arrest during laparotomy for acute massive hemoperitoneum. J Trauma. 1975;15(4):290-296. doi: 10.1097/00005373-197504000-00005 [DOI] [PubMed] [Google Scholar]
- 10.Markov NP, Percival TJ, Morrison JJ, et al. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery. 2013;153(6):848-856. doi: 10.1016/j.surg.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 11.Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma. 2011;71(6):1869-1872. doi: 10.1097/TA.0b013e31823fe90c [DOI] [PubMed] [Google Scholar]
- 12.White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150(3):400-409. doi: 10.1016/j.surg.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 13.Morrison JJ, Ross JD, Markov NP, Scott DJ, Spencer JR, Rasmussen TE. The inflammatory sequelae of aortic balloon occlusion in hemorrhagic shock. J Surg Res. 2014;191(2):423-431. doi: 10.1016/j.jss.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 14.Brenner ML, Moore LJ, DuBose JJ, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75(3):506-511. doi: 10.1097/TA.0b013e31829e5416 [DOI] [PubMed] [Google Scholar]
- 15.Martinelli T, Thony F, Decléty P, et al. Intra-aortic balloon occlusion to salvage patients with life-threatening hemorrhagic shocks from pelvic fractures. J Trauma. 2010;68(4):942-948. doi: 10.1097/TA.0b013e3181c40579 [DOI] [PubMed] [Google Scholar]
- 16.Saito N, Matsumoto H, Yagi T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2015;78(5):897-903. doi: 10.1097/TA.0000000000000614 [DOI] [PubMed] [Google Scholar]
- 17.Brenner M, Teeter W, Hoehn M, et al. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg. 2018;153(2):130-135. doi: 10.1001/jamasurg.2017.3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuBose JJ, Scalea TM, Brenner M, et al. ; AAST AORTA Study Group . The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409-419. doi: 10.1097/TA.0000000000001079 [DOI] [PubMed] [Google Scholar]
- 19.Moore LJ, Brenner M, Kozar RA, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79(4):523-530. doi: 10.1097/TA.0000000000000809 [DOI] [PubMed] [Google Scholar]
- 20.Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg. 2015;78(4):721-728. doi: 10.1097/TA.0000000000000578 [DOI] [PubMed] [Google Scholar]
- 21.Joseph B, Zeeshan M, Sakran JV, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg. 2019;154(6):500-508. doi: 10.1001/jamasurg.2019.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekdache O, Paradis T, Shen YBH, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): a scoping review protocol concerning indications—advantages and challenges of implementation in traumatic non-compressible torso haemorrhage. BMJ Open. 2019;9(2):e027572. doi: 10.1136/bmjopen-2018-027572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borger van der Burg BLS, van Dongen TTCF, Morrison JJ, et al. A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination. Eur J Trauma Emerg Surg. 2018;44(4):535-550. doi: 10.1007/s00068-018-0959-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castellini G, Gianola S, Biffi A, et al. ; Italian National Institute of Health guideline working group on major trauma . Resuscitative endovascular balloon occlusion of the aorta (REBOA) in patients with major trauma and uncontrolled haemorrhagic shock: a systematic review with meta-analysis. World J Emerg Surg. 2021;16(1):41. doi: 10.1186/s13017-021-00386-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manzano Nunez R, Naranjo MP, Foianini E, et al. A meta-analysis of resuscitative endovascular balloon occlusion of the aorta (REBOA) or open aortic cross-clamping by resuscitative thoracotomy in non-compressible torso hemorrhage patients. World J Emerg Surg. 2017;12(1):30. doi: 10.1186/s13017-017-0142-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016;80(2):324-334. Published correction appears in J Trauma Acute Care Surg. 2016;80(3):554. doi: 10.1097/TA.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 27.Cannon J, Morrison J, Lauer C, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) for hemorrhagic shock. Mil Med. 2018;183(suppl 2):55-59. doi: 10.1093/milmed/usy143 [DOI] [PubMed] [Google Scholar]
- 28.Brenner M, Bulger EM, Perina DG, et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA). Trauma Surg Acute Care Open. 2018;3(1):e000154. doi: 10.1136/tsaco-2017-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen JO, Cochran C, Boyers D, et al. ; UK-REBOA Trial grantholders . The effectiveness and cost-effectiveness of resuscitative endovascular balloon occlusion of the aorta (REBOA) for trauma patients with uncontrolled torso haemorrhage: study protocol for a randomised clinical trial (the UK-REBOA trial). Trials. 2022;23(1):384. doi: 10.1186/s13063-022-06346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnard EBG, Morrison JJ, Madureira RM, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): a population based gap analysis of trauma patients in England and Wales. Emerg Med J. 2015;32(12):926-932. doi: 10.1136/emermed-2015-205217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . Addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical analysis of clinical trials. Published November 20, 2019. Accessed April 16, 2023. https://database.ich.org/sites/default/files/E9-R1_Step4_Guideline_2019_1203.pdf
- 32.StataCorp . Stata Statistical Software: Release 17. StatCorp LLC; 2021. [Google Scholar]
- 33.Holcomb JB, Tilley BC, Baraniuk S, et al. ; PROPPR Study Group . Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi: 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran CG, Lecky F, Bouamra O, et al. Changing the system—major trauma patients and their outcomes in the NHS (England) 2008-17. EClinicalMedicine. 2018;2-3:13-21. doi: 10.1016/j.eclinm.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Participating major trauma centers and enrollment by site
eTable 2. Inclusion and exclusion criteria in list form
eTable 3. Definitions of outcomes and sources of data
eTable 4. Adjusted intention to treat analysis for 90 day mortality (primary outcome)
eTable 5. Causes of death
eTable 6. Complications
eMethods. Training information
eFigure 1. Two-day course, sample program
eFigure 2. Distribution of ED systolic blood pressure (mmgHG) on arrival, by group
eFigure 3. Distribution of Head Abbreviated Injury Scales (AIS), by group
eFigure 4. Distribution of Thorax Abbreviated Injury Scales (AIS), by group
eFigure 5. Distribution of Abdomen Abbreviated Injury Scales (AIS), by group
eFigure 6. Distribution of Pelvis Abbreviated Injury Scales (AIS), by group
eFigure 7. Distribution of Limbs Abbreviated Injury Scales (AIS), by group
eAppendix 1. Supplementary analysis: principal stratum/complier average causal effect (PS/CACE) analyses
eAppendix 2. Supplementary analysis: learning curve analysis
eReference
Trial protocol
Statistical analysis plan
Data sharing statement