Abstract
MAS825, a bispecific IL-1β/IL-18 monoclonal antibody, could improve clinical outcomes in COVID-19 pneumonia by reducing inflammasome-mediated inflammation. Hospitalized non-ventilated patients with COVID-19 pneumonia (n = 138) were randomized (1:1) to receive MAS825 (10 mg/kg single i.v.) or placebo in addition to standard of care (SoC). The primary endpoint was the composite Acute Physiology and Chronic Health Evaluation II (APACHE II) score on Day 15 or on the day of discharge (whichever was earlier) with worst-case imputation for death. Other study endpoints included safety, C-reactive protein (CRP), SARS-CoV-2 presence, and inflammatory markers. On Day 15, the APACHE II score was 14.5 ± 1.87 and 13.5 ± 1.8 in the MAS825 and placebo groups, respectively (P = 0.33). MAS825 + SoC led to 33% relative reduction in intensive care unit (ICU) admissions, ~1 day reduction in ICU stay, reduction in mean duration of oxygen support (13.5 versus 14.3 days), and earlier clearance of virus on Day 15 versus placebo + SoC group. On Day 15, compared with placebo group, patients treated with MAS825 + SoC showed a 51% decrease in CRP levels, 42% lower IL-6 levels, 19% decrease in neutrophil levels, and 16% lower interferon-γ levels, indicative of IL-1β and IL-18 pathway engagement. MAS825 + SoC did not improve APACHE II score in hospitalized patients with severe COVID-19 pneumonia; however, it inhibited relevant clinical and inflammatory pathway biomarkers and resulted in faster virus clearance versus placebo + SoC. MAS825 used in conjunction with SoC was well tolerated. None of the adverse events (AEs) or serious AEs were treatment-related.
Keywords: MAS825, COVID-19, pneumonia, impaired respiratory function, efficacy and safety
This Phase 2 study evaluated the efficacy and safety of MAS825 and current standard of care (SoC) versus placebo and SoC in hospitalized patients presenting with COVID-19 pneumonia and impaired respiratory function. Although the study did not meet its primary endpoint, compared with placebo, MAS825 inhibited relevant cytokine pathways, with faster SARS-CoV-2 virus clearance. MAS825 was well-tolerated and no drug-related serious adverse events were reported.
Graphical Abstract
Graphical Abstract.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was first reported in December 2019 from the Wuhan province in China and rapidly spread worldwide, resulting in a global pandemic infecting hundreds of millions of people leading to premature deaths, a heavy disease burden on healthcare systems and the society in general [1]. SARS-CoV-2 infection may present with a range of clinical symptoms; however, patients with mild illness are often asymptomatic or have minimal symptoms affecting the upper respiratory tract, such as fever, cough, sore throat, and loss of taste and smell which are usually self-limiting. In severe cases, patients may require hospitalization for respiratory support, and approximately one-third of hospitalized patients develop pneumonia and pulmonary fibrosis, ultimately resulting in respiratory failure and death [2–4].
Current evidence suggests that a dysregulated host immune response to SARS-CoV-2 may play a significant role in the pathogenesis and subsequent development of a hyperinflammatory syndrome [5]. Several studies suggest that SARS-CoV-2 directly or indirectly activates inflammasomes (large multiprotein cytosolic assemblies) in response to an infection, resulting in the secretion of pivotal inflammasome effector cytokine mediators such as IL-1β and IL-18 [6–8]. Although IL-1β and IL-18 may be potentially protective in the early phase of infection, they can become detrimental in later phases by causing tissue injury due to a dysregulated hyperinflammatory response [9]. The NLRP3 inflammasome contributes to the hyperinflammatory response by driving the secretion and activation of the IL-1β and IL-18, which promote the release of additional downstream proinflammatory cytokines, including IL-6 and IFN-γ [6–8] in severe pulmonary and end-organ damage in patients with severe COVID-19. Further release of downstream cytokines and subsequent immune cell recruitment may amplify the immune response via positive feedback loops, culminating in a hyperinflammatory microenvironment associated with severe disease [6]. Lung infiltration and activation of pro-inflammatory myeloid cells such as monocytes, macrophages, and neutrophils are also thought to play a key role in the hyperinflammatory syndrome observed in severe cases of COVID-19 [10–14].
The standard of care (SoC) for the management of COVID-19 is continuously evolving and includes high-dose corticosteroids, antivirals, and anticoagulants [3], the same was used during this study. Considering the emerging role of the NLRP3 inflammasome in the progression of COVID-19 pathogenesis, IL-1β, and IL-18 neutralization was considered a potential therapeutic option that could be evaluated in this clinical study.
MAS825 is a novel bispecific antibody that combines the anti–IL-1β and anti–IL-18 monoclonal antibodies (mAbs) inhibition domains in a single bispecific high-affinity cytokine capture molecule. By simultaneously targeting and neutralizing the key inflammasome effectors, IL-1β and IL-18, MAS825 may potentially have superior clinical efficacy via more effective downmodulation of the cytokine-mediated inflammasome cascade in inflammatory diseases. The purpose of this study was to evaluate the efficacy and safety of MAS825 in addition to the current SoC (MAS825+SoC) compared with placebo and SoC (placebo+SoC) in controlling the inflammatory response in hospitalized patients presenting with COVID-19 pneumonia and impaired respiratory function.
Materials and methods
Study design
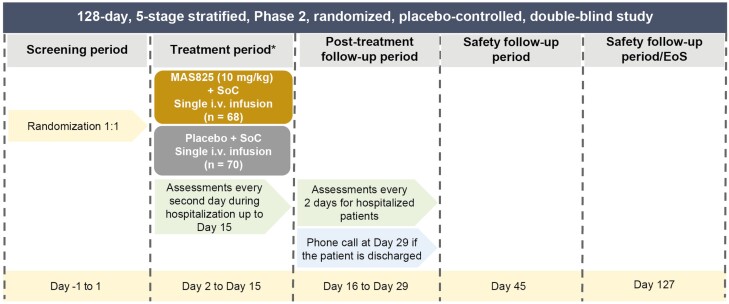
The MAS-COVID study was a Phase 2, randomized, placebo-controlled, double-blind, multicenter study to assess the efficacy and safety of MAS825 in conjunction with SoC for the treatment of SARS-CoV-2-infected patients with COVID-19 pneumonia and impaired respiratory function (NCT04382651). The study consisted of five stages spanning a total of 127 days (Fig. 1).
Figure 1.
Study design. *Endpoint calculated on the day of discharge for patients discharged prior to Day 15. EoS: end of study; SoC: standard of care.
Participants
Eligible participants included patients with clinically diagnosed SARS-CoV-2, aged ≥18 years at screening, hospitalized with COVID-19-induced pneumonia as evidenced by chest X-ray, computed tomography (CT) scan, or magnetic resonance (MR) scan (taken within 5 days prior to randomization). Included patients also had impaired respiratory function, defined as peripheral oxygen saturation (SpO2) ≤93% on room air or partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) <300 mmHg at the time of screening. Additional inclusion criteria included an Acute Physiology and Chronic Health Evaluation (APACHE) II score of ≥10 and C-reactive protein (CRP) ≥20 mg/l or ferritin level ≥600 μg/l. Key exclusion criteria included suspected active or chronic bacterial (including Mycobacterium tuberculosis), fungal, viral, or other infection (besides SARS-CoV-2); imminent and inevitable progression to death within the next 24 h in the opinion of the investigator; intubation prior to randomization; and treatment with anti-rejection and immunomodulatory drugs within the past 2 weeks, or within the past 30 days or five half-lives (whichever is longer for immunomodulatory therapeutic antibodies or prohibited drugs with the exception of antiviral therapies or corticosteroids). For COVID-19 infection, ongoing corticosteroid treatment was permitted at doses per local SoC and for non-COVID-19 disorders, ongoing corticosteroid treatment was permitted at doses up to and including prednisolone 10 mg daily or equivalent. The complete inclusion and exclusion criteria are listed in Supplementary Table S1.
Randomization and blinding
Eligible patients were randomized in a 1:1 ratio to receive a single i.v. infusion of MAS825 10 mg/kg or matching placebo in addition to SoC on Day 2 and were assessed until Day 15 and followed up until Day 29 (safety follow-up until Day 127). Patients were randomized within a maximum of 24 h after screening. Randomization was stratified by age (≤65 and >65 years), administration of any anti-viral therapy (e.g. hydroxychloroquine, chloroquine, convalescent plasma, remdesivir, favipiravir, ritonavir, and lopinavir) as SoC, and presence of any of the following comorbidities: diabetes, hypertension, cardiovascular disease, or chronic lung disease. Participants, investigator staff, persons performing the assessments, and the clinical trial team remained blinded to the identity of the treatment from the time of randomization until the end-of-study visit.
Assessments
Study assessments were conducted every 2 days for hospitalized patients. After completion of the treatment period (Day 15), patients were observed until Day 29 or until the day of discharge from the hospital, whichever was sooner. If a patient was discharged from the hospital prior to Day 15, assessments on the day of discharge were performed according to the schedule listed under Day 15, and the patient was instructed to return to the site for the Day 15 assessment or was visited at home by a home health nurse for the Day 15 assessment. For patients who were discharged from the hospital prior to Day 29, a telephone call was conducted on Day 29. A safety follow-up visit was conducted on Day 45 if the patient was still hospitalized. For patients who were discharged from the hospital prior to Day 45, a study visit was conducted by telephone on Day 45. An end-of-study safety follow-up visit was conducted on Day 127 if the patient was hospitalized. For patients who were discharged from the hospital prior to Day 127, a study visit was conducted by telephone on Day 127.
Outcomes
The primary endpoint of the study was the change in the APACHE II score on Day 15 or on the day of discharge (whichever was earlier) in the MAS825 + SoC group compared with the placebo + SoC group. Secondary endpoints included serum CRP and ferritin levels (to determine the effect on inflammatory status); survival without the need for invasive mechanical ventilation on Day 15 and Day 29; at least one-level improvement in clinical status on Day 15 and Day 29; clinical status over time; number of participants with adverse events (AEs), serious adverse events (SAEs), and clinically significant changes in laboratory results and vital signs (safety). Key exploratory endpoints included the presence of SARS-CoV-2 virus after treatment measured using polymerase chain reaction; mortality rate and hospital outcomes up to Day 29; change in clinical scores such as Sequential Organ Failure Assessment (SOFA) score that determined the extent of organ function and Simplified Acute Physiology Score II (SAPS II) that determined severity of disease; and measurement of IL-1β and IL-18 pathway biomarker inhibition such as IL-6, IFN-γ and neutrophils known to be linked to inflammation and disease. For the complete list of exploratory endpoints, please refer to Supplementary Table S2.
Statistical analysis
The primary endpoint was evaluated by an analysis of covariance (ANCOVA) model including treatment group and the three stratification groups (age group, administration of any anti-viral therapy at baseline, and presence of comorbidities ≥1) as factors and baseline APACHE II score as a covariate. The mean differences of MAS825 in addition to SoC vs. placebo in addition to SoC were reported with a 90% confidence interval (CI) and one-sided P value for the overall treatment was reported. To establish clinical efficacy based on APACHE II scores, a prior sample size of 60 patients per treatment group provided an 80% power when tested on a 10% one-sided alpha level under the assumption that MAS825 in addition to SoC reduces the APACHE II score by 3.6 points more than placebo. The primary estimate was based on this score to provide a comprehensive structured assessment of the clinical, physiological, and laboratory parameters that have been routinely employed by physicians to access the overall clinical status of COVID-19 patients with pneumonia and respiratory failure [15]. The safety analysis set included all randomized patients who had received any study drug and was used for the analysis of the primary and secondary endpoints. For the secondary endpoints, descriptive statistics (mean, standard deviation [SD], median, minimum, and maximum) were provided for numeric or continuous variables, while frequency distributions (with number and percentage) were provided for categorical variables. Safety summaries (tables and figures) included only data from the on-treatment period except for baseline data, which were summarized where appropriate (e.g. change from baseline summaries). The number (and percentage) of participants with treatment-emergent AEs (defined as events that started after the first dose of study medication or events present prior to the start of randomized treatment but that increased in severity based on preferred term) were summarized by treatment, primary system organ class and preferred term and by treatment, primary system organ class, preferred term, and maximum severity. For other data points, such as vital signs, electrocardiogram (ECG) results, and clinical laboratory evaluations, data were listed by treatment group, patient, and visit/ time and summary statistics were provided. Data were listed by treatment group, summary statistics, and graphical summaries were provided, and the number of values outside the limits of quantification were reported in each table. Biomarker data (CRP, IL-6, neutrophils, and IFN-γ) were reported as concentration results defined by lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ). Values that fell below the LLOQ and ULOQ were reported as <LLOQ * dilution factor. Parameters with values below the LLOQ were imputed as LLOQ/2 and values above the ULOQ are imputed as ULOQ. Biomarker endpoints were assumed to follow a log-normal distribution. The log-transformed data were analyzed by fitting a mixed-effect repeated measures (MMRM) model. The model included treatment, visit, and their interaction as well as three stratification factors as fixed factors and log-transformed baseline parameter as a covariate.
Study approval
The study was designed, executed, and reported in accordance with the International Conference on Harmonisation (ICH) E6 guideline for Good Clinical Practice (GCP), applicable local regulations, and the ethical principles laid down in the Declaration of Helsinki. Before participation, signed informed consent was obtained by a patient capable of giving consent or his or her legal/authorized representative.
Results
Patient disposition and baseline demographics
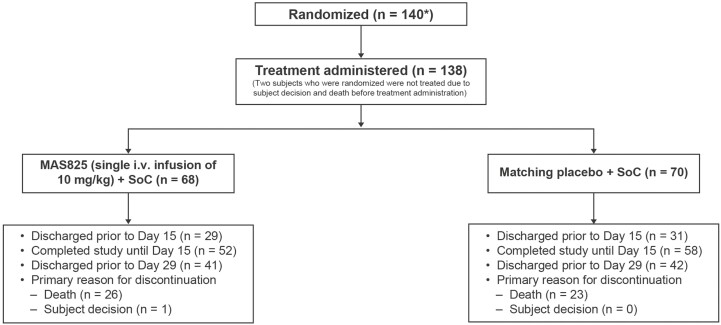
This study was conducted across 21 sites in the USA between June 11, 2020, and April 24, 2021, and included 138 subjects; 68% (n = 95) of the patients completed the study until Day 29, approximately 43% of the participants were discharged from the hospital until Day 15, and 59% of the participants were discharged by Day 29 (Fig. 2).
Figure 2.
CONSORT flowchart of patient disposition. SoC: standard of care.
The mean age of participants in the MAS825 + SoC group was slightly higher than that in the placebo group (65.3 versus 63.8 years). The MAS825 + SoC group also had poorer respiratory status (with one patient requiring mechanical ventilation, greater use of non-invasive ventilation, and higher flow oxygen at baseline) compared with the placebo + SoC group. At baseline, 37 patients required high-flow oxygen in the MAS825 + SoC arm compared with 34 in the placebo + SoC arm (54.4% versus 48.6%). Both groups had a high presence of comorbidities with hypertension and diabetes being the most common (Table 1). A slightly lower proportion of participants in the MAS825 + SoC arm received daily high-dose dexamethasone equivalent doses of ≥12 mg (10.3% versus 15.7%), and a higher proportion of participants received daily dexamethasone equivalent at lower doses of 6 to <12 mg (75% versus 62.9%) compared with the placebo + SoC arm. Similarly, concomitant therapies to treat COVID-19 were administered less to the MAS825 + SoC arm compared with the placebo + SoC arm, with less use of anti-viral medications (55.9% versus 65.7%), anti-coagulation therapies (70.6% versus 84.3%), and anti-infective therapies in the MAS825 + SoC arm (50.0% versus 61.4%).
Table 1.
Baseline characteristics
| Characteristic | MAS825 (10 mg/kg) + SoC (n = 68) |
Placebo + SoC (n = 70) |
Total (N = 138) |
|---|---|---|---|
| Age (years) | 65.3 ± 12.54 | 63.8 ± 12.97 | 64.6 ± 12.74 |
| BMI (kg/m2) | 31.2 ± 6.53 | 30.8 ± 6.53 | 31.0 ± 6.51 |
| Male, n (%) | 38 (55.9) | 47 (67.1) | 85 (61.6) |
| Days from symptom onset to randomization | 9.5 ± 4.84 | 10.3 ± 4.69 | 9.9 ± 4.76 |
| Days from diagnosis to randomization | 3.6 ± 3.25 | 3.5 ± 3.21 | 3.6 ± 3.22 |
| Days from hospital admission to randomization | 3.4 ± 4.97 | 2.5 ± 2.74 | 3.0 ± 4.01 |
| Presence of comorbidities, n (%) | |||
| Any comorbidities | 59 (86.8) | 63 (90.0) | 122 (88.4) |
| Cerebrovascular disorder | 2 (2.9) | 4 (5.7) | 6 (4.3) |
| Cardiac disorder | 11 (16.2) | 8 (11.4) | 19 (13.8) |
| Hypertension | 51 (75.0) | 58 (82.9) | 109 (79.0) |
| Chronic kidney disease | 6 (8.8) | 6 (8.6) | 12 (8.7) |
| Neoplasm malignant | 5 (7.4) | 5 (7.1) | 10 (7.2) |
| Diabetes | 27 (39.7) | 37 (52.9) | 64 (46.4) |
| Chronic lung disease | 12 (17.6) | 10 (14.3) | 22 (15.9) |
| APACHE II scores (mean ± SD) | 11.2 ± 3.06 | 11.5 ± 3.79 | 11.4 ± 3.44 |
| Leucocyte level (109/L) | 9.8 ± 4.51 | 9.5 ± 4.44 | 9.7 ± 4.47 |
| CRP (mg/L) | 118.8 ± 80.36 | 143.4 ± 137.81 | 131.1 ± 113.06 |
| Ferritin (μg/L) | 1134.6 ± 945.10 | 1069.2 ± 918.88 | 1101.7 ± 929.13 |
| Oxygen support, n (%) | |||
| Any baseline oxygen support | 68 (100.0) | 69 (98.6) | 137 (99.3) |
| Low-flow nasal oxygen | 10 (14.7) | 16 (22.9) | 26 (18.8) |
| Oxygen via face mask | 2 (2.9) | 4 (5.7) | 6 (4.3) |
| High-flow nasal oxygen | 37 (54.4) | 34 (48.6) | 71 (51.4) |
| Non-invasive ventilation | 18 (26.5) | 15 (21.4) | 33 (23.9) |
| Mechanical ventilation | 1 (1.5) | 0 | 1 (0.7) |
| Clinical status (9-point ordinal scale), n (%) | |||
| Hospitalized: no oxygen | 0 | 1 (1.4) | 1 (0.7) |
| Hospitalized: oxygen mask/nasal prongs | 12 (17.6) | 19 (27.1) | 31 (22.5) |
| Hospitalized: non-invasive ventilation/high-flow oxygen | 56 (82.4) | 50 (71.4) | 106 (76.8) |
| Use of corticosteroids at randomization, n (%) | |||
| Any corticosteroids | 61 (89.7) | 59 (84.3) | 120 (87.0) |
| ≥20 mg | 61 (89.7) | 59 (84.3) | 120 (87.0) |
| Anti-viral treatment (prior therapy) at randomization, n (%) | 49 (72.1) | 54 (77.1) | 103 (74.6) |
| Anti-coagulant treatment (prior therapy) at randomization, n (%) | 60 (88.2) | 60 (85.7) | 120 (87.0) |
| Anti-infective treatment (prior therapy) at randomization, n (%) | 26 (38.2) | 33 (47.1) | 59 (42.8) |
Data are presented as mean ± SD unless specified otherwise.
Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation II; BMI: body mass index; CRP: C-reactive protein; SoC: standard of care.
Primary efficacy outcomes
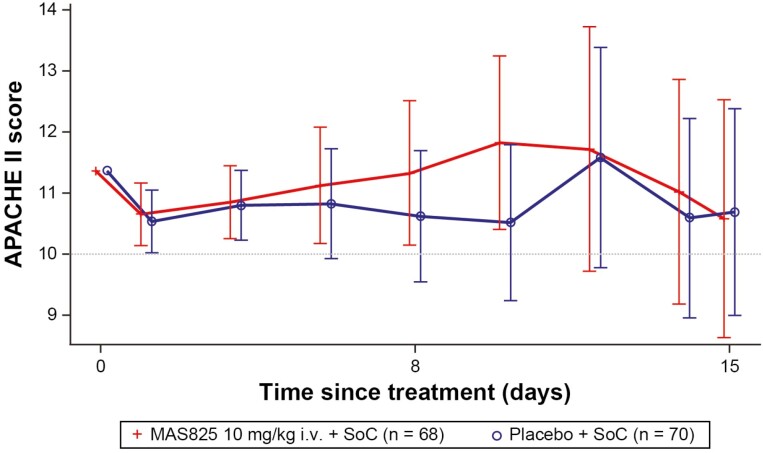
In safety analysis set, patients receiving MAS825 + SoC or placebo + SoC did not show a significant difference in their primary endpoint, composite APACHE II scores at Day 15 or on day of discharge (whichever was earlier) with worst-case imputation for death (adjusted LSM ± SE: 14.5 ± 1.87 versus 13.5 ± 1.8; LSM difference, 0.98, 90% CI −2.7 to 4.7; P = 0.33). The APACHE II score (range 0–71), a widely used ICU prognostic scoring model, has been shown to be an accurate measurement of patient severity and correlates strongly with the outcome in critical patients [15]. A mixed-effects repeated measures analysis of APACHE II scores over time showed a decrease from baseline to Day 15 in both MAS825 + SoC and placebo + SoC treatment arms. The mean APACHE II scores up to Day 15 are presented in Fig. 3. When the treatment effects were adjusted for baseline respiratory support, it was evident that there was a greater reduction in APACHE II scores in patients requiring more intensive respiratory support (Supplementary Fig. S1).
Figure 3.
APACHE II scores up to Day 15 (safety analysis set). Data presented as mean ± SE. APACHE II scores are modeled using a mixed-effects model with treatment, visit, stratification factors, visit * treatment, and visit * stratification factors as fixed effects and baseline score and visit * baseline as continuous covariates. Stratification factors were age group (≤65 years, >65 years), administration of any anti-viral therapy (yes/no), and presence of comorbidities ≥1 (yes/no). One-sided P-value for the overall treatment factor is P = 0.373. The safety analysis set included all randomized patients who received any study drug. APACHE II: Acute Physiology and Chronic Health Evaluation II; i.v.: intravenous; SoC: standard of care.
Other assessments
Categorical changes in clinical status (i.e. “worsening,” “no change,” and “improvement”) showed that about half of the participants were improving and thus discharged at Day 15 in both the arms (50% in the MAS825 + SoC arm versus 52.9% in the placebo + SoC arm), and this percentage increased to 57.4% after 4 weeks in the MAS825 + SoC arm versus 61.4% in placebo + SoC arm with no evidence of a treatment effect. No treatment differences were observed in the SOFA score and in the SAPS II score.
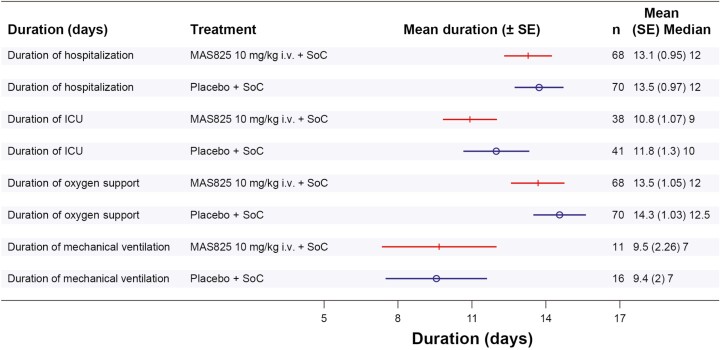
Hospital outcomes were also assessed and a reduction in ICU admission by up to 33% in the MAS825 + SoC arm (21.1% ICU admission) compared to the placebo + SoC arm (29.3% ICU admission) was observed on Day 29 (Supplementary Fig. S2). In addition, the mean duration of ICU stay was lower by approximately a day in MAS825 + SoC arm versus placebo + SoC arm, i.e. 10.8 days versus 11.8 days, respectively. The mean duration of oxygen support in participants (general and ICU care) was 13.5 days in the MAS825 + SoC arm, whereas it was 14.3 days in the placebo + SoC arm (Fig. 4). A higher percentage of patients receiving MAS825 achieved SARS-CoV-2 virus clearance compared with those receiving placebo (SARS-CoV-2 positive at Day 8: 60.7% versus 85.3%, Day 15: 52% versus 79.2%, respectively) (Supplementary Table S3).
Figure 4.
Forest plot of in-hospital outcomes in patients receiving MAS825 versus placebo at Day 29. ICU: intensive care unit; i.v.: intravenous; SE: standard error; SoC: standard of care.
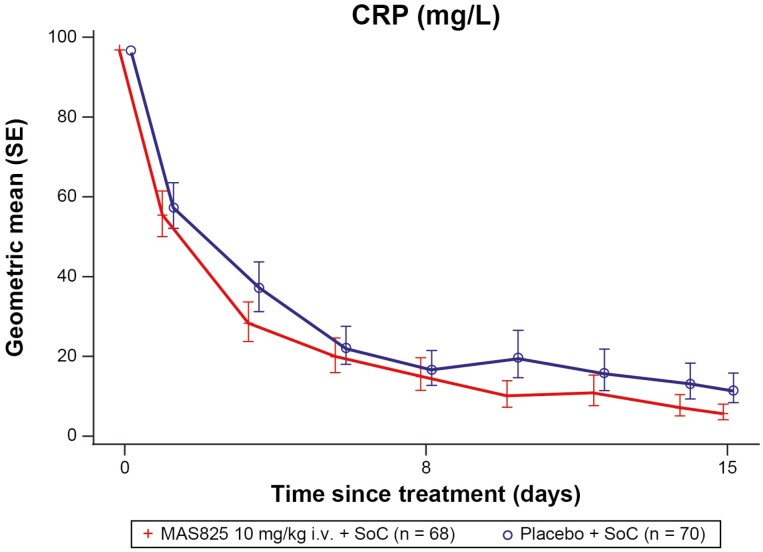
The mean CRP levels decreased over time in both treatment arms compared to baseline. There was a 51% decrease ([1 − Geometric mean ratio] * 100 i.e. [1 − 0.49] * 100 = 51%) in the MAS825 + SoC arm compared to the placebo + SoC arm on Day 15 (5.7 mg/L versus 11.6 mg/L) with normalization of the mean CRP in the MAS825 treated patient group only (<10 mg/L) (Fig. 5; Supplementary Table S4).
Figure 5.
CRP levels in patients receiving MAS825 + SoC versus placebo during the treatment period (PD analysis set). Data presented as estimated geometric mean ratio to baseline in CRP ± SE. Log-transformed CRP data is modeled using MMRM model with treatment, visit, stratification factors, visit * treatment and visit * stratification factors as fixed effects and log-transformed baseline score and visit * log-transformed baseline score as a continuous covariate. Stratification factors = age group, administration of any anti-viral therapy, presence of comorbidities. Results were back transformed to obtain adjusted geo-mean, geo-mean ratio, and 90% CI. CRP: C-reactive protein; i.v.: intravenous; MMRM: mixed-effect repeated measures; SoC: standard of care.
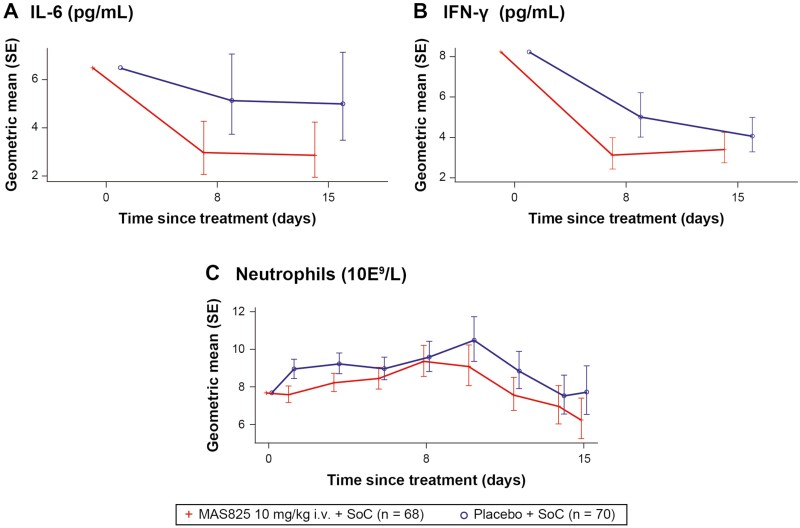
There was a reduction in IL-1β pathway biomarkers with mean IL-6 levels and neutrophil counts decreasing over time in both the arms during the study (Fig. 6A and C). Approximately 42% ([1 − 0.58] * 100 = 42%) lower mean IL-6 levels were observed with MAS825 + SoC versus placebo + SoC on Day 8 (2.99 pg/ml versus 5.15 pg/ml) and on Day 15 (2.88 pg/ml versus 5.00 pg/ml). Similarly, 19% ((1 − 0.81) * 100 = 19%) lower mean neutrophil levels were observed in the MAS825 + SoC arm compared to the placebo + SoC arm on Day 15 (Fig. 6C; Supplementary Table S4). In terms of IL-18 pathway biomarkers, IFN-γ levels were decreased in both the arms during the study. In addition, 38% ([1 − 0.62] * 100 = 38%) lower mean IFN-γ levels were observed in the MAS825 + SoC arm on Day 8 (3.12 pg/ml versus 5.01 pg/ml) and 16% ([1 - 0.84] * 100 = 16%) lower on Day 15 (3.41 pg/ml versus 4.07 pg/ml) compared to placebo + SoC arm (Fig. 6B; Supplementary Table S4).
Figure 6.
Analysis of IL-6, neutrophils, and IFN-γ in COVID-19 patients receiving MAS825 + SoC versus placebo during treatment period (PD analysis set). Data presented as estimated geometric mean ± SE. Biomarker data was reported as concentration results defined by the lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ). Values that fell below the LLOQ and ULOQ were reported as <LLOQ * dilution factor. Parameters with values below the LLOQ are imputed as LLOQ/2 and values above the ULOQ are imputed as ULOQ. Biomarker parameters were analyzed by MRMM. Log-transformed biomarker data is modeled using mixed-effects model with treatment, visit, stratification factors, visit * treatment, and visit * stratification factors as fixed effects and log-transformed baseline score and visit * log-transformed baseline score as continuous covariate. Stratification factors = age group, administration of any anti-viral therapy, presence of comorbidities. Results were back transformed to obtain adjusted geometric mean, geometric mean ratio, and 90% CI. COVID-19: coronavirus disease-19; IFN-γ: interferon gamma; IL-6: interleukin-6; i.v.: intravenous; MMRM: mixed-effect repeated measures; SoC: standard of care.
Other inflammatory markers, including IL-8, IL-10, IP-10, TNF-α, and IL-2RA levels, were similar in both treatment groups with the mean values of 65.34 pg/ml, 1.17 pg/ml, 746.42 pg/ml, 2.20 pg/ml, and 3508.62 pg/ml, respectively, for the MAS825 + SoC group and 52.58 pg/ml, 0.74 pg/ml, 636.53 pg/ml, 2.27 pg/ml, and 3169.02 pg/ml, respectively, for the placebo + SoC group at Day 15. Other cellular and liquid inflammatory, coagulation, and cardiac biomarkers such as ferritin, D-dimers, lactate dehydrogenase, and troponin were similar in both treatment groups with mean values of 502.90 µg/l, 1.6227 mg/l, 309.9301 U/l, and 0.0134 µg/l, respectively, for MAS825 and 504.20 µg/l, 1.5829 mg/l, 280.1892 U/l, and 0.0070 µg/l, respectively, for the placebo group, at Day 15.
Safety
The total number of AEs reported in the MAS825 + SoC group was lower than that in the placebo group (61.8% [n = 42] versus 75.7% [n = 53]), with slightly more SAEs in the MAS825 + SoC group [45.6% [n = 31] versus 40.0% [n = 28]). Fatal events up to Day 29 were 30.9% (n = 21) in the MAS825 + SoC arm versus 27.1% (n = 19) in the placebo + SoC arm. In both arms, most deaths were reported in the context of progressive worsening of the underlying COVID-19 infection. In addition, no AEs or SAEs were related to MAS825, as reported by the blinded investigators in this COVID-19 population (Table 2). Overall, the most frequent SAEs (reported in ≥5% of patients) in any of the treatment groups by primary system organ class included respiratory, thoracic, and mediastinal disorders (n = 39, 28.3%), infections and infestations (n = 15, 10.9%), cardiac disorders (n = 11, 8.0%), and renal and urinary disorders (n = 9, 6.5%). No unexpected fatal events or SAEs were reported in the MAS825 or placebo group, with respiratory failure being the most common fatal event (n = 9, 6.5%) (Table 3). Based on the safety data, MAS825 at 10 mg/kg i.v. was well tolerated by patients with COVID-19 respiratory failure and inflammation.
Table 2.
Overall incidence of adverse events
| MAS825 + SoC (N = 68) |
Placebo + SoC (N = 70) |
Total (N = 138) |
||||
|---|---|---|---|---|---|---|
| nE | nS (%) | nE | nS (%) | nE | nS (%) | |
| AE, patients with AE | 188 | 42 (61.8) | 225 | 53 (75.7) | 413 | 95 (68.8) |
| Mild AE | 54 | 28 (41.2) | 102 | 33 (47.1) | 156 | 61 (44.2) |
| Moderate AE | 65 | 24 (35.3) | 66 | 27 (38.6) | 131 | 51 (37.0) |
| Severe AE | 69 | 31 (45.6) | 57 | 29 (41.4) | 126 | 60 (43.5) |
| Study-drug–related AE | 0 | 0 | 2 | 2 (2.9) | 2 | 2 (1.4) |
| Study-drug–related SAE | 0 | 0 | 0 | 0 | 0 | 0 |
| SAE | 66 | 31 (45.6) | 48 | 28 (40.0) | 114 | 59 (42.8) |
| AE leading to discontinuation of study treatment | 0 | 0 | 0 | 0 | 0 | 0 |
| Study-drug-related AE leading to discontinuation of study treatment | 0 | 0 | 0 | 0 | 0 | 0 |
Percentages are based on the number of subjects. Only treatment-emergent AEs, i.e. from the date of administration of study treatment to Day 127, are considered.
Abbreviations: AE: adverse event; N: number of subjects studied; nE: number of AEs in the category; nS: number of subjects with at least one AE in the category; SAE: serious adverse event; SoC: standard of care.
Table 3.
Serious AEs by system organ class and preferred term
| Primary system organ class Preferred term |
MAS825 + SoC N = 68 n (%) |
Placebo +SoC N = 70 n (%) |
Total N = 138 n (%) |
|---|---|---|---|
| Number of subjects with at least one AE | 31 (45.6) | 28 (40.0) | 59 (42.8) |
| Blood and lymphatic system disorders | 2 (2.9) | 0 | 2 (1.4) |
| Pancytopenia | 2 (2.9) | 0 | 2 (1.4) |
| Cardiac disorders | 4 (5.9) | 7 (10.0) | 11(8.0) |
| Acute coronary syndrome | 1 (1.5) | 0 | 1 (0.7) |
| Atrial fibrillation | 0 | 2 (2.9) | 2 (1.4) |
| Cardiac arrest | 2 (2.9) | 3 (4.3) | 5 (3.6) |
| Cardiac failure acute | 1 (1.5) | 0 | 1 (0.7) |
| Cardiorespiratory arrest | 0 | 2 (2.9) | 2 (1.4) |
| Cardiogenic shock | 1 (1.5) | 1 (1.4) | 2 (1.4) |
| Myocarditis | 1 (1.5) | 0 | 1 (0.7) |
| General disorders | 3 (4.4) | 1 (1.4) | 4 (2.9) |
| Hypothermia | 1 (1.5) | 0 | 1 (0.7) |
| Multiple organ dysfunction syndrome | 2 (2.9) | 1 (1.4) | 3 (2.2) |
| Infections and infestations | 9 (13.2) | 6 (8.6) | 15 (10.9) |
| COVID-19 | 2 (2.9) | 0 | 2 (1.4) |
| COVID-19 pneumonia | 2 (2.9) | 4 (5.7) | 6 (4.3) |
| Pneumonia | 0 | 1 (1.4) | 1 (0.7) |
| Sepsis | 2 (2.9) | 1(1.4) | 3 (2.2) |
| Sepsis shock | 3 (4.4) | 0 | 3 (2.2) |
| Gastrointestinal disorders | 0 | 1 (1.4) | 1 (0.7) |
| Upper gastrointestinal hemorrhage | 0 | 1 (1.4) | 1 (0.7) |
| Investigations | 1 (1.5) | 1 (1.4) | 2 (1.4) |
| Alanine aminotransferase increased | 1 (1.5) | 0 | 1 (0.7) |
| Aspartate aminotransferase increased | 1 (1.5) | 0 | 1 (0.7) |
| Electrocardiogram QT prolonged | 0 | 1 (1.4) | 1 (0.7) |
| Metabolism and nutrition disorders | 1 (1.5) | 1 (1.4) | 2 (1.4) |
| Hyperkalemia | 0 | 1 (1.4) | 1 (0.7) |
| Metabolic acidosis | 1 (1.5) | 0 | 1 (0.7) |
| Respiratory, thoracic, and mediastinal disorders | 24 (35.3) | 15 (21.4) | 39 (28.3) |
| Acute respiratory distress syndrome | 6 (8.8) | 0 | 6 (4.3) |
| Acute respiratory failure | 11 (16.2) | 8 (11.4) | 19 (13.8) |
| Hypoxia | 5 (7.4) | 1 (1.4) | 6 (4.3) |
| Interstitial lung disease | 0 | 1 (1.4) | 1 (0.7) |
| Pneumomediastinum | 1 (1.5) | 0 | 1 (0.7) |
| Respiratory arrest | 1 (1.5) | 0 | 1 (0.7) |
| Respiratory failure | 5 (7.4) | 5 (7.1) | 10 (7.2) |
| Vascular disorders | 2 (2.9) | 4 (5.7) | 6 (4.3) |
| Distributive shock | 1 (1.5) | 0 | 1 (0.7) |
| Hypertension | 0 | 1 (1.4) | 1 (0.7) |
| Hypotension | 1 (1.5) | 1 (1.4) | 2 (1.4) |
| Shock hemorrhagic | 0 | 2 (2.9) | 2 (1.4) |
| Renal and urinary disorders | 4 (5.9) | 5 (7.1) | 9 (6.5) |
| Acute kidney injury | 3 (4.4) | 4 (5.7) | 7 (5.1) |
| Nephropathy | 1 (1.5) | 0 | 1 (0.7) |
| Renal failure | 0 | 1 (1.4) | 1 (0.7) |
| Nervous system failure | 2 (2.9) | 3 (4.3) | 5 (3.6) |
| Cerebrovascular accident | 1 (1.5) | 2 (2.9) | 3 (2.2) |
| Guillain-Barre syndrome | 0 | 1 (1.4) | 1 (0.7) |
Abbreviations: AE: adverse event; COVID-19: coronavirus disease 2019; SAE: serious adverse events; SoC: standard of care.
Discussion
This Phase 2, randomized, double-blind, multicenter study was conducted to evaluate the efficacy and safety of a novel bispecific antibody that inhibits both IL-1β and IL-18 simultaneously in COVID-19-infected and COVID-19-hospitalized patients. This study supports the assessment of preliminary efficacy and safety of MAS825 in addition to SoC in the critically ill COVID-19 population. The study did not meet its primary endpoint of showing a significant difference in APACHE II scores from baseline compared with placebo. However, MAS825 reduced the levels of pro-inflammatory cytokines (CRP, IL-6, and IFN-γ) and neutrophil counts and resulted in more rapid clearance of the SARS-CoV-2 virus, which might have potentially resulted in lesser duration of oxygen support, lower ICU admissions, and shorter stay in ICU as compared with those in the placebo arm. MAS825 was well tolerated in patients with COVID-19 respiratory failure and inflammation.
This study provides proof of concept for dual pathway engagement of IL-1β and IL-18 by MAS825 in patients with hyperinflammation. Patients treated with MAS825 had lower IL-6 and neutrophil levels compared with placebo, indicative of IL-1β pathway engagement. In parallel, there was evidence of a sustained reduction in IFN-γ levels, as compared with placebo, providing evidence of IL-18 pathway engagement. Despite the MAS-COVID study being adequately powered for primary endpoints, the study was relatively small to detect significant changes in all exploratory outcomes evaluated. The observed promising reductions of key biomarkers warrant further investigation in a larger study.
The overall results from the MAS-COVID study are similar to several small randomized controlled studies in hospitalized COVID-19 patients with tocilizumab or sarilumab (both anti-IL-6 receptor mAbs) where despite pathway engagement, reduction in CRP, and some improvement in clinical parameters, there was little impact on overall survival [16–18]. An overall benefit on mortality was observed only in much larger studies in COVID-19 patients with more severe disease, where both arms included high-dose steroid treatment, such as the RECOVERY trial, which randomized 4116 patients (1:1) with severe COVID-19 pneumonia and CRP ≥75 mg/l to receive either tocilizumab + SoC or SoC alone [19]. This suggests that despite the MAS-COVID study being a randomized, placebo-controlled that which was adequately powered, the study was relatively small to detect an MAS825 treatment effect. In addition, most of the clinical variables captured in APACHE II, such as system organ failure, kidney failure, and parameters such as white blood cells, creatinine, sodium, potassium, or arterial pH were not exacerbated in this population. The variables mainly contributing to the change in the APACHE II score were limited to oxygen support, respiratory rate, and temperature. Additionally, the optimal timing of IL inhibition remains elusive. Inflammasomal modulation may be more effective if initiated prior to recalcitrant end-organ damage. Determining reliable clinical indicators of an impending hyperinflammatory state and preventing the resultant tissue damage may prove beneficial. Our analysis did adjust for age but not for potential confounding factors such as sex and type of respiratory support, due to the limited sample size. Hence, the results should be interpreted with caution for a larger set of population.
Additional limitations of this study include the great use of high-dose steroids, and concomitant therapies (e.g. anti-infectives) in the placebo arm during the study; and the poor respiratory status at baseline in the MAS825 arm which may have contributed to lower observation of the MAS825 treatment effect. In addition, the researchers also faced multiple challenges, such as the rapidly evolving treatment landscape, the heterogenicity of the COVID-19 patient population, and geographical spread of the disease in the United States. Despite these challenges, this study provided evidence that MAS825 may simultaneously engage the IL-1β and IL-18 pathways and can reduce key markers of inflammation and further studies are now underway to explore the potential role of MAS825 as a therapeutic option in diseases where both IL-1β and IL-18 contribute to disease pathophysiology, such as NLRC4-GOF (NCT04641442) and hidradenitis suppurativa (NCT03827798).
Conclusion
The MAS-COVID study did not meet the primary efficacy endpoint of an improvement in APACHE II score; however, MAS825 used in conjunction with SoC inhibited relevant cytokine pathways, with faster SARS-CoV-2 virus clearance, and improved some clinical outcomes in patients with COVID-19 pneumonia and impaired respiratory function compared with placebo. MAS825 was well-tolerated and none of the AEs/SAEs were drug-related SAEs.
Supplementary Material
Acknowledgments
The study was funded by Novartis Pharma AG, Basel, Switzerland. We thank the investigators (Richard Nathan, Afsoon Roberts, Elaine Schwartz, Mihran Shirinian, Peter Hou, Charles Mahan, Jamie Meng, Brian Ross Zeno, and Aric Gregson) and patients at the investigation sites for their support during the conduct of the study. Under the direction of authors, Anupama B, Preethi B, and Priyanka Guru (professional medical writers; Novartis) assisted in the preparation of this article in accordance with the third edition of Good Publication Practice guidelines.
Glossary
Abbreviations
- AE
adverse event
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- CRP
C-reactive protein
- CT
computed tomography
- ECG
electrocardiogram
- FiO2
fraction of inspired oxygen
- ICU
intensive care unit
- LLOQ
lower limit of quantification
- mAbs
monoclonal antibodies
- MMRM
mixed-effect repeated measures
- PaO2
partial pressure of oxygen
- SAEs
serious adverse events
- SAPS II
Simplified Acute Physiology Score II
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SoC
standard of care
- SOFA
Sequential Organ Failure Assessment
- SpO2
peripheral inspired oxygen
- ULOQ
upper limit of quantification
Contributor Information
Alex D Hakim, Providence Little Company of Mary Medical Center, Torrance, CA, USA.
Mustafa Awili, Dallas Regional Medical Center, Mesquite, TX, USA.
Hollis R O’Neal, Louisiana State University Health Sciences Center and Our Lady of the Lake Regional Medical Center, Baton Rouge, LA, USA.
Omar Siddiqi, Boston Medical Center, Boston, MA, USA.
Naseem Jaffrani, Rapides Regional Medical Center, Alexandria, LA, USA.
Richard Lee, University of California, Irvine, CA, USA.
Jeffrey S Overcash, Sharp Grossmont Hospital, La Mesa, CA, USA.
Ann Chauffe, Louisiana State University Health Sciences Center, Lafayette, LA, USA.
Terese C Hammond, Saint Johns Cancer Institute, Santa Monica, CA, USA.
Bela Patel, University of Texas McGovern Medical School, Houston, TX, US.
Michael Waters, Sharp Chula Vista Medical Center, Chula Vista, CA, USA.
Gerard J Criner, Department of Thoracic Medicine and Surgery, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA.
Alok Pachori, MorphoSys AG, Boston, MA, USA.
Guido Junge, Novartis Pharma AG, Basel, Switzerland.
Rafael Levitch, Novartis Pharma AG, Basel, Switzerland.
Jen Watts, Oculis, Boston, MA, USA.
Philip Koo, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Tirtha Sengupta, Novartis Healthcare Pvt Ltd, Hi-Tech City, Hyderabad, India.
Lili Yu, Novartis Institutes for BioMedical Research, Cambridge, MA, USA.
Michael Kiffe, Novartis Institutes for BioMedical Research, Basel, Switzerland.
Anne Pinck, Novartis Institutes for BioMedical Research, Basel, Switzerland.
Richard R Stein, Novartis Institutes for BioMedical Research, Basel, Switzerland.
Jamie Bendrick-Peart, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Janet Jenkins, Novartis Institutes for BioMedical Research, Cambridge, MA, USA.
Marianna Rowlands, Novartis Institutes for BioMedical Research, Cambridge, MA, USA.
Frank Waldron-Lynch, Novartis Institutes for BioMedical Research, Cambridge, MA, USA.
Jesse Matthews, Hospital Medicine, St Charles Health System, Bend, OR, USA.
Ethical Approval
The study was conducted in accordance ICH GCP and Declaration of Helsinki. Patients or their legal representatives provided signed informed consent. The study was approved by the institutional review board and ethical committees of participating institutes. Additional details have been provided in the manuscript.
Conflict of Interests
JSO reports research funding from Novartis, paid to his institution. AP was a former employee of Novartis and reports stock and stock options from Novartis AG and MorphoSys AG. PK was a former employee of Novartis Pharmaceuticals Corporation and reports stock from Novartis Pharmaceuticals Corporation during the conduct of the study and stocks from Bristol Myers Squibb after the conduct of the study but prior to publication. MK and RRS reports patents relevant to their work and RRS is an employee of Novartis Institutes for BioMedical Research (Basel).
GJ and RL are employees of Novartis Pharma AG. JW and JBP are employees of Novartis Pharmaceuticals Corporation. TS is an employee of Novartis Healthcare Pvt Ltd. LY, JJ, and MR are employees of Novartis Institutes for BioMedical Research (Cambridge). MK and AP are employees of Novartis Institutes for BioMedical Research (Basel). FWL was a former employee of Novartis Institutes for BioMedical Research (Cambridge).
ADH, MA, HON, OS, NJ, RL, AC, HT, BP, MW, GJC, and JM reports no confict of interest.
Funding
The study was funded by Novartis Pharma AG, Basel, Switzerland.
Data Availability
Novartis is committed to sharing access to patient-level data and supporting documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
Author Contributions
The study was designed by G.J., M.K., R.R.S., M.R., R.L., T.S. and F.W.L. A.D.H., M.A., H.O.N., O.S., N.J., R.L., J.O., A.C., T.H., B.P., M.W., G.J.C., A.P., J.W., P.K., T.S., A.P., J.B.P., J.J., G.J., M.K., R.R.S., M.R., R.L., T.S., and F.W.L. contributed to the conduct of these studies. Data were acquired by A.D.H., M.A., H.O.N., O.S., N.J., R.L.,J.O., A.C., T.H., B.P., M.W., G.J.C. and analyzed by A.D.H., G.J., M.K , P.K., M.R., T.S., F.W.L. All authors contributed equally to the interpretation of data. A.D.H. is the principal investigator for this study. All authors contributed to the intellectual content of the manuscript and approved it for publication.
Permission to Reproduce
This manuscript does not contain data that requires permission to reproduce.
Clinical Trial Registration
ClinicalTrials.gov, NCT04382651
References
- 1. World Health Organization. Weekly epidemiological update on COVID-19—21 September 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---21-september-2021 (28 November 2021, date last accessed).
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395, 1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC.. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020, 324, 782–93. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4. Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M.. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care 2020, 24, 516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gustine JN, Jones D.. Immunopathology of hyperinflammation in COVID-19. Am J Pathol 2021, 191, 4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vora SM, Lieberman J, Hao W.. Inflammasome activation at the crux of severe COVID-19. Nat Rev Immunol 2021, 21, 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med 2021, 218, e20201707. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vecchie A, Bonaventura A, Toldo S, Dagna L, Dinarello CA, Abbate A.. IL-18 and infections: Is there a role for targeted therapies?. J Cell Physiol 2021, 236, 1638–57. doi: 10.1002/jcp.30008. [DOI] [PubMed] [Google Scholar]
- 9. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al.; Yale IMPACT Team. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–9. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomez-Rial, J, Rivero-Calle I, Salas A, Martinón-Torres F.. Role of monocytes/macrophages in COVID-19 pathogenesis: Implications for therapy. Infect Drug Resist. 2020, 13, 2485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, et al.; Deutsche COVID-19 OMICS Initiative (DeCOI). Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020, 182, 1419–1440.e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Merad M, Martin JC.. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020, 20, 355–62. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiang CC, Korinek M, Cheng W-J, Hwang T-L.. Targeting neutrophils to treat acute respiratory distress syndrome in coronavirus disease. Front Pharmacol 2020, 11, 572009. doi: 10.3389/fphar.2020.572009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mann ER, Menon M, Knight SB, Konkel JE, Jagger C, Shaw TN, et al.; NIHR Respiratory TRC,. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol 2020, 5, eabd6197. doi: 10.1126/sciimmunol.abd6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akavipat P, Thinkhamrop J, Thinkhamrop B, Sriraj W.. Acute physiology and chronic health evaluation (APACHE) II Score—the clinical predictor in neurosurgical intensive care unit. Acta Clin Croat. 2019, 58, 50–6. doi: 10.20471/acc.2019.58.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med 2021, 384, 20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med 2021, 384, 1503–16. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, et al.; Sarilumab COVID-19 Global Study Group. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021, 9, 522–32. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Group RC. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Novartis is committed to sharing access to patient-level data and supporting documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.