Abstract
PROBLEM:
Ascending bacterial infection is associated with ~ 40% of spontaneous preterm birth (PTB), and Ureaplasma spp. is one of the most common bacteria isolated from the amniotic fluid. Developing novel in vitro models that mimic in vivo uterine physiology is essential to study microbial pathogenesis. We utilized the feto-maternal interface organ-on-chip (FMi-OOC) device and determined the propagation of Ureaplasma parvum, and its impact on cell signaling and inflammation.
METHOD OF STUDY:
FMi-OOC is a microphysiologic device mimicking fetal membrane/decidua interconnected through microchannels. The impact of resident decidual CD45+ leukocytes was also determined by incorporating them into the decidual chamber in different combinations with U. parvum. We tested the propagation of live U. parvum from the decidual to the amniochorion membranes ( immunocytochemistry and quantitative PCR), determined its impact on cytotoxicity (LDH assay), cell signaling (JESS™ Western Blot), cellular transition (immunostaining for vimentin and cytokeratin), and inflammation (cytokine bead array).
RESULTS:
U. parvum transversed the chorion and reached the amnion epithelium after 72 hours but did not induce cell signaling kinases (p38MAPK and JNK) activation, or cellular transition (epithelial-mesenchymal), regardless of the presence of immune cells. The inflammatory response was limited to the choriodecidual interface and did not promote inflammation in the amnion layer.
CONCLUSIONS:
Our data suggest that U. parvum is poorly immunogenic and does not produce massive inflammatory changes at the feto-maternal interface. We speculate that the presence of U. parvum may still compromise the feto-maternal interface making it susceptible to other pathogenic infection.
Keywords: infection, inflammation, fetal membrane, decidua, pregnancy, organ-on-chip, preterm birth
Introduction
Preterm birth (PTB), the delivery before 37 weeks of gestation, is the leading cause of neonatal mortality and morbidity worldwide. Annually, 15 million babies are born preterm1, corresponding to 5% to 18% of total pregnancies2. Most of the PTBs are spontaneous with unknown etiology; however, ascending infection contributes to 40% of those cases3. In most cases, the infection is polymicrobial, and the most common bacteria isolated associated with PTB include genital mycoplasmas, such as Ureaplasma spp.4.
The ambiguity in the association of Ureaplasma spp. with adverse pregnancy outcomes, such as preterm premature rupture of membranes (pPROM), preterm labor (PTL), and PTB, was demonstrated recently in a systematic review5. Most associations, or lack thereof, can be explained by heterogeneities in studies establishing Ureaplasma parvum as a ‘causal’ pathogen. Various factors contribute to heterogeneities but are not limited to the differences in the timing (gestational age) and type of sampling for microbial detection, microbial load, type of approach used for microbial identification, and whether the infection was detected on the maternal or fetal side5. An association between the Ureaplasma spp. and inflammation has been shown in the literature; however, these data also remain inconclusive 6–9 for the reasons mentioned above. In summary, the systematic review and other studies reported that Ureaplasma spp. alone cannot produce the massive inflammation required to trigger the events leading to preterm labor5,10–12. This systematic review’s conclusion is consistent with a study on fetal membranes that reported a lack of proinflammatory response by genital mycoplasmas in the absence of a polymicrobial etiology that should also include Gardnerella vaginalis, Escherichia coli 10. Similar conclusions were made on ascending infection and PTB using animal model studies13,14. Conversely, direct administration of U. parvum into the amniotic fluid can cause PTB suggesting that feto-maternal interface (FMi) is a major barrier for the propagation of a load of microbe needed to reach the amniotic cavity to cause a fetal inflammatory response needed to drive parturition signals15. The cell signaling associated with infection that leads to adverse gestational outcomes has also been studied in recent years. Both p38 MAPK and Jun N-terminal kinase (JNK) are known to be involved in both physiologic and pathologic labor in crucial mechanisms, such as regulation of inflammatory cytokines genes, and apoptosis 16–18. Group B Streptococcus (GBS) and lipopolysaccharide (LPS) infection were shown to increase the phosphorylated levels of p38 MAPK and JNK 19,20.
Better in vitro models are needed to explain the inconclusive role of Ureaplasma spp. in PTB and other adverse outcomes after it ascends from the vagina through the cervical barriers. Recent advancements in microphysiologic models, like organ-on-chip (OOC) devices, have helped mimic different intrauterine organs. Here, multiple cell types can be cultured in an interconnected multiwell device mimicking an organ system as seen in utero 21–23. Our group has established several models of OOC recently14,24–26, and the fetal membrane feto-maternal interface organ-on-chip (FMi-OOC) 27 recreated both maternal (i.e., decidua) and fetal sides (i.e., amniochorion). This model has been used previously to test pathogenic changes caused by infectious agents (Lipopolysaccharides [LPS]) 27, environmental chemicals at the FMi 28, and the pharmacokinetics of drugs 26. In addition, an OOC device consisting of vaginal-cervical-decidual regions has reported U. parvum infection in the lower genital tract 14,29, showing that these models can be used to study infection-associated pathophysiological processes.
Current in vitro 2D and in vivo animal models have limitations in generating supportive evidence for the hypothesis that Ureaplasma is mechanistically associated with producing inflammation required at the FMi tissues. To better understand the pathogenesis of the bacteria, it is essential to know the role of immune cells in ascending infection since pregnancy shows a balanced immune response at the FMi. Studies have shown that CD45+ leukocytes (e.g., ~66 % T cells, ~13% NK cells, ~4% macrophages, ~4% B cells, ~1.2% neutrophils) 30 are increased in fetal membranes from at-term labor compared to a term not in labor subjects 31. The objective of this study is to recreate the FMi in vitro using an OOC device to test the pathogenic properties of Ureaplasma and its ability to cause FMi cell inflammation if it propagates from decidua to the fetal membranes. Using the FMi-OOC that contained decidual and three types of fetal membrane cells (chorion trophoblast, amnion mesenchyme, and epithelium), this study tested: 1) propagation of U. parvum from decidual cells to the fetal membrane cells, 2) the impact of U. parvum infection on cell signaling indicative of pathophysiology resulting in inflammation, and 3) the influence of decidual CD45+ cells in the immune response at the FMi in contributing to U. parvum pathology.
Methods
1. Cell culture
1.1. Decidual and fetal membrane cells
The collection of the placenta to be used in this study as a discarded human specimen, was approved by the Institutional Review Board (IRB) at the University of Texas Medical Branch at Galveston, TX (IRB approval number IRB16.0058, January 2020). Primary decidual and fetal membrane cells were isolated from elective term Cesarean section, not in labor placenta, and immortalized as reported previously 32. These cell lines were obtained from different fetal membrane samples included in our study. Human Fetal Membrane – Decidual cells hFM-DEC (DEC) and hFM-AMC - Human amnion mesenchymal cells (AMC) were cultured in Dulbecco’s Modified Eagle Media (DMEM)/F12 (Mediatech Inc., USA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (Mediatech Inc., USA), and 1% amphotericin B (Sigma-Aldrich, USA). hFM-CTC - Human chorion trophoblast cells (CTC) were cultured in DMEM/F12 supplemented with 0.20% FBS, 0.1 mM β-mercaptoethanol, 0.5% penicillin/streptomycin, 0.3% bovine serum albumin (BSA), 1× ITS-X, 2 μM CHIR99021, 0.05 μM A83-01, 1.5 μg/mL L-ascorbic acid, 50 ng/mL−1 epithelial growth factor, 0.08 mM valproic acid (VPA), and 1× Y27632 (Rock inhibitor). hFM-AEC - Human amnion epithelial cells (AEC) were cultured in Keratinocyte serum-free medium (KSFM) supplemented with bovine pituitary extract (30 μg mL), epidermal growth factor (0.1 ng mL), CaCl2 (0.4 mM), and primocin (0.5 mg mL). All cell types were grown at 37 °C and 5% CO2.
1.2. CD45+ leukocyte cells
CD45+ cells were isolated from the decidua to incorporate in our model. We collected placenta from elective term Cesarean section, not in labor, as described in previous studies 33,34. Decidual tissue was removed from the chorion and digested with DNAse (Sigma-Aldrich, USA) and collagenase (Sigma-Aldrich, USA). After digestion, red blood cells were lysed, washed, and CD45+ cells were isolated using Ficoll plaque density gradient (Cytiva, USA). CD45+ cells were maintained in RPMI-1640 (Gibco, USA) media supplemented with 10% FBS and 5% penicillin/streptomycin (Mediatech Inc., USA). The purity of the CD45+ cells isolated from decidua was confirmed by flow cytometry and immunocytochemistry using an Anti-CD45 antibody (dilution 1:200, ab30470, Abcam, Inc.) (data not shown).
2. Fetal Maternal interface organ-on-chip (FMi-OOC)
2.1. Device design
The FMi-OOC devices were designed and manufactured by our laboratory as previously described 27. The device has four chambers made of Poly(dimethylsiloxane) (PDMS) which are connected through 24 microchannels. Each chamber was designed for one of the four cell types that compose the FMi: the center chamber (1) contains maternal decidual cells (purple), chamber 2 contains CTC (blue), followed by chamber 3, which contains AMC (green), and finally, the outer chamber (4) has AEC (orange) (Figure 1). A reservoir block also made of PDMS is aligned on top of the cell culture chamber, matching the outlets and inlets. The devices were sterilized with 70% ethanol and washed with phosphate-buffered saline (PBS) before use. Microchannels between the AEC/AMC and AMC/CTC layers were filled with type IV basement membrane collagen Matrigel (Corning Matrigel , 1:25 in serum-free media) and incubated at 37°C with 5% CO2 for 4 hours to mimic the basement membranes in utero. All cell chambers were rinsed with serum-free media , and the devices were loaded as follows: 45,000 DEC in chamber 1; 200,000 CTCs + 5% primary collagen + 25% Matrigel in chamber 2; 60,000 AMCs + 20% primary collagen + 25% Matrigel for chamber 3; and 100,000 AECs for chamber 4. Finally, the FMi-OOCs were incubated at 37°C with 5% CO2 for 24 hours before treatments.
Figure 1. Schematic design of the FMi-OOC and experimental design.

A) The device is composed of four circular chambers connected by microchannels. The chambers correspond as follows: decidual chamber (purple), chorion trophoblast chamber (blue), amnion mesenchymal chamber (green), and amnion epithelial chamber (orange). By adding U. parvum in the decidua chamber, it will mimic the ascending infection model. The schematic design was created using BioRender. B) Bright field images of each type of cell in each chamber of the device – 20x magnification. Scale bar: 25 μm.
2.2. U. parvum culture
U. parvum (ATCC® 700970™) was obtained from the American Tissue Culture Collection (ATCC). U. parvum was propagated in UMCHs medium 35: Mycoplasma broth base (Becton, Dickinson and Co., Baltimore, MD) 1.47% (wt/vol) supplemented with horse serum (Biowhittaker, USA) 20% (vol/vol), yeast extract (Becton, Dickinson and Co., USA) 2.5% (wt/vol), L-cysteine hydrochloride 0.01% (wt/vol), urea 0.04% (wt/vol), phenol red 0.001% (wt/vol), and penicillin G 1000 U/ml. U. parvum cultures were incubated for 12 – 14 hours, and the amounts of Ureaplasma DNA were verified using a genesig Std Real-time PCR detection kit (Z- Path- U. parvum- std, American Research Products Inc., USA) and they amounted to 1.54 × 10E07 copy numbers/ml.
3. Experiment setup and assays
3.1. CD45+ cells
After isolation, 1×10E06 CD45+ cells were seeded in a 6-well plate with the appropriate media and treated with 1×10E05 DNA copies/mL of U. parvum. CD45+ cell culture and media without any cells were used as controls. After 24 hours, the supernatant was collected, centrifuged to remove cell debris, and stored at −80°C. The supernatant from CD45+ cells treated with U. parvum was included in subsequent models to recreate the contributions of immune cells in the decidual region in our FMi-OOC models. Media from untreated cells and blank media (no cells) were included as a control to confirm the contributions of decidual immune cells during an infectious process of the FMI. To note, CD45+ cells were not included in our FMi-OOCs.
3.2. FMi-OOC experiment
The FMi-OOC devices were seeded with specific cell types. After 24 hours, different types of treatments (Table 1) (n=5) were added to the decidual chamber: (1) regular DEC media as a negative control, mimicking a scenario with no infection in the FMi(CTL); (2) live U. parvum to determine the ascending infection (UP+); (3) supernatant from decidual CD45+ immune cells along with live U. parvum. This combination tested decidual response to live bacteria when immune cell secreted factors are present; (4) supernatant of CD45+ cells treated with U. parvum. This combination tested whether metabolites from immune cells exposed to U. parvum would cause changes at the FMi-OOC (CD45+), and (5) supernatant of CD45 previously treated with U. parvum and live infection of U. parvum to test if live infection of U. parvum would be affected by immune cells with previous contact with the microorganism (CD45+UP+). For the propagation experiment, U. parvum at a concentration of 1×10E05 DNA copies/mL was added in the decidua chamber. Media from all the chambers was collected at 24 hours, 48 hours, and 72 hours for the rt-PCR detection of U. parvum (Z- Path- U. parvum- std, American Research Products Inc., USA). The cells on the devices were fixed with ethanol and used for immunocytochemical staining of multiple banded antigen (MBA) (#MA5-17010, Invitrogen), a virulence factor of U. parvum.
Table 1.
Different types of treatments in the decidua chamber of the FMi-OOC.
| Groups | Treatment | Specifications | DEC media | U. parvum | CD45+ cells supernatant | CD45+ cells U. parvum treated supernatant |
|---|---|---|---|---|---|---|
| Group 1 | CTL | Regular DEC media (control) | + | − | − | − |
| Group 2 | UP+ | Live U. parvum at a concentration of 1x10E05 DNA copies/mL | + | + | − | − |
| Group 3 | CD45−UP+ | Supernatant of CD45+ cells only and live U. parvum | + | + | + | − |
| Group 4 | CD45+ | Supernatant of CD45 cells treated with U. parvum | + | − | − | + |
| Group 5 | CD45+UP+ | Supernatant of CD45 cells treated with U. parvum and live U. parvum | + | + | − | + |
The treatments mentioned above (Table 1) were added to the decidua chamber to check for cell morphology, inflammatory cytokine production, and protein analysis of cell signaling markers. After 72 hours, media was collected, and cells were either lysed or fixed from all chambers and stored at −80° for further analysis (Supplementary Figure 1).
4. Immunocytochemistry
Immunocytochemical staining for MBA (dilution 1:200, #MA5-17010, Invitrogen) in the FMi-OOC was performed 24 hours, 48 hours, and 72 hours post-infection with U. parvum to confirm propagation. To determine cell morphology and cellular transition status (i.e., epithelial to mesenchymal transition [EMT] or mesenchymal to epithelial transition [MET]) vimentin (dilution 1:300, #ab92547; Abcam, Inc.) and cytokeratin 18 (dilution 1:200, # ab668, Abcam, Inc.) were used for the staining of DEC/AMC and CTC/AEC, respectively.
After each time point, cells were fixed with 70% ethanol overnight, washed with PBS, and blocked with 3% bovine serum albumin in PBS before incubation with primary antibodies overnight at 4°C. After washing with PBS , slides were incubated with Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies (Life Technologies, Carlsbad, CA) and diluted 1:1000 in PBS for 1 hour in the dark. Slides were washed with PBS, treated with NucBlue Fixed Cell Stains ReadyProbes Reagent (R37606; Thermo Fisher Scientific) for 5 minutes, and then mounted using Mowiol 4 to 88 mounting medium (475904–100GM-M; Sigma-Aldrich, Inc.).
5. Automated Western immunoblotting – JESS™
The lysates of each chamber in the FMi-OOC were collected as follows: 40ul of the combination of radioimmunoprecipitation assay (RIPA) supplemented with 1% PTC (phosphatase inhibitor cocktail), 1% PIC (phosphatase inhibitor cocktail), and 1% PMSF (phenylmethylsulfonyl fluoride) was added in each chamber, collected after 10 minutes of incubation on ice, vortexed, sonicated for 30 seconds, and stored at −80°C. The protein concentration of the samples was calculated using Bicinchoninic Acid Method (BCA, Pierce, Rockford) after centrifuging at 12,000 RPM at 4°C. for 20 minutes.
Protein levels of p38 mitogen-activated protein kinase (MAPK) total (dilution 1:50, #9212, Cell Signaling Technology), phospho-p38 MAPK (dilution 1:50, #9211, Cell Signaling Technology, Danvers, MA), and phospho- Jun N-terminal kinase (JNK) (dilution 1:50, #9251, Cell Signaling Technology, Danvers, MA) were analyzed using the capillary-based instrument Simple Western JESS™ (Protein Simple). After optimization of the dilution of the antibodies and the sample concentration (0.2ug/ul), the assays were performed following the manufacturer’s protocol. Samples were diluted in 0.1x sample buffer (#042-195), and Fluorescent Master Mix (#PS-ST01EZ-8) was added for denaturation for 5 min at 95°C. Anti-rabbit (#042-206) or Anti-mouse secondary (#042-205) horseradish peroxidase (HRP)-labeled antibodies were used according to the species of the primary antibody. Replex (ProteinSimple, RP-001) module and β-actin (dilution 1:25, #ab8226, Abcam, Cambridge, MA) were used to normalize each plate. Data were analyzed using Compass for Simple Western software v4.0.0. The resulting area of each specific molecular weight peak was divided by either the β-actin value in the same capillary or the total protein value of the respective antibody for normalization.
6. LDH assay
Lactate dehydrogenase (LDH) cytotoxicity detection kit (#11644793001, Roche Diagnostics, IN, USA) was used according to the manufacturer’s instructions to check the cytotoxic effect of the treatments in the cell types. Approximately 10μl media from each chamber was collected after 72 hours, and 90ul of LDH solution was added to each well in a 96-well plate. Specific cell culture media of each cell type was used as the negative control, and cell supernatant treated with the mixture of 12% of Triton X-100 in 1X PBS was used as a positive control. After incubation at room temperature for 20 minutes in the dark, absorbance was measured at 450nm using a microplate reader.
7. Enzyme-linked immunosorbent assay (ELISA)
After 72 hours, supernatants were collected of all cell types. Multiplex assays were performed for tumor necrosis factor (TNF)-α, Interleukin (IL)-1β, IL-6, IL-8, IL-10, granulocyte-macrophage colony-stimulating factor (GM-CSF) (#HCYTA-60K, Millipore, Merck), and competitive ELISA kit for progesterone (#EIAP4C21, Invitrogen, Thermo Fisher). The assays were performed according to the manufacturer’s instructions. Standard curves were developed from known quantities of recombinant proteins provided. Sample concentrations were determined by relating the fluorescence values obtained to the standard curve by linear regression analysis.
8. Statistical analysis
Data were analyzed using GraphPad Prism software version 8 (GraphPad Software, San Diego, CA). To check the normality, the Shapiro-Wilk test was performed. Parametric data were analyzed by t-test or one-way ANOVA followed by Tukey comparison post hoc. The Kruskal-Wallis test, followed by Dunn’s multiple comparisons or Mann-Whitney U test, was used for non-parametric data. Statistical significance differences are indicated by p < 0.05.
Results
1. Cytotoxic effects of U. parvum in the FMi cells
Prior to testing the pathogenic properties of U. parvum, cytotoxicity (if any), induced by U. parvum colonization of the cells of the FMi-OOC were tested. LDH assay was conducted with the media collected from all device chambers for this. Although higher in CD45+ and CD45+UP+ treated, the cytotoxicity < 15% in AECs is in the acceptable biological range expected in these experiments (Figure 2). None of the other treatments or cells showed cytotoxicity.
Figure 2. Cytotoxic effects of U. parvum infection in the FMi-OOC.
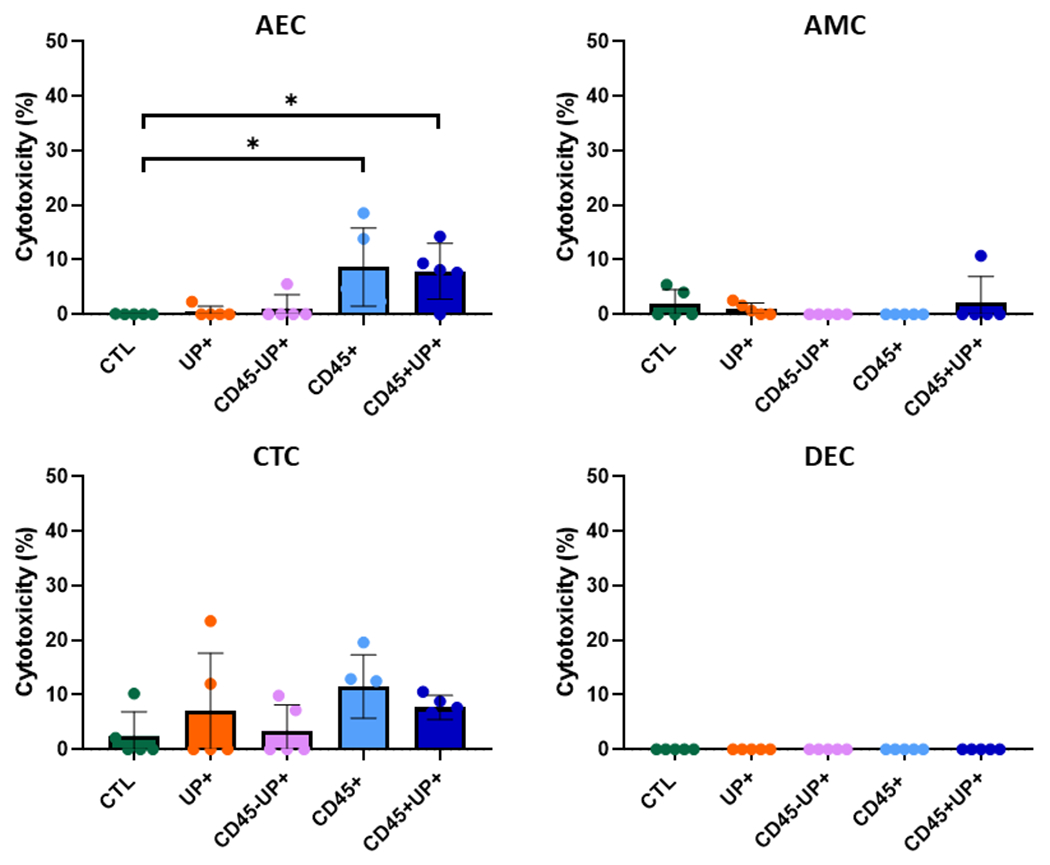
LDH assay was performed with the media of each type of cell in every treatment after 72 hours. Data are presented as mean±SEM. N=5. *, p < 0.05. **, <0.01. ***,<0.001
2. Propagation of U. parvum across the FMi
We recreated the fetal membrane-decidual interface using a microphysiologic device to test the pathogenic properties of an ascending U. parvum. This model is expected to mimic in utero environment and, most importantly, maintain intercellular interaction between cellular and collagen layers of the maternal-fetal interface organ system. To determine if U. parvum can transverse the FMi-OOC, mimicking in utero ascending infection, 1×10E05 DNA copies/mL of U. parvum were added to the DEC chamber after establishing an adherent cell culture in each chamber. Quantitative PCR detected U. parvum only in the DEC chamber after 24 hours (55,118 ± 7,981 DNA copies); after 48 hours, both CTC and AMC chambers tested positive for U. parvum; and after 72 hours, U. parvum was detected in all chambers, including the AEC chamber (Figure 3) which was the farthest from decidua. To confirm the presence of bacterial antigen (and not just DNA), immunostaining for MBA, a virulence factor of Ureaplasma spp., was performed. Figure 3B (yellow circle) shows that MBA localization in cells in all chambers confirmed PCR results. In summary, our data showed that U. parvum colonized all layers of the FMi-OOC after 72 hours. Hence, this time point was chosen for the rest of the experiments to determine the pathogenic effects of U. parvum in all the FMi-OOC cells.
Figure 3. Ascending infection of U. parvum in the FMi-OOC.

A) Results of the qPCR for U. parvum in the culture media of each chamber after each time point. According to the Cq Mean value of the standard curve and the manufacturer’s protocol, DNA copy number was calculated. If Cq >35, the sample was considered negative and the amount of DNA was not calculated. N=3/treatment/time point; Data are presented as mean±SEM. B) Immunocytochemical staining of multiple banded antigen (MBA- red) (dotted yellow circle), a virulence factor of U. parvum. Nuclei are stained with DAPI. Scale bar 10 um. Nuclei are stained with DAPI, n=3 technical replicates. Cq: quantification cycle. Scale bar, 25 μm.
3. Cell signaling mechanisms activated by U. parvum infection in the FMi cells.
Different treatments were established in this study to mimic different scenarios (Table 1): negative control (CTL), live infection with U. parvum (UP+), and decidual immune cells in multiple combinations with U. parvum. To determine cell signaling associated with U. parvum infection, stress-activated signaling kinases p38 MAPK and JNK levels were measured in the cells collected from the FMi-OOC by multiplexed western immunoblotting using JESS™. p38 MAPK activation via phosphorylation is a well-documented process critical for the induction of preterm and term labor onset 16,17. This stress-signaling kinase is a major contributor to fetal membrane dysfunction through various cellular derangements 36,37. This study determined the phosphorylated p38 MAPK (P-p38 MAPK -Thr180/Tyr182) and total p38 MAPK levels after the treatments. Although the total p38 MAPK in CTC (p=0.037) and AMC (p=0.012) treated with primed immune cells (CD45+) was increased compared to the control, the active P-p38 MAPK/total p38 MAPK ratio was not altered by the different U. parvum treatments compared to control (Figure 4). These findings suggest that U. parvum, irrespective of the presence of decidual immune cells, did not activate p38 MAPK. JNK is in the family of MAPK related to cellular senescence, apoptosis, and oxidative stress-induced inflammation, all critical cell fates and processes within fetal membrane cells during pathologic preterm or physiologic term labor 18,38. Compared to the control, there was no difference in P-JNK (threonine 183/tyrosine 185 residues) levels in the other treatments, regardless of the type of cells. (Figure 5). These findings showed that U. parvum infection did not affect the P-JNK, suggesting no apoptosis or JNK-derived inflammation induction. In summary, two MAPKs, generally associated with cellular stress and inflammatory signaling, are not impacted by U. parvum infection of the FMi cells.
Figure 4. p38 MAPK levels in cell lysates collected from FMiOOC after treatments.

JESS analysis and quantification of phosphorylated p38 MAPK (P-p38 MAPK) and total-p38 MAPK. β-actin was used to normalize the protein concentration. Error bars represent mean ± SEM, n=5. *, p < 0.05. **, <0.01. ***,<0.001
Figure 5. Phospho-JNK levels in cell lysates collected from FMiOOC after treatments.

JESS analysis and quantification of phosphorylated JNK (pJNK). β-actin was used as a control. Error bars represent mean ± SEM, n=5. p < 0.05. **,p <0.01. ***,<0.001.
4. U. parvum infection does not affect cell morphology or cellular transition status in the FMi cells.
As infectious stimulants (i.e., LPS) and infectious inflammation (i.e., TNFα) have been shown to induce changes in fetal membrane cell morphology and cause cellular transition (i.e., induction of EMT or MET) in amnion cells, we investigated the potential for U. parvum to induce similar cellular level changes by immunocytochemistry for cytoskeletal markers (i.e., vimentin [mesenchymal] and cytokeratin-18 [epithelial]) after treatments. Figure 6 shows that regardless of treatment, DECs maintained their elongated mesenchymal morphology and vimentin dominance, CTCs remained cuboidal and cytokeratin-18 dominated cell population, AMCs expressed vimentin and had fibroblastoid morphology, while AECs expressed cytokeratin-18 and a coble stone cuboidal cell shape. These data suggest that infection of U. parvum with or without immune cell components neither altered the cell morphology nor induced cellular transitions of the FMi cells.
Figure 6. Cell morphology after treatment with U. parvum and immune cells.

Immunocytochemical staining of the cells in FMi-OOC after 72 hours of treatment. CK-18 (red) was used for amnion epithelial and chorion trophoblast cells while vimentin (green) was used as a marker of amnion mesenchymal and decidual cells. Nuclei were stained with DAPI. N=3 per treatment; 20x magnification. Scale bar: 30 μm. AEC: amnion epithelial cells; AMC: amnion mesenchymal cell; CTC: chorion trophoblast cell; DEC: decidual cells.
5. Inflammatory effects of U. parvum in the FMi cells
After confirming the propagation of U. parvum across the FMi and verifying the absence of cellular or cytotoxic effects, we determined the impact of U. parvum and immune cells in the inflammatory response. The media from all the individual cell layers of the FMi-OOC were used to measure the concentrations of TNFα, IL-1β, IL-6, IL-8, IL-10, and GM-CSF (Figure 7). Except for IL-1β (data not shown), all other cytokines tested showed measurable levels in all cell types. In summary, U. parvum infection alone did not increase inflammatory cytokine production in FMi cells. However, live U. parvum infection of the DEC in the presence of immune cells in DECs significantly increased both pro- and anti-inflammatory cytokines in DEC and CTC. To note, DEC contained supernatant from immune cells either primed or unprimed by U. parvum.
Figure 7. Inflammatory profile of FMiOOC cells after 72 hours in different treatments.

Levels of TNF-α, IL-6, IL-8, GM-CSF, and IL-10 in the FMi-OOC cells measured by Luminex assay. n=5. *, p < 0.05. **, <0.01. ***,<0.001.
The presence of U. parvum alone (Group 2 - UP+) increased the levels of IL-8 (p=0.018) in DEC cells. Group 3 (CD45−UP+) had significantly higher levels of TNFα (p=0.033), IL-6 (p=0.018), and IL-10 (p=0.014) in the DEC chamber, and higher levels of IL-6 (p=0.006) and GM-CSF (p=0.002) in the CTC chamber compared to the control treatment. In AMC cells, this treatment increased IL-10 production (p=0.017). The treatment with immune cells previously treated with U. parvum (Group 4 - CD45+) only increased levels of GM-CSF (p=0.005) in DEC cells and levels of IL-8 (p=0.035) in CTC cells. Finally, Group 5 (CD45+UP+) significantly increased GM-CSF (p=0.027) in DEC cells, TNFα (p<0.001) , and IL-8 (p=0.026) in CTC cells compared to the control group after 72 hours. In addition, progesterone assay (P4) in CTC cells did not show a significant difference after the treatments of the study (Supplementary Figure 2). The inflammatory response observed in the DEC and CTC was not observed in the AMC and AEC, suggesting that maternal U. parvum infection induced a mild localized inflammatory response (Supplementary Table 1). Overall, U. parvum infection did not promote inflammation in the amnion layer but, interestingly, promoted an anti-inflammatory response (i.e., increased IL-10 production), suggesting a compensatory effect seen at the choriodecidua interface.
These findings showed that although U. parvum can ascend from the maternal to the fetal side, it did not cause cytotoxicity, cellular transitions, signaling kinase activation capable of activating inflammation, and a massive inflammatory response. The presence of U. parvum-treated decidual immune cell components was insufficient to mount an inflammatory response. These data support the systematic review and prior reports using fetal membrane explants and organ-on-a-chip devices 5,10,14.
Discussion
Studies about U. parvum have reported that they have poor immunogenicity, does not trigger a massive inflammatory response in human samples, and does not promote PTB in mouse models, which could be explained by this specie being a commensal of rodents, resulting in an immune response that may be different compared to humans 5,11,14. Using the FMi-OOC model, we tested the ascending infection of U. parvum and its pathogenic properties on the FMi . Our main findings include : 1) U. parvum propagated from the maternal decidua to the fetal amnion epithelial side within 72 hours; 2) U. parvum did not exhibit cytotoxicity in any cell types; 3) U. parvum infection was not associated with the activation of p38 MAPK and JNK pathways; 4) U. parvum, either alone or combined with immune cells promoted an increase in pro- and anti-inflammatory cytokine levels in the choriodecidual layer, but the amnion layer was refractory to the presence of U. parvum. In summary, U. parvum can ascend and propagate through the feto-maternal interface; however, it did not result in cell signaling or cellular derangement to cause a massive inflammatory response. Our model is unique in that we included decidual immune cells and tested its contribution during U. parvum infection of the FMi. Lack of any significant immune response, supported the hypothesis that U. parvum is not a powerful immunogenic bacterium at the FMi.
U. parvum effects in response to ascending infection and its role in preterm parturition were based on in vitro 2D models that use a single cell culture or animal studies 11,39–42. Differences between human and animal models of pregnancy and PTB have raised concerns regarding the use of data. Specifically, rodent models are often used for PTB and infection model studies. The chorion trophoblast layer is rather obscure at the mouse FMi, compared to the human chorion layer. In humans, multilayered chorion trophoblast forms a barrier and shows constitutive expression of HLA-G. Chorion also produces large quantities of progesterone, an anti-inflammatory factor, and expresses key membrane progesterone receptors (PGRMC1 and 2) 43 to create a major barrier function. In addition, in vitro 2D cultures have some limitations as they lack cell-cell interactions, especially when studying an interface with different types of cells 40,44,45 from two physiologically separate individuals (mother and the fetus). The FMi-OOC overcomes these challenges and provide a better scenario to study this interface. U. parvum has already been studied in another type of OOC. Tantengco et al. developed the vagina-cervix-decidua OOC and reported the ascending infection of U. parvum from the vagina to the decidua, showing that U. parvum can ascend, but not cause either cell death or inflammation 14. Our results showed that FMi-OOC is a good model for studying ascending infection of U. parvum past the decidua. Together, these findings propose a better model to study ascending infection that mimics the propagation of infectious agents in vivo. As reported by Romero et al. almost three decades ago, ascending infection is characterized by vaginitis, deciduitis, chorionitis, and amnionitis, leads to intraamniotic infection and inflammation predisposing to PTB 46,47. The two OOC devices can recreate the path of infection and inflammation. In summary, we demonstrated that Ureaplasma can ascend from the vagina to the amnion layer and interact with decidual immune cells, yet their propagation is not associated with an inflammatory response. It is well known that decidual immune cells have an important role in maintaining the maternal-fetal immunotolerance throughout pregnancy48,49. Changes in decidual immune cells have been associated with adverse gestational outcomes, such as preeclampsia50. Localized inflammation by specific cell types is observed, but overall, it is balanced. Cell death and other inflammation-producing cell fate-associated signaling molecules were also not changed, suggesting that the U. parvum infection alone is insufficient to elicit a massive inflammation required to trigger labor-associated changes. We have already reported a disease model with LPS using this model where propagation of LPS was associated with massive inflammation mimicking Escherichia coli27. We did not use LPS in this model as we have already reported that and physiologically validated using animal models 14,27. These data provided immense credence to this model system.
The localized inflammation in specific cell types is likely due to the presence of an antigen and stress induced by the U. parvum resulting in activation of stress kinases such as p38 MAPK or JNK. Previous studies showed that p38 MAPK could be activated by cigarette smoke extract, an oxidative stress inducer, and lead to cellular senescence 51, though this is not observed in this model. We also studied JNK, which is vital in apoptotic signaling, though U. parvum did not cause significant activation of JNK regardless of the type of cell. This suggests that U. parvum does not induce JNK-associated changes in fetal membrane cells. We speculate that U. parvum infection, inflammation, and other pathogenic consequences are location and load dependent. In endometrium or even fetal lung and brain, it is likely to cause an inflammatory response and associated pathologies 52–54; however, colonization of the amniochorionic membrane or amniotic fluid by U. parvum alone is unlikely to induce labor. Location shifts from amniotic fluid to fetal tissues can be detrimental to the fetal organs, as often observed 55. Infection of the amniotic cavity and the fetus with Ureaplasma spp. can promote bronchopulmonary dysplasia, intraventricular hemorrhage, and necrotizing enterocolitis 55,56.
This is the first time we showed U. parvum colonization and its pathologic effects in fetal membrane cells using an FMi-OOC platform. However, this study had some limitations, including 1) not mimicking long term infection and migration of leukocytes from maternal and fetal sides and analyzing a short time infection (72 hours), 2) using a single dose of U. parvum as treatment, though the dose chosen was based on the amount of U. parvum DNA copies found in the amniotic fluid 57, and 3) lack of CD45+ cells co-culture that did not allow for visualized of immune cell migration. Further studies will be conducted to address the short comings of this study. U. parvum alone may not be sufficient to cause PTB, but a combination of other pathogenic microbes can exaggerate the immune response leading to PTB.
Conclusion
We conclude that microphysiologic models can recreate in utero environments like choriodecidual interfaces to study pathophysiologic changes associated with PTB. We report that the load of U. parvum reported being associated with PTB is incapable of an inflammatory response required to drive PTB pathways. Our data support the systematic review based on clinical studies 5,12 and animal model studies reported by Pavlidis, Tantengco et al. 11,12,14. However, we speculate that the presence of U. parvum in the FMi may still compromise the integrity of the structure, making the FMi more susceptible to infection with other pathogenic species that could trigger pPROM, PTL, and PTB.
Supplementary Material
Acknowledgments:
This study was supported by UH3 TR003283 (NIH/NCATs/NICHD) to Drs. Han and Menon and R01HD100729-01S1 (NIH/NICHD) to Dr. Menon, and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 to Giovana Fernanda Cosi Bento. Dr. Richardson is supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program-BIRCWH; Berenson, PI) from the National Institutes of Health/Office of the Director (OD)/National Institute of Allergy and Infectious Diseases (NIAID), and Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations List
- AEC
hFM-AEC - Human amnion epithelial cells
- AMC
hFM-AMC - Human amnion mesenchymal cells
- ATCC
American Tissue Culture Collection
- BCA
Bicinchoninic Acid Method
- BSA
Bovine serum albumin
- CTC
hFM-CTC - Human chorion trophoblast cells
- DEC
hFM-DEC - Decidual cells
- DMEM
Dulbecco’s Modified Eagle Media
- EMT
Epithelial-to-mesenchymal transition
- FBS
Fetal bovine serum
- FMi
Feto-maternal interface
- FMi-OOC
Feto-maternal interface organ-on-chip
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HRP
horseradish peroxidase
- ICC
Immunocytochemistry
- IL
Interleukin
- JNK
Jun N-terminal kinase
- KSFM
Keratinocyte serum-free medium
- LDH
Lactate dehydrogenase
- MAPK
Mitogen-activated protein kinase
- MBA
Multiple banded antigen
- MET
Mesenchymal-to-epithelial transition
- OOC
Organ-on-chip
- P4
Progesterone assay
- PBS
Phosphate-buffered saline
- PDMS
Poly(dimethylsiloxane)
- PIC
Phenylmethylsulfonyl fluoride
- pPROM
Preterm premature rupture of membranes
- PTB
Preterm birth
- PTC
Phosphatase inhibitor cocktail
- PTL
Preterm labor
- RIPA
Radioimmunoprecipitation assay
- TNFα
Tumor necrosis factor alpha
- VPA
Valproic acid
Footnotes
Ethics Statement: The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received (Institutional Review Board (IRB) approval number IRB16.0058, January 2020). The study conformed to the US Federal Policy for the Protection of Human Subjects.
Conflict of Interest: The authors declare no conflict of interest in this study.
References
- 1.Organization W-WH. Preterm Birth. https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth
- 2.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. Aug 15 2014;345(6198):760–5. doi: 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. Jan 05 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. Feb 2012;17(1):2–11. doi: 10.1016/j.siny.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 5.Noda-Nicolau NM, Tantengco OAG, Polettini J, et al. Genital Mycoplasmas and Biomarkers of Inflammation and Their Association With Spontaneous Preterm Birth and Preterm Prelabor Rupture of Membranes: A Systematic Review and Meta-Analysis. Front Microbiol. 2022;13:859732. doi: 10.3389/fmicb.2022.859732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon BH, Romero R, Moon JB, et al. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol. Nov 2001;185(5):1137–42. doi: 10.1067/mob.2001.118162 [DOI] [PubMed] [Google Scholar]
- 7.Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. May 1999;78(5):379–82. [PubMed] [Google Scholar]
- 8.Kim M, Kim G, Romero R, Shim SS, Kim EC, Yoon BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31(2):146–52. doi: 10.1515/JPM.2003.020 [DOI] [PubMed] [Google Scholar]
- 9.Perni SC, Vardhana S, Korneeva I, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. Oct 2004;191(4):1382–6. doi: 10.1016/j.ajog.2004.05.070 [DOI] [PubMed] [Google Scholar]
- 10.Noda-Nicolau NM, Polettini J, Peltier MR, da Silva MG, Menon R. Combinations and loads of bacteria affect the cytokine production by fetal membranes: An in vitro study. Am J Reprod Immunol. December 2016;76(6):504–511. doi: 10.1111/aji.12596 [DOI] [PubMed] [Google Scholar]
- 11.Tantengco OAG, Kechichian T, Vincent KL, Pyles RB, Medina PMB, Menon R. Inflammatory response elicited by Ureaplasma parvum colonization in human cervical epithelial, stromal, and immune cells. Reproduction. Dec 09 2021;163(1):1–10. doi: 10.1530/REP-21-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kletzel HH, Rotem R, Barg M, Michaeli J, Reichman O. Ureaplasma urealyticum: the Role as a Pathogen in Women’s Health, a Systematic Review. Curr Infect Dis Rep. Jun 29 2018;20(9):33. doi: 10.1007/s11908-018-0640-y [DOI] [PubMed] [Google Scholar]
- 13.Pavlidis I, Spiller OB, Sammut Demarco G, et al. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat Commun. Jan 10 2020;11(1):199. doi: 10.1038/s41467-019-14089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tantengco OAG, Richardson LS, Radnaa E, et al. Modeling ascending Ureaplasma parvum infection through the female reproductive tract using vagina-cervix-decidua-organ-on-a-chip and feto-maternal interface-organ-on-a-chip. FASEB J. 10 2022;36(10):e22551. doi: 10.1096/fj.202200872R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motomura K, Romero R, Xu Y, et al. Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. mBio. Jun 23 2020;11(3)doi: 10.1128/mBio.00797-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoji T, Yoshida S, Mitsunari M, et al. Involvement of p38 MAP kinase in lipopolysaccharide-induced production of pro- and anti-inflammatory cytokines and prostaglandin E(2) in human choriodecidua. J Reprod Immunol. Oct 2007;75(2):82–90. doi: 10.1016/j.jri.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Sheller-Miller S, Richardson L, Martin L, Jin J, Menon R. Systematic review of p38 mitogen-activated kinase and its functional role in reproductive tissues. Am J Reprod Immunol. Dec 2018;80(6):e13047. doi: 10.1111/aji.13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. Oct 20 2008;27(48):6245–51. doi: 10.1038/onc.2008.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaherty RA, Magel M, Aronoff DM, Gaddy JA, Petroff MG, Manning SD. Modulation of Death and Inflammatory Signaling in Decidual Stromal Cells following Exposure to Group B. Infect Immun. 12 2019;87(12)doi: 10.1128/IAI.00729-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa M, Iriyama T, Suzuki K, et al. ASK1 promotes uterine inflammation leading to pathological preterm birth. Sci Rep. 02 05 2020;10(1):1887. doi: 10.1038/s41598-020-58653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balijepalli A, Sivaramakrishan V. Organs-on-chips: research and commercial perspectives. Drug Discov Today. 02 2017;22(2):397–403. doi: 10.1016/j.drudis.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 22.Haderspeck JC, Chuchuy J, Kustermann S, Liebau S, Loskill P. Organ-on-a-chip technologies that can transform ophthalmic drug discovery and disease modeling. Expert Opin Drug Discov. 01 2019;14(1):47–57. doi: 10.1080/17460441.2019.1551873 [DOI] [PubMed] [Google Scholar]
- 23.Caballero D, Reis RL, Kundu SC. Boosting the Clinical Translation of Organ-on-a-Chip Technology. Bioengineering (Basel). Oct 14 2022;9(10)doi: 10.3390/bioengineering9100549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson L, Jeong S, Kim S, Han A, Menon R. Amnion membrane organ-on-chip: an innovative approach to study cellular interactions. FASEB J. Aug 2019;33(8):8945–8960. doi: 10.1096/fj.201900020RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tantengco OAG, Richardson LS, Medina PMB, Han A, Menon R. Organ-on-chip of the cervical epithelial layer: A platform to study normal and pathological cellular remodeling of the cervix. FASEB J. 04 2021;35(4):e21463. doi: 10.1096/fj.202002590RRR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson LS, Emezienna N, Burd I, et al. Adapting an organ-on-chip device to study the effect of fetal sex and maternal race/ethnicity on preterm birth related intraamniotic inflammation leading to fetal neuroinflammation. Am J Reprod Immunol. Oct 29 2022:e13638. doi: 10.1111/aji.13638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson LS, Kim S, Han A, Menon R. Modeling ascending infection with a feto-maternal interface organ-on-chip. Lab Chip. Nov 24 2020;20(23):4486–4501. doi: 10.1039/d0lc00875c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, Richardson L, Radnaa E, et al. Molecular mechanisms of environmental toxin cadmium at the feto-maternal interface investigated using an organ-on-chip (FMi-OOC) model. J Hazard Mater. January 15 2022;422:126759. doi: 10.1016/j.jhazmat.2021.126759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tantengco OAG, Richardson LS, Radnaa E, et al. Exosomes from. Front Cell Dev Biol. 2022;10:931609. doi: 10.3389/fcell.2022.931609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson L, Radnaa E, Lintao RCV, et al. A Microphysiological Device to Model the Choriodecidual Interface Immune Status during Pregnancy. J Immunol. Mar 15 2023;doi: 10.4049/jimmunol.2200821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs SO, Sheller-Miller S, Richardson LS, Urrabaz-Garza R, Radnaa E, Menon R. Characterizing the immune cell population in the human fetal membrane. Am J Reprod Immunol. May 2021;85(5):e13368. doi: 10.1111/aji.13368 [DOI] [PubMed] [Google Scholar]
- 32.Radnaa E, Urrabaz-Garza R, Elrod ND, et al. Generation and characterization of human Fetal membrane and Decidual cell lines for reproductive biology experiments†. Biol Reprod. March 19 2022;106(3):568–582. doi: 10.1093/biolre/ioab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Plazyo O, Romero R, Hassan SS, Gomez-Lopez N. Isolation of Leukocytes from the Human Maternal-fetal Interface. J Vis Exp. May 21 2015;(99):e52863. doi: 10.3791/52863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farine T, Parsons M, Lye S, Shynlova O. Isolation of Primary Human Decidual Cells from the Fetal Membranes of Term Placentae. J Vis Exp. April 30 2018;(134)doi: 10.3791/57443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namba F, Hasegawa T, Nakayama M, et al. Placental features of chorioamnionitis colonized with Ureaplasma species in preterm delivery. Pediatr Res. Feb 2010;67(2):166–72. doi: 10.1203/PDR.0b013e3181c6e58e [DOI] [PubMed] [Google Scholar]
- 36.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. Sep 2016;22(5):535–60. doi: 10.1093/humupd/dmw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson LS, Radnaa E, Urrabaz-Garza R, Lavu N, Menon R. Stretch, scratch, and stress: Suppressors and supporters of senescence in human fetal membranes. Placenta. Sep 15 2020;99:27–34. doi: 10.1016/j.placenta.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papa S, Choy PM, Bubici C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene. Mar 2019;38(13):2223–2240. doi: 10.1038/s41388-018-0582-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amabebe E, Richardson LS, Bento GFC, et al. Ureaplasma parvum infection induces inflammatory changes in vaginal epithelial cells independent of sialidase. Mol Biol Rep. Apr 2023;50(4):3035–3043. doi: 10.1007/s11033-022-08183-6 [DOI] [PubMed] [Google Scholar]
- 40.Silwedel C, Speer CP, Haarmann A, et al. Species Modulate Cytokine and Chemokine Responses in Human Brain Microvascular Endothelial Cells. Int J Mol Sci. Jul 22 2019;20(14)doi: 10.3390/ijms20143583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida K, Nakahira K, Mimura K, et al. Effects of Ureaplasma parvum lipoprotein multiple-banded antigen on pregnancy outcome in mice. J Reprod Immunol. Dec 2013;100(2):118–27. doi: 10.1016/j.jri.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 42.Allam AB, von Chamier M, Brown MB, Reyes L. Immune profiling of BALB/C and C57BL/6 mice reveals a correlation between Ureaplasma parvum-Induced fetal inflammatory response syndrome-like pathology and increased placental expression of TLR2 and CD14. Am J Reprod Immunol. Mar 2014;71(3):241–51. doi: 10.1111/aji.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lozovyy V, Richardson L, Saade G, Menon R. Progesterone receptor membrane components: key regulators of fetal membrane integrity. Biol Reprod. Feb 11 2021;104(2):445–456. doi: 10.1093/biolre/ioaa192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potts LC, Feng L, Seed PC, et al. Inflammatory Response of Human Gestational Membranes to Ureaplasma parvum Using a Novel Dual-Chamber Tissue Explant System. Biol Reprod. May 2016;94(5):119. doi: 10.1095/biolreprod.115.137596 [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki T, Matsuo J, Nakamura S, Oguri S, Yamaguchi H. Effect of Ureaplasma parvum co-incubation on Chlamydia trachomatis maturation in human epithelial HeLa cells treated with interferon-γ. J Infect Chemother. Aug 2014;20(8):460–4. doi: 10.1016/j.jiac.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. Dec 2006;113 Suppl 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci. 1991;622:355–75. doi: 10.1111/j.1749-6632.1991.tb37880.x [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Li Y, Sang Y, Li DJ, Du M. Crosstalk Between Trophoblasts and Decidual Immune Cells: The Cornerstone of Maternal-Fetal Immunotolerance. Front Immunol. 2021;12:642392. doi: 10.3389/fimmu.2021.642392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim JYH. The role of decidual immune cells on human pregnancy. J Reprod Immunol. Nov 2017;124:44–53. doi: 10.1016/j.jri.2017.10.045 [DOI] [PubMed] [Google Scholar]
- 50.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. Jun 2010;63(6):534–43. doi: 10.1111/j.1600-0897.2010.00831.x [DOI] [PubMed] [Google Scholar]
- 51.Menon R, Boldogh I, Hawkins HK, et al. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol. Jun 2014;184(6):1740–51. doi: 10.1016/j.ajpath.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 52.Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thébaud B. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. Apr 2009;65(4):430–6. doi: 10.1203/PDR.0b013e31819984ce [DOI] [PubMed] [Google Scholar]
- 53.Hannaford K, Todd DA, Jeffery H, John E, Blyth K, Gilbert GL. Role of ureaplasma urealyticum in lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. Nov 1999;81(3):F162–7. doi: 10.1136/fn.81.3.f162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lozano FM, Bernabeu A, Lledo B, et al. Characterization of the vaginal and endometrial microbiome in patients with chronic endometritis. Eur J Obstet Gynecol Reprod Biol. Aug 2021;263:25–32. doi: 10.1016/j.ejogrb.2021.05.045 [DOI] [PubMed] [Google Scholar]
- 55.Viscardi RM. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed. Jan 2014;99(1):F87–92. doi: 10.1136/archdischild-2012-303351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pomini F, Caruso A, Challis JR. Interleukin-10 modifies the effects of interleukin-1beta and tumor necrosis factor-alpha on the activity and expression of prostaglandin H synthase-2 and the NAD+-dependent 15-hydroxyprostaglandin dehydrogenase in cultured term human villous trophoblast and chorion trophoblast cells. J Clin Endocrinol Metab. Dec 1999;84(12):4645–51. doi: 10.1210/jcem.84.12.6188 [DOI] [PubMed] [Google Scholar]
- 57.Kacerovsky M, Stranik J, Kukla R, et al. Intra-amniotic infection and sterile intra-amniotic inflammation in women with preterm labor with intact membranes are associated with a higher rate of. J Matern Fetal Neonatal Med. Dec 2022;35(25):7344–7352. doi: 10.1080/14767058.2021.1947231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


