Key Points
Question
Does transitioning from standard-volume to small-volume blood collection tubes for laboratory testing in intensive care units (ICUs) reduce red blood cell (RBC) transfusion?
Findings
After transition to small-volume tubes in this stepped-wedge cluster randomized trial, RBC transfusion was not significantly different in the primary analysis of 21 201 patients, excluding 6210 admitted during the COVID-19 pandemic (relative risk, 0.91), but it was significantly lower in the secondary analysis of all 27 411 patients (RR, 0.88; absolute decrease, 9.84 RBC units/100 patients). The frequency of insufficient specimens was not different (≤0.03%).
Meaning
Small-volume blood collection tubes in the ICU may decrease RBC transfusions without affecting laboratory analysis.
Abstract
Importance
Blood collection for laboratory testing in intensive care unit (ICU) patients is a modifiable contributor to anemia and red blood cell (RBC) transfusion. Most blood withdrawn is not required for analysis and is discarded.
Objective
To determine whether transitioning from standard-volume to small-volume vacuum tubes for blood collection in ICUs reduces RBC transfusion without compromising laboratory testing procedures.
Design, Setting, and Participants
Stepped-wedge cluster randomized trial in 25 adult medical-surgical ICUs in Canada (February 5, 2019 to January 21, 2021).
Interventions
ICUs were randomized to transition from standard-volume (n = 10 940) to small-volume tubes (n = 10 261) for laboratory testing.
Main Outcomes and Measures
The primary outcome was RBC transfusion (units per patient per ICU stay). Secondary outcomes were patients receiving at least 1 RBC transfusion, hemoglobin decrease during ICU stay (adjusted for RBC transfusion), specimens with insufficient volume for testing, length of stay in the ICU and hospital, and mortality in the ICU and hospital. The primary analysis included patients admitted for 48 hours or more, excluding those admitted during a 5.5-month COVID-19–related trial hiatus.
Results
In the primary analysis of 21 201 patients (mean age, 63.5 years; 39.9% female), which excluded 6210 patients admitted during the early COVID-19 pandemic, there was no significant difference in RBC units per patient per ICU stay (relative risk [RR], 0.91 [95% CI, 0.79 to 1.05]; P = .19; absolute reduction of 7.24 RBC units/100 patients per ICU stay [95% CI, −3.28 to 19.44]). In a prespecified secondary analysis (n = 27 411 patients), RBC units per patient per ICU stay decreased after transition from standard-volume to small-volume tubes (RR, 0.88 [95% CI, 0.77 to 1.00]; P = .04; absolute reduction of 9.84 RBC units/100 patients per ICU stay [95% CI, 0.24 to 20.76]). Median decrease in transfusion-adjusted hemoglobin was not statistically different in the primary population (mean difference, 0.10 g/dL [95% CI, −0.04 to 0.23]) and lower in the secondary population (mean difference, 0.17 g/dL [95% CI, 0.05 to 0.29]). Specimens with insufficient quantity for analysis were rare (≤0.03%) before and after transition.
Conclusions and Relevance
Use of small-volume blood collection tubes in the ICU may decrease RBC transfusions without affecting laboratory analysis.
Trial Registration
ClinicalTrials.gov Identifier: NCT03578419
This cluster randomized trial compares use of standard- vs small-volume blood collection tubes in decreasing rates of anemia and red blood cell transfusions among patients treated in intensive care units.
Introduction
Among patients admitted to intensive care units (ICUs), approximately 40% receive red blood cell (RBC) transfusions, which are costly, in scarce supply, and have associated health risks.1,2,3,4 More than half of RBC transfusions in the ICU are given in the absence of active bleeding.3 Critically ill patients typically have multiple daily blood draws for laboratory testing resulting in substantial iatrogenic blood loss, which is estimated to be equivalent to a unit of whole blood every 8 days and increases the risk of needing RBC transfusion.2,3,5 Although the need to reduce blood loss, anemia, and transfusion in ICU patients is widely recognized, evidence-based effective interventions are limited.6
Standard blood collection tubes, available worldwide, use a calibrated vacuum to withdraw a specific volume of blood, usually between 4 to 6 mL. Modern laboratory analytic equipment, however, requires only a very small fraction of the blood collected (usually <0.5 mL); thus, more than 90% of the blood withdrawn from patients is discarded.7 Small-volume vacuum blood collection tubes are designed to draw approximately 50% less blood than standard-volume tubes by using less vacuum strength.8 They have the same physical dimensions and collection technique as standard-volume tubes and are compatible with standard laboratory analytic equipment.
Small-volume tubes are available from suppliers of standard-volume tubes at no additional cost but are not commonly used in adult patients.9,10,11 Although routine use of small-volume tubes could reduce iatrogenic blood loss, they are not considered standard of care, nor are they specifically recommended by practice guidelines.6 This is due, at least in part, to a lack of high-quality evidence showing that transition to small-volume tubes improves clinical outcomes.8 We therefore designed a stepped-wedge, cluster randomized trial to test the hypothesis that transitioning from standard-volume to small-volume tubes for blood collection in adult ICUs would reduce RBC transfusion without compromising laboratory testing procedures.
Methods
Trial Design
We performed a pragmatic multicenter, stepped-wedge, cluster randomized trial, known as the Small-Volume Tubes to Reduce Anemia and Transfusion (STRATUS), to evaluate the effect of transitioning from standard-volume to small-volume blood collection vacuum tubes for all blood collection (except blood cultures and blood gases). We used cluster randomization, which is well-suited to assess the effectiveness of interventions that are incorporated into routine clinical care. Individual patient randomization was not considered feasible due the high risk of contamination within control and intervention groups in a busy ICU setting. A stepped-wedge, cluster randomized trial design was chosen because it is more statistically efficient than parallel cluster design and because multiple switches between interventions were considered infeasible given ordering and stocking of the tubes conducted by hospital staff. The stepped-wedge design is ideal for studying the effectiveness and implementation of interventions that have a high likelihood of benefit and low risk of harm; as such, a benefit of this design is that all sites have implemented the intervention by the end of the study. At the start of the study, participating ICUs were using standard-volume tubes (eTable 1 in Supplement 1). Every 6 weeks, 2 ICUs transitioned to small-volume tubes according to a predetermined (concealed) randomization schedule until all ICUs had transitioned (Figure 1).
Figure 1. Recruitment, ICU Randomization, and Patient Flow in the STRATUS Trial.
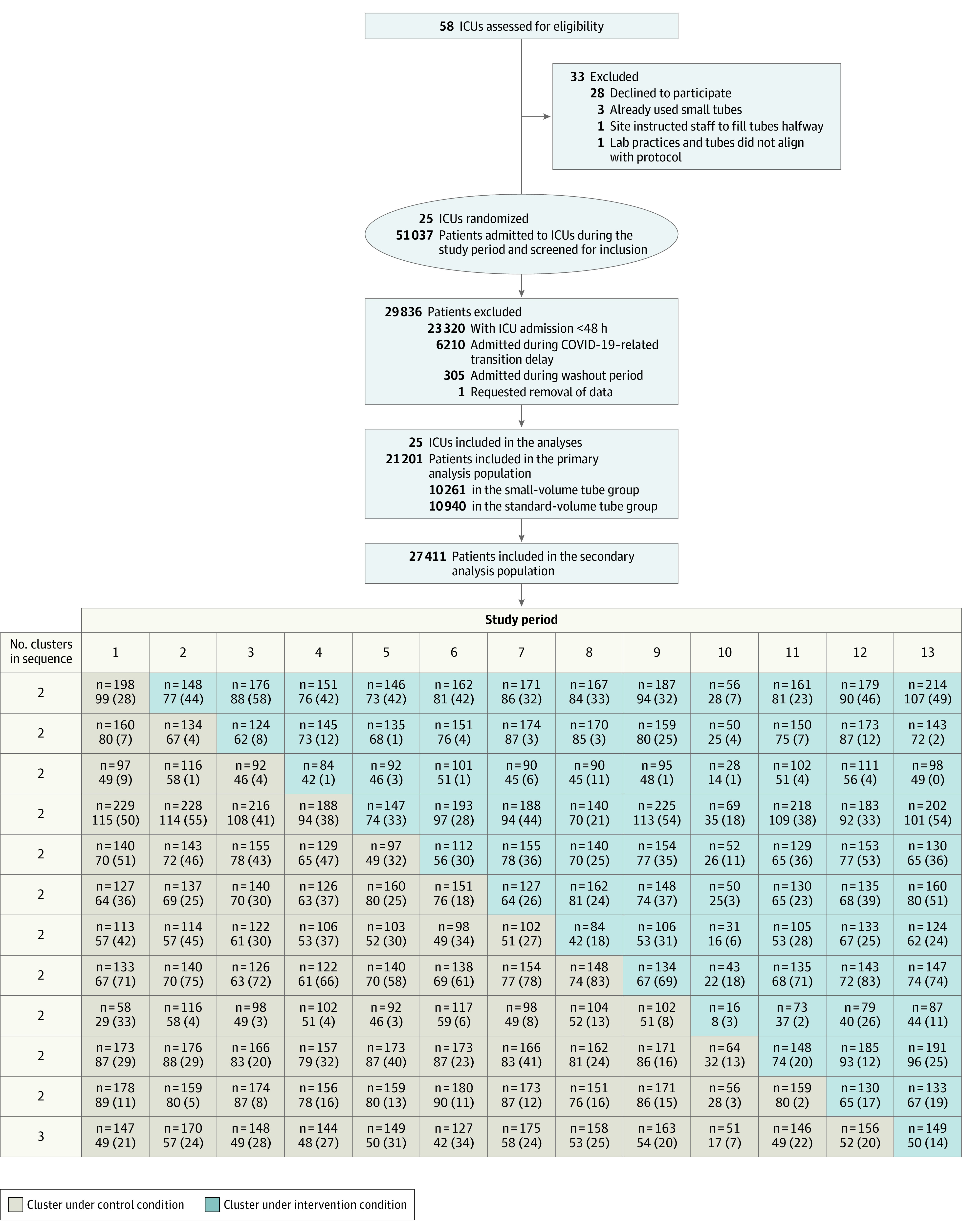
Numeric values in each cell indicate the number of patients and the mean (SD) size of included clusters. ICU indicates intensive care unit.
Research ethics boards at all participating centers approved the study under a waiver of individual patient consent due to the low-risk nature of the intervention and clinical use of both tube types (see trial protocol in Supplement 2).12,13 The trial was managed by the Population Health Research Institute, a joint institute of McMaster University and Hamilton Health Sciences (Hamilton, Ontario, Canada).
Trial Centers and Patients
ICUs were eligible to participate if they met the following criteria: 1, admitted adult medical-surgical critically ill patients; 2, had at least 14 ICU beds; 3, had capacity for invasive mechanical ventilation; 4, used standard-volume blood collection tubes for blood collection; and 5, had capacity for electronic data sharing. In 2 hospitals, 2 ICUs located in different areas and with separate storage facilities (low risk of cross-contamination of tube types) participated as separate sites. All patients admitted to participating ICUs during the study period were registered in the trial.
Intervention and Randomization
We used a computer-generated randomization schedule to determine the order in which ICUs would transition from standard-volume (4.0-6.0 mL) to small-volume (1.8-3.5 mL) tubes. Tube sizes at individual sites are shown in eTable 1 in Supplement 1. Investigators and personnel were blinded to this schedule, which was maintained by the unblinded study statistician. Sites were notified 2 weeks before their scheduled transition date, at which point short standardized education sessions, including posters and a video,14 were presented to local nursing and laboratory staff; adherence to local standard operating procedures regarding blood collection was reinforced. Standard- and small-volume blood collection tubes were procured by the hospital purchasing departments from their usual suppliers. On the transition date, standard-volume tubes were replaced with small-volume tubes. Random audits were done to evaluate adherence to allocated tubes.
COVID-19 Pandemic
The study began on February 5, 2019. Thirteen months later on March 2, 2020, due to the health care and research constraints and uncertainty related to the COVID-19 pandemic, transition to small-volume tubes at the 7 sites that had not yet transitioned to small-volume tubes was delayed for 5.5 months (March 2, 2020, to August 17, 2020). During this period, all sites continued using the tube size to which they were currently allocated by the study, and data collection continued. Due to uncertainty about effects of the COVID-19 pandemic, without reviewing or analyzing data, we specified that a special analysis would exclude patients admitted during the pandemic-related pause in transitions and decided that this would be the primary study analysis. Transitions to small-volume tubes at the 7 sites that had not transitioned resumed on August 18, 2020, according to the randomization schedule. This plan to handle extenuating circumstances and its reporting is in keeping with recommendations from the CONSERVE Group—Guidelines for Reporting Trial Protocols and Completed Trials Modified Due to the COVID-19 Pandemic and Other Extenuating Circumstances.15
Outcome Measures
The primary efficacy outcome was the number of RBC units transfused per patient during ICU admission. Secondary outcomes were the proportion of patients receiving at least 1 RBC transfusion, decrease in adjusted hemoglobin concentration during the ICU stay (adjustment was to decrease hemoglobin value by 1 g/dL for each RBC unit received), length of stay in the ICU and hospital, and mortality in the ICU and hospital. To assess the effect of transition to small-volume tubes on laboratory analysis, we measured the number of blood specimens reported as insufficient volume for testing (as defined at each hospital determined prior to trial initiation) from laboratory information systems using the 2 most frequently used tubes (ethylenediaminetetraacetic acid and sodium/lithium heparin). We performed exploratory analyses evaluating the change in hemoglobin concentration during the ICU stay (not adjusted for transfusion) in all patients and separately in patients who did not receive RBC transfusion.
We collected demographic, diagnostic, laboratory, and transfusion data electronically from hospital databases; these data were merged and validated in a central database.
Sample Size
To determine the number of sites and duration of data collection required, we used the following estimates: 1, average admission duration of 5 days for patients in the primary analysis (those with ICU stay of ≥48 hours); 2, that 40% of patients would receive RBC transfusion during admission; 3, average transfusion of 2 RBC units per patient per ICU admission; and 4, intraclass correlation coefficient of 0.01. We estimated that 18 240 patients distributed across 25 ICUs would be required to have 90% power (2-sided type I error of 5%) to detect a 2.5% relative reduction in the primary outcome (from 2.00 to 1.95 RBC units per patient per ICU admission). The final number of ICUs (N = 25) was determined based on the sample size required for individual patient randomization of (n = 6641) multiplied by the design effect of 2.73 and then divided by the cluster size of 729. The design effect was computed assuming an intraclass correlation coefficient of 0.01, 13 steps, a cluster period of 56 (ie, cluster size of 729), and a constant autocorrelation.16 We estimated that periods of 6 weeks’ duration (including a transition week or “washout period” for sites transitioning to the intervention) were required to ensure an adequate number of patients admitted to the ICU for 48 hours or more per cluster period received RBC transfusion. To complete the study within 18 months and thereby minimize the possibility of time-varying confounding that may occur with longer study durations, we designed the study such that 2 sites transitioned per period over 13 periods.
Statistical Analysis
The statistical analysis plan is included in Supplement 2. The population for the primary analysis included all patients admitted to the participating ICUs during the trial except the following: those admitted to the ICU for less than 48 hours, those admitted during one of the prespecified washout periods, or those admitted during the 4 periods in which implementation of the intervention was delayed due to the COVID-19 pandemic. The primary analysis focused on patients admitted to ICU for at least 48 hours because these patients are more likely to experience the cumulative effects of laboratory testing and associated blood loss and more likely to receive transfusion.2 Patients admitted prior to the transition and whose admission spanned the transition date were analyzed in the pretransition group. Data were collected until hospital discharge, death, or up to 30 days after ICU admission, whichever was earliest. For patients with multiple ICU admissions, only the first admission was included in the analysis.
For the primary analysis, the number of RBC transfusions was modeled as the outcome measure, and the ICU stay was incorporated as an offset term to account for variations in the number of RBC transfusions administered during ICU stays of varying duration. We used a negative binomial mixed model (hierarchical model adjusted for age and sex) to analyze the effect of the intervention on RBC units transfused per patient per ICU admission with periods modeled as fixed effects and ICU units modeled as a random effect for the stepped-wedge design. We conducted post hoc sensitivity analyses exploring the primary outcome by adjusting the model for imbalanced baseline characteristics (those with an SD >0.1) and by conducting the analysis using the generalized estimating equation model approach. We assessed whether there was a confounding effect of the COVID-19 pandemic on the relative risk (RR) of RBC units transfused by including interaction terms between study periods and the treatment variable, and COVID-19 and non–COVID-19 phase and the treatment variable. We further investigated for heterogeneity of treatment effects among ICUs by plotting the treatment effect for each ICU and evaluating heterogeneity using an interaction term between treatment effect and ICUs. We analyzed continuous or binary secondary outcomes with linear or logistic mixed models accounting for the stepped-wedge design by incorporating a random intercept for clustering and treating periods as fixed effects. We report mean differences with 95% CIs for continuous outcomes and RR or mean difference and 95% CIs for count or binary outcomes. We conducted analyses using SAS version 9.4 and considered a 2-sided P value of ≤.05 significant.
Results
Trial Centers and Patients
Of 58 Canadian ICUs contacted, 3 already used small-volume tubes; 25 met eligibility criteria and agreed to participate (Figure 1). Cluster characteristics are reported in Table 1 .The study began on February 5, 2019, and ended on January 21, 2021 (eFigure 1 in Supplement 1). During this time, 51 037 patients were admitted to the 25 participating ICUs, of whom 27 411 patients who stayed 48 hours or more were analyzed. The primary analysis population included 21 201 patients (6210 patients admitted during the 5.5-month pandemic-related trial hiatus were excluded) of whom 10 261 (48%) were in the small-volume tube group and 10 940 (52%) were in the standard tube group. Baseline characteristics of the primary and secondary populations (Table 2; eTable 2 in Supplement 1) were generally similar before and after transition, with standardized differences of 0.1 or less for most parameters. The baseline characteristics of patients admitted during the transition delay and those admitted during other study periods were also similar (eTable 3 in Supplement 1). eTable 4 in Supplement 1 shows selected baseline characteristics (age, sex, and initial hemoglobin concentration by sex) across individual ICUs.
Table 1. Cluster Characteristics.
| Characteristics | No. |
|---|---|
| Overall | 25 |
| Province in Canada | |
| Quebec | 14 |
| Ontario | 9 |
| Manitoba | 1 |
| New Brunswick | 1 |
| Total cluster size, median (IQR), patients | 854 (594-1037) |
| Cluster-period size, median (IQR), patients | 63 (43-85) |
Table 2. Baseline Characteristics of Patients Admitted to the Intensive Care Unit for 48 Hours or More.
| Small-volume tubes (n = 10 261) | Standard-volume tubes (n = 10 940) | |
|---|---|---|
| Age, mean (SD), y | 63.5 (15.9) | 63.0 (16.1) |
| Age >70 y, No./total (%) | 3832/10 261 (37.3) | 4083/10 940 (37.3) |
| Sex, No. (%) | n = 10 260 | n = 10 911 |
| Female | 4090 (39.9) | 4178 (38.3) |
| Male | 6170 (60.1) | 6733 (61.7) |
| Most responsible diagnosis, No. (%)a | n = 9078 | n = 8515 |
| Cardiovascular | 2245 (24.7) | 1762 (20.7) |
| Nervous system | 1156 (12.7) | 1155 (13.6) |
| Respiratory | 1047 (11.5) | 881 (10.3) |
| Injury | 965 (10.6)b | 1577 (18.5)b |
| Other | 912 (10.0) | 641 (7.5) |
| Infection | 807 (8.9) | 676 (7.9) |
| Cancer | 779 (8.6) | 801 (9.4) |
| Digestive | 787 (8.7) | 728 (8.5) |
| Genitourinary | 216 (2.4) | 191 (2.2) |
| Endocrine | 164 (1.8) | 103 (1.2) |
| Female patients | ||
| Initial hemoglobin, g/dLc | ||
| Mean (SD) | 10.47 (2.10) | 10.59 (2.04) |
| Median (IQR) | 10.40 (8.90-11.90) | 10.60 (9.10-12.00) |
| Initial hemoglobin by level, No. (%), g/dLc | n = 3853 | n = 3912 |
| <7.0 | 128 (3.3) | 114 (2.9) |
| 7.0-<9.0 | 853 (22.1) | 771 (19.7) |
| 9.0-<11.0 | 1329 (34.5) | 1337 (34.2) |
| 11.0-13.0 | 1114 (28.9) | 1241 (31.7) |
| >13.0 | 429 (11.1) | 449 (11.5) |
| Male patients | ||
| Initial hemoglobin, g/dLc | ||
| Mean (SD) | 11.12 (2.45) | 11.20 (2.32) |
| Median (IQR) | 11.10 (9.30-12.90) | 11.20 (9.50-12.90) |
| Initial hemoglobin by level, No. (%), g/dLc | n = 5852 | n = 6335 |
| <7.0 | 196 (3.3) | 160 (2.5) |
| 7.0-<9.0 | 1030 (17.6) | 1002 (15.8) |
| 9.0-<11.0 | 1575 (26.9) | 1745 (27.5) |
| 11.0-13.0 | 1723 (29.4) | 1998 (31.5) |
| >13.0 | 1328 (22.7) | 1430 (22.6) |
| Creatinine, mg/dLc | ||
| Mean (SD) | 1.52 (1.48) | 1.40 (1.37) |
| Median (IQR) | 1.00 (0.74-1.57) | 0.95 (0.72-1.43) |
| Creatinine, No. (%), mg/dLc | n = 9025 | n = 10 113 |
| <1.5 | 6570 (72.8) | 7775 (76.9) |
| 1.5-3.0 | 1541 (17.1) | 1470 (14.5) |
| >3.0 | 914 (10.1) | 868 (8.6) |
SI conversion factor: To convert creatinine to μmol/L, multiply values by 88.4.
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes. Data were provided by 20 of 25 participating intensive care units.
Standardized difference was greater than 0.1.
Data were provided by 24 of 25 participating intensive care units.
Each site underwent 1 to 3 random audits depending on the timing of transition. A total of 4 out of 50 audits documented a small number of standard-volume tubes in ICU storage areas (0.02%-2.9% of total tubes counted) for overall adherence of 92%.
RBC Transfusion
A total of 28 549 RBC units were given to 6362 patients (30%) in the primary analysis population and 35 687 were given to 8136 patients (30%) in the secondary analysis population. In the primary analysis population, which excluded 6210 patients admitted during the pandemic-related trial hiatus, least-squares mean RBC units transfused per patient was 0.79 (95% CI, 0.58 to 1.07) before transition and 0.72 (95% CI, 0.52 to 0.98) after transition to small-volume tubes (RR, 0.91 [95% CI, 0.79 to 1.05]; P = .19) (Table 3). The absolute difference in RBC units given per 100 patients per ICU stay was 7.24 RBC units (95% CI, −3.28 to 19.44). The estimated intraclass correlation coefficient was 0.054, compared with the assumed intraclass correlation coefficient of 0.01, resulting in a slight increase in the design effect to 2.82, which had a minor impact on statistical power.
Table 3. Outcomes for the Study of Small-Volume vs Standard-Volume Tubes for Blood Collection.
| Primary population (n = 21 201)a | ||||
|---|---|---|---|---|
| Small-volume tubes (n = 10 261) | Standard-volume tubes (n = 10 940) | Relative risk (95% CI)b | Mean difference (95% CI)b | |
| Primary outcome | ||||
| RBC units transfused in ICU per patient per median ICU stay | ||||
| Least-squares mean (95% CI) | 0.72 (0.52 to 0.98) | 0.79 (0.58 to 1.07) | 0.91 (0.79 to 1.05)c | −0.07 (−0.19 to 0.03) |
| Crude mean (SD) | 0.78 (2.23) | 0.88 (2.79) | ||
| Secondary outcomes | ||||
| Specimens with insufficient quantity for analysis, No. (%)d | 42 (0.022)e | 60 (0.031)e | −0.009 (−0.02 to 0.001)f | |
| Patients received ≥1 units RBC transfusion in ICU, No. (%) | 2975 (29.0) | 3387 (31.0) | 1.01 (0.93 to 1.09) | 0.16 (−2.19 to 2.34)f |
| Change in hemoglobin from ICU admission to ICU discharge adjusted for RBC transfusions, median (IQR), g/dLg,h,i | −1.40 (−3.00 to −0.20) | −1.50 (−3.20 to −0.40) | 0.10 (−0.04 to 0.23) | |
| Duration of ICU admission, median (IQR), d | 4.0 (3.0 to 8.0) | 4.0 (3.0 to 7.0) | 0.97 (0.93 to 1.02) | −0.18 (−0.47 to 0.10) |
| Duration of hospital admission, median (IQR), d | 11.0 (7.0 to 20.0) | 11.0 (7.0 to 18.0) | 1.03 (1.00 to 1.06) | 0.35 (−0.05 to 0.74) |
| Mortality in ICU, No. (%) | 1492 (14.5) | 1294 (11.8) | 0.97 (0.86 to 1.10)j | −0.37 (−2.13 to 1.20)f |
| Mortality in hospital, No. (%) | 1812 (17.7) | 1631 (14.9) | 0.98 (0.88 to 1.09)j | −0.34 (−2.22 to 1.35)f |
| Post hoc exploratory outcomes | ||||
| Hemoglobin within 48 h of ICU discharge, median (IQR), g/dLg,i | 9.50 (8.30 to 11.20) | 9.50 (8.30 to 11.10) | 1.01 (1.00 to 1.02) | 0.09 (0.002 to 0.18) |
| Change in hemoglobin from ICU admission to ICU discharge, median (IQR), g/dLg,i | −0.80 (−1.90 to 0.20) | −0.90 (−2.10 to 0.10) | 0.10 (0.02 to 0.18) | |
| Change in hemoglobin from ICU admission to ICU discharge in patients without RBC transfusions, median (IQR), g/dLg,k | −0.80 (−1.90 to 0.10) | −1.00 (−2.10 to 0.00) | 0.10 (0.01 to 0.19) | |
Abbreviations: ICU, intensive care unit; RBC, red blood cell.
Patients admitted during the COVID-19 pandemic-related delay in transitions to small-volume tubes (March 2, 2020, -August 17, 2020) were excluded.
Values were adjusted for age and sex and accounted for the stepped-wedge design with periods modeled as fixed effects, ICU units as a random effect, and for the primary outcome, length of ICU stay as an offset.
P = .19 for this relative risk, which represents the result of the hypothesis test for the significance of the intervention (transition to small-volume tubes) effect and was calculated by generalized linear mixed model, adjusted for age and sex, and accounted for the stepped-wedge design.
Number of tubes with insufficient quantity for analysis per total number of specimens sent for hemoglobin (ethylenediaminetetraacetic acid tubes) and creatinine (lithium or sodium heparin tubes) tests during ICU admission; analyzed using χ2 test of equality of 2 proportions.
Denominators were 193 695 for the small-volume tube group vs 195 383 for the standard-volume tube group.
Difference in % was presented for binary outcomes and was adjusted for age and sex and accounted for the stepped-wedge design. Absolute difference in % without any adjustment was presented for specimens with insufficient quantity outcome.
Analyses were adjusted for baseline admission hemoglobin in addition to adjusting for age and sex.
Hemoglobin was adjusted for RBC transfusion (1 transfusion = Hb – 1 g/dL). Values less than 0 were substituted with 0 (3.9% of values).
Denominators were 9260 for the small-volume tube group vs 9820 for the standard-volume tube group.
Unadjusted relative risk for mortality in ICU was 1.23 (95% CI, 1.15 to 1.32), and for mortality in hospital it was 1.18 (95% CI, 1.11 to 1.26).
Denominators were 6377 for the small-volume tube group vs 6645 for the standard-volume tube group.
In the secondary analysis population (whole cohort with ICU stay ≥48 hours), the least-squares mean number of RBC units transfused per patient during ICU stay was 0.80 (95% CI, 0.61 to 1.06) before transition to small-volume tubes and 0.71 (95% CI 0.53 to 0.93) after transition (RR, 0.88 [95% CI, 0.77 to 1.00]; P = .04), corresponding to an absolute difference of 9.84 (95% CI, 0.24 to 20.76) RBC units transfused per 100 patients during their ICU stay (eTable 5 in Supplement 1). There were no differences among patients admitted to the ICU for any duration (eTable 6 and eTable 7 in Supplement 1). No interactions were identified in prespecified subgroup analyses based on age, sex, and baseline hemoglobin concentration (eTable 8 and eTable 9 in Supplement 1).
In a post hoc analysis to examine for time-varying confounding, no significant interactions were found between the treatment variable and study periods (P = .71) or COVID-19 pandemic phases (P = .92). Mean RBC units per patient per ICU stay before and after transition to small-volume tubes for each ICU and overall is shown in Figure 2 for the primary population and in eFigure 2 in Supplement 1 for the secondary population. Treatment effect across ICUs showed significant heterogeneity (P = .002). Sensitivity analyses of the primary outcome were conducted in which the model was adjusted for imbalanced baseline characteristics (eTable 10 in Supplement 1), and the model incorporated time-decay correlation (eTable 11 in Supplement 1), both of which yielded similar results to the primary analysis. Another sensitivity analysis of the primary outcome using the generalized estimating equation approach resulted in a similar estimate of treatment effect but a wider CI (eTable 12 in Supplement 1).
Figure 2. RBC Units per Patient per ICU Stay Before and After Transition to Small-Volume Tubes at Individual ICUs (Primary Population).
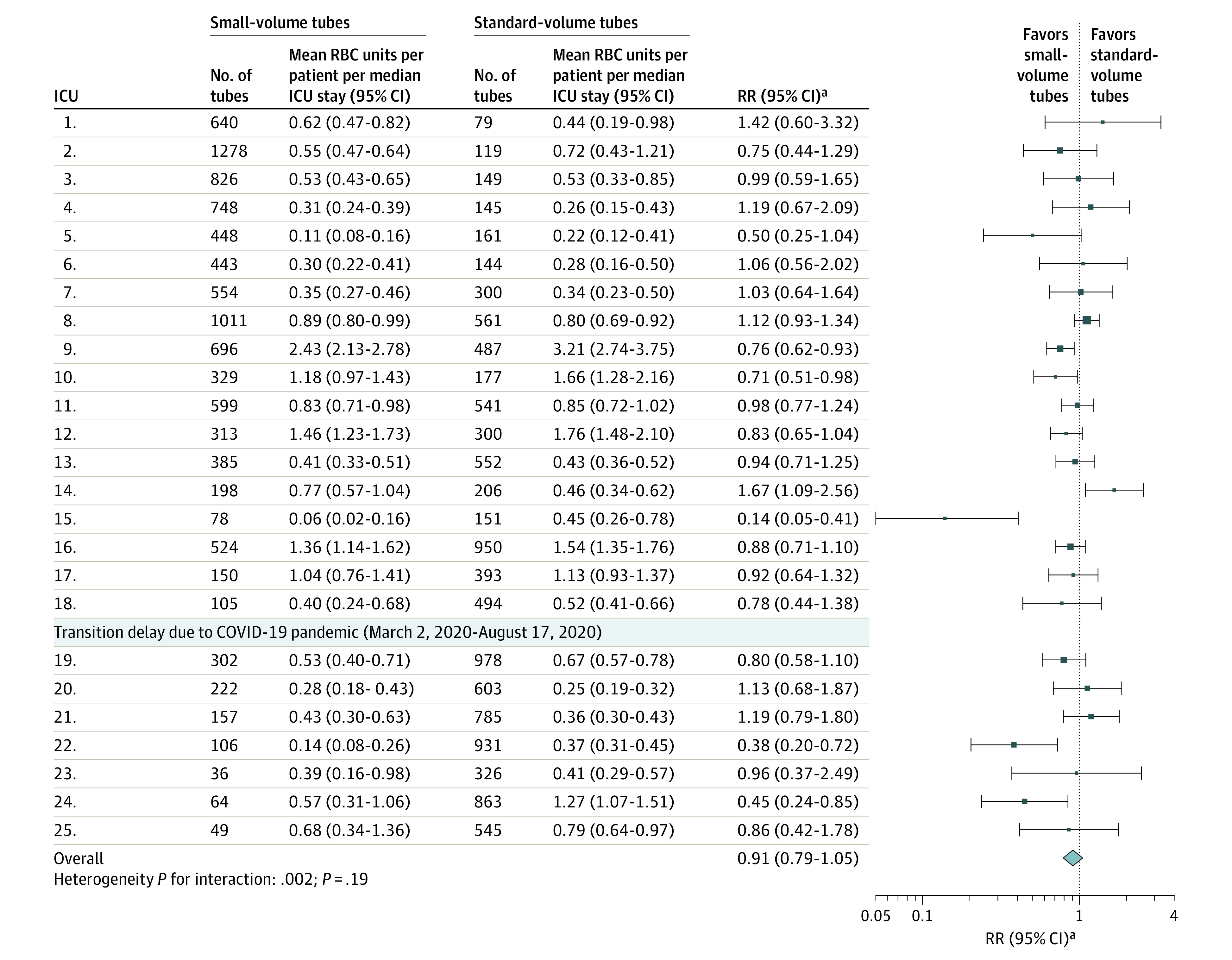
aRelative risk (RR) is adjusted for age and sex.
Plot shows the mean (95% CI) number of red blood cell (RBC) units per patient per median intensive care unit (ICU) stay at individual ICUs. The mean area of the squares is proportional to the corresponding total sample size of the ICU, and error bars indicate the 95% CIs. Patients admitted during the transition delay due to the COVID-19 pandemic were excluded. The square boxes denote the relative risk (RR) of RBC transfusion before and after transition to small-volume tubes adjusted for age and sex. Each ICU is represented by a single row and displayed in the order of transition to small-volume tubes.
Hemoglobin Reduction During ICU Admission
In a prespecified secondary analysis, the median transfusion-adjusted reduction in hemoglobin during ICU admission was 1.50 g/dL (IQR, 0.40-3.20) before transition and 1.40 g/dL (IQR, 0.20-3.00) after transition (mean difference, 0.10 g/dL [95% CI, −0.04 to 0.23]; P = .16) (Table 3). In the secondary analysis population, the median reduction in transfusion-adjusted hemoglobin decreased from 1.50 g/dL (IQR, 0.40-3.20) before transition to 1.40 g/dL (IQR, 0.30-3.10) after transition (mean difference, 0.17 g/dL [95% CI, 0.05 to 0.29]; P = .009). In exploratory analyses of all patients admitted for 48 hours or more, the median hemoglobin reduction (not adjusted for transfusion) decreased from 0.90 g/dL (IQR, 0.10-2.10) before transition to 0.80 g/dL (IQR, 0.20-2.00) after transition (mean difference, 0.12 g/dL [95% CI, 0.05 to 0.19) (eTable 5 in Supplement 1). Among patients admitted for 48 hours or more who received no RBC transfusions (70%), the median hemoglobin reduction decreased from 1.00 g/dL (IQR, 0.00-2.10) before transition to 0.90 g/dL (IQR, 0.00-2.00) after transition (mean difference, 0.10 g/dL [95% CI, 0.02 to 0.18).
Specimens With Insufficient Quantity for Analysis
A total of 510 141 ethylenediaminetetraacetic acid and sodium/lithium heparin tubes were sent for hemoglobin and creatinine testing during the study. In a prespecified secondary analysis, the number of specimens reported as having insufficient quantity for analysis was 65 out of 285 273 (0.02%) before transition and 64 out of 224 868 (0.03%), after transition (P = .21).
Discussion
In this pragmatic stepped-wedge, cluster randomized trial, RBC transfusions did not differ significantly before and after transition from standard- to small-volume blood collection tubes in the primary analysis population of ICU patients admitted for 48 hours or more, which excluded those admitted during a COVID-19 pandemic-related trial hiatus. However, in secondary analyses, RBC transfusion was lower among the larger cohort of all patients admitted for 48 hours or more, as was the decrease in hemoglobin concentration in both populations after the transition to small-volume tubes. While the magnitude and direction of effect on RBC transfusion was similar in the primary and secondary analyses, the P value associated with the analysis of the larger secondary population was nominally significant; whereas that of the smaller cohort was not. Taken together, these findings support a possible small reduction in RBC transfusion and ICU-related decrease in hemoglobin with transitioning to small-volume tubes. Importantly, we showed no adverse effect on laboratory testing measured as specimen insufficiency, the most important potential harm associated with use of small-volume tubes.
Reducing RBC transfusion, a scarce and costly intervention with well-described risks, is viewed widely as a clinical priority for hospitalized patients, particularly those in ICU who are frequently transfused.6,17,18 Blood loss for laboratory testing increases the likelihood of RBC transfusion and is a target of Patient Blood Management, a framework that aims to improve patient outcomes designed by maintaining hemoglobin, optimizing hemostasis, and minimizing blood loss. In the first randomized clinical trial to test and demonstrate possible benefit of small-volume blood collection tubes on RBC transfusion and hemoglobin in ICU patients, we add to the findings of previous small observational studies, which suggested that their use reduced the volume of blood taken for testing but reported an unclear effect on transfusion and laboratory testing.8 Although the effects of transitioning to small-volume tubes on RBC transfusion and hemoglobin were modest at the individual patient level, if applied broadly, they could have an impact on hospitals and health systems supporting efforts to preserve the limited supply of blood products amidst worldwide shortages. In this study alone, 30% of the studied ICU patients received about 36 000 RBC transfusions in less than 2 years and the use of small-volume tubes may have saved approximately 1500 RBC units.
Our study has several strengths. We used a stepped-wedge design, which is ideal for evaluating interventions that have a high likelihood of benefit and low risk of harm, particularly in complex care areas such as the ICU where contamination of treatment groups with individual patient randomization is a significant concern.
Small-volume tubes were implemented within routine clinical practice using hospital processes and in-service training of clinical and laboratory staff via simple, brief educational resources that reinforced the potential for broader implementation outside a clinical trial.
Both standard-volume and small-volume tubes were obtained by local hospital purchasing departments from usual commercial suppliers at essentially the same cost. We included medical-surgical ICUs located in different geographical regions and practice settings (both academic and community hospitals). Although sites in the study used tubes manufactured by Becton Dickinson (the main supplier in Canada), small-volume tubes are also available through other manufacturers worldwide and all major laboratory analyzer manufacturers were represented across sites, further supporting the generalizability of the results to other settings.
Limitations
First, the COVID-19 pandemic impacted care and disrupted clinical research in Canada and most countries due to increased hospitalization volumes, infection control precautions, and staffing shortages. At the onset of the pandemic, we temporarily suspended transitions to small-volume tubes at the 7 sites that had not yet transitioned. During this time, all sites continued to use the tube size (standard- or small-volume) already assigned. With significant uncertainty, we decided to modify our planned primary analysis to exclude the patients admitted during the pandemic-related study delay, although we still collected data throughout this period. We assessed for but did not find a confounding effect of the COVID-19 pandemic on the RR of RBC units transfused. Although there were some differences in baseline characteristics between groups, these did not appear to affect the results based on a sensitivity analysis that adjusted for imbalanced baseline characteristics.
Second, in this pragmatic open-label trial, we did not standardize tube volumes, waste practices during blood collection, or transfusion thresholds, which could affect the variability of treatment effect seen across sites. However, the use of institutional standard operating procedures for blood collection and evidence-based thresholds for transfusion were reinforced. While it is possible that knowledge of the intervention could influence transfusion use, given the complex clinical environment, high-acuity patients managed by multiple treating physicians, and standardized evidence-based approach to transfusion in ICU, it seems unlikely that this might have had a significant impact at the individual patient level. Arterial and central venous catheter use was also at the discretion of the treating clinicians.
Third, we collected administrative and electronic medical record data exclusively, which limits the information available on baseline characteristics and co-interventions and the subgroup and exploratory analyses that could be performed.
Fourth, the primary analysis population was defined using duration of ICU admission, which is a postrandomization variable that could increase the potential for bias. However, the nature of the study design with cluster randomization, implementation at the cluster level, and collection of data from all admitted ICU patients mitigates selection bias. Although possible, we think it is unlikely that a small intervention effect during the first 48 hours after admission would influence ICU length of stay, which is supported by a lack of difference seen in length of stay between groups.
Fifth, while we showed that the frequency of samples with insufficient volume for testing was low and similar between groups, we cannot rule out an effect on other aspects of testing including turnaround time for testing, analytes requiring a larger sample volume, requests for add-on orders for analytes not ordered at the time of collection, and specimen integrity (eg, hemolysis). A recent study, however, showed reduced hemolysis with small-volume vacuum collection tubes in the emergency department.19
Conclusion
The transition from standard-volume to small-volume tubes for blood collection in the ICU may reduce RBC transfusion without impacting biospecimen sufficiency for laboratory analysis.
Educational Objective: To identify the key insights or developments described in this article.
-
What type of small-volume blood collection tubes were used in this study?
Physically smaller tubes that collected approximately 0.5 mL of blood needed for most laboratory analyses.
Standard-sized collection tubes with decreased vacuum strength designed to draw approximately 50% of standard volume.
Standard-sized collection tubes with leak-proof connectors that allowed the operator to discontinue the pull when approximately 50% full.
-
What were the results of the trial?
Paradoxically, small-volume blood collection reduced the need for transfusion only in patients with shorter intensive care unit (ICU) stays.
Small-volume tubes led to practically prohibitive increases in specimens reported as having quantity insufficient for analysis.
The primary analysis, which excluded patients admitted during the COVID-19 pandemic, showed no significant difference in red blood cell transfusion with small-volume tubes, while secondary analysis in a larger population was nominally significant.
-
What do authors suggest might prohibit meaningful preservation of limited blood supply products through the use of small-volume blood collection in ICUs?
Effects are modest at the individual patient level, necessitating broad implementation for meaningful impact.
Small-volume blood collection tubes were substantially more expensive than standard collection tubes.
Special laboratory practices required for small-volume testing could limit generalizability.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
eFigure 1. Study Timeline and Randomized Transition Scheme
eFigure 2: RBC Units per Patient per ICU Stay Before and After Transition to Small-Volume Tubes at Individual ICUs (Secondary Analysis Population)
eTable 1. Blood Collection Tube Volumes Before and After Transition To Small-Volume Tubes at Participating ICUs
eTable 2. Cluster Characteristics and Baseline Characteristics of All Patients Admitted to ICU ≥48 Hours (Secondary Population)
eTable 3. Baseline Characteristics of Patients Admitted During the Pandemic-Related Delay and Other Study Periods
eTable 4. Selected Baseline Characteristics in Individual ICUs
eTable 5. Study Outcomes of All Patients Admitted to ICU ≥48 Hours (Secondary Population)
eTable 6. Study Outcomes Among Patients With Any Duration of ICU Admission (Excluding Patients Admitted During Pandemic-Related Delay)
eTable 7. Study Outcomes Among Patients With Any Duration of ICU Admission (Including Patients Admitted During Pandemic-Related Delay)
eTable 8. Subgroup Analyses for the Effect of Transition to Small-Volume Tubes on RBC Units Transfused in ICU (Primary Population)
eTable 9. Subgroup Analysis for the Effect of Intervention on RBC Units Transfused on ICU (Secondary Population)
eTable 10. Sensitivity Analysis of Primary Outcome Adjusted for Imbalanced Baseline Characteristics (Primary Population)
eTable 11. Sensitivity Analysis of Primary Outcome Without Adjusting for Age and Sex, and Primary Outcome Model With Time-Decay Correlation
eTable 12. Analysis of Primary Outcome Using Prespecified Generalized Linear Mixed Model (GLMM) and Post-Hoc Generalized Estimating Equation Model (GEE)
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.Hod EA, Francis RO. Noninfectious complications of blood transfusion. In: Cohn CS, Delaney M, Johnson ST, eds. AABB Technical Manual. 20th ed. HPC International; 2020:627. [Google Scholar]
- 2.Jackson Chornenki NL, James TE, Barty R, et al. Blood loss from laboratory testing, anemia, and red blood cell transfusion in the intensive care unit: a retrospective study. Transfusion. 2020;60(2):256-261. doi: 10.1111/trf.15649 [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Baron JF, Reinhart K, et al. ; ABC (Anemia and Blood Transfusion in Critical Care) Investigators . Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507. doi: 10.1001/jama.288.12.1499 [DOI] [PubMed] [Google Scholar]
- 4.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med. 2004;32(1):39-52. doi: 10.1097/01.CCM.0000104112.34142.79 [DOI] [PubMed] [Google Scholar]
- 5.Bodley T, Levi O, Chan M, Friedrich JO, Hicks LK. Reducing unnecessary diagnostic phlebotomy in intensive care: a prospective quality improvement intervention. BMJ Qual Saf. 2023;32(8):485-494. doi: 10.1136/bmjqs-2022-015358 [DOI] [PubMed] [Google Scholar]
- 6.Vlaar AP, Oczkowski S, de Bruin S, et al. Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2020;46(4):673-696. doi: 10.1007/s00134-019-05884-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale JC, Ruby SG. Specimen collection volumes for laboratory tests. Arch Pathol Lab Med. 2003;127(2):162-168. doi: 10.5858/2003-127-162-SCVFL [DOI] [PubMed] [Google Scholar]
- 8.Siegal DM, Manning N, Jackson Chornenki NL, Hillis CM, Heddle NM. Devices to reduce the volume of blood taken for laboratory testing in ICU patients: a systematic review. J Intensive Care Med. 2020;35(10):1074-1079. doi: 10.1177/0885066618810374 [DOI] [PubMed] [Google Scholar]
- 9.Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost. 2014;12(10):1591. doi: 10.1111/jth.12642 [DOI] [PubMed] [Google Scholar]
- 10.Ranasinghe T, Freeman WD. ‘ICU vampirism’—time for judicious blood draws in critically ill patients. Br J Haematol. 2014;164(2):302-303. doi: 10.1111/bjh.12613 [DOI] [PubMed] [Google Scholar]
- 11.Levi M. Twenty-five million liters of blood into the sewer. J Thromb Haemost. 2014;12(10):1592. doi: 10.1111/jth.12656 [DOI] [PubMed] [Google Scholar]
- 12.Weijer C, Grimshaw JM, Eccles MP, et al. ; Ottawa Ethics of Cluster Randomized Trials Consensus Group . The Ottawa Statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 2012;9(11):e1001346. doi: 10.1371/journal.pmed.1001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Institutes of Health Research; Natural Sciences and Engineering Research Council; Social Sciences and Humanities Research Council of Canada . Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2) 2018. Accessed October 4, 2023. https://ethics.gc.ca/eng/documents/tcps2-2018-en-interactive-final.pdf
- 14.Siegal DM, Belley-Côté EP, Lee SF, et al. Small-volume tubes to reduce anemia and transfusion (STRATUS): a pilot study. Can J Anaesth. Published online July 28, 2023. doi: 10.1007/s12630-023-02548-6 [DOI] [PubMed] [Google Scholar]
- 15.Orkin AM, Gill PJ, Ghersi D, et al. ; CONSERVE Group . Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 Statement. JAMA. 2021;326(3):257-265. doi: 10.1001/jama.2021.9941 [DOI] [PubMed] [Google Scholar]
- 16.Hooper R, Teerenstra S, de Hoop E, Eldridge S. Sample size calculation for stepped wedge and other longitudinal cluster randomised trials. Stat Med. 2016;35(26):4718-4728. doi: 10.1002/sim.7028 [DOI] [PubMed] [Google Scholar]
- 17.Meybohm P, Froessler B, Goodnough LT, et al. “Simplified International Recommendations for the Implementation of Patient Blood Management” (SIR4PBM). Perioper Med (Lond). 2017;6:5. doi: 10.1186/s13741-017-0061-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shander A, Javidroozi M, Lobel G. Patient blood management in the intensive care unit. Transfus Med Rev. 2017;31(4):264-271. doi: 10.1016/j.tmrv.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 19.Ramakers C. BD Vacutainer Barricor tube in the emergency department: reduced hemolysis rates using partial draw tubes with reduced vacuum. Clin Chem Lab Med. 2018;56(2):e31-e32. doi: 10.1515/cclm-2017-0411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study Timeline and Randomized Transition Scheme
eFigure 2: RBC Units per Patient per ICU Stay Before and After Transition to Small-Volume Tubes at Individual ICUs (Secondary Analysis Population)
eTable 1. Blood Collection Tube Volumes Before and After Transition To Small-Volume Tubes at Participating ICUs
eTable 2. Cluster Characteristics and Baseline Characteristics of All Patients Admitted to ICU ≥48 Hours (Secondary Population)
eTable 3. Baseline Characteristics of Patients Admitted During the Pandemic-Related Delay and Other Study Periods
eTable 4. Selected Baseline Characteristics in Individual ICUs
eTable 5. Study Outcomes of All Patients Admitted to ICU ≥48 Hours (Secondary Population)
eTable 6. Study Outcomes Among Patients With Any Duration of ICU Admission (Excluding Patients Admitted During Pandemic-Related Delay)
eTable 7. Study Outcomes Among Patients With Any Duration of ICU Admission (Including Patients Admitted During Pandemic-Related Delay)
eTable 8. Subgroup Analyses for the Effect of Transition to Small-Volume Tubes on RBC Units Transfused in ICU (Primary Population)
eTable 9. Subgroup Analysis for the Effect of Intervention on RBC Units Transfused on ICU (Secondary Population)
eTable 10. Sensitivity Analysis of Primary Outcome Adjusted for Imbalanced Baseline Characteristics (Primary Population)
eTable 11. Sensitivity Analysis of Primary Outcome Without Adjusting for Age and Sex, and Primary Outcome Model With Time-Decay Correlation
eTable 12. Analysis of Primary Outcome Using Prespecified Generalized Linear Mixed Model (GLMM) and Post-Hoc Generalized Estimating Equation Model (GEE)
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement


