Abstract
Therapeutic genome editing has the potential to cure diseases by directly correcting genetic mutations in tissues and cells. Recent progress in the CRISPR-Cas9 systems has led to breakthroughs in gene editing tools because of its high orthogonality, versatility, and efficiency. However, its safe and effective administration to target organs in patients is a major hurdle. Extracellular vesicles (EVs) are endogenous membranous particles secreted spontaneously by all cells. They are key actors in cell-to-cell communication, allowing the exchange of select molecules such as proteins, lipids, and RNAs to induce functional changes in the recipient cells. Recently, EVs have displayed their potential for trafficking the CRISPR-Cas9 system during or after their formation. In this review, we highlight recent developments in EV loading, surface functionalization, and strategies for increasing the efficiency of delivering CRISPR-Cas9 to tissues, organs, and cells for eventual use in gene therapies.
Keywords: MT: Exploiting Extracellular Vesicles as Therapeutic Agents Special Issue, extracellular vesicles, CRISPR-Cas9, gene therapy, delivery, tissue targeting
Graphical abstract
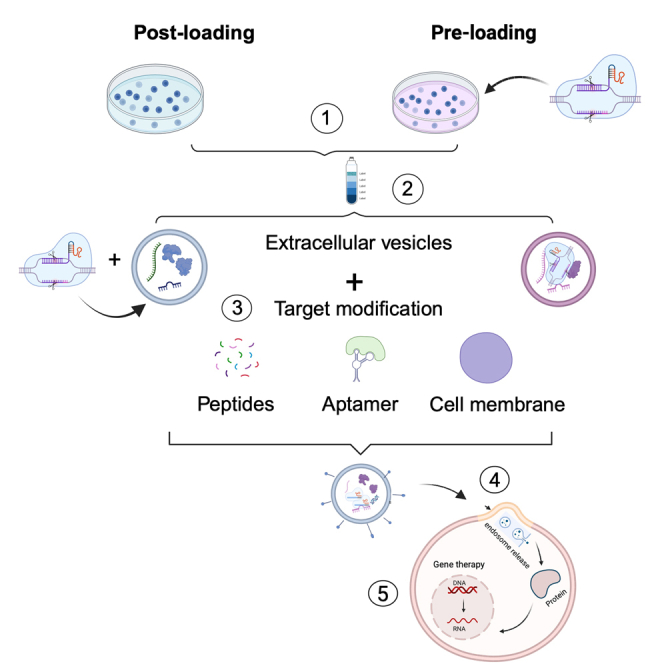
Lu and colleagues highlight recent developments in engineered EVs for CRISPR-Cas9 delivery in the context of gene therapy. We explain the main methods to load this system into EVs. We also present different strategies for EV surface functionalization to promote target delivery to specific organs.
Introduction
Genetic engineering is a sophisticated science that modifies genes at the molecular level. External genes can be replicated, transcribed, and expressed in receptor cells to produce a variety of valuable proteins. This expression can then achieve a functional modification of the receptor cells. The discovery of CRISPR-Cas systems has ushered in a new era of genome editing. The underlying CRISPR-Cas gene editing technology comes from the bacteria’s defense system against phages. There it evolved to cut invading nucleic acids and confer resistance to phages that previously infected the cell. In practice, the CRISPR-Cas protein needs to combine with a single artificial synthetic guide RNA (sgRNA) generated by fusing the CRISPR RNA (crRNA) and trans-activating crRNA. This forms the Cas and sgRNA ribonucleoprotein (RNP), which targets and cleaves specific DNA,1,2 which is programming of CRISPR-based gene editing tools. Type II CRISPR-Cas9 is now widely used in multiple fields requiring gene editing, as it is simple to use and highly efficient.1,2 To date, it has been used in basic biology, healthcare, and agriculture research. However, the widespread use of this technology in therapies is hindered by its accidental activity on non-target gene effects when editing genes in cells, the human autoimmune response against Cas9, and the danger associated with inefficient delivery approaches. To implement the use of CRISPR-Cas9 in gene therapies, developing a safe and efficient in vivo delivery system remains the most challenging aspect.3,4
Extracellular vesicles (EVs) are endogenous membranous particles secreted spontaneously by all cells. They function as messengers by exchanging cargo between cells, allowing the transport of various signaling chemicals, such as bioactive lipids, proteins, and nucleic acids (DNA and RNA).5 There are three primary types of EVs classified based on their size, function, and biogenesis pathway: exosomes, ectosomes, and apoptotic bodies.6 Exosomes (30–150 nm) are formed as intraluminal vesicles through the inward budding of early endosomes and are subsequently released into the extracellular space. Ectosomes are formed by the outward budding or pinching of the plasma membrane of cells. They contain different types of vesicles, which range from less than 100 nm to several micrometers. Ectosomes are mainly divided into microvesicles (0.2–1 μm) and large oncosomes (>1 μm). Apoptotic bodies (1–5 μm) contain harmful cellular components left over from apoptosis.7 Additional EV subtypes, such as migrasomes, have recently been described and are still being studied.6,8 The evidence accumulating reveals significant differences in the characteristics, composition, and function between these distinct EV types. However, they are involved in pathological and physiological states by transmitting bioactive components and overcoming biological barriers.9,10 Based on these characteristics, EVs are increasingly being used to deliver drugs. The EVs mentioned below represent exosomes and microvesicles.
In this review, we discuss current developments in the delivery of CRISPR-Cas9 using EVs while also outlining strategies to increase the effectiveness of EV delivery approaches.
CRISPR-Cas9 delivery approaches
The RNP complex has difficulty passing the cell membrane because Cas9 is a large protein (160 kDa), and the sgRNA is negatively charged. To date, many strategies have been developed to deliver CRISPR-Cas9 to target cells. These can be categorized as viral vectors (adenovirus, adeno-associated viruses ([AVs] and lentivirus) and non-viral vector-based delivery strategies. Non-viral vector approaches can be further subdivided into natural/synthetic materials (e.g., EVs, penetrating peptides, lipid nanoparticles [LNPs], and polymer nanoparticles) and physical approaches (e.g., cell microinjection, electroporation, sonoporation, and laser irradiation).11 To date, viral vectors have demonstrated the best CRISPR-Cas9 delivery and gene editing efficiency. AAVs, as the most widely used viral vector, display unique advantages such as reduced immunogenicity and mild toxicity compared with other viruses.12,13,14 However, the packaging capacity of AVVs (<5.2 kb) is a limiting factor when delivering the components of CRISPR-Cas9 and its derived gene editing technologies.15,16 Indeed, the open reading frame encoding Streptococcus pyogenes Cas9 is approximately 4.2 kb.4 Furthermore, the CRISPR-Cas9-derived gene editing technologies, including base editors (4.2–5.2 kb)17 and the prime editor (~6.3 kb)18 are larger than the capacity of AVVs. Moreover, this issue is compounded when sgRNAs and prime editing guide RNAs must be delivered in parallel.19 Although adenoviruses and lentiviruses have a greater capacity than AAVs, they demonstrate their own limitations and adverse effects. Adenoviruses induce an intense immune response, and lentiviruses are known to integrate into the host’s genome.
To increase the efficacy, safety, and targeting of gene editing, the method of delivering the CRISPR-Cas9 system must be actively considered. New strategies such as LNPs, EVs, and virus-like particles have been developed to deliver gene therapies.20 The clinical translation of these vectors have two primary challenges: safety and quick clearance by the reticuloendothelial system or the mononuclear phagocyte system. Cell-derived nanovesicles outperform manufactured nanocarriers in both aspects. EVs can also encapsulate dsDNA ranging from 100 bp to 20 kb with low immunogenicity.21,22 Additionally, the proinflammatory cytokines induced by EVs are both smaller in quantity and in effect when compared with inflammatory responses reported with LNPs.23,24 When considering the above and that the EV membrane structure can shield proteins and nucleic acids from the immune system and serum endonucleases,25 EVs are an excellent candidate for CRISPR-Cas9 delivery.
The advantages and limitations of EV delivery
When delivering therapeutic cargo, EVs provide several advantages including a larger capacity, higher biocompatibility, lower host immune response, and immunogenicity compared with their leading competitors.26,27 Furthermore, their naturally occurring lipid and surface protein composition allows them to avoid phagocytosis, increases their half-life in blood, and reduces long-term safety concerns after their administration. The unique structure of EVs also protects their contents from destruction in the extracellular environment for prolonged periods.7,28 Additionally, the small size of EVs makes it easier for them to extravasate, move across physical barriers, and pass through the extracellular matrix. Studies on the impact of EVs and their cargo have been conducted on numerous disorders, including cardiovascular disease,29 type 2 diabetes mellitus,30 and cancer.31 Meanwhile, EVs were reportedly loaded with therapeutic agents, such as small interfering RNA (siRNA),32 microRNA,33 mRNA/proteins,34,35 CRISPR-Cas9,25 and drugs36 for treating disorders in vitro and in vivo. Phase I and II clinical trials based on EVs are being carried out to evaluate the efficiency of using bone marrow mesenchymal stem cell (MSC)-derived exosomes to deliver drugs in patients hospitalized with severe acute respiratory syndrome coronavirus 2 pneumonia and COVID-19 within 28 days of the first treatment (NCT04602442 and NCT04276987). The result showed that, in seven COVID-19 patients, MSC-derived exosomes were feasible to deliver drugs in clinic and well tolerated, with no evidence of prespecified adverse events, acute clinical instability, or dose-relevant toxicity at any of the doses examined.37
Conversely, various obstacles must be cleared before EV-based therapies may be implemented in clinics. First, many characteristics and mechanisms relating to EV biology remain elusive, such as how to increase the purity of the EVs by distinguishing the overlap size among EVs categories or other substances like lipoprotein, pili, tamm-Horsfall protein, and cell dribs.38 Second, the mechanism of production or absorption is still poorly understood. EVs from the same cell types may have paradoxical effects because of the variation in cell culture conditions and differences in purification techniques.39 Moreover, no universal sample collection protocol exists and there are no universal biomarkers for validation.40 Finally, solutions for the difficulties faced during cargo-loading, EV uptake, cargo release, and endosomal escape cell/tissue targeting efficiency are all still in their infancy. These factors need to be further investigated before the widespread adoption of EVs-based therapies.
Methods to load cargo into EVs
Loading cargo into EVs is classified into endogenous and exogenous post-isolation loading methods.41 As the name implies, endogenous methods involve loading the cargo before the isolation of the EVs. This is accomplished by adding new genetic material or selecting molecules to interact with or modify parent cells, such as drugs, RNAs, DNAs, proteins, or select molecules that can be incorporated into their EVs.42 Exogenous post-isolation loading approaches encompass various techniques to load therapeutic cargo into EVs after their isolation.43,44 Each approach has its advantages and limitations.
During the endogenous loading method, EVs maintain their form and characteristics regardless of their cargo’s physical and chemical properties. However, this method has a relatively low loading efficiency, which limits its application as a standalone method. It is, therefore, effective when delivering small molecular proteins into EVs but ineffective when delivering biomacromolecules (proteins and nucleic acids) such as Cas9 protein or the dystrophin gene. Furthermore, proteins of interest can be incorporated in both the extra-vesicular and the intra-vesicular sides simultaneously, which could lead to immune responses against the EVs or the denaturation of external proteins during transport.
Compared with endogenous loading methods, exogenous post-isolation techniques allow a broader array of therapeutics to be loaded without altering the parental cell line. Since the cell types are not restricted to those that are easily transfected or have intrinsic cell regulation that allows chemicals to be inserted into cells, exogenous post-isolation methods are amenable to a wider range of EV-producer cells and can additionally be standardized and controlled. This is in contrast with endogenous loading, which depends on EV biogenesis and cell states.45
Whether one is using endogenous or exogenous loading techniques, the cargo is loaded either passively or actively. Passive approaches involve attracting bioactive substances into EVs by simple incubation. This is appropriate for small compounds like chemotherapeutic drugs and small nucleic acids. Hydrophobic chemicals have the best loading efficacy because they can bind to the cell membrane and the EV lipid bilayer in a stable fashion. However, the cargo enrichment in EVs by the passive loading approach depends on the condition of source cells and demonstrates poor repeatability.
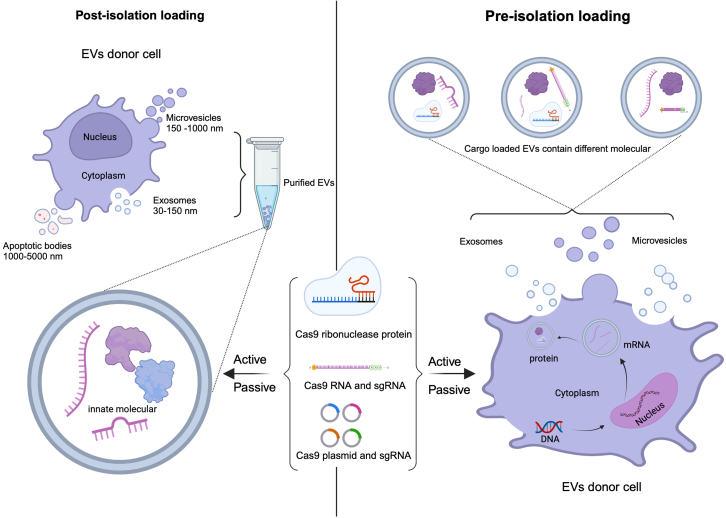
Active loading involves loading cargo into EVs by sonication, electroporation, freeze-thaw cycles, extrusion, and transfection (extensively reviewed elsewhere46,47). These methods generate higher loading efficiency than passive loading. However, these approaches share a common drawback in that the membrane of EVs can be damaged, affecting their structure and potentially affecting their ability to transport their materials. Based on those loading methods, the methods for loading the components of CRISPR-Cas9 into EVs are shown in Figure 1.
Figure 1.
Methods for loading CRISPR-Cas9 components into EVs
(Left) Post-isolation loading method. EVs are produced by unmodified parent cells. The components of the CRISPR-Cas9 system are loaded into EVs after their isolation and purification. (Right) Pre-isolation loading method. The components of the CRISPR-Cas9 system are transfected into parent cells. EVs are harvested and will contain the exogenous nucleic acid material by natural EV production route.
Delivery of CRISPR-Cas9 using EVs
The CRISPR-Cas9 system can be used in mammalian cell cultures by incorporating it into the cells in the form of plasmids, mRNA, and protein. Plasmids are the most complex form, but are inexpensive to produce and simple to use. Once the Cas9 plasmid is delivered into the cytoplasm of target cells, it is sent to the nucleus and transcribed into mRNAs. Subsequently, the Cas9 mRNAs are transported to the cytoplasm and translated into Cas9 proteins. Part of the plasmid also encodes a sgRNA that later interacts with the Cas9 protein to form the Cas9/sgRNA RNP complex. Given the complexity of transfection, expression of Cas9 and its sgRNA, and gene editing, many techniques have been developed to deliver the Cas9/sgRNA complex at different stages of its development. Here, we further describe the details of delivering the different formats of CRISPR-Cas9 components in vivo and in vitro by EVs.
EV delivering CRISPR-Cas9 plasmid
Kim et al.48 electroporated the CRISPR-Cas9 plasmid and sgRNA into SKOV3 cancer cell-derived exosomes to target the poly (ADP-ribose) polymerase-1 (PARP-1) gene in SKOV3 tumor cells and SKOV3 xenograft mice. The authors showed that their treatment-induced 55% insertions or deletions (indels) in PARP-1-positive SKOV3 cells and induced SKOV3 cell apoptosis in mice with ovarian cancer. They also demonstrated that cancer cells preferentially take up cancer cell-derived exosomes compared with exosomes produced by epithelial cells. This result shows that specific tropism can influence the ability of EVs to target specific cell types.
Furthermore, McAndrews et al.49 used a transfection reagent (Exo-Fect) to transfect the Cas9/sgRNA plasmid into exosomes derived from human embryonic kidney (HEK293T) epithelial cells. This allowed them to target the KrasG12D oncogenic allele in pancreatic cancer cells and led to a 58% knockdown of the target KrasG12D transcript levels in vitro. The authors also observed a decrease in the proliferation of tumor cells in vitro and of tumor growth inhibition in vivo. In a similar vein, exosomes are currently being investigated in phase 1 clinical trials in patients with metastatic pancreatic cancer with the KrasG12D gene mutation by delivering KrasG12D siRNA (NCT03608631).
Cell uptake efficiency is another consideration for exosome-mediated gene modification. Lin et al.50 described a simple incubation-based exosome-liposome hybrid system. They incubated exosomes with a mixture of liposomes and Cas9/sgRNA plasmids for 12 h at 37°C. They found that an incubation time of 12 h was best for balancing the fusion efficiency and stability. This strategy gave a higher loading capacity and a more effective delivery than native exosomes for delivering CRISPR-Cas9 to MSCs. Based on their outcomes, Liang et al.51 constructed chondrocyte affinity peptide-targeting exosomes (CAP-Exo). Those EVs are made by genetically adding a CAP at the N-terminus of the exosome surface protein Lamp2b. They transfected the CAP-FLAG-Lamp2b plasmid with Lipofectamine 2000 into dendritic cells to produce the CAP-Exo. The CAP peptide present on the exosome surface enables the exosomes to target chondrocytes and keep them in the joint following intra-articular injection. CAP-Exo was incubated with liposomes to encapsulate the CRISPR-Cas9 plasmid to knock down the matrix metalloproteinase 13 (MMP-13) in chondrocytes. The hybrid CAP-Exo successfully suppressed the production of MMP-13 in chondrocytes, penetrated the deep region of the cartilage matrix in osteoarthritis rats, and attenuated the hydrolytic breakdown of the extracellular matrix proteins in the cartilage.
To target spinal cord injuries (SCI), Wang et al.52 modified human umbilical cord MSC-derived exosomes to display the CAQK peptide (a sequence that can specifically bind to the injured site after SCI). This system achieved tissue recovery by inhibiting the tumor necrosis factor α (TNF-α)-induced inflammatory response and restraining the activation of the TNF-α/nuclear factor κB signaling pathway.
Moreover, Xu et al.53 transduced the anti-CD19 chimeric antigen receptor (CAR) lentivirus into the HEK293T cells. EVs derived from anti-CD19-CAR-HEK293T cells express anti-CD19-CAR on the surface showed selective tropism for tumors. The outcome demonstrated that CAR-EVs accumulated in cancer tumors more rapidly than ordinary EVs. Their results also showed that the CRISPR-Cas9 system was effectively released both in vitro and in vivo, and precisely targeted the MYC oncogene.
We have compiled the research on post-isolation loading of CRISPR-Cas9 plasmids into EVs in Table 1. More information on how to modify the plasmids for delivering this cargo into EVs is provided elsewhere.54,55,56,57,58
Table 1.
Delivery of CRISPR-Cas9 plasmid by EVs
| EV source | Target organ/cells | Target gene | Loading method | Reference |
|---|---|---|---|---|
| SKOV3/HEK293T | tumor generated by SKOV3 xenografts | PARP-1 | electroporation | Kim et al.48 |
| HEK293FT | MSCs | hCTNNB1 | liposomes | Lin et al.50 |
| HEK293T | tumor generated by Raji-bearing xenograft | MYC | electroporation | Xu et al.53 |
| HEK293T | pancreatic cancer | KrasG12D | Exo-Fect | McAndrews et al.49 |
| DCs | chondrocytes | MMP-13 | Lipofectamine 2000 | Liang et al.51 |
| MSC | spinal cord | TNF-α | electroporation | Wang et al.52 |
EV delivering CRISPR-Cas9 mRNA
Delivering Cas9 to cells as an mRNA minimizes immunogenicity and carcinogenicity. In this form, Cas9 mRNA does not need to go to the nucleus or go through the transcription step. Translation of the mRNA can take place directly in the cytoplasm. However, when compared with delivering plasmids, mRNA is unstable. Its short half-life may decrease the effectiveness of the gene editing treatment. Furthermore, numerous studies have demonstrated that the bulk of mRNA in EVs are degraded to some extent. Besides, the EVs from different cell sources primarily include short (100 nt) RNA molecules and only trace amounts of full-length mRNA.59,60,61 Notably, exosomes exhibit poor encapsulation effectiveness when delivering bulky CRISPR-Cas9 mRNA, even when using efficient electroporation loading methods.61 Wei et al.62 reported that exosomes from glioma cells only contain endogenous mRNA copies varying from 1/10 to 1/100,000 exosomes are detected.
Despite the difficulties, CRISPR-Cas9 mRNA has been delivered to target cells by loading it into EVs via electroporation. One study63 demonstrated that EVs derived from red blood cells (RBCEVs) enabled editing in leukemia and breast cancer cells in vitro and in vivo. The author electroporated HA-tag Cas9 mRNA into RBCEVs to treat MOLM13 leukemia cells. After 48 h of incubation, the Cas9 protein was detected using immunostaining in 50% of MOLM13 cell nuclei. This study demonstrated an effective RBCEV treatment without generating any observable cytotoxicity. In addition, RBCEVs do not pose a risk for horizontal gene transfer; RBCs are enucleated cells lacking their own DNA.
To insert Cas9 mRNA into exosomes, Li et al.64 used surface-functionalized exosomes. Their EVs were decorated with a fusion protein, consisting of the exosome membrane protein CD9 fused to the RNA binding protein HuR. Through the interaction of HuR and AU-rich elements of the RNA, CD9-HuR exosomes efficiently loaded functional CRISPR-dCas9 mRNA into exosomes. The result showed that CRISPR-dCas9 dramatically decreased C/ebpα endogenous expression in the presence of C/ebpα gRNA.
More recently, de Jong et al.65 developed a CROSS-FIRE reporter system to validate the transfer of RNA into cells that already express the Cas9 protein. With this system, they demonstrated that the deletion of the exosome-related genes Alix and Rab27A, which are involved in exosome release and production, respectively, drastically decreased reporter cell activation.65
The studies to load Cas9 mRNA into EVs have been summarized in Table 2. In addition, other modifications shown in reference that succeeded in delivering mRNA into EVs could be considered when attempting to deliver Cas9 mRNA.66,67,68,69,70,71,72
Table 2.
Delivery of CRISPR-Cas9 mRNA by EVs
| EV source | Target organ/cells | Target gene | Loading method | Reference |
|---|---|---|---|---|
| Red blood cells | MOLM13 cells | miR-125b | electroporation | Usman et al.63 |
| MDA-MB-231 cells | HEK293T | Reporter cell (mCherry) | transfection (Lipofectamine2000) | de Jong et al.65 |
| HEK293T cells | Liver | C/ebpα | transfection (Lipofectamine2000) | Li et al.64 |
Delivering CRISPR-Cas9 RNPs using EVs
The most efficient approach to deliver Cas9 for use in gene therapies involves trafficking the RNP directly. This circumvents the process of transcription and translation in target cells.73 Moreover, fewer off-target effects are detected when delivering RNPs directly.74 However, Cas9 is a large protein that hinders its loading into EVs. Current approaches to load RNPs into EVs can be broadly divided into four categories: (i) creating a fusion protein by linking the Cas9 protein to transmembrane or luminal proteins that are expressed specifically in EVs; (ii) performing post-translational modifications (PTMs) of the Cas9 protein such as ubiquitylation, phosphorylation, and glycosylation to load them into EVs; (iii) assisting by virus-derived protein like vesicular stomatitis virus glycoprotein (VSVG), help Cas9 load onto exosomes and increase the protein deliver efficiency via a pseudotyping mechanism; and (iv) incubating the Cas9 protein with EVs either with or without a transfection reagent to favor their incorporation.
Several teams have succeeded in loading the Cas9 protein into EVs by fusing it to a protein involved in EV proteins. For the protein mediating the surface and luminal engineering, Yao et al.75 use RNA aptamer Com sequence integrated into sgRNA loop and subsequently fused aptamer-binding protein Com into both terminals of CD63. The Com/com interaction enriched CRISPR-Cas9 and adenine base editor RNPs into EVs. They demonstrated that those EVs generated a 0.2% indel rate after being injected into the tibialis anterior muscle of Duchenne muscular dystrophy X-linked (DMD/mdx) mouse by intramuscular injection. Similarly, Osteikoetxea et al.76 reported that reversible heterodimerization of Cas9-fusions with EV sorting partners (cryptochrome 2 in combination with CD9 or a myristylation-palmitoylation-palmitoylation lipid modification) led to Cas9 being encapsulated into EVs. This approach efficiently loaded approximately 25 Cas9 molecules per EV and knocked down the PCSK9 gene with 6% indel efficiency in HEK293T cells.
Many methods have been developed to load Cas9 into EVs using PTMs. Whitley et al.77 fused an octapeptide derived from the leading sequence of the N-terminus of Src kinase to the N-terminal of Cas9. This led to the myristylation of the Cas9 and its subsequent packaging into EVs. This generated an eGFP knock-out efficiency of up to 42% when using VSVG-coated EVs. Wang et al.78 reported that they could pack ubiquitinated proteins into ARRDC1-mediated micro-vesicles (ARMM). To apply this to Cas9, they fused the WW domain from Nedd4 family members (ITCH, Nedd4-L, wwp2, and wwp1) to the protein, triggering its ubiquitination. The WW domain then interacted with the PPXY motif of ARRDC1 to load the Cas9 into ARMMs. This approach restored 12% of GFP expression in U2OS GFP-negative cells. In recent years, many other PTMs have been shown to mediate the entry of proteins into EVs. However, those modifications have not yet been used to deliver Cas9 into EVs.79,80
Virus proteins can also be used to facilitate the loading of the Cas9 into EVs while simultaneously increasing EV uptake by target cells.81,82 For example, Meyer et al.81 studied the impact of VSVG expression in cells internalizing EV. The VSVG protein is incorporated into the viral envelope when the membrane rafts are budding at the plasma membrane.83 When VSVG was expressed, the loading of the cargo protein was enhanced, and EV uptake was increased. Similarly, Gee et al.84 developed a new approach called "NanoMEDIC," which packed a Cas9 and sgRNA into EVs using an HIV-derived Gag protein. To recruit the Cas9 RNP into EVs, the authors used a chemical-induced dimerization system named FKBP12 and FRB. To attract the Cas9 protein into the EVs, the Cas9 and Gag proteins were fused to a heterodimerizer and conditionally dimerized by the addition of an inducible chemical ligand. Their results showed that NanoMEDIC achieved more than 90% exon-skipping efficiency in skeletal muscle cells derived from DMD patient iPS cells. Recently, Watanabe et al.85 published a protocol for large-scale production of NanoMEDIC, which brought them one step closer to the possibility of in vivo applications.
Some teams have encapsulated the unaltered CRISPR-Cas9 protein into purified EVs that were purified from serum by physical and non-virus approaches and further modified EVs with tissue-specific peptides to target the desired organs or tissues as described below.88,89 Moreover, Busatto et al.43 incubated Cas9 protein with di-octadecyl-amido-glycyl-spermine, a cationic lipid that binds to protein to form synthetic LNPs, and subsequently incubated with EVs to form a hybrid system to delivery Cas9 protein. The result showed that the Cas9-loaded EVs were efficiently taken up by receipt cells, and 83% of receipt cells were positive for Cas9-Alexa Fluor 488 at a dose of 2 × 104 EV/cell.
There are other studies mentioned that use Cas9 RNP complexes loaded into EVs to measure and track their distribution and uptake in cells. Ye et al.86 engineered tumor cells to release EVs containing a Cas9 protein and sgRNA. The RNPs deleted a stop codon before a fluorescent protein, leading to the fluorescence of the reporter cells or reporter animal models. The authors showed that tumor cells and normal cells could communicate with one another through EVs in vitro. Moreover, they demonstrated in vivo that intravenously injected EVs were absorbed by the liver. They also showed that intravenously injected EVs produced from melanoma xenografts preferentially target the brain and the liver. In addition, Strohmeier et al.87 overexpressed CRISPR-Cas9-GFP and GFP-CD63 into 293T cells. Their results demonstrated that there were more single-GFP-labeled EVs (83%) in the EVs separated from CRISPR-Cas9-modified cells than in the EVs derived from GFP-CD63 over-expressing cells (36%). This approach provides a strategy to track tumor derived EVs.
The approaches for helping the RNPs into EVs are summarized in Table 3. Likewise, we also provide some references that review how to modify and deliver proteins into EVs.80,90,91,92,93,94
Table 3.
Delivery of CRISPR-Cas9 RNP by EVs
| Method | EV source | Target organ/cells | Target gene | Loading method | Reference |
|---|---|---|---|---|---|
| Pre-isolation | Expi293F-derived EVs | HEK293T | fluorescent protein | transfection (PEI Max) | Osteikoetxea et al.76 |
| HEK293T-derived EVs | HEK293T-eGFP cells | fluorescent protein | transfection (calcium phosphate) | Whitley et al.77 | |
| HEK293T cells derived EVs | U2OS GFP-negative cells | fluorescent protein | Fugene 6 (Promega) and TurboFect | Wang et al.78 | |
| HEK293T cells derived EVs | HEK293T-derived HBB-IL2RG EGFP reporter cells | HBB/IL2RG and DMD exon 53 | Fugene HD (Promega)/polyethylenimine | Yao et al.75 | |
| 293T cells derived Gectosomes | MEF cells | PINK1 or PCSK9 | transfection (polyethylenimine) | Zhang et al.135 | |
| HEK 293T cell-derived exosome | A549stop-DsRed reporter cell line | stop-DsRed | transfection (Lipofectamine 2000) | Ye et al.136 | |
| Hepatic stellate cells-derived exosome | Liver | PUMA, CcnE1,(KAT5 | electroporation | Wan et al.137 | |
| HEK293T cells | Skeletal muscle cells | DMD | transfection (Lipofectamine 2000) | Gee et al.84 | |
| MSCs-derived exosome | HEK293T-EGFP cells | fluorescent protein | Lipofection (chemical) and electroporation (physical) | Hazrati et al.138 | |
| Tumor cells | Liver | fluorescent protein | transfection (Lipofectamine 2000) | Ye et al.86 | |
| HEK293T | HeLa cell | fluorescent protein | transfection (Endofectin Max reagent) | Strohmeier et al.87 | |
| Post-isolation | HEK293T cells-derived | Muscle | DMD | transfection (Lipofectamine 2000) | Watanabe et al.85 |
| C2C12 cells | Muscle | miR-29b | transfection (PEI MAX) | Chen et al.89 | |
| HEK293T cells-derived | Tumor | WNT10B | sonication/freeze-thaw cycles | Zhuang et al.139 | |
| Human or mouse serum | Mouse Muscle | DMD | protein transfectant | Majeau et al.88 |
EV surface functionalization
Cell type
The origin of an EV is an important factor to consider when planning to use it to deliver gene therapies.95 Depending on which cells they originated from, EVs will have different contents, exert different functions, and distribute differently.96 First, EVs naturally inherit parent cell features.95 Since EVs are frequently used between cells to communicate within the same organ, EVs are more likely to go to the same type of cell from which they originated. EVs also prefer to go to cells that are histogenetically close to them. For example, EVs from microglia tend to reach the CNS, EVs from Schwann cells tend to target the peripheral nerve, and EVs from tumor cells tend to reach homologous tumor cells.97,98,99 EVs are also used by cells to communicate between cells from different organs or types of cells. For example, EVs derived from mature dendritic cells (DCs) can reach tumor cells.100
Wiklander et al.96 studied the biodistribution of EVs derived from C2C12 (mouse muscle cell line), B16F10 (melanoma cell line), immature bone marrow-derived DCs, oligodendrocytes from rat (OLN-93), primary human MSCs, and HEK293T in mice following their intravenous injection. C2C12-derived EVs displayed a high liver accumulation (71%) and a low accumulation in the lungs (5%). They were also distributed into the gastrointestinal tract at 8% and into the spleen at 12%. Of B16F10-EVs, 56% were distributed to the liver, 15% to the gastrointestinal tract, 12% to the spleen, and 13% to the lungs. DC-EVs had the lowest liver accumulation at 46%; 28% were distributed into the spleen, 10% into the lungs, and 10% into the gastrointestinal tract. Curiously, EVs from another animal (rat or human) were distributed similarly from the mouse-derived EVs; 65% of the OLN-93-EVs reached the liver and 11% reached the gastrointestinal tract. MSC-EVs were distributed at 71% in the liver and at only 3% in the gastrointestinal tract. HEK293T-EVs accumulated at 60% in the liver and at 16% in the gastrointestinal tract. In their study, Wiklander et al.96 showed that DC-EVs have the most prominent distribution to the spleen. Thus, EVs seem to adopt the homing pattern of the parental cell of origin. The fact that EVs may share surface receptors and matrix-binding proteins with their parent cell can explain this natural tropism. For example, immunological cells preferentially target the spleen and other sites exhibiting immunological activity.101 Aside from that, another study showed that the epithelial cell-derived micro-vesicles delivered intravenously had demonstrated organ-specific tropism and are known to preferentially aggregate in a few important organs, including the liver, lung, and pancreas.102
Peptides
Decorating the surface of EVs with proteins or peptides can favor their delivery to specific cells or organs. To engineer the surface of EVs, different strategies can be used. The first strategy can be done before EV isolation. It consists of fusing a transmembrane EV protein to a peptide that can target specific cells. Integrins, lactadherin, lysosome-associated membrane protein-2b, and tetraspanins (CD9, CD63, and CD81 and CD82) are some examples of transmembrane proteins in EVs that can be fused to the desired peptide.103 The second strategy to decorate EVs is used after the isolation of the EVs and consists of attaching the peptide to the vesicle surface through adsorption. Copper-catalyzed azide-alkyne cycloaddition (click chemistry)104,105 and cloaking103 are some examples of methods that can be used to add peptides to the surface of EVs.
Brain targeting
RGE, RVG, T7, ApoB, and ApoA-I mimetic peptides are some examples of peptides that can be added to the surface of EVs to target the CNS (Table 4). Jia et al.104 bound RGE peptides (RGERPPR) to the surface of EVs by click chemistry. Their engineered EVs showed a strong glioma-targeting ability.104 This was explained by the fact that RGE peptides are specific for the neuropilin-1 receptor which is expressed on glioma. Alvarez-Erviti et al.106 decorated EVs with the rabies viral glycoprotein (RVG) peptide. To do so, they cloned it into Lamp2b. Their engineered EVs showed a high tropism for the CNS. Kim et al.107 used the T7 peptide which is a type of transferrin receptor (TfR)-binding peptide. The sequence of this peptide is HAIYPRH. The authors conjugated the T7 to Lamp2b for targeting glioblastoma. Their engineered EVs showed a higher targeting of intracranial glioblastoma in rat models than unmodified exosomes or even RVG-labeled exosomes.107 Choi et al.108 decorated exosomes with ApoB by conjugating it with CD9. The authors observed a higher accumulation of their engineered EVs in the brain compared to control exosomes.108 Those EVs even showed prolonged retention in the brain for 24 h.108 Ye et al.109 used an active targeting strategy by adding ApoA-I mimetic peptides on the surface of EVs. Those peptides can bind to low-density lipoprotein receptors expressed on the glioblastoma cells, and thus target brain cancer cells.
Table 4.
Some examples of peptides that can be added on EV surface to target specific organs/cells
| Targeted cells/organs | Peptide | Reference |
|---|---|---|
| Glioma | RGE | Jia et al.104 |
| CNS | RVG | Alvarez-Erviti et al.106 |
| Intracranial glioblastoma | T7 | Kim et al.107 |
| Brain | ApoB | Choi et al.108 |
| Glioblastoma | ApoA-I mimetic peptide | Ye et al.109 |
| Muscles | CP05 | Gao et al.110 |
| Muscles and heart | T9 | Wiklander et al.46 |
| Muscles and heart | M12 | Nance et al., Gao et al.140,141 |
| Cardiomyocytes | IL-3 | Bellavia et al.115 |
| Cardiomyocytes | cardiomyocyte-specific peptide | Mentkowski et al.114 |
| Heart | CTP | Zahid et al.116 |
| Heart | PCM | Nam et al.117 |
| Heart | ischemic myocardium-targeting peptide | Zhu et al.112 |
| Heart | cardiac homing peptide | Vandergriff et al.113 |
| Cartilage | chondrocyte affinity peptide | Liang et al.51 |
| Pancreas | tetraspanin-8 | Nazarenko et al., Rana et al.118,119 |
| Cancer cells | GE11 | Ohno et al.120 |
| Cancer cells | TfR | Song et al.103 |
| Cancer cells | glycans | Berenguer et al.122 |
| Monocyte-derived DCs | MUC1 | Koning et al.123 |
Muscles targeting
Gao et al.110 designed EVs targeting muscles in vivo. They fused the CP05 peptide (CRHSQMTVTSRL) with M12 (a muscle-targeting peptide) and conjugated the CP05 peptide with phosphorodiamidate morpholino oligomer (PMO, a treatment for DMD currently approved by the US Food and Drug Administration). The two constructions were co-incubated with exosomes. M12-CP05 and CP05-PMO were able to bind to EVs because of the intrinsic property of CP05 to bind to CD63 on the surface of EVs. Those EVs were injected intravenously into the mdx mouse. Their engineered EVs effectively targeted muscles in vivo and even led to a functional improvement in the mdx mice.110 The T9 peptide46,111 (SKTFNTHPQSTP) can also be used to target skeletal muscles and the heart.
Heart targeting
Zhu et al.112 put an ischemic myocardium-targeting peptide (CSTSMLKAC) on EVs to preferentially target ischemic injured cardiomyocytes. Vandergriff et al.113 decorated EVs with the cardiac homing peptide (CSTSMLKAC). The authors improved the efficacy of delivery using their engineered EVs. Mentkowski et al.114 fused a cardiomyocyte-specific peptide (WLSEAGPVVTVRALRGTGSW) to Lamp2b. Their engineered EVs showed an increased cardiomyocyte uptake. Interleukin-3 can be fused to Lamp2b in EVs to target cardiomyocytes.115 To target the heart, EVs could be decorated with the CTP peptide116 and the PCM peptide.117
Cartilage targeting
Liang et al.51 fused a CAP with Lamp2b to generate engineered EVs for cartilage arthritis. By intra-articular injection of their engineered EVs containing the plasmid of Cas9 and sgRNA target to MMP-13, they successfully targeted the deep region of the cartilage matrix in arthritic rats and knocked down the MMP-13 in chondrocytes. It resulted in the ablation of MMP-13 expression and in the attenuation of the hydrolytic degradation of the extracellular matrix proteins in the cartilage.
Pancreas targeting
In the pancreas, CD54+ acts as a key ligand that could attract the EVs modified with tetraspanin-8 on the surface, a multimodular protein involved in clathrin-mediated. Its specificity can be explained by its capacity to form a complex with the integrin α4 or CD49d.118,119
Tumor cell targeting
Tumor cells express specific peptides on their surface. By finding peptides that bind specifically to those molecules and by adding them to the EVs’ surface, the resulting EVs could target cancer cells. For example, the epidermal growth factor receptor (EGFR) is highly expressed on the surface of tumor cells. The GE11 peptide can bind specifically to EGFR. Thus, the GE11 peptide can be added to the EV surface to target cancer cells.120
Zuo et al.121 used exosomes derived from DCs and decorated them with an HCC-targeting peptide (P47-P), an α-fetoprotein epitope and a functional domain of high-mobility group nucleosome-binding protein 1 (N1ND-N). N1ND-N can be used to recruit and activate DCs.
EVs can also be decorated with glycans.122 Cancer cells have the chemokine (C–C motif) receptor 8, and the soluble ligand chemokine (C–C motif) ligand 18 on their surface, and glycans can bind to those proteins.122 Another protein that is present in cancer cells is Tf. TfRs can be found naturally on the surface of EVs.103 Thus, we can hypothesize that non-engineered EVs can naturally target cancer cells.
Monocyte targeting
Monocyte-derived DCs present intercellular adhesion molecule-grabbing non-integrin on their surface. This protein can bind to soluble mucin 1 (MUC1). Thus, EVs can be decorated with peptides from MUC1 to target monocyte-derived DCs.123
Antibody-based surface functionalization
Antibody-based peptides are another type of peptide that has been used to increase the targeting ability of EVs, especially when targeting cancer cells. For example, Cheng et al.124 developed synthetic multivalent antibody-targeted exosomes. These EVs expressed the monoclonal antibody αCD3 UCHT1, which is specific for CD3 T cells, and single-chain variable fragments of αEGFR cetuximab, which is specific for cancer cell-associated EGFR. The authors demonstrated that these engineered EVs could efficiently target T cells and EGFR-expressing breast cancer cells.
Aptamer
Another way to make EV delivery more accurate is by using aptamers. Aptamers bind to specific ligands with high affinity.103 For example, aptamers have been used to target cancer cells and even the brain. Han et al.125 decorated EVs with E3 aptamers and succeeded in targeting prostate cancer cells in vitro as well as in vivo. Xiang et al.126 added epithelial cell adhesion molecule aptamers on the surface of EVs and succeeded in penetrating tumors effectively. Macdonald et al.127 used a dual-functional aptamer combining EpA and TfR. Their EVs successfully passed the blood-brain barrier (and delivered the drug to the brain. Ren et al.128 used aptamer targeting α-synuclein aggregates to target the brain.
Increasing EV encapsulation and delivery efficiency
Increasing circulation time
To decrease phagocytosis by immune cells, CD47, an integrin protein, can be added to the surface of EVs.103 This also improves the stability of the EVs in the circulatory system. CD47 allows the release of a "do not eat me" signal by binding to signal regulatory protein-α on the macrophage surface.129 Polyethylene glycol (PEG) is also known to allow an increased circulation time when added to a particle.130 It was shown that PEGylation decreases nonspecific interactions with cells and increases EV circulation time.131
Autophagy pathway inhibitors
The lysosomal degradation of EVs can decrease the efficiency of EV delivery. In lysosomes, the cargo of EVs might be enzymatically degraded.132 This phenomenon happens mostly through the autophagy pathway.133 To overcome this obstacle, Zhang et al.134 inhibited the autophagy pathway. With this strategy, they improved the drug delivery of EVs in recipient cells. They studied the delivery of plasmid DNA and proteins, and the delivery of the two forms was enhanced by using an inhibitor of the autophagy pathway.134
Conclusion and perspective
Efficient in vivo CRISPR-Cas9 delivery is a significant challenge. Fortunately, there has been a notable advancement in the use of EVs for CRISPR-Cas9 delivery in the past few years. EV-based delivery approaches have several advantages over traditional virus delivery systems. For instance, EVs have greater stability in blood, enabling long distance transmission. EVs are often specific to cells or tissues and can get through a variety of physiological barriers and cellular obstacles. EVs can even be further functionalized to provide improved targeting capability. Given these benefits, EVs are regarded as a powerful method of delivering therapeutic substances in vivo.
However, there remain many technological obstacles to overcome before EV-mediated gene therapies may be used in a clinical setting. Even though EVs can be functionalized to provide targeting capability, the EVs injected by intravenous injection generally go to the liver. Thus, further studies to improve cell and tissue targeting must be completed. In addition, the various modifications that can load cargo into EVs, most of these have not yet been used to load CRISPR-Cas9. Currently, there are no clinical trials using EVs that deliver CRISPR-Cas9 for treatments. To increase the effectiveness of gene editing in vivo, higher efficiency loading methods specific to encapsulating CRISPR-Cas9 system into EVs are required. Solutions to increase EVs uptake, cargo release into the cytoplasm, and endosomal escape must be found to improve gene editing efficacy in a therapeutic context. Together, EVs are promising vectors for delivering the CRISPR-Cas9 system in vivo, but multiple technical hurdles await.
Acknowledgments
The work is supported by grants from the Canadian Institutes of Health Research (CIHR) (OGB-185747) and the Foundation for Cell and Gene Therapy (SIRUL-125794). Y.Y.L. is supported by the China Scholarship Council (CSC) Grant. K.G. and G.L. are both supported by the Fonds de Recherche – Santé (FRQS) and CIHR.
Author contributions
Y.Y.L. and K.G. conceived the manuscript; Y.Y.L., K.G., G.L., and J.P.T. wrote the manuscript; J.P.T. supervised the work.
Declaration of interests
The authors declare that the manuscript was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict.
References
- 1.Gasiunas G., Sinkunas T., Siksnys V. Molecular mechanisms of CRISPR-mediated microbial immunity. Cell. Mol. Life Sci. 2014;71:449–465. doi: 10.1007/s00018-013-1438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang F., Doudna J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017;46:505–529. doi: 10.1146/annurev-biophys-062215-010822. [DOI] [PubMed] [Google Scholar]
- 3.Liu W., Li L., Jiang J., Wu M., Lin P. Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precis. Clin. Med. 2021;4:179–191. doi: 10.1093/pcmedi/pbab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behr M., Zhou J., Xu B., Zhang H. In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges. Acta Pharm. Sin. B. 2021;11:2150–2171. doi: 10.1016/j.apsb.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quesenberry P.J., Aliotta J., Deregibus M.C., Camussi G. Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res. Ther. 2015;6:153. doi: 10.1186/s13287-015-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixson A.C., Dawson T.R., Di Vizio D., Weaver A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023;24:454–476. doi: 10.1038/s41580-023-00576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., He D., Cen H., Chen H., Li L., Nie G., Zhong Z., He Q., Yang X., Guo S., et al. Nucleic acid functionalized extracellular vesicles as promising therapeutic systems for nanomedicine. Extracell Vesicles Circ Nucl Acids. 2022 doi: 10.20517/evcna.2021.21. [DOI] [Google Scholar]
- 8.Jiang Y., Liu X., Ye J., Ma Y., Mao J., Feng D., Wang X. Migrasomes, a new mode of intercellular communication. Cell Commun. Signal. 2023;21:105. doi: 10.1186/s12964-023-01121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.C., Liu L., Ma F., Wong C.W., Guo X.E., Chacko J.V., Farhoodi H.P., Zhang S.X., Zimak J., Ségaliny A., et al. Elucidation of Exosome Migration Across the Blood–Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padmakumar S., D’Souza A., Parayath N.N., Bleier B.S., Amiji M.M. Nucleic acid therapies for CNS diseases: Pathophysiology, targets, barriers, and delivery strategies. J. Contr. Release. 2022;352:121–145. doi: 10.1016/j.jconrel.2022.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Yip B.H. Recent Advances in CRISPR/Cas9 Delivery Strategies. Biomolecules. 2020;10:839. doi: 10.3390/biom10060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller-Carter P.I., Basiri H., Harvey A.R., Carvalho L.S. Focused Update on AAV-Based Gene Therapy Clinical Trials for Inherited Retinal Degeneration. BioDrugs. 2020;34:763–781. doi: 10.1007/s40259-020-00453-8. [DOI] [PubMed] [Google Scholar]
- 13.Crudele J.M., Chamberlain J.S. AAV-based gene therapies for the muscular dystrophies. Hum. Mol. Genet. 2019;28:R102–R107. doi: 10.1093/hmg/ddz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au H.K.E., Isalan M., Mielcarek M. Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Front. Med. 2021;8:809118. doi: 10.3389/fmed.2021.809118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrone L., Marchi P.M., Azzouz M. Circumventing the packaging limit of AAV-mediated gene replacement therapy for neurological disorders. Expet Opin. Biol. Ther. 2022;22:1163–1176. doi: 10.1080/14712598.2022.2012148. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z., Yang H., Colosi P. Effect of Genome Size on AAV Vector Packaging. Mol. Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L., Su J., Long J., Tao R., Tang W., Qin F., Liu N., Wang Y., Jiao Y., Hu Y., et al. A universal strategy for AAV delivery of base editors to correct genetic point mutations in neonatal PKU mice. Mol. Ther. Methods Clin. Dev. 2022;24:230–240. doi: 10.1016/j.omtm.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anzalone A. v, Randolph P.B., Davis J.R., Sousa A.A., Koblan L.W., Levy J.M., Chen P.J., Wilson C., Newby G.A., Raguram A., Liu D.R. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu P., Liang S.-Q., Zheng C., Mintzer E., Zhao Y.G., Ponnienselvan K., Mir A., Sontheimer E.J., Gao G., Flotte T.R., et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. Nat. Commun. 2021;12:2121. doi: 10.1038/s41467-021-22295-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banskota S., Raguram A., Suh S., Du S.W., Davis J.R., Choi E.H., Wang X., Nielsen S.C., Newby G.A., Randolph P.B., et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell. 2022;185:250–265.e16. doi: 10.1016/j.cell.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahlert C., Melo S.A., Protopopov A., Tang J., Seth S., Koch M., Zhang J., Weitz J., Chin L., Futreal A., Kalluri R. Identification of Double-stranded Genomic DNA Spanning All Chromosomes with Mutated KRAS and p53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. J. Biol. Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elzanowska J., Semira C., Costa-Silva B. DNA in extracellular vesicles: biological and clinical aspects. Mol. Oncol. 2021;15:1701–1714. doi: 10.1002/1878-0261.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleh A.F., Lázaro-Ibáñez E., Forsgard M.A.-M., Shatnyeva O., Osteikoetxea X., Karlsson F., Heath N., Ingelsten M., Rose J., Harris J., et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019;11:6990–7001. doi: 10.1039/C8NR08720B. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V., Qin J., Jiang Y., Duncan R.G., Brigham B., Fishman S., Nair J.K., Akinc A., Barros S.A., Kasperkovitz P.V. Shielding of Lipid Nanoparticles for siRNA Delivery: Impact on Physicochemical Properties, Cytokine Induction, and Efficacy. Mol. Ther. Nucleic Acids. 2014;3 doi: 10.1038/mtna.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Y., Iqbal Z., Wang J., Xu L., Xu X., Ouyang K., Zhang H., Lu J., Duan L., Xia J. Cell-derived extracellular vesicles for CRISPR/Cas9 delivery: engineering strategies for cargo packaging and loading. Biomater. Sci. 2022;10:4095–4106. doi: 10.1039/D2BM00480A. [DOI] [PubMed] [Google Scholar]
- 26.Zhu X., Badawi M., Pomeroy S., Sutaria D.S., Xie Z., Baek A., Jiang J., Elgamal O.A., Mo X., Perle K.L., et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles. 2017;6 doi: 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y., Dong S., Li X., Kim B.Y.S., Yang Z., Jiang W. Extracellular Vesicles: An Emerging Nanoplatform for Cancer Therapy. Front. Oncol. 2020;10:606906. doi: 10.3389/fonc.2020.606906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boukouris S., Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteonomics Clin. Appl. 2015;9:358–367. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han C., Yang J., Sun J., Qin G. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol. Ther. 2022;233 doi: 10.1016/j.pharmthera.2021.108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., Zhao W., Peng L., Li Y., Nie S., Yu H., Qin Y., Zhang H. Elevated serum extracellular vesicle arginase 1 in type 2 diabetes mellitus: a cross-sectional study in middle-aged and elderly population. BMC Endocr. Disord. 2022;22:62. doi: 10.1186/s12902-022-00982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S.E. Extracellular vesicles in cancer therapy. Semin. Cancer Biol. 2022;86:296–309. doi: 10.1016/j.semcancer.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roerig J., Mitrach F., Schmid M., Hause G., Hacker M.C., Wölk C., Schulz-Siegmund M. Synergistic siRNA Loading of Extracellular Vesicles Enables Functional Delivery into Cells. Small Methods. 2022;6 doi: 10.1002/smtd.202201001. [DOI] [PubMed] [Google Scholar]
- 33.Jeyaram A., Lamichhane T.N., Wang S., Zou L., Dahal E., Kronstadt S.M., Levy D., Parajuli B., Knudsen D.R., Chao W., Jay S.M. Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles. Mol. Ther. 2020;28:975–985. doi: 10.1016/j.ymthe.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orefice N.S. Development of New Strategies Using Extracellular Vesicles Loaded with Exogenous Nucleic Acid. Pharmaceutics. 2020;12:705. doi: 10.3390/pharmaceutics12080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong J.P.K., Stevens M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018;130:12–16. doi: 10.1016/j.addr.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y.-G., Shi M.M., Monsel A., Dai C.X., Dong X., Shen H., Li S.K., Chang J., Xu C.L., Li P., et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Res. Ther. 2022;13:220. doi: 10.1186/s13287-022-02900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Y., Yu L., Ma T., Xu W., Qian H., Sun Y., Shi H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics. 2022;12:6548–6575. doi: 10.7150/thno.74305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gudbergsson J.M., Johnsen K.B., Skov M.N., Duroux M. Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology. 2016;68:579–592. doi: 10.1007/s10616-015-9913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sil S., Dagur R.S., Liao K., Peeples E.S., Hu G., Periyasamy P., Buch S. Strategies for the use of Extracellular Vesicles for the Delivery of Therapeutics. J. Neuroimmune Pharmacol. 2020;15:422–442. doi: 10.1007/s11481-019-09873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Manrique P., Matos M., Gutiérrez G., Pazos C., Blanco-López M.C. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2017.1422676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busatto S., Iannotta D., Walker S.A., di Marzio L., Wolfram J. A Simple and Quick Method for Loading Proteins in Extracellular Vesicles. Pharmaceuticals. 2021;14:356. doi: 10.3390/ph14040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carnino J.M., Ni K., Jin Y. Post-translational Modification Regulates Formation and Cargo-Loading of Extracellular Vesicles. Front. Immunol. 2020;11:948. doi: 10.3389/fimmu.2020.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou L., Kodidela S., Godse S., Thomas-Gooch S., Kumar A., Raji B., Zhi K., Kochat H., Kumar S. Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles. Pharmaceuticals. 2022;15:358. doi: 10.3390/ph15030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiklander O.P.B., Brennan M.Á., Lötvall J., Breakefield X.O., EL Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu S., Wang Y., Xia X., Zheng J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact. 2020;20 doi: 10.1016/j.impact.2020.100261. [DOI] [Google Scholar]
- 48.Kim S.M., Yang Y., Oh S.J., Hong Y., Seo M., Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Contr. Release. 2017;266:8–16. doi: 10.1016/j.jconrel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 49.McAndrews K.M., Xiao F., Chronopoulos A., LeBleu V.S., Kugeratski F.G., Kalluri R. Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic Kras G12D in pancreatic cancer. Life Sci. Alliance. 2021;4 doi: 10.26508/lsa.202000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. 2018;5 doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Y., Xu X., Xu L., Iqbal Z., Ouyang K., Zhang H., Wen C., Duan L., Xia J. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12:4866–4878. doi: 10.7150/thno.69368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B., Chang M., Zhang R., Wo J., Wu B., Zhang H., Zhou Z., Li Z., Zhang F., Zhong C., et al. Spinal cord injury target-immunotherapy with TNF-α autoregulated and feedback-controlled human umbilical cord mesenchymal stem cell derived exosomes remodelled by CRISPR/Cas9 plasmid. Biomater. Adv. 2022;133 doi: 10.1016/j.msec.2021.112624. [DOI] [PubMed] [Google Scholar]
- 53.Xu Q., Zhang Z., Zhao L., Qin Y., Cai H., Geng Z., Zhu X., Zhang W., Zhang Y., Tan J., et al. Tropism-facilitated delivery of CRISPR/Cas9 system with chimeric antigen receptor-extracellular vesicles against B-cell malignancies. J. Contr. Release. 2020;326:455–467. doi: 10.1016/j.jconrel.2020.07.033. [DOI] [PubMed] [Google Scholar]
- 54.Duan L., Ouyang K., Wang J., Xu L., Xu X., Wen C., Xie Y., Liang Y., Xia J. Exosomes as Targeted Delivery Platform of CRISPR/Cas9 for Therapeutic Genome Editing. Chembiochem. 2021;22:3360–3368. doi: 10.1002/cbic.202100359. [DOI] [PubMed] [Google Scholar]
- 55.Kanada M., Kim B.D., Hardy J.W., Ronald J.A., Bachmann M.H., Bernard M.P., Perez G.I., Zarea A.A., Ge T.J., Withrow A., et al. Microvesicle-Mediated Delivery of Minicircle DNA Results in Effective Gene-Directed Enzyme Prodrug Cancer Therapy. Mol. Cancer Therapeut. 2019;18:2331–2342. doi: 10.1158/1535-7163.MCT-19-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanada M., Bachmann M.H., Hardy J.W., Frimannson D.O., Bronsart L., Wang A., Sylvester M.D., Schmidt T.L., Kaspar R.L., Butte M.J., et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA. 2015;112:E1433–E1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulz-Siegmund M., Aigner A. Nucleic acid delivery with extracellular vesicles. Adv. Drug Deliv. Rev. 2021;173:89–111. doi: 10.1016/j.addr.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Dooley K., McConnell R.E., Xu K., Lewis N.D., Haupt S., Youniss M.R., Martin S., Sia C.L., McCoy C., Moniz R.J., et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. 2021;29:1729–1743. doi: 10.1016/j.ymthe.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim K.M., Abdelmohsen K., Mustapic M., Kapogiannis D., Gorospe M. Vol. 8. Wiley Interdiscip Rev RNA; 2017. p. e1413. (RNA in Extracellular Vesicles). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y., Zhao J., Yu S., Wang Z., He X., Su Y., Guo T., Sheng H., Chen J., Zheng Q., et al. Extracellular Vesicles Long RNA Sequencing Reveals Abundant mRNA, circRNA, and lncRNA in Human Blood as Potential Biomarkers for Cancer Diagnosis. Clin. Chem. 2019;65:798–808. doi: 10.1373/clinchem.2018.301291. [DOI] [PubMed] [Google Scholar]
- 61.Yang Z., Shi J., Xie J., Wang Y., Sun J., Liu T., Zhao Y., Zhao X., Wang X., Ma Y., et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020;4:69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei Z., Batagov A.O., Schinelli S., Wang J., Wang Y., el Fatimy R., Rabinovsky R., Balaj L., Chen C.C., Hochberg F., et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017;8:1145. doi: 10.1038/s41467-017-01196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B., Chan Y.S., Wei L., Chin S.M., Azad A., et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018;9:2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z., Zhou X., Wei M., Gao X., Zhao L., Shi R., Sun W., Duan Y., Yang G., Yuan L. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19–28. doi: 10.1021/acs.nanolett.8b02689. [DOI] [PubMed] [Google Scholar]
- 65.de Jong O.G., Murphy D.E., Mäger I., Willms E., Garcia-Guerra A., Gitz-Francois J.J., Lefferts J., Gupta D., Steenbeek S.C., van Rheenen J., et al. A CRISPR-Cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA. Nat. Commun. 2020;11:1113. doi: 10.1038/s41467-020-14977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Martin R., Wang G., Brandão B.B., Zanotto T.M., Shah S., Kumar Patel S., Schilling B., Kahn C.R. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446–451. doi: 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wozniak A.L., Adams A., King K.E., Dunn W., Christenson L.K., Hung W.-T., Weinman S.A. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J. Cell Biol. 2020;219 doi: 10.1083/jcb.201912074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D.J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dellar E.R., Hill C., Melling G.E., Carter D.R., Baena-Lopez L.A. Unpacking extracellular vesicles: RNA cargo loading and function. J. Extracell. Biol. 2022;1 doi: 10.1002/jex2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolukbasi M.F., Mizrak A., Ozdener G.B., Madlener S., Ströbel T., Erkan E.P., Fan J.-B., Breakefield X.O., Saydam O. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol. Ther. Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cecchin R., Troyer Z., Witwer K., Morris K.v. Extracellular vesicles: The next generation in gene therapy delivery. Mol. Ther. 2023;31:1225–1230. doi: 10.1016/j.ymthe.2023.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Z., Liu Z., Wu J., Li B. Cell-Derived Vesicles for mRNA Delivery. Pharmaceutics. 2022;14:2699. doi: 10.3390/pharmaceutics14122699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foley R.A., Sims R.A., Duggan E.C., Olmedo J.K., Ma R., Jonas S.J. Delivering the CRISPR/Cas9 system for engineering gene therapies: Recent cargo and delivery approaches for clinical translation. Front. Bioeng. Biotechnol. 2022;10:973326. doi: 10.3389/fbioe.2022.973326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zischewski J., Fischer R., Bortesi L. Detection of on-target and off-target mutations generated by CRISPR/Cas9 and other sequence-specific nucleases. Biotechnol. Adv. 2017;35:95–104. doi: 10.1016/j.biotechadv.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Yao X., Lyu P., Yoo K., Yadav M.K., Singh R., Atala A., Lu B. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J. Extracell. Vesicles. 2021;10:e12076. doi: 10.1002/jev2.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osteikoetxea X., Silva A., Lázaro-Ibáñez E., Salmond N., Shatnyeva O., Stein J., Schick J., Wren S., Lindgren J., Firth M., et al. Engineered Cas9 extracellular vesicles as a novel gene editing tool. J. Extracell. Vesicles. 2022;11:e12225. doi: 10.1002/jev2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitley J.A., Kim S., Lou L., Ye C., Alsaidan O.A., Sulejmani E., Cai J., Desrochers E.G., Beharry Z., Rickman C.B., et al. Encapsulating Cas9 into extracellular vesicles by protein myristoylation. J. Extracell. Vesicles. 2022;11:e12196. doi: 10.1002/jev2.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Q., Yu J., Kadungure T., Beyene J., Zhang H., Lu Q. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 2018;9:960. doi: 10.1038/s41467-018-03390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chamberlain L.H., Shipston M.J. The Physiology of Protein S- acylation. Physiol. Rev. 2015;95:341–376. doi: 10.1152/physrev.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathivanan S., Fonseka P., Nedeva C., Atukorala I., editors. New Frontiers: Extracellular Vesicles. Springer International Publishing; 2021. [DOI] [Google Scholar]
- 81.Meyer C., Losacco J., Stickney Z., Li L., Marriott G., Lu B. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int. J. Nanomed. 2017;12:3153–3170. doi: 10.2147/IJN.S133430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Campbell L.A., Coke L.M., Richie C.T., Fortuno L.v., Park A.Y., Harvey B.K. Gesicle-Mediated Delivery of CRISPR/Cas9 Ribonucleoprotein Complex for Inactivating the HIV Provirus. Mol. Ther. 2019;27:151–163. doi: 10.1016/j.ymthe.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Dongen H.M., Masoumi N., Witwer K.W., Pegtel D.M. Extracellular Vesicles Exploit Viral Entry Routes for Cargo Delivery. Microbiol. Mol. Biol. Rev. 2016;80:369–386. doi: 10.1128/MMBR.00063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gee P., Lung M.S.Y., Okuzaki Y., Sasakawa N., Iguchi T., Makita Y., Hozumi H., Miura Y., Yang L.F., Iwasaki M., et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat. Commun. 2020;11:1334. doi: 10.1038/s41467-020-14957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe K., Gee P., Hotta A. Preparation of NanoMEDIC Extracellular Vesicles to Deliver CRISPR-Cas9 Ribonucleoproteins for Genomic Exon Skipping. Methods Mol. Biol. 2023;2587:427–453. doi: 10.1007/978-1-0716-2772-3_22. [DOI] [PubMed] [Google Scholar]
- 86.Ye Y., Shi Q., Yang T., Xie F., Zhang X., Xu B., Fang J., Chen J., Zhang Y., Li J. In Vivo Visualized Tracking of Tumor-Derived Extracellular Vesicles Using CRISPR-Cas9 System. Technol. Cancer Res. Treat. 2022;21 doi: 10.1177/15330338221085370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strohmeier K., Hofmann M., Hauser F., Sivun D., Puthukodan S., Karner A., Sandner G., Le Renard P.-E., Jacak J., Mairhofer M. CRISPR/Cas9 Genome Editing vs. Over-Expression for Fluorescent Extracellular Vesicle-Labeling: A Quantitative Analysis. Int. J. Mol. Sci. 2021;23:282. doi: 10.3390/ijms23010282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Majeau N., Fortin-Archambault A., Gérard C., Rousseau J., Yaméogo P., Tremblay J.P. Serum extracellular vesicles for delivery of CRISPR-CAS9 ribonucleoproteins to modify the dystrophin gene. Mol. Ther. 2022;30:2429–2442. doi: 10.1016/j.ymthe.2022.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen R., Yuan W., Zheng Y., Zhu X., Jin B., Yang T., Yan Y., Xu W., Chen H., Gao J., et al. Delivery of engineered extracellular vesicles with miR-29b editing system for muscle atrophy therapy. J. Nanobiotechnol. 2022;20:304. doi: 10.1186/s12951-022-01508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nasiri Kenari A., Cheng L., Hill A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods. 2020;177:103–113. doi: 10.1016/j.ymeth.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Anand S., Samuel M., Kumar S., Mathivanan S. Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochim. Biophys. Acta, Proteins Proteomics. 2019;1867 doi: 10.1016/j.bbapap.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Vagner T., Chin A., Mariscal J., Bannykh S., Engman D.M., di Vizio D. Protein Composition Reflects Extracellular Vesicle Heterogeneity. Proteomics. 2019;19 doi: 10.1002/pmic.201800167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pathan M., Fonseka P., Chitti S. v, Kang T., Sanwlani R., Van Deun J., Hendrix A., Mathivanan S. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47:D516–D519. doi: 10.1093/nar/gky1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mir B., Goettsch C. Extracellular Vesicles as Delivery Vehicles of Specific Cellular Cargo. Cells. 2020;9:1601. doi: 10.3390/cells9071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meng W., He C., Hao Y., Wang L., Li L., Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wiklander O.P.B., Nordin J.Z., O’Loughlin A., Gustafsson Y., Corso G., Mäger I., Vader P., Lee Y., Sork H., Seow Y., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hercher D., Nguyen M.Q., Dworak H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp. Neurol. 2022;350 doi: 10.1016/j.expneurol.2021.113968. [DOI] [PubMed] [Google Scholar]
- 98.Zhang L., Wei W., Ai X., Kilic E., Hermann D.M., Venkataramani V., Bähr M., Doeppner T.R. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway. Cell Death Dis. 2021;12:1068. doi: 10.1038/s41419-021-04363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qiao L., Hu S., Huang K., Su T., Li Z., Vandergriff A., Cores J., Dinh P.-U., Allen T., Shen D., et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics. 2020;10:3474–3487. doi: 10.7150/thno.39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pitt J.M., André F., Amigorena S., Soria J.-C., Eggermont A., Kroemer G., Zitvogel L. Dendritic cell–derived exosomes for cancer therapy. J. Clin. Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kupiec-Weglinski J.W., Austyn J.M., Morris P.J. Migration patterns of dendritic cells in the mouse. Traffic from the blood, and T cell-dependent and -independent entry to lymphoid tissues. J. Exp. Med. 1988;167:632–645. doi: 10.1084/jem.167.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He C., Jaffar Ali D., Xu H., Kumaravel S., Si K., Li Y., Sun B., Ma J., Xiao Z. Epithelial cell -derived microvesicles: A safe delivery platform of CRISPR/Cas9 conferring synergistic anti-tumor effect with sorafenib. Exp. Cell Res. 2020;392 doi: 10.1016/j.yexcr.2020.112040. [DOI] [PubMed] [Google Scholar]
- 103.Song H., Chen X., Hao Y., Wang J., Xie Q., Wang X. Nanoengineering facilitating the target mission: targeted extracellular vesicles delivery systems design. J. Nanobiotechnol. 2022;20:431. doi: 10.1186/s12951-022-01638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jia G., Han Y., An Y., Ding Y., He C., Wang X., Tang Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi: 10.1016/j.biomaterials.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 105.Smyth T., Petrova K., Payton N.M., Persaud I., Redzic J.S., Graner M.W., Smith-Jones P., Anchordoquy T.J. Surface Functionalization of Exosomes Using Click Chemistry. Bioconjugate Chem. 2014;25:1777–1784. doi: 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 107.Kim G., Kim M., Lee Y., Byun J.W., Hwang D.W., Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Contr. Release. 2020;317:273–281. doi: 10.1016/j.jconrel.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 108.Choi H., Choi K., Kim D.-H., Oh B.-K., Yim H., Jo S., Choi C. Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges. Pharmaceutics. 2022;14:672. doi: 10.3390/pharmaceutics14030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ye Z., Zhang T., He W., Jin H., Liu C., Yang Z., Ren J. Methotrexate-Loaded Extracellular Vesicles Functionalized with Therapeutic and Targeted Peptides for the Treatment of Glioblastoma Multiforme. ACS Appl. Mater. Interfaces. 2018;10:12341–12350. doi: 10.1021/acsami.7b18135. [DOI] [PubMed] [Google Scholar]
- 110.Gao X., Ran N., Dong X., Zuo B., Yang R., Zhou Q., Moulton H.M., Seow Y., Yin H. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aat0195. [DOI] [PubMed] [Google Scholar]
- 111.Seow Y., Yin H., Wood M.J.A. Identification of a novel muscle targeting peptide in mdx mice. Peptides. 2010;31:1873–1877. doi: 10.1016/j.peptides.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 112.Zhu L.-P., Tian T., Wang J.-Y., He J.-N., Chen T., Pan M., Xu L., Zhang H.X., Qiu X.-T., Li C.-C., et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163–6177. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vandergriff A., Huang K., Shen D., Hu S., Hensley M.T., Caranasos T.G., Qian L., Cheng K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8:1869–1878. doi: 10.7150/thno.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mentkowski K.I., Lang J.K. Exosomes Engineered to Express a Cardiomyocyte Binding Peptide Demonstrate Improved Cardiac Retention in Vivo. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-46407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bellavia D., Raimondo S., Calabrese G., Forte S., Cristaldi M., Patinella A., Memeo L., Manno M., Raccosta S., Diana P., et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017;7:1333–1345. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zahid M., Feldman K.S., Garcia-Borrero G., Feinstein T.N., Pogodzinski N., Xu X., Yurko R., Czachowski M., Wu Y.L., Mason N.S., Lo C.W. Cardiac Targeting Peptide, a Novel Cardiac Vector: Studies in Bio-Distribution, Imaging Application, and Mechanism of Transduction. Biomolecules. 2018;8:147. doi: 10.3390/biom8040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nam H.Y., McGinn A., Kim P.-H., Kim S.W., Bull D.A. Primary cardiomyocyte-targeted bioreducible polymer for efficient gene delivery to the myocardium. Biomaterials. 2010;31:8081–8087. doi: 10.1016/j.biomaterials.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nazarenko I., Rana S., Baumann A., McAlear J., Hellwig A., Trendelenburg M., Lochnit G., Preissner K.T., Zöller M. Cell Surface Tetraspanin Tspan8 Contributes to Molecular Pathways of Exosome-Induced Endothelial Cell Activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 119.Rana S., Yue S., Stadel D., Zöller M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 120.Ohno S.i., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zuo B., Zhang Y., Zhao K., Wu L., Qi H., Yang R., Gao X., Geng M., Wu Y., Jing R., et al. Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J. Hematol. Oncol. 2022;15:46. doi: 10.1186/s13045-022-01266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berenguer J., Lagerweij T., Zhao X.W., Dusoswa S., van der Stoop P., Westerman B., de Gooijer M.C., Zoetemelk M., Zomer A., Crommentuijn M.H.W., et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1446660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koning N., Kessen S.F.M., van der Voorn J.P., Appelmelk B.J., Jeurink P.v., Knippels L.M.J., Garssen J., van Kooyk Y. Human Milk Blocks DC-SIGN–Pathogen Interaction via MUC1. Front. Immunol. 2015;6:112. doi: 10.3389/fimmu.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheng Q., Shi X., Han M., Smbatyan G., Lenz H.-J., Zhang Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018;140:16413–16417. doi: 10.1021/jacs.8b10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Han Q., Xie Q.R., Li F., Cheng Y., Wu T., Zhang Y., Lu X., Wong A.S.T., Sha J., Xia W. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics. 2021;11:6526–6541. doi: 10.7150/thno.53886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiang D., Zheng C., Zhou S.-F., Qiao S., Tran P.H.-L., Pu C., Li Y., Kong L., Kouzani A.Z., Lin J., et al. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics. 2015;5:1083–1097. doi: 10.7150/thno.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Macdonald J., Henri J., Goodman L., Xiang D., Duan W., Shigdar S. Development of a Bifunctional Aptamer Targeting the Transferrin Receptor and Epithelial Cell Adhesion Molecule (EpCAM) for the Treatment of Brain Cancer Metastases. ACS Chem. Neurosci. 2017;8:777–784. doi: 10.1021/acschemneuro.6b00369. [DOI] [PubMed] [Google Scholar]
- 128.Ren X., Zhao Y., Xue F., Zheng Y., Huang H., Wang W., Chang Y., Yang H., Zhang J. Exosomal DNA Aptamer Targeting α-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Mol. Ther. Nucleic Acids. 2019;17:726–740. doi: 10.1016/j.omtn.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koh E., Lee E.J., Nam G.-H., Hong Y., Cho E., Yang Y., Kim I.-S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 130.Murphy D.E., de Jong O.G., Brouwer M., Wood M.J., Lavieu G., Schiffelers R.M., Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019;51:1–12. doi: 10.1038/s12276-019-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kooijmans S.A.A., Fliervoet L.A.L., van der Meel R., Fens M.H.A.M., Heijnen H.F.G., van Bergen en Henegouwen P.M.P., Vader P., Schiffelers R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Contr. Release. 2016;224:77–85. doi: 10.1016/j.jconrel.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 132.Minakaki G., Menges S., Kittel A., Emmanouilidou E., Schaeffner I., Barkovits K., Bergmann A., Rockenstein E., Adame A., Marxreiter F., et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy. 2018;14:98–119. doi: 10.1080/15548627.2017.1395992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sahay G., Querbes W., Alabi C., Eltoukhy A., Sarkar S., Zurenko C., Karagiannis E., Love K., Chen D., Zoncu R., et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 2013;31:653–658. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y., Huang M., Jiang L., Li T., Wang J., Zhao L., Zhou J. Autophagy inhibitors enhance biomolecular delivery efficiency of extracellular vesicles. Biochem. Biophys. Res. Commun. 2022;603:130–137. doi: 10.1016/j.bbrc.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 135.Zhang X., Xu Q., Zi Z., Liu Z., Wan C., Crisman L., Shen J., Liu X. Programmable Extracellular Vesicles for Macromolecule Delivery and Genome Modifications. Dev. Cell. 2020;55:784–801.e9. doi: 10.1016/j.devcel.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ye Y., Zhang X., Xie F., Xu B., Xie P., Yang T., Shi Q., Zhang C.-Y., Zhang Y., Chen J., et al. An engineered exosome for delivering sgRNA:Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomater. Sci. 2020;8:2966–2976. doi: 10.1039/D0BM00427H. [DOI] [PubMed] [Google Scholar]
- 137.Wan T., Zhong J., Pan Q., Zhou T., Ping Y., Liu X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci. Adv. 2022;8:eabp9435. doi: 10.1126/sciadv.abp9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hazrati A., Malekpour K., Soudi S., Hashemi S.M. CRISPR/Cas9-engineered mesenchymal stromal/stem cells and their extracellular vesicles: A new approach to overcoming cell therapy limitations. Biomed. Pharmacother. 2022;156 doi: 10.1016/j.biopha.2022.113943. [DOI] [PubMed] [Google Scholar]
- 139.Zhuang J., Tan J., Wu C., Zhang J., Liu T., Fan C., Li J., Zhang Y. Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy. Nucleic Acids Res. 2020;48:8870–8882. doi: 10.1093/nar/gkaa683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nance M.E., Duan D. Perspective on Adeno-Associated Virus Capsid Modification for Duchenne Muscular Dystrophy Gene Therapy. Hum. Gene Ther. 2015;26:786–800. doi: 10.1089/hum.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao X., Zhao J., Han G., Zhang Y., Dong X., Cao L., Wang Q., Moulton H.M., Yin H. Effective Dystrophin Restoration by a Novel Muscle-Homing Peptide–Morpholino Conjugate in Dystrophin-Deficient mdx Mice. Mol. Ther. 2014;22:1333–1341. doi: 10.1038/mt.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]