Abstract
Expression of the multidrug efflux system MexC-MexD-OprJ in nfxB mutants of Pseudomonas aeruginosa contributes to resistance to fluoroquinolones and the “fourth-generation” cephems (cefpirome and cefozopran), but not to most β-lactams, including the ordinary cephems (ceftazidime and cefoperazone). nfxB mutants also express a second multidrug efflux system, MexA-MexB-OprM, due to incomplete transcriptional repression of this operon by the mexR gene product. To characterize the contribution of the MexC-MexD-OprJ system to drug resistance in P. aeruginosa, a site-specific deletion method was employed to remove the mexA-mexB-oprM region from the chromosome of wild-type and nfxB strains of P. aeruginosa. Characterization of mutants lacking the mexA-mexB-oprM region clearly indicated that the MexC-MexD-OprJ efflux system is involved in resistance to the ordinary cephems as well as fluoroquinolones and the fourth-generation cephems but not to carbenicillin and aztreonam. Rabbit polyclonal antisera and murine monoclonal antibody against the components of the MexA-MexB-OprM system were prepared and used to demonstrate the reduced production of this efflux system in the nfxB mutants. Consistent with this, transcription of the mexA-mexB-oprM operon decreased in an nfxB mutant. This reduction appears to explain the hypersusceptibility of the nfxB mutant to β-lactams, including ordinary cephems.
The clinically important opportunistic pathogen Pseudomonas aeruginosa exhibits intrinsic multiple-antibiotic resistance, which has been assumed to result from the low permeability of its outer membrane (21, 35). The OprM-overproducing nalB-type mutants show increased resistance to quinolones, β-lactams, tetracycline, and chloramphenicol (14) as well as trimethoprim and sulfamethoxazole (9). Recent identification of the mexA-mexB-oprM operon on the P. aeruginosa chromosome (24, 25), whose products show significant homology to other bacterial efflux systems such as those encoded by acrAB (16) and mtrCDE (23), suggests that antibiotic resistance is caused by this system via drug efflux. Indeed, overexpression of this operon by a mutation in the regulator gene mexR, which exists upstream of mexA-mexB-oprM operon and transcribed in the opposite direction, affords nalB-type multidrug resistance to P. aeruginosa (27). Moreover, disruption of each gene of this operon, which is expressed in wild-type cells, increases susceptibility of both wild-type and nalB-type strains to the same level, suggesting that the intrinsic resistance of this bacterium results, in part, from the function of this efflux system (4, 12, 13, 24).
Two homologs of the mexA-mexB-oprM operon, mexC-mexD-oprJ and mexE-mexF-oprN, have recently been identified and are overexpressed in nfxB and nfxC mutant cells, respectively (10, 26). Transcription of the mexC-mexD-oprJ operon is strictly repressed by a negative regulator encoded by the nfxB gene (22, 26, 29), and overexpression of this operon, as a result of the nfxB mutation, confers on cells resistance to fluoroquinolones, the “fourth-generation” cephems, tetracycline, and chloramphenicol and hypersusceptibility to most other β- lactams (19). The nfxC mutants expressing the mexE-mexF-oprN operon are resistant to fluoroquinolones and imipenem (2, 18).
Expression of the mexC-mexD-oprJ and mexE-mexF-oprN operons is not detectable in the wild-type P. aeruginosa (10, 26), while the mexA-mexB-oprM operon is expressed constitutively (4). Therefore, resistance profiles characterized in the nfxB and nfxC mutants might be attributed to a combination of the MexA-MexB-OprM system and either the MexC-MexD-OprJ or MexE-MexF-OprN system.
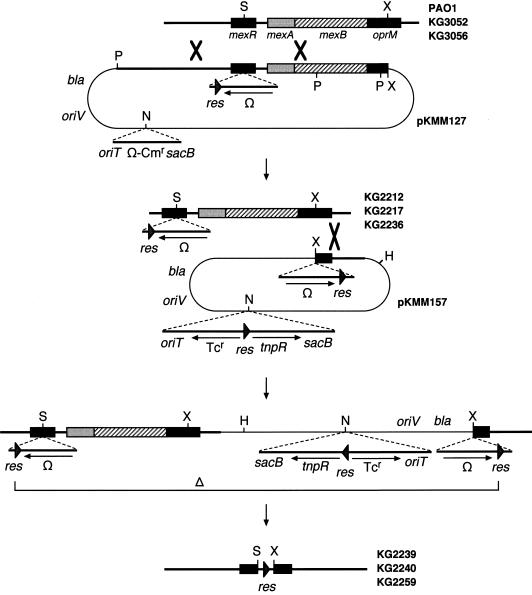
One of us has recently devised a general mutagenesis system to delete a large and defined chromosomal fragment by using the site-specific resolution system encoded by a class II transposon (34). In this system, two copies of the site-specific resolution (res) site are inserted at two defined chromosomal positions so that the res sites are in the same orientation. Provision of the site-specific TnpR recombinase leads to very efficient excision of the DNA fragment flanked by the two res sites. An improved version of this system was developed in the present study and used to isolate mutants specifically lacking the mexR-mexA-mexB-oprM region from the wild-type and nfxB mutant strains of P. aeruginosa. The study of such deletion mutants elucidated the role of the MexC-MexD-OprJ efflux system in resistance to various antibiotics.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used in this study are listed in Table 1. Bacterial cells were grown in L broth (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, and 0.5% [wt/vol] NaCl) or L agar (L broth plus 1.5% [wt/vol] agar) at 37°C. BM2 minimal medium (3) was used for selection of P. aeruginosa since Escherichia coli cannot utilize citrate. The following antibiotics were added to media at the indicated concentrations: ampicillin, 100 μg/ml for E. coli; carbenicillin, 200 μg/ml for P. aeruginosa; streptomycin, 30 μg/ml for E. coli and 100 μg/ml for P. aeruginosa; tetracycline, 10 μg/ml for E. coli and 100 μg/ml for P. aeruginosa; and chloramphenicol, 30 μg/ml for E. coli and 200 μg/ml for P. aeruginosa. L agar was supplemented with 5% (wt/vol) sucrose as required.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | Prototroph | |
| OCR1 | MexA-MexB-OprM-overproducing, nalB-type multidrug-resistant derivative of PAO1 | 17 |
| KG2212 | mexR::res-Ω of PAO1 | This study |
| KG2239 | ΔmexR-mexA-mexB-oprM of PAO1 | This study |
| KG3052 | Intermediately MexC-MexD-OprJ-overproducing, type-A nfxB multidrug-resistant derivative of PAO1 (formerly COR2) | 19 |
| KG2217 | mexR::res-Ω of KG3052 | This study |
| KG2240 | ΔmexR-mexA-mexB-oprM of KG3052 | This study |
| KG3056 | Highly MexC-MexD-OprJ-overproducing, type-B nfxB multidrug-resistant derivative of PAO1 (formerly COR6) | 19 |
| KG2236 | mexR::res-Ω of KG3056 | This study |
| KG2259 | ΔmexR-mexA-mexB-oprM of KG3056 | This study |
| Plasmids | ||
| pMT5059 | pBend2 derivative carrying the multiple-cloning site and NotI site; Apr | 33 |
| pKMM089 | pMT5059 derivative carrying a 2.6-kb XhoI-HindIII fragment from pPV20; Apr | This study |
| pKMM091 | pKMM089 derivative carrying NotI-flanked res-tnpR block from pMT5085; Apr Tcr | This study |
| pKMM102 | pMT5059 derivative carrying mexR, mexA, mexB, and a part of oprM on a 24-kb PvuII-XhoI fragment; Apr | This study |
| pKMM127 | pKMM102 derivative carrying SacI-flanked res-Ω cassette from pMT5096 and NotI-flanked Mob cassette from pMT5071; Apr Cmr | This study |
| pKMM151 | pMT5059 ori-carrying plasmid rescued from KG2213 chromosome; Apr | This study |
| pKMM157 | pKMM089 derivative carrying SacI-flanked res-Ω cassette from pMT5095 and NotI-flanked res-tnpR block from pMT5085; Apr Smr Tcr | This study |
| pMP190 | Broad-host-range, low-copy-number lacZ fusion vector; Cmr Smr | 31 |
| pKMM301 | pMP190 derivative carrying the mexR-mexA intergenic region on a 276-bp PCR product amplified with mexR7-mexA3, with the mexA promoter oriented towards the promoterless lacZ gene; Cmr Smr | This study |
Abbreviations: Apr, ampicillin resistant; Cbr, carbenicillin resistant; Cmr, chloramphenicol resistant; Kmr, kanamycin resistant; Smr, streptomycin resistant; and Tcr, tetracycline resistant.
Recombinant DNA techniques.
Transformation of E. coli, Southern hybridization, isolation of chromosomal DNA and plasmids, and restriction endonuclease digestions were carried out according to standard protocols (28). For PCR amplification of chromosomal DNA sequences (6), a bacterial colony was directly suspended in reaction mixture (6) lacking the primers and Taq polymerase. The mixture was boiled for 10 min and used as the template for amplification. The primer pairs used were as follows: mexR1 (5′-ATGAACTACCCCGTGAATCCC-3′) and mexR2 (5′-TTAAATATCCTCAAGCGGTTGC-3′) for mexR, 1611 and 1612 for mexA, 1613 and 1614 for mexB, and 1615 and 1680 for oprM, all of which have been described previously (6). The sizes of the amplified DNA fragments obtained by using these primer pairs were about 0.7, 1.5, 3.2, and 1.4 kb, respectively.
Cloning of chromosomal regions flanking the mexR-mexA-mexB-oprM operon.
To clone the chromosomal region located downstream of mexR, the chromosomal DNA of PAO1 was digested completely with XhoI and then partially with PvuII. The restriction fragments with sizes around 20 kb were collected and ligated with pMT5059 (33) that had been treated with XhoI and PvuII. The ligation mixture was used for transformation of E. coli DH5α (1) to select Apr clones on L agar plates. The mexR gene was successfully amplified by PCR (see above) from one of the 48 Apr clones, and the plasmid obtained from such a clone was termed pKMM102. This plasmid carried an ∼24-kb chromosomal fragment extending from a PvuII site downstream of mexR to the XhoI site in oprM. The chromosomal region located downstream of oprM was cloned as follows. Since the KG2213 (see below) chromosome carried a part of the pMT5059-derived oriV and bla gene, the chromosomal DNA of KG2213 was digested with SacI, self-ligated, and used to transform DH5α to obtain Apr clones. One of the plasmids thus recovered, termed pKMM151, contained an ∼10-kb chromosomal fragment extending from the XhoI site in oprM to a SacI site located downstream of oprM.
Construction of recombinant plasmids.
The SacI-digested res-Ω cassette from pMT5096 (34) was inserted into the SacI site in the mexR gene of pKMM102, followed by insertion of the pMT5071-derived, NotI-flanked Mob cassette (34) into the NotI site to generate pKMM127 (see Fig. 1). The 8.4-kb SacI-HindIII fragment encompassing a mexA-mexB-oprM region on pPV20 (25) was inserted into pAK1900 (25), and the resulting plasmid contains a 2.6-kb XhoI-HindIII fragment that covers the 3′ part of oprM and its downstream region. A NotI site in this fragment was disrupted by blunt ending with T4 DNA polymerase, and such a modified fragment was inserted into pMT5059 to construct pKMM089. pKMM096 was constructed by insertion of the pMT5095-derived, XhoI-flanked res-Ω cassette into the XhoI site of pKMM089. The NotI-flanked res-tnpR block from pMT5085 (34) was inserted into the pMT5059-derived NotI sites of pKMM089 and pKMM096 to construct pKMM091 and pKMM157, respectively.
FIG. 1.
Schematic models of the TnpR-mediated site-specific deletion based on an improvement of the deletion system developed by Tsuda (34). Specifically, the plasmid carrying an additional res-Ω cassette and a res-tnpR block (pKMM157) was integrated into the oprM gene of the chromosome containing another res-Ω cassette in the mexR gene. Deletion between the two copies of the res site led to removal of the intervening chromosomal region and the integrated plasmid sequence, leaving one copy of the res site. Abbreviations for restriction endonuclease sites: P, PvuII; S, SacI; X, XhoI; H, HindIII; and N, NotI.
The chromosomal region between the start codon of mexR and mexA was amplified by using primers mexR7 (5′-ATTGTTTGGCCGAGTAAACC-3′) and mexA3 (5′-TAGCGTTGTCCTCATGAGCG-3′) and inserted into the blunt-ended SalI site of pMP190 (31) to yield pKMM301, such that the promoterless lacZ gene was transcribed from the mexA promoter.
Mobilization of recombinant plasmids to P. aeruginosa chromosome.
An appropriate plasmid residing in an E. coli strain, S17-1 (30), was conjugationally mobilized to P. aeruginosa cells. After mating on L agar at 37°C for 4 h, the cell mixture was suspended in 0.4 ml of physiological saline. Aliquots (0.1 ml) of the 10- or 100-fold-diluted suspensions were plated on BM2 minimal agar plates (3) supplemented with appropriate antibiotics and incubated at 30°C for 2 days. The transconjugants thus obtained were purified once on the same selective plates, and examined for resistance to streptomycin, tetracycline, chloramphenicol, and/or sucrose on L agar plates. Clones indicating appropriate resistance to these selective markers were used in subsequent experiments.
Deletion of chromosomal mexR-mexA-mexB-oprM.
Plasmid pKMM127 (Fig. 1 and Table 1) carries a res-Ω cassette in the mexR gene and a Mob cassette, and this plasmid was mobilized from S17-1 to PAO1 (a wild-type strain), KG3052 (a type-A nfxB strain), and KG3056 (a type-B nfxB strain) to select Smr transconjugants. Among the transconjugants, those showing resistance to sucrose (e.g., KG2212, KG2217, and KG2236 from PAO1, KG3052, and KG3056, respectively) were presumed to have been formed by allelic exchange of the wild-type mexR gene with the mutant allele. This was confirmed by the fact that the mexR region could not be amplified by PCR from KG2212, KG2217, or KG2236 chromosome, consistent with an increase in the size of the mexR region in these strains as a result of insertion of the res-Ω cassette (data not shown). The argument for the expected allelic exchange was further supported by Southern hybridization experiments (data not shown).
To obtain the chromosomal deletion by using the original TnpR-mediated deletion system (34), pKMM091 (Table 1) carrying a pMT5085-derived res-tnpR block was transferred from E. coli S17-1 to KG2212 (PAO1 mexR::res-Ω) to select Tcr transconjugants on BM2 agar plates. These plasmid integrants were unstable, and their cultivation on L agar plates generated segregants which commonly lost the Smr and Tcr markers but not the sacB gene (e.g., KG2213).
To eliminate, in the process of deletion mutagenesis, the plasmid-derived sequence including the Cbr marker (bla), we developed a new improved method that involved mobilization of pKMM157 (Fig. 1 and Table 1) from S17-1 to the three mexR::res-Ω mutants of P. aeruginosa (KG2212, KG2217, and KG2236) and selection of Tcr transconjugants on BM2 agar plates. Phenotypic characterization and Southern analysis indicated that such transconjugants were formed by integration of pKMM157 into the chromosomal oprM gene (Fig. 1) (data not shown). These transconjugants gave rise to Tcs segregants at high frequencies after single-colony isolation on L agar plates. Each of three segregants obtained from the three crosses were resistant to sucrose and sensitive to streptomycin and carbenicillin. Such segregants derived from KG2212, KG2217, and KG2236 were designated KG2239, KG2240, and KG2259, respectively (Fig. 1).
Design of oligopeptides and development of polyclonal antisera specific to MexA and MexB.
To avoid potential problems with the purification of MexA and MexB, oligopeptides based on the deduced amino acid sequences of these proteins (24, 25) were synthesized and used to immunize rabbits. Hydropathy analysis was performed with the Analysis Plot program (the Kyte and Doolittle algorithm [11]) included in the GeneWorks software package (Intelligenetics), and the amino acid sequences predicted to belong to the hydrophilic region of each protein were used for design of the oligopeptides. Multiple antigen peptides (32) composed of these oligopeptides linked to poly-lysin carrier were manually synthesized on a Multiple Peptide Synthesizer (Shimadzu Model PSSM-8) by solid-phase peptide synthesis on TAK08-WTGS resin (Shimadzu). New Zealand White rabbits (female; 10 weeks old) were immunized with 20 μg of the prepared antigen at weeks 1, 3, and 6. The first injection was in Freund’s complete adjuvant, the second and third ones were in Freund’s incomplete adjuvant, and in all cases the antigen was injected subcutaneously. Titers were determined by an enzyme-linked immunosorbent assay using total membranes of the P. aeruginosa OCR1 cells, prepared by sonication and subsequent centrifugation (see below), as the antigen.
Development of murine monoclonal antibody specific to OprM.
BALB/c mice were immunized with 20 μg of purified OprM (4) on days 1, 7, 14, and 17 and an anti-OprM antibody-producing clone was prepared as described previously (5). A monoclonal antibody secreted by this clone was termed TM001.
Isolation of total membranes, SDS-PAGE, and immunoblot assay.
Cells grown in L broth were harvested by centrifugation at 5,000 × g for 10 min at 4°C. The cells were resuspended in 10 mM Tris-HCl, pH 8.0, and were broken by sonication. After the removal of unbroken cells by centrifugation, total membranes (cell envelopes) were pelleted from the resulting supernatant by centrifugation at 20,000 × g for 30 min. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described previously (4), with 10% (wt/vol) acrylamide in the running gel. Proteins fractionated by SDS-PAGE were electrophoretically transferred to a nitrocellulose membrane (0.45-μm pore size; Bio-Rad) as described previously (5). Binding of the primary antibodies was detected as described previously (4), with alkaline phosphatase-conjugated goat antibodies to rabbit immunoglobulin G (Cappel) or alkaline phosphatase-conjugated goat antibodies to mouse immunoglobulin G (Cappel) as the secondary antibodies and an Ap Conjugate Substrate Kit (Bio-Rad) for color development.
β-Galactosidase assays.
Bacteria harboring the plasmid pKMM301 were cultured overnight at 37°C in A medium (28) supplemented with 0.4% (wt/vol) glucose, thiamine (1 μg/ml), 1 mM MgSO4, and chloramphenicol (12.5 μg/ml for KG2239 or 400 μg/ml for KG2259) and subsequently diluted 50-fold into fresh medium consisting of the same solutes. Following growth to mid-log phase (A600, 0.3 to 0.6), cultures were assayed for β-galactosidase activity as described previously (20).
RESULTS AND DISCUSSION
Construction of ΔmexR-mexA-mexB-oprM strains.
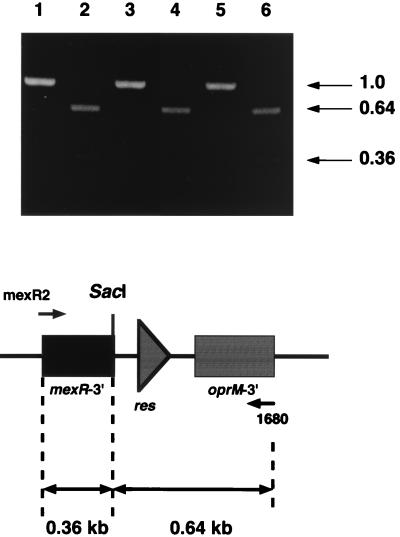
To characterize the precise nature of the contribution of MexC-MexD-OprJ to antibiotic resistance, we attempted to delete the mexA-mexB-oprM operon and the mexR gene from the chromosomes of two types of nfxB mutants that have different expression levels of mexC-mexD-oprJ (19). An improved method developed in this study facilitated our subsequent isolation of the deletion mutants (KG2239, KG2240, and KG2259 from PAO1, KG3052 [type-A nfxB], and KG3056 [type-B nfxB], respectively) that carried only a single copy of the res site (Fig. 1). The PCR amplification of the chromosomal DNAs of the three segregants by using mexR2 (antisense primer for mexR) and 1680 (antisense primer for oprM) generated 1.0-kb fragments, which generated 0.36- and 0.64-kb fragments by SacI digestion (Fig. 2). In contrast, no PCR products were detected when the primer pairs for mexR, mexA, mexB, and oprM were used (data not shown). Southern hybridization experiments of SacI-digested chromosomal DNAs of the three segregants further demonstrated the presence of the res site and the absence of the tnpR-sacB-oriV-bla region (data not shown). All of these results confirm that the segregants did in fact contain the chromosomal structures as depicted in Fig. 1. This improved system will be of use in many bacterial species to construct chromosomal deletion mutants that contain only the res site, and such mutant strains should prove suitable for biotechnological use in environmental bacteria.
FIG. 2.
Amplification of chromosomal fragments containing deletion endpoints. Approximately 1-kb fragments containing the remaining res site were amplified by PCR using primer sets that annealed at the 3′ ends of mexR and oprM (primers mexR2 and 1680, respectively). These fragments were digested with SacI and electrophoresed on a 1% (wt/vol) agarose gel. Lanes: 1 and 2, KG2239; 3 and 4, KG2240; 5 and 6, KG2259. Lanes 1, 3, and 5 show the ca. 1-kb amplified fragments, and lanes 2, 4, and 6 show the SacI-digested PCR products. Boxes labeled mexR-3′ and oprM-3′ refer to the 3′ ends of each gene. The sizes of the fragments are indicated in kilobases at the right of the figure.
Development of antibodies specific to MexA, MexB, and OprM.
To detect the production of MexA-MexB-OprM in the mutants isolated in this study, we prepared a murine monoclonal antibody specific to OprM and rabbit antisera specific to MexA and MexB. For this purpose, purified OprM (4) and synthetic oligopeptides were used as antigens for immunization. An immunoblot assay using an OprM-specific monoclonal antibody, TM001, as the primary antibody showed slight production of 49-kDa OprM in PAO1 and its overproduction in the nalB-type mutant OCR1 derived from PAO1 (data not shown). Similar results were obtained with antisera from rabbits immunized with oligopeptide 92106 (YQIDPATYEADYQSA) for MexA and oligopeptide 423437 (EGLSPREAARKSMGQ) for MexB (data not shown).
Susceptibility testing and substrate specificity of the two efflux systems.
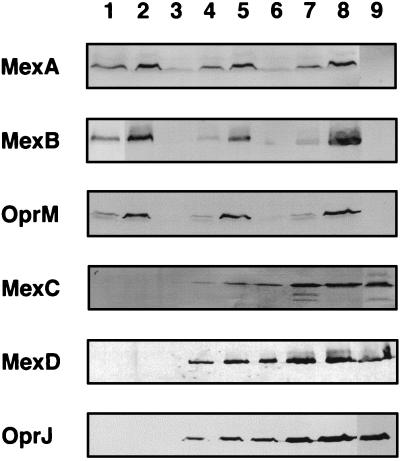
Table 2 shows the susceptibilities of the constructed mutants and their parent strains. Expression levels of various components of multidrug efflux systems were confirmed by immunoblot assays using rabbit polyclonal antisera for MexA (92106 [see above]) for MexB (423437 [see above]) for MexC (MEXC7 [7]) and for MexD (MEXD7 [7]), and murine monoclonal antibodies for OprM (TM001 [see above]) and for OprJ (HJ001 [8]). The nfxB mutants KG3052 (type A) and KG3056 (type B) showed resistance to fluoroquinolones, the fourth-generation cephems, tetracycline, and chloramphenicol and hypersusceptibility to ordinary cephems, carbenicillin, and aztreonam, concomitant with production of MexC-MexD-OprJ (Fig. 3, lanes 4 and 7). Elimination of mexR by insertion of res-Ω resulted in overproduction of MexA-MexB-OprM in the nfxB strains and PAO1 (Fig. 3, lanes 2, 5, and 8), as reported by Poole et al. (27), and increased resistance to all antimicrobials tested (see KG2212 [Table 2]). In contrast, the susceptibility to all antimicrobials tested increased in the mexR-mexA-mexB-oprM-deficient PAO1 strain, KG2239.
TABLE 2.
Susceptibilities of constructed mutants to various antibiotics
| Strain | Relevant genotypea | MIC (μg/ml) ofb:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPFX | CPFX | NFLX | LVFX | CZOP | CPR | CAZ | CPZ | CBPC | AZT | TC | CP | ||
| PAO1 | Prototroph | 0.39 | 0.10 | 0.39 | 0.39 | 0.39 | 1.56 | 1.56 | 6.25 | 12.5 | 1.56 | 6.25 | 12.5 |
| OCR1 | nalB | 1.56 | 0.78 | 3.13 | 3.13 | 1.56 | 6.25 | 6.25 | 25 | 100 | 12.5 | 25 | 100 |
| KG2212 | PAO1 mexR::res-Ω | 1.56 | 0.39 | 1.56 | 1.56 | 0.78 | 3.13 | 3.13 | 12.5 | 50 | 6.25 | 12.5 | 50 |
| KG2239 | PAO1ΔRABM | 0.05 | 0.012 | 0.05 | 0.05 | 0.10 | 0.39 | 0.20 | 0.39 | 0.39 | 0.10 | 0.20 | 0.78 |
| KG3052 | Type-A nfxB | 6.25 | 1.56 | 6.25 | 3.13 | 3.13 | 6.25 | 0.78 | 3.13 | 6.25 | 0.78 | 12.5 | 25 |
| KG2217 | KG3052 mexR::res-Ω | 6.25 | 1.56 | 6.25 | 3.13 | 3.13 | 6.25 | 3.13 | 12.5 | 50 | 6.25 | 25 | 50 |
| KG2240 | KG3052ΔRABM | 6.25 | 1.56 | 6.25 | 3.13 | 3.13 | 6.25 | 0.39 | 0.78 | 0.39 | 0.10 | 3.13 | 12.5 |
| KG3056 | Type-B nfxB | 12.5 | 3.13 | 12.5 | 6.25 | 6.25 | 12.5 | 0.78 | 3.13 | 1.56 | 0.39 | 25 | 50 |
| KG2236 | KG3056 mexR::res-Ω | 12.5 | 3.13 | 12.5 | 6.25 | 6.25 | 12.5 | 3.13 | 25 | 50 | 6.25 | 25 | 50 |
| KG2259 | KG3056ΔRABM | 12.5 | 3.13 | 12.5 | 6.25 | 6.25 | 12.5 | 0.78 | 1.56 | 0.39 | 0.10 | 25 | 50 |
ΔRABM, deletion of mexRAB-oprM operon.
Abbreviations: SPFX, sparfloxacin; CPFX, ciprofloxacin; NFLX, norfloxacin; LVFX, levofloxacin; CZOP, cefozopran; CPR, cefpirome; CAZ, ceftazidime; CPZ, cefoperazone; CBPC, carbenicillin; AZT, aztreonam; TC, tetracycline; and CP, chloramphenicol.
FIG. 3.
Detection of MexA-MexB-OprM and MexC-MexD-OprJ component proteins with antisera directed against synthetic oligopeptides containing part of the amino acid sequences of MexA, MexB, MexC, or MexD or monoclonal antibodies specific to OprM or OprJ. Each lane contains 30 μg of cell envelope protein, as determined by the method of Lowry et al. (15). Lanes: 1, PAO1; 2, KG2212 (PAO1 mexR::res-Ω); 3, KG2239 (PAO1 ΔmexR-mexA-mexB-oprM); 4, KG3052 (PAO1 type-A nfxB); 5, KG2217 (KG3052 mexR::res-Ω); 6, KG2240 (KG3052 ΔmexR-mexA-mexB-oprM); 7, KG3056 (PAO1 type-B nfxB); 8, KG2236 (KG3056 mexR::res-Ω); and 9, KG2259 (KG3056 ΔmexR-mexA-mexB-oprM).
Susceptibility tests also produced information on the substrate specificity of the MexA-MexB-OprM and MexC-MexD-OprJ efflux systems. Comparison of the levels of resistance to tetracycline and chloramphenicol between KG2239 and KG2240 (Table 2) revealed that the MexC-MexD-OprJ system could contribute to the extrusion of these agents. However, the resistance levels to tetracycline and chloramphenicol, but not those to the fluoroquinolones or the fourth-generation cephems, differed among the three type-A nfxB strains, KG3052, KG2217, and KG2240 (Table 2), that were distinguished from one another by the amounts of each component of the MexA-MexB-OprM system (Fig. 3). These results suggested that the MexC-MexD-OprJ system had, in comparison with the MexA-MexB-OprM system, a higher specificity to cause the extrusion of the fluoroquinolones and the fourth-generation cephems and a lower specificity to cause the extrusion of tetracycline and chloramphenicol. The three strains KG2239, KG2240, and KG2259 commonly lacked the mexA-mexB-oprM operon, and the last two of these strains, which express the MexC, MexD, and OprJ components showed higher resistance to ordinary cephems but not to carbenicillin and aztreonam (Table 2). This suggests the involvement of the MexC-MexD-OprJ system in the extrusion of ordinary cephems.
Expression of MexA-MexB-OprM in nfxB strains.
Immunoblot assays unexpectedly demonstrated that the amount of MexA-MexB-OprM produced in both nfxB mutants KG3052 and KG3056 was less than that produced in PAO1 (Fig. 3, lanes 1, 4, and 7). To confirm this, pKMM301 carrying the promoterless lacZ gene downstream of the mexA promoter was constructed and introduced into KG2239 (PAO1 ΔmexR-mexA-mexB-oprM) and KG2259 (KG3056 ΔmexR-mexA-mexB-oprM). The latter strain showed lower β-galactosidase activity (474 ± 59 Miller units) than the former strain (1,013 ± 59 Miller units). This indicated that the decreased production of MexA-MexB-OprM in the nfxB mutant KG3056 is at least partly due to the reduced transcription of the mexA-mexB-oprM operon. Decreased production of MexA-MexB-OprM explains the hypersusceptibility of the nfxB mutants to β-lactams, because in the absence of MexA-MexB-OprM, nfxB mutation did not confer hypersusceptibility to the β-lactams tested (KG2239, KG2240, and KG2259 [Table 2]).
In the present study, the characterization of the MexC-MexD-OprJ efflux system in nfxB strains that lacked the mexA-mexB-oprM region revealed that (i) the MexC-MexD-OprJ efflux system works to cause the extrusion of not only fluoroquinolones, fourth-generation cephems, tetracycline, and chloramphenicol but also ordinary cephems (KG2240 and KG2259 [Table 2]); (ii) this system apparently does not function in the efflux of carbenicillin and aztreonam (KG2240 and KG2259 [Table 2]); and (iii) hypersusceptibility to β-lactams, including ordinary cephems, in nfxB mutants is due to decreased expression of MexA-MexB-OprM rather than being a direct function of MexC-MexD-OprJ. Previous studies (8, 18, 19, 26) suggested that the ordinary cephems are saved from efflux by the MexC-MexD-OprJ efflux system, because those studies used nfxB mutants that still expressed MexA-MexB-OprM. By construction of MexA-MexB-OprM-lacking nfxB mutants, we have demonstrated that the MexC-MexD-OprJ system also functions for extrusion of the ordinary cephems. In addition, nfxC mutants exhibit hypersusceptibility to β-lactams, including ordinary cephems (2, 10). The nfxC mutation causes expression of a third efflux system, MexE-MexF-OprN, in MexA-MexB-OprM-producing (i.e., wild-type) strains. Construction of MexA-MexB-OprM-deficient mutants may be required to characterize the precise nature of the contribution of MexE-MexF-OprN to antibiotic resistance.
ACKNOWLEDGMENTS
We thank J. Yamagishi and K. Fukui for synthesizing the oligopeptides used in the development of antisera and T. Yamasaki for development of the monoclonal antibody.
This research was supported by Grants-in-Aid for Scientific Research to N.G. and M.T. from the Ministry of Education, Science, Sports and Culture, Japan, and by a grant to N.G. (Study of Drug-Resistant Bacteria [1996]) funded by the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley and Sons; 1992. [Google Scholar]
- 2.Fukuda H, Hosaka M, Hirai K, Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990;34:1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilleland H E, Jr, Stinnett J D, Eagon R G. Ultrastructural and chemical alteration of cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974;117:302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotoh N, Itoh N, Tsujimoto H, Yamagishi J, Oyamada Y, Nishino T. Isolation of OprM-deficient mutants of Pseudomonas aeruginosa by transposon insertion mutagenesis: evidence of involvement in multiple antibiotic resistance. FEMS Microbiol Lett. 1994;122:267–274. doi: 10.1111/j.1574-6968.1994.tb07179.x. [DOI] [PubMed] [Google Scholar]
- 5.Gotoh N, Nagino K, Wada K, Tsujimoto H, Nishino T. Burkholderia (formerly Pseudomonas) cepacia porin is an oligomer composed of two component protein. Microbiology. 1994;140:3285–3291. doi: 10.1099/13500872-140-12-3285. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh N, Tsujimoto H, Poole K, Yamagishi J, Nishino T. The outer membrane protein OprM of Pseudomonas aeruginosa is encoded by oprK of the mexA-mexB-oprK multidrug resistance operon. Antimicrob Agents Chemother. 1995;39:2567–2569. doi: 10.1128/aac.39.11.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotoh, N., H. Tsujimoto, A. Nomura, K. Okamoto, M. Tsuda, and T. Nishino. Functional replacement of OprJ by OprM in the MexC-MexD-OprJ multidrug efflux system of Pseudomonas aeruginosa. FEMS Microbiol. Lett., in press. [DOI] [PubMed]
- 8.Hosaka M, Gotoh N, Nishino T. Purification of a 54-kilodalton protein (OprJ) produced in NfxB mutants of Pseudomonas aeruginosa and production of a monoclonal antibody specific to OprJ. Antimicrob Agents Chemother. 1995;39:1731–1735. doi: 10.1128/aac.39.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köhler T, Kok M, Michea-Hamzehpour M, Plesiat P, Gotoh N, Nishino T, Curty L K, Pechère J-C. Multidrug efflux in intrinsic resistance to trimethoprim and sulfamethoxazole in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:2288–2290. doi: 10.1128/aac.40.10.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Köhler T, Michéa-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechère J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 11.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 12.Li X-Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X-Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to β-lactam resistance. Antimicrob Agents Chemother. 1994;38:1742–1752. doi: 10.1128/aac.38.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 17.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda N, Gotoh N, Ohya S, Nishino T. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:909–913. doi: 10.1128/aac.40.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J H. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 72–74. [Google Scholar]
- 21.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okazaki T, Hirai K. Cloning and nucleotide sequence of the Pseudomonas aeruginosa nfxB gene, conferring resistance to new quinolones. FEMS Microbiol Lett. 1992;97:197–202. doi: 10.1016/0378-1097(92)90386-3. [DOI] [PubMed] [Google Scholar]
- 23.Pan W, Spratt B G. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol. 1994;11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 24.Poole K, Heinrichs D, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 25.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X-Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 27.Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs D, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40:2021–2028. doi: 10.1128/aac.40.9.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory Manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Shiba T, Ishiguro K, Takemoto N, Koibuchi H, Sugimoto K. Purification and characterization of the Pseudomonas aeruginosa NfxB protein, the negative regulator of the nfxB gene. J Bacteriol. 1995;177:5872–5877. doi: 10.1128/jb.177.20.5872-5877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–790. [Google Scholar]
- 31.Spaink H P, Okker R J H, Wijffelman C A, Pees E, Lugtenberg B J J. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- 32.Tam J P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuda M, Miyazaki H, Nakazawa T. Genetic and physical mapping of genes involved in pyoverdine production in Pseudomonas aeruginosa PAO. J Bacteriol. 1995;177:423–431. doi: 10.1128/jb.177.2.423-431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuda M. Use of a transposon-encoded site-specific resolution system for construction of large and defined deletion mutations in bacterial chromosome. Gene. 1998;207:33–41. doi: 10.1016/s0378-1119(97)00601-x. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura F, Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982;152:636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]