Abstract
It is unclear whether racial/ethnic disparities in triple-negative breast cancer (TNBC) mortality remain after accounting for clinical characteristics, treatment, and access-to-care-related factors. In this study, women with a primary diagnosis of TNBC during 2010–2014 were identified from the National Cancer Database. Hazard ratios (HR) and 95% confidence intervals (CI) for 3- and 5-year all-cause mortality associated with race/ethnicity were estimated using Cox proportional hazards models with stepwise adjustments for age, clinical characteristics, treatment, and access-to-care-related factors. Of 78,708 patients, non-Hispanic (NH) black women had the lowest 3-year overall survival rates (79.4%), followed by NH-whites (83.1%), Hispanics (86.0%), and Asians (87.1%). After adjustment for clinical characteristics, NH-blacks had a 12% higher risk of dying 3 years post-diagnosis (HR=1.12, 95%CI: 1.07 to 1.17), while Hispanics and Asians had a 24% (HR=0.76, 95%CI: 0.70 to 0.83) and 17% (HR=0.83, 95%CI: 0.73 to 0.94) lower risk than their NH-white counterparts. The black-white disparity became non-significant after combined adjustment for treatment and access-to-care-related factors (HR=1.04, 95%CI: 0.99 to 1.09), while the white-Hispanic and white-Asian differences remained. Stratified analyses revealed that among women aged less than or equal to 50 with stage III cancer, the elevated risk among NH-blacks persisted (HR=1.20, 95%CI: 1.04 to 1.39) after full adjustments. Similar results were seen for 5-year mortality. Overall, clinical characteristics, treatment, and access-to-care-related factors accounted for most of the white-black differences in all-cause mortality of TNBC but explained little about Hispanic- and Asian-white differences.
Keywords: Race, Ethnic groups, Triple Negative Breast Neoplasms, Healthcare Disparities, Mortality
Introduction
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer (1), characterized by negative expressions of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), and accounting for 12%−17% of all breast cancers globally (2). Due to the lack of effective targets for treatment, prognosis for TNBC is poor, with a 5-year breast cancer-specific survival rate of 73% compared with 91% for luminal breast cancers (3,4).
Racial/ethnic disparities in TNBC incidence have been well-documented, especially between African and white Americans (5). The age-adjusted incidence of TNBC among non-Hispanic (NH) black women was 27.2/100,000 in the United States (U.S.) in 2011, almost doubles that for NH-white women (14.4/100,000) (6). As a result, despite only 15% of overall breast cancers classified as TNBC in the U.S. (5), TNBC accounts for 24% of all breast cancers among African Americans (7). This proportion increases to 39% among premenopausal African Americans (8). Studies also show a higher proportion of TNBC among Hispanic women compared with their NH-white counterparts (9). Because TNBC has an unfavorable prognosis, it has been suggested that the disproportionately high burden of TNBC among racial/ethnic minorities may contribute to the observed overall mortality gap for breast cancer (10), which has increased substantially over the past 30 years (11).
However, data on racial/ethnic disparities for TNBC outcomes are scant, and results are inconclusive. Studies based on the California Cancer Registry showed that African American women with TNBC had a 21% higher mortality than white patients after accounting for age, tumor characteristics, treatment, socioeconomic status and insurance (12). However, this disparity was only observed in patients with stage III disease when data were evaluated stage-by-stage. On the other hand, two other studies reported that African American women with TNBC or basal-like breast cancer had similar recurrence risk or mortality as white women, although the sample sizes of these studies were much smaller (13,14). In addition, to date, few studies have evaluated the mortality disparities between NH-white women and Hispanic and Asian Americans, two fastest-growing racial/ethnic groups in the U.S.
To fill this knowledge gap, we utilized data from the National Cancer Database (NCDB), to comprehensively investigate the impact of race/ethnicity on the mortality disparity in women with TNBC and evaluate the influences of clinical characteristics, treatment regimens and factors related to access-to-care.
Materials and Methods
Women with a primary diagnosis of TNBC between 2010 and 2014 were identified from the NCDB. The NCDB is a clinical oncology database sourced from hospital registry data, which collects data from more than 1,500 facilities and represents over 70% of newly-diagnosed cancer cases nationwide and more than 34 million historical records (15). Patients with concomitant diagnosis or history of other malignancies, ambiguous records of TNM stages or incomplete records of the follow-up period were excluded from evaluation. This study was approved as Exempt Human Research by the Institutional Review Board of Vanderbilt University Medical Center.
Following the 2010 Census of Population and Housing (16), the NCDB defines race/ethnicity categories by a combination of race and Hispanic/Spanish origin. Patients recorded as white or black and as non-Hispanic/non-Spanish (NH) were categorized as NH-white and NH-black. Patients who reported Hispanic or Spanish origin, or had Spanish surnames, were categorized as Hispanic, and patients of Asian origin were categorized as Asian. Patients recorded as American Indian, Aleutian, Eskimo, Pacific Islander, or other unspecified origin, or those with missing information on race/ethnicity were excluded from this study due to a small sample size (n=3,945). We also excluded patients who died within 90-days after primary surgery (n=543, including 340 NH-white, 138 NH-black, 24 Hispanic and 12 Asian patients).
In the NCDB, ER and PR status were categorized as positive when 1% or greater tumor cells stained positive using immunohistochemistry, while HER2 was considered positive when immunohistochemistry, fluorescence in situ hybridization (FISH) or chromogenic in situ hybridization (CISH) tests were positive. Accordingly, patients with negative tests for ER, PR and HER2, as recorded in the NCDB, were defined as having TNBC and included in the final analyses.
Information on age at diagnosis, clinical characteristics, treatment and factors related to access-to-care was collected from the NCDB. Clinical characteristics, obtained from the NCDB, included TNM stage, histology type, Nottingham combined histologic grade, lymphovascular invasion (LVI) status and Charlson/Deyo score. Charlson/Deyo score was employed as a measure of comorbidity, with a score of zero indicating no comorbidity at cancer diagnosis. Available information on receipt of treatment included primary surgery, chemotherapy, radiotherapy, year of diagnosis, and interval from diagnosis to the first treatment. Detailed chemotherapy regimens were not recorded in the NCDB, and thus, were coded as yes or no in this study. Factors related to access-to-care in this analysis were defined as neighborhood-level educational attainment, neighborhood-level annual household income, primary insurance at initial diagnosis (not insured, private insurance, Medicaid, Medicare, or other government insurance), urban/rural residence, facility type (community cancer program, comprehensive community cancer program, academic/research program, integrated network cancer program, or other), region of case reporting facility (Northeast, Midwest, South and West), and distance to care facility. In the NCDB, both educational attainment and annual household income were recorded at the neighborhood level by matching patients’ resident zip codes against American Community Survey data, while distance to care facility was estimated as miles between the patient’s residential zip code and the case reporting facility’s street address. Facility types were classified following the category classification by the Commission on Cancer (CoC) Accreditation program, which is based on the type of facility, program structure, services provided, and the number of cases accessioned each year (17).
Statistical Analysis
The primary outcome was 3- and 5-year all-cause mortality, defined as months from cancer diagnosis to death of any cause or to the last contact, and survival status at 3-, 5-years post-diagnosis. Patients lost to follow-up before reaching the time markers were censored at last contact. In the datasets to which we had access, follow-up data was available up to 2017. Cause of death and events of cancer recurrence were not recorded in the NCDB, and thus, breast cancer-specific mortality could not be evaluated in this study.
Descriptive characteristics of different racial/ethnic groups were compared using chi-square test for categorical variables and ANOVA for continuous variables. Differences in 3-year and 5-year survival rates across racial/ethnic groups were estimated and compared using the Kaplan-Meier method and log-rank tests. Stratified analysis by stage was also carried out. Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the associations of racial/ethnic groups with 3-year and 5-year all-cause mortality. The HRs and 95% CIs were derived with 1) adjustment for age at diagnosis alone, 2) additional adjustment for clinical characteristics, 3) additional adjustment for treatment, and 4) further adjustment for factors related to access-to-care, as defined above. Stage-specific Cox models were carried out to examine whether association patterns differed across stages. The abovementioned Cox regression analyses were also conducted with stratification by age at diagnosis (≤50 years and >50 years). Analyses stratified by types and regions of facility were also conducted in order to account for potential different pattens of access-to-care for racial/ethnic groups across facilities. Proportional hazard assumptions were examined by plotting scaled Schoenfeld residuals, and no violations of the assumptions were observed.
All statistical tests were based on 2-sided probability with significance levels set at P <0.05, with no adjustment for multiple comparisons. All statistical analyses were performed using R 3.5.1 (R Foundation, Vienna, Austria).
Results
A total of 78,708 patients were included in the final analyses (Supplementary Figure 1). NH-white patients accounted for 68.5% of the total study population, followed by NH-black (22.0%), Hispanic (6.5%) and Asian (3.0%) patients (Table 1). Overall, invasive ductal carcinoma accounted for a large majority across all racial/ethnic groups. Compared with NH-white patients, NH-black, Hispanic and Asian patients tended to have large tumors (>20mm), lymph node metastasis, and TNM stage II/III diseases. The proportions of distant metastasis at diagnosis and comorbidity were higher in NH-black patients than in NH-white patients. These proportions were similar between NH-white and Hispanic patients, but lower in Asian patients. Though almost 80% of all study patients received chemotherapy, chemotherapy was more frequently reported in NH-black, Hispanic and Asian patients than their NH-white counterparts. However, intervals between diagnosis and treatment initiation were longer in these minority patients than their NH-white counterparts. In comparison to NH-white women, patients from racial/ethnic minority groups were more likely to be young, uninsured, reside in metropolitan areas and to be treated in academic/research facilities; more NH-black and Hispanic patients resided in neighborhoods with low annual household incomes and education attainment, while more Asian patients resided in high-income neighborhoods (Table 1, Supplementary Table 1).
Table 1.
Clinical and Treatment-related Characteristics of Female Patients with Triple Negative Breast Cancer by Race/ethnicity
| Characteristics | NH White | NH Black | Hispanic | Asian | P |
|---|---|---|---|---|---|
| N=53,908 (%) | N=17,350 (%) | N=5,116 (%) | N=2,334 (%) | ||
| Age (years) | <0.001 | ||||
| 18–50 | 27.9 | 34.4 | 47.5 | 40.5 | |
| >50 | 72.1 | 65.6 | 52.5 | 59.4 | |
| Histology Grade | <0.001 | ||||
| I | 5.0 | 4.3 | 4.9 | 3.8 | |
| II | 16.3 | 12.1 | 11.4 | 16.0 | |
| III | 65.5 | 68.4 | 67.3 | 63.8 | |
| Unknown | 13.3 | 15.3 | 16.3 | 16.4 | |
| Histology type | <0.001 | ||||
| Ductal | 84.2 | 86.0 | 84.2 | 83.0 | |
| Lobular | 1.2 | 0.7 | 0.9 | 1.6 | |
| Other | 14.6 | 13.3 | 14.8 | 15.4 | |
| TNM Stage | <0.001 | ||||
| 0 | 3.5 | 2.6 | 3.2 | 4.0 | |
| I | 40.9 | 32.1 | 31.7 | 38.4 | |
| II | 38.1 | 42.6 | 42.3 | 41.1 | |
| III | 11.8 | 15.3 | 16.1 | 11.6 | |
| IV | 4.6 | 6.1 | 4.7 | 3.6 | |
| Unknown | 1.2 | 1.3 | 2.0 | 1.3 | |
| Comorbidity (Charlson Score) | <0.001 | ||||
| No | 84.4 | 78.2 | 84.6 | 87.9 | |
| Yes | 15.6 | 21.8 | 15.4 | 12.1 | |
| Surgery | <0.001 | ||||
| No or biopsy only | 6.7 | 10.7 | 8.6 | 7.5 | |
| Breast Conserving | 48.5 | 50.1 | 42.1 | 42.0 | |
| Mastectomy | 44.6 | 39.0 | 49.0 | 50.3 | |
| Unknown | 0.2 | 0.3 | 0.3 | 0.2 | |
| Radiation | <0.001 | ||||
| No | 39.9 | 36.7 | 41.6 | 43.3 | |
| Yes | 59.6 | 62.6 | 57.4 | 56.1 | |
| Unknown | 0.6 | 0.7 | 1.0 | 0.6 | |
| Chemotherapy | <0.001 | ||||
| No | 22.0 | 16.9 | 13.9 | 19.3 | |
| Yes | 76.7 | 81.5 | 84.2 | 79.1 | |
| Unknown | 1.4 | 1.7 | 1.9 | 1.6 | |
| Time-to-Treatment (Months) | 27.7 ± 24.7 | 33.3 ± 36.1 | 35.0 ± 33.7 | 29.2 ± 27.8 | <0.001 |
|
Follow-up
(Median, IQR; months) |
38.0 (24.5–54.9) |
35.9 (22.8–53.1) |
36.7 (23.7–54.0) |
36.8 (24.0–54.0) |
<0.001 |
Overall, at 3-years post-diagnosis, Asian TNBC patients showed the highest survival rate (87.1%), followed by Hispanic (86.0%) and NH-white (83.1%) groups, while NH-black patients had the lowest survival rate (79.4%, P<0.001; Table 2). Across patients with stage I-III diseases, Asian and Hispanic patients had higher survival rates, while NH-black and NH-white patients had similar lower survival rates. Among patients with stage IV disease, Asian groups showed the 3-year lowest survival rate of 14.4%, lower than those observed in Hispanic (32.5%), NH-white (17.5%), and NH-black (15.9%) counterparts. Similar comparisons were observed in terms of 5-year survival rates (Table 2).
Table 2.
Survival Rates of Female Patients with Triple Negative Breast Cancer by Race/ethnicity and Stage
| Deaths/ Patients | 3-year Survival | 5-year Survival | Overall Survival | P | |
|---|---|---|---|---|---|
| Overall Population | <0.001 | ||||
| White | 10,218/53,908 | 83.1 | 75.7 | 67.8 | |
| Black | 3,783/17,350 | 79.4 | 72.5 | 65.6 | |
| Hispanic | 770/5,116 | 86.0 | 79.7 | 75.3 | |
| Asian | 318/2,334 | 87.1 | 82.1 | 80.2 | |
| Stage I | <0.001 | ||||
| White | 1,697/22,068 | 94.1 | 88.9 | 81.6 | |
| Black | 451/5,563 | 93.5 | 88.0 | 85.0 | |
| Hispanic | 85/1,622 | 95.9 | 93.6 | 89.8 | |
| Asian | 39/896 | 95.9 | 93.7 | 93.7 | |
| Stage II | <0.001 | ||||
| White | 3,552/20,520 | 85.4 | 76.7 | 67.4 | |
| Black | 1,253/7,388 | 84.8 | 77.5 | 69.3 | |
| Hispanic | 219/2,162 | 91.0 | 85.7 | 83.5 | |
| Asian | 94/960 | 91.5 | 85.9 | 84.1 | |
| Stage III | 0.00 | ||||
| White | 2,710/6,343 | 60.3 | 48.5 | 41.6 | |
| Black | 1,129/2,647 | 58.1 | 48.4 | 46.0 | |
| Hispanic | 276/824 | 68.1 | 56.4 | 52.5 | |
| Asian | 108/270 | 62.7 | 53.2 | 47.0 | |
| Other/Unknown | 200/486 | 60.7 | 52.0 | 47.8 | |
| Stage IV | <0.001 | ||||
| White | 1,984/2,478 | 17.5 | 10.3 | 9.6 | |
| Black | 852/1,067 | 15.9 | 11.7 | 10.6 | |
| Hispanic | 157/238 | 32.5 | 19.9 | 19.9 | |
| Asian | 68/85 | 14.4 | 3.9 | 3.9 |
Racial/ethnic disparities in total mortality remained after adjustment for age and clinical characteristics. NH-black patients had a 12% higher 5-year mortality (HR=1.12, 95%CI: 1.07 to 1.17), while Hispanic patients had a 24% (HR=0.76, 95%CI: 0.70 to 0.83) and Asian patients had an 17% (HR=0.83, 95%CI: 0.73 to 0.94) lower 3-year mortality than NH-white counterparts. Additional adjustment for treatment resulted in slight attenuation of point estimates for racial/ethnic minorities versus NH-white patients. However, after further adjustment for factors related to access-to-care, the black-white disparity became non-significant (HR=1.04, 95%CI: 0.99 to 1.09), while the white-Hispanic (HR=0.69, 95%CI: 0.64 to 0.76) and white-Asian (HR=0.83, 95%CI: 0.73 to 0.94) differences persisted (Table 3).
Table 3.
HRs (95%CI) for 3-year and 5-year All-cause Mortality Associated with Race/ethnicity Among Female Patients with TNBC
| Deaths /Patients | HR (95% CI) 0 | HR (95% CI) 1 | HR (95% CI) 2 | HR (95% CI) 3 | |
|---|---|---|---|---|---|
| 3-year All-cause mortality | |||||
| White | 8,103/53,908 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Black | 3,158/17,350 | 1.35 (0.29 to 1.40) | 1.12 (1.07 to 1.17) | 1.09 (1.04 to 1.13) | 1.04 (0.99 to 1.09) |
| Hispanic | 619/5,116 | 0.94 (0.87 to 1.02) | 0.76 (0.70 to 0.83) | 0.75 (0.69 to 0.81) | 0.69 (0.64 to 0.76) |
| Asian | 263/2,334 | 0.83 (0.74 to 0.94) | 0.83 (0.73 to 0.94) | 0.80 (0.71 to 0.90) | 0.83 (0.73 to 0.94) |
| 5-year All-cause mortality | |||||
| White | 9,923/53,908 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Black | 3,702/17,350 | 1.31 (1.26 to 1.36) | 1.09 (1.05 to 1.14) | 1.07 (1.03 to 1.11) | 1.03 (0.98 to 1.07) |
| Hispanic | 753/5,116 | 0.95 (0.88 to 1.02) | 0.77 (0.72 to 0.83) | 0.75 (0.70 to 0.81) | 0.70 (0.65 to 0.76) |
| Asian | 312/2,334 | 0.82 (0.73 to 0.91) | 0.82 (0.73 to 0.91) | 0.79 (0.70 to 0.88) | 0.82 (0.73 to 0.92) |
Abbreviation: HR, Hazard Ratio; CI, Confidence Interval; TNBC, Triple Negative Breast Cancer.
Model 0. Adjusted for age at diagnosis (continuous).
Model 1. Additionally adjusted for clinical characteristics (histologic grade, histology type, tumor size, lymph node metastasis, distant metastasis, lymphovascular invasion, comorbidity).
Model 2. Additionally adjusted for treatment (surgery, radiation, chemotherapy, year of diagnosis and time-to-treatment).
Model 3. Additionally adjusted for factors related access to care (income, urban/rural residence, education, insurance, distance to care, facility type, and region of facility location).
These association patterns were largely consistent across cancer stages, except that Asian patients with stage III (HR=1.01, 95%CI: 0.81 to 1.25) or stage IV (HR=1.02, 95%CI: 0.79 to 1.32) diseases had similar mortality risk as their NH-white counterparts after full adjustments (Figure 1). Among women aged ≤50 years with stage III cancer, NH-blacks showed higher mortality than NH-whites (fully adjusted HR=1.20, 95%CI: 1.04 to 1.39; Figure 2). These patterns were very similar for 5-year mortality (Supplementary Tables 2–3). Consistent association patterns were observed across all types and regions of facility, though the estimates for white-Asian disparities were not significant in several subgroups (Supplementary Table 4). However, the latter analysis was based on a small sample size.
Figure 1. Multivariable-adjusted HRs (95% CI) for 3-year All-Cause Mortality in Triple Negative Breast Cancer Associated with Race/Ethnicity by Stage.
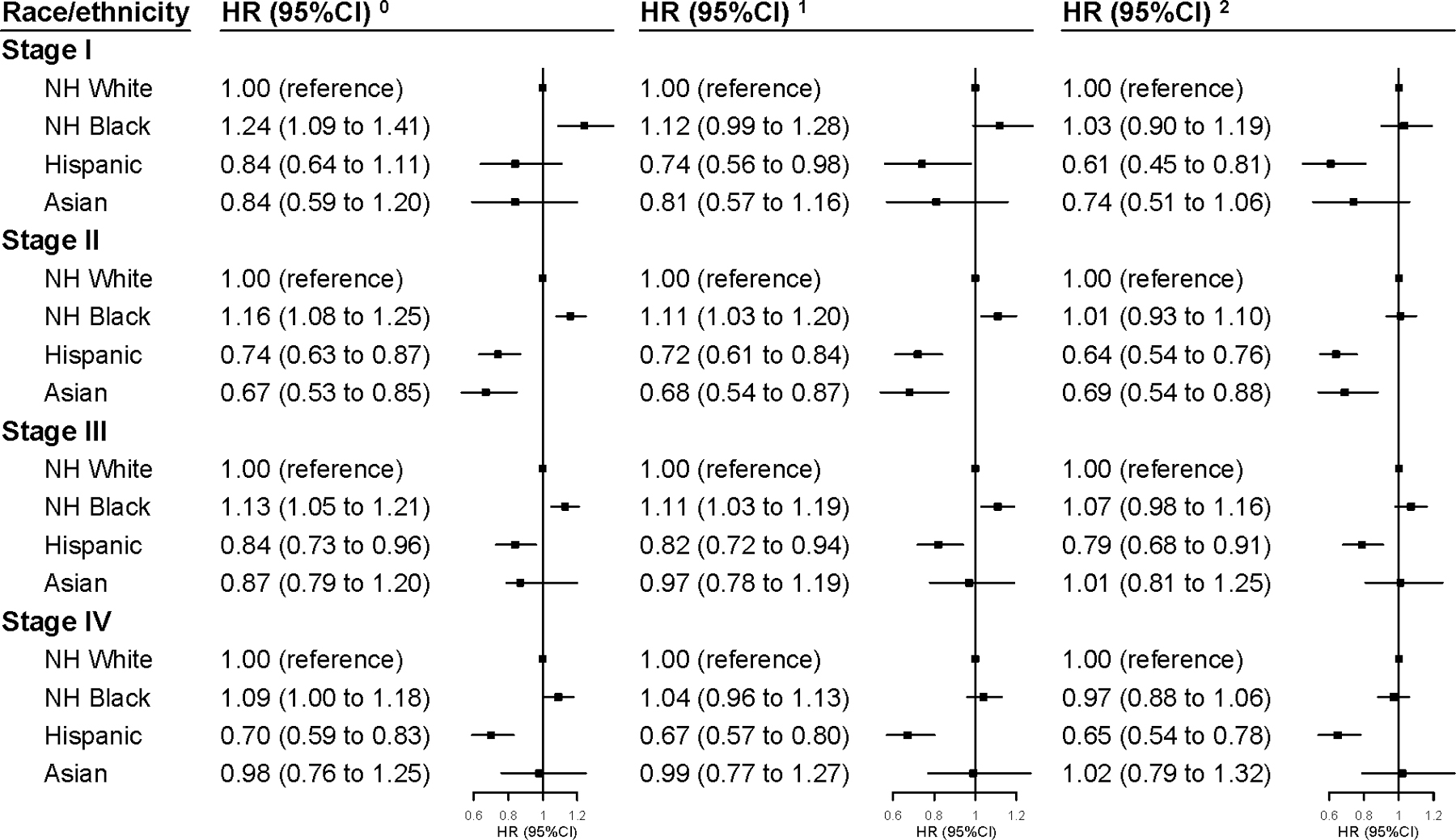
The HRs and 95% CIs derived from:
0. Model 0. Adjusted for age at diagnosis (continuous).
1. Model 1. Additionally adjusted for clinical characteristics (histologic grade, histology type, tumor size, lymph node metastasis, distant metastasis, lymphovascular invasion, comorbidity).
2. Model 2. Additionally adjusted for treatment (surgery, radiation, chemotherapy, year of diagnosis and time-to-treatment) and factors related access to care (income, urban/rural residence, education, insurance, distance to care, facility type, and region of facility location).
Abbreviation: HR, Hazard Ratio; CI, Confidence Interval.
Figure 2. Multivariable-adjusted HRs (95% CI) for 3-Year All-Cause Mortality in Triple Negative Breast Cancer Associated with Race/Ethnicity by Stage and Age.
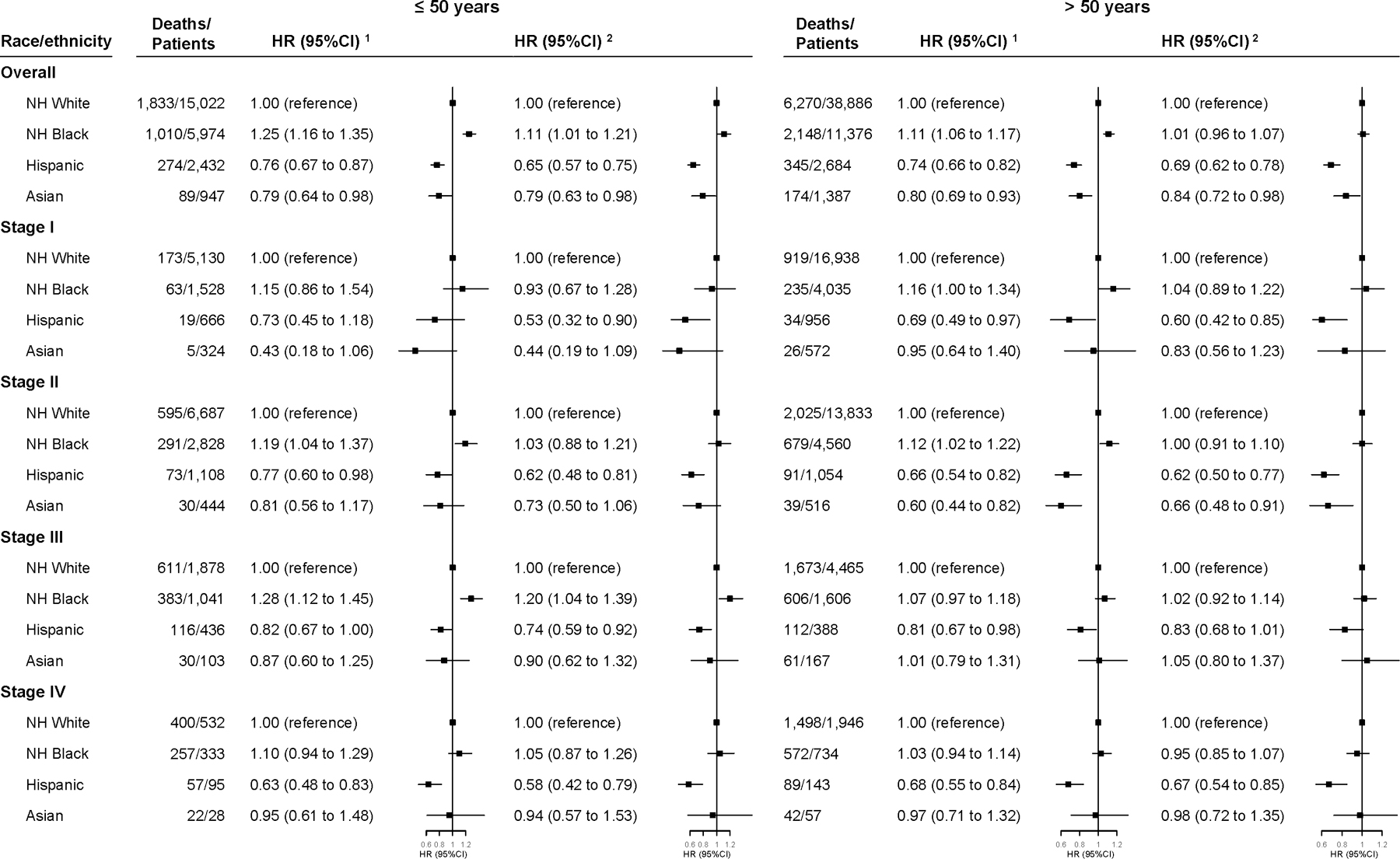
The HRs and 95% CIs derived from:
1. Model 1. Additionally adjusted for clinical characteristics (histologic grade, histology type, tumor size, lymph node metastasis, distant metastasis, lymphovascular invasion, comorbidity).
2. Model 2. Additionally adjusted for treatment (surgery, radiation, chemotherapy, year of diagnosis and time-to-treatment) and factors related access to care (income, urban/rural residence, education, insurance, distance to care, facility type, and region of facility location).
Abbreviation: HR, Hazard Ratio; CI, Confidence Interval.
Discussion
In this nationwide registry-based study, after accounting for age and clinical characteristics, we found that NH-black patients with TNBC had higher, while Hispanic and Asian patients had lower 3-year and 5-year mortality than NH-white counterparts. However, after further adjustment for treatment and access-to-care related factors in particular, little black-white disparity remained, with the exception of younger patients (<50 years) with stage III cancer, of whom black patients fared worse. Hispanic and Asian TNBC patients overall had a lower 3-year and 5-year all-cause mortality than NH-whites, and these associations persisted even after the aforementioned full adjustments. Asian patients with stage III/IV cancers had a similar mortality risk to their NH-white counterparts.
Racial/ethnic mortality disparities after diagnosis of TNBC have not been adequately investigated, and most studies focused on comparisons between NH-white and NH-black populations. Our finding for the NH black-white mortality difference is consistent with data from the California Cancer Registry, which was the largest study to date (12). With 10,485 patients evaluated, Tao et al. showed that NH-black patients with TNBC had higher mortality than NH-white patients among the stage III subgroup, but no mortality differences were observed among patients with stage I, II, and IV diseases. Our finding was also supported by two smaller studies (13,18), both of which showed similar mortality disparities between NH-white and NH-black TNBC patients after adjustment for tumor characteristics and treatment. By contrast, another study showed that African Americans had higher risk of mortality compared with white patients, even after accounting for stage, treatment, delay of treatment and poverty index (19). However, this study included only 135 patients with TNBC. It has been suggested that cancer outcome disparities may be, at least partly, attributed to socioeconomic disadvantages, which are more prevalent in NH-black populations and may, therefore, confer worse quality of cancer care (5,20–23). In our study, higher proportions of NH-black patients lived in neighborhoods with low socioeconomic status, i.e., higher proportions of residents with low incomes, low educational attainment and/or uninsured, in comparison to NH-white counterparts. Furthermore, despite a higher proportion of patients who received chemotherapy, NH-black patients had longer intervals between cancer diagnosis and treatment initiation, indicating potential treatment delays. Previous studies have also reported NH-black patients had low adherence to guideline-recommended locoregional or systemic treatment (24,25) and may have fewer and less effective communications with their healthcare providers (26), which may further contribute to worsened survival.
It is noteworthy that, despite no overall white-black disparities in TNBC mortality, we observed significantly higher mortality among NH-black patients with stage III cancer who were younger than 50 years compared with their NH-white counterparts, but not among those aged >50 years. This is supported by the abovementioned California study (12) in which higher mortality was observed among NH-black patients with stage III TNBC, relative to NH-whites, though no age-stratified results were reported. The reasons for such age- and stage-specific mortality disparities are unclear. Stage III TNBC is a relatively advanced disease, but still potentially curable with more advanced systemic treatments (e.g., dose-dense or high-dose regimens) and higher quality care (1), which may be more strongly influenced by factors related to access-to-care. Patients with an early-stage breast cancer have high 5-year survival rates (>90%), while fewer than 30% of patients with metastatic cancers can survive beyond 5 years despite optimal systemic chemotherapy (20). Treatment and quality of access-to-care may play less important roles for patients with advanced stage cancer. In addition, compared with NH-white patients with TNBC, NH-black patients had a younger age distribution (27,28). In our study, overall, 34.4% of NH-black and 27.8% of NH-white patients were diagnosed before age 50. This difference was even greater among patients with stage III cancer (39.3% in NH-black patients versus 29.6% in NH-white patients). Thus, the significant age-specific disparity found in our study may reflect, at least in part, the higher statistical power we had for the younger group. In addition, despite a higher prevalence of BRCA mutation among TNBC patients (29), the majority of NH-black women were not referred for BRCA counseling and/or testing, thus may be less likely to benefit from targeted therapy such as poly (ADP-ribose) polymerase inhibitors (30,31). Prior studies also reported additional differences in biologic characteristics between racial/ethnic groups with TNBC, such as methylation and histone modifications (32,33). Unfortunately, the above information was not recorded in the NCDB. Thus, these hypotheses could not be evaluated in our study. Furthermore, the observed higher mortality among young NH-black patients with advanced TNBC also suggested the key role of early diagnosis and detection among this population. This finding also provided some evidence and support to the viewpoint that mammography screening among NH-black populations should be initiated at an earlier age than that recommended by current guidelines (34).
Disparities in TNBC outcomes between NH-white and Hispanic, as well as Asian patients have been rarely reported. Using data from Pathway and Life After Cancer Epidemiology cohorts, Kroenke et al. found that both Hispanic and Asian patients with basal-like breast cancer had lower risk, although statistically significant, of recurrence than other racial/ethnic patients (14). However, this study only included 37 Hispanic and 16 Asian patients. In our study with over 5,000 Hispanic patients and 2,300 Asian patients evaluated, we found consistently lower mortality for these two minorities than for NH-white patients, even after accounting for treatment and factors related to access-to-care. This is different from the association patterns observed between NH-white and NH-black patients, suggesting that additional factors other than healthcare may contribute to racial/ethnic disparities in TNBC outcomes. It has been proposed that differences in lifestyle (e.g., dietary habits, physical activity and obesity), traditional medicine use and social support may contribute to different health outcomes (35–38). In addition, genetic/biological differences across racial/ethnic groups and their interactions with treatment may also play roles (39). It is interesting that, in our study, Asian patients with advanced diseases had similar mortality to NH-white patients, in contrast to the patterns observed for all and early-stage TNBC. One possible explanation is that Asian cancer patients usually have worse tolerance to cancer treatment and experience a higher prevalence of toxicity than white counterparts, even for some less aggressive treatments or after dose reductions (40–42). Thus, Asian patients with advanced stage TNBC are less likely to receive sufficient treatment or suffer more treatment-related adverse effects, which may offset their overall survival advantages. Nonetheless, we cannot rule out the possibility of chance finding due to the relatively small sample size of Asian patients with stage III/IV cancers. More research is warranted to fully understand the complexities of underlying mechanisms contributing to the racial disparity. In addition, because we were only able to evaluate all-cause mortality, we also cannot rule out the possibility that the lower mortality among Asians versus NH-whites is due to the difference in cause of death other than TNBC. According to data from the Center for Disease Control, in general, Asian Americans outlive NH-white Americans by an average of approximately 8 years (43). Future studies on breast cancer-specific mortality are needed.
Two of the most important advantages of our study are the large sample size and excellent generalizability because of the use of national registry data, which covers approximately 70% of all cancer patients across the U.S. We included nearly 80,000 patients from the NCDB who were diagnosed with TNBC between 2010 and 2014. This broad coverage of the NCDB enhances the representativeness and generalizability of our findings. In addition, we systematically evaluated mortality disparities across four major racial/ethnic groups and comprehensively accounted for a wide range of factors including clinical characteristics, treatment, as well as factors related to access-to-care. There are also some limitations of our study. First, due to the nature of the registry-based design, the potential misclassification of TNBC, as well as racial/ethnic categories from medical records, could not be ruled out. Also, due to the lack of more detailed molecular information (e.g., intrinsic subtype) in the NCDB, the molecular heterogeneity of TNBC could not be evaluated in our study (1,44). Second, race/ethnicity was evaluated in this study as aggregated groups, which consisted of several subgroups of heterogeneous cultural backgrounds and, therefore, we were unable to capture underlying heterogeneities. Third, breast cancer recurrence and cause of death were not recorded in the NCDB, and thus, cancer-specific mortality could not be evaluated in our study. In addition, the despite disparity patterns being quite similar in 5-year survival analyses, overall, the follow-up periods of our study population were relatively short. Finally, detailed treatment (agent type, duration, adherence, etc.), lifestyle (dietary habits, obesity, etc.) and other socioeconomic determinants (culture, provider/patient communication, etc.) could not be accounted for due to a lack of relevant information.
In conclusion, after accounting for clinical characteristics, treatment and access-to-care related factors, there is little black-white difference, but substantial Hispanic-white and Asian-white disparities in 5-year all-cause mortality for TNBC. Despite the lack of white-black disparity, research is critically needed to understand why NH-black women have a higher incidence of this aggressive type of breast cancer than white women and to reveal the underlying biological mechanisms. Our findings support the importance for providing equal healthcare to eliminate the black-white disparity in TNBC mortality and call for investigations on additional determinants for TNBC outcomes.
Supplementary Material
Significance:
These findings highlight the need for equal healthcare to mitigate the black-white disparity and for investigations of contributors beyond healthcare for lower mortality among Asians and Hispanics.
Acknowledgements:
All information was derived from the American College of Surgeons’ National Cancer Database. The American College of Surgeons and the Commission on Cancer are not responsible for conclusions drawn from the data. We would like to thank Dr. Mary Shannon Byers, Ph.D., M.S., for her assistance in editing this manuscript. This study is partially supported by the Ingram Cancer Professorship fund to Dr. XO Shu. Dr. F Wang is supported by the program of China Scholarships Council.
Footnotes
Conflict of interest disclosures: The authors indicate no potential conflicts of interest.
Reference
- 1.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016;13:674–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938–48. [DOI] [PubMed] [Google Scholar]
- 3.Malorni L, Shetty PB, De Angelis C, Hilsenbeck S, Rimawi MF, Elledge R, et al. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat 2012;136:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheang MCU, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-Like Breast Cancer Defined by Five Biomarkers Has Superior Prognostic Value than Triple-Negative Phenotype. Clin Cancer Res 2008;14:1368–76. [DOI] [PubMed] [Google Scholar]
- 5.Newman LA, Kaljee LM. Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA Surg 2017;152:485–93. [DOI] [PubMed] [Google Scholar]
- 6.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst 2015;107:djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plasilova ML, Hayse B, Killelea BK, Horowitz NR, Chagpar AB, Lannin DR. Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine (Baltimore) 2016;95:e4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502. [DOI] [PubMed] [Google Scholar]
- 9.Scott LC, Mobley LR, Kuo T-M, Il’yasova D. Update on triple-negative breast cancer disparities for the United States: A population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer 2019;125:3412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363–85. [DOI] [PubMed] [Google Scholar]
- 11.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 2016;66:31–42. [DOI] [PubMed] [Google Scholar]
- 12.Tao L, Gomez SL, Keegan THM, Kurian AW, Clarke CA. Breast Cancer Mortality in African-American and Non-Hispanic White Women by Molecular Subtype and Stage at Diagnosis: A Population-Based Study. Cancer Epidemiol Biomarkers Prev 2015;24:1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, et al. Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer 2013;13:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroenke CH, Sweeney C, Kwan ML, Quesenberry CP, Weltzien EK, Habel LA, et al. Race and breast cancer survival by intrinsic subtype based on PAM50 gene expression. Breast Cancer Res Treat 2014;144:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCabe RM. National Cancer Database: The Past, Present, and Future of the Cancer Registry and Its Efforts to Improve the Quality of Cancer Care. Semin Radiat Oncol 2019;29:323–5. [DOI] [PubMed] [Google Scholar]
- 16.2010 Census Summary File 1 [United States] prepared by the U.S. Census Bureau, 2012. [Internet] Available from: https://www.census.gov/prod/cen2010/doc/sf1.pdf
- 17.About Cancer Program Categories [Internet] American College of Surgeons. [cited 2020 Oct 27]. Available from: https://www.facs.org/quality-programs/cancer/coc/accreditation/categories
- 18.Sachdev JC, Ahmed S, Mirza MM, Farooq A, Kronish L, Jahanzeb M. Does Race Affect Outcomes in Triple Negative Breast Cancer? Breast Cancer (Auckl) 2010;4:23–33. [PMC free article] [PubMed] [Google Scholar]
- 19.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat 2009;113:357–70. [DOI] [PubMed] [Google Scholar]
- 20.American Cancer Society. Cancer Facts & Figures 2019 Atlanta: American Cancer Society; 2019. [Google Scholar]
- 21.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA: A Cancer Journal for Clinicians 2004;54:78–93. [DOI] [PubMed] [Google Scholar]
- 22.Saini G, Ogden A, McCullough LE, Torres M, Rida P, Aneja R. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes Control 2019;30:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jemal A, Robbins AS, Lin CC, Flanders WD, DeSantis CE, Ward EM, et al. Factors That Contributed to Black-White Disparities in Survival Among Nonelderly Women With Breast Cancer Between 2004 and 2013. J Clin Oncol 2018;36:14–24. [DOI] [PubMed] [Google Scholar]
- 24.Green AK, Aviki EM, Matsoukas K, Patil S, Korenstein D, Blinder V. Racial disparities in chemotherapy administration for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 2018;172:247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 2002;94:334–57. [DOI] [PubMed] [Google Scholar]
- 26.Check DK, Chawla N, Kwan ML, Pinheiro L, Roh JM, Ergas IJ, et al. Understanding Racial/Ethnic Differences in Breast Cancer-Related Physical Well-Being: The role of patient-provider interactions. Breast Cancer Res Treat 2018;170:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien KM, Cole SR, Tse C-K, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res 2010;16:6100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tano SV, Kavanaugh MM, Peddi P, Mansour RP, Shi R, Burton GV. Triple negative breast cancer (TNBC): Analysis of age and stage distribution and survival between African American and Caucasian women in a predominant low-income population. JCO 2017;35:e12586–e12586. [Google Scholar]
- 29.Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol 2014;15:e625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenup R, Buchanan A, Lorizio W, Rhoads K, Chan S, Leedom T, et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol 2013;20:3254–8. [DOI] [PubMed] [Google Scholar]
- 31.Pal T, Bonner D, Kim J, Monteiro ANA, Kessler L, Royer R, et al. Early onset breast cancer in a registry-based sample of African-american women: BRCA mutation prevalence, and other personal and system-level clinical characteristics. Breast J 2013;19:189–92. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosone CB, Young AC, Sucheston LE, Wang D, Yan L, Liu S, et al. Genome-wide methylation patterns provide insight into differences in breast tumor biology between American women of African and European ancestry. Oncotarget 2014;5:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddharth S, Sharma D. Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers (Basel) 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin N, Wingfield J. USPSTF screening recommendations for breast cancer: the potential impact on the African American community. J Health Care Poor Underserved 2012;23:91–7. [DOI] [PubMed] [Google Scholar]
- 35.Kagawa‐Singer M, Dadia AV, Yu MC, Surbone A. Cancer, Culture, and Health Disparities: Time to Chart a New Course? CA: A Cancer Journal for Clinicians 2010;60:12–39. [DOI] [PubMed] [Google Scholar]
- 36.Kushi LH, Kwan ML, Lee MM, Ambrosone CB. Lifestyle Factors and Survival in Women with Breast Cancer. J Nutr 2007;137:236S–242S. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke CH, Michael YL, Poole EM, Kwan ML, Nechuta S, Leas E, et al. Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer 2017;123:1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev 2012;21:1260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinh Q-D, Nguyen PL, Leow JJ, Dalela D, Chao GF, Mahal BA, et al. Cancer-Specific Mortality of Asian Americans Diagnosed With Cancer: A Nationwide Population-Based Assessment. J Natl Cancer Inst [Internet] 2015. [cited 2019 May 24];107. Available from: https://academic.oup.com/jnci/article/107/6/djv054/870476 [DOI] [PubMed] [Google Scholar]
- 40.O’Donnell PH, Dolan ME. Cancer Pharmacoethnicity: Ethnic Differences in Susceptibility to the Effects of Chemotherapy. Clin Cancer Res 2009;15:4806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han HS, Reis IM, Zhao W, Kuroi K, Toi M, Suzuki E, et al. Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur J Cancer 2011;47:2537–45. [DOI] [PubMed] [Google Scholar]
- 42.Chow LWC, Biganzoli L, Leo AD, Kuroi K, Han HS, Patel J, et al. Toxicity profile differences of adjuvant docetaxel/cyclophosphamide (TC) between Asian and Caucasian breast cancer patients. Asia-Pacific Journal of Clinical Oncology 2017;13:372–8. [DOI] [PubMed] [Google Scholar]
- 43.Acciai F, Noah AJ, Firebaugh G. Pinpointing the Sources of the Asian Mortality Advantage in the United States. J Epidemiol Community Health 2015;69:1006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills MN, Yang GQ, Oliver DE, Liveringhouse CL, Ahmed KA, Orman AG, et al. Histologic heterogeneity of triple negative breast cancer: A National Cancer Centre Database analysis. Eur J Cancer 2018;98:48–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


