Abstract
Exposure of cells to adverse environmental conditions invokes a genetically programmed series of events resulting in the induction of specific genes. The fluoroquinolone antibiotic ciprofloxacin has recently been reported to upregulate interleukin-2 (IL-2) gene induction. In the present investigation, the effect of ciprofloxacin at supratherapeutic concentrations on immediate-early (<2 h) gene expression in primary human peripheral blood lymphocytes was studied with Northern blots. In addition, transcriptional activity of IL-2 and metallothionein enhancer and promoter regions and transcription factors AP-1, NF-κB, and NF-AT were analyzed by chloramphenicol acetyltransferase (CAT) and electrophoretic mobility shift assays, respectively. The concentration of c-fos, c-jun, c-myc, junB, and fra-1 mRNAs was increased in activated peripheral blood lymphocytes incubated with ciprofloxacin compared to that in untreated controls. Ciprofloxacin increased CAT activity in stimulated lymphocytes transfected with plasmids containing either the IL-2 or metallothionein enhancer. Furthermore, among the transcription factors tested, AP-1 activity was increased in stimulated purified T helper lymphocytes incubated with ciprofloxacin compared to drug-free controls. Taken together, ciprofloxacin increased the levels of immediate-early transcripts, enhanced IL-2 and metallothionein promoter induction, and upregulated AP-1 concentrations in primary lymphocytes, reflecting a program commonly observed in mammalian stress responses.
Fluoroquinolones, which perform their bactericidal effect by inhibiting DNA gyrase (a type II topoisomerase) (5), are known to interfere with certain immune functions. Ciprofloxacin and other quinolones at >20 μg/ml inhibit peripheral blood lymphocyte (PBL) cell growth by 30 to 35%, causing impaired cell cycle progression through the S phase (8). Cell cycle analysis thus indicates DNA synthesis to be inhibited by fluoroquinolones at these concentrations. Despite this adverse effect on cell growth, fluoroquinolone antibiotics at therapeutic concentrations (between 1.56 and 6.25 μg/ml) are able to enhance [3H]thymidine incorporation two- to threefold in phytohemagglutinin (PHA)-stimulated PBLs, compared to that in control cells unexposed to antibiotics, the effects being most pronounced after 4 to 5 days of lymphocyte incubation (8, 38).
In addition to the stimulatory effects on thymidine incorporation, ciprofloxacin (range, 5 to 80 μg/ml) and other fluoroquinolones potentiate interleukin-2 (IL-2) synthesis by PHA-stimulated PBLs (26, 42). Experiments with the human T-cell lymphoma cell line Jurkat and the murine equivalent EL-4, transiently transfected with a plasmid containing the IL-2 promoter region, show that ciprofloxacin enhances IL-2 promoter activation (27, 30). An earlier and stronger ciprofloxacin-dependent activation of the transcriptional regulation factors nuclear factor of activated T cells 1 (NF-AT-1) and activator protein 1 (AP-1) is observed in these immortalized cell lines. In accordance with these findings, under certain in vitro conditions, ciprofloxacin (20 to 80 μg/ml) counteracts the effect of the immunosuppressive agent cyclosporine A that normally inhibits the phosphatase activity of calcineurin, causing impaired NF-AT-1 activity (29). Ciprofloxacin thus interferes with a regulative pathway common to several cytokines. Indeed, analysis of cytokine mRNAs in ciprofloxacin (20 to 80 μg/ml)-treated PBLs revealed that not only IL-2 mRNA is enhanced but so are an array of other cytokine mRNAs, including gamma interferon (IFN-γ) and IL-4 (30). Finally, there are several reports on ciprofloxacin-dependent immunomodulation in vivo, strongly indicating that the cytokine upregulation observed is not an in vitro artifact (19, 36, 38, 41).
Exposure of cells to DNA-damaging agents induces numerous genes that facilitate the repair of such lesions. This response is essential for an organism to adapt to life-threatening environmental conditions and to duplicate its genetic material with the highest fidelity. This sensory network has been thoroughly characterized in bacteria. Treatment of Escherichia coli with agents that damage DNA or inhibit replication (for example, quinolone antibacterials) induces a set of physiological responses that have collectively been called the SOS response (40). These responses promote cell survival, and blocking them genetically leads to DNA damage sensitivity. Numerous genes that are transcriptionally activated in response to DNA damage have been identified, including several involved in excision repair, recombinational repair, SOS repression, mutagenesis, and cell cycle arrest.
In eukaryotic cells, a network of overlapping systems seems to be activated following exposure to DNA-damaging agents. Many genes are induced specifically by UV and gamma rays, while others also respond to alkylating agents and to growth arrest (7, 10, 17). In several cases, the cellular response to genotoxic treatment is triggered by signal transduction pathways which are not DNA damage specific (i.e., many of the genes triggered by DNA damage are also induced by agents such as phorbol esters and by metabolic or oxidative stress) (5, 10, 11, 34). The activation of primary stress-inducible genes (e.g., the immediate-early expressed genes c-fos, c-jun, and c-myc) is rapid and independent of protein synthesis, whereas secondary induced genes (e.g., early expressed cytokines and genes involved in DNA repair) are expressed later and are dependent on the primary ones (10, 11).
The eukaryotic UV response is the most well-characterized stress-induced pathway and resembles in many ways the bacterial SOS response (7, 16). Following UV exposure, the membrane-associated Src tyrosine kinases are induced, leading to activation of cytoplasmic protein kinases that eventually increase AP-1 activity and induce nuclear translocation of NF-κB. The enhancement in AP-1 activity is mediated both by induction of c-jun and c-fos expression and by posttranslational modification of c-Jun. The majority of genes identified during a UV response are not specifically linked to DNA repair. However, the gene product of the UV- and gamma-ray-inducible GADD45 gene stimulates excision repair as well as inhibits DNA replication by blocking the cell cycle at the G1 checkpoint (for a review, see reference 37). Metallothionein is another well-studied example of a UV and DNA damage-inducible gene (15). Overexpression of metallothionein protects mammalian cells against oxidative stress and can dramatically reduce the level of intracellular oxygen radicals (35).
Topoisomerase II inhibitors have also been shown to trigger DNA damage responses (20, 23, 31, 39). We have demonstrated that ciprofloxacin at high concentrations (80 μg/ml) interferes with topoisomerase II in human lymphoblastoid Raji cells (2). This phenomenon, in addition to the fact that IL-2 and other cytokines are enhanced in ciprofloxacin-treated cells (26, 30), led us to examine whether ciprofloxacin induces a stress response in primary human lymphoid cells. Surprisingly, ciprofloxacin superinduced both IL-2 and metallothionein gene induction in PHA-activated PBLs compared to that in untreated controls. Ciprofloxacin was also found to increase the concentrations of immediate-early gene transcripts without influencing mRNA stability. Finally, the transcription factor AP-1 was strongly induced by ciprofloxacin, whereas binding of NF-AT-1 and NF-κB was unaffected. Taken together, our data indicate that the increased cytokine production observed in the presence of ciprofloxacin is most likely related to a mammalian stress response.
MATERIALS AND METHODS
Reagents.
Preservative-free ciprofloxacin was kindly provided by Bayer (Wuppertal, Germany). PHA (Wellcome, Dartford, England) and phorbol myristate acetate (PMA; Sigma, Stockholm, Sweden) were dissolved in RPMI 1640 medium and dimethylsulfoxide, respectively. Actinomycin D was purchased from Boehringer Mannheim (Mannheim, Germany) and used at a final concentration of 10 μg/ml.
Cells.
Human PBLs were isolated from buffy coats with citrate or from heparinized blood from healthy donors by centrifugation on a step gradient of Ficoll-Isopaque (Lymphoprep; Pharmacia, Uppsala, Sweden) (26). PBLs (106/ml) were incubated in a humidified 5% CO2 atmosphere in RPMI 1640 medium containing HEPES buffer (Gibco, Paisley, Scotland) supplemented with 10% heat-inactivated fetal calf serum, glutamine, and gentamicin (12 μg/ml). A pure population (>98%) of CD4+ T cells was isolated with Dynabeads (Dynal, Oslo, Norway). A protocol consisting of a negative selection procedure was used based upon the manufacturer’s instructions. The biological activity of IL-2 in supernatants was analyzed by means of IL-2-dependent stimulation of proliferation of the murine cytolytic T-lymphocyte line CTLL-2 as previously described (26).
RNA isolation, Northern blots, and DNA.
Total RNA was prepared as previously reported (30). RNA (10 to 20 μg) was loaded onto formaldehyde-agarose gels and blotted to nylon filters (Hybond-N+; Amersham, Buckinghamshire, England) as described by the manufacturer. Filters were hybridized according to standard protocols and exposed for 24 to 72 h to preflashed X-ray film (XAR-5; Kodak, Rochester, N.Y.) at −70°C by using intensifying screens. Autoradiographs were quantified by scanning laser densitometry. The gene-specific probes used to probe RNA blots were isolated from agarose gels after digestion of the plasmids in which they were propagated with the appropriate restriction endonucleases. The c-myc probe was a 1,000-bp ClaI-EcoRI fragment isolated from a human c-myc cDNA. The fra-1 cDNA was a 900-bp EcoRI fragment from pfraI (1). The c-fos probe was a 900-bp ScaI-NcoI fragment from pc-fos-1 (American Type Culture Collection), and the c-jun probe was an ≈1,400-bp NotI-HindIII fragment from pRSV-c-jun (provided by Michael Karin) (9). The junB probe (murine) was a 1,800-bp EcoRI fragment from pjunB (1). The fra-1, junB, and c-myc cDNAs were kindly provided by T. Lindsten. The IL-2- and IFN-γ-specific probes and β-actin cDNA have been described previously (30). DNA fragments were labeled with [α-32P]dCTP (specific activity 3,000 Ci/mmol; PB 10205; Amersham) by random priming (Amersham). Free nucleotides were separated on spin columns (Costar, Cambridge, Mass.) containing Sephadex G-50 fine (Pharmacia). For transfection experiments, expression vectors MT-CAT-SV and SV-IL-2-CAT were used (reference 30 and references therein). DNA concentrations were estimated by DNA dipstick (Invitrogen, San Diego, Calif.) or by examination on agarose gels.
Transfections and CAT assay.
PBLs were stimulated in the presence of PHA (1 μg/ml) and PMA (50 ng/ml) for 18 h before transfection by electroporation with a Gene Pulser apparatus (Bio-Rad, Richmond, Calif.). Before transfection, cells in log phase were washed in Tris-buffered saline. For each electroporation, 107 cells were resuspended in 350 μl of serum-free medium and transferred to a 1-ml electroporation cuvette with a 0.4-cm space between the electrodes (Bio-Rad). After addition of 10 μg of the appropriate plasmid DNA, the samples were gently shaken and kept at room temperature for 5 min. The samples were subjected to electroporation at 960 μF and 450 V. The pulse generated an exponential decay pulse with time constants at 36 to 43 ms. In all experiments, cells were mixed after the transfections and aliquoted to different culture flasks. Cells were rested for 36 h followed by addition of ciprofloxacin and 1 μg of PHA per ml. After activation for 15 h, cells were washed, extracts were prepared, and the chloramphenicol acetyltransferase (CAT) reaction, including separation of products by thin-layer chromatography, was performed as previously reported (30). No CAT activity was detected in untransfected cells with or without ciprofloxacin.
Electrophoretic mobility shift assay.
Whole-cell protein extracts were prepared from stimulated CD4+ T cells (33), and protein concentrations were determined with a commercial protein assay (Pierce bicinchoninic acid reagent; Tecator, Sollentuna, Sweden). Protein extract (3.5 to 5.0 μg/reaction) was mixed with 1.0 μg of poly(dI-dC) (Boehringer Mannheim) and 5× binding buffer (100 mM phosphate buffer [pH 6.0], 50 mM MgCl2, 0.5 mM EDTA, 10 mM dithiothreitol, 0.05% Nonidet P-40, 0.5 M NaCl, 500 μg of bovine serum albumin per ml, 20% Ficoll) in a final volume of 15 μl. After incubation for 10 min at room temperature, a 10,000- or 15,000-cpm 32P-kinased probe (60 × 10−6 to 90 × 10−6 μg) was added, and the reaction mixtures were incubated for a further 20 min at 37°C. Samples were separated by 5% Tris-borate-EDTA–polyacrylamide gel electrophoresis (PAGE) were fixed, dried, and autoradiographed. The probes used were the AP-1 consensus binding site (5′-ctagtgatgagtcagccggatc-3′), an Oct-1 binding site (5′-cgtctcatgcgatgcaaatcacttgagatc-3′), an NF-κB consensus binding site (5′-gatcgaggggactttccctagc-3′), and, finally, an NF-AT-1 binding sequence (5′-ggaggaaaaactgtttcatacagaaggcgt-3′) (30). Competition experiments with 10 to 1,000× molar excess of unlabeled oligonucleotides were carried out to identify the specific complexes. For supershift analyses, polyclonal antibodies (1 μg/reaction) directed against the c-Fos family (K-25), c-Fos (4-G), Fra-1 (N-17), the c-Jun family (D), c-Jun/AP-1 (N), JunB (210), and JunD (329) were used. All antibodies were purchased as gel supershift reagents from Santa Cruz Biotechnology (Santa Cruz, Calif.). Rabbit immunoglobulin was used as a negative control (Dakopatts, Gentofte, Denmark).
Statistics.
Student’s t test for paired data was used for statistical calculations. Statistical significance was set at P ≤ 0.05.
RESULTS
Superinduced early gene transcription by ciprofloxacin in primary lymphocytes.
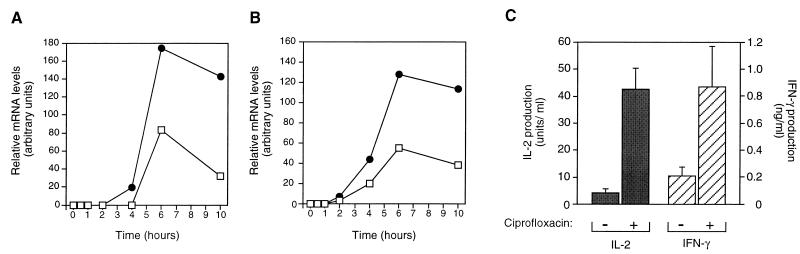
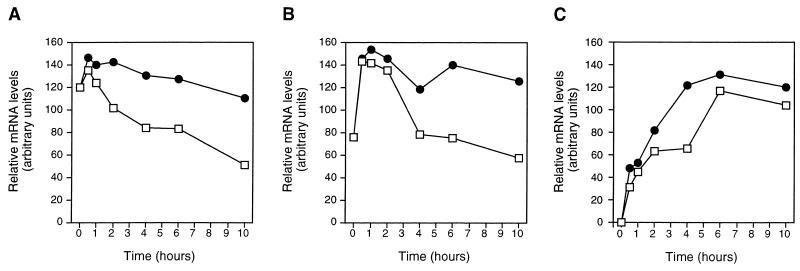
We have earlier demonstrated that ciprofloxacin enhances several cytokine mRNAs, including IL-2 and IFN-γ in PHA-activated PBLs incubated for 24 to 72 h (30). In order to characterize the early kinetics of IL-2 and IFN-γ transcripts, total RNA was isolated from PHA-activated PBLs incubated with ciprofloxacin for up to 10 h. Specific cytokine mRNAs in addition to the housekeeping β-actin message were analyzed with Northern blots with radioactive cDNA probes. At 4 to 10 h of culture, IL-2 and IFN-γ mRNA levels were significantly increased in PBLs incubated with ciprofloxacin compared to in untreated controls. Up to 2.2-fold-more IL-2 mRNA was detected in ciprofloxacin-treated cells as early as 6 h after PHA activation (Fig. 1A). A similar increase was observed when IFN-γ mRNA was examined (Fig. 1B). The ciprofloxacin-dependent superinduction of cytokine mRNAs resulted in enhanced cytokine secretion (Fig. 1C). In PBL cultures incubated with ciprofloxacin, the IL-2 and IFN-γ production increased 10- and 4-fold, respectively.
FIG. 1.
Kinetics of IL-2 and IFN-γ gene expression in PBLs exposed to ciprofloxacin. IL-2 (A) and IFN-γ (B) mRNA concentrations were analyzed at 30 min to 10 h for human PHA-activated PBLs incubated with ciprofloxacin (80 μg/ml) (•) and compared to those in drug-free controls (□). At 24 h, IL-2 and IFN-γ concentrations (C) in culture supernatants were analyzed. Total RNA was isolated at the indicated time points, and Northern blotting was performed with comparable samples of RNA. Radioactive cDNA probes specific for the indicated genes were sequentially hybridized to the filters. Scanning densitometry data are shown. The specific cytokine values were divided by the corresponding values for β-actin mRNA. An arbitrary unit was defined as equal amounts of cytokine mRNA and β-actin mRNA. No cytokine mRNA was detected in unstimulated PBLs at time zero and was set as 0 arbitrary units. The data presented in panels A and B are representative of three independent experiments and blood donors. (C) IL-2 and IFN-γ were analyzed with an IL-2-dependent cytotoxic T-cell line and enzyme-linked immunosorbent assay, respectively. Results from four separate experiments are shown. Error bars in panel C indicate the standard deviation. P < 0.01 and P < 0.001 for IL-2 and IFN-γ, respectively.
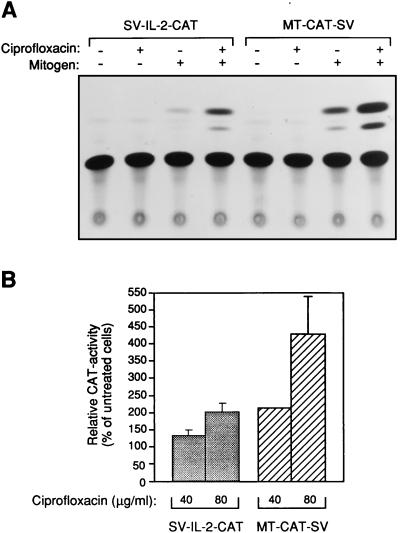
Collective data from our previous investigations demonstrated that ciprofloxacin enhances IL-2 transcriptional activity in T cells (27, 30). However, those studies were performed with immortalized cell lines that in many ways differ from primary cells. To characterize gene transcription in ciprofloxacin-treated primary lymphocytes, human PBLs were transiently transfected with a construct containing the IL-2 promoter and enhancer in conjunction with a simian virus 40 (SV40) enhancer element linked to the bacterial reporter gene coding for CAT. In addition, a CAT plasmid consisting of the metallothionein enhancer and promoter (instead of the corresponding part of IL-2) was used as a control. IL-2 promoter activity was enhanced (i.e., an increased CAT activity was detected in PBLs incubated in the presence of ciprofloxacin at 80 μg/ml) (Fig. 2A). Interestingly, when metallothionein enhancer activity was examined in ciprofloxacin-treated PBLs, CAT activity was found to be upregulated. Metallothionein expression in PHA-activated PBLs increased 2.2- and 4.3-fold at ciprofloxacin concentrations of 40 and 80 μg/ml, respectively, compared to that in controls without ciprofloxacin (Fig. 2B). The superinduced metallothionein gene activity was considerably stronger than the IL-2 gene induction. For comparison, ciprofloxacin at 40 and 80 μg/ml increased IL-2 gene induction 1.3- and 2.1-fold, respectively. Thus, the augmented gene transcription by ciprofloxacin was not specific for lymphocyte-derived cytokines.
FIG. 2.
Ciprofloxacin-dependent increase of IL-2 and metallothionein enhancer and promoter regions in stimulated PBLs. Raw data are shown for IL-2 and metallothionein, indicated by SV-IL-2-CAT and MT-CAT-SV, respectively (A). Relative CAT activities for ciprofloxacin-treated PBL are also presented (B). PBLs were stimulated (1 μg of PHA per ml and 50 ng of PMA per ml) for 18 h, at which time, cells were transfected with the indicated plasmids and rested. After 36 h, PBLs were rechallenged with 1 μg of PHA per ml and incubated with or without ciprofloxacin (40 or 80 μg/ml). After an additional 15 h, cells were lysed and CAT activity was analyzed as described in Materials and Methods. For quantification, areas containing the acetylated and nonacetylated forms of chloramphenicol were cut out from thin-layer chromatography plates and measured with a scintillation counter. Six independent donors were examined on six different occasions. Duplicates were included in all experiments. Error bars indicate the standard error of the mean. Values for ciprofloxacin-treated PBLs were significantly different from those of the controls. SV-IL-2-CAT, P < 0.05 and P < 0.01 for ciprofloxacin at 40 and 80 μg/ml, respectively. MT-CAT-SV, P < 0.001 and P < 0.05 for ciprofloxacin at 40 and 80 μg/ml, respectively.
Effect of ciprofloxacin on transcription factor activity in primary lymphocytes.
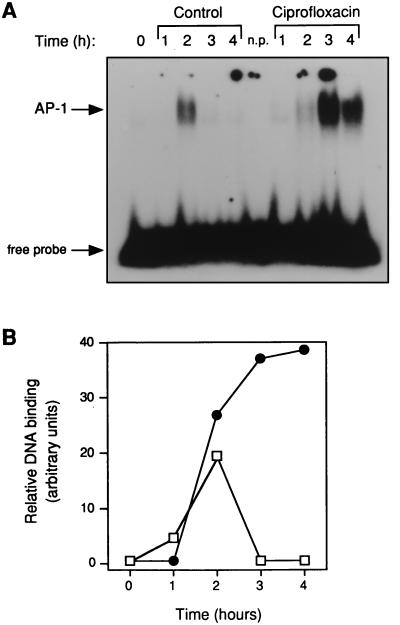
Since ciprofloxacin has been shown to interfere with transcription factors in the two T-cell lymphoma cell lines Jurkat and EL-4 (27, 30), the next step was to examine the transcription factors AP-1, NF-AT-1, and NF-κB in T cells purified from peripheral blood. To exclude monocytes and B lymphocytes, CD3+ CD4+ lymphocytes (T helper cells) were isolated by negative selection with magnetic beads. Total cell protein was isolated from PHA- and PMA-activated CD4+ T cells at 1 to 4 h and incubated with a 32P-labelled oligonucleotide corresponding to an AP-1 consensus binding site. When the protein-DNA complexes were analyzed by PAGE, a distinct pattern appeared (Fig. 3A). At ≥3 h of incubation, a higher level of AP-1 binding was detected in CD4+ T-cell extracts purified from activated cells incubated with ciprofloxacin compared to that in drug-free controls. To verify that equal amounts of protein were added to the reaction mixtures, cell extracts were also analyzed for Oct-1 binding that is considered to be invariable during the cell cycle (not shown). Mean AP-1 binding activities divided by corresponding values for Oct-1 are illustrated in Fig. 3B. In contrast, when the same cell extracts were examined for NF-AT-1 or NF-κB binding, we did not observe any difference between ciprofloxacin-treated cells and controls incubated without any drug (data not shown).
FIG. 3.
Increased AP-1 binding activity in stimulated CD4+ T cells incubated with ciprofloxacin. A typical AP-1 shift is shown for activated purified T helper cells incubated with (•) or without (█)) ciprofloxacin (80 μg/ml) (A). Scanning densitometry data from three independent experiments are also shown (B). Total cell protein was isolated from a pure population of T helper cells that had been activated with PHA (1 μg/ml) and PMA (50 ng/ml) for the indicated times. Protein extracts were incubated with radioactive oligonucleotides and analyzed by PAGE. n.p., a reaction without any protein. In panel B, 1 arbitrary unit was defined as equal amounts of AP-1 and Oct-1. No AP-1 binding activity was detected in unstimulated cells incubated with ciprofloxacin.
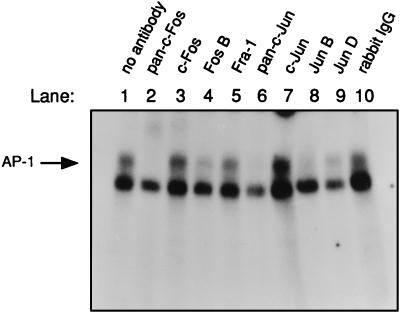
To further characterize the different proteins included in the AP-1 complex detected in T cells exposed to ciprofloxacin, supershifts were performed. The reaction mixtures were supplemented with polyclonal antibodies directed against the specific members of the c-Fos and c-Jun families 30 min prior to addition of the radioactive AP-1 oligonucleotide. Cytoplasmic protein isolated from the 3-h time point with ciprofloxacin, as shown in Fig. 3A, was analyzed for AP-1 components. The upper band showed the specific AP-1 binding, as can be seen in Fig. 4, lane 1. The AP-1 shift disappeared when the polyclonal antiserum against the c-Fos and c-Jun family of proteins was added, as illustrated in lane 2 (pan-c-Fos) and lane 6 (pan-c-Jun), respectively. Rabbit immunoglobulin, a negative antibody control, did not interfere with the specific AP-1 band (Fig. 4, lane 10). Interestingly, the AP-1 complex mainly consisted of the FosB, JunB, and JunD proteins. A similar pattern, however, was seen in ciprofloxacin-free control extracts. Taken together, the upregulated AP-1 activity observed in ciprofloxacin-treated T cells was indeed specific because it consisted of proteins from the c-Fos and c-Jun families.
FIG. 4.
The superinduced AP-1 complex in ciprofloxacin-treated CD4+ T cells contained proteins from the c-Fos and c-Jun families. Cell extract from the CD4+ T cells exposed to ciprofloxacin for 3 h displayed in Fig. 3A was examined in supershifts. The upper band shows the specific AP-1 binding (see, for example, lanes 1 and 3). The lower band shows the unspecific binding to the AP-1 oligonucleotide sometimes observed. Rabbit polyclonal antibodies were preincubated with the extracts for 30 min on ice prior to addition of the AP-1 oligonucleotide. Unspecific rabbit immunoglobulins, which were used as a control serum, are indicated by rabbit immunoglobulin G (IgG) in lane 10. The free probe (shown in Fig. 3A) was run out from the gel. Similar results were obtained with the 2-h extract from control T cells shown in Fig. 3A (data not presented). The results are representative for two different blood donors.
Increased concentrations of immediate-early expressed mRNAs in ciprofloxacin-treated PBLs.
Metallothioneins that protect cells against oxidative stress are induced by a wide range of different stimuli, including metals and UV C radiation (14). The observed hyperinduced metallothionein transcription in addition to increased AP-1 activity suggested that a phenomenon similar to a stress response occurred in PBLs incubated with ciprofloxacin. This urged us to examine the expression of c-fos, c-jun, and c-myc mRNAs, which is commonly related to mammalian stress responses. To investigate the effect of ciprofloxacin on these immediate-early gene transcripts, PBLs were stimulated with PHA and incubated with ciprofloxacin (80 μg/ml), followed by analysis of steady-state mRNA levels at various time points. No major differences were observed between ciprofloxacin-treated and control PBLs during the first 20 min of culture (not shown). However, after 30 to 120 min of incubation, more c-fos and c-jun mRNAs were detected in cells incubated with ciprofloxacin than in drug-free controls (Fig. 5A and B). The increased mRNA levels found in cells incubated with ciprofloxacin were present up to 10 h of incubation. After 4 h of incubation, ciprofloxacin did not, however, enhance steady-state mRNA concentrations above the initial mRNA levels detected at 30 to 60 min. In contrast to c-fos and c-jun mRNAs, c-myc mRNA was expressed earlier in ciprofloxacin-treated PBLs (at 2 to 6 h) than in the drug-free controls (Fig. 5C). Interestingly, the kinetics of these immediate-early induced genes were in accordance with the kinetics for IL-2 and IFN-γ mRNAs, whose expression is dependent on, among other proteins, c-Fos and c-Jun. The steady-state cytokine mRNA levels increased at ≥4 h (Fig. 1A and B), a time point when the immediate-early mRNAs already had started accumulating (Fig. 5). Thus, in ciprofloxacin-treated cells, the activated AP-1 found at ≥2 h (Fig. 3B) paralleled the increased c-jun and c-fos mRNA steady-state levels detected at ≥30 min.
FIG. 5.
Kinetics of immediate-early gene transcripts in stimulated PBLs incubated with ciprofloxacin. Northern blot results are shown for c-fos (A), c-jun (B), and c-myc (C) mRNA expression between 30 min and 10 h with (•) or without (□) ciprofloxacin. PBLs were isolated from healthy donors, and PHA (1 μg/ml) in addition to ciprofloxacin (80 μg/ml) was added at culture initiation. Specific mRNAs were analyzed as described in the legend to Fig. 1. One typical experiment out of three is shown.
Ciprofloxacin did not stabilize the immediate-early expressed gene transcripts.
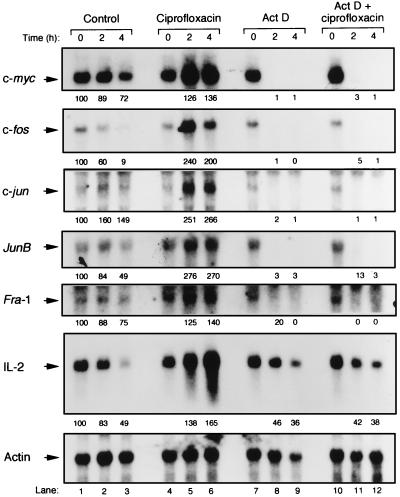
To exclude whether ciprofloxacin influenced c-fos, c-jun, and c-myc mRNA degradation, ciprofloxacin with or without the transcriptional inhibitor actinomycin D was added to PBLs that had been stimulated with PHA for 6 h. Different mRNAs were analyzed after 2 and 4 h of the actinomycin D and/or ciprofloxacin chase (Fig. 6, lanes 7 to 12). Irrespective of the presence or absence of ciprofloxacin, c-myc, c-fos, c-jun, junB, fra-1, and IL-2 mRNAs were all degraded in actinomycin D-treated cells (i.e., when new mRNA transcription was inhibited by actinomycin D). Thus, ciprofloxacin did not influence the half-life of the immediate-early transcripts. In the absence of actinomycin D, the various mRNAs were found to be upregulated by ciprofloxacin after both 2 and 4 additional h of culture, indicating that even a short exposure to the drug resulted in increased mRNA concentrations (Fig. 6, lanes 1 to 6). For example, 2.6- and 2.7-fold increases of c-jun and junB mRNA levels, respectively, were detected after a 4-h ciprofloxacin pulse. In contrast, the control mRNA, β-actin, was unaffected by ciprofloxacin.
FIG. 6.
Ciprofloxacin does not influence the half-lives of immediate-early gene transcripts in activated PBLs. Northern blot results are shown for c-myc, c-fos, c-jun, junB, and fra-1 mRNAs. For comparison, IL-2 mRNA was included. Scanning densitometry data are shown below the specific mRNA bands and were obtained by dividing all values by the corresponding values for β-actin mRNA. Control cell cultures (without any addition of drugs) were set to 100 arbitrary units for the different mRNAs. Lane numbers are indicated at the bottom. Human PBLs were stimulated with PHA (4 μg/ml) for 6 h, at which time ciprofloxacin (80 μg/ml) and/or actinomycin D (Act D [10 μg/ml]) was added. mRNAs were analyzed at 0, 2, and 4 h of additional lymphocyte incubation as described in the legend to Fig. 1. The data presented are representative of three independent experiments including different blood donors.
DISCUSSION
In the present investigation, we show that the quinolone antibiotic ciprofloxacin induces a mammalian stress response similar to the SOS response seen in bacteria. The extent to which the implications of the effects of fluoroquinolones on PBLs observed in the present study are of clinical interest is difficult to establish, since the effects were observed at intermediate and high quinolone concentrations not reached in patients. However, the most likely parallels can be drawn with the effects of clinically achievable concentrations of ciprofloxacin. It is well established that ciprofloxacin and other quinolones at 5 μg/ml increase IL-2 production in both human and murine in vitro systems, but also that a maximal effect is observed at 80 μg/ml (26, 38). Due to the relatively insensitive methods presently available, we did not detect any IL-2 mRNA increase at 5 μg/ml but only detected increases at 20 and 80 μg/ml (30). In parallel, the augmented induction of the IL-2 and metallothionein genes was observed at ≥40 μg/ml (Fig. 2). Recently published reports on immunomodulatory effects of ciprofloxacin in vivo support our hypothesis. For example, when ciprofloxacin at clinically relevant doses was administered to sublethally irradiated mice, hematopoiesis was stimulated, most probably due to induction of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-3 (19, 36). Despite the high concentrations of ciprofloxacin (80 μg/ml) that were required for us to sense a significant response in vitro, augmented GM-CSF and IL-3 mRNA levels were observed in mitogen-activated PBLs exposed to ciprofloxacin (30). Another example of a relationship between in vitro and in vivo findings was recently published by Wrishko et al. (41). In their study, a pharmacodynamic interaction between cyclosporine A and ciprofloxacin was demonstrated in renal transplant patients, suggesting that the antibacterial agent may be immunomodulatory, particularly when T cells were activated (41). The corresponding in vitro finding, i.e., a cyclosporine A-dependent immunosuppressive effect on cytokine synthesis counteracted by the quinolone, was observed at ≥20 μg of ciprofloxacin per ml (29). Taken together, the collective data suggest that the upregulated IL-2 synthesis observed in PBLs incubated with low concentrations of ciprofloxacin may reflect a stress response. However, ciprofloxacin at high concentrations was required in vitro to clearly demonstrate the relationship.
During treatment of mammalian cells with, for example, cytotoxic drugs or heavy metals, a stress response is generally triggered, resulting in induction of several different genes (10, 11). Increased concentrations of transcriptional regulation factors (27, 30) (Fig. 2) and upregulated c-fos, c-jun, and cytokine gene transcripts in stimulated eukaryotic cells exposed to ciprofloxacin suggest that ciprofloxacin might have induced a stress response similar to a mammalian DNA damage response (genotoxic stress). However, since ciprofloxacin did not increase mutation frequency (DNA damage) in a shuttle vector plasmid mutation test (3), direct interference with topoisomerase II might be another mechanism by which ciprofloxacin induced a stress response. Ciprofloxacin at high concentrations (80 μg/ml) has in fact been shown to interfere with topoisomerase II in Raji cells when investigated by a neutral elution technique (2). Interestingly, the topoisomerase II inhibitor vepeside (VP-16) induces vimentin expression and activates the GADD153 promoter in the monocytic cell line U-937 (20, 31). In addition, vepeside affects protein kinase C in the same cell line, causing enhanced AP-1 activity, but without influencing NF-κB binding (23). Inhibition of topoisomerase II in thymocytes by teniposide (VM-26) leads to superinduction of c-jun mRNA expression and the c-Jun N-terminal kinase, resulting in increased AP-1 activity (39). It has also been shown that teniposide induces the transcription of heat shock genes in HeLa cells (32). However, despite the fact that vepeside has been thoroughly documented to induce genotoxic stress, it did not stimulate IL-2 gene expression in our experimental models (28). Thus, in addition to triggering a genotoxic response, DNA-damaging agents may directly inhibit topoisomerase II, thus facilitating transcription. This would be another mechanism that accounts for the effects seen in ciprofloxacin-treated cells.
DNA-damaging agents and genotoxic stress may also increase the stability of certain gene transcripts. Recently it was reported that photodynamic treatment of HeLa cells resulted in the prolonged half-life of c-jun and c-fos transcripts (18). UV radiation or methylmethane sulfonate (MMS) also enhanced mRNA stability for the growth arrest and DNA damage-inducible (gadd) genes in hamster cells compared to that in untreated exponentially growing cells (12). Furthermore, Mallardo and collaborators described an extensive IL-6 and IL-1 mRNA stabilization in human monocytes incubated with the alkylating agents MMS and mitomycin (22). In contrast, ciprofloxacin did not appear to increase the stability of immediate-early mRNAs: no difference in mRNA turnover was detected in PBLs incubated with or without ciprofloxacin in the presence of the transcriptional inhibitor actinomycin D (Fig. 6).
Activation of T lymphocytes results in the induction of a number of genes, such as immediate-early (e.g., c-fos and c-jun), early (IL-2), and late-response (histone H3) genes (4). In cells incubated with ciprofloxacin, a time relationship appeared to exist between the increased c-fos and c-jun mRNA levels on one hand and the superinduced IL-2 and IFN-γ mRNA concentrations on the other (Fig. 1 and 5). In contrast to ciprofloxacin-treated Jurkat cells that display increased AP-1 concentrations at 15 min (30), any upregulated AP-1 binding by ciprofloxacin was not detected at earlier time points than 2 h in primary lymphocytes (Fig. 3). Thus, in PBLs, the upregulated expression of c-fos and c-jun mRNAs was not preceded by increased AP-1 activity. The mRNAs of at least six different Fos and Jun proteins included in the AP-1 protein complex are detected in stimulated T cells (13). Interestingly, in our highly purified CD4+ T cells activated with PHA and PMA, the induced AP-1 detected at 2 and 3 h of activation mainly consisted of the proteins FosB, JunB, and JunD (Fig. 4). The immediate-early genes encoding members of the Jun and Fos families are tightly controlled by transcription factors other than AP-1. The regulation of c-fos is mediated by factors binding to a cyclic AMP-responsive element (CRE), Sis-inducible enhancer (SIE), and, finally, a serum-response element (SRE) (see reference 16 for a review). Numerous transcriptional regulation factors, including CRE-binding proteins and the signal transducer and activator of transcription factors, including CRE-binding proteins and the signal transducer and activator of transcription (STAT) group, bind to the c-fos enhancer and promoter region. In contrast, c-jun is induced via a tetradecanoyl phorbol acetate-responsive element (TRE), which has been proposed to be recognized by c-Jun-activating transcription factor 2 (ATF2) heterodimers in addition to other presently unknown factors. Taken together, ciprofloxacin induced transcripts consisting of members of the Jun and Fos families, resulting in dimeric protein complexes that in turn constituted AP-1.
Depending on the composition of the AP-1 dimer, different sequence elements are preferentially recognized. Two AP-1 binding sequences exist in the promoter of the T-cell cytokine IL-2 (4, 6). In contrast to a less important distal AP-1 site, the proximal AP-1 sequence is crucial for IL-2 gene induction and represents a major site of protein kinase C responsiveness (14). Deletion of the proximal site diminishes the IL-2 promoter activity by approximately 75%, compared to that of the wild type (6). We investigated the effect of ciprofloxacin on IL-2 promoter induction in a well-defined murine system by using different constructs consisting of mutated IL-2 enhancer and promoter regions (27). No upregulated CAT conversion was found with an IL-2 promoter construct lacking the proximal AP-1 site in the presence of ciprofloxacin (25a). In contrast, in experiments with IL-2 promoter constructs lacking the NF-AT-1 or Oct-1/OAP40 binding sequences, ciprofloxacin increased CAT activity regardless of the mutated sequence compared to that in drug-free controls. Moreover, in the present study, it was shown that ciprofloxacin increased expression for both constructs MT-CAT-SV and IL-2-CAT-SV containing the promoters metallothionein and IL-2, respectively (Fig. 2). To reach the detection limit for CAT in primary lymphocytes, we had to include the SV40 enhancer. It is well known that AP-1 is crucial for the activity of the SV40 enhancer and the IL-2 promoter (6, 25). In contrast, AP-1 seems to be less important for the induction of the metallothioneins (24). When the AP-1 site was mutated in the human metallothionein promoter, the low basal activity disappeared, while the metallothionein gene still could be induced by Zn2+ (21). Unpublished observations from our laboratory reveal that in contrast to an intact promoter, the metallothionein promoter with a mutated AP-1 site (21) was not enhanced by ciprofloxacin in stimulated murine EL-4 cells. Most likely the ciprofloxacin-dependent upregulation of MT-CAT-SV activity in PBLs was not caused by the non-AP-1-related transcription factors (24) but was due to a combination of the AP-1 binding sequences in the metallothionein promoter and the SV40 enhancer element. Ciprofloxacin appeared to mainly interfere with AP-1 activity when transcriptional activity was examined with CAT constructs.
In conclusion, an association between stress response and cytokine superinduction triggered by ciprofloxacin has been described in the present communication. Several gene transcripts included in the c-Fos and c-Jun families in addition to most T-cell cytokines (30) were augmented in primary lymphocytes exposed to ciprofloxacin. Moreover, AP-1 binding activity was increased in ciprofloxacin-treated PBLs, which is a commonly described phenomenon during mammalian stress. Despite the available evidence of immunomodulatory effects of ciprofloxacin in vivo (36, 41), the clinical significance of our findings warrants further studies.
ACKNOWLEDGMENTS
We thank Michael Karin, Carsten Jonat, and Tullia Lindsten for providing us with plasmids.
This investigation was supported by grants from the Anna and Edwin Berger Foundation, the Greta and Johan Kock Foundation, the Cancer Foundation at University Hospital MAS, the Swedish Medical Research Council, and the Österlund Foundation.
REFERENCES
- 1.Boise L H, Petryniak B, Mao X, June C H, Wang C-Y, Lindsten T, Bravo R, Kovary K, Leiden J M, Thompson C B. The NFAT-1 DNA binding complex in activated T cells contains Fra-1 and JunB. Mol Cell Biol. 1993;13:1911–1919. doi: 10.1128/mcb.13.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredberg A, Brant M, Jaszyk M. Ciprofloxacin-induced inhibition of topoisomerase II in human lymphoblastoid cells. Antimicrob Agents Chemother. 1991;35:448–450. doi: 10.1128/aac.35.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredberg A, Brant M, Riesbeck K, Azou Y, Forsgren A. 4-Quinolone antibiotics: positive genotoxic screening tests despite an apparent lack of mutation induction. Mutat Res. 1989;211:171–181. doi: 10.1016/0027-5107(89)90117-6. [DOI] [PubMed] [Google Scholar]
- 4.Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and the nucleus of T-lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 5.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durand D B, Shaw J-P, Bush M R, Replogle R E, Balagaje R, Crabtree G R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelberg D, Klein C, Martinetto H, Struhl K, Karin M. The UV response involving the Ras signaling pathway and AP-1 transcription factors is conserved between yeast and mammals. Cell. 1994;77:381–390. doi: 10.1016/0092-8674(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 8.Forsgren A, Schlossman S F, Tedder T F. 4-Quinolone drugs affect cell cycle progression and function of human lymphocytes in vitro. Antimicrob Agents Chemother. 1987;31:768–773. doi: 10.1128/aac.31.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori K, Angel P, LeBeau M M, Karin M. Structure and chromosomal localization of the functional intronless human protooncogene JUN. Proc Natl Acad Sci USA. 1988;85:9148–9152. doi: 10.1073/pnas.85.23.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrlich P, Ponta H, Rahmsdorf H J. DNA damage-induced gene expression: signal transduction and relation to growth factor signaling. Rev Physiol Biochem Pharmacol. 1992;119:187–223. doi: 10.1007/3540551921_7. [DOI] [PubMed] [Google Scholar]
- 11.Holbrook N J, Liu Y, Fornace A J., Jr . Signaling events controlling the molecular response to genotoxic stress. In: Feige U, Morimoto R I, Yahara I, Polla B, editors. Stress-inducible cellular responses—1996. Basel, Switzerland: Birkhäuser Verlag; 1996. pp. 273–288. [DOI] [PubMed] [Google Scholar]
- 12.Jackman J, Alamo I, Jr, Fornace A J., Jr Genotoxic stress confers preferential and coordinate messenger RNA stability on the five gadd genes. Cancer Res. 1994;54:5656–5662. [PubMed] [Google Scholar]
- 13.Jain J, Valge-Archer V E, Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992;148:1240–1250. [PubMed] [Google Scholar]
- 14.Jain J, Valge-Archer V E, Sinskey A J, Rao A. The AP-1 site at −150 bp, but not the NF-κB site, is likely to represent the major target of protein kinase C in the interleukin 2 promoter. J Exp Med. 1992;175:853–862. doi: 10.1084/jem.175.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kägi J H. Overview of metallothionein. Methods Enzymol. 1991;205:613–626. doi: 10.1016/0076-6879(91)05145-l. [DOI] [PubMed] [Google Scholar]
- 16.Karin M, Zheng-Gang L, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 17.Kharabanda S, Yuan Z-M, Weichselbaum R, Kufe D. Functional role for the c-Abl protein tyrosine kinase in the cellular response to genotoxic stress. Biochim Biophys Acta. 1997;1333:O1–O7. doi: 10.1016/s0304-419x(97)00020-6. [DOI] [PubMed] [Google Scholar]
- 18.Kick G, Messer G, Plewig G, Kind P, Goetz A E. Strong and prolonged induction of c-jun and c-fos proto-oncogenes by photodynamic therapy. Br J Cancer. 1996;74:30–36. doi: 10.1038/bjc.1996.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kletter Y, Riklis I, Shalit I, Fabian I. Enhanced repopulation of murine hematopoietic organs in sublethally irradiated mice after treatment with ciprofloxacin. Blood. 1991;78:1685–1691. [PubMed] [Google Scholar]
- 20.Luethy J D, Holbrook N J. Activation of the gadd153 promoter by genotoxic agents: a rapid specific response to DNA damage. Cancer Res. 1992;52:5–10. [PubMed] [Google Scholar]
- 21.Makarov S S, Jonat C, Haskill S. Hyperinducible human metallothionein promoter with a low level basal activity. Nucleic Acids Res. 1994;22:1504–1505. doi: 10.1093/nar/22.8.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallardo M, Giordano V, Dragonetti E, Scala G, Quinto I. DNA damaging agents increase the stability of interleukin-1 alpha, interleukin-1 beta, and interleukin-6 transcripts and the production of the relative proteins. J Biol Chem. 1994;269:14899–14904. [PubMed] [Google Scholar]
- 23.Perez C, Vilaboa N E, Garcia-Bermejo L, de-Blas E, Creighton A M, Aller P. Differentiation of U-937 promonocytic cells by etoposide and ICRF-193, two antitumour DNA topoisomerase inhibitors with different mechanisms of action. J Cell Sci. 1997;110:337–343. doi: 10.1242/jcs.110.3.337. [DOI] [PubMed] [Google Scholar]
- 24.Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, Schaffner W. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 1993;12:1355–1362. doi: 10.1002/j.1460-2075.1993.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahmsdorf H J, Herrlich P. Regulation of gene expression by tumor promoters. Pharmacol Ther. 1990;48:157–188. doi: 10.1016/0163-7258(90)90079-h. [DOI] [PubMed] [Google Scholar]
- 25a.Riesbeck, K. Unpublished observations.
- 26.Riesbeck K, Andersson J, Gullberg M, Forsgren A. Fluorinated 4-quinolones induce hyperproduction of interleukin 2. Proc Natl Acad Sci USA. 1989;86:2809–2813. doi: 10.1073/pnas.86.8.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riesbeck K, Forsgren A. Increased interleukin 2 transcription in murine lymphocytes by ciprofloxacin. Immunopharmacology. 1994;27:155–164. doi: 10.1016/0162-3109(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 28.Riesbeck K, Forsgren A. CP-115,953 stimulates cytokine production by lymphocytes. Antimicrob Agents Chemother. 1995;39:476–483. doi: 10.1128/aac.39.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riesbeck K, Gullberg M, Forsgren A. Evidence that the antibiotic ciprofloxacin counteracts cyclosporine-dependent suppression of cytokine production. Transplantation. 1994;57:267–272. doi: 10.1097/00007890-199401001-00020. [DOI] [PubMed] [Google Scholar]
- 30.Riesbeck K, Sigvardsson M, Leanderson T, Forsgren A. Superinduction of cytokine gene transcription by ciprofloxacin. J Immunol. 1994;153:343–352. [PubMed] [Google Scholar]
- 31.Rius C, Zorilla A R, Cabans C, Mata F, Bernabeu C, Aller P. Differentiation of human promonocytic leukemia U-937 cells with DNA topoisomerase II inhibitors: induction of vimentin gene expression. Mol Pharmacol. 1991;39:442–448. [PubMed] [Google Scholar]
- 32.Schaak J, Schedl P, Schenk T. Transcription of adenovirus and HeLa cell genes in the presence of drugs that inhibit topoisomerase I and II function. Nucleic Acids Res. 1990;18:1499–1508. doi: 10.1093/nar/18.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schöler H R, Hatzopoulos A K, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989;8:2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultze-Osthoff K, Bauer M K A, Vogt M, Wesselborg S. Oxidative stress and signal transduction. Int J Vitam Nutr Res. 1997;67:336–342. [PubMed] [Google Scholar]
- 35.Schwartz M A, Lazo J S, Yalowich J C, Reynolds I, Kagan V E, Tyurin V, Kim Y-M, Watkins S C, Pitt B R. Cytoplasmic metallothionein overexpression protects NIH 3T3 cells from tert-butyl hydroperoxide toxicity. J Biol Chem. 1994;269:15238–15243. [PubMed] [Google Scholar]
- 36.Shalit I, Kletter Y, Weiss K, Gruss T, Fabian I. Enhanced hematopoiesis in sublethally irradiated mice treated with various quinolones. Eur J Haematol. 1997;58:92–98. doi: 10.1111/j.1600-0609.1997.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 37.Smith M L, Fornace A J., Jr Mammalian DNA damage-inducible genes associated with growth arrest and apoptosis. Mutat Res. 1996;340:109–124. doi: 10.1016/s0165-1110(96)90043-3. [DOI] [PubMed] [Google Scholar]
- 38.Stünkel K G E, Hewlett G, Zeiler H J. Ciprofloxacin enhances T cell function by modulating interleukin activities. Clin Exp Immunol. 1991;86:525–531. doi: 10.1111/j.1365-2249.1991.tb02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Testolin L, Carson C, Wang Y, Walker P R, Armato U, Sikorska M. Jun and JNK kinase are activated in thymocytes in response to VM26 and radiation but not glucocorticoids. Exp Cell Res. 1997;230:220–232. doi: 10.1006/excr.1996.3419. [DOI] [PubMed] [Google Scholar]
- 40.Walker G C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984;48:60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrishko R E, Levine M, Primmett D R N, Kim S, Partovi N, Lewis S, Landsberg D, Keown P A. Investigation of a possible interaction between ciprofloxacin and cyclosporine in renal transplant patients. Transplantation. 1997;64:996–999. doi: 10.1097/00007890-199710150-00011. [DOI] [PubMed] [Google Scholar]
- 42.Zehavi-Willner T, Shalit I. Enhancement of interleukin-2 production in human lymphocytes by two new quinolone derivatives. Lymphokine Res. 1989;3:35–46. [PubMed] [Google Scholar]