Abstract
Background
Aedes albopictus and Aedes aegypti are known for their potential as vectors of dengue (DENV) and chikungunya (CHIKV) viruses. However, entomological surveys are mostly carried out during epidemics. In Gabon where outbreaks of both viruses have occurred, there is no vector control program targeting these arboviruses. Therefore, we assessed the presence of Aedes species along a rural–urban gradient in Lambaréné (Gabon) and its surroundings and determined ecological factors associated to their presence.
Methods
An entomological survey was conducted in Lambaréné and its surrounding rural areas. Mosquitoes were collected with aspirators around human dwellings, and ecological and environmental data were collected from each study area. Morphological identification keys were used to identify Aedes species. RNA was extracted from pools of female mosquitoes and amplified by RT-qPCR to detect the presence of DENV and CHIKV.
Results
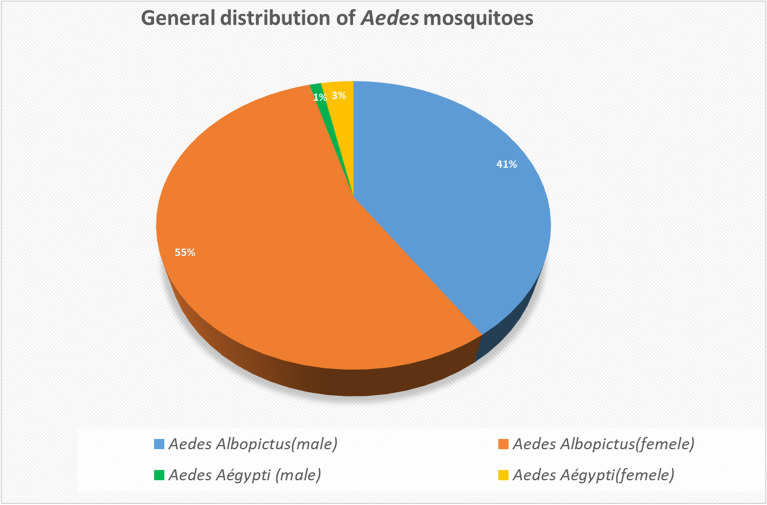
Overall, the most common vector collected was Aedes albopictus (97%, 4236/4367 specimens), followed by Aedes aegypti (3%, 131/4367). Albopictus vectors was more abundant in the rural area (Wilcoxon signed-rank test, Z = 627, P = 0.043) than in the urban area. In the urban area, a higher number of mosquitoes (45%) were recorded in the economic zone (zone 3) than in the historical and administrative zones (zone 1 and 2). In the rural area, the proportions of species numbers were significantly higher along the south rural transect (92%) compared to the north rural transect (Wilcoxon signed-rank test, Z = 43, P ˂ 0.016). We also noted a high abundance of vectors in environments characterized by monocultures of Hevea brasiliensis (Hevea) and Manihot esculenta (cassava) (Kruskal–Wallis H-test, H = 25.7, df = 2, P < 0.001). Finally, no mosquito pools were positive for either DENV or CHIKV.
Conclusion
Aedes albopictus was the dominant vector across the study sites due to its high invasiveness capacity. This presence re-affirms the potential for local transmission of both DENV and CHIKV, as indicated previously by serological surveys conducted in our study area, even though no transmission was detected during the current study. These findings underscore the need for regular arbovirus surveillance in the study region, with the aim of supporting vector control efforts in the event of outbreaks.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-023-05901-2.
Keywords: Aedes. albopictus, Aedes. aegypti, Hevea brasiliensis, Rural area, Urban area, Lambaréné, Gabon
Background
Dengue and chikungunya are two widely distributed arboviral diseases that cause acute episodes characterized by symptoms such as fever and joint pain [1–3]. The major vectors of transmission are mosquitoes of the genus Aedes. Until recently, Aedes aegypti was considered to be the main epidemic vector for both dengue virus (DENV) and chikungunya virus (CHIKV) [4, 5], but in recent years the invasive species Aedes albopictus has been identified as the major vector during outbreaks in Asia and Africa [6]. Aedes albopictus, a species originating from Southeast Asia, was recently introduced in Africa, the Americas, Australia and Europe [7, 8] where in some cases it has gradually replaced the local species, Ae. aegypti [9–11].
In Gabon, a country located in Central Africa, Ae. albopictus was first reported in 2006 in the city of Port-Gentil [12]. Its presence coincided with the occurrence of concomitant outbreaks of DENV serotype 2 (DENV2) and CHIKV. During these outbreaks, hundreds of patients were infected with both DENV2 and CHIKV in different towns of Gabon, with eight cases of co-infections reported [13–16]. Aedes albopictus was identified as the main vector species during this outbreak based on its higher prevalence compared to other vector species, such as Ae. aegypti and Aedes simpsoni, and the highest infection rates for both DENV2 and CHIKV, including one record of an Ae. albopictus positive for both CHIKV and DENV2 [17]. Aedes albopictus was also identified as the main vector species for CHIKV in a remote village of Gabon (Dangui). These studies demonstrated the spread of this mosquito species and its potential to drive arbovirus epidemics across the country [18].
Little data are available on these two arboviruses during inter-epidemic periods in Gabon, particularly in terms of the vectors involved in their transmission. Recent seroprevalence surveys in Moyen Ogooué province revealed a seroprevalence of 23% and 62% for DENV and CHIKV, respectively, among samples collected from 462 patients between 2014 and 2017 [19], while Lim et al. [20] reported a seroprevalence of 17.4% for DENV in Lambaréné for samples collected from 682 patients between 2015 and 2016. During this period, the presence of both DENV2 and DENV serotype 3 (DENV3) was confirmed by reverse transcription-quantitative PCR (RT-qPCR) in human samples [21].
These seroprevalences suggest the circulation of these arboviruses in the region and raise questions about the drivers of this transmission during non-epidemic periods. Assuming that DENV and CHIKV were preferentially transmitted by Ae. albopictus during past epidemics [22], we hypothesize that this vector is also responsible for the maintenance and circulation of viral strains in Moyen Ogooué province. Little information is currently available on the distribution of these vectors, or on the ecological factors favoring their expansion, thereby increasing the risk of future outbreaks, especially in the absence of vector control interventions against these vectors. This study therefore aims to assess the presence of Aedes mosquito species in Lambaréné and its surroundings to determine the ecological factors that influence their distribution range and the possible implication of these mosquito vectors in the spread of diseases.
Methods
Study areas
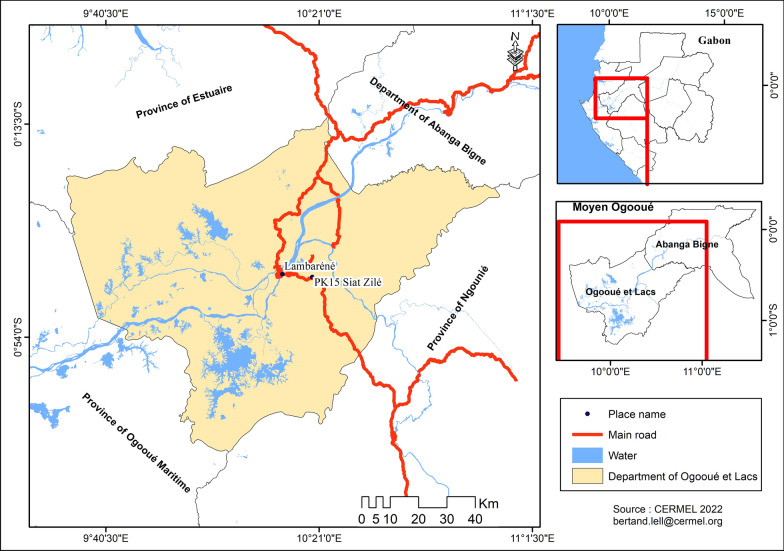
The study was carried out in different areas of Moyen Ogooué province which is located in the center of Gabon. The main city in the province is Lambaréné, located 250 km south of the capital Libreville, on the banks of the Ogooué river. Lambaréné has a population of 38,775 inhabitants [20] and is flanked by two major agricultural areas, with palm tree plantations 56 km north of the city and rubber tree plantations about 25–30 km south of the city (Fig. 1).
Fig. 1.
Map of Moyen Ogooué region showing the geographical location of Lambaréné
Gabon is subjected to four seasons annually, two dry and two rainy. Recently, however, changes in the climate have resulted in changes the rain patterns, with heavy rains recorded during months of dry seasons. Therefore, for this study we defined rainy seasons as those periods with monthly precipitation > 200 mm (October–December and March–June) and the dry seasons as periods with monthly rainfalls of < 200 mm (January–February and July–September).
Mosquito collection
The mosquitoes were collected in Lambaréné (urban) and its surroundings areas (rural) from November 2019 to March 2021 during repetitive cross-sectional surveys across multiple consecutive (wet and dry) seasons. Rural and urban areas were classified according to specific community conditions, such as the lack/absence (rural) or presence (urban) of public administrative structures and infrastructure. Lambaréné, the urban area, is the provincial capital with all administrative structures, including three referral hospitals, businesses, supermarkets, secondary-level schools, police stations, town hall, governor's office and modern water and electricity supply facilities. In contrast, the surrounding areas were defined as rural areas, characterized by the absence of almost all of the above mentioned structures and infrastructure.
In the rural area, collections were performed along two transects: Lambaréné-Makouké north towards Libreville (rural zone 1 [Rz1]) and Lambaréné—PK30 south towards Fougamou (rural zone 2 [Rz2]).
For the urban collections in Lambaréné, collection sites were divided into three zones separated by the natural physical barrier of the Ogooué river that divides the town into three distinct areas: zone 1 (historical zone, around the Albert Schweitzer museum); zone 2 (administrative zone, home to the main administration facilities); zone 3 (economic zone, characterized by trading and industrial activities). Each area in each zone was divided into neighborhoods. Capture sessions were performed in each village and neighborhood in areas covering a radius of 50 to 100 m and identified as conducive to the presence of Aedes spp. according to information reported in the literature (presence of gutters, trees, shaded areas in the vicinity of human dwellings).
Mosquitoes were collected using electrical aspirators (Rule 4″ In-Line Blower, 240 V, 7.0 amperes). The collections were carried out in open air in the morning from 6:00 a.m. to 10:00 a.m. in a discontinuous manner around human dwellings by two collectors who generally were separated from each other by 10 to 20 m. The capture points were not fixed in the area, and the collectors captured mosquitoes within the pre-defined radius. Mosquitoes were caught either sitting on the foliage of flowers and grasses or flying around the collector. Microhabitats with shade and dead leaves on the ground were favored for the capture of mosquitoes. All mosquitoes collected within one collection session by each collector were grouped in the same cage with access to a 10% sugar solution and then transferred to the Medical Entomology Laboratory of the Centre de Recherches Médicales de Lambaréné (CERMEL). Mosquito collections were carried out at least twice per week at each study site. Geographic information system (GIS) data were recorded for each collection site using the Global Positioning System (GPS) Essentials GPS tool (version 2.0). The geographical coordinates (latitudes and longitudes) of the recorded areas were exported in GPX 1.1 format and saved in QGIS software for mapping.
Ecological and environmental data were also collected, including information on habitat, presence of water access points, vegetation type, type of crops, breeding site, presence of fruit trees (such as wild apples and mangoes on which mosquitoes feed on sugar when the fruit is ripe), human activity and climatic conditions during the collection.
Identification of Aedes vectors
The mosquitoes were identified morphologically using a Zeiss Stemi 508 stereo microscope equipped with a binocular lens (Carl Zeiss AG,Oberkochen, Germany)) and the identification keys from Leopoldo [23]. All collected mosquitoes identified as Aedes spp. were grouped into separate pools of 15–20 male and female Ae. aegypti or Ae. albopictus mosquitoes. These mosquito pools were transferred into 2-ml Eppendorf tubes (Eppendorf AG, Hamburg, Germany) containing RNAlater solution (Thermo Fisher Scientific, Waltham, MA, USA) and stored at − 80 °C until further processing
Viral RNA extraction and detection by RT-qPCR
Female mosquitoes of both Aedes spp. were homogenized using the protocol from Frentiu et al. [24] with a slight modification. The pools of Aedes spp. were transferred into tubes containing 2-mm beads (Lysing Matrix Z; MP Biomedicals, Santa Ana, CA, USA) and ground in phosphate-buffered saline (PBS) a FastPrep-24™ 5G homogenizer (MP Biomedical). RNA was then extracted using the Qiagen RNA Mini Kit following the manufacturer’s instructions (Qiagen, Hilden, Germany).
We used previously described primer and probe sequences from Santiago et al. [25]. For the detection of DENV, we performed a multiplex RT-qPCR that detects the four DENV serotypes (DENV1, DENV2, DENV3, DENV4). RT-qPCR was also used for the detection of CHIKV. The RT-qPCR reactions were performed in a 20-µl reaction mixture using a 2× One-Step PrimeScript RT-PCR kit (Takara Bio., Kusatsu, Japan). Each reaction mix contained 10 µl of 2× One-Step PrimeScript RT-qPCR Mix, 10 µM of Primer Probe mix (primer plus probe), 0.2 µl of a passive Rox fluorochrome, pure water without RNase and 2 µl of RNA template. The RT-qPCR was performed using a LightCycler 480 Instrument II PCR platform (Roche Applied Science, Penzberg, Germany) at cycling conditions of 5 min at 52 °C; 10 s at 95 °C and 45 cycles of 5 s at 95 °C and 35 s at 60 °C. Samples with cycle threshold (Ct) values ≤ 40 were considered to be positive.
Statistical analysis
The overall distribution of vectors was determined by the frequencies of the different vectors in the study area using the formula % = (A/ N) × 100, where A is the number of Aedes mosquitoes per site collected and N is the total population of vectors collected.
The Capture Effort Index (CEI), defined as the number of mosquitoes caught per collector per hour (m/c/h), was used to determine vector abundance. This index was calculated over four parameters, including A (number of Aedes mosquitoes per site), F (frequency of site visits), C (number of collectors) and D (duration of each collection session according to the following formula: CEI = A/(F × C × D). The difference in vector abundance was assessed by comparing the median CEI in each zone or environment using the Wilcoxon signed-rank test and Krustal-Wallis test. Interquartile range (IQR) was taken into consideration to account for the asymmetric distribution of our data in the study. As the data collected were count data, we performed a multiple linear regression to determine the association between various ecological factors (access to water, vegetation type, type of crops, breeding site and fruit tree) on the outcome (abundance of vectors) in each study area. All analyses were performed using R software, and the significance level was set at α ˂ 0.05.
Results
A total of 4367 Aedes mosquitoes were collected in the study areas. The number of mosquitoes collected ranged from two to 1042 by site. The median number of mosquitoes collected was 50. Aedes albopictus represented approximately 97% (4236/4367) of vectors collected, with the remaining 3% (131/4367) comprising Ae. aegypti.
Vector distribution by CEI in the study areas
Aedes albopictus was present in roughly equal proportions in the rural and urban areas, with CEI of 110.5 m/c/h in the rural area and CEI of 122 m/c/h in the urban area (Table 1). The median CEI of Ae. albopictus by collection site in the rural area (3.6 m/c/h, IQR 0.2–9.9 m/c/h) differed significantly from that in the urban area (0.5 m/c/h, IQR 0–3.5 m/c/h) (Wilcoxon signed-rank test, Z = 627, P = 0.043); in contrast, Ae. aegypti was only found in urban areas.
Table 1.
Distribution of Aedes species across the study areas
| Study areas/zones | Aedes spp. | Aedes albopictus | Aedes aegypti | |||||
|---|---|---|---|---|---|---|---|---|
| Total N (%) | Total CEI | CEI | Median (IQR) | Statistic test | CEI | Median (IQR) | Statistic test | |
| Study areas | ||||||||
| Rural | 2028 (47) | 110.5 | 110.5 | 3.6 (0.2–9.9) | Z = 627, P = 0.043 | 0 | 0 (0–0) | Z = 406, P = 0.15 |
| Urban | 2323 (53) | 124.9 | 122 | 0.5 (0–3.5) | 3 | 0 (0–0) | ||
| Urban zones | ||||||||
| LBN-zone 1 (historical zone) | 493 (21) | 13.6 | 10.8 | 0.33 (0–0.8) | H = 1.65, df = 2, P = 0.44 | 3 | 0 (0 -0.2) | H = 324, df = 2, P ˂ 0.001 |
| LBN-zone 2 (administrative zone) | 796 (34) | 46.7 | 46.6 | 0.9 (0–4.4) | 0.1 | 0 (0–0) | ||
| LBN-zone 3 (economic zone) | 1034 (45) | 64.6 | 64.6 | 1.5 (0–4.8) | 0 | 0 (0–0) | ||
| Rural zones | ||||||||
| PK-LBN-route south FGM (Rz2) | 1871 (92) | 100.7 | 100.7 | 10 (8–17) | Z = 43, P ˂ 0.016 | 0 | 0 (0–0) | - |
| PK-LBN-route north LBV (Rz1) | 157 (7.7) | 9.8 | 9.8 | 0 (0–2) | 0 | 0 (0–0) | ||
CEI Capture Effort Index, FGM Fougamou, IQR inter-quartile range, LBN Lambaréné, LBV Libreville, PK route PK30, Rz1, Rz2 rural zones 1, 2
In the rural area, the collection of Aedes mosquitoes along the transects (zones) where the collections were carried out revealed that there was a higher abundance of Ae. albopictus (CEI 100.7 m/c/h) in villages located in Rz2 compared to Rz1 (CEI 9.8). Aedes albopictus vectors were significantly more abundant (Wilcoxon signed-rank test, Z = 43, P ˂ 0.016) in Rz2 (median 10 m/c/h, IQR 8–17 m/c/h) than in Rz1 (median 0 m/c/h, IQR 0–2 m/c/h). The distribution of Ae. albopictus along these two transects was heterogeneous, with this vector more prevalent in villages such as Zilé for the Lambaréné-PK30-Fougamou transect (Rz2) and Bindo for the Lambaréné-Makouké transect (Rz1).
In the three distinct zones of the urban area, the distribution of Aedes species showed that urban zone 3 (economic) had the highest CEI of Ae. albopictus (CEI 64.6 m/c/h) followed by urban zone 2 (CEI 46.6 m/c/h) and then by urban zone 1 (CEI 10.8 m/c/h) (Table 1). Despite this difference, the abundance of Ae. albopictus in these three zones was essentially the same, with no statistically significant difference (Kruskal–Wallis H-test, H = 1.65, df = 2, P = 0.44) between urban zone 3 (median 1.5 m/c/h, IQR 0.0–4.8 m/c/h) compared to urban zone 2 (median 0.9 m/c/h, IQR 0.0–4.4 m/c/h) and urban zone 1 (median 0.3 m/c/h, IQR 0.0–0.8 m/c/h).
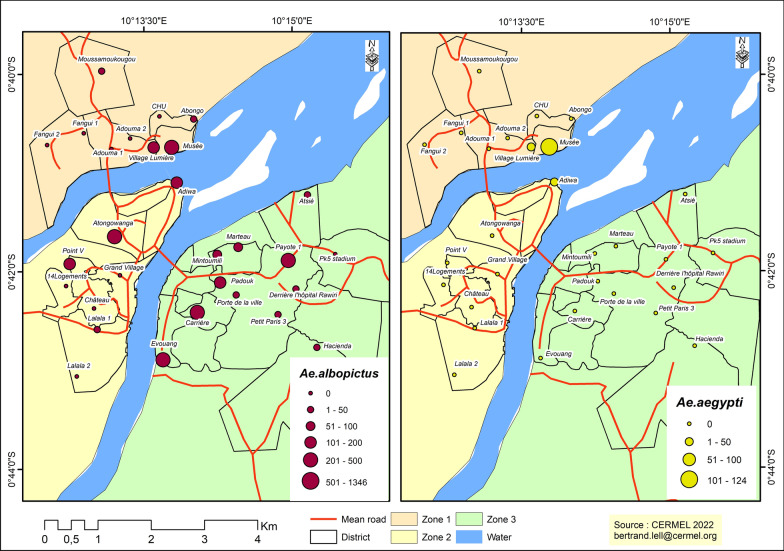
Figure 2 shows the spatial distribution of vectors in the three different urban zones. Aedes albopictus vectors were clearly more abundant in urban zone 3 with at least four neighborhoods (Carriere, Evouang, Paillote, Padoouk) in which a minimum of 201 mosquitoes were collected by site. However, two other neighborhoods, in urban zone 2 and 1, respectively, had comparable mosquito numbers. Although poorly represented, Ae. aegypti mosquitoes were mainly found in the Museum (n = 124) district in urban zone 1 and sporadically in the surrounding districts (Lumière) with fewer individuals (n = 6).
Fig. 2.
Distribution of Aedes albopictus (left) and Aedes aegypti (right) caught from sampling sites in urban areas
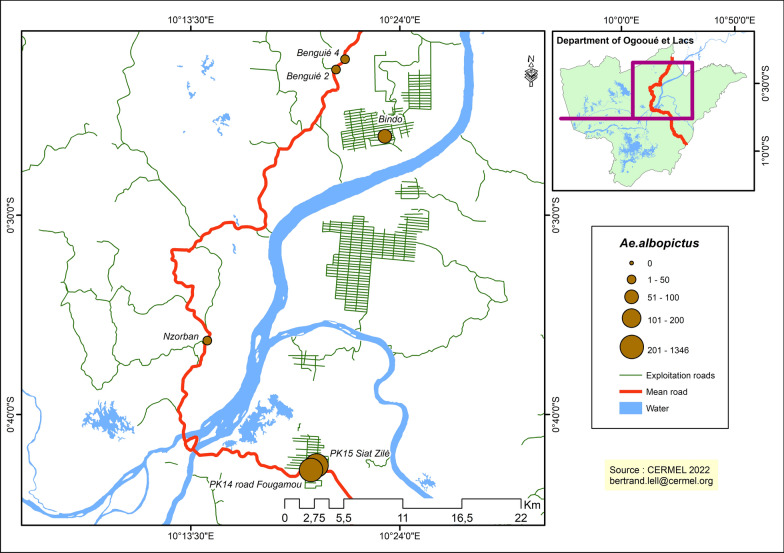
The data reported in Fig. 2 also show that the spread of Aedes mosquitoes in the city remains heterogeneous although, importantly, sites with high recorded numbers of mosquitoes were located in areas near the Ogooué river and its tributaries. By contrast, in rural areas (Fig. 3), the spread of Ae. albopictus vector populations was localized in specific agricultural sites, such as rubber plantations along the Lambaréné-PK30-Fougamou transect (Rz2), with a minimum of 501 mosquitoes by collection site.
Fig. 3.
Distribution of Aedes albopictus in rural zones
Influence of ecological factors on the abundance of vectors
The distribution of vectors was also evaluated according to a set of defined ecological and environmental factors, such as season, access to various types of water sources, type of vegetation, crops grown, presence of fruit trees and presence and quality of breeding sites.
Aedes mosquitoes were collected in both the dry (CEI 87.9 m/c/h) and rainy seasons (CEI 182.4 m/c/h) (Table 2). Despite the high CEI in the rainy season, there was no difference in the abundance of Ae. albopictus between seasons (Wilcoxon signed-rank test, Z = 837.5, P = 0.47), with a comparable median number of Ae. albopictus captured during the dry season (CEI: median 1.0 m/c/h, IQR 0.0–5.6 m/c/h) and the rainy season (CEI: median 0.5 m/c/h, IQR 0.0–3.5 m/c/h). Similarly, for Aedes aegypti, no difference between seasons, (Wilcoxon signed-rank test, Z = 706, P = 0.3) was observed.
Table 2.
Assessment of vectors distribution and abundance according to ecological factors
| Ecological factors | Aedes spp. | Ae. albopictus | Ae. aegypti | |||||
|---|---|---|---|---|---|---|---|---|
| Total N (%) | Total CEI | CEI | Median (IQR) | Statistic test | CEI | Median (IQR) | Statistic test | |
| Seasons | ||||||||
| Dry | 1432 (33) | 87.9 | 87.8 | 1.0 (0–5.6) | Z = 837.5, P = 0.47 | 0.1 | 0 (0–0) | Z = 706, P = 0.3 |
| Rain | 2919 (67) | 182.4 | 179.2 | 0.5 (0–3.5) | 3.1 | 0 (0–0) | ||
| Access to water | ||||||||
| Rain | 2358 (33) | 131.2 | 131.2 | 0.6 (0–6.8) | H = 2.36, df = 3, P = 0.5 | 0 | 0 (0–0) | H = 455, df = 3, P < 0.01 |
| Tap | 2105 (29) | 111.3 | 108.4 | 2.1 (0.4–4.7) | 3 | 0 (0–0) | ||
| River | 2551 (35) | 143.2 | 143.2 | 0.4 (0–5.9) | 0 | 0 (0–0) | ||
| Wells | 215 (3) | 13.4 | 13.4 | 0.0 (0–3.7) | 0 | 0 (0–0) | ||
| Vegetation type | ||||||||
| Herbaceous | 2003 (41) | 104.9 | 102 | 0.8 (0–4.4) | H = 21, df = 3, P < 0.001 | 3 | 0 (0–0) | H = 580, df = 3, P < 0.04 |
| Plantation | 1871 (38) | 100.7 | 100.7 | 9.8 (8–17) | 0 | 0 (0–0) | ||
| Tropical forest | 715 (15) | 28.5 | 28.5 | 0.1 (0–3.3) | 0 | 0 (0–0) | ||
| Urban forest | 281 (5.8) | 17.6 | 17.6 | 0.0 (0–0) | 0 | 0 (0–0) | ||
| Type of crops | ||||||||
| Hevea brasiliensis | 1761 (37) | 97.3 | 97.3 | 14.5 (9.1- 31) | H = 25.7, df = 2, P < 0.001 | 0 | 0 (0–0) | H = 464, df = 3, P = 0.2 |
| Manihot esculenta | 1778 (37) | 93.9 | 93.8 | 5.0 (3.1–7.5) | 0.1 | 0 (0–0) | ||
| No cultivated crops | 1210 (25) | 56.4 | 53.5 | 0.3 (0 -2.0) | 2.9 | 0 (0–0) | ||
| Presence of fruit trees | ||||||||
| No | 1926 (44) | 115.8 | 114.4 | 0.0 (0–2.1) | Z = 559, P = 0.05 | 1.4 | 0 (0–0) | Z = 792, P = 0.5 |
| Yes | 2425 (56) | 119.6 | 118.1 | 1.2 (0–5) | 1.6 | 0 (0–0) | ||
| Presence of breeding site | ||||||||
| No | 837 (19) | 51.3 | 51.3 | 0.1 (0 -3.1) | Z = 620.5, P = 0.10 | 0 | 0 (0–0) | Z = 644, P < 0.05 |
| Yes | 3514 (81) | 184.2 | 181.2 | 0.9 (0–5.3) | 3 | 0 (0–0) | ||
| Status of breeding sitea | ||||||||
| Negative | 686 (20) | 29.8 | 26.9 | 0.2 (0–0.8) | Z = 107.5, P < 0.001 | 2.9 | 0 (0–0.6) | Z = 349, P < 0.06 |
| Positive | 2828 (80) | 154.4 | 154.3 | 5.0 (1.1–8.4) | 0.1 | 0 (0–0) | ||
CEI Capture Effort Index, IQR inter-quartile range
aNegative or positive status means absence or presence, respectively, of larvae in the breeding site
Table 2 show a significant difference in the abundance of Ae. albopictus collected across areas with different vegetation types, with the median CEI of Ae. albopictus collected in areas with rubber fields being higher than the median CEIs of Ae. albopictus from areas with wild forest, urban forest and herbs or shrubs (Kruskal–Wallis H-test, H = 21, df = 3, P < 0.001). The median CEI of Ae. albopictus collected was statistically different according to the types of crops grown in the areas where the mosquitoes were collected (Kruskal–Wallis H-test, H = 25.7, df = 2, P < 0.001). Specifically, the median CEI of mosquitoes was higher in areas where the Brazilian rubber tree Hevea brasiliensis (14.5 m/c/h, IQR 9.1–31 m/c/h) was the main crop grown compared to areas where the dominant crop was cassava Manihot esculenta (5 m/c/h, IQR 3.1–7.5 m/c/h) or where there was no agriculture (0.3 m/c/h, IQR 0.0–2 m/c/h).
Although the number of Ae. albopictus collected tended to be higher in areas where the main source of water supply was rainwater (2374/4367; 33%) than in areas where the main water access was either tap water, river or wells, the difference was not statistically significant overall (Kruskal–Wallis H-test, H = 2.36, df = 3, P = 0.5). Likewise, the median CEI of Ae. albopictus was significantly higher in areas with fruit trees compared to an environment without fruit trees (Wilcoxon signed-rank test, Z = 559, P = 0.05). Moreover, no difference in abundance of Ae. albopictus mosquitoes was observed in sites with or without the presence of breeding sites (Wilcoxon signed-rank test, Z = 620.5, P = 0.10). However, the presence of positive Culicidae breeding sites significant influenced the median CEI of Aedes mosquitoes collected (2.5 m/c/h, IQR 0.9–6.7 m/c/h) in the study area (Wilcoxon signed-rank test, Z = 107.5, P < 0.001).
Regarding the association of Ae. aegypti and the ecological and environmental factors, no conclusion on the results can be provided. The low values of the CEI and the calculated median CEI linked to an extremely low number and a localized distribution only in zone 1 of the urban area of the vector did not allow us to draw a conclusion, despite the P-values reported in Table 2.
Associations between ecological factors and vector abundance
The impact of ecological factors on the abundance of Ae. albopictus mosquitoes in the study areas was assessed. Table 3 reports the results of the linear regression obtained from the analysis of six out the seven pre-determined ecological factors. For most of these factors, with the exception of those related to the presence of fruit trees (regression coefficient [β] = − 1.69) and well water (β = − 0.29), β was positive, although not statistically significant. Access to water showed no association with vector abundance.
Table 3.
Factors associated with the abundance of Aedes albopictus mosquitoes
| Ecological predictors | Number of observations | Estimate of regression coefficient β | 95% Confidence interval | P-value |
|---|---|---|---|---|
| Access to water | ||||
| Tap | 44 | 1 | – | – |
| Rain | 26 | 2.51 | [− 2.0, 7.0] | 0.274 |
| River | 33 | 1.81 | [− 2.4, 6.0] | 0.397 |
| Wells | 6 | − 0.29 | [− 8.3, 7.7] | 0.943 |
| Vegetation type | ||||
| Urban forest | 20 | 1 | – | – |
| Herbaceous | 49 | 1.26 | [-2.0, 4.6] | 0.448 |
| Rubber field | 6 | 15.9 | [10, 22 | < 0.001 |
| Tropical forest | 20 | 0.55 | [− 3.4, 4.5] | 0.783 |
| Type of crops | ||||
| No cultivated crops | 50 | 1 | – | – |
| Hevea brasiliensis | 4 | 23.19 | [17, 30] | < 0.001 |
| Manihot esculenta | 21 | 3.34 | [0.18, 6.5] | 0.038 |
| Presence of fruit trees | ||||
| No | 29 | 1 | – | – |
| Yes | 52 | − 1.69 | [− 5.3, 1.9] | 0.346 |
| Presence of breeding sites | ||||
| No | 33 | 1 | – | – |
| Yes | 48 | 2.28 | [− 1.2, 5.7] | 0.192 |
| Status of breeding sitea | ||||
| Negative | 25 | 1 | – | – |
| Positive | 23 | 5.52 | [0.02, 11] | 0.049 |
aNegative or positive status means absence or presence, respectively, of larvae in the breeding site
In contrast, regarding factors related to vegetation type, only rubber tree forests were associated with vector abundance (linear regression, β = 15.9, P ˂ 0.001). Similarly, in the crop-related factor group, Manihot esculenta (linear regression, β = 3.34, P = 0.038) and Hevea brasiliensis (linear regression, β = 23.19, P ˂0.001) were strongly associated with Ae. albopictus abundance in the study area.
Finally, although there was no association between the abundance of adult mosquitoes and the presence of breeding sites, we found an association between abundance of mosquitoes and status of breeding sites in the study area (linear regression, β = 5.52, P = 0.049).
Characterization of DENV and CHIKV in Aedes species collected
A total of 122 pools of adult female Ae. albopictus and Ae. aegypti mosquitoes, totaling approximately 2240 mosquitoes, were extracted and screened by RT qPCR. The presence of neither DENV nor CHIKV was detected in any of the mosquito pools.
Discussion
Regular surveillance of potential arbovirus vectors and assessment of ecological factors that play a role in their distribution and spread are important for designing control strategies that are tailored to local settings.
The entomological surveys based on the collection of adult mosquitoes carried out in this study allowed us to identify Ae. albopictus as the most aggressive species and potentially the most abundant vector in rural areas, with Ae. aegypti found in low numbers and only in urban areas of Lambaréné. This predominance of Ae. albopictus is in line with collections carried out in Libreville and Lastrouville during the DENV and CHIKV outbreaks in 2007 and 2010, respectively, during which this species identified as the main vector of the infections [14, 18, 26]. These results indicate the expansion and establishment of Ae. albopictus in many countries of the Central African region, to the detriment of Ae. aegypti, believed to have originated in Africa, as the main vector of various arboviruses [6, 18, 27–30]. Satyrization, which is a form of reproductive competition in which males of one species mate with female of another species, resulting in the production of less-fit hybrid offspring, could explain the progressive replacement of Ae. aegypti by the recently introduced Ae. albopictus [31]. In addition, interspecific competition for resources during larval stages can sometimes contribute to the displacement of some species to the detriment of another. Previous studies showed that sympatry between these two main vectors in the urban environment contributes to the displacement of Ae. aegypti [32, 33], as previously reported in Cameroon and the Central African Republic [34]. In the present study, Ae. albopictus was not evenly distributed across all zones, with larger numbers collected in urban zone 3, where commercial and industrial activities such as markets are concentrated. The proximity of this area to Rz2 as well as the availability of suitable breeding sites could further explain the abundance of Ae. albopictus in such environments [35].
Although Ae. albopictus was present in all environments, the survey also showed that these mosquitoes were more abundant along the Lambaréné-PK30-Fougamou transect (Rz2). This transect is home to a vast scheme of rubber tree plantations that may provide suitable conditions for the development of Aedes larvae. In particular, harvesting pots used for the collection of the rubber sap are potential breeding sites for Aedes species and contribute to the maintenance of Aedes spp. populations in this environment, which further points to an anthropological role in setting up favorable conditions for vector development [1]. On the other hand, despite the presence of a palm-oil plantation scheme on the Lambaréné–Bindo–Libreville transect, fewer Aedes were collected in this area. Therefore, the presence of rubber crops appears to be a major driver for the development of Aedes mosquitoes in the rural area studied. Similar results were reported in an entomological survey assessing the effect of changes in land-usage on the distribution and abundance of vectors in environments dominated by oil palm (Elaesis guineensis), cocoa and cassava plantations in the southern part of the Ivory Coast [36].
Several studies have demonstrated the role of environmental conditions in the development and invasion of vectors [37]. The presence of larval breeding sites, the influence of seasons, humidity, type of vegetation, presence of fruit trees and the influence of crops are some of the factors investigated in our survey, with the aim to determine their association with vector abundance. We found no difference in the distribution of vectors between the dry season and the rainy season. The rainfall recorded (Additional file 1: Fig. S1) during these dry periods, although lower compared to the rainy season, provides the necessary conditions for vector development.
The survey data (Additional file 2: Dataset) also provided insight into the influence of breeding sites and their status on the presence and abundance of Aedes mosquitoes. Our results show that the presence of these breeding sites and their positivity is weakly associated with the abundance of vectors in the area, as often described in studies where distribution and abundance were assessed on the basis of mosquito egg collection [35, 38]. In contrast to these earlier studies, our collections consisted of adult mosquitoes, which could explain the weak association we observed, in addition to the fact that culicine larvae were not reared to the adult stage and therefore not identified specifically as Aedes.
It also appears from this survey that the vectors have a specific tropism for a type of vegetation and for a certain type of crop, as already reported by Zahouli [36] in the Ivory Coast, indicating that Aedes spp. mosquitoes were widely abundant in polycultures in which the Euphorbiaceae family was highly represented. In our survey, several collections were made in areas with Manihot esculenta crops, including urban zone 3 and part of urban zone 2 where a relatively higher number of Aedes mosquitoes were collected.
Finally, no viral carriage was found from the pools of mosquitoes analyzed. This is surprising if we consider the high seroprevalences of 20.4% found by Yuri and collaborators in the Lambaréné population [19] where we found a high abundance of Ae. albopictus. This result could be explained by the fact that the collections in our study were performed during the non-epidemic period when viral carriage and the circulation of strains may be low; higher numbers of virus-carrying vectors would be expected when there is an active and intense circulation of the viruses in the human population [14, 18]. The viral carriage reported in the Ae. albopictus mosquitoes during the epidemic period of 2007 to 2010 in Gabon was obtained by collecting mosquitoes in and around the houses of people found to be infected with arboviruses [13]. A similar approach could be used in surveys during inter-epidemic period by performing collections in the surroundings of people with a non-malaria fever or those who are seropositive for arboviruses.
One limitation in this study was the use of mosquito vacuums, such as those used in our collections. The power of the hoover may result in the disappearance of some of the distinctive features of the vector of interest, as the suction airflow may be intense during collection. This could be a confounding factor when discriminating between two vectors that are morphologically close and thus create an under- or over-estimation of one or the other vector.
Conclusion
Integrated vector control strategies against the transmission of arboviruses and other vector-borne diseases are an important public health priority for Gabon. In the present study, we have provided evidence identifying Ae. albopictus as the most widespread potential arbovirus vector in Moyen Ogooué province. Some of the ecological factors listed in this survey to be associated with the vector population demonstrate the important role of humans in the proliferation of this species in rural and urban areas. Activities associated with industrial rubber plantations to the south of Lambaréné, as well as those situated in two other areas of Gabon (Kango and Mitzic), are potential hotspots for the invasion of these vectors into other areas, as highlighted in this study.
Supplementary Information
Additional file 1: Fig S1. Rainfall data on two years, November 2019 to April2021 to Lambaréné.
Additional file 2. Dataset. Entomological survey in Lambaréné and its surroundings.
Acknowledgements
We are grateful to Theo Nzoughe-Nzeng, Eddy Wilfrid Mangaboula, Pakouda Souleymane and all the collectors for helping with the mosquito collection. The authors thank the MTN coordination team project for technical and administrative assistance. Finally, we thank the Immunology and molecular Biology Laboratory staff for help in the laboratory work.
Abbreviations
- CEI
Capture Effort Index
- CHIKV
Chikungunya virus
- DENV
Dengue virus
- RT-qPCR
Reverse transcription-quantitative PCR
Author contributions
AAA, BL, SB, JY, HA, STBS and RB conceived and planned the study and its design. RB, STBS, AGDN and BN performed the field activities including the collection and entomological identification of species. RB, AM, HA and YU performed the molecular analyses of the samples. BR, SM and RBM analyzed the data. YD and BL designed the maps for the study. RB drafted the manuscript. AAA, STBS, HA, SB and BL critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the OCEAC MTN projet funded by KFW (BMZ–Nr 2015.69.227 + BMZ–Nr 2016.68.797). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
All data supporting the conclusions of the study are included in the manuscript and its supplementary information.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of the Centre de Recherches Médicales de Lambaréné (CERMEL) (CEI-008/2019), and the National Ethics Committee of Gabon (PROT No. 0085/2019/PR/SG/CNER). It was also reviewed by the Scientific Review Committee of the CERMEL (RSC No. 2019–10).
Consent for publication
All authors concur with the submission presented by the corresponding author.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abílio AP, Abudasse G, Kampango A, Candrinho B, Sitoi S, Luciano J, et al. Distribution and breeding sites of Aedes aegypti and Aedes albopictus in 32 urban/peri-urban districts of Mozambique: implication for assessing the risk of arbovirus outbreaks. PLoS Negl Trop Dis. 2018;12:e0006692. doi: 10.1371/journal.pntd.0006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuya-Kanamori L, Liang S, Milinovich G, Soares Magalhaes RJ, Clements AC, Hu W, et al. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis. 2016;16:84. doi: 10.1186/s12879-016-1417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman A, Istúriz RE. Update on the global spread of dengue. Int J Antimicrob Agents. 2010;36:S40–S42. doi: 10.1016/j.ijantimicag.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Rezza G. Dengue and chikungunya: long-distance spread and outbreaks in naïve areas. Pathog Glob Health. 2014;108:349–355. doi: 10.1179/2047773214Y.0000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezza G, El-Sawaf G, Faggioni G, Vescio F, Al Ameri R, De Santis R, et al. Co-circulation of dengue and chikungunya viruses, Al Hudaydah, Yemen, 2012. Emerg Infect Dis. 2014;20:1351–1354. doi: 10.3201/eid2008.131615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngoagouni C, Kamgang B, Kazanji M, Paupy C, Nakouné E. Potential of Aedes aegypti and Aedes albopictus populations in the Central African Republic to transmit enzootic chikungunya virus strains. Parasit Vectors. 2017;10:164. doi: 10.1186/s13071-017-2101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontenille D, Toto JC. Aedes (Stegomyia) albopictus (Skuse), a potential new dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7:1066–1067. doi: 10.3201/eid0706.010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter P. Aedes albopictus and the world trade in used tires, 1988–1995: the shape of things to come? J Am Mosq Control Assoc. 1998;14:83–94. [PubMed] [Google Scholar]
- 9.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 10.Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010;10:259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- 11.Savage HM, Ezike VI, Nwankwo AC, Spiegel R, Miller BR. First record of breeding populations of Aedes albopictus in continental Africa: implications for arboviral transmission. J Am Mosq Control Assoc. 1992;8:101–103. [PubMed] [Google Scholar]
- 12.Coffinet T, Mourou JR, Pradines B, Toto JC, Jarjaval F, Amalvict R, et al. First record of Aedes albopictus in Gabon. J Am Mosq Control Assoc. 2007;23:471–472. doi: 10.2987/5636.1. [DOI] [PubMed] [Google Scholar]
- 13.Caron M, Paupy C, Grard G, Becquart P, Mombo I, Nso BB, et al. Recent introduction and rapid dissemination of Chikungunya virus and Dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, Central Africa. Clin Infect Dis. 2012;55:e45–53. doi: 10.1093/cid/cis530. [DOI] [PubMed] [Google Scholar]
- 14.Leroy EM, Nkoghe D, Ollomo B, Nze-Nkogue C, Becquart P, Grard G, et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis. 2009;15:591–593. doi: 10.3201/eid1504.080664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nkoghe D, Kassa RF, Bisvigou U, Caron M, Grard G, Leroy EM. No clinical or biological difference between Chikungunya and Dengue fever during the 2010 Gabonese outbreak. Infect Dis Rep. 2012;4:e5. doi: 10.4081/idr.2012.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nkoghe D, Kassa RF, Caron M, Grard G, Mombo I, Bikié B, et al. Clinical forms of chikungunya in Gabon, 2010. PLoS Negl Trop Dis. 2012;6:e1517. doi: 10.1371/journal.pntd.0001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caron M, Grard G, Paupy C, Mombo IM, Bikie Bi Nso B, KassaKassa FR, et al. First evidence of simultaneous circulation of three different dengue virus serotypes in Africa. PLoS ONE. 2013;8:e78030. doi: 10.1371/journal.pone.0078030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paupy C, Kassa Kassa F, Caron M, Nkoghé D, Leroy EM. A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector Borne Zoonotic Dis. 2012;12:167–169. doi: 10.1089/vbz.2011.0736. [DOI] [PubMed] [Google Scholar]
- 19.Ushijima Y, Abe H, Nguema Ondo G, Bikangui R, Massinga Loembé M, Zadeh VR, et al. Surveillance of the major pathogenic arboviruses of public health concern in Gabon, Central Africa: increased risk of West Nile virus and dengue virus infections. BMC Infect Dis. 2021;21:265. doi: 10.1186/s12879-021-05960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim JK, Fernandes JF, Yoon IK, Lee JS, Mba RO, Lee KS, et al. Epidemiology of dengue fever in Gabon: results from a health facility-based fever surveillance in Lambarene and its surroundings. PLoS Negl Trop Dis. 2021;15:e0008861. doi: 10.1371/journal.pntd.0008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe H, Ushijima Y, Loembe MM, Bikangui R, Nguema-Ondo G, Mpingabo PI, et al. Re-emergence of dengue virus serotype 3 infections in Gabon in 2016–2017, and evidence for the risk of repeated dengue virus infections. Int J Infect Dis. 2020;91:129–136. doi: 10.1016/j.ijid.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Vazeille M, Moutailler S, Pages F, Jarjaval F, Failloux AB. Introduction of Aedes albopictus in Gabon: what consequences for dengue and chikungunya transmission? Trop Med Int Health. 2008;13:1176–1179. doi: 10.1111/j.1365-3156.2008.02123.x. [DOI] [PubMed] [Google Scholar]
- 23.Rueda LM. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue Virus Transmission. zootaxa. 2004;589:1–60. 10.11646/zootaxa.589.1.1
- 24.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8(2):e2688. [DOI] [PMC free article] [PubMed]
- 25.Santiago GA, Vergne E, Quiles Y, Cosme J, Vazquez J, Medina JF, et al. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis. 2013;7(7):e2311. [DOI] [PMC free article] [PubMed]
- 26.Pagès F, Peyrefitte CN, Mve MT, Jarjaval F, Brisse S, Iteman I, et al. Aedes albopictus mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLoS ONE. 2009;4:e4691. doi: 10.1371/journal.pone.0004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngoagouni C, Kamgang B, Nakouné E, Paupy C, Kazanji M. Invasion of Aedes albopictus (Diptera: Culicidae) into Central Africa: what consequences for emerging diseases? Parasit Vectors. 2015;8:191. doi: 10.1186/s13071-015-0808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paupy C, Brengues C, Kamgang B, Hervé JP, Fontenille D, Simard F. Gene flow between domestic and sylvan populations of Aedes aegypti (Diptera: Culicidae) in North Cameroon. J Med Entomol. 2008;45:391–400. doi: 10.1093/jmedent/45.3.391. [DOI] [PubMed] [Google Scholar]
- 29.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Weetman D, Kamgang B, Badolo A, Moyes CL, Shearer FM, Coulibaly M, et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int J Environ Res Public Health. 2018;15:220. 10.3390/ijerph15020220. [DOI] [PMC free article] [PubMed]
- 31.Ribeiro JM. Can satyrs control pests and vectors? J Med Entomol. 1988;25:431–440. doi: 10.1093/jmedent/25.6.431. [DOI] [PubMed] [Google Scholar]
- 32.Lounibos LP, Juliano SA. Where vectors collide: the importance of mechanisms shaping the realized niche for modeling ranges of invasive Aedes mosquitoes. Biol Invasions. 2018;20:1913–1929. doi: 10.1007/s10530-018-1674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro AF, Marques GR, Voltolini JC, Condino ML. Association between dengue incidence and climatic factors. Rev Saude Publica. 2006;40:671–676. doi: 10.1590/S0034-89102006000500017. [DOI] [PubMed] [Google Scholar]
- 34.Tedjou AN, Kamgang B, Yougang AP, Njiokou F, Wondji CS. Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. PLoS Negl Trop Dis. 2019;13:e0007137. doi: 10.1371/journal.pntd.0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Custódio JMO, Nogueira LMS, Souza DA, Fernandes MF, Oshiro ET, Oliveira EF, et al. Abiotic factors and population dynamic of Aedes aegypti and Aedes albopictus in an endemic area of dengue in Brazil. Rev Inst Med Trop Sao Paulo. 2019;61:e18. doi: 10.1590/s1678-9946201961018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahouli JBZ, Koudou BG, Müller P, Malone D, Tano Y, Utzinger J. Effect of land-use changes on the abundance, distribution, and host-seeking behavior of Aedes arbovirus vectors in oil palm-dominated landscapes, southeastern Côte d'Ivoire. PLoS ONE. 2017;12:e0189082. doi: 10.1371/journal.pone.0189082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diallo D, Diagne CT, Hanley KA, Sall AA, Buenemann M, Ba Y, et al. Larval ecology of mosquitoes in sylvatic arbovirus foci in southeastern Senegal. Parasit Vectors. 2012;5:286. doi: 10.1186/1756-3305-5-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson-Bahun TA, Kamgang B, Lenga A, Wondji CS. Larval ecology and infestation indices of two major arbovirus vectors, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazzaville, the capital city of the Republic of the Congo. Parasit Vectors. 2020;13:492. doi: 10.1186/s13071-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig S1. Rainfall data on two years, November 2019 to April2021 to Lambaréné.
Additional file 2. Dataset. Entomological survey in Lambaréné and its surroundings.
Data Availability Statement
All data supporting the conclusions of the study are included in the manuscript and its supplementary information.