Abstract
HMR 3647, a new ketolide, is active upon intracellular pathogens. We previously demonstrated that HMR 3004 (RU 64004), another ketolide, is highly concentrated by human polymorphonuclear neutrophils (PMNs). This prompted us to evaluate whether the presence of a 3-keto group instead of an l-cladinose, a neutral sugar characteristic of erythromycin A derivatives, confers peculiar pharmacokinetic properties with regard to cellular accumulation and efflux. After incubation with the radiolabelled drug, HMR 3647 uptake was determined by a velocity gradient centrifugation technique. HMR 3647 was avidly concentrated by PMNs, without saturation, over a 3-h incubation period, with cellular-to-extracellular concentration ratios of 31 ± 4.2 at 5 min and up to 348 ± 27.1 at 180 min. About 60% of HMR 3647 was located in the granular compartment; less than 6% was associated with the membranes. HMR 3647 gradually egressed from loaded cells placed in drug-free medium. Uptake was dependent on environmental temperature (activation energy, 128 ± 9.4 kJ/mol) but not on extracellular pH. HMR 3647 displayed Michaelis-Menten saturation kinetics with a mean Vmax of 2315 ng/2.5 × 106 PMNs/5 min and a mean Km of 117 mg/liter (144 μM). As already observed with erythromycin A-derived macrolides, extracellular Ca2+ was necessary for optimal uptake of HMR 3647. Interestingly, verapamil increased the uptake of HMR 3647 at 5 min, but this was followed by gradual inhibition at later incubation times, a phenomenon probably related to stimulation of drug efflux. The impact of intracellular accumulation of HMR 3647 on PMN functions was also investigated. In contrast to other erythromycin A derivatives, HMR 3647 only weakly triggered granule exocytosis, but it inhibited superoxide anion production in a time- and concentration-dependent manner, with concentrations which inhibited 50% of control response of 55 (67 μM) (5 min) and 30 (36 μM) (30 min) mg/liter for formyl-methionyl-leucyl-phenylalanine stimulation and 117 (143 μM) (5 min) and 44 (54 μM) (30 min) mg/liter for phorbol myristate acetate stimulation.
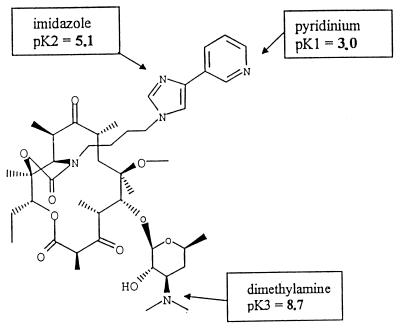
HMR 3647 belongs to a new class of semisynthetic erythromycin A derivatives, the ketolides, characterized by a 3-keto group in place of the l-cladinose moiety at position C-3 of the lactone ring (8). HMR 3647 was one of the most active compounds on respiratory pathogens, among a series of 11,12-cyclo-disubstitued ketolides synthesized by Roussel-Uclaf (4). In addition, HMR 3647 is active against intracellular microorganisms (e.g., Chlamydia pneumoniae, Legionella pneumophila, and Toxoplasma gondii) (5, 31, 32). We recently reported that HMR 3004 (formerly RU 64004) is strongly accumulated by human polymorphonuclear neutrophils (PMNs) (35). HMR 3647 (Fig. 1) differs from HMR 3004 by having two azolium moieties (imidazolium and pyridinium) linked by an alkyl chain to the C-11–C-12 carbamate ring. This structure confers 3 pKas on HMR 3647, above and below the physiological pH: pK1 for the pyridinium ring, 3.0; pK2 for the imidazolium ring, 5.1; and pK3 for the d-desosamine, 8.7. It was therefore interesting to investigate whether HMR 3647 displays uptake kinetics similar to those obtained with HMR 3004 and other erythromycin A derivatives, to complete our preliminary classification of macrolides (25). In addition, we investigated whether cellular accumulation of HMR 3647 interferes with two functional activities of PMNs, exocytosis and the oxidative burst, which are strongly modified by all l-cladinose-possessing macrolides (1).
FIG. 1.
Chemical structure of HMR 3647.
(Results of this investigation were presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 28 September to 1 October, 1997 [23].)
MATERIALS AND METHODS
Antibacterial agents.
HMR 3647, erythromycin, roxithromycin, levofloxacin (Hoechst-Marion-Roussel, Romainville, France), josamycin (Pharmuka, Paris, France), and azithromycin (Laboratoire Pfizer, Paris, France) were provided by the manufacturers. Ampicillin sodium was from ICN Pharmaceuticals. The radiolabelled drug 3H-HMR 3647 (35.4 Ci/mmol) in ethanol-water (7/3, vol/vol) was provided by Hoechst-Marion-Roussel. The tritiated drug (2.5 μl; about 30 μg/ml) was mixed with 25 μl of unlabelled HMR 3647 (1 mg/ml of Hanks balanced salt solution [HBSS]; Diagnostic Pasteur, Paris, France) and 222.5 μl of HBSS. Stock solutions were further diluted in HBSS to the desired concentrations.
Human neutrophils (PMNs).
PMNs were obtained from the venous blood of healthy volunteers, by Ficoll-Paque centrifugation followed by 2% dextran sedimentation and osmotic lysis of residual erythrocytes. The viability and purity of the PMN preparation, assessed by Trypan blue exclusion, were both greater than 96%.
Macrolide uptake.
A radiometric assay was used to measure macrolide uptake (35). Briefly, 2.5 × 106 PMNs were incubated at 37°C with the radiolabelled drug and were then centrifuged at 12,000 × g for 3 min at 22°C through a water-impermeable silicone-paraffin (86/14, vol/vol) oil barrier. The pellet was solubilized in Hionic fluor (Packard), and cell-associated radioactivity was quantified by liquid scintillation counting (LS-6000-S; Beckman). Standard dilution curves were used to determine the amounts of cell-associated drug. The results are expressed as nanograms per 2.5 × 106 PMNs. The concentration of macrolide used in the assays was 2.5 mg/liter except when indicated otherwise. A previously determined intracellular volume of 0.6 μl/2.5 × 106 PMNs (29) was used to determine the cellular/extracellular concentration ratio (C/E ratio). The various experimental conditions used here (temperature, pH, inhibitors) did not significantly modify this value.
Characteristics of macrolide uptake.
We first analyzed uptake kinetics over a 3-h incubation period. The influences of extracellular pH and temperature were assessed after incubation for 5 to 180 min. The effects of metabolic inhibitors (10-min pretreatment with sodium cyanide, NaCN [1 mM], potassium fluoride, KF [1 mM], sodium azide, NaN3 [1 mM], or 2,4-dinitrophenol [1 mM]) were assessed over a 60-min incubation period. The effects of competitive inhibitors (10-min pretreatment with puromycin [1 mM]; amino acid [1 mM] l-methionine, l-phenylalanine, l-tyrosine, or l-arginine; glucose [1 mM]; various macrolides [10 to 100 mg/liter, 12 to 136 μM]; levofloxacin [10 to 100 mg/liter, 28 to 280 μM]; or ampicillin [100 mg/liter, 287 μM]) were assessed at 5 min. All chemical solutions were buffered to pH 7.4 to avoid any influence of pH on macrolide uptake (29). The influence of extracellular concentration (0.5 to 300 mg/liter) was assessed during the first 5 min of incubation, i.e., when the rate of uptake is optimal.
Cellular location.
Macrolide-loaded PMNs (30 min at 37°C) were centrifuged through the silicone-paraffin oil barrier, and the cell pellet was sonicated in the presence of 0.5% Triton (three 15-s bursts) or 0.73 M sucrose (three 5-s bursts) to protect granules (35). After centrifugation (100,000 × g, 30 min), the amounts of the marker enzymes lactate dehydrogenase (LDH) (7), β-glucuronidase (34), and lysozyme (28) in the pellet and supernatant, together with the amounts of radiolabelled drugs, were determined.
Macrolide efflux.
Aliquots of macrolide-loaded PMNs were centrifuged through the silicone-paraffin oil barrier; one aliquot was used to quantify cell-associated macrolides (total associated drug). The other cell pellets were placed in drug-free HBSS and, at various times, were again centrifuged through the oil barrier; radioactivity was then measured in the cell pellet and supernatant. We verified that the sum of the radioactivity (that in the pellet plus that in the supernatant) did not significantly differ from the total load. Efflux of macrolides was expressed as the percentage of drug remaining associated with the cell pellet compared to the sum of the radioactivities (radioactivity in the pellet plus radioactivity in the supernatant).
Influence of Ca2+ on macrolide uptake and efflux.
The uptake kinetics of macrolides was assessed first in Ca2+-depleted HBSS (Gibco), supplemented with 1 mM EGTA (Merck), 1 mM magnesium chloride (Merck), and 4.2 mM sodium bicarbonate (NaHCO3) (Diagnostic Pasteur). Uptake kinetics was also assessed in the presence of nickel chloride (Ni2+; 0.5 to 5 mM) (Sigma) or l-type Ca2+ channel inhibitors (verapamil hydrochloride [5 to 250 μM] [Sigma], nifedipine, and diltiazem [100 μM] [Calbiochem]). All reagents were buffered at pH 7.4 before use. Efflux of macrolides was measured in Ca2+-free HBSS or in the presence of verapamil (250 to 125 μM) or Ni2+ (5 mM).
PMN viability.
PMNs were incubated in the presence of HMR 3647 (10 to 100 mg/liter) at 37°C for 5 to 180 min. PMN viability was assessed by measuring the amount of LDH released in the supernatant.
PMN exocytosis.
PMNs were incubated at 37°C for 30 to 180 min in the presence of HMR 3647 (10 to 100 mg/liter). The exocytosis of specific and azurophilic granules was measured as previously described (3).
Superoxide anion production.
PMNs were incubated at 37°C for 5 to 30 min in the presence of HMR 3647 (10 to 100 mg/liter). Superoxide anion production was measured in terms of superoxide dismutase-inhibitable cytochrome C reduction, as previously described (1). Formyl-methionyl-leucyl-phenylalanine (FMLP) (10−6 M) and cytochalasin B (5 μg/ml) or phorbol myristate acetate (PMA) (100 ng/ml) were used as the stimulating agent.
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEM) for n experiments conducted with PMNs from different volunteers. Analysis of variance, regression analysis, and Student’s t test for paired data were used to determine statistical significance. All tests were performed with the Statworks program, version 1.2, Cricket software (1985 release).
RESULTS
Effect of HMR 3647 on cell viability.
PMNs were incubated with HMR 3647 (10 to 100 mg/liter) for 30 to 180 min at 37°C (Table 1). Only high concentrations (100 to 50 mg/liter) of the macrolide impaired PMN viability after a 3-h incubation period. Regression analysis of the data indicated the following concentration dependency: percent LDH release = 0.23 (concentration in milligrams per liter) + 5.5 (P < 0.001; r, 0.833). The concentration which resulted in 50% LDH release was 193.5 mg/liter (238 μM). None of the experimental conditions used for the analysis of drug uptake altered PMN viability (LDH release < 10%).
TABLE 1.
Effect of HMR 3647 on PMN viability
| HMR 3647 concn (mg/liter) | Mean % LDH release ± SEM after the following incubation perioda:
|
|||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |
| 100 | 6 ± 0.9 (3) | 7 ± 4.6 (2) | 19 ± 8.6 (2) | 31 ± 8.3b (3) |
| 50 | 5 ± 0.4 (5) | 7 ± 0.9b (3) | 14 ± 3.5 (2) | 14 ± 2.1b (4) |
| 25 | 4 ± 0.0 (2) | 6 ± 0.0 (2) | 10 ± 2.5 (2) | 12 ± 0.6 (3) |
| 10 | 5 ± 0.0 (2) | 6 ± 0.0 (2) | 10 ± 2.5 (2) | 8 ± 0.9 (3) |
| Control | 5 ± 0.6 (6) | 5 ± 0.6 (4) | 6 ± 0.9 (4) | 6 ± 0.9 (4) |
Numbers of experiments are given in parentheses.
P < 0.05 versus control (Student’s t test for paired data).
Accumulation of HMR 3647.
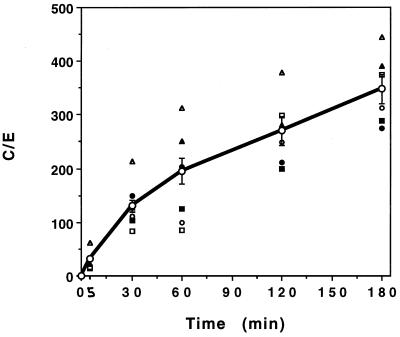
HMR 3647 was gradually accumulated by PMNs, without saturation, over a 3-h incubation period (Fig. 2). The mean C/E ratios were about 31 ± 4.2 (mean for 17 experiments) at 5 min and about 348 ± 27.1 at 180 min (mean for 6 experiments). This profile is similar to that observed with dibasic macrolides (e.g., azithromycin, dirithromycin, and erythromycylamine) (29, 35) and differs from that observed with monobasic macrolides and HMR 3004 (34). As already noted with other erythromycin A derivatives (27, 29, 35), HMR 3647 uptake kinetics differed among PMN samples from different individuals (Fig. 2). The lowest and highest C/E ratios were 14 and 61 at 5 min and 275 and 445 at 180 min.
FIG. 2.
Uptake kinetics of HMR 3647. Results are expressed as means ± SEM of the C/E ratio for 6 to 17 experiments. The values obtained with PMN samples from six different individuals are shown as unconnected symbols.
Factors influencing macrolide uptake. (i) Extracellular pH.
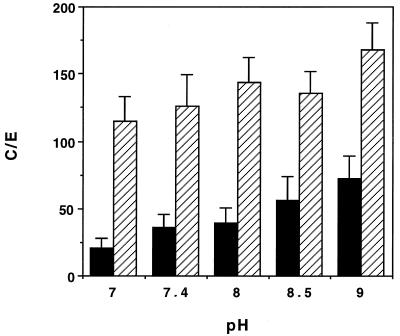
The uptake of HMR 3647 was little influenced by changes in extracellular pH (Fig. 3) (analysis of variance [ANOVA] P = 0.110 at 5 min and P > 0.5 at 30 min).
FIG. 3.
Effect of extracellular pH on HMR 3647 uptake. Results are expressed as means ± SEM for four (5 min) (solid bars) or five (30 min) (hatched bars) experiments.
(ii) Metabolic inhibitors.
None of the metabolic inhibitors tested (KF, an inhibitor of anaerobic glycolysis; NaCN, an inhibitor of mitochondrial oxidative respiration; 2,4-dinitrophenol, a phosphorylative oxidation uncoupler; or NaN3 an inhibitor of cytochrome electron transfer) inhibited HMR 3647 uptake (data not shown).
(iii) Effects of temperature.
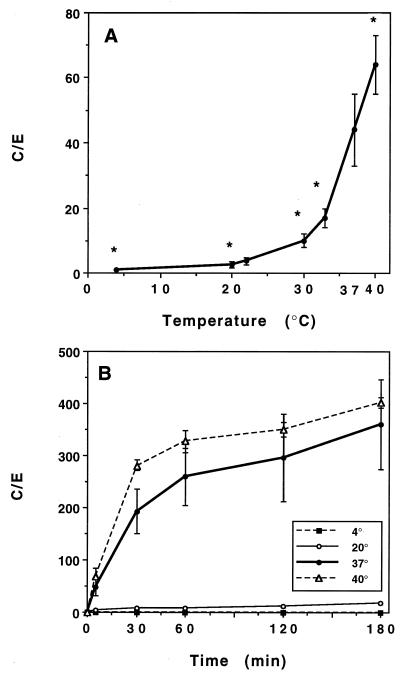
HMR 3647 was incubated for 5 min at temperatures ranging from 4 to 40°C (Fig. 4A). Activation energy was calculated as previously described (26, 34), by using the Arrhenius equation: ΔG = −RT ln Keq, where ΔG is the activation energy (calories per mole), T is the temperature (in degrees Kelvin), R is a constant (equal to 1.98), and ln Keq is the Napierian logarithm of the C/E ratio at 5 min (the time when uptake rates are maximal). ΔG can be obtained from the slope of the curve by using the Van’t Hoff plot representation of data: ln Keq = −ΔG/RT.
FIG. 4.
Effect of temperature on HMR 3647 uptake. (A) PMNs were incubated for 5 min with HMR 3647. Results are expressed as mean C/E ratios ± SEM for three to five experiments. (B) PMNs were incubated for 5 to 180 min with HMR 3647 (two experiments). ∗, P < 0.005 (ANOVA followed by Student’s t test for paired data) versus uptake at 37°C.
The activation energy of HMR 3647 was high (128 ± 9.4 kJ/mol [mean for four experiments]) a value similar to that obtained previously with azithromycin (35).
Uptake kinetics of HMR 3647 was measured at 4, 20, 37, and 40°C (Fig. 4B).
At 4°C, HMR 3647 uptake was nil (C/E ≤ 1 throughout the incubation period); at 20°C, uptake was moderate and gradual (C/E ratios of 3 at 5 min and 18 at 180 min). At 40°C, HMR 3647 uptake was slightly increased compared to that observed at 37°C, and the difference was significant at 5 min only (an increase of about 40% P < 0.05) (Fig. 4A).
(iv) Effect of extracellular concentration on HMR 3647 uptake.
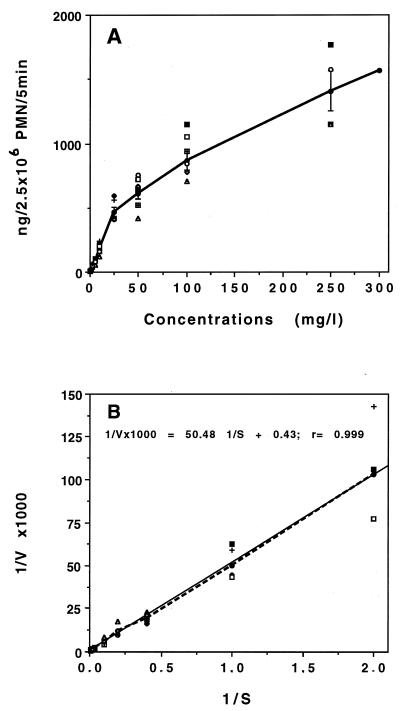
The influence of extracellular concentration was studied in the range 0.5 to 300 mg/liter during the first 5 min of incubation (Fig. 5A). HMR 3647 uptake displayed saturation kinetics characteristic of a carrier-mediated transport process. Kinetic analyses of macrolide uptake (Lineweaver-Burk reciprocal plots) are shown in Fig. 5B. Calculation of constants from the curve gave a mean Vmax of 2,315 ng/2.5 × 106 PMNs/5 min and a mean Km of 117 mg/liter (144 μM). It was interesting that, as with uptake kinetics, there was strong interindividual variability in the saturation kinetics of HMR 3647 (Fig. 5). Individual calculation of kinetic constants for PMN samples from eight different donors gave Vmax values ranging from 588 to 2,702 ng/2.5 × 106 PMNs/5 min and Km values ranging from 34 to 118 mg/liter (95% confidence interval for Vmax, 878 to 1,812 ng/2.5 × 106 PMNs/5 min; for Km, 44 to 88 mg/liter).
FIG. 5.
Effect of extracellular concentration on HMR 3647 uptake at 5 min. (A) Accumulation of HMR 3647. The data are means ± SEM for four to nine experiments and values obtained with eight PMN samples from eight individuals (unconnected symbols). (B) Lineweaver-Burk plot of the data in panel A.
We further studied whether macrolide transport into PMNs was mediated by a common carrier system. The uptake of HMR 3647 (2.5 mg/liter) was measured at 5 min in the presence of various macrolides (10 to 100 mg/liter) or other antimicrobial agents (levofloxacin, a fluoroquinolone which passively enters PMNs [36], and ampicillin, a β-lactam antibiotic which does not enter PMNs). Macrolides, irrespective of the size of the lactone ring, inhibited HMR 3647 uptake in a concentration-dependent manner. The most effective compound was azithromycin, with 25% inhibition at 10 mg/liter (P, 0.016) and 50% at 100 mg/liter (P < 0.001) (five experiments). Erythromycin, roxithromycin, and josamycin inhibited HMR 3647 uptake by about 25% at 100 mg/liter only (P < 0.05; four to six experiments). In three complementary experiments we studied the concentration-dependent uptake of HMR 3647 at 5 min in the presence of azithromycin at 10 mg/liter (two experiments) or roxithromycin at 10 and 100 mg/liter (one experiment). Azithromycin did not modify the Vmax of HMR 3647 (842 ± 142 [mean ± standard deviation] versus 1,015 ± 7 ng/2.5 × 106 PMNs/5 min, respectively, for control uptake and azithromycin inhibition) but increased the Km (42 ± 2.3 versus 67 ± 11.3 mg/liter). Similarly, roxithromycin (100 mg/liter) did not impair Vmax (1,368 [control] versus 1,471 [roxithromycin] ng/2.5 × 106 PMNs) but markedly increased the Km (90 versus 199 mg/liter). At 10 mg/liter, roxithromycin did not modify these constants. Taken together, these data argue for competitive inhibition of HMR 3647 uptake by macrolides. In contrast, neither levofloxacin nor ampicillin (100 mg/liter) significantly impaired HMR 3647 uptake (P > 0.05 [three to four experiments]). Competitive inhibitors of known transport systems on the PMN membrane (1 mM glucose; 1 mM amino acid l-phenylalanine, l-methionine, l-tyrosine, or l-arginine; or 1 mM puromycin) did not inhibit HMR 3647 uptake: 103% ± 16.9% relative to control for glucose (three experiments), 99% ± 11.5% for l-phenylalanine (three experiments), 100% ± 12.5% for l-methionine (three experiments), 100% for l-tyrosine, 80% for l-arginine, and 85% for puromycin (mean for two experiments at 5 min).
(v) Effects of Ca2+ chelators and Ca2+ channel blockers on HMR 3647 accumulation.
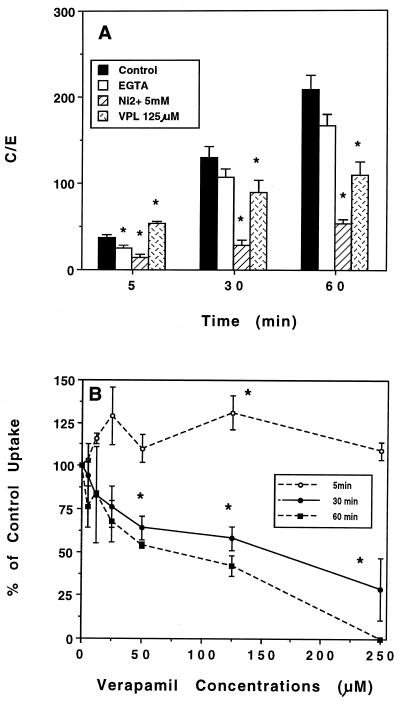
We have previously reported that the intracellular accumulation of various erythromycin A derivatives is highly dependent on the presence of extracellular Ca2+ and particularly on the correct functioning of the Na+ and Ca2+ exchanger (29). In addition, verapamil (but not other organic Ca2+ channel blockers) impaired roxithromycin, erythromycin, and HMR 3004 uptake but increased that of azithromycin at 5 min (34). We therefore investigated whether HMR 3647 accumulation also depended on Ca2+ entry into PMNs. Chelation of extracellular Ca2+ by EGTA significantly impaired HMR 3647 accumulation at 5 min (C/E ratio, 25 ± 2.5; P, 0.002 [versus control value, 37 ± 4.3] [five experiments]), but the inhibition was not significant at longer incubation times (Fig. 6A). Ni2+ (5 mM) strongly impaired HMR 3647 uptake at 5, 30, and 60 min (P ≤ 0.005) (Fig. 6A). Logarithmic regression analysis was used to determine the concentration which inhibited 50% of HMR 3647 uptake (IC50) of Ni2+. Values were as follows: 5 min, 3.1 mM (P, 0.003; r, 0.781); 30 min, 2.2 mM (P < 0.001; r 0.923); 60 min, 2.1 mM (P < 0.001; r, 0.976). The effect of verapamil was interesting (Fig. 6). At 5 min of incubation, 125 μM verapamil significantly increased HMR 3647 uptake by about 30% (P < 0.05), whereas it impaired it at longer incubation times (65% ± 7.5% of control uptake at 30 min [P, 0.018], and 49% ± 5.9% at 60 min [P, 0.019] [means ± SEM of four experiments]). At 5 min of incubation, only the concentration of 125 μM verapamil increased HMR 3647 uptake (Fig. 6B), whereas at 30 and 60 min of incubation the inhibitory effect was concentration dependent. Regression analysis performed at 30 and 60 min gave mean IC50 of 140 μM (P, 0.001; r, 0.699) and 56 μM (P < 0.001, r, 0.802) for verapamil. Neither 100 μM nifedipine nor 100 μM diltiazem impaired HMR 3647 accumulation at 5 min (96% ± 7.4% and 86% ± 9.8% of control uptake, respectively [four experiments]).
FIG. 6.
Effect of Ca2+ chelation and Ca2+ channel inhibitors on HMR 3647 uptake. (A) PMNs were incubated for 5 to 60 min in the presence of 1 mM EGTA, 5 mM Ni2+, 125 μM verapamil (VPL), or control HBSS. Results are means ± SEM for four to six experiments. ∗, P < 0.005 (ANOVA followed by Student’s t test for paired data). (B) PMNs were incubated for 5, 30, or 60 min in the presence of verapamil, 5 to 250 μM. Results are means ± SEM for three to seven experiments.
Intracellular location of HMR 3647.
The intracellular location of HMR 3647 was studied after 5 and 30 min of incubation. In the presence of 0.5% Triton X-100, sonication resulted in the breakage of all cytoplasmic and granular membranes, resulting in the release of about 97% of lysozyme, β-glucuronidase, and LDH in the supernatant. HMR 3647 was not strongly associated with the membrane pellet after 5 and 30 min (6% ± 1.5% and 7% ± 1.7% of the total amount of cell-associated drug, respectively). In contrast, in the granular pellet obtained in the presence of 0.73 M sucrose, as indicated by the release of less than 10% of granule enzymes and more than 95% of LDH in the supernatant, there was a strong accumulation of HMR 3647. Intragranular accumulation of this compound was maximal as early as the first 5 min (56% ± 10.9% of total intracellular HMR 3647, a value not significantly different from that obtained at 30 min, 59% ± 9.2%).
Efflux of HMR 3647.
HMR 3647 gradually egressed from loaded cells, with 17% ± 2.0% of total macrolide released at 5 min, 33% ± 2.0% at 30 min, and 45% ± 6.1% at 60 min. The release of the drug followed a linear regression curve: percent cell-associated HMR 3647 = −0.67(time in minutes) + 92.3 (P < 0.001; r, 0.875).
As already reported for HMR 3004, roxithromycin, and azithromycin, neither 1 mM EGTA nor 5 mM Ni2+ modified HMR 3647 efflux, whereas verapamil strongly increased it. After 5-min incubation in the presence of 125 μM verapamil, the percentage of cell-associated HMR 3647 was 41% ± 3.9% versus 75% ± 3.8% for the control (P, 0.001; three experiments).
Effect of HMR 3647 on PMN functions. (i) Exocytosis.
PMNs pretreated for 5 min with cytochalasin B were incubated for 30 to 180 min in the presence of ≤50 mg of HMR 3647 per liter, due to the cell toxicity observed at 100 mg/liter. Enzyme release was measured as described in Materials and Methods. HMR 3647 (50 mg/liter) slightly triggered the release of lysozyme and lactoferrin at 120 min (22% ± 1.5% [P, 0.042 versus control, 11% ± 0.9%; three experiments] and 42% ± 8.6% [P, 0.005 versus control, 21% ± 2.9%; three experiments], respectively) and 180 min (30% ± 2.6% [P, 0.008 versus 13 ± 0.4%; four experiments] and 42% ± 5.7% [P, 0.012 versus 26% ± 4.5%, respectively). By regression analysis, we found concentrations which resulted in 50% enzyme release of 181 mg/liter (P < 0.001; r, 0.940) and 127 mg/liter (P < 0.001; r, 0.819) for the release of lysozyme and 64 mg/liter (P, 0.001; r, 0.821) and 72 mg/liter (P, 0.005; r, 0.701) for the release of lactoferrin, at 120 and 180 min, respectively. In contrast, at concentrations of ≤100 mg/liter, HMR 3647 did not induce the release of β-glucuronidase.
(ii) Oxidative metabolism.
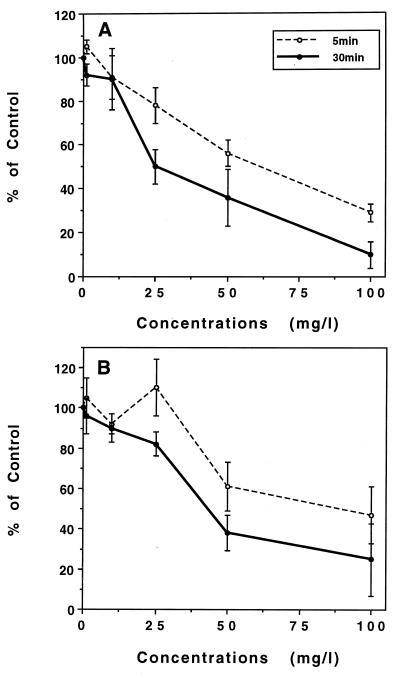
HMR 3647 moderately impaired oxidant production by PMNs in a time- and concentration-dependent manner (Fig. 7). The inhibitory effect was similar whatever the stimulus (FMLP [Fig. 7A] or PMA [Fig. 7B] at HMR 3647 concentrations of ≥50 mg/liter. At the lowest concentrations, the inhibition for the FMLP-induced response was stronger than that for the PMA-induced response. Logarithmic regression analysis gave IC50 of 55 mg/liter (67 μM; P < 0.001; r, 0.825) and 30 mg/liter (36 μM; P < 0.001; r, 0.806) for FMLP stimulation after incubation for 5 and 30 min, respectively, and 117 mg/liter (143 μM; P, 0.008; r, 0.553) and 44 mg/liter (54 μM; P < 0.001, r, 0.745) for PMA stimulation after 5 and 30 min of incubation, respectively.
FIG. 7.
Effect of HMR 3647 on the oxidative response of PMNs. PMNs were incubated for 5 or 30 min with HMR 3647 (1 to 100 mg/liter) before stimulation with FMLP (5 × 10−6 M) (A) or with PMA (100 ng/ml) (B). Results are mean percentages of control responses ± SEM (five or six experiments). Control superoxide anion production levels (in nanomoles per 107 PMNs per minute) were 94 ± 6.1 (5 min) or 38 ± 4.8 (30 min) for FMLP and 46 ± 6.3 (5 min) or 24 ± 5.5 (30 min) for PMA.
DISCUSSION
A major property of macrolides, which largely underlies their use in infections caused by intracellular pathogens, is their ability to enter and concentrate within host cells (17, 19). In addition, the cellular accumulation of macrolides may modify cell functions. This phenomenon is particularly well documented in the case of phagocytes (16, 25) and could, partly at least, contribute to the anti-inflammatory activity of these antibiotics (18, 25). It is thus of particular interest to understand how macrolides accumulate within host cells and the chemical structures involved, in order to propose chemical innovations aimed at increasing the antibacterial activity of these drugs and/or their anti-inflammatory potential. We recently proposed a preliminary classification of erythromycin A-derived macrolides according to their cellular pharmacokinetic profile in human neutrophils (e.g., accumulation kinetics, cellular location, efflux) and their structure (mono-dibasic compounds) (25). We further extended this classification by studying HMR 3004 (RU 64004), which belongs to a new subgroup of erythromycin A derivatives, the ketolides, which possess a 3-keto group in place of the l-cladinose (8). HMR 3004 displays a peculiar cellular kinetic profile, with some aspects characteristic of group 1 (dibasic) drugs, e.g., granular location and moderate efflux, and others characteristic of group 2 (monobasic) drugs such as the saturable accumulation process, although it was far more strongly accumulated than the other macrolides (35). In addition, whereas extracellular Ca2+ is required for optimal uptake of all macrolides (30, 35), verapamil, the l-type Ca2+ channel inhibitor, exerted different effects on the 5-min uptake of group 1 compounds (increased accumulation) and HMR 3004 and group 2 compounds (decreased accumulated (35). The accumulation process of all erythromycin A derivatives appeared to be mediated by a common carrier system, with HMR 3004 having the highest affinity for the carrier (Km, two- to threefold lower than those obtained with azithromycin and roxithromycin). It was thus interesting to analyze whether the 3-keto group confers peculiar uptake characteristics. We studied a new ketolide, HMR 3647, whose chemical structure differs slightly from that of HMR 3004, by the nature of the substituents linked to the C-11–C-12 carbamate ring (Fig. 1). HMR 3647 was gradually accumulated by PMNs, without saturation, over a 3-h incubation period (Fig. 2), a profile similar to that of azithromycin but differing from that of HMR 3004 (34). As already reported with other macrolides (27, 29, 35), there was strong interindividual variability with regard to cellular kinetics of accumulation (Fig. 2). Another characteristic shared with azithromycin was the high activation energy (128 kJ/mol), whereas group 2 macrolides and HMR 3004 have somewhat lower activation energy (35). However, at variance with group 1 macrolides, HMR 3647 uptake was little influenced by variations in the extracellular pH (Fig. 3). Despite strong intragranular accumulation (about 60% of total cellular drug), HMR 3647 was gradually released from loaded cells placed in drug-free medium. It was interesting that Ni2+ and EGTA impaired HMR 3647 accumulation but not its efflux, a feature common to all erythromycin A derivatives, whether or not they have an l-cladinose substituent (30, 35). As already observed with azithromycin, verapamil exerted a dual effect: it rapidly (5 min) increased HMR 3647 uptake, and this was followed by significant gradual inhibition (Fig. 6), an effect probably related to increased drug efflux. Despite reports suggesting an active entry process of macrolides (13, 26, 35), the underlying mechanism is still poorly understood. No carrier system has yet been identified, although preliminary data from our group have demonstrated that protein kinase A-dependent phosphorylation is required for macrolide uptake (24). The possibility that HMR 3647 was taken up by an active transport system was suggested by several observations. At low temperatures, 4 and 22°C, when the cells are metabolically inactive, HMR 3647 uptake was insignificant, although it may be argued that the lipophilic membrane structure was altered in these conditions (15). The failure of metabolic inhibitors, particularly fluoride (an inhibitor of anaerobic glycolysis, i.e., the essential ATP production pathway in PMNs) to reduce HMR 3647 uptake could be due to the use of low concentrations of inhibitors to preserve cell viability in our system. The interindividual variability observed with HMR 3647 and other macrolides (27, 29, 35) is intriguing. The PMN samples of various volunteers have now been controlled over a 3-year period, and they conserve the same characteristics whatever the macrolide, i.e., rapid or slow accumulation of the drugs. Such interindividual variability was not observed with antibacterial agents whose uptake is mainly passive (e.g., some quinolones [36]). The reason(s) for this variability is unknown, but the existence of a carrier protein whose number and/or activity may vary among individuals is one possible explanation. The essential feature which characterizes active processes was the observed saturation kinetics of drug uptake (Fig. 5). The Km of HMR 3647 (about 144 μM) was slightly higher than that obtained previously with azithromycin and roxithromycin (62 to 108 μM) and HMR 3004 (20 μM) (35), indicating a lower affinity for the carrier than with the other macrolides. In addition, the fact that various macrolides impaired the uptake of HMR 3647 in a competitive manner, whereas other types of antibacterial agents and competitive inhibitors of carrier systems on the PMN membrane were ineffective, strongly supports the existence of a macrolide carrier in PMNs.
A major argument for the existence of an active process in erythromycin A derivative uptake by neutrophils comes from a previous report that extracellular Ca2+, and particularly its entry via the Na+-Ca2+ exchanger, is required for maximal uptake by neutrophils (29), while macrolide efflux does not require extracellular Ca2+ or a functional Na+-Ca2+ exchanger. The data reported here confirm this work in the case of HMR 3647. These results strongly suggest that Na+-Ca2+ exchanges are required for cellular entry of all macrolides derived from erythromycin A. To our knowledge, the influence of Ca2+ on the uptake of 16-membered-ring macrolides has not been envisaged but would help to understand the similarities and differences among members of the macrolide family.
We have recently proposed that efflux of erythromycin A-derived macrolides also depends on an active transport system which is greatly potentiated by verapamil (35). Besides its effect on l-type Ca2+ channels (14), whose existence has not been demonstrated in the cytoplasmic membrane in neutrophils, verapamil has many effects including inhibition of various protein kinases and elevation of cyclic AMP (10). Also, verapamil has been shown to reverse the multiple drug resistance phenotype of various tumor cells by acting on a membrane pump, a protein belonging to the P-glycoprotein (PgP) family involved in the active efflux of various hydrophobic compounds (11). Some macrolides, as well as the macrolidic immunosuppressants FK 506 and rapamycin, have been shown to interfere with PgP activity in various cell lines (6, 9). These results suggest that macrolides might also use this PgP system either to enter or to egress from PMNs. As already observed with other macrolides (35), the verapamil-mediated increase in HMR 3647 efflux was stronger than the decrease in drug accumulation: the accumulation of HMR 3647 was almost doubled at 5 min in the presence of 125 μM verapamil, whereas efflux was significantly stimulated over the same incubation time. This suggests that verapamil could have a stimulating effect on the entry mechanism of macrolides.
As HMR 3647 is highly concentrated in PMNs, we further analyzed whether this drug could also modify PMN functions. We have recently demonstrated that l-cladinose, the neutral sugar linked at C-3 of the lactone ring, is critical for the effect of erythromycin A derivatives on PMN oxidative metabolism and exocytosis (1). Only l-cladinose-bearing macrolides both triggered granule exocytosis and impaired the oxidative response of PMNs. However, we have observed that HMR 3004 impairs oxidant production by PMNs (2), an effect probably related to the presence of a quinoline ring, a structure also present in various antimalarials which inhibit oxidant production by PMNs (20, 22, 33) and also induce PMN exocytosis (12). Unpublished observations by our group show that HMR 3004 is also able to promote PMN degranulation. In this paper we demonstrate that HMR 3647 also altered PMN functions. High concentrations and long incubation times were required to moderately trigger specific (not azurophilic) granule exocytosis. However, HMR 3647 inhibited the oxidative response of PMNs (Fig. 7). The inhibition was time and concentration dependent, with an IC50 slightly lower than that reported with erythromycin A derivatives (1, 2). These data confirm that, whereas l-cladinose confers peculiar properties to macrolides with regard to interference with the functional activities of PMNs, other substituents are likely able to modify the properties of erythromycin A-derived macrolides (21). The mechanism which underlies the inhibitory property of HMR 3647 is being investigated. The clinical relevance of these data depends first on the concentrations of this drug in serum and tissue and second on the possibility that HMR 3647-mediated inhibition of superoxide production also impairs the bactericidal activity of these cells. There are no published data on the pharmacokinetic profile of HMR 3647. Tissue drug concentrations should be in the same range as those observed with various macrolides; in this case, concentrations of 30 to 40 mg/liter (the IC50 calculated here) may be reached in tissues, after a prolonged administration. Whether and to which extent the inhibition of oxidant production by this drug also impairs the bactericidal activity of PMNs, which have many compensatory mechanisms (particularly natural antibiotic peptides and proteins), should, however, be investigated.
In summary, HMR 3647, a new ketolide, gradually enters and accumulates within PMNs, by using a transport system which seems to be common to all macrolides. It also impairs oxidant production by PMNs in a time-dependent manner. The presence of a 3-keto group instead of an l-cladinose neither changed the cellular pharmacokinetic profile nor interfered with the functional activities of PMN. Rather, the various substituents on the erythronolide ring may confer different affinities for the carrier system(s) involved in entry and efflux of macrolides and for various intracellular targets in PMN signalling pathways.
ACKNOWLEDGMENT
This work was supported in part by a grant from Hoechst-Marion-Roussel.
REFERENCES
- 1.Abdelghaffar H, Vazifeh D, Labro M T. Erythromycin A-derived macrolides modify the functional activities of human neutrophils by altering the phospholipase D-phosphatidate phosphohydrolase transduction pathway. J Immunol. 1997;159:3995–4005. [PubMed] [Google Scholar]
- 2.Abdelghaffar H, Labro M T. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Effect of RU 64004 on oxidant production by human neutrophils, abstr. F-225; p. 139. [Google Scholar]
- 3.Abdelghaffar H, Mtairag E M, Labro M T. Effects of dirithromycin and erythromycylamine on human neutrophil degranulation. Antimicrob Agents Chemother. 1994;38:1548–1554. doi: 10.1128/aac.38.7.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agouridas C, Bonnefoy A, Chantot J F. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro antibacterial activity of HMR 3647, a novel ketolide highly active against respiratory pathogens, abstr. F-112; p. 165. [Google Scholar]
- 5.Araujo-Khan F, Bryskier A, Remington J. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Ketolides are active against Toxoplasma gondii, abstr. F-251; p. 188. [Google Scholar]
- 6.Arceci R J, Stieglitz K, Bierer B E. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood. 1992;80:1528–1536. [PubMed] [Google Scholar]
- 7.Bergmeyer H U, Bernt E. Lactate dehydrogenase. In: Bergmeyer H U, editor. Methods in enzymatic analysis. New York, N.Y: Academic Press, Inc.; 1963. pp. 737–739. [Google Scholar]
- 8.Bryskier A, Agouridas C, Chantot J F. Ketolides: new semisynthetic 14-membered-ring macrolides. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides, and streptogramins. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 39–50. [Google Scholar]
- 9.Crosta L, Candiloro V, Meli M, Tolomeo M, Rausa L, Dusonchet L. Lacidipine and josamycin: two new multidrug resistance modulators. Anticancer Res. 1994;14:2685–2690. [PubMed] [Google Scholar]
- 10.Della Bianca V, Greskoviak M, De Togni P, Cassatella M, Rossi F. Inhibition by verapamil of neutrophil responses to formyl methionyl leucyl phenylalanine and phorbol myristate acetate. Mechanisms involving Ca2+ changes, cyclic AMP and protein kinase C. Biochim Biophys Acta. 1985;845:223–236. doi: 10.1016/0167-4889(85)90180-6. [DOI] [PubMed] [Google Scholar]
- 11.Endicott J A, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 12.Fontagne J, Roch-Arveiller M, Giroux J P, Lechat P. Effects of some antimalarials on rat inflammatory polymorphonuclear leukocyte function. Biomed Pharmacother. 1989;43:43–51. doi: 10.1016/0753-3322(89)90190-x. [DOI] [PubMed] [Google Scholar]
- 13.Hand W L, King-Thompson N, Holman J W. Entry of roxithromycin (RU 965), imipenem, cefotaxime, trimethoprim, and metronidazole into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987;31:1553–1557. doi: 10.1128/aac.31.10.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry P D. Comparative pharmacology of calcium antagonists: nifedipine, verapamil and diltiazem. Am J Cardiol. 1980;46:1047–1058. doi: 10.1016/0002-9149(80)90366-5. [DOI] [PubMed] [Google Scholar]
- 15.Koga H. High-performance liquid chromatography measurement of antimicrobial concentrations in polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987;30:137–142. doi: 10.1128/aac.31.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labro M T. Effect of macrolides on host natural defenses. In: Bryskier A, Butzler J P, Neu H C, Tulkens P M, editors. Macrolides: chemistry, pharmacology and clinical use. Paris, France: Arnette-Blackwell; 1993. pp. 389–408. [Google Scholar]
- 17.Labro M T. Intraphagocytic penetration of macrolide antibiotics. In: Bryskier A, Butzler J P, Neu H C, Tulkens P M, editors. Macrolides: chemistry, pharmacology and clinical use. Paris, France: Arnette-Blackwell; 1993. pp. 379–388. [Google Scholar]
- 18.Labro M T. Immunomodulatory action of antibacterial agents. Clin Immunother. 1996;6:454–464. [Google Scholar]
- 19.Labro, M. T. 1996. Intracellular bioactivity of macrolides. Clin. Microbiol. Infect. 1(Suppl. 1):24–30. [DOI] [PubMed]
- 20.Labro M T, Babin-Chevaye C. Effects of amodiaquine, chloroquine, and mefloquine on human polymorphonuclear neutrophil function in vitro. Antimicrob Agents Chemother. 1988;32:1124–1130. doi: 10.1128/aac.32.8.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labro M T, Abdelghaffar H, Kirst H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Structure-activity relationships among erythromycylamine derivatives for their effects on human neutrophils in vitro, abstr. F-268; p. 191. [Google Scholar]
- 22.Labro M T, El Benna J. Effect of monodesethyl amodiaquine on human polymorphonuclear leukocyte functions in vitro. Antimicrob Agents Chemother. 1991;35:824–830. doi: 10.1128/aac.35.5.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labro M T, Vazifeh D, Abdelghaffar H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Effect of the new ketolide HMR 3647 on human neutrophil function in vitro, abstr. F-257; p. 189. [Google Scholar]
- 24.Labro M T, Abdelghaffar H, Vazifeh D, Bryskier A. Program and abstracts of the 7th International Congress for Infectious Diseases. 1996. Roxithromycin uptake by human neutrophils is mediated by a protein kinase A-dependent mechanism, abstr. 110-018; p. 278. [Google Scholar]
- 25.Labro M T. Effects of macrolides on leukocytes and inflammation. In: Zinner S H, Young L S, Acar J F, editors. Expanding indications of the new macrolides, azalides and streptogramins. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 101–116. [Google Scholar]
- 26.Laufen H, Wildfeuer A, Lach P. Kinetics of the uptake of antimicrobial agents by human polymorphonuclear leucocytes. Arzneim Forsch. 1989;39:233–235. [PubMed] [Google Scholar]
- 27.Laufen H, Wildfeuer A, Lach P. Mechanism of azithromycin uptake in human polymorphonuclear leucocytes. Arzneim Forsch. 1990;40:686–689. [PubMed] [Google Scholar]
- 28.Litwack G. Photometric determination of lysozyme activity. Proc Soc Exp Biol Med. 1955;89:401–403. doi: 10.3181/00379727-89-21824. [DOI] [PubMed] [Google Scholar]
- 29.Mtairag E M, Abdelghaffar H, Labro M T. Investigation of dirithromycin and erythromycylamine uptake by human neutrophils in vitro. J Antimicrob Chemother. 1994;33:523–536. doi: 10.1093/jac/33.3.523. [DOI] [PubMed] [Google Scholar]
- 30.Mtairag E M, Abdelghaffar H, Douhet C, Labro M T. Role of extracellular calcium in in vitro uptake and intraphagocytic location of macrolides. Antimicrob Agents Chemother. 1995;39:1676–1682. doi: 10.1128/aac.39.8.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roblin P M, Hammerschlag M R. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro activity of a new ketolide antibiotic, HMR 3647, against Chlamydia pneumoniae, abstr. F-248; p. 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith R P, Baltch A L, Ritz W, Franke M, Michelsen P. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Antibacterial effect of ketolides HMR3004 and HMR3647 against intracellular Legionella pneumophila, abstr. F-249; p. 188. [Google Scholar]
- 33.Styrt B, Klempner M S. Inhibition of neutrophil oxidative metabolism by lysomotropic weak bases. Blood. 1986;67:334–342. [PubMed] [Google Scholar]
- 34.Talalay P, Fishman W H, Huggins C. Chromogenic substrate. II. Phenolphthalein glucuronic acid as substrate for the assay of glucuronidase activity. J Biol Chem. 1946;166:756–772. [PubMed] [Google Scholar]
- 35.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the new ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vazifeh D, Bryskier A, Labro M T. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Investigation of the mechanism underlying levofloxacin uptake by human neutrophils, abstr. A-74; p. 15. [Google Scholar]