Abstract
Heat shock factor 1 (HSF1) is a cellular stress-protective transcription factor exploited by a wide range of cancers to drive proliferation, survival, invasion, and metastasis. Nuclear HSF1 abundance is prognostic for cancer severity, therapy-resistance, and shortened patient survival. Here we demonstrated that the HSF1 gene was amplified, and nuclear HSF1 abundance was dramatically elevated in prostate cancers and particularly in neuroendocrine prostate cancer (NEPC) patients, who have no available treatment options. Despite genetic validation of HSF1 as a therapeutic target in a range of cancers, a direct and selective small-molecule HSF1 inhibitor has not been validated or developed for use in the clinic. We presented the identification of a direct HSF1 inhibitor, Direct Targeted HSF1 InhiBitor (DTHIB), which physically engages HSF1 and selectively stimulates nuclear HSF1 degradation. DTHIB robustly inhibited the HSF1 cancer gene signature and prostate cancer cell proliferation. DTHIB potently attenuated tumor progression in four therapy-resistant prostate cancer animal models, including the profound potentiation of tumor regression in a NEPC model. This study reports the identification and validation of a direct HSF1 inhibitor and provides a path forward for the development of a small molecule HSF1-targeted therapy for prostate cancers and other therapy-resistant cancers.
One Sentence Summary:
A direct small molecule inhibitor of HSF1 causes profound tumor regression in animal models of therapy-resistant prostate cancer.
Introduction
Cancer cells are chronically exposed to stressful conditions as they proliferate, invade, metastasize and colonize distinct tissues and compartments (1). The malignant phenotype is associated with numerous stresses including the need to cope with elevated demand for protein expression, the accumulation of metastable mutated proteins, and the support of conformationally labile oncogenic signaling proteins such as receptors, protein kinases and transcription factors(1, 2).
In human cancers, heat shock factor 1 (HSF1) is a central stress-protective transcription factor driving a unique oncogenic transcription program known as the HSF1 cancer gene signature (HSF1 CaSig), in which HSF1 activates expression of genes encoding protein folding chaperones, DNA replication and repair factors, cell cycle drivers, metabolic enzymes and pro-survival proteins, while repressing genes that promote immune surveillance, inflammation, cell adhesion and cell death (1–5). In addition, HSF1 plays critical supportive roles in stroma and in the tumor microenvironment (1–3). Although HSF1 is dispensable for overall growth and development in mammals, pioneering studies demonstrate that a wide range of cancers exhibit a “non-oncogene addiction” to HSF1 (5–11). Indeed, elevated HSF1 expression and activity are associated with disease severity, therapy-resistance and shortened disease-free survival in cancer patients (2, 7, 12).
In normal cells HSF1 is found as an inactive monomer in the cytoplasm that oligomerizes and accumulates in the nucleus in response to acute stress. However, in the chronic proteotoxic and broadly stressful environment of cancer cells, HSF1 is highly expressed, constitutively activated, oligomerized and localized to the nucleus via diverse mechanisms (3, 8–10, 13). The constitutive activation of HSF1 in cancer, and its prognostic value, has prompted genetic experiments to validate the role of HSF1 in the initiation and progression of cancer (1, 2, 6). HSF1 knockout mice are protected from cancer caused by mutations in RAS, inactivation of the P53 tumor suppressor and to HER2-induced breast cancer (6, 14–16). Genetic HSF1 knockout or knockdown studies have shown strong efficacy in a broad spectrum of animal cancer models (2, 9, 10, 15).
Prostate cancer development and progression is strongly driven by androgen receptor (AR) signaling, which activates the expression of genes that underlie prostate cancer biology (17, 18). AR is highly dependent on the HSF1-activated multi-chaperone complex composed of HSP90, 70, 40 and other proteins for AR stability, androgen hormone binding, nuclear translocation, dimerization and target gene DNA binding (17, 19). While AR antagonists and androgen deprivation are well-established therapies for prostate cancer, virtually all patients inevitably become therapy-resistant and progress to castration-resistant prostate cancer (CRPC) or neuroendocrine prostate cancer (NEPC) (18). The traditional AR-targeted therapeutic approach has significant limitations due to multiple mechanisms of drug resistance including AR gene amplification (17), the emergence of drug-resistant AR mutations (18), the expression of constitutively active AR splice variants that lack the ligand binding domain (19, 20), and the development of AR-negative NEPC (21, 22). Hence, alternative, AR-dependent and -independent therapies are urgently needed for patients with therapy-resistant prostate cancer.
Despite compelling genetic data demonstrating the strict in vivo requirement for HSF1 in a broad spectrum of cancers, a direct and selective HSF1 inhibitor has not been comprehensively validated. While “HSF1 pathway” inhibitors have been identified and evaluated in cellular and mouse xenograft cancer models, they either act in an indirect manner in the HSF1 pathway or have an unknown mechanism of action (MoA) (2, 11). Here we describe the identification and characterization of a Direct, Targeted, HSF1 InhiBitor (DTHIB), by screening for physical interactions between the structurally well-ordered HSF1 DNA-binding domain (DBD) and small molecules. DTHIB selectively engaged with HSF1 and inhibited core HSF1 functions by accelerating the rate of nuclear HSF1 degradation. By exploiting the dependence of AR and AR-v7 on the multi-chaperone machine, DTHIB suppressed AR/AR-v7 signaling pathways. Moreover, DTHIB also acted independently of AR and potently attenuated multiple prostate cancer progression in mouse models, including highly aggressive NEPC. Taken together, these results identified a direct HSF1 inhibitor for further development for therapy-resistant prostate cancer and other cancers.
Results
Differential Scanning Fluorimetry Identifies Direct HSF1 Inhibitors
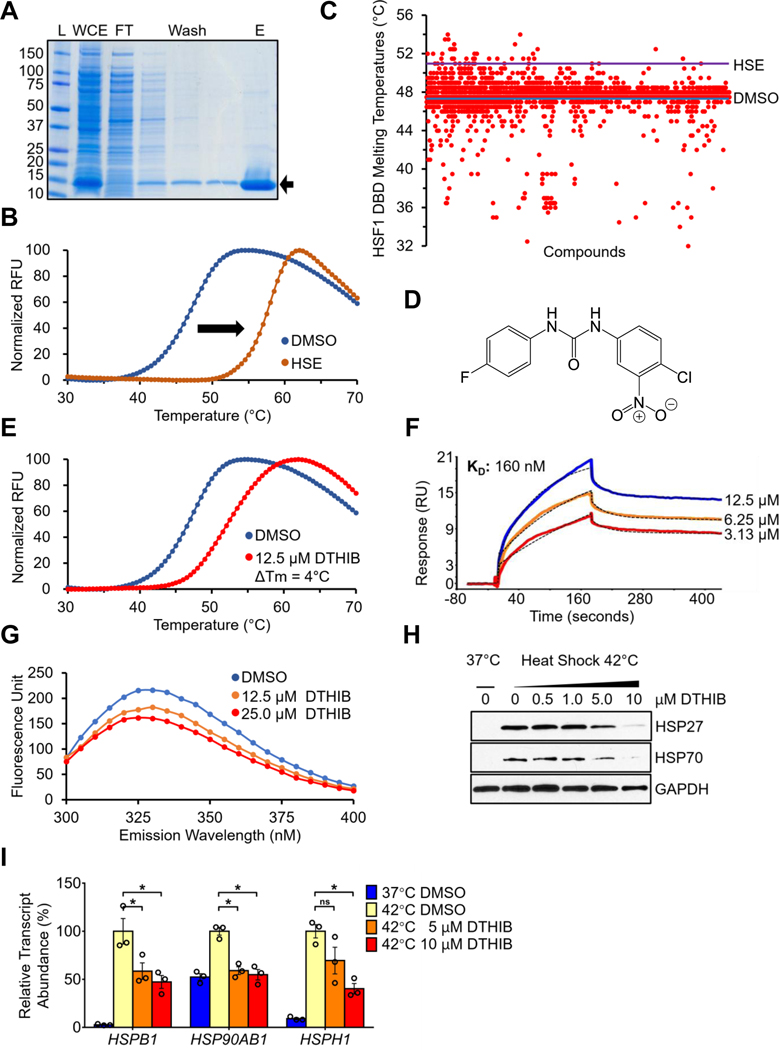
While genetic approaches have underscored the requirement for HSF1 in a variety of cancer models, current small molecule HSF1 inhibitors suffer from a lack of evidence for a direct, high affinity interaction with HSF1. Instead, most existing HSF1 inhibitors function indirectly through protein kinases, the general transcription/translation machinery, or via other proteins of unclear mechanistic relationship to HSF1 function (1–3, 11). To identify small molecules that directly target human HSF1, an in vitro screening platform was established based on differential scanning fluorimetry (DSF) (23, 24). DSF reveals direct protein-ligand interactions by monitoring changes in the melting temperature of the target protein as a function of ligand binding. Direct protein-ligand interactions could either increase or decrease the thermal stability of the target, leading to a change in the observed melting temperature (Tm) (23). We previously demonstrated that DSF serves as a robust assay to evaluate the binding of recombinant full-length HSF1, or the HSF1 DBD to its cognate DNA-binding site, the heat shock element (HSE) (24).
Recombinant human 6xHis-tagged HSF1 DBD was expressed in E. coli and purified via Ni-NTA enrichment (Fig. 1A). The purified HSF1 DBD bound its DNA ligand HSE in vitro, as shown by DSF, indicating that it was properly folded and functional (Fig. 1B). A collection of structurally diverse small molecules was screened against the HSF1 DBD by DSF. To monitor the quality and inter-plate variability of HSF1 DBD-small molecule interactions, DMSO and the HSE oligonucleotide were included as negative and positive controls respectively. While 98.87% of the ~5,500 screened compounds were negative for HSF1 DBD binding, positive compounds either increased or decreased the thermal stability of the HSF1 DBD relative to DMSO (Fig. 1C). All positive compounds were subjected to secondary DSF validation screens to identify those molecules positive for HSF1 DBD binding but negative for binding to the HSC70 chaperone, as a preliminary measure of non-specific interactions (fig. S1A).
Fig. 1.
In vitro ligand binding screen identifies a direct small-molecule HSF1 inhibitor. (A) Coomassie stained SDS-PAGE protein gel showing the purification of recombinant human HSF1 DBD. L, molecular weight ladder; WCE, whole-cell extract; FT, flow-through faction; Wash, washing fractions; E, elution fraction. (B) DSF melting curves demonstrating the shift in thermal stability of the HSF1 DBD upon interaction with the HSE (n = 3). (C) A scatter plot summary of the primary DSF screen results. Each red dot represents the observed Tm of the HSF1 DBD in the presence of a screened compound. Blue line, Tm of HSF1 DBD with DMSO solvent. Purple line, Tm of HSF1 DBD with HSE DNA ligand. (D) The chemical structure of compound DTHIB. (E) DTHIB physically binds to the HSF1 DBD and increased its melting temperature by 4˚C (n = 3). (F) SPR sensorgram of DTHIB binding to a surface coated with HSF1 DBD, with DTHIB concentrations and calculated Kd for binding shown. (G) HSF1 DBD fluorescence emission spectra obtained from the TFQ assay in response to increased concentrations of DTHIB (n = 3). (H) Immunoblot showing DTHIB dose-dependently inhibited heat shock-induced HSP25 and HSP70 expression in MEFs. (I) qRT-PCR data showing impact of DTHIB on indicated transcript levels in response to heat shock (n = 3).
Two structurally similar molecules, I06 and P06, were identified in the screen that selectively interacted with HSF1 DBD (fig. S1, B and C). As these two compounds were not available from commercial sources at high purity, a structurally similar derivative, denoted DTHIB, was synthesized and purified in-house (Fig. 1D and fig. S1, D, E, and F). As demonstrated with the DSF assay, DTHIB strongly interacted with the HSF1 DBD (Fig. 1E) and was further investigated to characterize its binding properties and potential impact on HSF1 function. Based on chemical structures, DTHIB, together with primary screen hits I06 and P06, represented a class of small molecules that has not been reported as interacting with HSF1 (2).
To further assess the specificity of DTHIB, DSF assays were perform using DTHIB against purified the RPA1 protein single stranded DNA binding domain or the HSC70 chaperone. DTHIB exhibited no sign of nonspecific interaction with either proteiin (fig. S2A). A structurally related molecule, A01, was synthesized, which contained the basic 1,3-diphenylurea scaffold. A01 did not interact with the HSF1 DBD and was included as a negative control for downstream analyses (fig. S2, B, C and D). Surface plasmon resonance (SPR) was used to independently and quantitatively assess DTHIB binding to the HSF1 DBD, demonstrating a Kd for DTHIB binding to the HSF1 DBD of 160 nM (Fig. 1F). Elucidated in the crystal structure (25), the HSF1 DBD are two solvent-exposed tryptophan residues in proximity that could enable the detection HSF1 DBD interactions based on intrinsic tryptophan fluorescence quenching (TFQ), an approach commonly used to monitor ligand binding (26). In the presence of solvent alone, the HSF1 DBD emitted an intrinsic fluorescence peak at λ = 330 nM after excitation at 280 nm. Titration of DTHIB into the in vitro reaction demonstrated dose-dependent quenching of the intrinsic tryptophan fluorescence of the HSF1 DBD (Fig. 1G), while DTHIB had no effect on tryptophan fluorescence for RPA1 ssDBD or HSC70 (fig. S2E). Moreover, consistent with DTHIB binding to the HSF1 DBD within the context of the full-length protein, incubation of DTHIB with full-length recombinant HSF1 conferred an altered limited-proteolytic pattern for HSF1, detected by silver staining (fig. S3, A and B). Several polypeptide species with increased abundance upon addition of DTHIB were excised and analyzed by mass-spectrometry (fig. S3B and table S1). Among the altered species, a 15~20kDa band was found to encompass the HSF1 DBD (amino acid residues 63 to 117) (fig. S3, C and D).
Unlike cell-based screens, the DSF screen is agnostic to in vivo activity. To ascertain the potential impact of DTHIB on HSF1, DTHIB was first evaluated in mouse embryonic fibroblasts (MEF) under acute heat shock conditions. In MEF, DTHIB attenuated the robust acute heat-shock induction of the HSP70 and HSP25 molecular chaperones in a dose-dependent manner. (Fig. 1H). Moreover, qRT-PCR results demonstrated that DTHIB attenuated the heat shock response (HSR) by reducing the steady state transcript abundance of multiple molecule chaperones (Fig. 1I). The structurally-related derivative, A01, which did not bind the HSF1 DBD, had no detectable impact on heat shock induction of the HSP70 or HSP25 proteins (fig. S4A).
DTHIB is a Direct HSF1 Inhibitor and targets the HSF1 CaSig
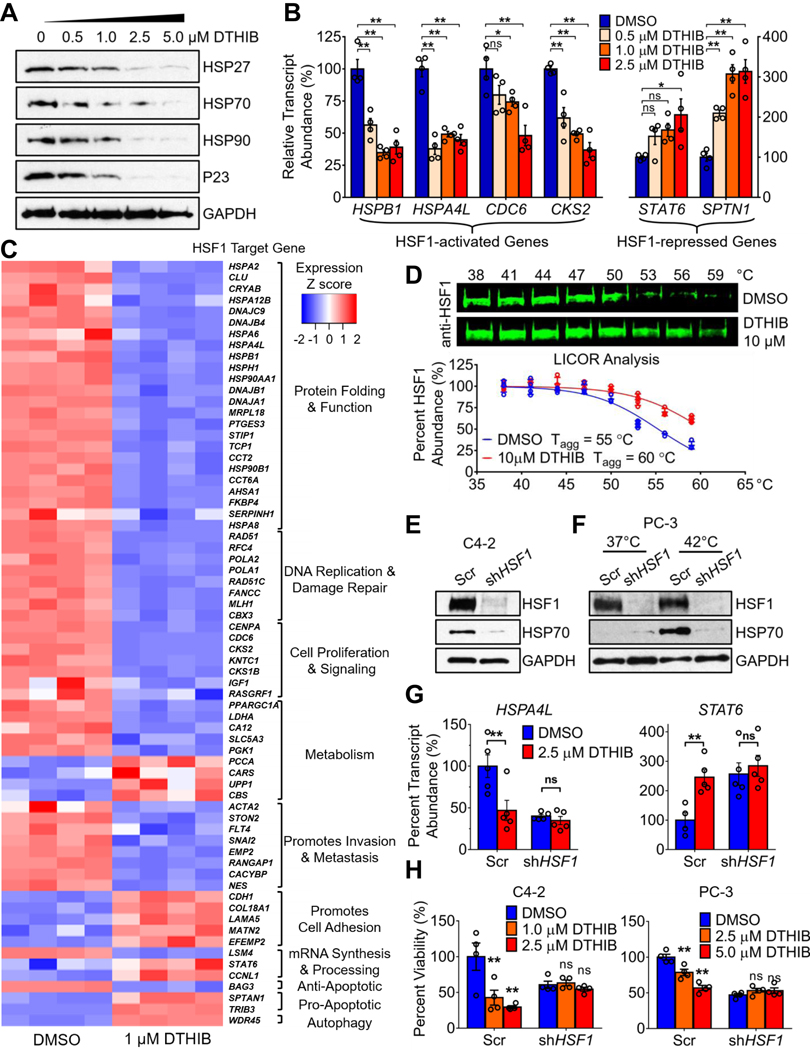
A wide variety of cancer cell lines and tissues exhibit chronic HSF1 activation and strongly elevated abundance of protein chaperones and other HSF1-activated genes (1–5, 12). To investigate the impact of DTHIB in cells with constitutive HSF1 activity, the human CRPC cell line C4–2 was used (27). In C4–2 cells, DTHIB reduced steady-state protein abundance of the molecular chaperones P23, HSP27, HSP70, and HSP90, all bona fide HSF1 targets (fig. 2A and quantitated in Fig. S4B) (3–5). Moreover, analysis of transcript abundance demonstrated that DTHIB repressed the expression of genes that are directly bound and activated by HSF1, and de-repressed expression of genes directly bound and repressed by HSF1 (Fig. 2B) (3–5). This latter observation suggested that DTHIB did not nonspecifically inhibit the transcription machinery at the concentrations used, since cells mounted a positive transcription response. In the PC-3 AR-negative human prostate cancer cell line (28), DTHIB also inhibited HSF1 (fig. S4C), suggesting that the activity of DTHIB was independent of AR status.
Fig. 2.
HSF1 is highly expressed in Human Prostate Cancer and is Inhibited by DTHIB. (A) DTHIB dose-dependently inhibited expression of molecular chaperones in C4–2 PCa cells after 48-hour treatment with the indicated concentrations of DTHIB. (B) Changes in HSF1 target gene transcript abundance after 48-hour DTHIB treatment with the indicated concentrations in C4–2 cells (n = 4). Relative abundance of transcripts was first normalized internally to GAPDH and then compared to the DMSO control. (C) RNA-Seq analysis of C4–2 cells after 48-hour treatment with 1 μM DTHIB or DMSO solvent (n= 4). Shown is a heat map for transcripts from direct HSF1 target genes grouped in functional categories. (D) HSF1 immunoblots and LICOR quantitation (n = 4) from CETSA with C4–2 lysates treated with DMSO or DTHIB at 10 μM. Shown are the temperatures used for lysate treatment (E) Generation of Scrambled shRNA (Scr) and shHSF1 cell lines from C4–2 and PC-3 cells. For C4–2-derived cell lines, basal abundance of HSF1 and HSP70 were assessed by immunoblotting. (F) For PC-3-derived cell lines, both basal (37˚C) and heat shock-induced HSF1 and HSP70 abundance were assessed by immunoblotting. (G) C4–2 Scr and shHSF1 cells exposed to 36-hour treatment with 2.5 μM DTHIB were evaluated for HSF1 target gene expression by qRT-PCR (n = 5). HSPA4L is a target gene activated by HSF1 and STAT6 is a target gene repressed by HSF1. (H) Cell viability readouts of Scr and shHSF1 cell lines derived from C4–2 and PC-3 cells after 72-hour treatment with the indicated concentration of DTHIB (n = 4).
The impact of DTHIB administration in C4–2 cells was further validated by RNAseq experiments, cross-referenced to HSF1-ChIPseq databases, to investigate the impact of DTHIB on the broader landscape of the HSF1 CaSig (Fig. 2C, fig. S4D, and table S2) (5, 29, 30). These studies demonstrated that DTHIB treatment broadly inhibited the HSF1 CaSig and HSF1-mediated transcriptional network (Fig. 2C). The observation that DTHIB administration de-repressed multiple HSF1-repressed genes further supported the notion that DTHIB was not acting as an inhibitor of the core transcription machinery.
In addition to the direct binding of DTHIB to the HSF1 DBD demonstrated by multiple biochemical assays in vitro, a cellular thermal shift assay (CETSA) in C4–2 whole cell lysate was perfomed. These experiments demonstrated that DTHIB increased the aggregation temperature (Tagg) of HSF1 by 5˚C (Fig. 2D), validating the engagement of DTHIB with full-length HSF1 in cell extracts (31). In contrast, DTHIB elicited no impact on the Tagg of another nuclear transcription factor, SP1 (fig. S4E).
The Activity of DTHIB is HSF1 Dependent
As the HSF1 inhibitor DTHIB was identified from an in vitro screen targeting recombinant human HSF1 DBD, it is critical to ascertain if the activity of DTHIB in cell culture is HSF1-dependent. To address this question scrambled shRNA (Scr) control and stable HSF1 knockdown (shHSF1) cell lines were generated from C4–2 and PC-3 cells, representing both AR positive and the AR negative prostate cancer models, respectively (Fig. 2, E and F). The genetic depletion of HSF1 led to loss of HSP70 basal expression in C4–2 cells and diminished the heat-shock response in PC-3 cells (Fig. 2, E and F).
The analysis of HSF1 target gene expression via qRT-PCR demonstrated that, in contrast to the C4–2 Scr line, the C4–2 shHSF1 cell line was not responsive to DTHIB for both the HSF1-activated gene HSPA4L and HSF1-repressed gene STAT6. (Fig. 2G). A similar result was observed in the isogenic pair of Scr and shHSF1 PC-3 cell lines when the HSF1 targets HSPA4L and HSP90AB1 were evaluated (fig. S4F). In cell viability assays, both the C4–2 and PC-3 shHSF1 cell lines showed reduced viability compared to control cells that was not further inhibited by DTHIB treatment (Fig. 2H). To further evaluate HSF1 dependency for DTHIB action in a distinct background, primary MEF isolated from Hsf1+/+, Nf1−/− or Hsf1−/−, Nf1−/− mice were used. The neurofibromatosis type 1 (NF1) tumor suppressor protein represses HSF1 activity and loss of NF1 leads to HSF1 activation and over-expression of several HSF1 target genes (8). In comparison to Hsf1+/+, Nf1−/− MEFs, the Hsf1−/−, Nf1−/− double knockout MEFs were resistant to DTHIB (fig. S4G).
DTHIB Mechanism of Action for HSF1 Inhibition
DTHIB is a direct HSF1 inhibitor that physically engaged with the HSF1 DNA-binding domain with nanomolar affinity in vitro. In cell culture, DTHIB inhibited both the acute heat shock response and the activation or repression of HSF1 CaSig targets.
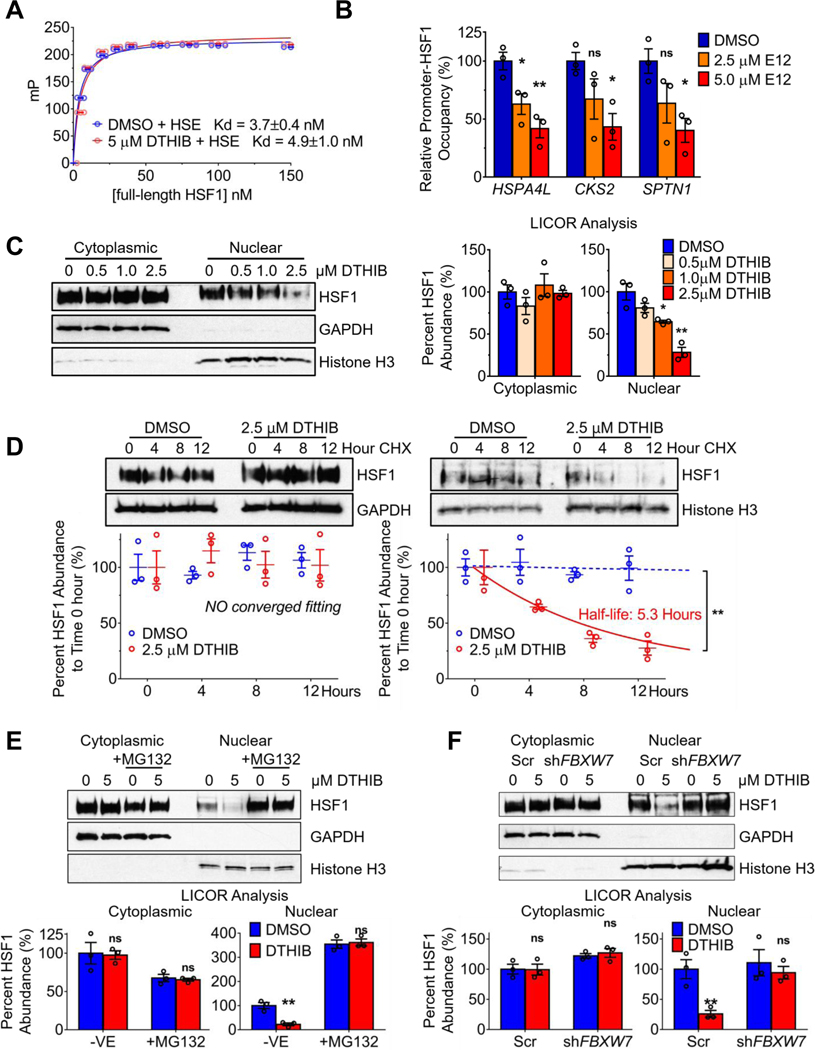
To evaluate whether DTHIB directly impacts HSF1 DNA binding in vitro, a fluorescence anisotropy (FA) assay was used to quantitate the DNA-binding affinity of recombinant HSF1 DBD, or full-length HSF1 homo-trimeric protein (24). Shown by FA, addition of DTHIB had no detectable impact on the in vitro DNA-binding affinity of either HSF1 DBD or full-length HSF1 homotrimer (Fig. 3A and fig. S5, A and B). To determine whether DTHIB impacted HSF1 DNA binding in cells, chromatin immunoprecipitation (ChIP)-qPCR was used to measure HSF1 occupancy at both activated and repressed loci in the absence or presence of DTHIB. Unexpectedly, DTHIB reduced promoter occupancy at both HSF1-activated and HSF1-repressed target genes (Fig. 3B).
Fig. 3.
DTHIB mechanism of action for HSF1 inhibition. (A) FA titration curves showing the impact of DTHIB on the binding of full-length oligomeric HSF1 to a 6-FAM conjugated HSE oligonucleotide in vitro (n = 3) Shown are millipolarization units (Y axis) and the concentration of HSF1 (nM) on the X-axis. (B) ChIP-qPCR data from experiments quantitatively assessing promoter-HSF1 occupancy in C4–2 cells after 48-hour DTHIB treatment at the indicated concentrations (n = 3). HSPA4L and CKS2 are HSF1-activated genes while SPTN1 is an HSF1-repressed gene. The relative promoter-HSF1 occupancy for each gene was normalized to the DMSO control. (C) Immunoblots and LICOR quantitation from subcellular fractionation experiments to assess cytosolic and nuclear HSF1 abundance from C4–2 cells treated with DTHIB for 48hr at the indicated concentrations (n = 3). (D) Immunoblots and LICOR quantitation from cycloheximide (CHX) pause-chase and subcellular fractionation experiments with C4–2 cells to assess cytosolic and nuclear HSF1 half-life in response to 2.5 μM DTHIB. (E) C4–2 cells were treated without and with proteasome inhibitor MG132, in the absence or presence of the indicated concentration of DTHIB for 48 hours and nuclear and cytosolic fractions isolated. Proteins of interest were analyzed by immunoblotting and quantitated with LICOR (n = 3). (F) After 48-hour treatment with 5 μM DTHIB, Scr and shFBXW7 C4–2 cell lines were analyzed for DTHIB-induced nuclear HSF1 degradation after subcellular fractionation. Proteins of interest were analyzed by immunoblotting and quantitated with LICOR (n = 3).
To understand the seemingly disparate in vitro and in vivo HSF1 DNA binding results, subcellular fractionation experiments were performed to separate and quantitate the abundance of cytosolic and nuclear HSF1. DTHIB reduced nuclear HSF1 steady-state protein abundance in a dose-dependent manner, while cytosolic HSF1 was unaffected (Fig. 3C). Similar results were observed in AR-negative PC-3 cells (fig. S5C). To evaluate whether the reduction in nuclear HSF1 abundance was due to a change in HSF1 stability, nuclear HSF1 abundance in C4–2 cells was quantitated over time after treatment with cycloheximide to arrest new protein synthesis, in the absence or presence of DTHIB. While there was no change in nuclear HSF1 stability in solvent treated cells over 12 hours, DTHIB destabilized nuclear HSF1, resulting in a shortened HSF1 half-life of ~5 hours (Fig. 3D). In contrast, the stability of another nuclear transcription factor, SP1, was not impacted by DTHIB (fig. S5D). Moreover, the inactive analogue A01, had no impact on either cytosolic or nuclear HSF1 stability (fig. S5E).
For both cancer and neurodegenerative diseases, nuclear HSF1 degradation is proteasome-dependent and mediated by the FBXW7, the F-box component of SCF E3 ligase complex, or the NEDD4 E3 ligase (3). To ascertain whether DTHIB-induced degradation of nuclear HSF1 was proteasome-dependent, C4–2 cells were treated with the proteasome inhibitor MG132 and the abundance of cytosolic and nuclear HSF1 assessed in the absence or presence of DTHIB. While cytosolic HSF1 abundance was unchanged comparing solvent to DTHIB treatment, the loss of nuclear HSF1 in response to DTHIB was ablated when the proteasome was inhibited (Fig. 3E). Additionally, co-treatment with DTHIB and MG132 led to the accumulation of poly-ubiquitinated HSF1 species, in comparison to the MG132 alone condition (fig. S5F). Stable FBXW7 or NEDD4 knockdown alleles were generated in C4–2 cells using shRNA (fig. S5G). When FBXW7 expression was knocked down, DTHIB was unable to accelerate nuclear HSF1 degradation (Fig. 3F), while NEDD4 knockdown had no impact on DTHIB activity. (fig. S5H). FBXW7 has a number of physiological targets including C-MYC (9). To investigate whether DTHIB perturbs the normal function of FBXW7, the steady-state abundance of nuclear C-MYC was evaluated and demonstrated that DTHIB had no impact on nuclear C-MYC abundance (fig. S5I). Taken together, these studies demonstrated that DTHIB inhibited HSF1 by preferentially stimulating nuclear HSF1 degradation via a proteasome- and FBXW7-dependent pathway, while sparing other targets of this degradation pathway. The loss of nuclear HSF1 led to depletion of promoter bound HSF1 and to inhibition of the HSF1 CaSig.
Targeting HSF1 in Therapy-resistant prostate cancer
Since the initial discovery of the critical roles HSF1 plays in regulating gene expression to support the malignant phenotype, this role has been validated in many cellular and animal cancer models. Accordingly, the high abundance of HSF1 in cancer cells and tissues, and particularly high nuclear HSF1 abundance, strongly correlate with poor clinical prognosis in a broad spectrum of human cancers (2, 7, 12). In prostate cancer, elevated nuclear HSF1 abundance significantly associated with higher Gleason grades (p = 0.014) and metastatic lymph node status (p = 0.017), suggesting HSF1 as a critical factor for prostate cancer progression and metastasis (12).
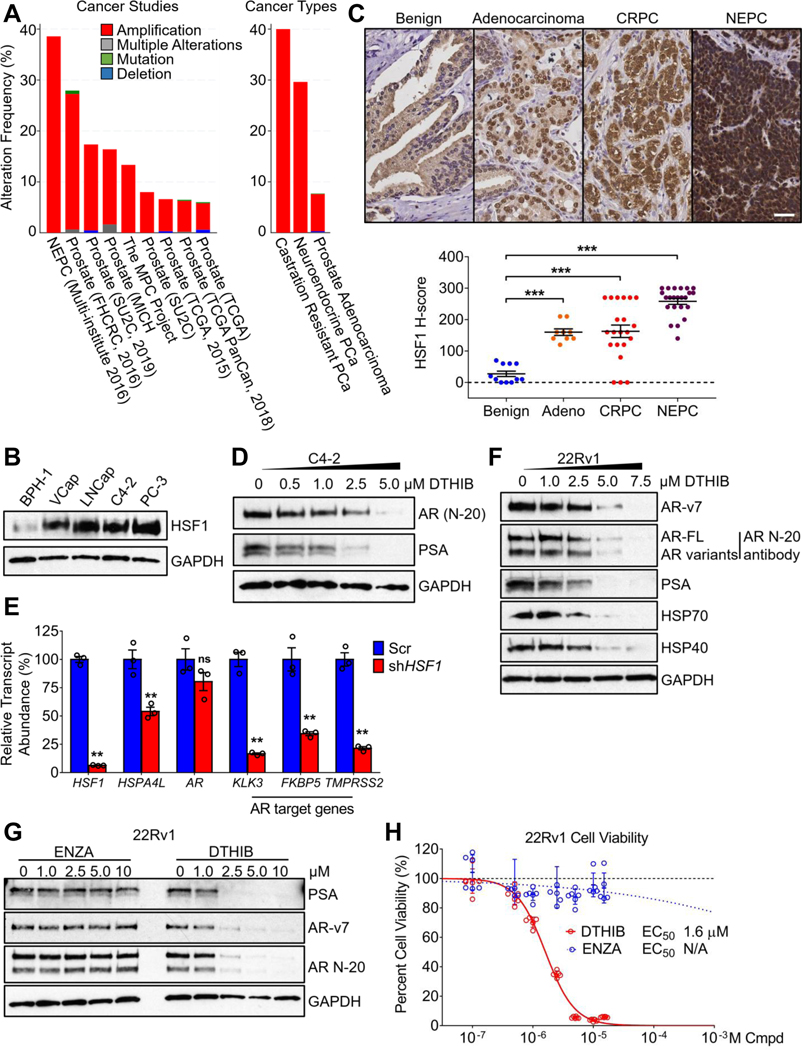
Analysis of the NCI TCGA database and published work (2, 13) demonstrated that the HSF1 gene was frequently amplified across a broad spectrum of human cancers. In prostate cancer (PCa), including CRPC and NEPC, the HSF1 gene was highly amplified and the abundance of nuclear HSF1 is prognostic for advanced disease, poor survival and therapy resistance (5, 7, 9, 12) (Fig. 4A). Examination of a panel of human prostate cancer cell lines demonstrated that HSF1 protein abundance was highly elevated in malignant cells, compared to the benign prostate cell lines BPH-1 or RWPE-1 (Fig. 4B and fig. S6A). To independently ascertain the clinical relevance of high levels of HSF1 in PCa, total HSF1 abundance was evaluated by immunohistochemistry (IHC) staining in PCa patient tumor microarrays (TMA). In comparison to benign samples (n=9), malignant prostate adenocarcinoma (n=11), CRPC (n=23), and NEPC (n=21) samples all show elevated HSF1 abundance. Notably, the most aggressive, AR-negative, NEPC samples had the most profound elevation in HSF1 abundance compared to the other three groups, accompanied by strong nuclear staining. (Fig. 4C).
Fig. 4.
Targeting HSF1 inhibits AR signaling. (A) Genetic alteration frequencies of the HSF1 gene in prostate cancer (http://www.cbioportal.org/), amplified across a collection of independent prostate cancer studies (left) and in the three major clinical types of prostate cancer, in aggregate (right). (B) Immunoblots of HSF1 in the malignant prostate cancer cell lines VCap, LNCap, C4–2 and PC-3, as compared to the benign prostate cell line BPH-1. (C) Representative images from hematoxylin and eosin (H&E) and HSF1 IHC stains of human prostate cancer specimens from TMA, with scale bar equal to 20 μM and HSF1 quantitation shown below. (D) Immunoblot showing 48-hour DTHIB treatment dose-dependently reduced the steady-state protein abundance of AR and PSA in C4–2 cells. (E) Stable knockdown of HSF1 in C4–2 cells led to reductions in HSF1 and AR activity, as ascertained for the HSF1 target gene HSPA4L AR target gene expression (KLK3, FKBP5 and TMPRSS2), as compared to Scr C4–2 cells, ascertained by qRT-PCR (n = 3). (F) 48-hour DTHIB treatment of 22Rv1 cells dose-dependently inhibited expression of molecular chaperones HSP40 and HSP70, and led to reduction in full-length AR and AR-v7 protein abundance and diminished PSA expression. (G) In comparison to enzalutamide (ENZA), 72-hour DTHIB treatment of 22Rv1 cells inhibited PSA expression and destabilized both full-length AR and AR-v7 protein, ascertained by immunoblotting. (H) Cell viability assay of 22Rv1 cells treated for 96 hours with enzalutamide or DTHIB at the indicated concentrations. Shown is the calculated EC50 value for DTHIB. N/A indicates a reliable EC50 value was unable to be estimated.
HSF1 Inhibition Suppresses Androgen Receptor Activity
Among the many roles that HSF1 target genes play in cancer, the critical functions of chaperones to fold, mature, stabilize, and sustain the activity of key signaling proteins such as nuclear hormone receptors is clear (17). This is particularly evident in hormonally driven cancers such as prostate cancer, in which the AR plays a pivotal role in the regulation of genes that drive prostate cancer survival, proliferation and progression (17, 19). As DTHIB potently inhibited HSF1 and coordinately diminished abundance of the multi-chaperone machine essential for AR stability and activity (Fig. 2A), the impact of DTHIB on AR function was evaluated. Indeed, DTHIB treatment of C4–2 PCa cells depleted AR protein and potently dampened expression of the AR target gene KLK3, encoding Prostate Specific Antigen (PSA), a key peripheral biomarker for prostate cancer progression (Fig. 4D and quantitated in fig. S6B). As a genetic validation, in comparison to shScr cells, shHSF1 C4–2 cells showed strong depletion of HSF1 protein and diminished expression of PSA and other AR targets (Fig. 4E and fig. S6C).
In response to AR antagonist administration, PCa patients frequently developed therapy-resistance through several mechanisms including AR gene amplification, AR drug-resistant mutations and the generation of AR splice variants (18). In particular, the constitutively active AR splice variant 7 (AR-v7) lacks both the Ligand Binding Domain (LBD) and the HSP90-binding motif, rendering AR-v7 resistant to AR antagonists such as enzalutamide, and independent of HSP90 function (20). However, the stability and activity of AR-v7 remains heavily reliant on other chaperones, such as HSP40 and HSP70 (19). Human PCa cell line 22Rv1, expressing both full-length AR and abundant AR-v7, was used to evaluate the efficacy of DTHIB. In 22Rv1 cells, DTHIB inhibited HSF1 and led to the coordinated depletion of molecular chaperones including HSP40 and HSP70. Simultaneously, the steady-state levels of both full-length AR, and AR-v7, were reduced in a dose-dependent manner, with a parallel loss of PSA expression (Fig. 4F and quantitated in fig. S6D). Importantly, while the clinically relevant AR antagonist enzalutamide (17, 18) was ineffective against AR-v7, DTHIB potently inhibited AR signaling, PSA expression, and 22Rv1 cell growth (Fig. 4, G and H). Similarly, in C4–2 cells DTHIB exhibited superior efficacy in the inhibition of AR signaling and cell growth, as compared to enzalutamide (fig. S6, E and F). These results demonstrated that DTHIB coordinately reduced expression of the multi-chaperone complex and attenuated AR and AR-v7 signaling in a human AR antagonist-resistant PCa cell line.
DTHIB Inhibits Therapy-resistant Prostate cancer cell proliferation
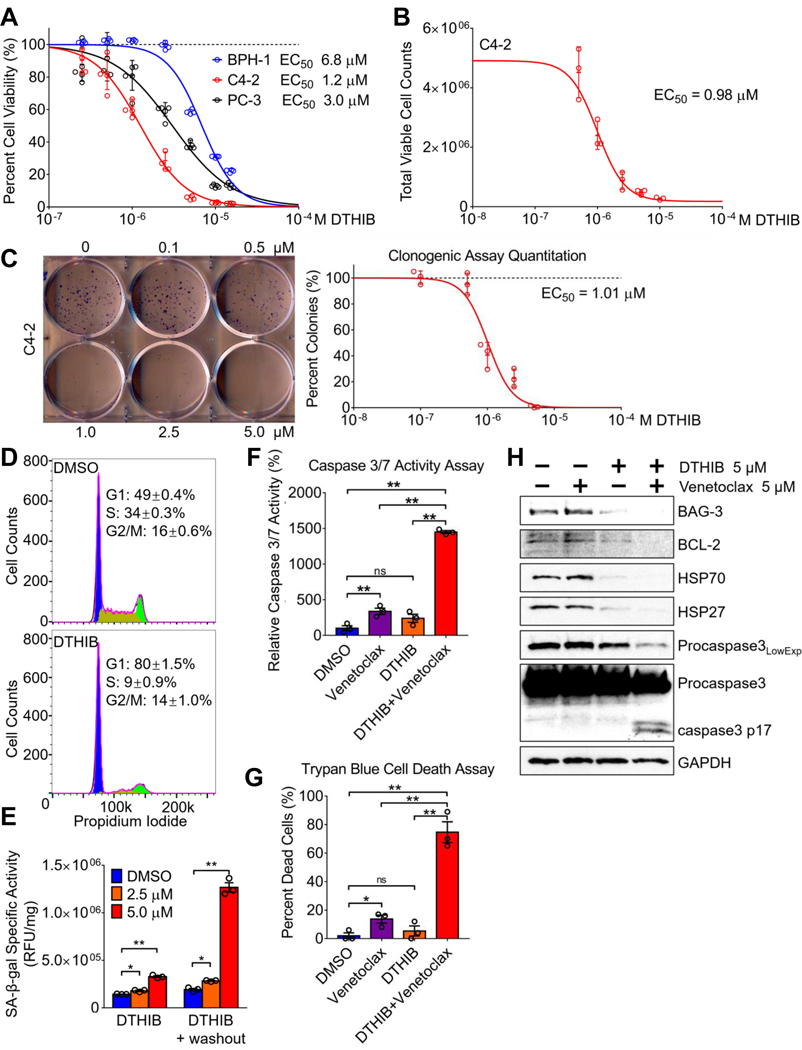
Consistent with the functional importance of HSF1 in cancer (1, 2, 4, 11), pharmacological HSF1 inhibition via DTHIB decreased the viability of an array of PCa cells encompassing both AR-positive cell lines LNCap, VCap, C4–2, TRAMP-C2, and 22Rv1, and the AR-negative cell line PC-3, as compared to a benign prostate cell line (Fig. 5A and fig. S7A) (27). The efficacy of DTHIB in C4–2 cell growth inhibition was further validated by trypan-blue exclusion assays and automated viable cell counting (Fig. 5B). In clonogenic assays, DTHIB dose-dependently reduced the clonal expansion of both C4–2 and PC-3 PCa cells (Fig. 5C and fig. S7B). Moreover, the inactive analogue A01 had no detectable impact on C4–2 cell viability (fig. S8A). In agreement with the broad dependency of cancers on HSF1 (1, 4), the efficacy of DTHIB was not limited to prostate cancers. Experiments demonstrated that DTHIB reduced cell viability in human breast cancer and melanoma cells, and inhibited the HSF1-CaSig in the melanoma cell line SK-MEL-5 (fig. S8B and S8C).
Fig. 5.
DTHIB inhibits human prostate cancer cell proliferation (A) AlamarBlue assays from 96-hour DTHIB treatment preferentially reduced the viability of the malignant prostate cancer cell lines C4–2 and PC-3, as compared to the benign prostate cell line BPH-1 (n = 5). Shown is the calculated EC50 for DTHIB in each cell line (B) Validation of DTHIB efficacy in the C4–2 PCa cell model using the trypan-blue exclusion assay (n = 3). Shown is the EC50 for DTHIB in C4–2 cells calculated with this assay (C) Clonogenic assay of C4–2 cells treated with the indicated concentrations of DTHIB for 10 days. A representative image of C4–2 colonies is shown. Shown is the EC50 for DTHIB in C4–2 cells calculated with the clonogenic assay (D) FACS analysis showing 48-hour 5 μM DTHIB treatment of C4–2 cells induced cell cycle arrest with accumulation in the G1 phase. (E) DTHIB treatment drives C4–2 cells into senescence, indicated by the induction in SA-β-galactosidase activity (n = 3). (F) C4–2 cells were treated with 5μM venetoclax, 5μM DTHIB, or 5μM of venetoclax and DTHIB for 48 hours. Apoptosis in each sample was quantitated by measuring caspase 3/7 enzymatic activity (n = 3). (G) C4–2 cells were treated as described in (F) and the percent dead cells was quantitated using the trypan-blue exclusion assay. (H) Immunoblotting was used to detect changes in key molecular markers involved in the synergy between DTHIB and venetoclax. C4–2 cells were treated the same as described in section (F).
Acute genetic knockdown of HSF1 in a variety of cancer cells resulted in cell cycle arrest and cellular senescence, a condition in which cells retain viability without further proliferation (14, 32, 33). As analyzed by Fluorescence-Activated Cell Sorting (FACS), DTHIB treatment of C4–2 cells induced cell cycle arrest, with accumulation in the G1-phase (Fig. 5D). Moreover, DTHIB treatment led to a significant elevation (P < 0.05) of SA-β-gal activity, indicating that DTHIB stimulated C4–2 PCa cell entry into senescence (Fig. 5E). When DTHIB was removed from C4–2 cell culture medium after an initial 48-hour exposure, cell growth did not resume, and a robust induction of SA-β-gal activity occurred during the 72-hour washout period (Fig. 5E and fig. S9A). Together, these results demonstrated that DTHIB triggered PCa cell senescence, and is consistent with the observed reprogramming of other cancer cell types into a senescence program after acute genetic HSF1 knockdown (15, 33).
Although senescent cells are arrested in the cell cycle and do not proliferate, they remain metabolically active (32). To evaluate downstream markers of HSF1 inhibition for the duration of the DTHIB response, levels of the HSP70, AR, and PSA proteins, and their recovery rate after DTHIB removal, were determined in C4–2 cells. After the initial 48-hour treatment, DTHIB resulted in strong depletion of HSP70, AR and PSA levels, with the time co-treatment with DTHIB and venetoclax strongly induced caspase 3/7 activity, an apoptotic marker, while treatment with each single agent had limited effect (Fig. 5F). This observation was corroborated using the trypan blue cell death assay (Fig. 5G). BAG3 is an HSF1-activated gene and the BAG3 protein critically stabilizes the anti-apoptotic protein BCL-2, thereby keeping apoptosis in check. Exposure of C4–2 cells to DTHIB reduced the expression of BAG3, HSP70 and HSP27, with a parallel reduction in BCL-2 protein abundance. Furthermore, co-treatment with DTHIB and venetoclax stimulated robust activation of caspase 3, as indicated by the enhanced cleavage of pro-caspase 3 (Fig. 5H).
Targeting HSF1 in Therapy-resistant Prostate Cancer Animal Models
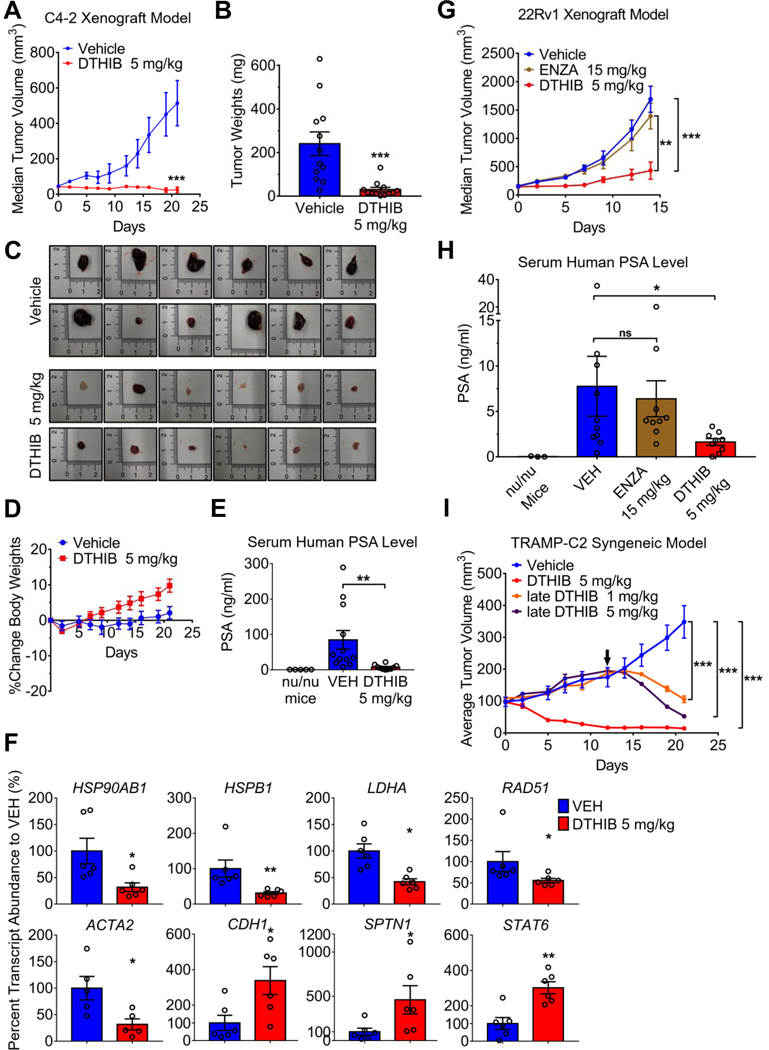
The critical nature of HSF1 has been demonstrated in a variety of animal cancer models by genetic knockdown (2, 6, 15). However, no direct HSF1 inhibitor has been used to establish pharmacological proof-of-concept for HSF1 as a druggable target in animals. To test this, a mouse xenograft model using C4–2 cells was evaluated after daily intraperitoneal (IP) administration of DTHIB at 5 mg/kg, as compared to vehicle alone. DTHIB potently attenuated tumor progression, with no visible tumor growth over a three-week period and a 40% reduction in median tumor volume (Fig. 6A and table S3). Longitudinal tumor measurements in situ were confirmed by postmortem quantitation of tumor weight (Fig. 6, A, B, and C and table S3). During the three-week course, DTHIB administration elicited no observable adverse effects on mouse behavior, weight or organ histopathology (Fig. 6D and fig. S10A). At the experimental endpoint circulating human PSA levels, secreted from C4–2 cells, showed a significant decrease (P < 0.01) in the DTHIB cohort, compared to vehicle-treated animals (Fig. 6E). Furthermore, qRT-PCR analysis of mRNA from the 6 largest tumors isolated from each cohort demonstrated that DTHIB inhibited the HSF1 CaSig in the tumor tissue (Fig. 6F), mirroring the impact of DTHIB in cultured C4–2 cells (Fig. 2C). In a pilot xenograft experiment, DTHIB was also efficacious against the AR-negative PC-3 cell PCa model (fig. S10, B and C and table S3). This agreed with the observation that the core activity of DTHIB was independent of AR (Fig. 5A, fig. S4C, fig. S7B, fig. S8, B and C), likely as a result of perturbation of the critical HSF1 CaSig (5).
Fig. 6.
Pharmacological inhibition of HSF1 attenuates prostate cancer progression in animal models. (A) Median tumor volume plot over 21 days of C4–2 xenograft study in which vehicle (blue) or DTHIB (red) at 5 mg/kg were administered daily by IP to independent mouse cohorts (n = 12). (B) Tumors from the C4–2 xenograft study (A) were excised from mice and weighed at the experimental endpoint (n = 12/cohort). (C) Images of tumors from the C4–2 xenograft study (A) harvested at the experimental endpoint. (D) Percent change in average body weight of mice from the C4–2 xenograft study (A) (n = 12/cohort). (E) Mouse serum samples from the C4–2 xenograft study (A) obtained at the experimental endpoint were assayed for human PSA levels by ELISA (n = 12/cohort), with serum from nu/nu mice (no tumor implantation and no drug treatment) as negative control (n = 5). (F) Pharmacodynamic analysis of transcript abundance corresponding to direct HSF1 target genes in C4–2 tumors (n = 6/cohort), by qRT-PCR. CDH2, SPTN1 and STAT6 are genes repressed by HSF1, all others are genes activated by HSF1. (G) Median tumor volume plot from a 22Rv1 xenograft study in which vehicle, enzalutamide (ENZA) at 15 mg/kg or DTHIB at 5 mg/kg were administered daily by IP to three animal cohorts (n = 10/cohort). (H) At the experimental endpoint, mouse serum samples (n = 10/cohort) from 22Rv1 xenograft study (G) were collected and assayed for human PSA levels by ELISA, using nu/nu mouse serum as blank (n = 3). (I) In the TRAMP-C2 syngeneic mouse prostate cancer model tumors from two cohorts of 8 mice/cohort were allowed to grow to an average tumor volume of 100 mm3 and mice then treated with vehicle or DTHIB at 5 mg/kg daily by IP. Tumors from two other cohorts of 4 mice/cohort (late cohorts) were allowed to grow to an average tumor volume of ~200 mm3, and treated with 5 mg/kg DTHIB or 1 mg/kg DTHIB daily by IP. Arrow indicated the treatment start date of the “late” cohorts.
Human 22Rv1 cells represent a widely used model to investigate AR-pathway targeted drug resistant prostate cancer, driven by the AR-v7 variant (27). The in vivo efficacy of DTHIB against AR-v7, as compared to the AR antagonist enzalutamide, was assessed in a 22Rv1 xenograft model. In agreement with a previous report (18) and our cell culture data (Fig. 4, G and H), 22Rv1-derived tumors were not responsive to enzalutamide in vivo. However, DTHIB administration at 5 mg/kg/day strongly attenuated the growth of 22Rv1 tumors. Circulating human PSA abundance was evaluated at the experimental endpoint, where the DTHIB-treated cohort had significantly smaller tumors (P < 0.01) and lower serum PSA ( P < 0.05) compared to either the vehicle or enzalutamide-treated cohort (Fig. 6, G and H, fig. S10D, and table S3). During the course of the experiment, DTHIB was well-tolerated and no weight loss was observed (fig. S10E and table S3).
While DTHIB imposed a strong suppression of tumor growth in nude mice, the host immune system can make profound contributions to tumor regression (34, 35). To investigate whether pharmacological HSF1 inhibition shows improved efficacy in animals with a functional immune system, DTHIB was evaluated in a syngeneic mouse PCa model. This model of NEPC, which is highly refractile to current therapies, makes use of TRAMP-C2 cells implanted in isogenic C57BL/6 mice (22, 27, 36). Implanted tumors were allowed to grow to ~100 mm3, after which either vehicle or DTHIB was administered at 5 mg/kg/day IP. In two additional cohorts, tumors were allowed to grow to ~200 mm3 and then mice received either 1 or 5 mg/kg/day DTHIB. For both cohorts administered 5 mg/kg DTHIB, robust tumor regression was observed after initiating treatment (Fig. 6I, fig. S10F, and table S3). Moreover, administration of 1 mg/kg/day of DTHIB also resulted in significant tumor regression (P < 0.001), demonstrating a dose-dependent activity for the HSF1 inhibitor in animals (Fig. 6I). In the syngeneic mouse PCa model, DTHIB was well-tolerated and neither weight loss nor organ damage was observed (fig. S10, G and H).
Discussion
Over a decade ago Professor Susan Lindquist predicted that HSF1 inhibitors would soon be identified and in early pre-clinical development for use in oncology (6). While the use of cell-based reporter screens has identified chemical probes to interrogate aspects of the heat shock response (2, 11), this approach has not yielded direct HSF1 inhibitors with a known mechanism of action. In contrast, the DSF screen detailed here provided a well-defined system, to interrogate a well-structured HSF1 DBD, which identified direct protein-ligand interactions. This approach was predicated on recent structural studies of HSF DBDs, which determined that the surface of the HSF1 DBD is highly solvent-exposed (25, 37, 38). The HSF1 DBD, one of the few structured regions of mammalian HSF1, embodies the critical DNA-binding activity and serves as a critical hub for protein-protein interactions that regulate the function of HSF1 in vivo (2, 38, 39). Here we demonstrated that DTHIB directly binds to HSF1 by DSF, SPR, TFQ and partial proteolysis in a purified system in vitro. Moreover, the ability of DTHIB to inhibit the HSF1 CaSig and cell viability, in multiple cancer cell lines, is dependent upon the presence of HSF1. Thus, DTHIB binds with high affinity to a distinct domain on HSF1, but the precise amino acid residues and side chains that DTHIB interacts with have yet to be structurally elucidated.
DTHIB prevented tumor growth in four therapy-resistant PCa models and inhibited the HSF1 CaSig within human tumors implanted in mice. This indicates that DTHIB was distributed to tumor tissue and engaged HSF1 in vivo, consistent with DTHIB binding to and stabilizing full-length HSF1 in PCa cell extracts by CETSA analysis. Furthermore, DTHIB was well tolerated in animals at doses of at least 25 mg/kg. Neither overt weight loss, behavioral abnormalities nor histopathology in all organs evaluated was observed in DTHIB-treated mice. As Hsf1−/− mice have been generated in distinct genetic backgrounds, the tolerance to DTHIB may also, in part, reflect the non-essentiality of HSF1 in normal tissue (40, 41). Moreover, the de-repression of HSF1-repressed genes by DTHIB indicated that DTHIB-treated cells were viable and that, in contrast to other HSF1 inhibitors, DTHIB did not inhibit the general transcription or translation machineries (2, 5). Given the time required for re-synthesis and accumulation of downstream targets of HSF1, and their impact on AR abundance and activity, the degradation of nuclear HSF1 by DTHIB may result in prolonged therapeutic activity.
DTHIB selectively accelerated the degradation of nuclear HSF1, while sparing the inactive cytosolic fraction of the protein. This MoA required the proteasome and depended on the E3 ligase Fbox component FBXW7. Dysregulation of FBXW7 in cancer and Huntington’s disease greatly impact nuclear HSF1 degradation and HSF1 activity (9, 42). In both scenarios, HSF1 degradation requires FBXW7 binding to the same phospho-degron region in the HSF1 regulatory domain (3). Since DTHIB binds the HSF1 DBD, it is possible that DTHIB functions to directly recruit FBXW7 to the HSF1 DBD and recent work suggests that FBXW7 can also interact with HSF1 through the carboxyl-terminal region of the DBD (43). Alternatively, DTHIB binding to the HSF1 DBD could alter the structure of full-length HSF1, enhancing the recruitment of FBXW7 to the phosphor-degron. Further structural and biochemical experiments are required to elucidate these molecular mechanisms.
The precise mechanism by which DTHIB drives nuclear compartment-specific HSF1 degradation remains to be elucidated. There are three splicing isoforms derived from the single FBXW7 gene, among which the FBXW7α isoform resides in the nucleus and is responsible for nuclear HSF1 degradation in melanoma (9, 44). Perhaps DTHIB preferentially recruited the FBXW7α isoform to nuclear HSF1 pools, thereby selectively driving nuclear HSF1 degradation. Furthermore, the inactive cytosolic HSF1 is bound and regulated by multi-chaperone complexes (3). HSF1 also binds and regulates the activity of AMPK (AMP-activated protein kinase) independent of its transcriptional function (45). The extensive protein-protein interactions between cytosolic HSF1 and other cellular components could preclude DTHIB and or cytosolic isoforms of FBXW7 from engaging cytosolic pools of HSF1. Due to large regions of HSF1 predicted to be intrinsically disordered (3, 46), this property of HSF1 poses a challenge to structural biology approaches. Therefore, little structural information is available for full-length HSF1, the HSF1-FBXW7 complex or the HSF1-DTHIB complex. These molecular details will be important for a more thorough understanding of DTHIB mechanism of action. In contrast to the findings from melanoma models (9), genetic ablation of FBXW7 in C4–2 PCa cells failed to elevate nuclear HSF1 abundance. Provided the greatly elevated nuclear HSF1 abundance in both prostate cancer cells and patient tumor samples, it is possible that key molecular processes needed for nuclear HSF1 degradation, such as the phosphorylation of HSF1 phosphodegron or the recruitment of FBXW7 to HSF1, are compromised in prostate cancer. Alternatively, other mechanisms controlling HSF1 stability, such as lysine acetylation, could also function in regulating HSF1 abundance in prostate cancer (47, 48).
Given the dependence of the Estrogen Receptor (ER), Progesterone Receptor (PR), and Glucocorticoid Receptor (GR) on protein chaperones (49), pharmacological HSF1 inhibition is likely to be effective against other hormone receptor-driven cancers and therapy resistance mechanisms driven by GR (50). It is important to emphasize that, while the HSF1 inhibitor DTHIB severely compromised AR and AR-v7 signaling, the efficacy of DTHIB in PCa cell and animal models was not limited by the AR status. This key feature could enable DTHIB to circumvent the challenges imposed by drug resistant AR LBD mutations, therapy-resistant AR variants such as AR-v7, and AR-negative NEPC (18, 28). The critical functions of HSF1 in driving human malignancy are multi-faceted, including but not limited to protein-folding and maintaining nuclear hormone receptor activity, supporting a large constellation of proteins involved in oncogenic signaling, metabolism, DNA replication and repair and preventing apoptosis. Thus, pharmacological HSF1 inhibition in PCa could have distinct advantages, and a broader spectrum of action, over traditional AR-targeted approaches by exploiting cancer cell addiction to HSF1 activity. While the efficacy of DTHIB was demonstrated here in animal models of PCa, the application of DTHIB could be broader as suggested by a cancer dependency map (DEPMAP) analysis (2). Apart from its potential for development as a therapeutic in oncology, a well-characterized, direct small molecule inhibitor of HSF1 could serve as a useful tool to investigate mechanistic questions on the regulation and role of HSF1 in basic stress biology and in cancer.
Materials and Methods
Study Design
The objective of this study was to identify a direct small-molecule inhibitor of HSF1 and conduct validation, characterization, and animal model studies of the HSF1 inhibitor. For in vitro or cell culture experiments, at least 3 biological replicates per condition were used for statistical analysis. The precise number of biological replicates varied between different types of experiments due to technical limitations. No outliers were excluded.
For animal experiments power analyses were conducted based on preliminary data and selected cohort sizes used for all experiments were sufficient to have a power of 0.8 and P<0.05. For all animal experiments tumor bearing animals were randomized prior to any treatment. No outliers were excluded unless explicitly mentioned, in which case the extreme outliers were identified and removed using 3xIQR as determining criteria. Detailed descriptions of experimental methods described in this manuscript are provided in the Supplementary Materials and Methods.
Statistical Analysis
Unless specifically indicated statistical analyses of data described in this manuscript were conducted using the unpaired student t test assuming equal variance for comparison between two groups and standard one-way ANOVA for comparison between multiple groups. The standard error of the mean (SEM) was used to depict the error bars. For p value < 0.05, one asterisk (*) was depicted. For p value < 0.01, two asterisks (**) were depicted. For p value < 0.001, three asterisks (***) were depicted. For p value > 0.05, ns (not significant) was depicted. Sample sizes (n) were incorporated in the text and figure legends, and individual data points from each replicate were depicted as small circles in all figures.
Supplementary Material
Acknowledgments:
We gratefully acknowledge advice from Eileen Burchfiel, Mehdi Mollapour, David Stokoe, Pei Zhou and Jiyong Hong. We thank Suzanne Wardell for guidance on xenograft models, Jeffrey Everitt for veterinary pathology analysis, Maria Schumacher for guidance on fluorescence anisotropy, Jason Gestwick for providing the human HSC70 expression vector, Susan Lindquist and Tyler Jacks for providing the NF1-related MEFs, Sean Cutler for providing the LATCA small molecule library and Jun Liu, Curtis Chong and David Sullivan for providing the Johns Hopkins Clinical Compound Library.
Funding:
This work was partially supported by an award from the Duke Cancer Institute as part of the P30 Cancer Center Support Grant (Grant ID: NIH CA014236) to B.D and D.J.T., a grant from the Prostate Cancer Foundation to J.H. and a Damon Runyon Cancer Research Foundation Postdoctoral Fellowship to A.J.M.
Footnotes
Competing interests: B.D., A.M.J., P.H., T.A.H., J.H., and D.J.T. are co-inventors on a technology related to HSF1 inhibitors (U.S. Provisional Patent Application # 62/777,831: COMPOSITIONS AND METHODS FOR THE TREATMENT OF CANCER). D.J.T. and J.H. have equity and a board relationship with Sisu Pharma, Inc. All other authors declare that they have no competing interests.
Data and materials availability:
All data associated with this study are available in the main text or the supplementary materials.
References and Notes:
- 1.Dai C, Sampson SB, HSF1: Guardian of Proteostasis in Cancer. Trends Cell Biol 26, 17–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong B, Jaeger AM, Thiele DJ, Inhibiting Heat Shock Factor 1 in Cancer: A Unique Therapeutic Opportunity. Trends Pharmacol Sci 40, 986–1005 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Pastor R, Burchfiel ET, Thiele DJ, Regulation of heat shock transcription factors and their roles in physiology and disease. Nat Rev Mol Cell Biol 19, 4–19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joutsen J, Sistonen L, Tailoring of Proteostasis Networks with Heat Shock Factors. Cold Spring Harb Perspect Biol 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S, HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 150, 549–562 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai C, Whitesell L, Rogers AB, Lindquist S, Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130, 1005–1018 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM, Lindquist S, Ince TA, High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A 108, 18378–18383 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai C, Santagata S, Tang Z, Shi J, Cao J, Kwon H, Bronson RT, Whitesell L, Lindquist S, Loss of tumor suppressor NF1 activates HSF1 to promote carcinogenesis. J Clin Invest 122, 3742–3754 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kourtis N, Moubarak RS, Aranda-Orgilles B, Lui K, Aydin IT, Trimarchi T, Darvishian F, Salvaggio C, Zhong J, Bhatt K, Chen EI, Celebi JT, Lazaris C, Tsirigos A, Osman I, Hernando E, Aifantis I, FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat Cell Biol 17, 322–332 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kourtis N, Lazaris C, Hockemeyer K, Balandrán JC, Jimenez AR, Mullenders J, Gong Y, Trimarchi T, Bhatt K, Hu H, Shrestha L, Ambesi-Impiombato A, Kelliher M, Paietta E, Chiosis G, Guzman ML, Ferrando AA, Tsirigos A, Aifantis I, Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia. Nat Med 24, 1157–1166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kijima T, Prince T, Neckers L, Koga F, Fujii Y, Heat shock factor 1 (HSF1)-targeted anticancer therapeutics: overview of current preclinical progress. Expert Opin Ther Targets 23, 369–377 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Björk JK, Ahonen I, Mirtti T, Erickson A, Rannikko A, Bützow A, Nordling S, Lundin J, Lundin M, Sistonen L, Nees M, Åkerfelt M, Increased HSF1 expression predicts shorter disease-specific survival of prostate cancer patients following radical prostatectomy. Oncotarget 9, 31200–31213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang CQ, Williams H, Prince TL, Ho ES, Overexpressed HSF1 cancer signature genes cluster in human chromosome 8q. Hum Genomics 11, 35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng L, Gabai VL, Sherman MY, Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene 29, 5204–5213 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabai VL, Meng L, Kim G, Mills TA, Benjamin IJ, Sherman MY, Heat shock transcription factor Hsf1 is involved in tumor progression via regulation of hypoxia-inducible factor 1 and RNA-binding protein HuR. Mol Cell Biol 32, 929–940 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi C, Hu Y, Buckhaults P, Moskophidis D, Mivechi NF, Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J Biol Chem 287, 35646–35657 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyatt AW, Gleave ME, Targeting the adaptive molecular landscape of castration-resistant prostate cancer. EMBO Mol Med 7, 878–894 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson PA, Arora VK, Sawyers CL, Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 15, 701–711 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moses MA, Kim YS, Rivera-Marquez GM, Oshima N, Watson MJ, Beebe KE, Wells C, Lee S, Zuehlke AD, Shao H, Bingman WE 3rd, Kumar V, Malhotra SV, Weigel NL, Gestwicki JE, Trepel JB, Neckers LM, Targeting the Hsp40/Hsp70 Chaperone Axis as a Novel Strategy to Treat Castration-Resistant Prostate Cancer. Cancer Res 78, 4022–4035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J, AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371, 1028–1038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Niu J, Huang J, Neuroendocrine differentiation in prostate cancer. Am J Transl Res 1, 148–162 (2009). [PMC free article] [PubMed] [Google Scholar]
- 22.Berman-Booty LD, Knudsen KE, Models of neuroendocrine prostate cancer. Endocr Relat Cancer 22, R33–49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niesen FH, Berglund H, Vedadi M, The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2, 2212–2221 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Jaeger AM, Makley LN, Gestwicki JE, Thiele DJ, Genomic heat shock element sequences drive cooperative human heat shock factor 1 DNA binding and selectivity. J Biol Chem 289, 30459–30469 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neudegger T, Verghese J, Hayer-Hartl M, Hartl FU, Bracher A, Structure of human heat-shock transcription factor 1 in complex with DNA. Nat Struct Mol Biol 23, 140–146 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Miyata Y, Ung PM, Bertelsen EB, McQuade TJ, Carlson HA, Zuiderweg ER, Gestwicki JE, Chemical screens against a reconstituted multiprotein complex: myricetin blocks DnaJ regulation of DnaK through an allosteric mechanism. Chem Biol 18, 210–221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Gong S, Roy-Burman P, Lee P, Culig Z, Current mouse and cell models in prostate cancer research. Endocr Relat Cancer 20, R155–170 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, Huang J, PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 71, 1668–1679 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santagata S, Mendillo ML, Tang YC, Subramanian A, Perley CC, Roche SP, Wong B, Narayan R, Kwon H, Koeva M, Amon A, Golub TR, Porco JA, Whitesell L, Lindquist S, Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science 341, 1238303 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vihervaara A, Sergelius C, Vasara J, Blom MA, Elsing AN, Roos-Mattjus P, Sistonen L, Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc Natl Acad Sci U S A 110, E3388–3397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundback T, Nordlund P, Molina D. Martinez, The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc 9, 2100–2122 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Ewald JA, Desotelle JA, Wilding G, Jarrard DF, Therapy-induced senescence in cancer. J Natl Cancer Inst 102, 1536–1546 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oda T, Sekimoto T, Kurashima K, Fujimoto M, Nakai A, Yamashita T, Acute HSF1 depletion induces cellular senescence through the MDM2-p53-p21 pathway in human diploid fibroblasts. J Cell Sci 131, (2018). [DOI] [PubMed] [Google Scholar]
- 34.Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M, Stemmer SM, Whitesell L, Lindquist S, The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell 158, 564–578 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaeger AM, Stopfer L, Lee S, Gaglia G, Sandel D, Santagata S, Lin NU, Trepel JB, White F, Jacks T, Lindquist S, Whitesell L, Rebalancing Protein Homeostasis Enhances Tumor Antigen Presentation. Clin Cancer Res, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelman IH, How the TRAMP Model Revolutionized the Study of Prostate Cancer Progression. Cancer Res 76, 6137–6139 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Jaeger AM, Pemble C. W. t., Sistonen L, Thiele DJ, Structures of HSF2 reveal mechanisms for differential regulation of human heat-shock factors. Nat Struct Mol Biol 23, 147–154 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakai A, Molecular basis of HSF regulation. Nat Struct Mol Biol 23, 93–95 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Dai S, Tang Z, Cao J, Zhou W, Li H, Sampson S, Dai C, Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J 34, 275–293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ, Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem 273, 7523–7528 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Jin X, Eroglu B, Moskophidis D, Mivechi NF, Targeted Deletion of Hsf1, 2, and 4 Genes in Mice. Methods Mol Biol 1709, 1–22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Pastor R, Burchfiel ET, Neef DW, Jaeger AM, Cabiscol E, McKinstry SU, Doss A, Aballay A, Lo DC, Akimov SS, Ross CA, Eroglu C, Thiele DJ, Abnormal degradation of the neuronal stress-protective transcription factor HSF1 in Huntington’s disease. Nat Commun 8, 14405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang T, Ren C, Lu C, Qiao P, Han X, Wang L, Wang D, Lv S, Sun Y, Yu Z, Phosphorylation of HSF1 by PIM2 induces PD-L1 expression and promotes tumor growth in breast cancer. Cancer Res, (2019). [DOI] [PubMed] [Google Scholar]
- 44.Welcker M, Clurman BE, FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 8, 83–93 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Su KH, Dai S, Tang Z, Xu M, Dai C, Heat Shock Factor 1 Is a Direct Antagonist of AMP-Activated Protein Kinase. Mol Cell 76, 546–561 e548 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hentze N, Le Breton L, Wiesner J, Kempf G, Mayer MP, Molecular mechanism of thermosensory function of human heat shock transcription factor Hsf1. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raychaudhuri S, Loew C, Korner R, Pinkert S, Theis M, Hayer-Hartl M, Buchholz F, Hartl FU, Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell 156, 975–985 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Westerheide SD, Anckar J, Stevens SM, Sistonen L, Morimoto RI, Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323, 1063–1066 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Echeverria PC, Picard D, Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim Biophys Acta 1803, 641–649 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis C, Zheng D, Sawyers CL, Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155, 1309–1322 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y, Chow TF, Puckrin RS, Alfred SE, Korir AK, Larive CK, Cutler SR, Chemical genetic interrogation of natural variation uncovers a molecule that is glycoactivated. Nat Chem Biol 3, 716–721 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Brady LS, Winsky L, Goodman W, Oliveri ME, Stover E, NIMH initiatives to facilitate collaborations among industry, academia, and government for the discovery and clinical testing of novel models and drugs for psychiatric disorders. Neuropsychopharmacology 34, 229–243 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ Jr., A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol 2, 415–416 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Neef DW, Turski ML, Thiele DJ, Modulation of heat shock transcription factor 1 as a therapeutic target for small molecule intervention in neurodegenerative disease. PLoS Biol 8, e1000291 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are available in the main text or the supplementary materials.