Abstract
This study purposed to investigate the alleviating effect of dietary curcumin supplementation on oxidative stress in the liver of broilers induced by diquat. One-day-old Cobb broilers (400) were selected and randomly divided into 5 groups, with 8 replicates and 10 broilers per replicate. The control group and the diquat group were fed the basal diet, while the curcumin supplementation groups were fed the basal diet supplemented with different amounts of curcumin (50, 100, and 150 mg/kg). On d 21 of the test, 1 broiler was randomly selected from each replicate and intraperitoneally injected with 20 mg/mL of diquat solution at a dose of 1 mL/kg BW or equivalent physiological saline (for the control group). After 48 h of feeding, the selected broilers were slaughtered for analysis. The results show that diquat treatment reduced the antioxidant capacity of the liver, caused oxidative stress, and affected its lipid metabolism. However, diet supplementation using curcumin completely or partially reversed the effect of diquat on the liver of broilers. The blood alanine aminotransferase activity, total bilirubin and total protein levels, and liver Caspase-3 mRNA abundance in broilers were lower or significantly lower in the curcumin supplementation group than in the diquat group (P < 0.05). The curcumin supplementation groups had significantly higher total antioxidant capacity activity but significantly lower malondialdehyde in the liver of broilers than the diquat group (P < 0.05). The blood triglyceride level of broilers was lower or significantly lower in the curcumin supplementation groups than in the diquat group (P < 0.05). The activities of cetyl coenzyme A carboxylase in the liver were significantly lower in the 150 mg/kg curcumin supplementation groups than in the DQ group (P < 0.05). In conclusion, dietary curcumin supplementation could ameliorate the effects of diquat-induced oxidative stress on the antioxidant capacity, tissue morphology, and lipid metabolism of the liver of broilers, thus protecting the liver. The recommended dosage for broiler diets is 100 to 150 mg/kg curcumin.
Key words: curcumin, broiler, diquat, liver, oxidative stress
INTRODUCTION
Reactive oxygen species (ROS) is one of the by-products of oxygen metabolism in cells. Under normal conditions, the cellular antioxidant system scavenges excessive ROS in time to prevent oxidative stress and injury (Sies et al., 2022). The large-scale and intensive development of the poultry breeding industry has gradually improved breeding efficiency but exacerbated oxidative stress in poultry (Brannan et al., 2022; Hu et al., 2022). Excessive breeding density, improper feeding management, and various feed problems, such as lipid oxidation, mycotoxin pollution, and excessive heavy metal content, are risk factors for oxidative stress (Wang et al., 2022; Chen et al., 2023; Lei et al., 2023). Since broilers exhibit fast growth, weak antistress ability, and low disease resistance, they are susceptible to various stressors and pathogens, causing excessive production of free radicals and antioxidant system imbalance, resulting in oxidative stress and injury (Xu et al., 2022). In addition to metabolic and synthetic functions, the liver is the center for redox reactions and can therefore produce ROS, making it the main target organ for ROS attack (Abakumova et al., 2022). Relevant studies showed that oxidative stress could increase the relative liver weight and the activities of glutamic oxaloacetic transaminase and glutamic pyruvic transaminase in blood. Oxidative stress can also result in larger hepatocyte gap with loose arrangement, nuclear coagulation, reduced ATP content in the liver, mitochondrial liver dysfunction, and increased hepatocyte apoptosis rate in broilers, thereby affecting the liver function and health of broilers (Chen et al., 2020, 2021; Nong et al., 2023). Antioxidants are often used in broiler production to alleviate the impacts of oxidative stress. The research development and application of natural antioxidants have attracted much attention.
Curcumin (C21H20O6) is mainly extracted from the rhizomes of Zingiberaceae or Araceae plants. It is a phenolic compound with a diketone structure and an additive approved by the World Health Organization and many countries (Sahu, 2016). Curcumin has antioxidant activities due to the presence of antioxidant groups, such as methylene hydrogen, o-methoxyphenol, carbon-carbon double bond, and β-diketone group, in its structure (Racz et al., 2022). Diquat (DQ) is a redox circulator, easily converted into free radicals in the presence of molecular oxygen to produce superoxide anion and other redox products, which induce oxidative stress and damage to the body (Jones and Vale, 2000). It is one of the most commonly used chemicals in oxidative stress research. In poultry research, diquat has been utilized to establish oxidative stress models of broilers (Chen et al., 2021).
Curcumin has a high development potential and research value in improving antioxidant performance, alleviating oxidative stress and injury, and protecting the liver and health of broilers. However, there are few reports on its effect on alleviating oxidative stress and damage in broiler liver. Therefore, this study evaluated the alleviating effects of different curcumin amounts on the diquat-induced oxidative stress and injury in the liver of broilers to provide a reference for the application of curcumin in broiler production.
MATERIALS AND METHODS
The experimental protocols were approved by the Animal Care and Use Committee of Hebei Agriculture University (Baoding, China) (Protocol: 2022003). All animal experiments complied with the ARRIVE guidelines were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
Test Diet
Curcumin with a purity guarantee value ≥95% was purchased from the Chenguang Biotechnology Group Co., Ltd. (Handan, China). The basal diet was a corn-soybean meal prepared according to the “Nutrition Requirements of Chinese Chicken (NY/T 33-2004).” The composition and nutrition level of the diet is shown in Table 1.
Table 1.
Composition and nutrient levels of the basal diet (air-dry basis, %).
| Items | Trial period |
|
|---|---|---|
| 1–21 d | 22–42 d | |
| Ingredients | ||
| Corn | 48.20 | 51.45 |
| Soybean meal | 38.50 | 33.30 |
| Vegetable oil | 5.00 | 7.05 |
| Premix1 | 5.00 | 5.00 |
| Limestone | 1.60 | 1.50 |
| Calcium phosphate | 1.25 | 1.25 |
| Sodium chloride | 0.35 | 0.35 |
| Choline chloride | 0.10 | 0.10 |
| Total | 100.00 | 100.00 |
| Nutrient levels2 | ||
| Metabolizable energy/(MJ/kg) | 12.76 | 13.39 |
| Crude protein | 24.14 | 22.91 |
| Crude fiber | 3.48 | 3.15 |
| Calcium | 0.83 | 0.81 |
| Available phosphorus | 0.42 | 0.37 |
| Total phosphorus | 0.60 | 0.58 |
| Lysine | 1.26 | 1.11 |
| Methionine | 0.53 | 0.51 |
| Threonine | 0.93 | 0.85 |
The premix provided the following per kg of diets: vitamin A, 10,000 IU; vitamin D3, 4,000 IU; vitamin E, 20 IU; vitamin K3, 2 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B6, 3 mg; vitamin B12, 0.02 mg; nicotinamide, 40 mg; calcium pantothenate, 10 mg; folic acid, 1 mg; biotin, 0.12 mg; Cu, 16 mg; Fe 80 mg; Zn, 110 mg; Mn, 120 mg; I, 1.5 mg; Se, 0.3 mg.
Metabolic energy, crude protein, calcium, and total phosphorus in nutrients are measured values, while the rest are calculated values.
Experimental Design and Animal Management
We selected 400 healthy 1-day-old male Cobb broilers with similar weight (44.18 ± 1.05 g) and randomly divided them into 5 groups, with 8 replicates per group and 10 broilers per replicate. The test period was 23 d. The control and the diquat groups were fed with the basal diet, but the curcumin supplementation groups were fed with the basic diet supplemented with 50, 100, and 150 mg/kg curcumin. The temperature of the chicken house was kept between 32°C and 34°C during the first week after hatching and decreased by 2°C to 3°C every other week until the final temperature reached 24°C. The lighting condition followed the standard of 23 h light and 1 h darkness.
Diquat (dibromide monohydrate; product ID: 45422; Sigma Aldrich, St Louis, MO) was dissolved in 0.86% normal saline to prepare a 20 mg/mL diquat solution. On d 21 of the test, 1 broiler with a weight close to the average value was selected from each replicate and intraperitoneally injected with 20 mg/mL diquat solution. The dosage was 1 mL/kg BW (DQ group and curcumin supplementation group) or an equivalent amount of physiological saline (control group). The broilers were then slaughtered for sampling after 48 h of feeding.
Growth Performance and Liver Index
At the beginning and end of the test, the fasting weight of broiler chickens in each group was recorded as the initial and final weights. In addition, the feed intake of the broilers in each group during the test was recorded. The average daily gain (ADG) and average daily feed intake (ADFI) were calculated according to the test days. Finally, the gain to feed ratio (F/G) was calculated based on ADG and ADFI. The broilers were weighed at 21-days old to calculate the body weight (BW) change rate within 48 h after challenge, which is determined by comparing the average BW at 23 d with that at 21 d.
The broiler chickens were also weighed before slaughter. Subsequently, the weight of liver was recorded after slaughter to calculate the liver index using the following equation: Liver index (g/kg) = liver weight (g)/BW before slaughter (kg).
Blood and Tissue Indexes
At the end of the test period, the broilers were left unfed for 12 h, and blood was sampled (10 mL) from the wing vein of the broilers treated with normal saline or diquat into the blood collection vessels. The samples were centrifuged at 3,000 × g for 10 min at 4°C to separate the serum. The serum samples were stored at −20°C until further analysis. Thereafter, the broilers were euthanized by cervical dislocation, and liver tissue samples were collected and cryopreserved at −80°C for analysis. The activities of total antioxidant capacity (TAOC), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), fatty acid synthase (FAS), acetyl coenzyme A carboxylase (ACC), and hormone-sensitive lipase (HSL) and malondialdehyde (MDA) content in serum and liver tissue were detected by ELISA. The TAOC, CAT, SOD, and GSH-Px kits were purchased from Jiangsu Meimian Industrial Co., Ltd. (Yancheng, China), while FAS, ACC, and HSL kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The assays were performed according to the manufacturer's instructions. Moreover, the alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), total protein (TP), glucose, total cholesterol, and triglyceride kits were bought from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) and the assays conducted according to the manufacturer's instructions.
Observation of Tissue Morphology
The liver samples were quickly fixed in 4% paraformaldehyde solution and dehydrated using the full-automatic dehydrator (TSJ-II, Zhongwei, Changzhou, China), followed by embedding and sectioning. The sections were dewaxed, stained with hematoxylin staining for 15 min, and rinsed with tap water for 2 min. After hydrochloric acid-alcohol differentiation for 10 s and tap water rinsing for 2 min, the solution from the sections turned back to blue when placed in 50°C warm water. The sections were then rinsed with tap water for 2 min and immersed in 85% alcohol for 4 min, followed by eosin staining for 4 min. After water rinsing for 5 s and alcohol gradient dehydration, the slices were cleared with xylene and sealed with neutral gum. The microscope camera system (BA200Digital, Motic, Xiamen, China) was used for slice observation and image acquisition.
Relative Gene Expression in the Liver
The relative content of mRNA in liver samples was determined via qRT-PCR. Specific primers were designed by Primer 6.0 software and synthesized by Shenggong Biotech Co., Ltd. (Shanghai, China) (Table 2). These primers targeted several chicken genes, including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Kelch-like ECH-associated protein 1 (Keap1), nuclear factor E2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD (P) H: quinone oxidoreductase 1 (NQO-1), GSH-Px, copper and zinc superoxide dismutase (SOD1), B-cell lymphoma/leukemia 2 (Bcl-2), BCL2-associated X protein (Bax), and cysteine-aspartic acid protease-3 (Caspase-3).
Table 2.
Sequence of primers for real-time PCR.
| Genes | Primer sequence (5′→3′) | Accession no. |
|---|---|---|
| Keap11 | F: TGCCCCTGTGGTCAAAGTG R: GGTTCGGTTACCGTCCTGC |
XM_015274015.1 |
| Nrf22 | F: CACCAAAGAAAGACCCTCCT R: GAACTGCTCCTTCGACATCA |
XM_015289381.3 |
| HO-13 | F: CCGCTATTTGGGAGACCT R: CTCAAGGGCATTCATTCG |
NM_205344.1 |
| NQO-14 | F: TCTCTGACCTCTACGCCAT R: TCTCGTAGACAAAGCACTCGG |
NM_001277621.1 |
| GSH-Px5 | F: GCTGTTCGCCTTCCTGAGAG R: GTTCCAGGAGACGTCGTTGC |
NM_001277853.1 |
| SOD16 | F: AGGGAGGAGTGGCAGAAGT R: GCTAAACGAGGTCCAGCAT |
NM_205064.1 |
| Bcl-27 | F: GCTGCTTTACTCTTGGGGGT R: CTTCAGCACTATCTCGCGGT |
NM_205339.2 |
| Bax8 | F: GGTGACAGGGATCGTCACAG R: TAGGCCAGGAACAGGGTGAAG |
XM_422067 |
| Caspase-39 | F: TGGTGGAGGTGGAGGAGC R: TGTCTGTCATCATGGCTCTTG |
NM_204725.1 |
| FAS10 | F: ATTTGTTCGTCATCACCGTTCTA R: GCATATTAAGGTTTCGTAGGCTC |
NM_001199487.1 |
| ACC11 | F: AGACAACCAACGCCAAAGTG R: TGGTAGAAAAAGTTGGGTAGCAC |
NM_205505.1 |
| GAPDH12 | F: GGCTGCTAAGGCTGTGGG R: ATCATCATACTTGGCTGGTTTC |
NM_204305.1 |
Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor E2-related factor 2; HO-1, heme oxygenase-1; NQO-1, NAD (P) H: quinone oxidoreductase; GSH-Px, glutathione peroxidase; SOD1, copper and zinc superoxide dismutase; Bcl-2, B-cell lymphoma/leukemia; Bax, BCL2-associated X protein; Caspase-3, cysteine-aspartic acid protease-3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. N = 8.
Total RNA was extracted using 50 to 100 mg of liver samples using the Trizol kit (Invitrogen, Carlsbad, CA). The RNA concentration meter (Nanodrop Lite, Thermo Fisher Scientific, Waltham, MA) was employed to measure the RNA concentration. Thereafter, 1 μg of RNA was used for cDNA synthesis using the HiScriptIII RT SuperMix for qPCR (+gDNA wiper) kit (No.: R323-01, Vazyme, Nanjing, China). The ChamQ Universal SYBR qPCR Master Mix (No.: Q711-02, Vazyme, Nanjing, China) was used for qRT-PCR on the CFX96 contact real-time PCR detection system (Bio-Rad, Hercules, CA), with GAPDH as the internal control to compare the amplification effect. The 20 μL qRT-PCR mixture included 10 μL of SYBR Green Master Mix, 0.8 μL of the forward and reverse primers (stock solution concentration was 10 μmol/L), 2 μL (200 ng) of template cDNA, and 7.2 μL of DNase/RNase-free H2O. Thermal cycle conditions for the qRT-PCR were: 95°C for 5 min, followed by 40 cycles at 95°C for 10 s and 60°C for 30 s. The experiment was conducted in triplicate. After the amplification, the melt curve analysis was conducted, and the target gene expression was calculated by the 2−△△ Ct method.
Statistical Analysis
The statistical data analysis was conducted using SPSS 26.0 software. The t test was performed to compare the control and DQ groups. One-way analysis of variance (ANOVA) and the Duncan method were utilized to determine the differences among the DQ and curcumin supplementation groups. Orthogonal polynomials were employed to detect the linear and quadratic effects of curcumin in the DQ and curcumin supplementation groups. A P value less than 0.05 (P < 0.05) indicated a significant difference between the groups.
RESULTS
Growth Performance and Liver Index
There were no significant differences in ADG, ADFI, F/G, and liver index of broilers among the 5 groups (Table 3). The BW change rate was significantly lower in the DQ group than in the control group (P < 0.05). The BW change rate in the 100 and 150 mg/kg curcumin supplementation groups were significantly higher than in the DQ group (P < 0.05). Moreover, the BW change rate increased linearly with the increasing curcumin concentration (P < 0.05).
Table 3.
Effects of curcumin on growth performance and liver index of diquat-challenged broilers.
| The amount of curcumin |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | Control group | DQ1 | 50 mg/kg | 100 mg/kg | 150 mg/kg | SEM2 | ANOVA | Linear | Quadratic |
| ADG3 (g) | 33.60 | 33.47 | 34.67 | 32.11 | 33.94 | 0.58 | 0.473 | 0.823 | 0.788 |
| ADFI4 (g) | 51.95 | 52.70 | 51.21 | 49.05 | 51.30 | 0.69 | 0.329 | 0.312 | 0.184 |
| F/G5 | 1.55 | 1.58 | 1.49 | 1.53 | 1.51 | 0.02 | 0.343 | 0.314 | 0.322 |
| BW6 change rate | 1.15* | 1.07a | 1.08a | 1.10b | 1.11b | 0.04 | <0.001 | <0.001 | 0.807 |
| Liver index (g/kg) | 21.43 | 19.32 | 20.51 | 21.40 | 21.16 | 0.48 | 0.437 | 0.147 | 0.466 |
There was significant difference (P < 0.05) between the control and DQ groups.
Within a row, means with different superscripts differ significantly (P < 0.05). Values are means (n = 8).
DQ, diquat group; SEM, the standard error of the means; ADG, average daily gain; ADFI, average daily feed intake; F/G, gain to feed ratio; BW, body weight.
Blood Indexes
As shown in Table 4, the blood ALT and AST activities, TB, and triglyceride levels were significantly higher in the DQ group than in the control group (P < 0.05). However, the effect of diquat was reversed in the curcumin supplementation group, whose TB level at 50 mg/kg curcumin was significantly lower than that in the DQ group (P < 0.05). The ALT activity and TB level in the 100 mg/kg curcumin supplementation group were significantly lower than those in the DQ group (P < 0.05). Moreover, the ALT activity, TB, TP, and triglyceride levels in the 150 mg/kg curcumin supplementation group were significantly lower than those in the DQ group (P < 0.05). The activity of ALT and the contents of TB, TP, albumin, and triglyceride in the blood decreased linearly with the increasing curcumin concentration (P < 0.05). Moreover, the content of TB presented quadratic curve changes (P < 0.05).
Table 4.
Effects of curcumin on blood indexes of diquat-challenged broilers.
| Items | Control group | DQ1 | The amount of curcumin |
SEM2 |
P value |
||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg/kg | 100 mg/kg | 150 mg/kg | ANOVA | Linear | Quadratic | ||||
| ALT3 (U/L) | 6.42* | 7.43b | 7.41ab | 6.63a | 6.49a | 0.13 | 0.044 | 0.005 | 0.809 |
| AST4 (U/L) | 42.24* | 49.21 | 47.71 | 44.86 | 45.98 | 0.76 | 0.220 | 0.071 | 0.362 |
| TB5 (μmol/L) | 4.72* | 15.07b | 8.28a | 5.73a | 6.81a | 0.74 | <0.001 | <0.001 | 0.001 |
| TP6 (g/L) | 30.59 | 34.45b | 34.00b | 29.06ab | 27.49a | 0.86 | 0.037 | 0.005 | 0.567 |
| Albumin (g/L) | 15.82 | 18.07 | 16.56 | 15.60 | 15.90 | 0.37 | 0.870 | 0.025 | 0.217 |
| Glucose (mmol/L) | 14.31 | 16.61 | 15.22 | 14.88 | 15.37 | 0.44 | 0.546 | 0.313 | 0.279 |
| Total cholesterol (mmol/L) | 5.66 | 6.26 | 5.52 | 5.39 | 5.98 | 0.18 | 0.273 | 0.536 | 0.063 |
| Triglyceride (mmol/L) | 0.53* | 0.68b | 0.65ab | 0.60ab | 0.56a | 0.02 | 0.046 | 0.023 | 0.996 |
There was significant difference (P < 0.05) between the control and DQ groups.
Within a row, means with different superscripts differ significantly (P < 0.05). Values are means (n = 8).
DQ, diquat group; SEM, the standard error of the means; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; TP, total protein.
Liver Antioxidant Indexes
Compared with the control group, the activities of TAOC, GSH-Px, and SOD in the broiler liver were significantly reduced, but the MDA content was significantly increased in the DQ group (P < 0.05) (Table 5). However, the effect of diquat was reversed in the curcumin supplementation group. The MDA content was significantly lower in the 50 and 100 mg/kg curcumin supplementation groups than that in the DQ group (P < 0.05). The 150 mg/kg curcumin supplementation group had significantly higher TAOC activity but significantly lower MDA content than the DQ group (P < 0.05). The activities of TAOC and SOD in the liver increased quadratically (P < 0.05) with the increase in curcumin supplementation amount, while MDA content decreased linearly (P < 0.05). Additionally, the activity of GSH-Px in the liver increased both linearly and quadratically (P < 0.05). The TAOC, SOD, and CAT activities increased, while MDA level decreased linearly (P < 0.05) with the increasing curcumin concentration.
Table 5.
Effects of curcumin on liver antioxidant indexes of diquat-challenged broilers.
| Items | Control group | DQ1 | The amount of curcumin |
SEM2 |
P value |
||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg/kg | 100 mg/kg | 150 mg/kg | ANOVA | Linear | Quadratic | ||||
| TAOC3 (U/mg prot) | 40.81* | 33.75a | 37.38a | 39.85ab | 40.03b | 0.93 | 0.030 | 0.006 | 0.295 |
| GSH-Px4 (IU/g prot) | 45.99* | 38.98 | 39.08 | 39.64 | 39.13 | 0.61 | 0.985 | 0.741 | 0.884 |
| SOD5 (IU/L prot) | 766.10* | 608.34a | 651.46ab | 699.93ab | 734.88b | 19.84 | 0.390 | 0.017 | 0.908 |
| CAT6(U/mL prot) | 6.90 | 6.52 | 6.77 | 6.79 | 7.38 | 0.13 | 0.128 | 0.031 | 0.504 |
| MDA7 (nmol/g prot) | 2.39* | 2.82b | 2.08a | 2.03a | 1.75a | 0.12 | 0.001 | <0.001 | 0.102 |
There was significant difference (P < 0.05) between the control and DQ groups.
Within a row, means with different superscripts differ significantly (P < 0.05). Values are means (n = 8).
DQ, diquat group; SEM, the standard error of the means; TAOC, total antioxidant capacity; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde.
Activities of the Liver Fat Metabolism-Related Enzymes
As shown in Table 6, the liver HSL activity was significantly higher in the DQ group than in the control group (P < 0.05). The 150 mg/kg curcumin supplementation group also had significantly lower ACC than the DQ group (P < 0.05). The activity of ACC decreased linearly with the increasing curcumin concentration (P < 0.05).
Table 6.
Effect of curcumin on activities of the liver fat metabolism-related enzymes of diquat-challenged broilers.
| Items | Control group | DQ1 | The amount of curcumin |
SEM2 |
P value |
||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg/kg | 100 mg/kg | 150 mg/kg | ANOVA | Linear | Quadratic | ||||
| FAS3 (U/mg prot) | 436.47 | 392.84 | 414.50 | 358.03 | 361.65 | 10.21 | 0.156 | 0.098 | 0.649 |
| ACC4 (mU/mg prot) | 30.98 | 28.50b | 26.01ab | 22.97ab | 21.95a | 1.21 | 0.046 | 0.040 | 0.757 |
| HSL5 (mU/mg prot) | 19.71 | 24.95 | 20.86 | 23.72 | 22.55 | 0.73 | 0.243 | 0.505 | 0.318 |
Within a row, means with different superscripts differ significantly (P < 0.05). Values are means (n = 8).
DQ, diquat group; SEM, the standard error of the means; FAS, fatty acid synthetase; ACC, acetyl CoA carboxylase; HSL, hormone-sensitive triglyceride lip.
Morphology of Liver Tissue
The liver tissue capsules of broilers in the control group showed no significant thickness, with underdeveloped connective tissue in the liver lobule (Figure 1). The endothelium of the central vein in the hepatic lobule was complete, and the hepatocytes radially extended around the central vein forming the hepatic plate. The liver plate comprised 2 rows of hepatocytes, and the adjacent liver plates were connected. Irregular and interconnected hepatic sinuses could be seen between the liver plates. Moreover, the portal canal area had an intact structure without obvious pathological changes. Compared with the control group, the liver of broilers in the DQ group showed focal necrosis of hepatocytes and had severely damaged local liver plates. Several cell fragments were also clustered in the necrotic area of the DQ group. In the 50 mg/kg curcumin supplementation group, focal necrosis of local hepatocytes was observed in the liver of broilers, and the hepatocytes were disintegrated with obscure morphologies and structures. Focal necrosis was also observed in a few hepatocytes of broilers in the 100 mg/kg curcumin supplementation group. There were no obvious pathological changes in the liver of broilers in the 150 mg/kg curcumin supplementation group. However, tissue morphology changes were observed in the liver of broilers after exposure to diquat, but adding curcumin alleviated the adverse effects on the liver caused by diquat treatment. The protective effect increased with the increase in the supplemented curcumin dosage. The liver of broilers in the 150 mg/kg curcumin supplementation group and the control group presented no significant tissue morphology changes.
Figure 1.
Effects of curcumin on the liver structure and morphology of diquat-challenged broilers. A (control group), B (diquat group), C (50 mg/kg curcumin + diquat group), D (100 mg/kg curcumin + diquat group), and E (150 mg/kg curcumin + diquat group). A–E images were taken at ×100 (left) and ×400 (right) magnification (n = 8). Key: green, hepatocyte necrosis. The magnification used was ×100 and ×400 and scales of 100 μm and 10 μm, respectively.
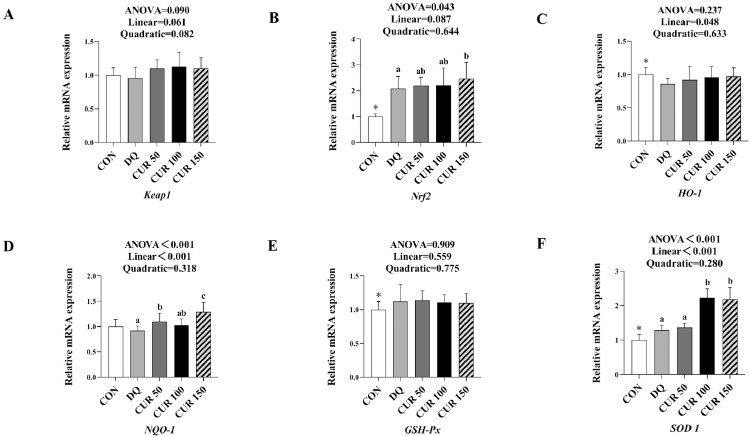
Expression Level of Antioxidant-Related Genes in Liver
As shown in Figure 2, the abundance of Nrf2, GSH-Px, and SOD1 mRNA increased significantly (P < 0.05), while that of HO-1 mRNA decreased significantly (P < 0.05) in the liver of broilers in the DQ group compared with the control group (P < 0.05). The abundance of Nrf2 mRNA in the 150 mg/kg curcumin supplementation group was significantly higher than in the DQ group (P < 0.05). Similarly, the abundance of NQO-1 mRNA in the 50 and 150 mg/kg curcumin supplementation groups were significantly higher than in the DQ group (P < 0.05). Unlike the DQ group, the abundance of SOD1 mRNA was significantly increased in the 100 and 150 mg/kg curcumin supplementation groups (P < 0.05). The abundance of HO-1, NQO-1, and SOD1 mRNA increased linearly with the increasing curcumin concentration (P < 0.05).
Figure 2.
Effects of curcumin on expression level of antioxidant-related genes in liver of diquat-challenged broilers. Keap1 gene (A), Nrf2 gene (B), HO-1 gene (C), NQO-1 gene (D), GSH-Px gene (E) and SOD1 gene (F). The mRNA level of genes was determined by qRT-PCR. Bars represent the means ± standard deviation (SD) (n = 8). *There was significant difference (P < 0.05) between the control and DQ groups. Above the bar no letter or the same letter mean no significant (P > 0.05), while with different letter mean significant difference (P < 0.05). Kelch-like ECH-associated protein 1 (Keap1), nuclear factor E2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD (P) H: quinone oxidoreductase 1 (NQO-1), glutathione peroxidase (GSH-Px), copper and zinc superoxide dismutase (SOD1).
Expression Level of the Apoptosis-Related Genes in the Liver
The diquat treatment significantly increased the abundance of Bax and Caspase-3 mRNA in the liver of broilers in the DQ group compared to the control group (P < 0.05) (Figure 3), but adding curcumin reversed this effect. The abundance of Caspase-3 mRNA in the liver of broilers in the curcumin supplementation groups was significantly lower than that in the DQ group (P < 0.05). Moreover, the abundance of Bcl-2 mRNA in the liver of broilers in the 150 mg/kg curcumin supplementation group was significantly higher than that in the DQ group (P < 0.05). The abundance of Bcl-2 mRNA increased linearly (P < 0.05), while that of Caspase-3 mRNA decreased linearly (P < 0.05) with the increasing curcumin concentration.
Figure 3.
Effects of curcumin on expression level of apoptosis-related genes in liver of diquat-challenged broilers. Bax gene (A), Bcl-2 gene (B), and Caspase-3 gene (C). The mRNA level of genes was determined by qRT-PCR. Bars represent the means ± standard deviation (SD) (n = 8). *There was significant difference (P < 0.05) between the control and DQ groups. Above the bar no letter or the same letter mean no significant (P > 0.05), while with different letter mean significant difference (P < 0.05). BCL2-associated X protein (Bax), B-cell lymphoma/leukemia 2 (Bcl-2), cysteine-aspartic acid protease-3 (Caspase-3).
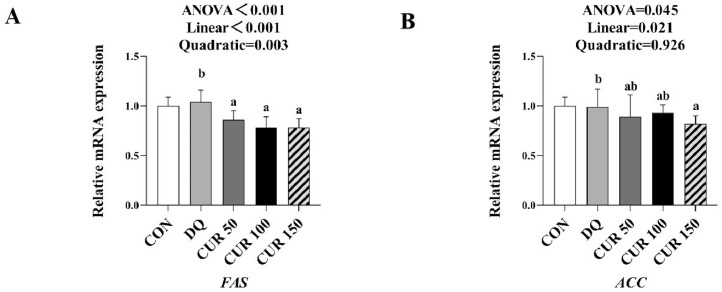
Expression Level of the Apoptosis-Related Genes in the Liver
As shown in Figure 4, the abundance of FAS mRNA in the liver of broilers in the curcumin supplementation groups was significantly reduced (P < 0.05) compared with the DQ groups. The abundance of ACC mRNA in the 150 mg/kg curcumin supplementation group was also significantly reduced (P < 0.05). The abundance of FAS and ACC mRNA decreased linearly with the increasing curcumin concentration (P < 0.05).
Figure 4.
Effects of curcumin on expression level of lipid metabolism-related genes in liver of diquat-challenged broilers. FAS gene (A) and ACC gene (B). The mRNA level of genes was determined by qRT-PCR. Bars represent the means ± standard deviation (SD) (n = 8). *There was significant difference (P < 0.05) between the control and DQ groups. Above the bar no letter or the same letter mean no significant (P > 0.05), while with different letter mean significant difference (P < 0.05). Fatty acid synthase (FAS), acetyl coenzyme A carboxylase (ACC).
DISCUSSION
The liver is the largest digestive gland and plays an important role in bile secretion, immunity, detoxification, blood coagulation, vitamin absorption and metabolism, and other physiological activities (Trefts et al., 2017). Diquat, one of the commonly used chemicals in studying the effects of oxidative stress, generates free radicals in cells through the redox cycle. The liver and intestine are the main target organs for diquat (Jones and Vale, 2000). We employed diquat to induce oxidative stress in the liver of broilers, and curcumin was fed to the broilers to investigate its protective effect against oxidative stress. The oxidative stress caused by diquat treatment in the liver and intestines could have resulted in reduced growth performance in broilers (Magalhães et al.,2018; Chen et al., 2020, 2021). These reports are partially consistent with our research results. The linear increase in the BW change rate with increasing curcumin concentration demonstrates that curcumin can reverse the effect of DQ on broiler growth performance. This may be due to the antioxidant activities and other biological activities of curcumin. The diquat treatment significantly reduced the activities of TAOC, GSH-Px, and SOD but increased the MDA content in the liver of broilers in the DQ group compared with the control group. This indicated that diquat treatment reduced the antioxidant capacity of the liver, causing oxidative stress. Nuclear factor E2-related factor 2 plays an important role in cellular redox homeostasis in a Keap1-dependent or independent manner by regulating the gene expression of various antioxidant and detoxification enzymes (Bryan et al., 2013). In this experiment, the abundance of Nrf2, GSH-Px, and SOD1 mRNA in the liver of broilers in the DQ group was significantly increased compared with the control group after the diquat treatment due to the acute reaction of oxidative stress induced by diquat (Wang et al., 2020). When 20-day-old Ross broilers were intraperitoneally injected with 20 mg/mL of diquat at a dose of 1 mL/kg of BW, the activity of SOD in the blood was significantly reduced 24 h after the treatment, with a significant increase in the MDA content in blood and liver and the abundance of Nrf2 mRNA in the liver (Chen et al., 2021). Diquat enters the cell mainly by diffusion, forming superoxide anion-free radicals upon catalysis with the reduced coenzyme II and cytochrome P450 reductase. These free radicals can form hydrogen peroxide and oxygen through SOD catalysis, and hydrogen peroxide forms water under the action of GSH-Px and CAT (Zielonka et al., 2006). In addition to ROS, diquat can induce oxidative stress by inducing reactive nitrogen species (RNS), such as peroxynitrite anion, in hepatocytes (Fu et al., 2001). Since diquat has a higher redox potential value, it can induce the production of numerous free radicals, significantly increasing the consumption of liver SOD, GSH-Px, and other antioxidant enzymes, thus leading to MDA accumulation (Magalhães et al., 2018). In addition, the liver injury caused by diquat treatment could have resulted in reduced antioxidant capacity in the broilers. After 24 h of the treatment, the 20-day-old Ross broilers intraperitoneally injected with 20 mg/mL diquat at the dose of 1 mL/kg BW had significantly reduced liver TAOC activity but increased MDA content (Chen et al., 2020). Another study also reported that TAOC and GSH-Px activities were significantly reduced, but the MDA content was increased in the liver of broilers after diquat treatment (Nong et al., 2023). These results are partially consistent with the results of this experiment because the effects on GSH-Px activity are different among the studies, probably due to the differences in the variety of the selected broiler and the time and frequency of diquat treatment.
In the curcumin supplementation groups, the effect of diquat treatment on the antioxidant capacity of the liver was reversed. Total antioxidant capacity and SOD activities were significantly higher in the 150 mg/kg curcumin supplementation group than in the DQ group. The MDA content was significantly lower in the curcumin supplementation groups than in the DQ group. One study demonstrated that curcumin could reverse the effect of aflatoxin B1 on the antioxidant enzyme activity in the liver of broilers, significantly increasing SOD and CAT activities and decreasing the MDA content (Li et al., 2022a). It was also reported that 100 mg/kg of curcumin could reverse the effect of heat stress on the antioxidant capacity of the liver, significantly increasing the SOD activity and reducing the MDA content (Zhang et al., 2018b). These reports are partially consistent with our research results, probably due to the antioxidant activity of curcumin. The structure of curcumin contains various antioxidant groups, which can eliminate various free radicals, including superoxide anion and hydroxyl radicals, thus reducing the oxidative stress caused by MDA, mercaptan, protein carbonyl, nitrotyrosine, etc. This reduces the consumption of antioxidant enzymes in the body (Abraham et al., 2019). We found that curcumin increased the abundance of Nrf2, NQO-1, and SOD1 mRNA in the broiler liver of the curcumin supplementation groups compared to the DQ and control groups. This indicated that the Nrf2 signal was involved in the curcumin-mediated alleviation of diquat-induced oxidative stress. Nuclear transcription factor Nrf2 plays an important role in cell resistance to oxidative stress and detoxification. Curcumin could stimulate the Nrf2 signaling pathway by inhibiting Keap1, regulating upstream medium and target gene expression of Nrf2, and improving Nrf2’s nuclear transfer, to enhance cellular antioxidant activity and alleviate the impact of oxidative stress (Ashrafizadeh et al., 2020). Curcumin has also been used to alleviate oxidative stress in broilers. Curcumin could reportedly regulate the activity of antioxidant enzymes through the Nrf2 signaling pathway to alleviate the reduction of antioxidant activity in broilers caused by heat stress (Zhang et al., 2018a). It was also demonstrated that curcumin could alleviate oxidative stress and liver injury caused by aflatoxin B1 in broilers through the Nrf2 signaling pathway (Muhammad et al., 2018).
In normal cellular physiological activities, an appropriate amount of ROS is produced to maintain the redox reaction required for cell metabolism, differentiation and survival, epigenetic status, regulation of transcription factor activity, and immune defense (Lennicke and Cochemé, 2021). However, when the amount of oxidants (ROS and active nitrogen) exceeds the buffer capacity of antioxidants (GSH-Px, CAT, SOD, etc.), they can cause oxidative stress. This may lead to DNA, protein, and lipid damage, cell apoptosis, necrosis and autophagy, and liver damage (Brieger et al., 2012). The liver is vulnerable to oxidative stress. Microsomes, mitochondria, and peroxidase in liver parenchyma cells can produce ROS, making the liver one of the main organs targeted by ROS (Cichoż-Lach and Michalak, 2014). Alanine aminotransferase and TB are sensitive indicators of hepatocyte injury, which usually increase when hepatocyte injury occurs (Senior, 2012). It has been reported that diquat treatment can induce oxidative stress in broilers, and damage normal liver function, significantly increasing blood ALT activity and TB levels (Chen et al., 2021). Some studies found that diquat treatment could induce oxidative stress in mice, causing changes such as vacuolization of hepatocytes, chromatin coagulation, and nuclear swelling, thereby significantly increasing the ALT activity in the blood (Zhang et al., 2021). In this study, the liver ALT and AST activities and TB level of broilers were significantly higher in the DQ group than in the control group after the diquat treatment, indicating that diquat treatment caused adverse effects on the liver of broilers. The tissue morphology changes in liver tissues further confirmed this result. The Bax and Caspase-3 are important pro-apoptotic genes, while Bcl-2 is an important antiapoptotic gene. These 3 genes play an important regulatory role in the process of apoptosis (Ola et al., 2011). It was discovered that diquat treatment significantly increased the apoptosis rate of chicken hepatocytes and the mRNA abundance of Caspase-3 but significantly reduced the abundance of Bcl-2 mRNA (Chen et al., 2020). Another study demonstrated that diquat treatment significantly increased the mRNA abundance of Bax and Caspase-3 in the liver of broilers (Chen et al., 2021). In the present study, the mRNA abundance of Caspase-3 in the liver of broilers was significantly higher in the DQ group than in the control group after the diquat treatment. This was partially consistent with the previous reports and further indicated the adverse effects of diquat treatment on the liver. The adverse effects caused by diquat to the liver were alleviated in the curcumin supplementation groups. This was demonstrated by the reduced ALT activity and TB and TP levels, reduced adverse effects changes in the liver, increased abundance of Bcl-2 mRNA, and decreased abundance of Caspase-3 mRNA. This may be due to the antioxidant activities and other biological activities of curcumin. A previous study showed that curcumin could alleviate the injury in the jejunum and mitochondrial dysfunction of piglets caused by diquat-induced oxidative stress (Li et al., 2022b). Additional studies also reported that curcumin could alleviate liver injury induced by carbon tetrachloride by inhibiting oxidative stress in rat liver. In addition, curcumin could protect liver tissue and alleviate liver injury by regulating the NF-κB signaling pathway (Li et al., 2021a), the expression of long-chain noncoding RNA (Li et al., 2021b), and DNA methylation (Wu et al., 2016).
Liver injury reduces the antioxidant capacity, prolonging the recovery time of the oxidative stress and injury of the liver, thus affecting liver function. Lipid metabolism is one of the important functions of the liver. In this study, the blood triglyceride content of broilers was significantly higher in the DQ group than in the control group. To investigate whether diquat treatment could affect the lipid metabolism of the liver and the effect of curcumin supplementation, we evaluated the activity of lipid metabolism-related enzymes in the liver and the abundance of their mRNA. The results showed that adding curcumin reversed the effect of diquat on triglycerides, and the inhibition effect increased with the increasing amount of curcumin added. We also found that ACC activity and the abundance of ACC and FAS mRNA in the liver were reduced. Fatty acid synthetase is a key enzyme for fatty acid synthesis, which can catalyze the conversion of acetyl coenzyme A and malonyl coenzyme A into fatty acids. Meanwhile, ACC is the rate-limiting enzyme for fatty acid synthesis, and increased activity can promote fat synthesis (Toussant et al., 1981). Therefore, our results indicated that curcumin had an inhibitory effect on liver fat deposition. Lipid deposition in the liver and oxidative stress can stimulate each other and cause liver injury. It was reported that being an effective inhibitor of FAS, curcumin could inhibit adipocyte differentiation and lipid accumulation by hindering FAS activity (Zhao et al., 2011). Other studies also confirmed that curcumin could significantly inhibit the abundance of ACC mRNA in the liver of broilers. Curcumin also reportedly reduced the total cholesterol and triglyceride levels significantly in blood and liver triglyceride, inhibiting fat deposition (Xie et al., 2019).
In conclusion, supplementing curcumin in the diet can ameliorate the effects of diquat-induced oxidative stress on the antioxidant capacity, tissue morphology, and lipid metabolism of the liver of broilers, thus protecting the liver. Based on data from the current study, the recommended dose range for diets of broiler chickens diets is between 100 and 150 mg/kg curcumin.
ACKNOWLEDGMENTS
This research was funded by Postdoctoral Research Project of Hebei Province, grant number (B2022003045).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abakumova T., Vaneev A., Naumenko V., Shokhina A., Belousov V., Mikaelyan A., Balysheva K., Gorelkin P., Erofeev A., Zatsepin T. Intravital electrochemical nanosensor as a tool for the measurement of reactive oxygen/nitrogen species in liver diseases. J. Nanobiotechnol. 2022;20:497. doi: 10.1186/s12951-022-01688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams S., Haylett W.L., Johnson G., Carr J.A., Bardien S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: a review. Neuroscience. 2019;406:1–21. doi: 10.1016/j.neuroscience.2019.02.020. [DOI] [PubMed] [Google Scholar]
- Ashrafizadeh M., Ahmadi Z., Mohammadinejad R., Farkhondeh T., Samarghandian S. Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr. Mol. Med. 2020;20:116–133. doi: 10.2174/1566524019666191016150757. [DOI] [PubMed] [Google Scholar]
- Brannan K.E., Helfrich K.K., Flentke G.R., Smith S.M., Livingston K.A., Jansen van Rensburg C. Influence of incubation, diet, and sex on avian uncoupling protein expression and oxidative stress in market age broilers following exposure to acute heat stress. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieger K., Schiavone S., Miller F.J., Krause K.H. Reactive oxygen species: from health to disease. Swiss. Med. Wkly. 2012;142:13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- Bryan H.K., Olayanju A., Goldring C.E., Park B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen Y., Zhang H., Wang T. Pterostilbene as a protective antioxidant attenuates diaquat-induced liver injury and oxidative stress in 21-day-old broiler chickens. Poult. Sci. 2020;99:3158–3167. doi: 10.1016/j.psj.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Dai G., Wu X., Li L., Tian Y., Tan L. Protective effects of Fagopyrum dibotrys on oxidized oil-induced oxidative stress, intestinal barrier impairment, and altered cecal microbiota in broiler chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Gu Y., Zhao H., Zhou Y. Dietary squalene supplementation alleviates diaquat-induced oxidative stress and liver damage of broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Sies H., Lei X.G. Opposite roles of selenium-dependent glutathione peroxidase-1 in superoxide generator diquat- and peroxynitrite-induced apoptosis and signaling. J. Biol. Chem. 2001;276:43004–43009. doi: 10.1074/jbc.M106946200. [DOI] [PubMed] [Google Scholar]
- Hu J., Mohammed A.A., Murugesan G.R., Cheng H. Effect of a synbiotic supplement as an antibiotic alternative on broiler skeletal, physiological, and oxidative parameters under heat stress. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.M., Vale J.A. Mechanisms of toxicity, clinical features, and management of diaquat poisoning: a review. J. Toxicol. Clin. Toxicol. 2000;38:123–128. doi: 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- Lei C., Huo Y., Ma F., Liao J., Hu Z., Han Q., Li Y., Pan J., Hu L., Guo J., Tang Z. Long-term copper exposure caused hepatocytes autophagy in broiler via miR-455-3p-OXSR1 axis. Chem. Biol. Interact. 2023;369 doi: 10.1016/j.cbi.2022.110256. [DOI] [PubMed] [Google Scholar]
- Lennicke C., Cochemé H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell. 2021;81:3691–3707. doi: 10.1016/j.molcel.2021.08.018. [DOI] [PubMed] [Google Scholar]
- Li W., Jiang L., Lu X., Liu X., Ling M. Curcumin protects radiation-induced liver damage in rats through the NF-κB signaling pathway. BMC Complement Med. Ther. 2021;1:10. doi: 10.1186/s12906-020-03182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Liu R., Wei G., Guo G., Yu H., Zhang Y., Ishfaq M., Fazilani S.A., Zhang X. Curcumin protects against Aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111725. [DOI] [PubMed] [Google Scholar]
- Li S., Liu R., Xia S., Wei G., Ishfaq M., Zhang Y., Zhang X. Protective role of curcumin on aflatoxin B1-induced TLR4/RIPK pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol. Environ. Saf. 2022;233 doi: 10.1016/j.ecoenv.2022.113319. [DOI] [PubMed] [Google Scholar]
- Li X., Zhu J., Lin Q., Yu M., Lu J., Feng J., Hu C. Effects of curcumin on mitochondrial function, endoplasmic reticulum stress, and mitochondria-associated endoplasmic reticulum membranes in the jejunum of oxidative stress piglets. J. Agric. Food Chem. 2022;70:8974–8985. doi: 10.1021/acs.jafc.2c02824. [DOI] [PubMed] [Google Scholar]
- Magalhães N., Carvalho F., Dinis-Oliveira R.J. Human and experimental toxicology of diaquat poisoning: toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum. Exp. Toxicol. 2018;37:1131–1160. doi: 10.1177/0960327118765330. [DOI] [PubMed] [Google Scholar]
- Muhammad I., Wang H., Sun X., Wang X., Han M., Lu Z., Cheng P., Hussain M.A., Zhang X. Dual role of dietary curcumin through attenuating AFB1-induced oxidative stress and liver injury via modulating liver phase-I and phase-II enzymes involved in AFB1 bioactivation and detoxification. Front. Pharmacol. 2018;9:554. doi: 10.3389/fphar.2018.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong K., Liu Y., Fang X., X.Qin Z.Liu, Zhang H. Effects of the vitamin D3 on alleviating the oxidative stress induced by diquat in wenchang chickens. Animals (Basel) 2023;13:711. doi: 10.3390/ani13040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola M.S., Nawaz M., Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- Racz L.Z., Racz C.P., Pop L.C., Tomoaia G., Mocanu A., Barbu I., Sárközi M., Roman I., Avram A., Tomoaia-Cotisel M., Toma V.A. Strategies for improving bioavailability, bioactivity, and physical-chemical behavior of curcumin. Molecules. 2022;27:6854. doi: 10.3390/molecules27206854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu P.K. Design, structure activity relationship, cytotoxicity and evaluation of antioxidant activity of curcumin derivatives/analogues. Eur. J. Med. Chem. 2016;121:510–516. doi: 10.1016/j.ejmech.2016.05.037. [DOI] [PubMed] [Google Scholar]
- Senior J.R. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury - past, present, and future. Clin. Pharmacol. Ther. 2012;92:332–339. doi: 10.1038/clpt.2012.108. [DOI] [PubMed] [Google Scholar]
- Sies H., Belousov V.V., Chandel N.S., Davies M.J., Jones D.P., Mann G.E., Murphy M.P., Yamamoto M., Winterbourn C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell. Biol. 2022;23:499–515. doi: 10.1038/s41580-022-00456-z. [DOI] [PubMed] [Google Scholar]
- Toussant M.J., Wilson M.D., Clarke S.D. Coordinate suppression of liver acetyl-CoA carboxylase and fatty acid synthetase by polyunsaturated fat. J. Nutr. 1981;111:146–153. doi: 10.1093/jn/111.1.146. [DOI] [PubMed] [Google Scholar]
- Trefts E., Gannon M., Wasserman D.H. The liver. Curr. Biol. 2017;27:1147–1151. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Huang H., Hu Y., Huang J., Yang H., Wang L., Chen S., Chen C., He S. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. J. Anim. Sci. 2020;8:112. doi: 10.1093/jas/skaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhu J., Cao Q., Zhang C., Dong Z., Feng D., Ye H., Zuo J. Dietary catalase supplementation alleviates deoxynivalenol-induced oxidative stress and gut microbiota dysbiosis in broiler chickens. Toxins (Basel) 2022;14:830. doi: 10.3390/toxins14120830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Huang R., Xiong Y., Wu C. Protective effects of curcumin against liver fibrosis through modulating DNA methylation. Chin. J. Nat. Med. 2016;14:255–264. doi: 10.1016/S1875-5364(16)30025-5. [DOI] [PubMed] [Google Scholar]
- Xie Z., Shen G., Wang Y., Wu C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019;98:422–429. doi: 10.3382/ps/pey315. [DOI] [PubMed] [Google Scholar]
- Xu X., Zhu Y., Wei Y., Chen X., Li R., Xie J., Wang G., Ming J., Yin H., Xiang J., Huang F., Yang Y. Dietary Se-enriched cardamine enshiensis supplementation alleviates transport-stress-induced body weight loss, anti-oxidative capacity and meat quality impairments of broilers. Animals (Basel) 2022;12:3193. doi: 10.3390/ani12223193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Bai K.W., He J., Niu Y., Lu Y., Zhang L., Wang T. Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J. Anim. Sci. 2018;96:867–879. doi: 10.1093/jas/sky009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang H., Liu Y., Fang X., Gu L., Luo C., Chen L., Wang Q. Vitamin D3 protects mice from diquat-induced oxidative stress through the NF-κB/Nrf2/HO-1 signaling pathway. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/6776956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Sun X.B., Ye F., Tian W.X. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol. Cell. Biochem. 2011;351:19–28. doi: 10.1007/s11010-010-0707-z. [DOI] [PubMed] [Google Scholar]
- Zielonka J., Rybak M., Celińska J., Adamus J., Marcinek A., Gebicki J. Effect of heparin on viologen-stimulated enzymatic NADH depletion. Chem. Res. Toxicol. 2006;19:668–673. doi: 10.1021/tx050336s. [DOI] [PubMed] [Google Scholar]