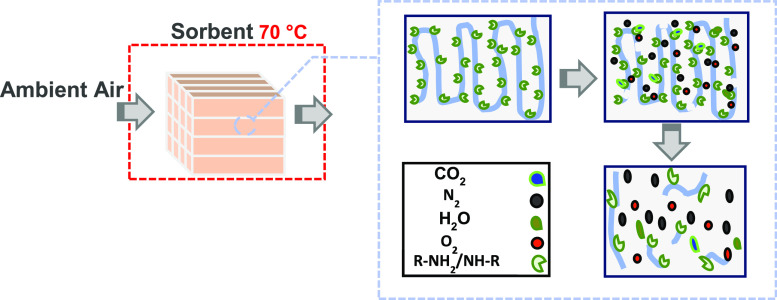
Abstract
Aminopolymer-based sorbents are preferred materials for extraction of CO2 from ambient air [direct air capture (DAC) of CO2] owing to their high CO2 adsorption capacity and selectivity at ultra-dilute conditions. While those adsorptive properties are important, the stability of a sorbent is a key element in developing high-performing, cost-effective, and long-lasting sorbents that can be deployed at scale. Along with process upsets, environmental components such as CO2, O2, and H2O may contribute to long-term sorbent instability. As such, unraveling the complex effects of such atmospheric components on the sorbent lifetime as they appear in the environment is a critical step to understanding sorbent deactivation mechanisms and designing more effective sorbents and processes. Here, a poly(ethylenimine) (PEI)/Al2O3 sorbent is assessed over continuous and cyclic dry and humid conditions to determine the effect of the copresence of CO2 and O2 on stability at an intermediate temperature of 70 °C. Thermogravimetric and elemental analyses in combination with in situ horizontal attenuated total reflection infrared (HATR-IR) spectroscopy are performed to measure the extent of deactivation, elemental content, and molecular level changes in the sorbent due to deactivation. The thermal/thermogravimetric analysis results reveal that incorporating CO2 with O2 accelerates sorbent deactivation using these sorbents in dry and humid conditions compared to that using CO2-free air in similar conditions. The in situ HATR-IR spectroscopy results of PEI/Al2O3 sorbent deactivation under a CO2-air environment show the formation of primary amine species in higher quantity (compared to that in conditions without O2 or CO2), which arises due to the C–N bond cleavage at secondary amines due to oxidative degradation. We hypothesize that the formation of bound CO2 species such as carbamic acids catalyzes C–N cleavage reactions in the oxidative degradation pathway by shuttling protons, resulting in a low activation energy barrier for degradation, as probed by metadynamics simulations. In the cyclic experiment after 30 cycles, results show a gradual loss in stability (dry: 29%, humid: 52%) under CO2-containing air (0.04% CO2/21% O2 balance N2). However, the loss in capacity during cyclic studies is significantly less than that during continuous deactivation, as expected.
Keywords: DAC, carbon capture, degradation, poly(ethylenimine), oxidation, radical
Introduction
Global greenhouse gas (GHG) emissions from various economic sectors have been growing rapidly for several decades.1 GHGs present in the atmosphere absorb and retain heat released from the earth increasing the global surface temperature, warming oceans, melting arctic ice, and causing sea level rise. CO2 emissions, which were 80% of the released GHGs in 2019, have increased the atmospheric CO2 concentration from 331 ppm (1975) to 416 ppm (current), raising the global average surface temperature by nearly 1 °C.1 Maintaining the current GHG emission profile will lead to an increase of the global surface temperature by more than 1.5 °C in the near future.1,2 Therefore, it is essential to develop robust technologies to limit future emissions of GHGs and reverse the already existing damage.
Direct air capture (DAC) technologies for the removal of CO2 from the atmosphere (negative emissions) and carbon capture technologies for large point sources (avoided emissions) such as coal-fired power plants are proposed technologies to address the continuously increasing greenhouse gas emissions.3 Considering the large amount of CO2 present in the atmosphere and the goal of limiting the global surface temperature increase below 2 °C, quickly developing and deploying DAC processes, alongside other negative emission technologies,4 is important to climate stabilization. To this end, developing practical sorbent materials with high CO2 selectivity, adsorption capacity, and cyclic stability is necessary.1,5,6
Amine-functionalized sorbents are components of promising DAC technologies that are in the early stages of commercialization due to their high CO2 selectivity, moderate regeneration energies, high CO2 adsorption capacities, and capability to perform under dry and humid conditions.7,8 Poly(ethylenimine) (PEI) supported in porous γ-Al2O3 (PEI/γ-Al2O3) is a well-known and well-studied supported aminopolymer sorbent in the DAC literature and is the focus of this study.7,9 One drawback of amine-functionalized adsorbents is their susceptibility to the loss of their high CO2 adsorption capacity when operating continuously over multiple adsorption/desorption cycles in DAC systems. In a typical DAC adsorption–desorption cycle, the sorbents are exposed to ambient air composed of several components such as O2, CO2, N2, H2O, and other small atmospheric molecules and particles. These components together with varying process parameters during different parts of the cycle (temperature, pressure, etc.) affect the long-term sorbent stability in a complex way.10–14 One main example of this is the copresence of high O2 concentration (∼21 mol %) and elevated temperatures associated with sorbent regeneration for short periods of time, which are considered to be the primary opportunity for oxidative degradation. The presence of other components of ambient air such as CO2 and H2O, in addition to the elevated O2 concentration, alongside constantly changing process conditions, leads to an array of factors that may affect the sorbent’s oxidative degradation behavior, mechanism(s), and the resulting longevity of the sorbent.
Studies of materials and processes for DAC to date have mostly focused on improving the CO2 adsorption capacities of solid sorbents. While the adsorption capacity is an important factor, several studies have indicated that a sorbent material that can be used for thousands of adsorption/desorption cycles would substantially reduce overall DAC technology costs.9 Of the studies on sorbent deactivation to date, most have focused on investigating the effects of exposure to O2, H2O, or CO2 at elevated temperatures.15–17 Furthermore, high temperatures that may not be directly relevant to the process are often used to accelerate the deactivation kinetics. The most explored deactivation paths include CO2-induced deactivation in simulated flue gas and oxidative degradation in simulated air (21% O2 balance N2, dry, CO2-free). In 2012, Heydari-Gorji and Sayari10 investigated the thermal, oxidative, and CO2-induced deactivation of PEI-impregnated mesoporous silica (SBA-15) with a platelet particle morphology and short pore channels under inert gas, simulated air, simulated flue gas, and various CO2/O2/N2 conditions. They found that the presence of humidity helped mitigate CO2-induced deactivation using a 5% CO2/N2 mixture. Similarly, in prehumidified CO2/O2/N2 mixtures, they observed improved sorbent stability, suggesting that CO2 and/or H2O protected them against oxidative degradation.10 In a follow-up work by the same group, CO2-induced deactivation under dry cyclic conditions using a flow of pure CO2 both for adsorption at 50 or 100 °C and desorption at 130–160 °C was explored.11 Several amine-grafted MCM-41 and wet impregnated PEI (branched and linear) mesoporous silica sorbents were explored to understand the reaction mechanism(s) and products formed. Both studies suggested urea formation as a result of CO2-induced deactivation at elevated temperatures and atmospheric pressure. As such, reaction pathways for open chain and cyclic urea formation were explored by others.10,11 Didas et al. explored the thermal, oxidative, and CO2-induced deactivation of primary amine-grafted mesoporous silica (SBA-15) adsorbents with alkyl linker lengths varying from methyl to propyl under pure CO2 at 135 °C. The ethyl and propyl linkers showed better resistance to oxidative and thermal deactivation; both, however, were vulnerable to CO2-induced deactivation.12
While these studies helped build foundational knowledge of sorbent deactivation, mainly on CO2-induced deactivation, they mostly explored flue gas CO2 capture conditions where the CO2, H2O, and O2 concentrations differ from DAC. As such, the roles of CO2, H2O, and O2 in sorbent stability might not be directly transferable to the DAC conditions where the concentrations are different and temperatures are lower. Notably, a recent study from our group on the impact of atmospheric humidity on the stability of the PEI/Al2O3 sorbent showed that H2O plays a significant role in accelerating degradation reactions on some sorbents, which is contrary to other reports in literature, as briefly mentioned above.15 To this end, a broad understanding of the impact of ambient air components (H2O/CO2/O2/N2) as they appear in the environment on sorbent degradation needs further in-depth exploration.
In this work, we investigate the environmental parameters that influence sorbent stability that have not been fully explored in the DAC literature. Specifically, we assess the impact of incorporating dry and humid CO2 in air on the stability of a model aminopolymer sorbent (PEI/Al2O3) and its influence on the oxidative degradation behavior at intermediate temperatures (70 °C) directly relevant to the CO2 desorption process. Furthermore, we explore the effect of humidity on the sorbent stability in the presence and absence of CO2 at the same temperature. In situ spectroscopic, thermal, and elemental characterizations of pristine and oxidized sorbents are performed using horizontal attenuated total reflection infrared (HATR-IR) spectroscopy, alongside thermal analysis via thermogravimetric analysis to achieve the experimental goals. Metadynamics simulations are performed to gain further insight into the role of CO2 during the oxidation degradation. The knowledge gained from this study will enable further identification of aminopolymer sorbent deactivation pathways and products.
Experimental Methods
Materials and Sorbent Synthesis
Aminopolymer sorbents were synthesized by wet impregnation of PEI (branched, 800 g/mol, Sigma-Aldrich) onto a mesoporous gamma-alumina (CATALOX HP 14/15 γ-Al2O3, Sasol) support (surface area 136 m2/g and pore volume 0.95 ± 0.05 cm3/g). A branched 45 wt % PEI/γ-Al2O3 sorbent was used for both infrared spectroscopic analysis and thermal analysis studies. Commercially available alumina (γ-Al2O3) is used in this study because it is a well-studied support in amine-functionalized sorbents in DAC due to its high surface area and pore volume and offers a more accessible pathway for scale-up and deployment due to the availability of alumina wash-coated monoliths.14 Furthermore, it has a higher hydrothermal stability compared to that of the other commercially available supports such as SiO2, mainly due to their crystallinity and lower hydrophilicity.18,19 Hydrothermal stability is essential considering the practical use of steam regeneration in large-scale DAC plants/processes.14,20 γ-Al2O3 has also been reported to have lower environmental impact in comparison to that of other support materials studied for DAC applications.21
N2 physisorption was used to characterize the pore volume and pore size of the sorbent (PEI/γ-Al2O3) and the support (γ-Al2O3). N2 physisorption was performed on a Micromeritics TriStar II 3020 Version 3.02 at 77 K using 100–150 mg of sorbent. The samples were pretreated under a vacuum at 100 °C (γ-Al2O3) or 60 °C (PEI/γ-Al2O3) for 10 h prior to the adsorption measurement. The pore size distribution was calculated using the Barrett–Joyner–Halenda method.22 Elemental analysis of the fresh and deactivated sorbents was carried out by Atlantic Microlabs to determine the CHN content of the samples before and after various treatments. Specifically, the C/N and H/(C + N) ratios were calculated to understand the evolution of the elemental composition as the sorbent deactivates.
Thermogravimetric combustion analysis was used to determine the total organic content (PEI) of the sorbent. Organic combustion analysis was performed on a thermogravimetric analyzer (TA Instrument Q550) by heating the sample from room temperature to 700 °C with a ramp rate of 10 °C/min under air (Airgas, UZG UZ300, 21% O2 balance N2). The weight loss of the sorbent from 160 to 700 °C was taken as organic content.
CO2 Adsorption Experiments
CO2 adsorption experiments were conducted using a thermogravimetric analyzer (TA Instrument Q500) with 22 ± 0.2 mg of sample on a 50 μL platinum pan to investigate the CO2 adsorption capacity. To pretreat the sample, He (Airgas UHP300) gas at 90 mL/min was flowed, and the temperature was increased to 100 °C from room temperature at a ramp rate of 10 °C/min and maintained for 1 h. Then, the temperature was decreased to 30 °C at a ramp rate of 10 °C/min. Once the system equilibrated at 30 °C, the gas was switched to 400 ppm of CO2 (balance He) at the same flow rate and held for 3 h to allow for CO2 adsorption. After 3 h of adsorption, the gas was switched back to He (Airgas UHP300) at 90 mL/min, and the temperature was increased to 100 °C at a ramp rate of 10 °C/min to desorb the CO2. The temperature was held at 100 °C for 1 h to allow the complete desorption of CO2. At the end of the desorption, the temperature was reduced to 30 °C at a ramp rate of 10 °C/min to cool the instrument and prepare for the next run.
Thermal Analysis Experiments
Oxidation experiments were performed using a thermogravimetric analysis–differential scanning calorimetry TGA-DSC (TA Instruments Q600) at 70 °C and 1 atm using 28−35 mg of sorbents (45% PEI/γ-Al2O3) on a 40 μL alumina ceramic pan. Prior to performing the deactivation study, the sample was purged with N2 (Airgas UHP 99.999%) at a flow rate of 100 mL/min at room temperature for 25 min followed by pretreatment at 100 °C for 1 h to remove the adsorbed moisture and CO2. After the pretreatment, the temperature was reduced to 70 °C at a ramp rate of 5 °C per minute. Then, the temperature was held at 70 °C (isothermal), and the gas was switched to air (Airgas, UZG UZ300, 21% O2 balance N2) (CO2-free air), 0.04% CO2/21% O2 balance N2 (0.04% CO2-air), or 0.04% CO2 balance N2 (0.04% CO2–N2) at 100 mL/min for the specified period of deactivation. For humid deactivation experiments, the deactivating gases were prehumidified by flowing through a saturated K2CO3 solution at a temperature of 23 °C, which provides ∼43% relative humidity (RH). The RH of the gas stream was monitored using a LI-COR 850 H2O/CO2 analyzer and was constant throughout the experiment. When the deactivation experiment was completed, the gas was switched back to N2, and the temperature was lowered to 25 °C to limit further deactivation of the sorbent.
In Situ ATR-IR Spectroscopy
In situ IR experiments were conducted using a Thermo Scientific Nicolet 8700 HATR-IR) spectrometer with a ZnSe crystal cell to observe the effect of oxidation on the PEI/γ-Al2O3 sorbent, tracking functional group formation due to the various thermal treatments. For each experiment, a slurry of 45 wt % PEI/Al2O3 in methanol was prepared by stirring the mixture overnight at room temperature (∼23 °C). After the slurry was well mixed, some of the methanol was removed by rotary evaporation at 50 °C and 218 mTorr for about 2 min. The remaining PEI/Al2O3/methanol slurry was added dropwise to an ATR-IR ZnSe crystal to form an ∼10 μm thin film. The thin film was purged under N2 (Airgas UHP 99.999%) at 100 mL/min for about 20 min at room temperature (∼23 °C) and preheated at 100 °C for 1 h to remove the methanol solvent and the other adsorbed species (such as H2O and CO2) acquired during sample storage or preparation steps. The thickness of the film was confirmed by scanning electron microscopy (SEM) imaging of the PEI/Al2O3-coated ZnSe crystal. After pretreatment, the temperature was reduced to 70 °C, and the samples were exposed to a continuous flow of deactivating gas [CO2-free air (Airgas UZG UZ300, 21% O2 balance N2), 0.04% CO2/21% O2 balance N2, or 0.04% CO2 balance N2] at 100 mL/min, while the cell temperature was held at 70 °C for 3 days. The HATR-IR spectra shown in this study were produced by subtraction of the spectrum of PEI/Al2O3 at time = 0 at 70 °C under the respective gas mixtures. As such, the spectra shown in the figures (with both positive and negative bands) are a result of the changes in the sample due to exposure to the experimental conditions. A period of 3 days of deactivation was chosen based on the sorbent deactivation data obtained from the thermal analysis study. Peak deconvolution analysis was performed using the SciPy optimize curve fit function in Python to distinguish among the peaks collected from the in situ HATR-IR experiments that are of interest and overlap with each other.
First-Principles Metadynamics Simulations
Ab initio molecular dynamics (AIMD) and metadynamics simulations were performed with the Vienna ab initio simulation package (VASP), version 5.4.4,23 using the projector-augmented wave treatment of the core–valence interactions24,25 with the Perdew–Burke–Ernzerhof (PBE) generalized gradient approximation26 for the exchange–correlation energy. The DFT-D3 method of Grimme et al. was employed for the van der Waals-dispersion energy corrections.27 Triethylenetetramine (TETA) was used in all the simulations as a small-molecule surrogate for PEI. A 15 Å × 15 Å × 15 Å cubic supercell was used to accommodate TETA and the CO2-bound TETA molecules, and the Brillouin zone was sampled at the Γ-point only. 10 ps of NVT AIMD simulations at 343 K were run to pre-equilibrate structures before initiating the metadynamics simulations. Coordination numbers as defined in VASP were used as the collective variables (CVs). To reduce the sampling errors, a fine set of Gaussian hill parameters were used: height 0.0025 eV and width 0.02 CV unit. The Gaussian hills were added to the underlying potential energy surface every 20 fs. Metadynamics simulations were terminated after the CVs crossed from the reaction basin into the target product basin following a previously reported protocol.28 The free-energy barrier was computed by summing the amounts of bias potentials accumulated in the reactant basin.
Results and Discussion
Thermal Analysis
Oxidative and thermal degradation of the 45% PEI/γ-Al2O3 sorbent: a model PEI/Al2O3 sorbent with a PEI loading of 45 wt % and about 98% pore fill (0.028 cm3/g) was used for the thermal analysis study. The physical characteristics of the sorbent are determined using N2 physisorption. TGA-DSC was used to follow the weight loss and heat flow of 28–35 mg of the 45 wt % PEI/γ-Al2O3 sorbent as a function of time. The sorbent was exposed to CO2-free air (21% O2) for 3, 7, 10, and 14 days and to 99+% N2 for 3, 7, and 14 days continuously at 70 °C for oxidative and thermal degradation experiments, respectively. The weight loss recorded at 70 °C was assumed to be a result of PEI deactivation. Figure S1 shows the thermogravimetric combustion measurement of the model sorbent (45 wt % PEI/γ-Al2O3). The CO2 adsorption capacities of the fresh and oxidized samples were determined using 400 ppm of CO2 balance He stream. Sorbent deactivation is defined as the percent loss in the CO2 adsorption capacity (mmol of CO2/g of sorbent) from a fresh sorbent after exposure to a specified gas mixture and a deactivation period at 70 °C. The CO2 adsorption capacity of a fresh sorbent was 1.34 mmol of CO2/g sorbent.
As the results in Figure 1 show, oxidative and thermal degradation appeared to have similar impact on the loss of adsorption capacity until day 7 (4% vs 3% loss in capacity, respectively). We assign this region of the degradation as “temperature dominant” as most of the impact can be attributed to thermal effects. After day 7, there was more significant sorbent deactivation under oxidative degradation conditions compared with the thermal-only conditions. This region is assigned as the “oxidative degradation”-dominant region.
Figure 1.

Oxidative and thermal degradation of the 45% PEI/γ-Al2O3 sorbent at 70 °C.
Building on these results as a baseline, we then investigated the presence of CO2 and H2O together and separately. As shown in Figure 2, after 3 and 7 days of continuous exposure to 0.04% CO2–N2 flow, the loss in the CO2 adsorption capacity was minimal (3 and 2%, respectively; purple diamonds), indicating limited deactivation. On the contrary, under 0.04% CO2-air, notable loss in CO2 adsorption capacity was observed, even in the early stages of deactivation (17% loss after 3 h, blue triangles). Though the deactivation rate is moderate, it eliminates the induction period observed under oxidative and thermal degradation conditions in the absence of CO2 (Figure 1). After 3 h, the deactivation continued to increase, reaching 80% loss after 7 days. Thus, the combination of CO2 and O2 leads to more rapid deactivation than with either species alone.
Figure 2.

Deactivation of the 45% PEI/γ-Al2O3 sorbent under CO2-free air (for 3, 7, 10, and 14 days), under N2 (for 3, 7, and 14 days), under 0.04% CO2-air (for 3 h, 18 h, 36 h, 72 h, and 7 days), and 0.04% CO2 balance N2 environments at 70 °C for 3 and 7 days.
In a humid environment [43% RH; olive triangles], a stream of 0.04% CO2-air yielded similar deactivation behavior as the dry 0.04% CO2-air mixture. The initial deactivation rate appeared slightly accelerated under dry conditions and slightly slower at longer times, relative to humid conditions, but these apparent differences are likely within the margin of experimental error. The data in Figure 2 show that in the absence of CO2, the differences between dry and humid environments were magnified (dry, red triangles; humid, black squares), with higher deactivation under humid conditions after 7 days. This difference suggests that CO2 has a bigger impact on oxidative degradation than H2O under the continuous deactivation conditions explored, with H2O enhancing degradation in the absence of CO2. The enhancement of oxidative degradation in the presence of H2O vapor is consistent with the impact of H2O in accelerating oxidative degradation reported in our recent work.15
The above results (Figure 2) show the implications of the copresence of CO2 and O2, as they appear in ambient air, on the stability of the amine-based sorbents at an intermediate temperature. As briefly mentioned above, these results differ from what has been reported in the literature for different amine sorbents under slightly different conditions. Previously, the presence of CO2 in O2-containing streams was reported to improve the stability of the amine-based sorbents based on the fact that the amine species react faster with CO2 compared to O2, forming carbamate and bicarbonate species that reportedly enhance stability.10 In the work of Heydari-Gorji and Sayari,10 the thermal, oxidative, and CO2-induced deactivation of PEI (branched and linear)-impregnated mesoporous silica (SBA-15) sorbents with the platelet particle morphologies and short pore channels was explored. In their specific study pertaining to the copresence of CO2 and O2, a PEI/SBA-15 sorbent was deactivated continuously under prehumidified CO2/O2/N2 streams at different concentrations (1–20%/10.5–17%/balance N2) and temperatures ranging from 50 to 120 °C for 30 h. See Table S1 for tabulated values. The study reported no significant deactivation below 100 °C for all of the CO2/O2/N2 mixtures. At 100 °C, 70% CO2 uptake loss was obtained for the 1%/17%/82% (CO2/O2/N2) gas mixture after 30 h, and the uptake loss decreased significantly as the CO2 concentration increased to 20% (2.6% loss Table S1).10 In contrast, in the current study, the 45 wt % PEI/Al2O3 sorbent deactivation treatments at 70 °C in both dry and humid 0.04% CO2-air mixtures yielded noticeable losses in CO2 adsorption capacity even after 3 h of deactivation (loss of 17% for dry and 10% for humid). Previously, the presence of H2O was reported to have a positive effect during the oxidative degradation, retarding oxidation;10 however, as seen in Figure 2 and as reported in our recent work, the outcome in our system is reversed.15 When rationalizing these differing results, it is important to note the key differences between the two studies, which include the deactivation temperature (50–120 °C for the Heydari-Gorji and Sayari study and 70 °C for this study), CO2 concentration (1–20% for the Heydari-Gorji and Sayari study and 0.04% for this study), CO2 uptake temperature (75 °C for the Heydari-Gorji and Sayari study and 30 °C for this study), and support type (laboratory-synthesized ordered mesoporous silica vs disordered, commercial mesoporous alumina). Further discussion comparing and contrasting our results to the literature will be presented after additional analysis in the forthcoming sections.
Spectroscopic Analysis
To investigate the accelerated sorbent deactivation in the copresence of CO2 and O2, in situ HATR-IR spectroscopy experiments were performed using a 45 wt % PEI/Al2O3 sample as a model sorbent. Experiments were conducted using different gas/vapor compositions, including dry CO2-free air, 0.04% CO2-air (21% O2 balance N2), and 0.04% CO2–N2 at 70 °C for 3 days (Figure 3 and S2). Complementing the TGA studies above, these experiments allow the elucidation of molecular level changes due to PEI/Al2O3 sorbent deactivation under the conditions described above and the investigation of possible changes in the PEI/Al2O3 structure. The thermal analysis results given above indicate significant deactivation (∼64%) under 0.04% CO2-air after 3 days and no major deactivation under CO2-free air, 0.04% CO2–N2 and N2 until after day 7. As such, all the IR spectroscopy experiments were conducted for 3 days by collecting spectra every 30 min. Figures 3 and S2 show the absorption spectra of PEI/Al2O3 over 3 days at 70 °C under the four experimental conditions.
Figure 3.
IR spectra (1750–1000 cm–1) of PEI/Al2O3 deactivation under (a) 0.04% CO2–N2, (b) 0.04% CO2-air, (c) CO2-free air, and (d) 100% N2 for 3 days (72 h) at 70 °C. (Full spectra (3500–1000 cm–1) in Figure S2.
The main characteristics of a PEI/Al2O3 sorbent in the infrared spectra are two N–H stretching bands of primary amines (3400–3300 and 3330–3250 cm–1), one of secondary amines (3350–3310 cm–1), C–H stretching bands (2950–2720 cm–1), an N–H bending band 1605–1590 cm–1, a C–H bending band ∼1454 cm–1, and C–N stretching bands at ∼1125 and ∼1050 cm–1.29,30
In the early phase of sorbent deactivation under 0.04% CO2–N2 at 70 °C, the adsorbed CO2 dominated the difference spectra, and the thermal deactivation of PEI/Al2O3 should contribute only minimally to the spectral changes under these conditions (see Figure 1). Figure 3a shows bands of species such as the COO– asymmetric stretch (∼1565 cm–1), NH3+ symmetric deformation (∼1540 cm–1), COO– symmetric stretch (∼1428 cm–1), C–N stretching band (∼1405 cm–1), N–COO (∼1330 cm–1), NCOO– skeletal vibration (∼1297 cm–1), and NH2+ at 1631 cm–1, which indicate the formation of ammonium carbamate ion pairs in the early stages of the experiment and their growth in intensity with time.30–43 In CO2 capture studies using amines like PEI, the dominant species that forms due to the interaction of CO2 with primary and secondary amines under dry conditions is the carbamate species.31,33,43–46
Figure 3a–d also shows the bands in the region of 1666 cm–1 that increase in intensity as the treatment time increases. This band has been discussed in the literature as a characteristic of amine-related oxidation products as a result of exposure to O2 and has been assigned to the vibrations of C=N (imines) and/or C=O (carbonyl) groups.29,39,41,47,48 A recent study from our group on the oxidative degradation of the PEI/γ-Al2O3 sorbent at high temperatures illustrated the individual contributions of the imine and carbonyl species to the band in the region around 1666 cm–1 for the first time.15 The study links the C–N bond cleavage events to the formation of carbonyl and imine species, new primary amine species, and other low-molecular-weight amine products.15 To briefly discuss the proposed mechanism(s), in dry conditions, degradation begins with the formation of radical species (α- or β-amino alkyl radicals) due to thermal stress, which leads to chain relaxation and breakage,15 or due to metal impurities within the sorbent that catalyze free radical formation.15,49 In the presence of O2, the radical species react with O2 forming peroxyl radical species (α- or β-amino peroxyl radicals). The peroxyl radicals extract hydrogen from the PEI chain, forming hydroperoxide species (ROOH), which decompose to form carbonyl species in the PEI chain.15 The formation of the carbonyl species requires the cleavage of the C–N bond, and depending on the location of the C–N bond (terminal primary amine, terminal secondary amine, or secondary amine along the PEI chain), volatile organic products (e.g., ammonia and 2-aminoacetaldehyde) and new primary amine species can form.15,50
A simplified deconvolution analysis was performed in the region between 1720 and 1485 cm–1 to distinguish the contributions of the imine and carbonyl species to the 1665 cm–1 band under 0.04% CO2-air conditions. Figure 4a shows the results of the deconvolution analysis under the 0.04% CO2-air condition.
Figure 4.
(a) Deconvoluted spectra of 0.04% CO2-air at the 72nd hour (end of day 3) from 1720 to 1485 cm–1. Integrated peak areas of (b) ∼ 1595 cm–1 (N–H bending band) and (c) ∼1666 cm–1 (C=O/C=N bands) under 0.04% CO2-air and CO2-free air.
Two peaks (1671 and 1648 cm–1) contributed to the 1665 cm–1 band, while only one peak contributed to the 1595 cm–1 band. The band ∼1595 cm–1 represents an N–H bending (N–H deformation) mode.15,29,42 The N–H bending/deformation vibration bands appear in the region between 1650 and 1580 cm–1 for primary and secondary amine species.29 Furthermore, in liquid amines, the N–H bending band gives rise to a shoulder/overtone band near the N–H stretching regions that can be seen from the band around 3135 cm–1 in Figure S2a–d.29 As briefly discussed above, the increased intensity of the N–H bending band has been reported in our recent work as an indication of the formation of new primary amine species as a result of the C–N bond cleavage near the secondary amine sites.15 Similarly, Figure 3b,c shows an increase in the intensity of the bands around 1595 cm–1 with time for 0.04% CO2-air and CO2-free air conditions. The integrated area of the N–H bending band (∼1595 cm–1) for the sample treated in 0.04% CO2-air was larger than that for the sample under CO2-free air (Figure 4b). This result suggests that the formation of new primary and secondary amine species and C–N bond cleavage reactions occurs more frequently in the copresence of CO2 and O2, which agrees well with the thermal analysis results shown in Figure 2, where deactivation of the PEI/γ-Al2O3 sorbent is more significant under a 0.04% CO2-air environment compared to that under a CO2-free air. The disproportional loss of the hydrogen and nitrogen species with respect to carbon species is shown by the C/N and H/(C + N) molar ratios in Figure 5a,b during the deactivation of the sorbent under both dry and humid 0.04% CO2-air conditions. The higher loss of N and H content compared to that of C content is an additional sign of the C–N bond cleavage, as indicated by the spectra in Figure 3b. Figure S3 also shows the loss of hydrogen and nitrogen species and an increase in carbon species under both dry and humid conditions.
Figure 5.
(a) C/N (b) H/(C + N) molar ratio of the deactivated 45 wt % PEI/γ-Al2O3 sorbent as a function of time under dry 0.04% CO2-air and humid 0.04% CO2-air.
The increase in the intensity of the band ∼1665 cm–1 shown in Figure 3b,c under CO2-free air and 0.04% CO2-air environments can be attributed to the formation of carbonyl and imine species based on the deconvolution results in Figure 4a and the evidence for the C–N bond cleavage reactions, as also indicated by the negative bands at 1123 and 1045 cm–1 for 0.04% CO2-air and at 1125 and 1049 cm–1 for CO2-free air. The 1671 cm–1 and the 1648 cm–1 bands, which are the two peaks that contribute to the 1665 cm–1 band, can be attributed to the carbonyl species (C=O) and imine species (C=N), respectively, based on the presence of the 1648 and 1650 cm–1 bands that indicate imine species formation under 0.04% CO2–N2 and N2 environments (O2 free) (Figure 3a,d). Under 0.04% CO2–N2 and under N2, the intensities of the imine bands and the primary amine bands that likely occur due to hydrogen abstraction followed by the C–N bond cleavage are much less pronounced compared to the imine/carbonyl and primary amine band intensities under 0.04% CO2-air. This result suggests similar deactivation under 0.04% CO2–N2 and N2 environments and agrees well with the thermal analysis results for the deactivation of the PEI/γ-Al2O3 sorbent in a 0.04% CO2–N2 and N2 environment at 70 °C, as shown in Figure 2.
The C–N bond cleavage leading to the formation of new primary amine species (as well as other products mentioned above) has been reported as paths of oxidative and thermal degradation at high temperatures in solid amine sorbents.15,16,50 As such, these results suggest the possible occurrence of oxidative and thermal degradation under CO2-free air and 0.04% CO2-air conditions and thermal degradation under 0.04% CO2–N2 and N2 conditions. However, the accelerated PEI/γ-Al2O3 sorbent deactivation and new primary amine species formation under 0.04% CO2-air, as shown in Figures 2 and 4b, indicate the presence of additional reaction mechanism(s) occurring in the presence of CO2.
Metadynamics Simulations
A previous modeling study by Li et al.16 used TETA as a molecular proxy for PEI and assessed the kinetic feasibility of C–N bond cleavage in the presence of radicals but without CO2 using enhanced sampling, i.e., metadynamics simulations.16 To this end, we performed first-principles metadynamics simulations using CO2-bound TETA as a molecular proxy to analogously evaluate the kinetics of the C–N bond cleavage of PEI/Al2O3 under CO2-air conditions.
To model the interactions between CO2 and amines, we constructed bimolecular structural models consisting of one TETA with CO2 adsorbed to a primary amine site, carbamic acid. CO2 adsorbed as an ammonium carbamate ion pair will behave similarly due to the dynamic equilibrium between a hydrogen-bonded carbamic acid–amine pair and the ammonium carbamate. The adsorbed CO2 further interacts with either a primary or secondary amine on another TETA with a preformed alkyl radical, presumably resulting from radical propagation under oxidative conditions. The AIMD-equilibrated structures are presented in Figure 6 and were prepared for metadynamic simulations with a temperature set at 70 °C to replicate the experimental conditions. The metadynamics approach adds periodic biasing potentials to the original potential surfaces along predefined CVs, which discourage the system from revisiting points in the configurational space, resulting in the acceleration of energy barrier crossing for the sampled reactions.51 To determine the kinetics of the C–N bond cleavage, two CVs were defined: C–N coordination number (CNC–N) and N–H coordination number (CNN–H) of amines interacting with the carbamic acid in proximity.
Figure 6.

Time evolution of CVs along the trajectories of metadynamics simulations of the CO2-catalyzed C–N bond cleavage near (a) the primary amine and (b) the secondary amine. Structural snapshots near transition states and product states are shown. Atom color code: C—black, N—blue, O—red, and H—pink.
Figure 6 displays the evolution of CVs during metadynamics simulations for the C–N bond cleavage near the primary and secondary amines. The successful cleavage of the C–N bonds is observed in both cases, as evidenced by sharp decreases in the level of CNC–N from approximately 1 to 0. Additionally, simultaneous increases in CNN–H and the structural snapshots near the transition states demonstrate that for both primary and secondary amines, H transfer occurs from a neighboring amine group to the affected amine, resulting in the formation of NH3 and ethylene diamine, respectively. Scheme 1 illustrates that the carbamic acids function as effective H-shuttles, facilitating the proton and electron transfer necessary to cleave the C–N bonds. The products consist of fragments of TETA and regenerated TETA with a carbamic acid tail. The formation of a new primary amine in the case of the C–N bond cleavage near a secondary amine is consistent with the observations from spectroscopic experiments. Significantly, the sum of the biasing potentials indicates that in the presence of CO2, both reactions of C–N bond cleavage have free-energy barriers of ∼20 kJ mol–1, which are considerably lower compared to that in the absence of CO2 (∼90 kJ mol–1).16 This manifests the strong catalyzing effect of CO2 on the C–N bond cleavage kinetics through acid–base interactions, consistent with the accelerated PEI/γ-Al2O3 sorbent deactivation under 0.04% CO2-air conditions.
Scheme 1. Reaction Pathways of the C–N Bond Cleavage near Primary and Secondary Amines in the Presence of CO2.
Based on the spectroscopic, thermal, and elemental analyses and metadynamics simulations performed in this study and the accumulated knowledge in the aminopolymer degradation literature, we rationalize the accelerated deactivation in the copresence of CO2 and O2. Figure 4b,c shows that the difference in the rate of formation of new primary amines and carbonyl/imine species is not significant for 0.04% CO2-air and CO2-free air. However, the thermal analysis data in Figure 2 showed that the deactivation under 0.04% CO2-air was substantially higher, even at the early stages of deactivation (3 and 18 h). The difference in deactivation between the two conditions suggests that under a CO2-free environment, the C–N cleavage process occurs at a slower rate (compared to 0.04% CO2-air). Based on the oxidative degradation pathway proposed in our recent work15 and the basic autoxidation mechanism,52 the slower rate of C–N cleavage is likely due to the substantial energetic barrier for the decomposition of hydroperoxide species to hydroxyl and alkoxyl radical species in the oxidative degradation process.52 The deactivation temperature studied here (70 °C), which is much lower than those of prior studies, is likely not high enough to allow the decomposition process to occur rapidly. In the copresence of CO2 and O2 (0.04% CO2-air), CO2 reacts with the amine sites forming carbamic acids that can further react with primary or secondary amines in the surrounding region via acid–base interactions. Some of these species become captured CO2 in the form of alkyl ammonium carbamates, while some facilitate H-transfer and C–N bond cleavage forming ammonia, primary amine, or secondary amine species, depending on the site of CO2 adsorption, as supported by the metadynamics simulations. This acid-catalyzed process requires the presence of alkyl radicals on the PEI chain, where C–N cleavage occurs. Oxygen, which is present in much higher concentration than CO2, can then participate in the formation of peroxyl radicals that abstract hydrogen atoms, leading to more alkyl radical species in what appears to be a carbamic acid-catalyzed decomposition reaction. As a result, the carbamic acid-catalyzed deactivation is proposed to provide an alternative pathway for the C–N bond cleavage that bypasses the hydroperoxide decomposition step and subsequently accelerates the deactivation and formation of the various species discussed above.
Adsorption–Desorption Cycles
Cyclic adsorption–desorption experiments were performed to develop relationships with the continuous deactivation study under dry and humid 0.04% CO2/air (21% O2 balance N2) conditions at 70 °C and to observe sorbent deactivation under close-to-realistic DAC process conditions. A typical cyclic process involves the adsorption of CO2 from the atmosphere, consuming most of the process time, followed by the desorption of CO2 captured from the atmosphere and finally cooling of the sorbent to the adsorption temperature. During these adsorption–desorption processes, sorbent materials are exposed to conditions and environments that cause deactivation over an extended period of operation. In practice, on occasions where process upsets occur due to the operational malfunctions or for other reasons, additional deactivation factors are introduced, such as the copresence of high temperatures and high oxygen concentrations.
In these cyclic studies, the 45 wt % PEI/γ-Al2O3 sorbent was exposed to dry or humid (43% RH or 12 mmol H2O/mol air absolute humidity) 0.04% CO2-air for 2 h, which represents roughly two-thirds of the laboratory process/cycle time. The captured CO2 is desorbed at 100 °C for 30 min under dry N2 or humid N2 [1.3% RH (absolute humidity of 12 mmol/mol)] to mimic the vacuum-assisted desorption during a temperature–vacuum swing desorption process. After 30 min of desorption, the temperature is ramped down to 70 °C under N2 (dry or humid). Following that, the sorbent is cooled to the adsorption temperature of 30 °C under dry or humid 0.04% CO2/air (21% w/w O2 balance N2). This cooling step is typically performed in an inert environment such as N2 in prior literature studies of amine sorbent stability; the conditions here more closely mimic those that might be encountered in practical operation. The cooling process takes about 30 min on average in the laboratory but would be more rapid in realistic, low-pressure drop gas–solid contactors. Cooling the sorbent under CO2-containing air (atmospheric concentration air) removes the use of inert gases in this step, which is common in the laboratory, and it allows for CO2 to be captured during the cooling process. In addition, cooling using atmospheric concentration air eliminates the use of inert gas, reducing the energy and process cost. Figure 7 shows the adsorption–desorption cycle used in this study.
Figure 7.

Adsorption–desorption cycle deployed in the TGA cycling.
During the adsorption and cooling process, the sorbent interacts with O2 and CO2 at low and intermediate temperatures after desorption in flowing N2 (simulating vacuum) at higher temperatures. In this cyclic process, the most important time when the oxidative deactivation would be a concern is during the cooling process where the sorbent is exposed to intermediate temperatures under the copresence of CO2 and O2, leading up to the next adsorption step. The results in Figure 8a,b show that 30 adsorption–desorption cycles result in a CO2 uptake loss of 23% under dry and 48% humid conditions as well as a gradual loss in the sorbent mass (∼6% for dry and ∼4% for humid). Consistent with the continuous deactivation study in Figure 2, the copresence of CO2 and O2 results in noticeable deactivation under cyclic conditions, and the use of consecutive cycles seeks to ascertain if numerous short exposures begin to approach the deactivation of extended exposure times.
Figure 8.
Sorbent mass and CO2 adsorption capacity change after 30 cycles under dry (a) and humid (b) 0.04% CO2-air.
Another interesting finding from this study is that during continuous deactivation, the presence of H2O only slightly affected the sorbent deactivation in the copresence of CO2 and O2, as shown in Figure 2. On the contrary, under cyclic conditions, the presence of H2O shows considerable deactivation after 30 cycles compared to the dry cyclic condition (about 2× higher). This might be due to H2O gaining more access to the PEI domain and radical species in the absence of CO2 (desorption and ramp down step), which can lead to accelerated deactivation.15 Another possible explanation for the difference in the CO2 capacity loss between dry and humid conditions could be the higher CO2 adsorption capacity obtained in the first few cycles under humid conditions. The presence of H2O during CO2 adsorption enhances the amine efficiency of the sorbent (CO2 adsorption capability of the sorbent) by accelerating the formation of hydronium carbamate and ammonium carbamate.37,53 However, once the sorbent deactivation starts to occur, the presence of humidity plays a reverse role, in which it participates in accelerating the loss of the CO2 adsorption capacity of the sorbent15 under some conditions. As such, the drop in CO2 adsorption capacity after deactivation starts is reduced in dry conditions compared to that in humid conditions. Notably, this result is consistent with our recent finding on the effect of humidity in accelerating the sorbent deactivation that shows 2× faster deactivation in the presence of H2O conditions.15
It is important to note that the cyclic CO2 uptake under humid conditions was determined by performing the same cyclic experiment under humid N2 for 15 cycles and then subtracting the H2O vapor uptake from that measured in the 0.04% CO2-air humid cyclic experiment. During the humid N2 cyclic experiment, the H2O adsorbed per cycle was around 2.2 mg (shown in Figure S4). In the 0.04% CO2-air humid cyclic experiment, this amount will most likely be less than 2.2 mg due to the competitive interaction between CO2 and H2O vapor with the amine sites during the adsorption step. As such, the CO2 uptake reported in Figure 8b for the humid cyclic conditions likely slightly underestimates the actual capacity.
The total time the sample was exposed to elevated temperature, during the desorption step at 100 °C and the cooling step from 100 to 70 °C, sums up to approximately 17 h across the 30 cycles. During these steps, the sample is only exposed to an inert atmosphere, and so, the thermal degradation due to the loss of sorbent mass is the dominant loss mechanism, 6 and 4% for the dry and humid cyclic conditions, respectively.
The total time the sample was exposed to oxygen, during the cooling step from 70 to 30 °C and the following 2 h adsorption step at 30 °C, sums up to approximately 3 days across the 30 cycles. Therefore, it is reasonable to compare the loss in capacity across the cyclic experiment with the 3 day continuous deactivation experiment. Under both conditions, the loss in capacity under the cyclic conditions is lower (dry: 29% vs 64%, humid: 52% vs 68%). This difference in capacity loss shows that continuous exposure exaggerates deactivation. However, continuous deactivation studies can help estimate the lifetime of a sorbent over an extended period of operation if suitable correlations can be developed for real operation. As such, below, we will use the deactivation mechanism(s) presented in Scheme 1 to rationalize the differences in capacity loss between the continuous and cyclic conditions.
One of the primary reaction intermediates in the deactivation of the sorbent, the peroxyl radicals, forms by the reaction of the alkyl radicals with molecular oxygen. The peroxyl radicals form at similar rates at any temperature once the alkyl radicals form and the sorbent is exposed to an O2-containing stream.52,54 However, the subsequent step of the reaction, the formation of hydroperoxide species, requires the abstraction of a hydrogen atom from a C–H bond, and this reaction is temperature-dependent. At higher temperatures, more energy is available to overcome the activation energy required for the hydrogen abstraction.52,54 Similarly, the rate of decomposition of the hydroperoxide species to alkoxyl and hydroxyl radical species increases with temperature.52,54 As such, during the cooling (from 70 to 30 °C) and adsorption step (at 30 °C) of the cyclic process, it is expected to have a lower concentration of hydroperoxide species and that the decomposition of hydroperoxide species occurs at a slower rate compared to continuous deactivation cases at 70 °C. It is also important to note that during the cyclic process, the limited coexistence of O2 and heat compared to the continuous deactivation likely plays a role in maintaining sorbent stability. Correspondingly, during the cooling and adsorption periods of the cyclic process, the recombination reactions of the radical species may occur, causing the sorbent to “recover” some stability. These reasons together lead to delayed deactivation in the cyclic processes. Furthermore, in industrial-scale adsorption–desorption units, the adsorption, desorption, and cooling time are much shorter than the conditions used in this cyclic study, leading to even slower sorbent deactivation. In the Supporting Information, we provide various simple mathematical fits based on the cyclic deactivation data to estimate the sorbent lifetime. However, high-fidelity predictions require a much larger data set than is available here.
Like the continuous deactivation studies, we compare and contrast our results from the cyclic experiments with those of the literature. To the best of our knowledge, there have not been any adsorption–desorption cyclic studies on the impact of the copresence of CO2 and O2 in amine sorbent degradation under dry and humid conditions. The closest relevant example is the study by Heydari-Gorji and Sayari, which performed cyclic studies under humid and dry pure CO2 (adsorption at 75 °C and desorption at 105 and 120 °C under N2 for 30 min each) and humid 15% CO2/N2 (adsorption at 50 °C and desorption at 85 °C under humid N2 for 30 min each) for 66 cycles. Their results showed a significantly higher loss in CO2 uptake under the dry conditions compared to that under the humid conditions studied (dry: 40 and 52% loss, humid: 2 and 3.5% loss, at 105 and 120 °C desorption conditions, respectively).10 Similar to the pure humid CO2 conditions, the sample treated in humid 15% CO2/N2 also showed relatively little loss after 66 cycles.10 The authors attributed the significant loss under dry conditions to urea formation due to the exposure to high CO2 concentrations. In the presence of humidity, however, urea formation is suppressed, and sorbent stability is maintained.10
To rationalize the discrepancy in the continuous deactivation behavior of the two studies, we consider the major differences between the two studies, including the support type, CO2 uptake temperature, and CO2 concentration. First, we note that γ-Al2O3, the mesoporous oxide support used in this study, is often produced by extracting alumina from metakaolin, a dehydroxylated form of the clay mineral kaolinite.55 As a result, γ-Al2O3 often has trace metal impurities in higher concentration than that of the laboratory-synthesized silica supports, as used by Heydari-Gorji and Sayari.10 Mesoporous silica SBA-15, often synthesized in the laboratory from Pluronic P-123 and tetraethyl orthosilicate (TEOS), has notably lower transition metal impurities due to the use of TEOS in the synthesis.56 Metal impurities are one of the key contributing factors in initiating alkyl radical species formation and accelerating sorbent deactivation.49 A recent study from our group reported higher metal impurities (mainly Fe and Ni) in γ-Al2O3 (similar to γ-Al2O3 used in this study) compared to that in SBA-15.15 The presence of such transition metals in higher concentrations can lead to O2 activation, accelerating oxidation reactions, thereby catalyzing the sorbent deactivation process. As such, the higher concentration of the metal impurities in γ-Al2O3 compared to that in SBA-15 is likely one reason for the differences observed in the loss in CO2 uptake between the two studies.
Additional factors that also may contribute to the differences in stability are the conditions used for the CO2 uptake measurement during and after the deactivation experiments (specifically CO2 concentration and CO2 uptake temperature). In the study by Heydari-Gorji and Sayari,10 long-term deactivation experiments were performed under different CO2/O2/N2 concentrations (1–20%/10.5–17%/balance N2) and temperatures ranging from 50 to 120 °C for 30 h, whereas in this work, deactivation experiments were performed under 0.04% CO2-air, 0.04% CO2–N2, and CO2-free air at 70 °C. Our first-principles modeling indicates that the adsorbed CO2 significantly decreases the free-energy barrier of the C–N bond cleavage, likely altering the oxidation rate-determining step from the C–N bond cleavage (either through direct cleavage or alkyl hydroperoxide decomposition50) to radical propagation that generates the necessary on-site alkyl radicals before cleaving the C–N bonds. In the CO2 concentrations explored in the Heydari-Gorji and Sayari study, the interaction of the amines and CO2 occurs quickly. This rapid interaction between CO2 and the amine sites due to the high concentration of CO2 interlocks the amine chains, slowing down radical propagation, likely preventing the carbamic acid-catalyzed C–N bond cleavage from occurring and the additional CO2 and O2 from fully accessing the polymer domains. Consequently, in the work of Heydari-Gorji and Sayari, the sorbent stability was more effectively maintained under the deactivation conditions explored. This hypothesis is consistent with the studies probing the diffusion of CO2 through supported PEI as a function of CO2 concentration and time, where concentrated CO2 rapidly cross-links the amine chains and limits further CO2 and O2 penetration into the bulk of the sorbent,57 as well as studies that invoke such behavior to rationalize the cases where comparable CO2 uptakes were achieved at both air and flue gas CO2 concentrations.58
Conclusions
We have investigated the long-term stability of the PEI/Al2O3 sorbent under various environments (O2, CO2, and H2O) at an intermediate temperature of 70 °C. The thermal analysis results for oxidative and thermal degradation show a similar impact on the sorbent stability until day 7. After day 7, the oxidative degradation condition becomes dominant, showing significant sorbent deactivation compared to the exclusively thermal conditions. In the copresence of CO2 and O2 (0.04% CO2/21% O2) in both humid and dry streams, sorbent deactivation is further accelerated, eliminating the induction period observed in the early stages of oxidative degradation experiments.
In situ HATR-IR experiments reveal that the presence of CO2, which leads to surface carbamic acid and ammonium carbamate species, contributes to accelerated oxidative degradation and formation of new primary amine, carbonyl, and imine species, which is the evidence for the C–N and C–H bond cleavages due to oxidative degradation. Based on metadynamics calculations, we hypothesize that the carbamic acid species can catalyze the cleavage of the C–N bonds on adjacent amine chains that contain a carbon-based radical, leading to loss of some amine binding sites.
Sorbent stability studies under close-to-realistic cyclic DAC conditions (30 adsorption–desorption cycles) show a gradual loss in stability (dry: 29%, humid: 52%) under 0.04% CO2-air. The loss in capacity during the sorption/desorption cycling is significantly less than the degradation under continuous deactivation conditions, as expected.
The main limitations of this study relative to practical DAC deployments include (i) the use of a TGA for cycling experiments, which differs in flow characteristics from practical DAC gas/solid contactor based on monoliths, fibers, or laminates and the (ii) differing cycle times that result from such TGA use, compared to the more practical contactors. Additionally, desorption is done under a gas purge in these studies, instead of through use of vacuum and/or steam flow, which are favored in some large-scale process designs. Finally, the work here and elsewhere15 demonstrate that sorbent degradation is impacted by the nature of the supports used, so further extrapolation is not facile to other systems. Nonetheless, performing studies under these close-to realistic conditions (compared to existing literature studies) aids close comparison to industrial scale DAC operations and provides means for the expedited development and implementation of the sorbent materials with significantly improved stability.
Acknowledgments
This work was initiated with financial support from Global Thermostat. This work was supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences, Materials Science and Engineering Division. Work at LLNL was done under the auspices of the U.S. DOE Contract DE-AC52-07NA27344. The National Energy Research Scientific Computing Center (BES-ERCAP0023097) and the LLNL Grand Challenge Program provided the computational resources. We also thank Dr. Pawel Chmielniak and Dominik Groh for their help with peak deconvolution and SEM imaging, respectively.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.3c08140.
45 wt % PEI/γ-Al2O3 sorbent thermogravimetric combustion data, IR spectra (3500–1000 cm–1) under various environments, changes in C, H, and N content after heating under dry and humid 0.04% CO2-air, cyclic H2O uptake under humid N2 and mathematical fits to cyclic deactivation data, literature values of CO2 uptake loss of 55 wt.% PEI (branched, Mn ∼ 600)-SBA-15 sorbent after 30 h under prehumidified CO2/O2/N2 mixtures, structures of TETA-carbamic acid/TETA complexes interacting at primary amine and secondary amine sites, and effect of CO2 concentration on sorbent stability in the copresence of CO2 and O2 (PDF)
The authors declare the following competing financial interest(s): C.W.J. has a financial interest in Global Thermostat, which seeks to commercialize CO2 capture from air. C.W.J. has a conflict-of-interest management plan in place at Georgia Tech.
Supplementary Material
References
- Corner S. P.The IPCC Sixth Assessment Report on Climate Change Impacts. Popul. Dev. Rev. 2022, 48 ( (2), ), 629–633. 10.1111/padr.12497. [DOI] [Google Scholar]
- Rogelj J.; Luderer G.; Pietzcker R. C.; Kriegler E.; Schaeffer M.; Krey V.; Riahi K. Energy system transformations for limiting end-of-century warming to below 1.5 °C. Nat. Clim. Change 2015, 5 (6), 519–527. 10.1038/nclimate2572. [DOI] [Google Scholar]
- Boot-Handford M. E.; Abanades J. C.; Anthony E. J.; Blunt M. J.; Brandani S.; Mac Dowell N.; Fernandez J. R.; Ferrari M. C.; Gross R.; Hallett J. P.; Haszeldine R. S.; Heptonstall P.; Lyngfelt A.; Makuch Z.; Mangano E.; Porter R. T. J.; Pourkashanian M.; Rochelle G. T.; Shah N.; Yao J. G.; Fennell P. S. Carbon Capture and Storage Update. Energy Environ. Sci. 2014, 7 (1), 130–189. 10.1039/c3ee42350f. [DOI] [Google Scholar]
- Med N. A. S. E.Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; National Academies Press, 2019; pp 1–495. [PubMed] [Google Scholar]
- Rosa L.; Sanchez D. L.; Mazzotti M. Assessment of Carbon Dioxide Removal Potential via BECCS in a Carbon-Neutral Europe. Energy Environ. Sci. 2021, 14 (5), 3086–3097. 10.1039/d1ee00642h. [DOI] [Google Scholar]
- Jones C. W. Metal-Organic Frameworks and Covalent Organic Frameworks: Emerging Advances and Applications. JACS Au 2022, 2 (7), 1504–1505. 10.1021/jacsau.2c00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Perez E. S.; Murdock C. R.; Didas S. A.; Jones C. W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116 (19), 11840–11876. 10.1021/acs.chemrev.6b00173. [DOI] [PubMed] [Google Scholar]
- Rim G.; Kong F. H.; Song M. Y.; Rosu C.; Priyadarshini P.; Lively R. P.; Jones C. W. Sub-Ambient Temperature Direct Air Capture of CO2 using Amine-Impregnated MIL-101(Cr) Enables Ambient Temperature CO2 Recovery. JACS Au 2022, 2 (2), 380–393. 10.1021/jacsau.1c00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. C.; Xie W. W.; Wu J. Y.; Miao Y. H.; Xiang C. J.; Chen C. P.; Ge B. Y.; Gan Z. Z.; Yang F.; Zhang M.; O’Hare D.; Li J.; Ge T. S.; Wang R. Z. Recent Advances in Direct Air Capture by Adsorption. Chem. Soc. Rev. 2022, 51 (15), 6574–6651. 10.1039/d1cs00970b. [DOI] [PubMed] [Google Scholar]
- Heydari-Gorji A.; Sayari A. Thermal, Oxidative, and CO2-Induced Degradation of Supported Polyethylenimine Adsorbents. Ind. Eng. Chem. Res. 2012, 51 (19), 6887–6894. 10.1021/ie3003446. [DOI] [Google Scholar]
- Sayari A.; Heydari-Gorji A.; Yang Y. CO2-Induced Degradation of Amine-Containing Adsorbents: Reaction Products and Pathways. J. Am. Chem. Soc. 2012, 134 (33), 13834–13842. 10.1021/ja304888a. [DOI] [PubMed] [Google Scholar]
- Didas S. A.; Zhu R. S.; Brunelli N. A.; Sholl D. S.; Jones C. W. Thermal, Oxidative and CO2 Induced Degradation of Primary Amines Used for CO2 Capture: Effect of Alkyl Linker on Stability. J. Phys. Chem. C 2014, 118 (23), 12302–12311. 10.1021/jp5025137. [DOI] [Google Scholar]
- Drage T. C.; Arenillas A.; Smith K. M.; Snape C. E. Thermal Stability of Polyethylenimine Based Carbon Dioxide Adsorbents and its Influence on Selection of Regeneration Strategies. Microporous Mesoporous Mater. 2008, 116 (1–3), 504–512. 10.1016/j.micromeso.2008.05.009. [DOI] [Google Scholar]
- Sakwa-Novak M. A.; Jones C. W. Steam Induced Structural Changes of a Poly(ethylenimine) Impregnated gamma-Alumina Sorbent for CO2 Extraction from Ambient Air. ACS Appl. Mater. Interfaces 2014, 6 (12), 9245–9255. 10.1021/am501500q. [DOI] [PubMed] [Google Scholar]
- Carneiro J. S. A.; Innocenti G.; Moon H. J.; Guta Y.; Proano L.; Sievers C.; Sakwa-Novak M. A.; Ping E. W.; Jones C. W. Insights into the Oxidative Degradation Mechanism of Solid Amine Sorbents for CO2 Capture from Air: Roles of Atmospheric Water. Angew. Chem., Int. Ed. 2023, 62, e202302887 10.1002/anie.202302887. [DOI] [PubMed] [Google Scholar]
- Racicot J.; Li S. C.; Clabaugh A.; Hertz C.; Akhade S. A.; Ping E. W.; Pang S. H.; Sakwa-Novak M. A. Volatile Products of the Autoxidation of Poly(ethylenimine) in CO2 Sorbents. J. Phys. Chem. C 2022, 126 (20), 8807–8816. 10.1021/acs.jpcc.1c09949. [DOI] [Google Scholar]
- Nezam I.; Xie J. W.; Golub K. W.; Carneiro J.; Olsen K.; Ping E. W.; Jones C. W.; Sakwa-Novak M. A. Chemical Kinetics of the Autoxidation of Poly(ethylenimine) in CO2 Sorbents. ACS Sustain. Chem. Eng. 2021, 9 (25), 8477–8486. 10.1021/acssuschemeng.1c01367. [DOI] [Google Scholar]
- Li W.; Bollini P.; Didas S. A.; Choi S.; Drese J. H.; Jones C. W. Structural Changes of Silica Mesocellular Foam Supported Amine-Functionalized CO2 Adsorbents Upon Exposure to Steam. ACS Appl. Mater. Interfaces 2010, 2 (11), 3363–3372. 10.1021/am100786z. [DOI] [PubMed] [Google Scholar]
- Chaikittisilp W.; Kim H. J.; Jones C. W. Mesoporous Alumina-Supported Amines as Potential Steam-Stable Adsorbents for Capturing CO2 from Simulated Flue Gas and Ambient Air. Energy Fuel. 2011, 25 (11), 5528–5537. 10.1021/ef201224v. [DOI] [Google Scholar]
- Grossmann Q.; Stampi-Bombelli V.; Yakimov A.; Docherty S.; Coperet C.; Mazzotti M. Developing Versatile Contactors for Direct Air Capture of CO2 through Amine Grafting onto Alumina Pellets and Alumina Wash-Coated Monoliths. Ind. Eng. Chem. Res. 2023, 62, 13594–13611. 10.1021/acs.iecr.3c01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutz S.; Bardow A. Life-cycle assessment of an industrial direct air capture process based on temperature-vacuum swing adsorption. Nat. Energy 2021, 6 (2), 203–213. 10.1038/s41560-020-00771-9. [DOI] [Google Scholar]
- Barrett E. P.; Joyner L. G.; Halenda P. P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73 (1), 373–380. 10.1021/ja01145a126. [DOI] [Google Scholar]
- Kresse G.; Furthmuller J. Efficient Iterative Schemes for ab initio Total-Energy Calculations using a Plane-Wave Basis Set. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 54 (16), 11169–11186. 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- Blochl P. E. Projector Augmented-Wave Method. Phys. Rev. B: Condens. Matter Mater. Phys. 1994, 50 (24), 17953–17979. 10.1103/physrevb.50.17953. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Joubert D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B: Condens. Matter Mater. Phys. 1999, 59 (3), 1758–1775. 10.1103/physrevb.59.1758. [DOI] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77 (18), 3865–3868. 10.1103/physrevlett.77.3865. [DOI] [PubMed] [Google Scholar]
- Grimme S.; Antony J.; Ehrlich S.; Krieg H. A Consistent and Accurate ab initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132 (15), 154104. 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Li S. C.; Zheng Y.; Gao F.; Szanyi J.; Schneider W. F. Experimental and Computational Interrogation of Fast SCR Mechanism and Active Sites on H-Form SSZ-13. ACS Catal. 2017, 7 (8), 5087–5096. 10.1021/acscatal.7b01319. [DOI] [Google Scholar]
- Colthup N. B.; Daly L. H.; Wiberley S. E.. Introduction to Infrared and Raman Spectroscopy; Academic Press, 1990; pp 339–348. [Google Scholar]
- Wilfong W. C.; Srikanth C. S.; Chuang S. S. C. In Situ ATR and DRIFTS Studies of the Nature of Adsorbed CO2 on Tetraethylenepentamine Films. ACS Appl. Mater. Interfaces 2014, 6 (16), 13617–13626. 10.1021/am5031006. [DOI] [PubMed] [Google Scholar]
- Didas S. A.; Sakwa-Novak M. A.; Foo G. S.; Sievers C.; Jones C. W. Effect of Amine Surface Coverage on the Co-Adsorption of CO2 and Water: Spectral Deconvolution of Adsorbed Species. J. Phys. Chem. Lett. 2014, 5 (23), 4194–4200. 10.1021/jz502032c. [DOI] [PubMed] [Google Scholar]
- Foo G. S.; Lee J. J.; Chen C. H.; Hayes S. E.; Sievers C.; Jones C. W. Elucidation of Surface Species through in Situ FTIR Spectroscopy of Carbon Dioxide Adsorption on Amine-Grafted SBA-15. ChemSusChem 2017, 10 (1), 266–276. 10.1002/cssc.201600809. [DOI] [PubMed] [Google Scholar]
- Said R. B.; Kolle J. M.; Essalah K.; Tangour B.; Sayari A. A Unified Approach to CO2-Amine Reaction Mechanisms. ACS Omega 2020, 5 (40), 26125–26133. 10.1021/acsomega.0c03727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Zhai Y. X.; Chuang S. S. C. Water Enhancement in CO2 Capture by Amines: An Insight into CO2-H2O Interactions on Amine Films and Sorbents. Ind. Eng. Chem. Res. 2018, 57 (11), 4052–4062. 10.1021/acs.iecr.7b05114. [DOI] [Google Scholar]
- Miller D. D.; Yu J.; Chuang S. S. C. Unraveling the Structure and Binding Energy of Adsorbed CO2/H2O on Amine Sorbents. J. Phys. Chem. C 2020, 124 (45), 24677–24689. 10.1021/acs.jpcc.0c04942. [DOI] [Google Scholar]
- Yu J.; Chuang S. S. C. The Structure of Adsorbed Species on Immobilized Amines in CO2 Capture: An in Situ IR Study. Energy Fuel. 2016, 30 (9), 7579–7587. 10.1021/acs.energyfuels.6b01423. [DOI] [Google Scholar]
- Yu J.; Chuang S. S. C. The Role of Water in CO2 Capture by Amine. Ind. Eng. Chem. Res. 2017, 56 (21), 6337–6347. 10.1021/acs.iecr.7b00715. [DOI] [Google Scholar]
- Zhai Y. X.; Chuang S. S. C. The Nature of Adsorbed Carbon Dioxide on Immobilized Amines during Carbon Dioxide Capture from Air and Simulated Flue Gas. Energy Technol. 2017, 5 (3), 510–519. 10.1002/ente.201600685. [DOI] [Google Scholar]
- Srikanth C. S.; Chuang S. S. C. Infrared Study of Strongly and Weakly Adsorbed CO2 on Fresh and Oxidatively Degraded Amine Sorbents. J. Phys. Chem. C 2013, 117 (18), 9196–9205. 10.1021/jp311232f. [DOI] [Google Scholar]
- Tumuluri U.; Isenberg M.; Tan C. S.; Chuang S. S. C. In Situ Infrared Study of the Effect of Amine Density on the Nature of Adsorbed CO2 on Amine-Functionalized Solid Sorbents. Langmuir 2014, 30 (25), 7405–7413. 10.1021/la501284y. [DOI] [PubMed] [Google Scholar]
- Zhai Y. X.; Chuang S. S. C. Enhancing Degradation Resistance of Polyethylenimine for CO2 Capture with Cross-Linked Poly(vinyl alcohol). Ind. Eng. Chem. Res. 2017, 56 (46), 13766–13775. 10.1021/acs.iecr.7b03636. [DOI] [Google Scholar]
- Miller D. D.; Chuang S. S. C. Control of CO2 Adsorption and Desorption Using Polyethylene Glycol in a Tetraethylenepentamine Thin Film: An In Situ ATR and Theoretical Study. J. Phys. Chem. C 2016, 120 (44), 25489–25504. 10.1021/acs.jpcc.6b09506. [DOI] [Google Scholar]
- Hedin N.; Bacsik Z. Perspectives on the Adsorption of CO2 on Amine-Modified Silica Studied by Infrared Spectroscopy. Curr. Opin. Green Sustainable Chem. 2019, 16, 13–19. 10.1016/j.cogsc.2018.11.010. [DOI] [Google Scholar]
- Hahn M. W.; Jelic J.; Berger E.; Reuter K.; Jentys A.; Lercher J. A. Role of Amine Functionality for CO2 Chemisorption on Silica. J. Phys. Chem. B 2016, 120 (8), 1988–1995. 10.1021/acs.jpcb.5b10012. [DOI] [PubMed] [Google Scholar]
- Cendak T.; Sequeira L.; Sardo M.; Valente A.; Pinto M. L.; Mafra L. Detecting Proton Transfer in CO2 Species Chemisorbed on Amine-Modified Mesoporous Silicas by Using 13C NMR Chemical Shift Anisotropy and Smart Control of Amine Surface Density. Chem.—Eur. J. 2018, 24 (40), 10136–10145. 10.1002/chem.201800930. [DOI] [PubMed] [Google Scholar]
- Bacsik Z.; Hedin N. Effects of Carbon Dioxide Captured from Ambient Air on the Infrared Spectra of Supported Amines. Vib. Spectrosc. 2016, 87, 215–221. 10.1016/j.vibspec.2016.10.006. [DOI] [Google Scholar]
- Morsch S.; Liu Y. W.; Lyon S. B.; Gibbon S. R.; Gabriele B.; Malanin M.; Eichhorn K. J. Examining the Early Stages of Thermal Oxidative Degradation in Epoxy-Amine Resins. Polym. Degrad. Stab. 2020, 176, 109147. 10.1016/j.polymdegradstab.2020.109147. [DOI] [Google Scholar]
- Srikanth C. S.; Chuang S. S. C. Spectroscopic Investigation into Oxidative Degradation of Silica-Supported Amine Sorbents for CO2 Capture. ChemSusChem 2012, 5 (8), 1435–1442. 10.1002/cssc.201100662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K.; Choi W.; Kim C.; Choi M. Oxidation-Stable Amine-containing Adsorbents for Carbon Dioxide Capture. Nat. Commun. 2018, 9, 726. 10.1038/s41467-018-03123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. C.; Ceron M. R.; Eshelman H. V.; Varni A. J.; Maiti A.; Akhade S.; Pang S. H. Probing the Kinetic Origin of Varying Oxidative Stability of Ethyl- vs. Propyl-spaced Amines for Direct Air Capture. ChemSusChem 2023, 16, e202201908 10.1002/cssc.202201908. [DOI] [PubMed] [Google Scholar]
- Laio A.; Parrinello M. Escaping Free-Energy Minima. Proc. Natl. Acad. Sci. U.S.A. 2002, 99 (20), 12562–12566. 10.1073/pnas.202427399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel H.; Maier R. D.; Schiller M.. Plastics Additives Handbook; Hanser Publications, 2009; pp 3–9. [Google Scholar]
- Li K. J.; Kress J. D.; Mebane D. S. The Mechanism of CO2 Adsorption under Dry and Humid Conditions in Mesoporous Silica-Supported Amine Sorbents. J. Phys. Chem. C 2016, 120 (41), 23683–23691. 10.1021/acs.jpcc.6b08808. [DOI] [Google Scholar]
- Smith L. M.; Aitken H. M.; Coote M. L. The Fate of the Peroxyl Radical in Autoxidation: How Does Polymer Degradation Really Occur?. Acc. Chem. Res. 2018, 51 (9), 2006–2013. 10.1021/acs.accounts.8b00250. [DOI] [PubMed] [Google Scholar]
- Salahudeen N.; Ahmed A. S.; Al-Muhtaseb A. H.; Dauda M.; Waziri S. M.; Jibril B. Y. Synthesis of Gamma Alumina from Kankara Kaolin using a Novel Technique. Appl. Clay Sci. 2015, 105–106, 170–177. 10.1016/j.clay.2014.11.041. [DOI] [Google Scholar]
- Pang S. H.; Lively R. P.; Jones C. W. Oxidatively-Stable Linear Poly(propylenimine)-Containing Adsorbents for CO2 Capture from Ultradilute Streams. ChemSusChem 2018, 11 (15), 2628–2637. 10.1002/cssc.201800438. [DOI] [PubMed] [Google Scholar]
- Vallace A.; Ren Y.; Jones C. W.; Lively R. P. Kinetic Model Describing Self-Limiting CO2 Diffusion in Supported Amine Adsorbents. Chem. Eng. J. 2023, 472, 144838. [Google Scholar]
- Kwon H. T.; Sakwa-Novak M. A.; Pang S. H.; Sujan A. R.; Ping E. W.; Jones C. W. Aminopolymer-Impregnated Hierarchical Silica Structures: Unexpected Equivalent CO2 Uptake under Simulated Air Capture and Flue Gas Capture Conditions. Chem. Mater. 2019, 31 (14), 5229–5237. 10.1021/acs.chemmater.9b01474. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.