Key Points
Question
Is intratumoral toll-like receptor 4 agonist glycopyranosyl lipid A in stable-emulsion formulation (GLA-SE) injection with radiotherapy an effective and feasible treatment for patients with advanced soft tissue sarcoma (STS)?
Findings
In this phase 1 nonrandomized controlled trial of 12 patients with advanced STS, intratumoral GLA-SE with radiotherapy was well tolerated, with all patients achieving local control of injected tumors. Durable local response was associated with expansion of intratumoral T-cell receptor clones, and these specific clones were detectable in the systemic circulation following intratumoral GLA-SE.
Meaning
These findings suggest that intratumoral IT GLA-SE with radiotherapy is a promising combination treatment associated with systemic expansion of putative antitumor T-cell clones in advanced STS.
This nonrandomized controlled trial of patients with advanced soft tissue sarcoma analyzed the immunomodulatory effects of intratumoral glycopyranosyl lipid A in stable-emulsion formulation with concurrent radiotherapy.
Abstract
Importance
Metastatic soft tissue sarcomas (STSs) have limited systemic therapy options, and immunomodulation has not yet meaningfully improved outcomes. Intratumoral (IT) injection of the toll-like receptor 4 (TLR4) agonist glycopyranosyl lipid A in stable-emulsion formulation (GLA-SE) has been studied as immunotherapy in other contexts.
Objective
To evaluate the safety, efficacy, and immunomodulatory effects of IT GLA-SE with concurrent radiotherapy in patients with metastatic STS with injectable lesions.
Design, Setting, and Participants
This phase 1 nonrandomized controlled trial of patients with STS was performed at a single academic sarcoma specialty center from November 17, 2014, to March 16, 2016. Data analysis was performed from August 2016 to September 2022.
Interventions
Two doses of IT GLA-SE (5 μg and 10 μg for 8 weekly doses) were tested for safety in combination with concurrent radiotherapy of the injected lesion.
Main Outcomes and Measures
Primary end points were safety and tolerability. Secondary and exploratory end points included local response rates as well as measurement of antitumor immunity with immunohistochemistry and T-cell receptor (TCR) sequencing of tumor-infiltrating and circulating lymphocytes.
Results
Twelve patients (median [range] age, 65 [34-78] years; 8 [67%] female) were treated across the 2 dose cohorts. Intratumoral GLA-SE was well tolerated, with only 1 patient (8%) experiencing a grade 2 adverse event. All patients achieved local control of the injected lesion after 8 doses, with 1 patient having complete regression (mean regression, −25%; range, −100% to 4%). In patients with durable local response, there were detectable increases in tumor-infiltrating lymphocytes. In 1 patient (target lesion −39% at 259 days of follow-up), TCR sequencing revealed expansion of preexisting and de novo clonotypes, with convergence of numerous rearrangements coding for the same binding sequence (suggestive of clonal convergence to antitumor targets). Single-cell sequencing identified these same expanded TCR clones in peripheral blood after treatment; these T cells had markedly enhanced Tbet expression, suggesting TH1 phenotype.
Conclusions and Relevance
In this nonrandomized controlled trial, IT GLA-SE with concurrent radiotherapy was well tolerated and provided more durable local control than radiotherapy alone. Patients with durable local response demonstrated enhanced IT T-cell clonal expansion, with matched expansion of these clonotypes in the circulation. Additional studies evaluating synergism of IT GLA-SE and radiotherapy with systemic immune modulation are warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT02180698
Introduction
Soft tissue sarcomas (STSs) are a heterogeneous group of more than 50 distinct mesenchymal neoplasms together composing 1% of all cancers. Despite incremental advances, median overall survival for patients with metastatic STS remains approximately 24 months.1 Although checkpoint inhibitors have activity in certain STS subtypes, the role of immunotherapy in STS is still being defined. Glycopyranosyl lipid A in stable-emulsion formulation (GLA-SE) is a toll-like receptor 4 (TLR4) agonist that is an established adjuvant for hepatitis B as well as human papillomavirus (serotypes 16 and 18) vaccines and has been tested intratumorally in several tumor types.2,3,4,5 Glycopyranosyl lipid A in stable-emulsion formulation is a potent activator of dendritic cells and induces a greater TH1 CD4+ cell response compared with other TLR agonists.6,7 Multiple investigators have been interested in potential synergy between radiotherapy and TLR4 agonists.8,9 Because patients presenting with metastatic sarcoma occasionally have symptomatic superficial tumors requiring radiotherapy,10,11,12 we sought to evaluate the feasibility and safety of intratumoral (IT) GLA-SE and radiotherapy in metastatic STS in this phase 1 pilot study.
As a key exploratory end point, we characterized changes in the T-cell receptor (TCR) repertoire following GLA-SE. Metrics evaluating TCR clonality and diversity in tumor-infiltrating lymphocytes (TILs) have been associated with patient outcome in a number of settings.13,14 Higher TCR clonality has been associated with immunotherapy response, as it may represent TCR skewing toward specificity against putative tumor antigens. We hypothesized that IT GLA-SE with radiotherapy might induce changes in the TCR repertoire within TILs cultured from STS tumors, consistent with an antigen-specific T-cell response.15,16 In this report, we present detailed analysis of the changing TCR repertoire within TILs and in the circulation in the context of a safe, well-tolerated IT TLR4 agonist injection with concurrent radiation.
Methods
Study Design and Patients
This open-label, phase 1 nonrandomized controlled trial of IT GLA-SE was conducted at the Seattle Cancer Center Alliance and Fred Hutchinson Cancer Research Center from November 17, 2014, to March 16, 2016, in accordance with International Conference on Harmonization Guidelines for Good Clinical Practice and the Code of Federal Regulations. The trial protocol (Supplement 1) was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. All patients provided written informed consent. The study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Eligible patients were 18 years or older with metastatic sarcoma and at least 1 palpable, superficial tumor safely accessible for bedside injection that would also be irradiated. Patients were required to have adequate hematologic, kidney, and hepatic function. Patient race and ethnicity information were collected by self-report. A complete list of eligibility criteria is provided in eFigure 1 in Supplement 2.
Treatment
Patients received planned, standard-of-care palliative radiotherapy to the injected superficial tumor as part of normal clinical care. Radiation was given within 2 weeks after starting GLA-SE injections. Although the goal radiation dose was 50 Gy or higher, the administered dose was at the discretion of the treating radiation oncologist.
The treatment schema is provided in eFigure 2 in Supplement 2. Intratumoral GLA-SE injections were performed on an approximately weekly basis in an outpatient clinic following administration of local anesthetic and under direct palpation of the target lesion. No radiographic image guidance was required. Cohorts 1 and 2 used 5 μg (1-mL injection) and 10 μg (2-mL injection) doses of GLA-SE, respectively.
Clinical Assessments
The primary objective was to assess the safety and tolerability of IT GLA-SE in combination with radiotherapy using the Common Terminology Criteria for Adverse Events, version 5.0. Secondary objectives were to assess the clinical efficacy of local and systemic disease control as well the immunologic effects. Assessment of tumor responses was performed for both target and noninjected lesions according to RECIST (Response Evaluation Criteria in Solid Tumors), version 1.1. Imaging and biopsies were performed at baseline before treatment and after the eighth dose. After this, imaging was performed every 6 weeks for 4 scans, then every 12 weeks after that, until progression.
TLR4 Staining Analysis
Slides from the formalin-fixed paraffin-embedded tumor blocks were stained with hematoxylin-eosin and with antibodies to TLR4 (MAB14783; R&D Systems) and analyzed by Mosaic Laboratories. A pathologist confirmed tumor presence using hematoxylin-eosin, and immunohistochemical findings were scored on a positivity scale of 0 (absent) to 3+ (strong). Banked tumor formalin-fixed paraffin-embedded blocks from previous institutional review board–approved protocols were also stained.
Immunologic Correlative Studies
Rapid expansion protocol of TILs,17 multiplex immunohistochemical analysis of core biopsy specimens,18 and TCR sequencing of rapid expansion protocol TILs and peripheral blood mononuclear cells (PBMCs)19 were performed in accordance with methods detailed in the respective references. For additional methodologic information, including methods for single-cell reverse gene transcription of PBMCs and flow cytometric sorting and sequencing of cytokine-producing T cells, see eFigure 3 in Supplement 2.
Statistical Analysis
Statistical analysis was conducted from August 2016 to September 2022. All enrolled patients who received at least 1 dose of IT GLA-SE were included in the safety analysis. Descriptive statistics were used to summarize baseline patient characteristics, safety, clinical response, and immunologic response variables. Local response was measured as percent change of the maximum dimension of the target lesion. For local response comparison between target lesions and concomitant irradiated or untreated lesions, means were calculated by pooling local response at last follow-up for all lesions among evaluable patients (n = 3). Statistical analysis was performed using Microsoft Excel, version 16 (Microsoft Corp) and GraphPad Prism, version 9 (GraphPad Software Inc).
Results
Patients
Twelve patients (median [range] age, 65 [34-78] years; 8 [67%] female and 4 [33%] male; 9 [75%] White, 1 [8%] Asian, and 2 [17%] with no race or ethnicity reported) with metastatic sarcoma with a superficial tumor amenable to bedside IT injection concurrent with radiotherapy given over 2 weeks were enrolled in the trial. Six patients were treated in the 5-μg cohort, and after observing no dose-limiting toxic effects, 6 additional patients were treated in the 10-μg cohort. One patient was not enrolled because of screening failure.
Six patients (50%) had leiomyosarcoma; the next most common subtype was synovial sarcoma (2 [17%]). The mean (SD) largest dimension of the injected tumor was 5.4 (3.6) cm. Patients had a median of 5 sites (range, 2-9 sites) of disease and 3.5 lines of treatment (range, 0-5 lines) before enrollment (Table 1; eTable in Supplement 2).
Table 1. Patient Characteristics at Baselinea.
| Characteristic | Patients (N = 12) |
|---|---|
| Sex | |
| Female | 8 (67) |
| Male | 4 (33) |
| Age, median (range), y | 65 (34-78) |
| Race and ethnicity | |
| Asian | 1 (8) |
| White | 9 (75) |
| Not reported | 2 (17) |
| ECOG performance status | |
| 1 | 11 (92) |
| 2 | 1 (8) |
| Histologic subtype | |
| Leiomyosarcoma | 6 (50) |
| Synovial sarcoma | 2 (17) |
| Epithelioid sarcoma | 1 (8) |
| Myxofibrosarcoma | 1 (8) |
| Chondrosarcoma | 1 (8) |
| Undifferentiated round cell sarcoma | 1 (8) |
| Previous No. of lines of systemic therapy, median (range) | 3.5 (0-5) |
| No. of disease sites, median (range) | 5 (2-9) |
| Injected lesion location | |
| Extremity | 5 (42) |
| Abdominal wall | 4 (33) |
| Trunk | 3 (25) |
| Size of lesion, mean (SD), cm | 5.4 (3.6) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Data are presented as number (percentage) of patients unless otherwise indicated.
Seven patients (58%) had at least a grade 1 adverse event (AE), with only 1 patient (8%) in the 5-μg cohort having grade 2 AEs (myalgia and fatigue). The most common AE reported was fatigue in 5 patients (42%), followed by injection site reaction (2 [17%]); all injection site reactions were pain related. No serious infections or rashes were reported. There was no association between the dose of IT GLA-SE and the frequency or severity of AEs (Table 2; eTable in Supplement 2). No grade 3 or higher AEs were seen, and no dose delays were required.
Table 2. Number of Patients With TRAEsa.
| TRAE | Patients, No. (%) | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Any grade | |
| Total patients with TRAEs | 7 (58) | 1 (8) | 0 | 7 (58) |
| Fatigue | 4 | 1 | 0 | 5 |
| Injection site reaction | 2 | 0 | 0 | 1 |
| Night sweats | 1 | 0 | 0 | 1 |
| Myalgia | 0 | 1 | 0 | 1 |
| Patients with TRAEs in the 5-μg cohort (n = 6) | 3 (50) | 1 (17) | 0 | 4 (67) |
| Patients with TRAEs in the 10-μg cohort (n = 6) | 3 (50) | 0 | 0 | 3 (50) |
Abbreviation: TRAE, treatment-related adverse event.
Grading of adverse events was based on Common Terminology Criteria for Adverse Events, version 5.0.
Local Response
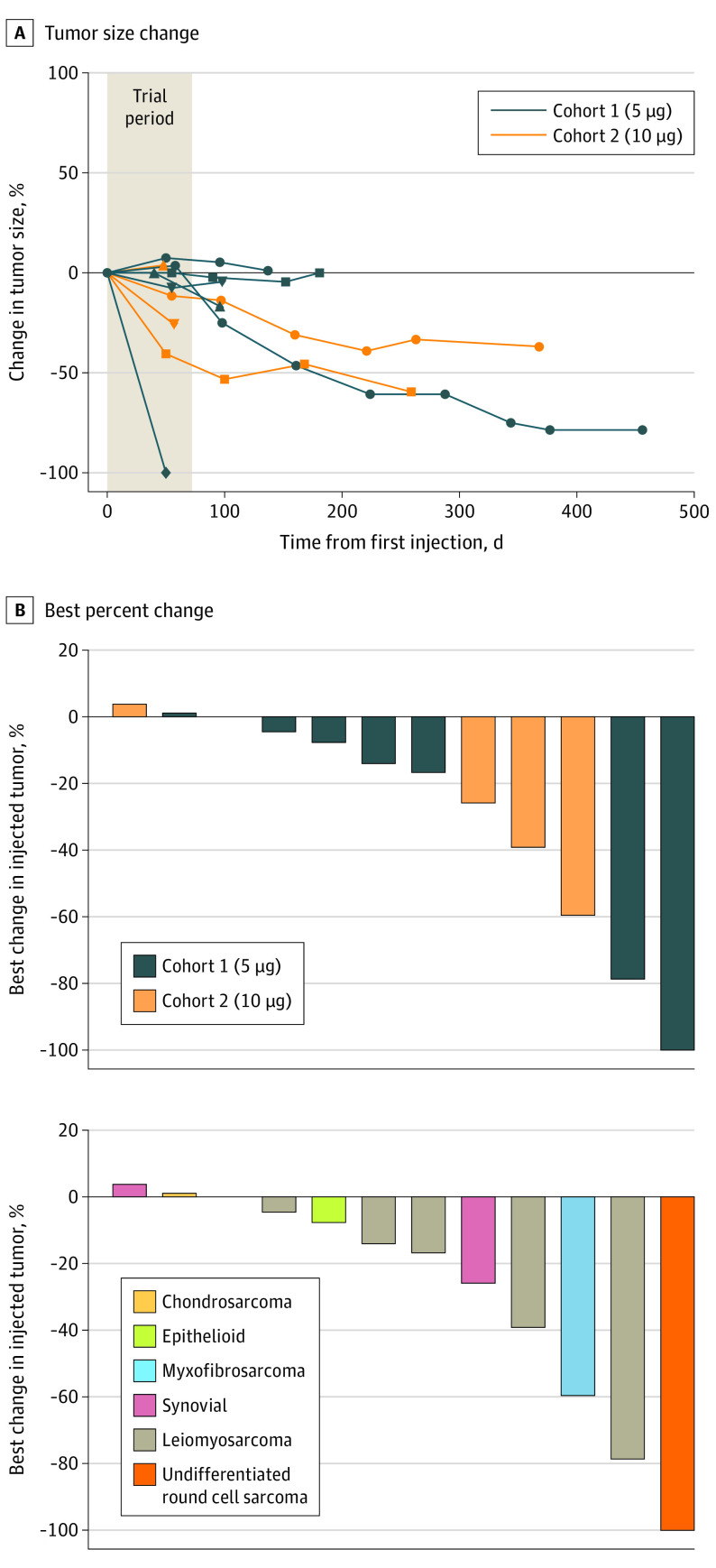
All patients achieved local control of the injected target lesions after 8 doses. One patient (8%) had complete response in the injected tumor, whereas 4 other patients (33%) had durable local response with greater than 25% size reduction. In this small cohort, there was no apparent association between local response and dosage, sarcoma subtype, previous therapies, number of metastatic sites, age, or sex (eTable and eFigure 4 in Supplement 2). The tumors injected with IT GLA-SE all showed durable response or stability, even in the setting of systemic progression; all patients with available imaging demonstrated their best local response at the last follow-up time point (median follow-up, 117.5 days after first injection; range, 50-456 days). The mean change in size overall (measured as percent change of the maximum dimension of the target lesion) was −25% (range, −100% to +4%) (Figure 1). With respect to their overall noninjected disease burden, 3 patients had stable disease) after 8 doses, whereas the remainder had disease progression (eTable in Supplement 2).
Figure 1. Local Response of Lesions Receiving Intratumoral Glycopyranosyl Lipid A in Stable-Emulsion Formulation.
A, Spider plot demonstrating change from baseline of tumor size and its durability beyond the trial period. B, Best percent change (which was at the end of follow-up time point for all patients) by cohort and histologic subtype.
To analyze the contribution of IT GLA-SE in combination therapy, we identified 3 patients with evaluable concomitant lesions during the trial follow-up period who received radiotherapy alone or no local therapy. Although other patients also had multiple sites of disease, they lacked lesions that underwent radiotherapy alone during the trial follow-up period. Durable local responses were observed only in irradiated lesions that also received IT GLA-SE. By pooled mean percent change at last follow-up, lesions treated with IT GLA-SE and radiotherapy had deep size reductions (−69%), single-modality irradiated lesions also decreased (−39%), and untreated lesions increased (+69%) (Figure 2; eFigure 5 in Supplement 2). For example, 1 patient who had a complete response in their injected tumor had a separate lesion that received radiotherapy alone and decreased in size by only 36%, whereas their untreated tumors grew.
Figure 2. Local Control After Intratumoral Glycopyranosyl Lipid A in Stable-Emulsion Formulation (IT GLA-SE) Compared With Concomitant Lesions.
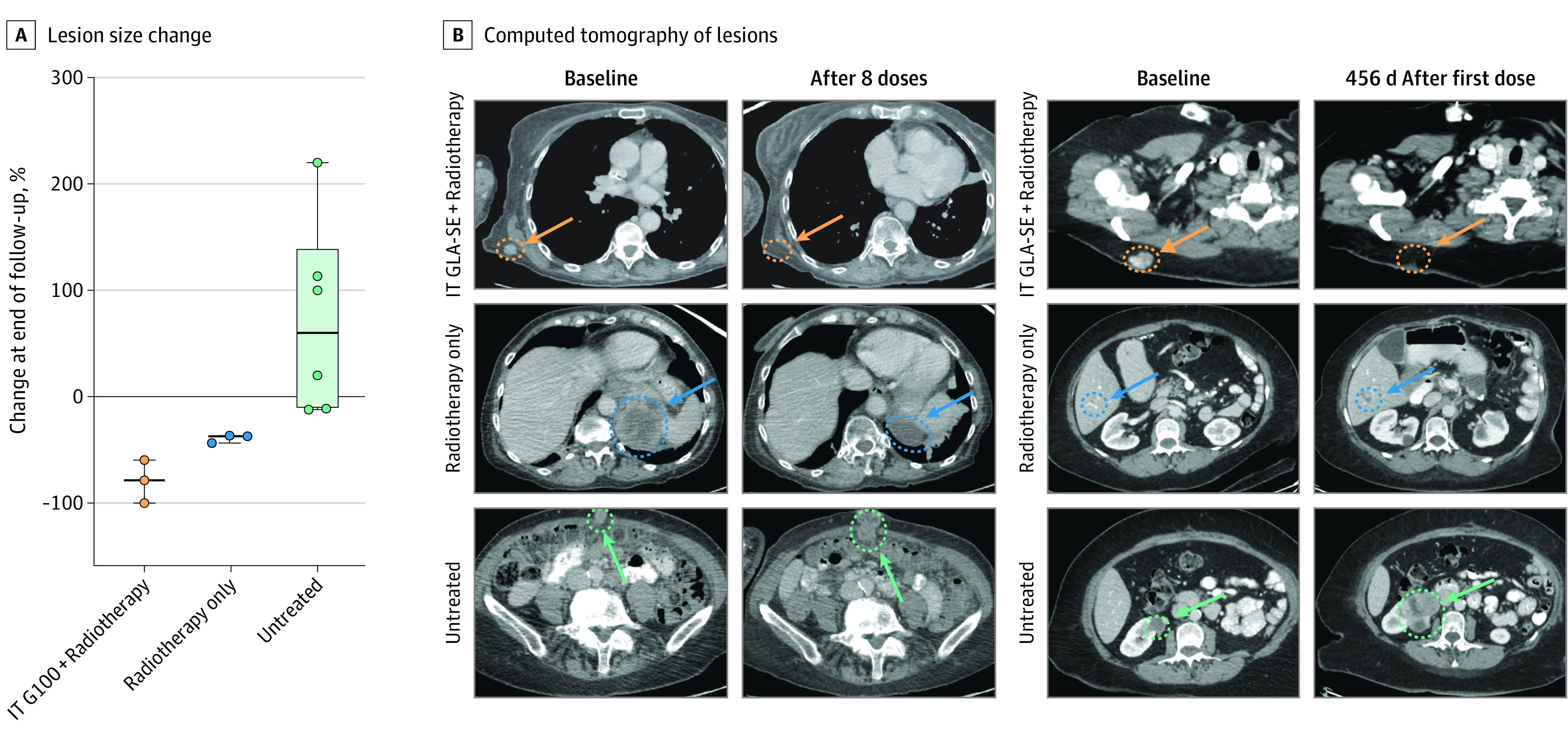
A, Percent change in size of lesions at end of same follow-up period for tumors receiving IT GLA-SE plus radiotherapy (orange) vs tumors receiving radiation only (blue) or no local treatment (green). B, Representative computed tomograms of lesions at baseline and at last follow-up in a patient with undifferentiated round cell sarcoma and complete regression of tumor after IT GLA-SE and patient with leiomyosarcoma −79% in size after IT GLA-SE.
Changes in the Tumor Microenvironment of Injected Tumor
Where sufficient quality tissue was available for evaluation of spatial architecture (n = 7), multiplex immunohistochemical analysis was performed on pretreatment and posttreatment samples (Figure 3A). CD4+ and CD8+ T-cell infiltration increased in patients with long-term local control. In 1 patient, who had excellent durable local response at the injected site (−79% from baseline at 456 days), there was a marked increase in the CD4+ T-cell infiltration into the tumor after injection (11%-34% of all stained cells) concomitant with an increase in the CD8+ T-cell fraction (0.3%-3.4%). Increased CD4+ T-cell infiltration was observed among patients with durable local control (mean infiltration, 10.7% pretreatment to 21.6% posttreatment), whereas the T-cell infiltration decreased in those with limited response (from 15.6% pretreatment to 9.2% posttreatment) (supporting data are given in Figure 3A; eFigure 6 in Supplement 2).
Figure 3. Local Immune Infiltration After Intratumoral Glycopyranosyl Lipid A in Stable-Emulsion Formulation (IT GLA-SE) in Durable Responders.
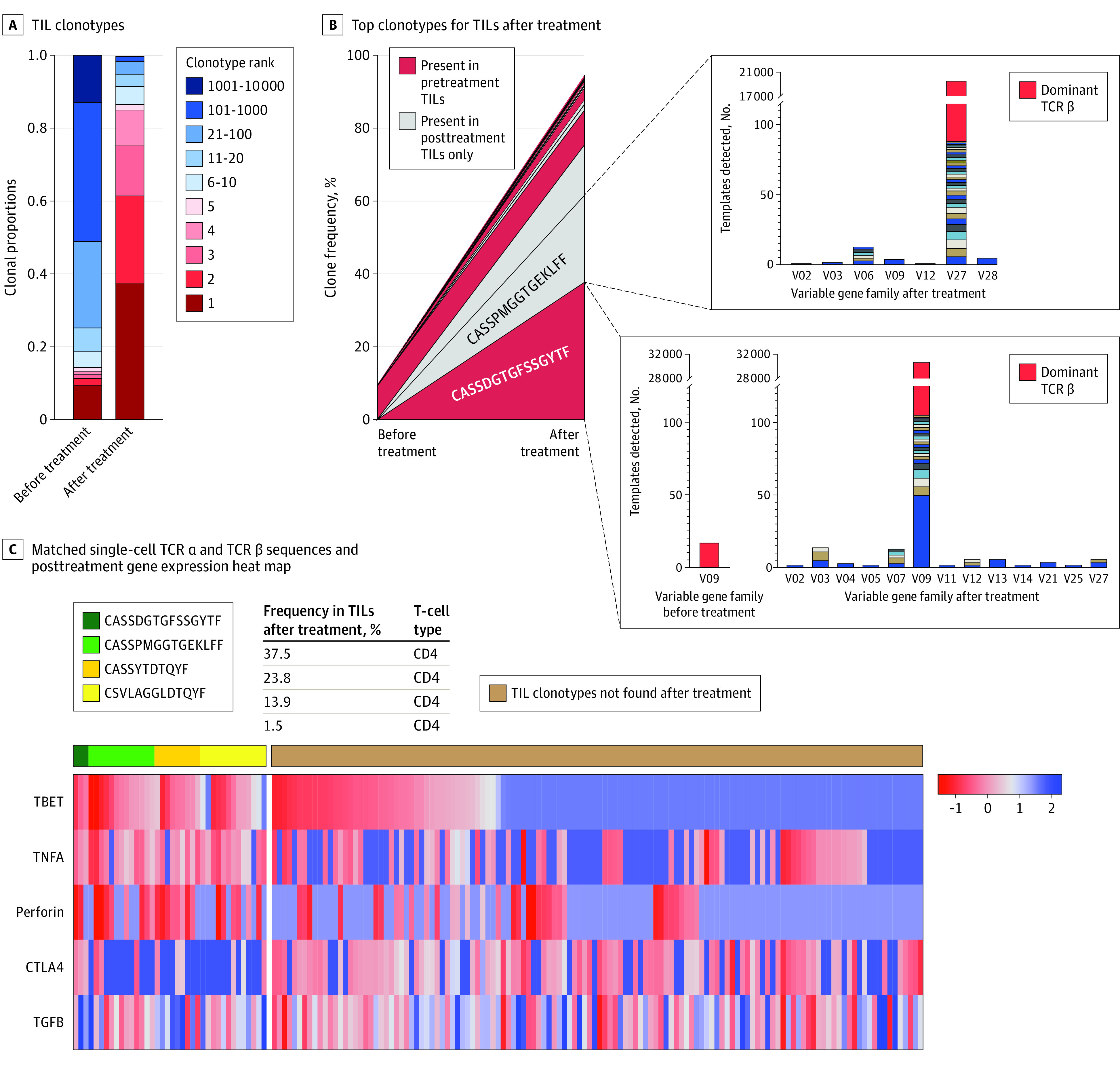
A, Multiplex immunohistochemical analysis found a detectable increase in CD4 and CD8 T-cell infiltration after treatment with IT GLA-SE. One patient had a detectable increase in CD4 T-cell infiltration from 11% to 34% of all cells counted, in addition to an increase in CD8 infiltration from 0.3% to 3.4%. Another patient had a detectable increase in CD8 T cells (3% to 22% of all cells counted) with a relative decrease in the proportion of T-regulatory cells (scale bars = 100 μm). B, Top clonotypes after IT GLA-SE. Tumor-infiltrating lymphocytes (TILs) were present in 1 patient, with red denoting clonotypes also present in pretreatment TILs. Inset boxes indicate breakdown of specific T-cell receptor (TCR) β DNA sequences coding for same variable amino acid region (red denotes dominant sequence). C, Matched single-cell TCR α and TCR β sequences and their gene expression heat map from posttreatment peripheral blood mononuclear cells. Green and yellow columns on left denote clonotypes expanded in posttreatment TILs. Color bar denotes standardized z score.
We investigated whether clinical outcomes were associated with the tumor’s TLR4 expression. We performed TLR4 immunohistochemical analysis across multiple histologic subtypes of banked untreated sarcomas (n = 34); the mean positivity rate was 59% (range, 0%-100%) (eFigure 7A-B in Supplement 2). This wide range of expression was also seen in pretreatment tissue when available (n = 5; mean, 73%; range, 2%-100%); 1 patient who had a complete response in the injected tumor had only 2% TLR4 positivity by immunohistochemical analysis, suggesting response did not depend on tumor TLR4 expression (eFigure 7C in Supplement 2) and instead could be related to TLR signaling by infiltrating immune cells.
Changes to TCR Repertoire in TILs Following GLA-SE
To delineate whether the increased T-cell infiltration was due to a generalized inflammation vs clonal expansion against putative tumor antigens, we analyzed TILs, generated from core biopsy samples and expanded ex vivo, using TCR sequencing. In total, 10 patients had evaluable TILs from both pretreatment and posttreatment samples. Although there was no discernible increase in TIL clonality after IT GLA-SE among all patients (mean [SD], 0.11 [0.09] before treatment and 0.16 [0.11] after treatment), increased clonality was observed in patients with local durable response (from 0.10 to 0.20), as opposed to those with limited response (from 0.07 to 0.06) (eFigure 8 in Supplement 2).
The greatest increase in TIL clonality was seen in a patient with durable local response) (eTable in Supplement 2), increasing from 0.09 to 0.37 after treatment; the most prevalent TCR amino acid sequence (or clonotype) after treatment represented more than 37% of all T cells sequenced. Although no dominant clones were seen before treatment for this patient, the top 4 clonotypes comprised more than 80% of sequences, with expansion of both preexisting and de novo clones represented (Figure 3B).
Of the dominant TCR clonotypes, CASSDGTGFSSGYTF was present at a very low frequency IT GLA-SE in only 1 unique DNA rearrangement (14 template reads, <0.01% relative frequency); after IT GLA-SE, this TCR rearrangement expanded to become the dominant clone, contributing more than 30 000 template reads using 13 different variable gene families (Figure 3B), suggesting clonal convergence to the same epitope. Another prominent clonotype after IT GLA-SE (CASSPMGGTGEKLFF) was not present in pretreatment TILs, but after IT GLA-SE, it expanded to 43 unique rearrangements from 7 different variable gene families, all coding for the same amino acid sequence (Figure 3B). These findings suggest pressure within the tumor microenvironment toward clonal expansion of both preexisting and de novo T cells.
To phenotype these dominant clones, we performed single-cell sorting and targeted gene reverse transcription using nested polymerase chain reaction in posttreatment PMBCs, where we found these TCRs frequently present. Multiple singlets with matching TCR β sequence were isolated from posttreatment PBMCs. By RNA sequencing, we found high levels of CD4+ T cells as well as high levels of T-bet, tumor necrosis factor α, and Ki-67, while showing lower levels of exhaustion markers, such as cytotoxic T-lymphocyte–associated protein 4 (Figure 3C; eFigure 9 in Supplement 2). These findings suggested a TH1 subtype that was present and detectable in circulating T cells after treatment.
Circulating T-Cell Response
We were interested in whether TCR sequences dominant in TILs became more prevalent in the circulation as part of a systemic antitumor response. The PBMCs from all patients underwent TCR sequencing before and after completion of study treatment. Of particular interest was 1 patient who had a complete local response with no radiographically detectable lesion at the end of the trial period (making posttreatment biopsy impossible). After localized injection with IT GLA-SE, the overall circulating PBMC clonality increased 5-fold (0.057 to 0.279). Three clones were present in the pretreatment TILs and PBMCs; these clones underwent clonal expansion in the posttreatment PBMCs and represented 11% of all circulating TCRs (supporting data in eFigure 10 in Supplement 2), suggesting that the combination of IT GLA-SE and radiotherapy resulted in systemic expansion of dominant TIL clones despite this patient’s systemic progressive disease.
Because 1 patient had dominant clones with markers for TH1 phenotype, we sought to test whether this was also the case for another patient. For the second patient, we used flow cytometry–based cell sorting for CD4, CD8, and TH1 surface markers (CD4+, CD19−, CXCR3+) and performed TCR sequencing on sorted populations. These sorted cells were TCR sequenced and matched back to the pretreatment TILs, allowing for phenotypic characterization. Of the matched CD4 clonotypes (90 [13%] of 695 unique clonotypes), 74 [82%] were clonotypes sorted into the TH1 population (eFigure 10C in Supplement 2), again suggesting a massive expansion in TH1-specific T cells as might be expected after use of a TLR4 agonist.20
We then asked whether the T cells with convergent TCRs constituted a functional effector population. To test this, PBMCs from durable local responders were stimulated with CD3/CD28 beads and then sorted using intracellular staining for tumor necrosis factor α and granzyme B along with CD4 and CD8 labeling (eFigure 11 in Supplement 2). Cells capable of producing both cytokines on stimulation underwent TCR sequencing and were compared with their original TIL TCR. In 1 patient with complete local response, the proportion of circulating CD8 T-cell clonotypes producing both cytokines increased from 9% to 58% after treatment with IT GLA-SE. This high proportion of dual cytokine-producing CD8 T cells was seen in durable responders but not in limited responders (supporting data in eFigure 11 in Supplement 2).
We sought to test for a difference in inflammatory proteins and circulating checkpoints in durable responders to evaluate whether this was different compared with minimal responders following IT GLA-SE treatment. Indeed, there were increased markers of both inflammation and checkpoint markers in the responders. For instance, 1 patient with complete regression of treated tumor had a log-fold increase of 0.6 for CD28, 0.6 for lymphocyte activation gene 3 protein, and 0.4 for programmed cell death ligand 1, whereas another patient (with best local response of −17%) had log-fold changes of 0.1, −0.1, and 0, respectively (eFigure 12 in Supplement 2).
Discussion
Radiation therapy is an effective tool in the management of symptomatic metastasis for STS.21,22 This phase 1 nonrandomized controlled trial suggests that the combination of GLA-SE with radiotherapy for symptomatic, superficial STS tumors is feasible and effective. The therapy was well tolerated, and although the study was not designed to compare doses, there was no obvious difference between the 5- and 10-μg cohorts. All patients had local control with deep, durable regression observed in one-third of patients, with 1 patient having 80% reduction in size and another having a complete response at the injected site. Although published local control rates of metastatic STS after radiotherapy are high (>80%),23,24 most studies concern visceral metastases (eg, pulmonary) and may not be directly applicable. In patients with concomitant evaluable lesions, IT GLA-SE with radiotherapy may cause more durable response than radiotherapy alone, although this study was not designed to make a direct comparison. Despite the increase in T-cell infiltration after IT GLA-SE seen in these durable responders, no systemic responses by RECIST criteria were observed. These results were similar to the finding of the recent PEMBROSARC (Combination of MK3475 and Metronomic Cyclophosphamide in Patients With Advanced Sarcomas: Multicentre Phase II Trial) trial testing intratumoral TLR4 injection with pembrolizumab in STS, which also saw poor systemic efficacy but did not include radiotherapy.25 Given the outstanding local control seen combining radiotherapy with IT GLA-SE, combination studies with systemic therapies are warranted.
We saw large increases in the clonality of PBMCs, including expansion of TCR sequences observed in TILs, suggesting additional systemic potential for this therapy.26 In 1 patient, the expanded clonotypes in posttreatment TILs (with evidence of clonal convergence) were isolated in posttreatment PBMCs; single-cell analysis revealed that these clonotypes expressed a TH1 phenotype. In another patient, though posttreatment biopsy could not be obtained due to complete response, there was expansion of preexisting top PBMC clones after IT GLA-SE. By sorting PBMCs via flow cytometry for TH1 cells and conducting TCR sequencing, we found that many of these were TH1 CD4 T cells as well. Although shared amino acid sequence of the complementarity-determining region 3 of the TCR composed of multiple DNA rearrangements are occasionally encountered, the induction of such sequences in TILs after immunotherapy is unique and suggests important functionality. The use of IT GLA-SE as a tool for identification of putative antitumor TH1 T cells warrants further exploration as potential targets for adoptive T-cell therapy across STS subtypes.27,28,29 Combination studies with adoptive cellular therapy30 may also hold promise.
Limitations
This study as some limitations. Because this was a phase 1 dose-escalation trial, the findings are preliminary. In addition, the number of patients was limited and not powered to evaluate clinical efficacy, and there was no placebo control group.
Conclusions
In this phase 1 nonrandomized controlled trial, IT GLA-SE with concurrent radiotherapy was safe, with preliminary evidence of efficacy in the injected tumor. However, further study is warranted. In patients with durable local response, there was evidence of intratumoral T-cell clonal expansion of both preexisting and novel TCRs; these unique TCRs were then detected in circulating PBMCs after treatment and had a TH1 effector phenotype. In some cases, this included a single dominant TCR composed of many individual gene rearranged clones.
Trial Protocol
eTable. Baseline Characteristics, Treatment Response, and Adverse Events
eFigure 1. Eligibility Criteria
eFigure 2. Trial Schema
eFigure 3. Detailed Immune Correlative Methodologies
eFigure 4. Size of Injected Tumor Versus Local Response
eFigure 5. Spider Plots of Concomitant Lesions Across Follow-Up Period
eFigure 6. Multiplex IHC Data Overview
eFigure 7. TLR4 Staining Across Sarcoma Subtypes
eFigure 8. T-Cell Receptor Sequencing Data Overview
eFigure 9. Schema for Single Cell Analysis of Circulating Tcrβ Clonotypes
eFigure 10. Expansion of Circulating Th1-Type Clonotypes
eFigure 11. Expansion of Circulating Polyfunctional CD8 T Cells
eFigure 12. Circulating Serum Expression Profiles of Immunophenotypic Proteins in Durable Local Responders Versus Minimal Responders
Data Sharing Statement
References
- 1.Lochner J, Menge F, Vassos N, Hohenberger P, Kasper B. Prognosis of patients with metastatic soft tissue sarcoma: advances in recent years. Oncol Res Treat. 2020;43(11):613-619. doi: 10.1159/000509519 [DOI] [PubMed] [Google Scholar]
- 2.Peters NC, Bertholet S, Lawyer PG, et al. Evaluation of recombinant Leishmania polyprotein plus glucopyranosyl lipid A stable emulsion vaccines against sand fly-transmitted Leishmania major in C57BL/6 mice. J Immunol. 2012;189(10):4832-4841. doi: 10.4049/jimmunol.1201676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones GJ, Steinbach S, Clifford D, et al. Immunisation with ID83 fusion protein induces antigen-specific cell mediated and humoral immune responses in cattle. Vaccine. 2013;31(45):5250-5255. doi: 10.1016/j.vaccine.2013.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S, Miller NJ, Lu H, et al. Intratumoral G100, a TLR4 agonist, induces antitumor immune responses and tumor regression in patients with Merkel cell carcinoma. Clin Cancer Res. 2019;25(4):1185-1195. doi: 10.1158/1078-0432.CCR-18-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halwani AS, Panizo C, Isufi I, et al. Phase 1/2 study of intratumoral G100 (TLR4 agonist) with or without pembrolizumab in follicular lymphoma. Leuk Lymphoma. 2022;63(4):821-833. doi: 10.1080/10428194.2021.2010057 [DOI] [PubMed] [Google Scholar]
- 6.Coler RN, Bertholet S, Moutaftsi M, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6(1):e16333. doi: 10.1371/journal.pone.0016333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider LP, Schoonderwoerd AJ, Moutaftsi M, et al. Intradermally administered TLR4 agonist GLA-SE enhances the capacity of human skin DCs to activate T cells and promotes emigration of Langerhans cells. Vaccine. 2012;30(28):4216-4224. doi: 10.1016/j.vaccine.2012.04.051 [DOI] [PubMed] [Google Scholar]
- 8.Walshaw RC, Honeychurch J, Choudhury A, Illidge TM. Toll-like receptor agonists and radiation therapy combinations: an untapped opportunity to induce anticancer immunity and improve tumor control. Int J Radiat Oncol Biol Phys. 2020;108(1):27-37. doi: 10.1016/j.ijrobp.2020.04.020 [DOI] [PubMed] [Google Scholar]
- 9.Frank MJ, Reagan PM, Bartlett NL, et al. in situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 2018;8(10):1258-1269. doi: 10.1158/2159-8290.CD-18-0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farshadpour F, Schaapveld M, Suurmeijer AJH, Wymenga ANM, Otter R, Hoekstra HJ. Soft tissue sarcoma: why not treated? Crit Rev Oncol Hematol. 2005;54(1):77-83. doi: 10.1016/j.critrevonc.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 11.Wang WL, Bones-Valentin RA, Prieto VG, Pollock RE, Lev DC, Lazar AJ. Sarcoma metastases to the skin: a clinicopathologic study of 65 patients. Cancer. 2012;118(11):2900-2904. doi: 10.1002/cncr.26590 [DOI] [PubMed] [Google Scholar]
- 12.Damron TA, Heiner J. Distant soft tissue metastases: a series of 30 new patients and 91 cases from the literature. Ann Surg Oncol. 2000;7(7):526-534. doi: 10.1007/s10434-000-0526-7 [DOI] [PubMed] [Google Scholar]
- 13.Yusko E, Vignali M, Wilson RK, et al. Association of tumor microenvironment T-cell repertoire and mutational load with clinical outcome after sequential checkpoint blockade in melanoma. Cancer Immunol Res. 2019;7(3):458-465. doi: 10.1158/2326-6066.CIR-18-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federico L, McGrail DJ, Bentebibel SE, et al. Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer. Ann Oncol. 2022;33(1):42-56. doi: 10.1016/j.annonc.2021.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valpione S, Mundra PA, Galvani E, et al. The T cell receptor repertoire of tumor infiltrating T cells is predictive and prognostic for cancer survival. Nat Commun. 2021;12(1):4098. doi: 10.1038/s41467-021-24343-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner MJ, Zhang Y, Cranmer LD, et al. A phase 1/2 trial combining avelumab and trabectedin for advanced liposarcoma and leiomyosarcoma. Clin Cancer Res. 2022;28(11):2306-2312. doi: 10.1158/1078-0432.CCR-22-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128(2):189-201. doi: 10.1016/0022-1759(90)90210-M [DOI] [PubMed] [Google Scholar]
- 18.Schroeder BA, LaFranzo NA, LaFleur BJ, et al. CD4+ T cell and M2 macrophage infiltration predict dedifferentiated liposarcoma patient outcomes. J Immunother Cancer. 2021;9(8):e002812. doi: 10.1136/jitc-2021-002812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robins HS, Srivastava SK, Campregher PV, et al. Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci Transl Med. 2010;2(47):47ra64. doi: 10.1126/scitranslmed.3001442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21(5):733-741. doi: 10.1016/j.immuni.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 21.Olivier T, Pop D, Chouiter Djebaili A, et al. Treating metastatic sarcomas locally: a paradoxe, a rationale, an evidence? Crit Rev Oncol Hematol. 2015;95(1):62-77. doi: 10.1016/j.critrevonc.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 22.Spałek MJ, Teterycz P, Borkowska A, Poleszczuk J, Rutkowski P. Stereotactic radiotherapy for soft tissue and bone sarcomas: real-world evidence. Ther Adv Med Oncol. 2022;14:17588359211070646. doi: 10.1177/17588359211070646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah NK, Yegya-Raman N, Jones JA, Shabason JE. Radiation therapy in metastatic soft tissue sarcoma: from palliation to ablation. Cancers (Basel). 2021;13(19):4775. doi: 10.3390/cancers13194775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farooqi A, Mitra D, Guadagnolo BA, Bishop AJ. The evolving role of radiation therapy in patients with metastatic soft tissue sarcoma. Curr Oncol Rep. 2020;22(8):79. doi: 10.1007/s11912-020-00936-5 [DOI] [PubMed] [Google Scholar]
- 25.Spalato-Ceruso M, Bouteiller F, Guegan JP, et al. Pembrolizumab combined with low-dose cyclophosphamide and intra-tumoral injection of the toll-like receptor 4 agonist G100 in patients with advanced pretreated soft tissue sarcoma: results from the PEMBROSARC basket study. J Hematol Oncol. 2022;15(1):157. doi: 10.1186/s13045-022-01377-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goff PH, Riolobos L, LaFleur BJ, et al. Neoadjuvant therapy induces a potent immune response to sarcoma, dominated by myeloid and B cells. Clin Cancer Res. 2022;28(8):1701-1711. doi: 10.1158/1078-0432.CCR-21-4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollack SM, Jones RL, Farrar EA, et al. Tetramer guided, cell sorter assisted production of clinical grade autologous NY-ESO-1 specific CD8+ T cells. J Immunother Cancer. 2014;2(1):36. doi: 10.1186/s40425-014-0036-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins PF, Kassim SH, Tran TLN, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1–reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21(5):1019-1027. doi: 10.1158/1078-0432.CCR-14-2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollack SM, Lu H, Gnjatic S, et al. First-in-human treatment with a dendritic cell-targeting lentiviral vector-expressing NY-ESO-1, LV305, induces deep, durable response in refractory metastatic synovial sarcoma patient. J Immunother. 2017;40(8):302-306. doi: 10.1097/CJI.0000000000000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullinax JE, Hall M, Beatty M, et al. Expanded tumor-infiltrating lymphocytes from soft tissue sarcoma have tumor-specific function. J Immunother. 2021;44(2):63-70. doi: 10.1097/CJI.0000000000000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Baseline Characteristics, Treatment Response, and Adverse Events
eFigure 1. Eligibility Criteria
eFigure 2. Trial Schema
eFigure 3. Detailed Immune Correlative Methodologies
eFigure 4. Size of Injected Tumor Versus Local Response
eFigure 5. Spider Plots of Concomitant Lesions Across Follow-Up Period
eFigure 6. Multiplex IHC Data Overview
eFigure 7. TLR4 Staining Across Sarcoma Subtypes
eFigure 8. T-Cell Receptor Sequencing Data Overview
eFigure 9. Schema for Single Cell Analysis of Circulating Tcrβ Clonotypes
eFigure 10. Expansion of Circulating Th1-Type Clonotypes
eFigure 11. Expansion of Circulating Polyfunctional CD8 T Cells
eFigure 12. Circulating Serum Expression Profiles of Immunophenotypic Proteins in Durable Local Responders Versus Minimal Responders
Data Sharing Statement