Abstract
Apical–basal progenitor cell polarity establishes key features of the radial and laminar architecture of the developing human cortex. The unique diversity of cortical stem cell populations and an expansion of progenitor population size in the human cortex have been mirrored by an increase in the complexity of cellular processes that regulate stem cell morphology and behaviour, including their polarity. The study of human cells in primary tissue samples and human stem cell-derived model systems (such as cortical organoids) has provided insight into these processes, revealing that protein complexes regulate progenitor polarity by controlling cell membrane adherence within appropriate cortical niches and are themselves regulated by cytoskeletal proteins, signalling molecules and receptors, and cellular organelles. Studies exploring how cortical stem cell polarity is established and maintained are key for understanding the features of human brain development and have implications for neurological dysfunction.
The cerebral cortex is highly expanded in humans when compared with other mammals and has several unique features relating to the organization, number and diversity of cell types that it contains. Many of these characteristics are established during development. The developing human cerebral cortex contains more diverse populations of progenitors, along with vastly increased numbers of progenitor cells, than that of other mammals1. The formation and function of the diverse progenitor cell types that create the human cortex are reliant upon the establishment and maintenance of their apical–basal cell polarity. In this Review, we will use ‘polarity’ to refer to any asymmetric spatial distribution of a cell’s morphology that is predominantly dependent on the apical–basal orientation of progenitor cells during cortical development.
Although foundational studies in animal model systems have illuminated the mechanisms guiding cortical polarity, the unique expansion in size and diversity of the progenitor populations presents a unique challenge to our understanding of these phenomena in the human cortex2,3. Further, features of human brain development implicated in multiple neurodevelopmental disorders may not be shared with animal models. It is therefore imperative to study the regulation of polarity in human cortical progenitors to understand the origins of neurological disease in the human brain. Recent years have seen advances in methods for culturing human neural cells while maintaining their polarity and organization. These studies have generated critical insights into how cytoskeletal regulators, organelle functions and division dynamics influence progenitor cell polarity and, in turn, increase progenitor cell diversity in the developing human brain.
In this Review, we provide an overview of these developments and findings, with a focus on those aspects that relate specifically to human neural progenitor polarity. In addition, we discuss the consequences of disrupted developmental progenitor polarity and the role of such disruptions in various human neurological diseases, including various cortical malformations, neuropsychiatric disorders and neurodegenerative diseases. We will show how recent innovations in methods for culturing human cells, as well as the discovery of human-enriched populations of cortical progenitors, provide an opportunity to better understand the development of the unique features of the human cortex and to evaluate how changes in progenitor polarity impact the human brain in neurological disease states.
Human cortical progenitor populations
In the developing human cerebral cortex, distinct populations of stem cells and progenitor cells proliferate, differentiate and migrate to establish the radial and laminar architecture of the cortex during neurogenesis4,5 (FIG. 1). The cerebral cortex originates from the invagination and expansion of the anterior end of the neural tube. The single layer of neuroepithelial progenitor cells forming the neural tube are the primary stem cell population from which all other cortical cell types arise. The polarity and radial organization of this founding progenitor population thus define the radial architecture of the developing human cortex6.
Fig. 1 |. Progenitor types and division programmes in the developing human cortex.
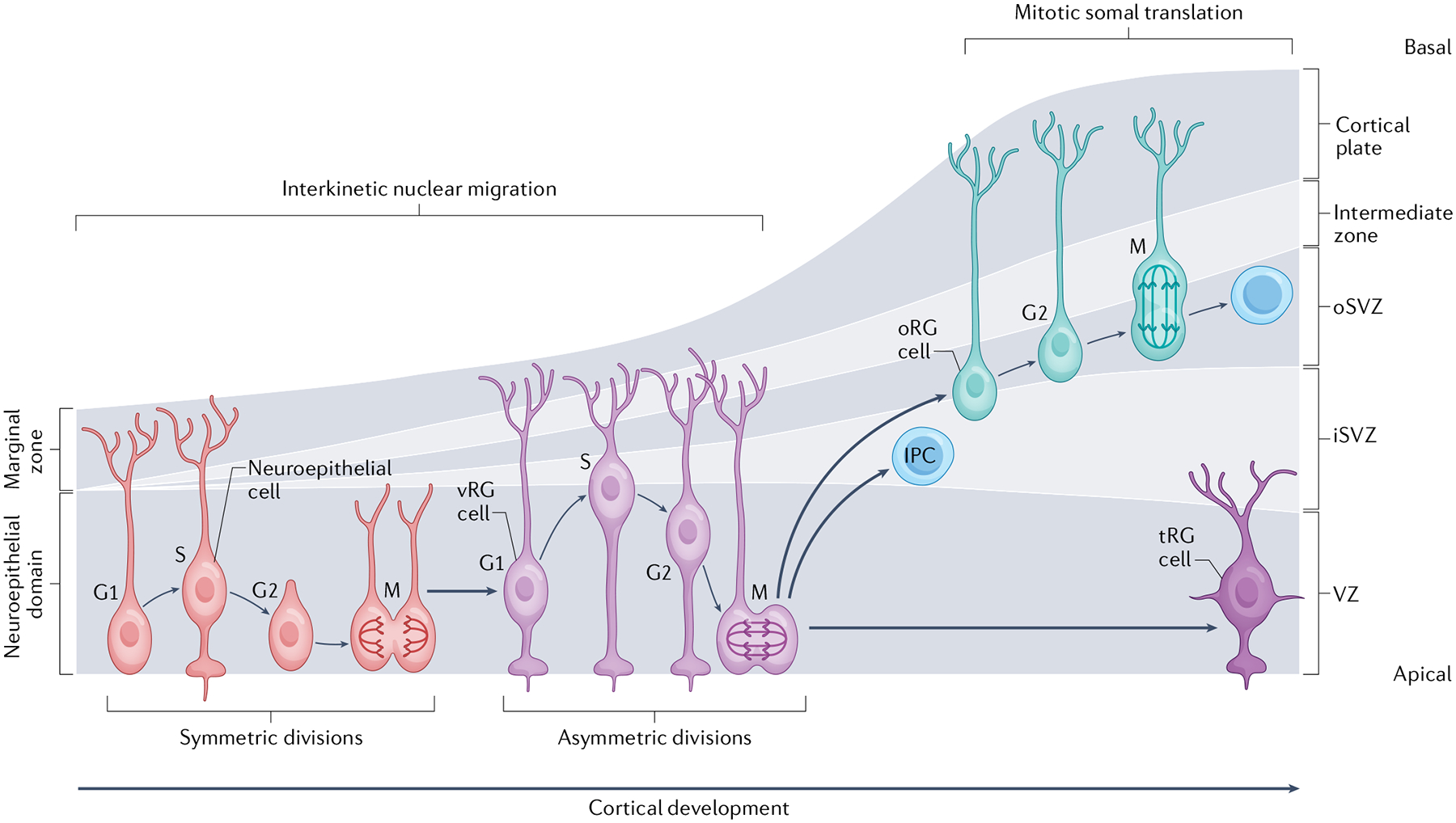
During early human forebrain development a pseudostratified layer of neuroepithelial cells populate the developing neural tube6. Neuroepithelial cells divide symmetrically at the ventricular surface in self-renewing divisions7. From the end of the first trimester, neuroepithelial cells differentiate into multiple subtypes of radial glial cells, including apically localized, early-born ventricular radial glial (vRG) cells and later-born outer radial glial (oRG) cells22,62,162. vRG cells divide in a process known as interkinetic nuclear migration, in which the nucleus moves in a basal direction during DNA synthesis (S phase) and then moves in an apical direction to undergo mitosis at the ventricular surface. oRG cells divide in a process known as mitotic somal translocation, in which the cell body ‘jumps’ (moves quickly) in a basal direction before dividing horizontally by mitosis29. Both vRG cells and oRG cells differentiate into neurogenic intermediate progenitor cells (IPCs) or directly into neurons (not shown)19,22,163–165. Later in neurogenesis, the apically localized radial glial cells become truncated radial glial (tRG) cells31. The cortex is divided into distinct zones, in which each of these cellular populations resides. Early in development, the cortex comprises the neuroepithelial domain and the marginal zone, whereas later — as the complexity of cell types and cortical size expands — the progenitor zone is divided into the ventricular zone (VZ), inner subventricular zone (iSVZ) and outer subventricular zone (oSVZ), and the neuronal domains are divided into the cortical plate, containing layers II–IV of excitatory neurons, and the marginal zone (which contains Cajal–Retzius cells), in layer I above the cortical plate166. As progenitor cells differentiate into neurons, they move basally along the radial glial scaffold to form the intermediate zone and neurons differentiate to contribute to the growing cortical plate167.
Stem cells.
A collective term for neuroepithelial cells and radial glial cells; multipotent cells that give rise to other progenitor cells and various neuronal and glial cell types.
Progenitor cells.
Multipotent cells (such as intermediate progenitor cells) that differentiate into postmitotic cell types.
Early in the first trimester of human development, cortical neuroepithelial cells extend from the apical side of the neural tube, which borders the lumen, to the basal lamina at the pial surface7,8. This apical–basal polarity is retained as the early neuroepithelial cells develop into a pseudostratified neuroepithelium. Through the process of interkinetic nuclear migration (INM) neuroepithelial cell nuclei move in a basal direction during G1 phase of the cell cycle, with S phase occurring at the basal (pial) surface. The nuclei then move apically during G2 phase prior to mitosis (M phase), which takes place at the apical (ventricular) surface (FIG. 1). During the first trimester, neuroepithelial cell numbers increase through symmetric division to expand the progenitor pool. During each mitotic division, parent neuroepithelial cells retract their basal processes and daughter cells then re-establish basal contact by extending new processes to the basal lamina9. Studies conducted in the mouse, chicken and zebrafish have suggested that, in these species, parent neuroepithelial cells split their basal process during cytokinesis; however, this has not been observed in human studies10. A prolonged expansion of neuroepithelial stem cells has been proposed to be the main driver of the increase in neuron numbers in the human cortex6. Although neuroepithelial cells are relatively uniform in terms of their morphology and behaviour, recent studies have identified transcriptional diversity and molecular variations within this population in the human brain, suggesting that even early stem cells may have distinct developmental trajectories and functions11.
Neuroepithelial cells.
Pseudostratified stem cells that establish the developing neocortex through their polar morphology.
Cell cycle.
A process of cell division, in which one cell becomes two; composed of stages that include cell growth (G1 phase, G2 phase), DNA synthesis (S phase) and mitosis/cytokinesis (M phase).
Symmetric division.
Cell division in which a parent cell gives rise to two identical daughter cells. This type of division can be self-renewing.
The general mitotic behaviour and pseudostratified morphology of neuroepithelial cells are typical of epithelial cell types, but these features become more specialized as the neuroepithelial cells differentiate. In the cortex of all mammals, including humans, neuroepithelial cells transform into ventricular radial glial (vRG) cells, also called apical radial glia (FIG. 1). In the human cortex, this cellular transformation occurs towards the end of the first trimester9,11,12. The apico-basal polarity of neuroepithelial cells defines the polarity of vRG cells, which are elongated and anchored apically at the ventricular surface with a long basal process attached to the pial surface. However, unlike neuroepithelial cells, the basal processes of vRG cells do not retract during mitosis and, instead, remain anchored to the basal lamina9. This change in mitotic behaviour presumably enables the radial glial scaffold to expand significantly over time and makes it possible for the radial fibre to guide the migration of newborn neurons13,14. vRG cells exhibit INM and divide at the ventricular surface, similar to neuroepithelial cells; however, although their basal fibres extend across the entire cortical mantle, the region of nuclear movement is restricted to the ventricular zone (VZ). Although vRG cells do undergo symmetric (proliferative) divisions, they mostly undergo asymmetric division to self-renew and either generate neurons directly or indirectly through production of a population of neurogenic transit-amplifying cells, called intermediate progenitor cells (IPCs)9,15,16. The dynamics of radial glial expansion, polarity and architecture have been studied extensively in the mouse, but the diversity of radial glia subtypes in the human brain has only been appreciated for the past 15 years17.
Radial glial scaffold.
A structure in the developing cerebral cortex that has an apical–basal orientation and is composed of the basal processes of radial glial cells. The scaffold is required to support neurons as they migrate through the developing cortex to reach their laminar position. Progenitor polarity is essential for scaffold integrity.
Ventricular zone.
(VZ). A progenitor zone located on the apical side of the developing cortex in close proximity to the lateral ventricle. The VZ is usually defined as the zone of interkinetic nuclear migration of radial glia.
Asymmetric division.
Cell division in which a parent cell gives rise to two different daughter cells. This can be a differentiating division.
Cortical neurogenesis and the increased diversity of radial glial subtypes begins towards the end of the first trimester. In humans, the progenitor domains expand into an inner subventricular zone (iSVZ) and an outer subventricular zone (oSVZ), in which distinct cell populations arise from vRG progenitors17–20 (FIG. 1). Asymmetric divisions of vRG cells contribute directly to early generated neurons that form the subplate and the marginal zone, as well as to IPCs that subsequently give rise to the neurons that will reside in the deep layers of the cortex21–25. vRG cells also give rise to a second population of radial progenitors that reside in the expanded oSVZ domain17. These cells, called outer radial glial (oRG) cells or basal radial glia, are enriched in humans compared with other species; they are a minor population in the mouse but are found in increased numbers in mammals with large brains, including carnivores and non-human primates26,27.
oRG cells have a unique transcriptional signature and polar morphology compared with other radial glia28. They lose their apical attachment to the ventricular surface, retaining only a long basal process extending towards the pial surface. They exhibit a unique division behaviour called mitotic somal translocation (MST), in which the nucleus of the parent cell moves rapidly (by translocating, or ‘jumping’) along the basal fibre immediately prior to division17 (FIG. 1). MST is driven by myosin motors, depends on the integrity of the oRG cell’s basal process19,29,30 and has been hypothesized to contribute to the expansion of the human oSVZ29. Similar to vRG cells, oRG cells undergo self-renewing divisions as well as asymmetric divisions giving rise to IPCs or directly to neurons.
By the middle of the second trimester, oRG cells become the predominant progenitor population giving rise to neurons in the human cortex28. During the horizontal divisions, by which oRG cells are generated from vRG cells, the basally positioned daughter cells inherit the basal process of the dividing vRG cell and become oRG cells, whereas the apically positioned daughter cells lose their basal processes but maintain their apical anchor and become truncated radial glial cells31. Owing to a surge in oRG cell generation, the radial glial scaffold is primarily composed of the basal processes of oRG cells at this stage31. The radial architecture of the human cortex during the second half of neurogenesis, when upper cortical layer neurons are being generated, is therefore determined by the polarity of oRG cells. At the ventricular surface, the truncated radial glial cells grow basal processes that do not extend to the pial surface but, instead, become truncated, often ending on blood vessels and contributing to a shortened radial glial scaffold31,32 (FIG. 1).
Horizontal divisions.
Cell divisions in which there is a horizontal plane of cytokinesis. These divisions are typically asymmetric.
IPCs, sometimes classified together with oRG cells as basal progenitors33, are, in rodents, typically defined by their expression of the transcription factor EOMES (also known as TBR2), indicating their neurogenic commitment, an expression pattern also present in human IPCs34,35. In contrast to neuroepithelial and radial glial cells, IPCs are often multipolar, without a defined polarity36. Various morphologies or morpho-types have been described for primate basal progenitors, based largely on the number and orientation of their processes37. Typically, these processes are shorter than those of the long basal radial glial fibres30,36,37. As noted above, IPCs derived from vRG cells are thought to give rise to the earlier-born deep-layer excitatory neurons, whereas oRG cell-derived IPCs produce the later-born upper-layer excitatory neurons31. Both the developmental timing and the expansion of the oRG cell and upper-layer neuron populations in the human brain support this hypothesis. Most postmitotic excitatory neurons maintain both a polar and a radial organization as they extend their primary dendrite towards the pial surface during migration and maintain apico-basal orientation as they develop their dendritic trees38. This complex neuronal polarity is maintained by axon guidance and dendritic formation signals, such as neurotrophins, cadherins and semaphorins, which regulate actin through Rho GTPases and microtubule stabilization through protein kinase A (PKA) and serine/threonine protein kinase (AKT) signalling39. oRG cells also differentiate into various glial cells. These include astrocytes and oligodendrocyte precursor cells40,41. It has been shown that human vRG cells and oRG cells give rise to distinct populations of astrocytes42, although the contribution of radial glial polarity to astrocyte fate and laminar position is unknown. In order to decipher how polarity of diverse progenitor types impacts human brain development, we need to study the molecular mechanisms regulating polarity in the human cortex.
Modelling human progenitor polarity
A major challenge to understanding many of the features of human cerebral cortex development described above is the lack of suitable models. Mouse models do not recapitulate the full diversity of progenitor types, extended developmental timelines or complex organization of the human brain26,30,43,44. Developing human brain tissue is largely inaccessible and cannot be experimentally manipulated throughout development. However, pluripotent stem cell (PSC)-derived in vitro model systems can provide access to developing human neural progenitors.
Pluripotent stem cell.
(PSC). A cell that has the potential to become any other cell type in the body. There are two types of PSC: embryonic stem cells are derived from the inner cell mass of blastocyst and induced PSCs are reprogrammed from somatic cells.
In two-dimensional adherent conditions, PSC-derived neural progenitors display similar morphological features to endogenous progenitor cells and form pseudostratified epithelial-like circular structures called neural rosettes. The cells in these rosettes consist of polarized radial glia with their apical processes making contact with the central lumen45. These adherent radial glia are capable of differentiating into various cell types, including neurons and astrocytes46. However, adherent cultures have limitations, including the need for passaging, challenges with long-term adherence and a lack of extracellular matrix proteins and three-dimensional architecture. With the innovation of three-dimensional culture methods, such as cerebral organoids, it has become more tractable to study early developmental events, including progenitor polarity, in a more complex, tissue-like structure45,47 (BOX 1). Organoids can therefore be a useful in vitro model system to evaluate the proliferation, morphology and polarity of human neuroepithelial cells and radial glial cells over time48.
Box 1 |. Progenitor diversity and cytoarchitecture in human cerebral organoids.
Cerebral organoids are pluripotent stem cell-derived cultures that resemble the developing human brain in terms of the cell types that they contain and their organizational features. Although cerebral organoids are smaller in scale than the brain, they exhibit similar features, including the generation of neuroepithelial structures consisting of proliferating progenitor cells arranged around a central lumen (see the figure)45,47,49,169. These three-dimensional ‘neural rosettes’, in which neuroepithelial-like cells express the tight-junction protein ZO1 (indicating that they express genes characteristic of neuroepithelial cells), exhibit neuroepithelial cell-like morphology and are stratified along an apical–basal orientation, mimicking the pseudostratified progenitor zone of the developing cortex that lies adjacent to the lateral ventricle11,51. Progenitor rosettes are consistently observed in cerebral organoid protocols45,47. Within the rosette structures, ventricular radial glial (vRG) cells (the major progenitor population present in the ventricular zone (VZ)) display the same bipolar morphology and broad marker expression that are seen in vRG cells in vivo9,51. As at the ventricular surface of the developing cortex, vRG cells in organoids undergo mitosis at the apical surface of the rosette (adjacent to the lumen). The vRG cells then extend basal processes to the outermost border of the progenitor rosette and create an apical–basal-oriented scaffold reminiscent of that present in the developing human cortex45. As vRG cells in the organoids differentiate, they give rise to intermediate progenitor cells (IPCs) and neurons, as well as to outer radial glial (oRG) cells that create a subventricular zone (SVZ)-like domain169. The specification of truncated radial glial cells and the discontinuity of the glial scaffold that occurs in later neurogenesis in vivo31 have not yet been described in organoid models. Instead, as the progenitors in the organoids differentiate into neurons, the radial scaffold-like structure begins to lose its orientation and overall cellular organization, rather than becoming increasingly large and complex as it does in vivo54. The differentiation of the progenitor cells produces separation of the progenitor and neuronal domains and a rudimentary inside-out organization arises169. oSVZ, outer subventricular zone.
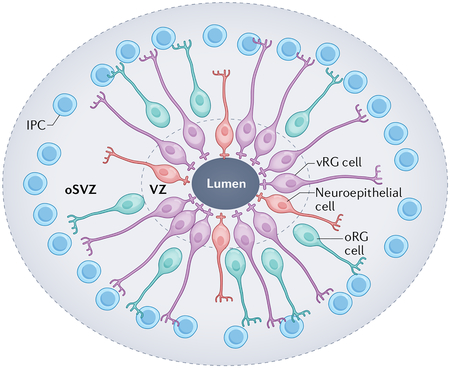
Cerebral organoids.
Three-dimensional neural structures resembling the developing cerebrum that spontaneously differentiate from pluripotent stem cells without manipulation of developmental signalling molecules.
Time-lapse imaging studies of progenitor cells in human cerebral organoids reveal similar cellular behaviour to that of the developing human cortex (BOX 1). Markers of their apical surfaces and of mitosis are strongly expressed along the lumen edge49. Neuroepithelial and radial glial cells both display characteristic apical to basal INM but differ from each other in the mode of cell division and rates of proliferation50. Similar to in vivo behaviour, symmetrically dividing mitotic neuroepithelial cells round up at the lumen and retract their basal processes and daughter cells regrow new basal processes following mitosis9. In a comparative study, an increase in organoid apical surface area, a shorter neuroepithelial cell cycle length and a delayed transition to neurogenesis were all observed in human cerebral organoids as compared with those derived from gorilla cells51, highlighting species-specific features of human organoid cultures. The distinctive proliferating and symmetric mitotic divisions of neuroepithelial cells were observed in younger organoids (from 5 days to 5 weeks in culture)9,51, whereas radial glial progenitors increased in number in older organoids (5–10 weeks in culture). These radial glial progenitors did not retract their basal processes during division and showed a range of distinct cleavage angles and types of division including vertical self-renewing, horizontal asymmetric and oblique divisions9,49. The basal processes of both neuroepithelial and radial glial cells extended from the lumen towards the outer edge of the rosette9,47,49. oRG cells were present in an expanded SVZ domain in cortical organoids after 10–15 weeks in culture and display stereotyped behaviours including MST division49. Thus, although it is challenging to study mitotic dynamics in the endogenous human brain over a range of developmental time points, human organoid models have made the study of these refined cellular behaviours experimentally tractable.
In organoids, the signalling molecules and protein complexes that regulate progenitor polarity can also be easily perturbed and assayed for their impact on early radial scaffold organization and radial glial polarity. For example, in human cortical organoids, glycogen synthase kinase 3 (GSK3) is required to maintain the establishment of vRG polarity, lumen formation and radial glial proliferation rates, whereas the serine/threonine protein kinase mTOR regulates oRG morphology and process length52,53. Organoids are thus a useful tool to study the impact of gene function, molecular signalling and cell polarity on developmental processes in the early human neuroepithelium.
Despite these advantages, cerebral organoid models do show limited specification and maturation of refined human cortical cell types54,55, making it important to validate findings regarding polarity and cell type transitions in primary samples, when possible. For example, recent studies utilizing non-human primate tissue suggest that both radial glial fibres and neuroepithelial cells may retract their basal processes prior to mitosis56. As studies in organoids have utilized the lack of this retraction in radial glial cells as a cell type-defining behaviour, this makes the transition state from neuroepithelial cells to radial glia less clear in this model. Additionally, a transcriptional study of first-trimester cortical development indicated significant differences in the temporal expression level of gene programmes defining neuroepithelial or radial glial cell types in organoids compared with primary tissue11.
Although these findings highlight some of the current limitations in the recapitulation of complex corticogenesis in organoids, the studies also show that many features are preserved, demonstrating their value as a human model system. For example, organoids have robust division and differentiation programmes and clearly defined early cytoarchitectural organization54,55 (BOX 1). This has allowed organoid models to be used to discover features of human progenitor morphology, behaviour and polarity in the context of both health and disease.
Mechanisms of progenitor polarity
As noted above, both neuroepithelial and vRG cell progenitors have highly polarized apico-basal orientation and are anchored to the apical surface of the developing cortex7,9, whereas oRG cells maintain their apico-basal orientation with a single primary process oriented towards the basal surface of the cortex17. IPCs are not defined by polarity or orientation to a particular cortical surface but are a delaminated population with variable morphology and great dynamic mobility22. The differences in polarity and orientation of these distinct progenitor populations enable their key functions. Neuroepithelial cells are fundamental in establishing the initial apico-basal organization and proliferative stem cell expansion of the emerging neocortex. vRG cells and oRG cells not only serve as neural stem cells but (via their radial fibres) also form a scaffold that guides neuronal migration57,58. Neurogenic IPCs, on the other hand, are not physically constrained and are able to migrate more freely within the progenitor zones, which may enable subsequent neurogenic contributions to appropriate laminae. Neuroepithelial cells and vRG cells contain a primary cilium, which is an important determinant of progenitor polarity. Neuroepithelial cells that contain a primary cilium on the basolateral, rather than an apical, surface are committed to IPC fate and delaminate from the apical surface59. Each distinct progenitor population not only has unique functions, guided by their specific polarity and morphology, but also has distinct regulatory mechanisms.
Primary cilium.
A slim microtubule-based organelle that is present in most eukaryotic cells. The primary cilium is made up of nine microtubule bundles (called an axoneme) and has a ciliary membrane. In neuroepithelial cells and radial glial cells, the primary cilia extend into the ventricular space.
Regulation of apical and basal progenitor attachment.
The establishment of apico-basal polarity in cortical progenitors requires the assembly and regulation of specialized junctional complexes — including tight junctions and adherens junctions — along the apical edge of neuroepithelial cells and vRG cells60–62. In humans the establishment of neuroepithelial cell polarity during the first trimester of development is particularly important9,11,51. Polarity-regulating junctional complexes have been predominantly studied in the mouse (FIG. 2; reviewed extensively in REFS.59–68); therefore, here we will focus on human-specific aspects of their regulation and their contributions to cortical progenitor polarity.
Fig. 2 |. Subcellular structures and organelles regulating progenitor polarity.
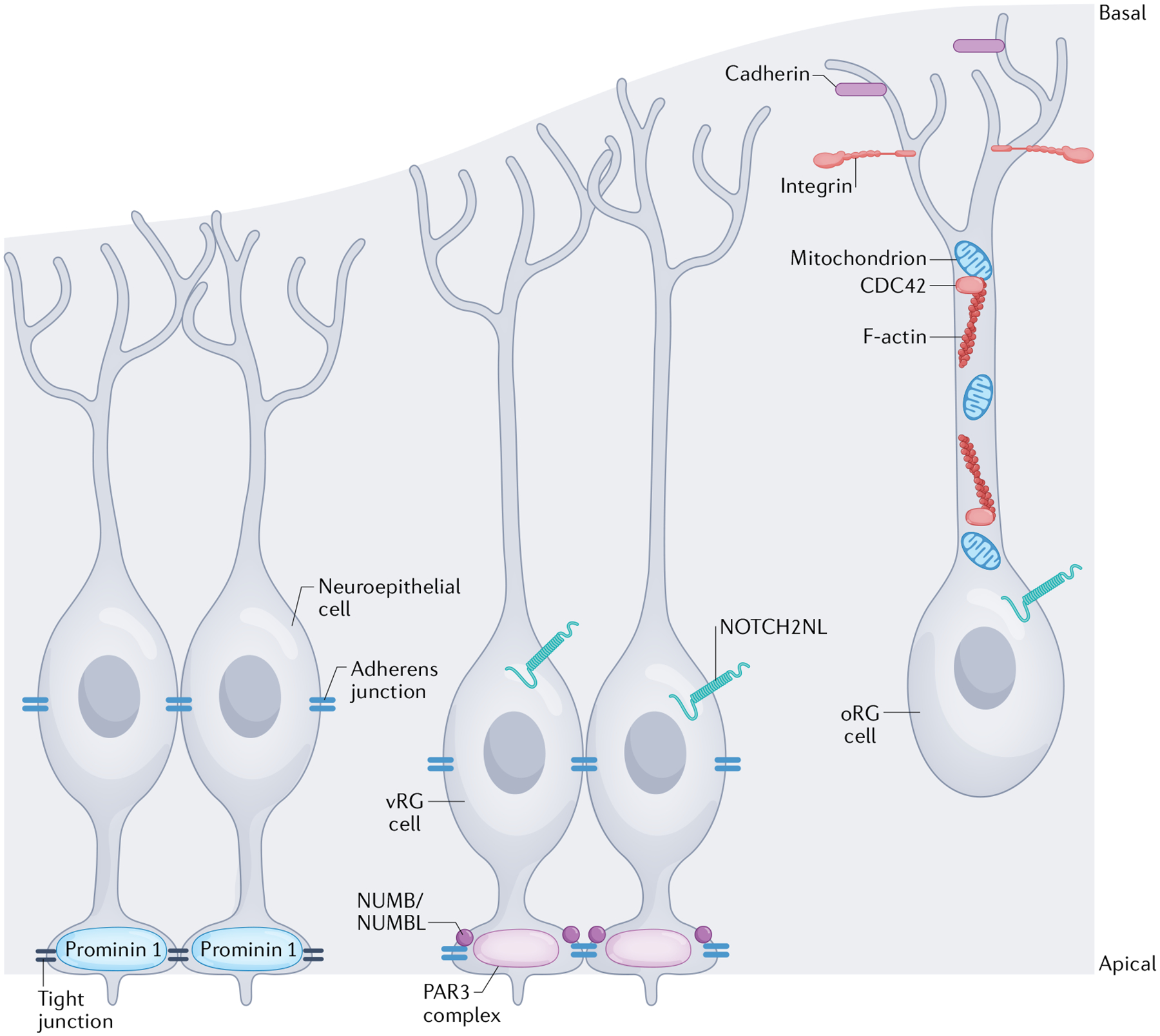
The specific morphology and polarity of different cortical progenitor subtypes are regulated by distinct molecular mechanisms. Although the dynamic regulation of human progenitor polarity is being revealed, most mechanistic studies have been performed in rodent or other model species. This figure represents a cumulative understanding of polarity from animal and human studies. The apical membranes of neuroepithelial cells form the ventricular surface of the cortex. Neuroepithelial cells express the transmembrane glycoprotein prominin 1 at the apical membrane168 and tight junctions form between neuroepithelial cells to establish the ventricular surface and establish their polarity. In a more basal location within the cells, along the cell body, adherens junctions are also formed between neuroepithelial cells60,61,63. Ventricular radial glial (vRG) cells have a similar apical–basal polarity, but their apical anchoring to the ventricular surface is regulated by a different set of proteins, including those that comprise the PAR3 complex, and the vRG cells are predominantly connected to one another by adherens junctions75. The proteins NUMB and/or NUMBL help maintain these adherens junctions at the apical surface74. Junctional complexes regulate receipt of NOTCH signals, which are involved in mediating proliferation and polarity. The human-specific receptor, NOTCH2NL, impacts radial glial cell proliferation76,78. Outer radial glial (oRG) cells retain a basal polarity, but without an apical attachment. Their connection to the basal lamina is regulated by cadherins and integrins65,67 in the oRG cell endfeet and the integrity of their basal processes is maintained by appropriate F-actin activity (regulated by the GTPase CDC42) and mitochondrial function52,82.
Tight junctions and adherens junctions are cell–cell adhesion complexes that have distinct functional roles in controlling the polarity of neuroepithelial cells and radial glial cells, respectively. Tight junctions are typically located more apically and associated with apical polarity, whereas adherens junctions are located at slightly more basal positions with respect to the tight junctions. However, studies in mouse models have shown that the localization of these junctional complexes to the apical membrane of the progenitors is controlled by similar intracellular transport mechanisms. Further, the proteins that comprise both of these junctional complexes regulate polarity. Briefly, tight junctions regulate polarity by coordinating transport of ions and small molecules, whereas adherens junctions coordinate polarity through their roles as transmembrane and scaffolding proteins (reviewed elsewhere2,69–73) (FIG. 2). Interestingly, the outcome of these disparate mechanisms of polarity regulation is the regulated asymmetrical transport of NUMB protein to the apical membrane, where it can regulate the local intercellular milieu via the NOTCH signalling cascade74,75. There is, however, evidence for the existence of human-specific mechanisms that influence these junctional complexes. Recent genomic studies have identified distinct human paralogs of mouse polarity and proliferation-regulating proteins of the NOTCH2 family in human neural progenitors76–78. In stem cell and organoid models, expression of one of the human-specific NOTCH2 paralogs, NOTCH2NL, increases progenitor proliferation76,78. The functional effects of allelic variations of NOTCH2NL in the human cortex also appear to be relevant to neurodevelopmental disorders: duplications of NOTCH2NL are present in patients with macrocephaly, whereas microcephaly is seen in patients with NOTCH2NL deletions78. However, whether these human-specific NOTCH proteins have a role in regulating progenitor polarity remains unclear and detailed studies in the human cortex are still needed to better understand these mechanisms.
Macrocephaly.
A cortical malformation in which the cortex is larger than normal, identified by an increase in head circumference.
Microcephaly.
A cortical malformation in which the cortex is smaller than normal.
In rodents, experimental disruption of any of the components of the junctional complexes results in delamination, with a loss of cell polarity and a separation of the progenitors from the apical surface, leading to cortical disorganization74,79,80. In humans, however, it has been suggested that the developmentally regulated dissolution of junctional complexes may increase the size of the developing cortex by contributing to the emergence of delaminated progenitor cells, such as oRG cells and IPCs28,32,58. In support of this idea, recent studies have shown that cellular pathways controlling epithelial–mesenchymal transition (EMT) via loss of cellular adhesion and polarity in epithelial cell types are enriched during oRG cell generation in the human brain. Proteins such as PTPRZ1 that are involved in EMT may regulate the production of oRG cells and changes in oRG cell morphology and polarity28,29,32. More broadly, EMTs and dissolution of junctional complexes impact the morphology and polarity both of neuroepithelial cells as they transition to radial glial cells and of vRG cells as they differentiate into neurogenic IPCs. Future studies probing the cell type-specific contributions of EMTs, junctional complex changes during transitions and the delamination of progenitors using human models, such as organoids, will further our understanding of the role of polarity regulation in long-term cortical organization and function.
Delamination.
A process in which epithelial cells lose contact with their neighbours and move out of the epithelial sheet. In the developing cerebral cortex, this occurs when neuroepithelial cells and radial glial cells lose junctional contacts and/or retract their cellular processes and then migrate into a different position in the developing cortex.
Intracellular trafficking.
The trafficking of organelles and junctional complex components by the cytoskeleton (see below) plays a role in the maintenance of progenitor polarity. Little is known about how this occurs in the developing human brain. However, in vRG cells in the mouse cerebral cortex, lipid signalling regulates apically directed trafficking of the Golgi complex through interaction with the actin cytoskeleton during neurogenesis81. This process requires phosphatidylinositol transfer proteins and is key to proper Golgi organelle distribution and overall polarity81. Mitochondrial transport is also needed for appropriate metabolic regulation of polar processes in mouse progenitor cells. Mitochondria are trafficked from the cell soma along the vRG basal process and accumulate in high-energy locations such as the endfeet82 (FIG. 2). When sugar levels in blood or cerebrospinal fluid (CSF) are disrupted, misregulated mitochondrial trafficking results in the production of inappropriate levels of Ca2+ and, ultimately, causes the degradation of the radial glial scaffold82. In human cell cultures, when the trans-Golgi Network is disrupted by mutations in the ADP-ribosylation factor guanine nucleotide-exchange factor 2 (ARFGEF2), the transport of Golgi complexes to the cell surface is disrupted83. Ultimately, mechanisms that interfere with polarized organelle trafficking can lead to decreased radial glial proliferation and migration3,82,83.
Cerebrospinal fluid.
(CSF). A fluid that contains the necessary nutrients for brain health. CSF is produced by the choroid plexus and flows through the ventricles. During development, radial glial apical and basal endfeet are exposed to CSF.
Cytoskeletal regulation.
Cytoskeletal proteins regulate cellular morphology, mitotic behaviour and polarity by establishing filamentous structures that provide cellular support and mediate protein and organelle trafficking (see above). The cytoskeleton of all cells, including progenitors, is composed of actin filaments and microtubules. The family of Rho GTPases regulate actin and microtubule motility and assembly in a dynamic manner84. The regulation of these cytoskeletal proteins can uniquely regulate aspects of cell polarity, as well as cellular structure, migration and the mitotic behaviour of radial glial cells29,32,52,85. In addition, microtubule motor proteins and associated proteins use microtubules to mediate nuclear movement, self-renewal and INM in radial glial cells. In rodents, disruptions in intracellular transport via the cytoskeleton can lead to changes in the polarity of radial glial cells86,87. Although many features of cytoskeletal function are conserved across mammalian species, the unique progenitor types present in the developing human cortex require different molecular regulation of the cytoskeleton.
For example, investigations in human oRG cells have revealed a novel division of labour amongst different cytoskeletal proteins and signalling regulators that drive polarity and division in this cell type (FIG. 3). MST, under-taken by oRG cells during mitosis, consists of three distinct stages: the migratory movement of the cell prior to the jump, the actual ‘jump’ (the rapid movement of the nucleus during S phase) and cytokinesis. The jump requires the activation of the actin-binding protein non-muscle myosin II, but does not require microtubule function, and is also independent of cytokinesis. This non-muscle myosin II-mediated behaviour requires signalling via the Rho GTPase ROCK29. By contrast, mitotic cell division in oRG cells requires the microtubule protein β-tubulin. Finally, the length of the oRG fibre, fibre orientation and corresponding jump distance are dependent on F-actin regulation by CDC42, which is in turn regulated by mTOR signalling52 (FIG. 3). oRG cells are an expanded progenitor population in humans, and this complex cytoskeletal regulation demonstrates how different aspects of division behaviour, and subsequent establishment of cellular identity and polarity, can be regulated by distinct molecular machinery.
Fig. 3 |. Human-specific regulation of the cytoskeleton in outer radial glial cells.
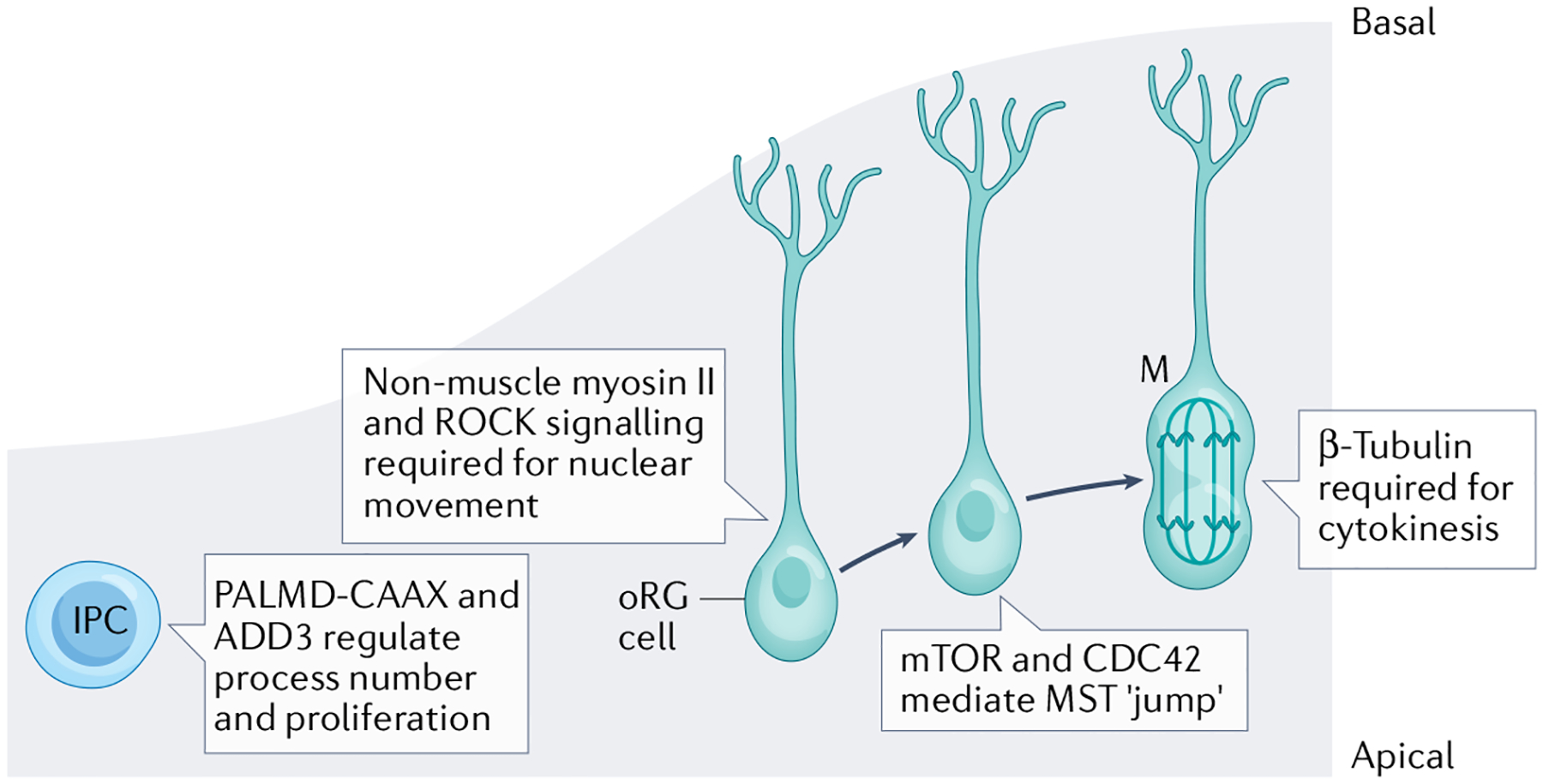
In the developing human cortex, outer radial glial (oRG) cells divide via a process known as mitotic somal translocation (MST). Appropriate oRG cell basal polarity is vital for the basal direction and appropriate migration of differentiating oRG cells during MST. Each phase of MST is regulated by distinct molecular components. Nuclear movement prior to division is regulated by non-muscle myosin II and requires ROCK signalling29. The distance of the MST ‘jump’ is regulated by mTOR-mediated CDC42 activity52. Finally, oRG cell cytokinesis requires β-tubulin29. Intermediate progenitor cell (IPC) multipolar process numbers are regulated by membrane-bound PALMD-CAAX and ADD3 (REF.30).
The polarity of multipolar IPCs in the human cortex is also maintained by the regulation of the actin cytoskeleton. Expression of the membrane-bound PALMDELPHIN protein (PALMD-CAAX) is enriched in the SVZ of the human and ferret, but not mouse, neocortex30. PALMD-CAAX controls the cytoskeleton and complexes with the cytoskeletal-associated proteins ADD3 to maintain the multipolar morphology of IPCs as well as their proliferative capacity, possibly via downstream effects of integrin signalling30. Future studies will be needed to explore the differential regulation of the cytoskeleton in diverse cortical cell types and how this regulates the dynamics of progenitor cell polarity during developmental transition states.
Unique regulation of polarity in human oRG cells.
ARHGAP11B, a Rho GTPase activating protein that regulates progenitor proliferation and polarity, is expressed in the developing human cortex, but not in the cortex of other mammals, including non-human primates88,89. ARHGAP11B arose from a partial duplication of the gene encoding a related protein, ARHGAP11A. ARHGAP11B gain-of-function experiments in mice and ferrets demonstrate that the presence of this protein leads to an increase in symmetric divisions of progenitors, as well as an increase in oRG cell numbers and changes in radial polarity (with an increase in monopolar and bipolar radial glial-like cells, compared with multipolar IPCs)88,89. The increase in oRG cell numbers in mice expressing ARHGAP11B is associated with increased proliferation of oRG cells, but not vRG cells, resulting in a subsequent increase in cortical neuron number and overall cortical size90.
Similarly, in the human cortex, but not in that of non-human primates, there is a specific increase in the expression of the gene encoding mTOR in oRG cells91,92 that is not observed in other human progenitor types52. Functional studies in human oRG cells demonstrate that dysregulation of mTOR signalling results in changes to oRG morphology, motility and basal process orientation52. Interestingly, despite the evolutionary divergence that leads to increased mTOR signalling in human oRG cells, the downstream mechanisms through which this signalling mediates oRG cell morphology and migration are conserved. mTOR regulates actin abundance in oRG cells through activation of the Rho GTPase protein CDC42, to maintain oRG fibre length and polarity.
Together, these studies demonstrate some of the unique features of recent evolution that have influenced the regulation of human progenitor number, cortical expansion and polarity. However, the downstream targets of the affected genes remain part of the shared cytoskeletal machinery. Many questions remain about the mechanisms driving conserved and divergent programmes altering cellular morphology and polarity in human progenitor cells.
Functions of progenitor polarity
Polarity governs response to developmental signals.
The progenitor niches (VZ, oSVZ) of the developing human cortex contain distinct progenitor cell types that are actively patterned by developmental signalling molecules vital for appropriate proliferation, fate determination and regionalization. The morphology and polarity of each of the progenitor subtypes found in the developing human cortex uniquely determine how the cells interact within various developmental niches, as well as how they receive and respond to patterning information from extracellular sources.
Developmental niches.
Uniquely defined extracellular micro-environments that are clearly distinguished from other parts of the cortex.
Neuroepithelial cells and radial glial cells receive extracellular signals from the ventricle93, the basement membrane and/or the pial surface, and local signals from neighbouring cells. In mouse models, these apical progenitors receive diffusible signals from the CSF through their primary cilia at the ventricular surface, initiating signalling cascades that can regulate their proliferation94–96; however, the influence of the CSF and/or ciliary interactions in the regulation of human cell types has not been directly explored94–96. There is evidence from animal studies that as the composition of the CSF changes with development, its effects on progenitor cells also change. For example, multiple studies demonstrate that rodent forebrain explants or neurospheres created using progenitor cells obtained at distinct developmental stages require age-matched CSF to optimally maintain appropriate progenitor identity, proliferation and neuronal differentiation. Further investigations are required to understand the influence of temporal changes in CSF during human development93,97.
In mouse models, as apical progenitors mature and gap junction complexes form between radial glia and migrating neurons, they begin to be influenced by neighbouring cells98,99. In addition, the basal processes of radial glial cells can integrate extracellular signals from the basement membrane, the meninges and the pial surface100,101. Several recent studies have highlighted the dynamic organization of basal processes and their ability to respond to local signals9,102, often through local translation occurring at the basal endfeet. The endfeet thus serve as both sensors and effectors of signals from the basal niche, and errors in these functions can result in neurodevelopmental disorders102–104.
Similar to vRG cells, oRG cells can receive niche signals from the pial surface via their basal processes, but unlike apical progenitors they do not receive direct signals from the ventricular surface (although they may receive small molecular weight signals from the CSF). In mice, ferrets and humans, both vRG cells and oRG cells receive developmental cues from their cortical neighbours or from the local environment via intercellular and intracellular signalling, blood vessels and the extracellular matrix19,76,78,101. Radial glial cells are also coupled to one another by gap junctions, and studies in mouse models have shown that changes in membrane potential can spread to neighbouring radial glia and impact progenitor cell identity105. Similarly, newborn neurons in mice are coupled to radial glial cells by gap junction contacts and can influence radial glial polarity via contact inhibition106–108. Additionally, radial glial cells express GABA and glutamate receptors and respond to neurotransmitter signals, presumably released by neurons, to regulate radial glia proliferation98,99,109.
IPCs can also receive signals from their cortical neighbours, including endothelial cells110,111, as well as from the extracellular matrix. As IPCs are delaminated, and often multipolar, the niches within which they reside change with time during human cortical development36. IPCs are less likely to receive long-range signals from their processes as radial glia do, but instead are more likely to respond to local cues from immediate cell neighbours.
Studies in the mouse highlight how polarity differences between progenitor cell types can regulate their ability to respond to signals from different developmental niches, and similar principles are likely to apply in the developing human brain. During early gestational stages in rodents, immature excitatory migrating neurons move radially along the basal processes of their parent or neighbouring progenitor cells, and do not disperse far laterally112–114. As a result, neurons retain their anteroposterior or medio-lateral position within the cortex at these early stages and the overall positional information of these cells is maintained in the maturing cortex115,116. This radial organization establishes overall network topology by establishing cortical columns. Thus, the polarity of progenitor cells contributes to the generation of the cortical protomap and the emergence of functional modules that define cortical networks117. This spatial map is particularly challenging to establish and maintain in human PSC-derived models, such as organoids. Future studies in which these models are refined to enable them to maintain a radial glial scaffold long-term will therefore be key for understanding the mechanisms that establish human cortical network topology and organizational dynamics.
Polarity influences gyrencephaly.
Junctional complexes anchor progenitors to one another, thus forming the apical (ventricular) surface of the epithelium. The mitotic expansion of progenitors at the apical surface is, however, limited by the available ventricular space. One way to accommodate increased progenitor cell numbers is via INM, which allows the relatively bulky cell nuclei to occupy the apical surface only during mitosis and permits a larger number of progenitor cells to contact the apical surface through their apical endfeet. It has also been proposed that the evolution of the expanded cerebral cortex in humans may result not only from INM and an increase in apical surface area through expansion of the ventricle, but also from a longer proliferative period118,119. In addition, some of the shifts in the mechanisms regulating progenitor polarity at the apical surface discussed above may have also contributed by leading to the generation of greater numbers of basally located progenitors3,120,121 capable of undergoing mitosis away from the ventricular surface. In support of this idea, the number of oRG cells is increased in large brain mammals, as is the degree of gyrencephaly122. True gyrencephaly has been described as the expansion and folding of the outer surface of the cortex, resulting in an increase in surface area, whereas the ventricular surface remains small and relatively smooth. This supports the hypothesis that progenitors not bound to the ventricular surface may contribute to human cortical expansion123. Radial and tangential tension produced by radial glial fibres and axon tracts, increased ventricular surface area and greater numbers of neurons and glial cells have all been hypothesized to contribute to cortical folding123–125. How oRG cells may contribute to cortical folding is, however, unclear. A recent study in non-human primates emphasized that neurogenesis is completed prior to cortical folding, and points to oRG cell production of glial cells as a feature that may increase cortical size and folding40. Thus, although it seems likely that changes in cell polarity have contributed to evolutionary changes in brain size and structure, much work remains to be done to understand the cell type-specific mechanisms driving evolutionary expansion and the contribution of unique cell types, molecular programmes and transition states to the overall composition of the human cortex.
Gyrencephaly.
The characteristic folding of the cerebral cortex, resulting in increased cortical surface area.
Polarity alterations in disease
Defects in progenitor development — and, in particular, progenitor polarity — are implicated in numerous neurological conditions. Mutations impacting the radial architecture of the developing cortex and its proliferation dynamics result in various cortical malformations and dysplasias accompanied by seizures and neuropsychiatric symptoms, and perhaps can even be linked to neurodegenerative diseases73,121. In animal models, disruptions of the microtubule-regulated and polar processes of cell division impact overall brain size and can lead to microcephaly or megalencephaly, whereas loss of the apical adherence that underlies progenitor polarity can result in changes to brain structure producing microcephaly, lissencephaly and cortical heterotopias, and changes in basal process adherence and polarity can impact neuronal migration and also result in heterotopias and epilepsy (TABLE 1).
Table 1 |.
Mutations in genes that regulate polarity in neural disease
| Mutated gene | Role in polarity regulation | Disease(s) | Refs. |
|---|---|---|---|
| MCPH1 | Centrosomal complex protein that has an impact on chromosome condensation, leading to premature differentiation | Microcephaly | 129,130 |
| ASPM | Centrosomal complex protein that anchors vRG cells to the apical surface | Microcephaly | 127,131 |
| CDK5RAP2 | Centrosomal complex protein involved in spindle pole positioning during neuroepithelial cell division | Microcephaly | 128 |
| CENPJ | Centrosomal complex protein involved in spindle pole positioning during neuroepithelial cell division | Microcephaly | 128 |
| WDR62 | Regulates cilium disassembly and length | Microcephaly | 133 |
| LIS1 | Regulates the microtubule motor, dynein | Lissencephaly | 49,134,137 |
| YWHAE | Impacts cell adhesion and microtubule organization | Lissencephaly | 136,138 |
| PTEN | Regulates progenitor pool expansion | Megalencephaly | 139,140 |
| LGI1 | Interacts with the PAR6/aPKC polarity complex | Hydrocephalus | 80,142 |
| CDC42 | Involved in the localization of apical polarity complex | Holoprosencephaly | 143 |
| RHOA | Cytoskeletal regulator that affects the radial glial scaffold and polarity | Cortical dysplasia | 145 |
| MTOR | Regulates cytoskeletal organization in oRG cells | Cortical dysplasia | 52 |
| FLNA | Actin binding protein in vRG cells impacting apical adhesion, apical/basal orientation and division | Periventricular heterotopia | 146,147 |
| ARFGEF2 | Regulates vesicle trafficking in radial glia impacting junctional complex localization and cytoskeletal interaction, which alters polarity | Periventricular heterotopia | 83,146,160 |
| MAP3K4 | Affects MAP kinase signalling, regulates the cytoskeleton and interacts with FLNA to impact radial glial polarity and migration | Periventricular heterotopia | 146,161 |
| PCDH19 | Adhesion molecule that increases co-localization of cytoskeletal proteins and cadherins impacting radial glial polarity | PCDH19 girls clustering epilepsy | 148 |
| DISC1 | Impacts WNT signalling affecting vRG polarity | Major depression, schizophrenia and bipolar disorder | 152–154 |
| FZD3 | Impacts WNT signalling affecting vRG polarity | Schizophrenia | 156 |
| CYFIP1 | Impacts adherens junctions and polarity in radial glia | Schizophrenia and autism | 157 |
| HTT | Involved in localization of junctional complexes and ciliary development | Huntington disease | 158,159 |
ARFGEF2, guanine nucleotide-exchange factor 2; DISC1, disrupted in schizophrenia 1; oRG, outer radial glial; PCDH19, Protocadherin 19; vRG, ventricular radial glial.
Megalencephaly.
A cortical malformation in which the brain is atypically large or heavy, defined by an increase in brain tissue.
Lissencephaly.
A cortical malformation in which the brain is smooth and does not have appropriate gyrification.
Heterotopias.
Cortical malformations in which neural cells are in the incorrect position.
Polarity changes in microcephaly.
Dysfunction of the centrosome in apical progenitors can result in a premature shift from symmetric to asymmetric divisions, depleting the progenitor pool and resulting in microcephaly. Several gene mutations identified in patients with microcephaly, including mutations in MCPH1, ASPM, CDK5RAP2 and CENPJ126–131, affect the centrosomal complex. The ASPM protein complex also anchors vRG cells to the apical surface, and recent studies in gene-edited ferrets132 have shown that loss of Aspm function results in delamination of apical vRG cells and a concomitant increase in the number of basally located oRG cells. In human cerebral organoids, deletion of the centrosomal protein WDR62 during progenitor proliferation specifically impacts oRG cells by affecting cilium disassembly, increasing cilia length and delaying cell cycle progression resulting in premature differentiation133. Future studies exploring how dysregulation of other centrosomal genes impact the polarity of apical and basal progenitor types in human PSC models will provide us with further information on the mechanisms regulating brain size and growth.
Centrosome.
A cellular structure that comprises microtubules and is involved in cell division.
Polarity changes in lissencephaly.
LIS1, the gene mutated in individuals with classic lissencephaly, and 14.3.3ε, a highly abundant adaptor protein encoded by YWHAE, maintain expression of cell adhesion molecules and microtubule organization in vRG cells in human organoid models through non-cell autonomous N-CADHERIN and/or β-CATENIN signalling134–136. LIS1 regulates the microtubule protein dynein, and is required to maintain radial glial cell polarity and appropriate division behaviour in the rat cortex137. In humans, LIS1 mutation alters the cleavage angle of vRG cell mitotic divisions, increases the number of multipolar SVZ progenitors (at the expense of vRG cells) and produces an increase in the number of deep-layer neurons, suggesting a premature shift towards differentiating divisions and depletion of the progenitor pool49,134. This leads to smaller size in brain organoids137. Miller–Dieker syndrome, caused by a heterozygous deletion of chromosome 17p13.3 that involves the genes LIS1 and YWHAE, leads to a more severe form of lissencephaly accompanied by microcephaly136,138. oRG cells in organoids derived from patients with Miller–Dieker syndrome have altered MST behaviour; they translocate farther and their mitosis is delayed or arrested. This change, as well as the altered spindle orientation of vRG cells, leads to increased differentiating divisions and a depletion of the radial glial cell pool49.
Deep-layer neurons.
Subcortically projecting excitatory neurons that reside in cortical layers V and VI.
Polarity changes in megalencephaly.
Megalencephaly arises due to a mutation in PTEN and is characterized by brain overgrowth. Megalencephaly is often associated with malformations of cortical development and increased risk of epilepsy, intellectual disability and autistic phenotypes139. Neural progenitor cells may contribute to these phenotypes. Indeed, abnormal expansion of the progenitor pool in the VZ and oSVZ, along with inappropriate migration, contribute to an increase in organoid surface folding following homozygous PTEN deletion140. However, whether alterations in radial glial cell polarity impact proliferation and differentiation in this human model remains to be explored in future studies.
Polarity changes in hydrocephalus.
Changes to the radial orientation of neuroepithelial cells and radial glial cells can result in hydrocephalus. For example, in mice, vRG cells that lack primary cilia show changes to the mitotic spindle and generate an increased number of IPCs141. This change in radial organization at the apical surface is associated with altered mTOR signalling and leads to an increase in ventricular size during embryogenesis resulting in postnatal hydrocephalus141. In mice, mutations in Lgl1, which encodes a protein that interacts with the PAR6/aPKC polarity complex, result in inability of neuroepithelial cells to apically localize NOTCH signalling inhibitors, leading to prolonged symmetric divisions80. Prolonged symmetric proliferative divisions, occurring at the expense of differentiation, can lead to progenitor cell death, increased ventricular size and hydrocephalus80,142. Future studies exploring how gene mutations altering ciliary phenotypes impact human neuroepithelial cells, vRG cells, oRG cells and IPCs will be key to increase our understanding of the relationship between division types, polarity and the integrity of the ventricular surface.
Hydrocephalus.
A condition in which there is increased cerebrospinal fluid volume in the ventricles.
Polarity changes in holoprosencephaly.
The appropriate development of forebrain midline structures is required for normal brain and craniofacial development. In holoprosencephaly, midline structures, including the eyes, do not develop correctly as a consequence of disruption to forebrain development during early gestation. When the Rho GTPase CDC42 is deleted in the developing rodent cortex, this leads to inappropriate localization of apical polarity complex proteins in neuroepithelial cells and altered radial glial fibre length. The consequences of these changes to neuroepithelial and radial glial polarity and structure include a lack of hemisphere separation and holoprosencephaly143. Future studies implementing patient-derived and gene-edited human stem cell models, such as organoids, may help explore this mechanism in human holoprosencephaly.
Polarity changes in cortical dysplasias and epilepsy.
Although several cortical malformations arise as a result of dysregulation of apical polarity, anchoring of the radial glial cell basal process to the pial surface is also vulnerable to disruption in cortical disease. Disruption of the radial processes of both vRG cells and oRG cells can be problematic for human brain development and function32,49,52,144. Similarly, when β1-class integrins that regulate basal polarity are deleted in the rodent brain, radial glial cell endfeet do not appropriately connect to the pial surface, impacting formation of the meninges at the pial surface as well as distribution of Cajal–Retzius neurons in the marginal zone68. Changes to the laminar organization of cortical neurons as a result of radial process disruption characterize a range of cortical dysplasias and malformations. For example, subcortical band heterotopia is associated with a mutation in the Rho GTPase RHOA, resulting in arrested neuronal migration145. In mice with mutations in RhoA, there is widespread disruption to the cytoskeleton, including both actin filaments and microtubules, leading to changes in the radial glial scaffold that alter the final positioning of neurons. Periventricular heterotopia occurs when neurons are mispositioned and do not migrate away from the lateral ventricle, resulting in a malformation that is often associated with seizures. This disorder is caused by mutations that disrupt vesicle trafficking and cell adhesion in radial glial cells, including mutations in FLNA, ARFGEF2 and MAP3K4 (REFS.146,147). PCDH19 girls clustering epilepsy is a form of epilepsy caused by loss of the X chromosome gene PCDH19, encoding Protocadherin 19), which regulates radial glial polarity. Loss of PCDH19 results in inappropriate and premature differentiation of radial glial cells into neurons148.
Cortical dysplasias that result in paediatric epilepsy, including focal cortical dysplasia and tuberous sclerosis, are caused by mutations in mTOR signalling genes139,149–151. As described above, mTOR regulates oRG cell morphology and basal process orientation52. Disruption of mTOR signalling in cortical organoids or primary cortical tissue leads to truncation of the oRG basal process, changes to oRG cell migration and division behaviour and, ultimately, broad disruption of the glial scaffold52. These effects arise owing to changes in cytoskeleton organization involving the Rho GTPase proteins, which are known regulators of cell polarity. Thus, cytoskeletal regulatory mechanisms maintain the radial architecture of the human cortex with implications for long-term effects on cortical cell migration and organization.
Polarity changes in neuropsychiatric disease.
Several lines of evidence indicate that loss of progenitor polarity is associated with the development of neuropsychiatric disease. For example, mutations of the disrupted in schizophrenia 1 gene (DISC1) increase the incidence of major depression, schizophrenia and bipolar disorder, and disrupt the WNT signalling pathway through abnormal GSK3 expression and loss of β-catenin activity152,153. Loss of DISC1 function leads to a decrease in radial glial cell proliferation with a corresponding increase in early differentiation154. In organoids, when GSK3 function is inhibited, vRG cell polarity and proliferation are disrupted. Cellular migration is also impacted by phosphorylation of DIXDC1, which regulates binding of DISC1 to its partner, NDEL1. Without these interactions, neuronal positioning is disorganized155. More broadly, in case studies, individuals with mutations in WNT signalling genes, such as FZD3, have an increased incidence of schizophrenia156.
The 15q11.2 copy number variant is a risk factor for both schizophrenia and autism. This mutation impacts the function of CYFIP1, which comprises one part of the actin-regulating WAVE protein complex. Changes to CYFIP1 and WAVE result in changes to the adherens junctions and polarity of radial glial cells derived from 15q11.2 patient induced PSC lines157. The loss of radial glial cell polarity results in inappropriate positioning of vRG cells at a distance from the ventricle with a corresponding increase in multipolar IPCs that leads to the generation of cortical neurons outside the appropriate proliferative zone157.
Overall, mechanistic studies in human stem cells and mice support the hypothesis that autism and schizophrenia risk genes may be associated, in part, with early defects in progenitor polarity, morphology and proliferation. Further study of these cellular processes in human model systems can clarify their contribution to the onset of specific disorders.
Polarity changes in neurodegeneration.
Recent intriguing studies have provided hints that foundational cortical progenitor polarity may influence the onset of neurodegeneration. Primary human developing cortical tissue from embryos carrying Huntington disease-related mutations in HTT are reported to exhibit inappropriate localization of radial glial junctional complexes, abnormal cilia development and changes to radial glial cell polarity. The changes to vRG cell morphology and polarity also impact INM division behaviour resulting in premature differentiation of radial glial cells into IPCs158. Cortical organoids created by differentiating induced PSCs derived from individuals with Huntington disease also demonstrate morphological abnormalities159. It is surprising, given these findings, that disruptions to the seminal developmental processes of cell polarity have no apparent effects on cortical organization in individuals with Huntington disease until the onset of symptoms several decades later. However, these observations support the fundamental importance of neural progenitor polarity in early development and suggest that progenitor polarity might play a role in maintaining a healthy cerebral cortex throughout the lifespan.
Conclusions
The establishment of neuroepithelial and radial glial cell polarity is vital for the ordered proliferation, differentiation, cytoarchitecture and structure of the developing human brain. Early-established progenitor polarity sets the stage for appropriate connectivity and organization of mature cell types in the adult brain. The increased diversity of progenitor types in the developing human cortex compared with other species makes the study of these processes more challenging, but also vitally important for a fundamental understanding of human brain function and health. The recent establishment of human stem cell-derived models enables functional studies of human cortical progenitor polarity and early cortical organization. However, the difficulty in establishing and maintaining cortical organization in vitro presents technical challenges for assessing the long-term impacts of polarity on human brain development. Future studies should explore cell type-specific mutations driving cortical malformations within diverse progenitor classes in order to understand subsequent impact on mature cortical organization, connectivity and function. Moreover, although defects in polarization of progenitor cells have consequences on their division programmes, cellular orientation and differentiation, it will be important to understand how these changes also impact neuronal and glial progeny. Continued studies of the contribution of cortical progenitor polarity to the establishment of the complex structure of the developing human brain promise to provide significant future insights into evolutionary adaptations as well as the origin of many neurological disorders.
Acknowledgements
The authors thank members of the Kriegstein laboratory for invaluable discussions about human cortical development. This article was supported by National Institutes of Health (NIH) awards U01MH114825 and R35NS097305 to A.R.K. and K99MH125329 to M.G.A., and a Brain & Behaviour Research Foundation Young Investigator Grant to M.G.A.
Footnotes
Competing interests
A.R.K. is a co-founder, consultant and member of the Board of Neurona Therapeutics. The other authors declare no competing interests.
References
- 1.Geschwind DH & Rakic P Cortical evolution: judge the brain by its cover. Neuron 80, 633–647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou F-S, Li R & Wang P-S Molecular components and polarity of radial glial cells during cerebral cortex development. Cell. Mol. Life Sci 75, 1027–1041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai Y & Taverna E Neural progenitor cell polarity and cortical development. Front. Cell. Neurosci 11, 384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Götz M & Huttner WB The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol 6, 777–788 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Mira H & Morante J Neurogenesis from embryo to adult — lessons from flies and mice. Front. Cell Dev. Biol 8, 533 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakic P Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci 10, 724–735 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HO & Norden C Mechanisms controlling arrangements and movements of nuclei in pseudostratified epithelia. Trends Cell Biol. 23, 141–150 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Sauer FC Mitosis in the neural tube. J. Comp. Neurol 62, 377–405 (1935). [Google Scholar]
- 9.Subramanian L, Bershteyn M, Paredes MF & Kriegstein AR Dynamic behaviour of human neuroepithelial cells in the developing forebrain. Nat. Commun 8, 14167 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies changes in mitotic behaviour between primary human neuroepithelial cells and radial glial cells, which impact the morphology and polarity of the radial glial scaffold.
- 10.Kosodo Y et al. Cytokinesis of neuroepithelial cells can divide their basal process before anaphase. EMBO J. 27, 3151–3163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eze UC, Bhaduri A, Haeussler M, Nowakowski TJ & Kriegstein AR Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nat. Neurosci 24, 584–594 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work transcriptionally characterizes primary human neuroepithelial cells from first-trimester cortical tissue and shows that there is distinct marker gene expression in different subpopulations.
- 12.Kriegstein AR & Götz M Radial glia diversity: a matter of cell fate. Glia 43, 37–43 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Tabata H & Nakajima K Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J. Neurosci 23, 9996–10001 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakic P Elusive radial glial cells: historical and evolutionary perspective. Glia 43, 19–32 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Noctor SC, Flint AC, Weissman TA, Dammerman RS & Kriegstein AR Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001). [DOI] [PubMed] [Google Scholar]; This study shows that cortical neurons arise from radial glial cells and, post differentiation, migrate towards the cortical plate along radial fibres to establish columns of excitatory neurons.
- 16.Malatesta P, Hartfuss E & Götz M Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127, 5253–5263 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Hansen DV, Lui JH, Parker PRL & Kriegstein AR Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561 (2010). [DOI] [PubMed] [Google Scholar]; This study identifies oRG cells in the oSVZ within human cortical tissue and demonstrates their role as neuronal progenitors.
- 18.Bayatti N et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb. Cortex 18, 1536–1548 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fietz SA et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci 13, 690–699 (2010). [DOI] [PubMed] [Google Scholar]; This study identifies basally expanded neurogenic progenitor cells in the oSVZ of the primary developing human cortex and demonstrates their maintenance by integrin signalling.
- 20.Smart IHM, Dehay C, Giroud P, Berland M & Kennedy H Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12, 37–53 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyata T et al. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133–3145 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Noctor SC, Martínez-Cerdeño V, Ivic L & Kriegstein AR Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci 7, 136–144 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Hoerder-Suabedissen A & Molnár Z Development, evolution and pathology of neocortical subplate neurons. Nat. Rev. Neurosci 16, 133–146 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Luskin MB & Shatz CJ Studies of the earliest generated cells of the cat’s visual cortex: cogeneration of subplate and marginal zones. J. Neurosci 5, 1062–1075 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haubensak W, Attardo A, Denk W & Huttner WB Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl Acad. Sci. USA 101, 3196–3201 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shitamukai A, Konno D & Matsuzaki F Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J. Neurosci 31, 3683–3695 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaid S et al. A novel population of Hopx-dependent basal radial glial cells in the developing mouse neocortex. Development 145, dev169276 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Pollen AA et al. Molecular identity of human outer radial glia during cortical development. Cell 163, 55–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work transcriptionally characterizes oRG cells in primary human cortical tissue using single-cell RNA sequencing, in which identified marker genes are utilized to identify cell types.
- 29.Ostrem BEL, Lui JH, Gertz CC & Kriegstein AR Control of outer radial glial stem cell mitosis in the human brain. Cell Rep. 8, 656–664 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalebic N et al. Neocortical expansion due to increased proliferation of basal progenitors is linked to changes in their morphology. Cell Stem Cell 24, 535–550 (2019). [DOI] [PubMed] [Google Scholar]; This study shows that basal progenitors in the human and ferret cortex have increased levels of PALMDELPHIN (compared with the mouse), which potentially regulates process growth and multipolar orientation and is hypothesized to contribute to human cortical expansion.
- 31.Nowakowski TJ, Pollen AA, Sandoval-Espinosa C & Kriegstein AR Transformation of the radial glia scaffold demarcates two stages of human cerebral cortex development. Neuron 91, 1219–1227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaMonica BE, Lui JH, Hansen DV & Kriegstein AR Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat. Commun 4, 1665 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attardo A, Calegari F, Haubensak W, Wilsch-Bräuninger M & Huttner WB Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS ONE 3, e2388 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the loss of apical–basal polarity in the transition from vRG cells to IPCs restricts the cell division type and leads to neuronal production.
- 34.Arnold SJ et al. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 22, 2479–2484 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv X et al. TBR2 coordinates neurogenesis expansion and precise microcircuit organization via Protocadherin 19 in the mammalian cortex. Nat. Commun 10, 3946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pebworth M-P, Ross J, Andrews M, Bhaduri A & Kriegstein AR Human intermediate progenitor diversity during cortical development. Proc. Natl Acad. Sci. USA 118, e2019415118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betizeau M et al. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 80, 442–457 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Magescas J, Fetter RD, Feldman JL & Shen K Inherited apicobasal polarity defines the key features of axon–dendrite polarity in a sensory neuron. Curr. Biol 31, 3768–3783 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Takano T, Funahashi Y & Kaibuchi K Neuronal polarity: positive and negative feedback signals. Front. Cell Dev. Biol 7, 69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rash BG et al. Gliogenesis in the outer subventricular zone promotes enlargement and gyrification of the primate cerebrum. Proc. Natl Acad. Sci. USA 116, 7089–7094 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang W et al. Origins and proliferative states of human oligodendrocyte precursor. Cells Cell 182, 594–608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen DE et al. Fate mapping of neural stem cell niches reveals distinct origins of human cortical astrocytes. Science 376, 1441–1446 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall JJ & Mason JO Mouse vs man: organoid models of brain development & disease. Brain Res. 1724, 146427 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Subramanian L, Calcagnotto ME & Paredes MF Cortical malformations: lessons in human brain development. Front. Cell. Neurosci 13, 576 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadoshima T et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl Acad. Sci. Usa 110, 20284–20289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This foundational study establishes regionally specified cortical organoid-like aggregates from human embryonic stem cells for the study of human neural development.
- 46.Otani T, Marchetto MC, Gage FH, Simons BD & Livesey FJ 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 18, 467–480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lancaster MA et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This pioneering study develops cerebral organoid models for the study of human neural development and disease.
- 48.Mariani J et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 109, 12770–12775 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bershteyn M et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435–449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using induced PSC lines derived from patients with Miller–Dieker syndrome differentiated into cortical organoids, this study identifies changes in neuroepithelial and radial glial cell division types, mitotic behaviour and migration.
- 50.Kosodo Y et al. Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 30, 1690–1704 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benito-Kwiecinski S et al. An early cell shape transition drives evolutionary expansion of the human forebrain. Cell 184, 2084–2102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using cerebral organoids, this study observes neuroepithelial cell morphology and polarity differences between human and non-human primate species.
- 52.Andrews MG, Subramanian L & Kriegstein AR mTOR signaling regulates the morphology and migration of outer radial glia in developing human cortex. eLife 9, 58737 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies the role of mTOR signalling, a risk factor for many comorbid neurodevelopmental disorders, in the morphology and basal process orientation of oRG cells in human cortical primary tissue and organoids.
- 53.López-Tobón A et al. Human cortical organoids expose a differential function of GSK3 on cortical neurogenesis. Stem Cell Rep. 13, 847–861 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses organoids to evaluate the influence of GSK3, a risk gene for neurodevelopmental disease; when GSK3 is inhibited, altered radial glial cell polarity and organization are observed.
- 54.Bhaduri A et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature 578, 142–148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrews MG & Nowakowski TJ Human brain development through the lens of cerebral organoid models. Brain Res. 1725, 146470 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arellano JI, Morozov YM, Micali N & Rakic P Radial glial cells: new views on old questions. Neurochem. Res 46, 2512–2524 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reillo I, de Juan Romero C, García-Cabezas MÁ & Borrell V A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb. Cortex 21, 1674–1694 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Borrell V & Götz M Role of radial glial cells in cerebral cortex folding. Curr. Opin. Neurobiol 27, 39–46 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Wilsch-Bräuninger M, Peters J, Paridaen JTML & Huttner WB Basolateral rather than apical primary cilia on neuroepithelial cells committed to delamination. Development 139, 95–105 (2012). [DOI] [PubMed] [Google Scholar]
- 60.Farquhar MG & Palade GE Junctional complexes in various epithelia. J. Cell Biol 17, 375–412 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veeraval L, O’Leary CJ & Cooper HM Adherens junctions: guardians of cortical development. Front. Cell Dev. Biol 8, 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aaku-Saraste E, Hellwig A & Huttner WB Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure–remodeling of the neuroepithelium prior to neurogenesis. Dev. Biol 180, 664–679 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Weigmann A, Corbeil D, Hellwig A & Huttner WB Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl Acad. Sci. USA 94, 12425–12430 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakaya Y, Sukowati EW, Wu Y & Sheng G RhoA and microtubule dynamics control cell–basement membrane interaction in EMT during gastrulation. Nat. Cell Biol 10, 765–775 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U & Götz M Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development 133, 3245–3254 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Colognato H & ffrench-Constant C Mechanisms of glial development. Curr. Opin. Neurobiol 14, 37–44 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Kosodo Y & Huttner WB Basal process and cell divisions of neural progenitors in the developing brain. Dev. Growth Differ 51, 251–261 (2009). [DOI] [PubMed] [Google Scholar]
- 68.Graus-Porta D et al. β1-Class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31, 367–379 (2001). [DOI] [PubMed] [Google Scholar]
- 69.Zihni C, Mills C, Matter K & Balda MS Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol 17, 564–580 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Harris TJC & Tepass U Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol 11, 502–514 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Campbell HK, Maiers JL & DeMali KA Interplay between tight junctions & adherens junctions. Exp. Cell Res 358, 39–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartsock A & Nelson WJ Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hakanen J, Ruiz-Reig N & Tissir F Linking cell polarity to cortical development and malformations. Front. Cell. Neurosci 13, 244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rašin M-R et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat. Neurosci 10, 819–827 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Bultje RS et al. Mammalian Par3 regulates progenitor cell asymmetric division via Notch signaling in the developing neocortex. Neuron 63, 189–202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki IK et al. Human-specific NOTCH2NL genes expand cortical neurogenesis through Delta/Notch regulation. Cell 173, 1370–1384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights a human-specific gene, NOTCH2NL, which regulates radial glial progenitor proliferation and a subsequent increase in neuron numbers.
- 77.Florio M et al. Evolution and cell-type specificity of human-specific genes preferentially expressed in progenitors of fetal neocortex. eLife 7, e32332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fiddes IT et al. Human-specific NOTCH2NL genes affect Notch signaling and cortical neurogenesis. Cell 173, 1356–1369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that NOTCH2NL regulates Notch signalling and increases expansion of the cortical progenitor pool, with implications for human cortical expansion.
- 79.Li HS et al. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron 40, 1105–1118 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Klezovitch O, Fernandez TE, Tapscott SJ & Vasioukhin V Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 18, 559–571 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie Z, Hur SK, Zhao L, Abrams CS & Bankaitis VA A golgi lipid signaling pathway controls apical golgi distribution and cell polarity during neurogenesis. Dev. Cell 44, 725–740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rash BG et al. Metabolic regulation and glucose sensitivity of cortical radial glial cells. Proc. Natl Acad. Sci. USA 115, 10142–10147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that appropriate metabolic regulation and trafficking of mitochondria into radial glial processes for energy is required to maintain radial glial cell morphology and radial scaffold integrity.
- 83.Sheen VL et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat. Genet 36, 69–76 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Bezanilla M, Gladfelter AS, Kovar DR & Lee W-L Cytoskeletal dynamics: a view from the membrane. J. Cell Biol 209, 329–337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gertz CC, Lui JH, LaMonica BE, Wang X & Kriegstein AR Diverse behaviors of outer radial glia in developing ferret and human cortex. J. Neurosci 34, 2559–2570 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai J-W, Lian W-N, Kemal S, Kriegstein AR & Vallee RB Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat. Neurosci 13, 1463–1471 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie Z et al. Cep120 and TACCs control interkinetic nuclear migration and the neural progenitor pool. Neuron 56, 79–93 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Florio M et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science 347, 1465–1470 (2015). [DOI] [PubMed] [Google Scholar]; This study shows that alterations in the Rho GTPase ARHGAP11B increase the numbers of basal progenitors by altering the cell polarity and proliferation of oRG cells.
- 89.Kalebic N et al. Human-specific ARHGAP11B induces hallmarks of neocortical expansion in developing ferret neocortex. eLife 7, e41241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work, with gain-of-function studies using ferret cortical tissue, shows that ARHGAP11B increases the numbers of basal progenitors by altering cell polarity and proliferation of oRG cells.
- 90.Xing L et al. Expression of human-specific ARHGAP11B in mice leads to neocortex expansion and increased memory flexibility. EMBO J. 40, e107093 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nowakowski TJ et al. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 358, 1318–1323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pollen AA et al. Establishing cerebral organoids as models of human-specific brain evolution. Cell 176, 743–756.e17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lehtinen MK et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893–905 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Higginbotham H et al. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat. Neurosci 16, 1000–1007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakagawa N et al. APC sets the Wnt tone necessary for cerebral cortical progenitor development. Genes Dev. 31, 1679–1692 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo J et al. Developmental disruptions underlying brain abnormalities in ciliopathies. Nat. Commun 6, 7857 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chau KF et al. Progressive differentiation and instructive capacities of amniotic fluid and cerebrospinal fluid proteomes following neural tube closure. Dev. Cell 35, 789–802 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng L & Heintz N Differentiating neurons activate transcription of the brain lipid-binding protein gene in radial glia through a novel regulatory element. Development 121, 1719–1730 (1995). [DOI] [PubMed] [Google Scholar]
- 99.Hartfuss E et al. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development 130, 4597–4609 (2003). [DOI] [PubMed] [Google Scholar]
- 100.Siegenthaler JA et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell 139, 597–609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Long KR & Huttner WB How the extracellular matrix shapes neural development. Open. Biol 9, 180216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pilaz L-J, Lennox AL, Rouanet JP & Silver DL Dynamic mRNA transport and local translation in radial glial progenitors of the developing brain. Curr. Biol 26, 3383–3392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pilaz L-J & Silver DL Moving messages in the developing brain-emerging roles for mRNA transport and local translation in neural stem cells. FEBS Lett. 591, 1526–1539 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lennox AL et al. Pathogenic DDX3X mutations impair RNA metabolism and neurogenesis during fetal cortical development. Neuron 106, 404–420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vitali I et al. Progenitor hyperpolarization regulates the sequential generation of neuronal subtypes in the developing neocortex. Cell 174, 1264–1276 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elias LAB & Kriegstein AR Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci. 31, 243–250 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bittman K, Owens DF, Kriegstein AR & LoTurco JJ Cell coupling and uncoupling in the ventricular zone of developing neocortex. J. Neurosci 17, 7037–7044 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sutor B & Hagerty T Involvement of gap junctions in the development of the neocortex. Biochim. Biophys. Acta 1719, 59–68 (2005). [DOI] [PubMed] [Google Scholar]
- 109.LoTurco JJ, Owens DF, Heath MJ, Davis MB & Kriegstein AR GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15, 1287–1298 (1995). [DOI] [PubMed] [Google Scholar]
- 110.Javaherian A & Kriegstein A A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb. Cortex 19, i70–i77 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stubbs D et al. Neurovascular congruence during cerebral cortical development. Cereb. Cortex 19, i32–i41 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gertz CC & Kriegstein AR Neuronal migration dynamics in the developing ferret cortex. J. Neurosci 35, 14307–14315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rakic P Experimental manipulation of cerebral cortical areas in primates. Philos. Trans. R. Soc. Lond. B Biol. Sci 331, 291–294 (1991). [DOI] [PubMed] [Google Scholar]
- 114.Rakic P Specification of cerebral cortical areas. Science 241, 170–176 (1988). [DOI] [PubMed] [Google Scholar]
- 115.Suter B, Nowakowski RS, Bhide PG & Caviness VS Navigating neocortical neurogenesis and neuronal specification: a positional information system encoded by neurogenetic gradients. J. Neurosci 27, 10777–10784 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delgado RN & Lim DA Maintenance of positional identity of neural progenitors in the embryonic and postnatal telencephalon. Front. Mol. Neurosci 10, 373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rakic P The radial edifice of cortical architecture: from neuronal silhouettes to genetic engineering. Brain Res. Rev 55, 204–219 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rakic P A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18, 383–388 (1995). [DOI] [PubMed] [Google Scholar]
- 119.Rakic P Neuronal migration and contact guidance in the primate telencephalon. Postgrad. Med. J 54, 25–40 (1978). [PubMed] [Google Scholar]; This study shows that radial glial cells form the radial scaffold, upon which neurons migrate, and newborn neurons localize to radial positions to form cortical columns.
- 120.de Juan Romero C, Bruder C, Tomasello U, Sanz-Anquela JM & Borrell V Discrete domains of gene expression in germinal layers distinguish the development of gyrencephaly. EMBO J. 34, 1859–1874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Florio M & Huttner WB Neural progenitors, neurogenesis and the evolution of the neocortex. Development 141, 2182–2194 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Herculano-Houzel S The evolution of human capabilities and abilities. Cerebrum 2018, cer-05–18 (2018). [PMC free article] [PubMed] [Google Scholar]
- 123.Llinares-Benadero C & Borrell V Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat. Rev. Neurosci 20, 161–176 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Van Essen DCA 2020 view of tension-based cortical morphogenesis. Proc. Natl Acad. Sci. USA 117, 32868–32879 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shinmyo Y et al. Localized astrogenesis regulates gyrification of the cerebral cortex. Sci. Adv 8, eabi5209 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jayaraman D, Bae B-I & Walsh CA The genetics of primary microcephaly. Annu. Rev. Genomics Hum. Genet 19, 177–200 (2018). [DOI] [PubMed] [Google Scholar]
- 127.Bond J et al. ASPM is a major determinant of cerebral cortical size. Nat. Genet 32, 316–320 (2002). [DOI] [PubMed] [Google Scholar]
- 128.Bond J et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat. Genet 37, 353–355 (2005). [DOI] [PubMed] [Google Scholar]
- 129.Jackson AP et al. Identification of microcephalin, a protein implicated in determining the size of the human brain. Am. J. Hum. Genet 71, 136–142 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhong X, Pfeifer GP & Xu X Microcephalin encodes a centrosomal protein. Cell Cycle 5, 457–458 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Zhong X, Liu L, Zhao A, Pfeifer GP & Xu X The abnormal spindle-like, microcephaly-associated (ASPM) gene encodes a centrosomal protein. Cell Cycle 4, 1227–1229 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Johnson MB et al. Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature 556, 370–375 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang W et al. Modeling microcephaly with cerebral organoids reveals a WDR62–CEP170–KIF2A pathway promoting cilium disassembly in neural progenitors. Nat. Commun 10, 2612 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using organoids, this study evaluates the role of WDR62 in cilium assembly and localization and identifies a contribution to appropriate radial glial proliferation.
- 134.Iefremova V et al. An organoid-based model of cortical development identifies non-cell-autonomous defects in Wnt signaling contributing to Miller–Dieker syndrome. Cell Rep. 19, 50–59 (2017). [DOI] [PubMed] [Google Scholar]; This study using organoids derived from patients with Miller–Dieker syndrome identifies alterations in vRG cell division and adhesion, which is regulated by B-catenin signalling, and shows that these phenotypes can be rescued by replacing this signalling.
- 135.Cornell B & Toyo-Oka K 14-3-3 proteins in brain development: neurogenesis, neuronal migration and neuromorphogenesis. Front. Mol. Neurosci 10, 318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reiner O et al. Isolation of a Miller–Dicker lissencephaly gene containing G protein β-subunit-like repeats. Nature 364, 717–721 (1993). [DOI] [PubMed] [Google Scholar]
- 137.Tsai J-W, Chen Y, Kriegstein AR & Vallee RB LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J. Cell Biol 170, 935–945 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lo Nigro C et al. Point mutations and an intragenic deletion in LIS1, the lissencephaly causative gene in isolated lissencephaly sequence and Miller–Dieker syndrome. Hum. Mol. Genet 6, 157–164 (1997). [DOI] [PubMed] [Google Scholar]
- 139.Mirzaa GM et al. Association of MTOR mutations with developmental brain disorders, including megalencephaly, focal cortical dysplasia, and pigmentary mosaicism. JAMA Neurol. 73, 836–845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Y et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 20, 385–396 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Foerster P et al. mTORC1 signaling and primary cilia are required for brain ventricle morphogenesis. Development 144, 201–210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jossin Y et al. Llgl1 connects cell polarity with cell–cell adhesion in embryonic neural stem cells. Dev. Cell 41, 481–495 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen L et al. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc. Natl Acad. Sci. USA 103, 16520–16525 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baburamani AA et al. Assessment of radial glia in the frontal lobe of fetuses with Down syndrome. Acta Neuropathol. Commun 8, 141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cappello S et al. A radial glia-specific role of RhoA in double cortex formation. Neuron 73, 911–924 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Ferland RJ et al. Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia. Hum. Mol. Genet 18, 497–516 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using post-mortem patient tissue and mouse models, this study shows that neuronal migration occurs normally but that there are alterations in cellular adhesion and vesicle trafficking in the VZ in periventricular heterotopia.
- 147.Carabalona A et al. A glial origin for periventricular nodular heterotopia caused by impaired expression of Filamin-A. Hum. Mol. Genet 21, 1004–1017 (2012). [DOI] [PubMed] [Google Scholar]
- 148.Homan CC et al. PCDH19 regulation of neural progenitor cell differentiation suggests asynchrony of neurogenesis as a mechanism contributing to PCDH19 girls clustering epilepsy. Neurobiol. Dis 116, 106–119 (2018). [DOI] [PubMed] [Google Scholar]
- 149.D’Gama AM et al. Somatic mutations activating the mTOR pathway in dorsal telencephalic progenitors cause a continuum of cortical dysplasias. Cell Rep. 21, 3754–3766 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lim JS et al. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat. Med 21, 395–400 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Møller RS et al. Germline and somatic mutations in the MTOR gene in focal cortical dysplasia and epilepsy. Neurol. Genet 2, e118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Muneer A Wnt and GSK3 signaling pathways in bipolar disorder: clinical and therapeutic implications. Clin. Psychopharmacol. Neurosci 15, 100–114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singh KK et al. Common DISC1 polymorphisms disrupt Wnt/GSK3β signaling and brain development. Neuron 72, 545–558 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mao Y et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/βa-catenin signaling. Cell 136, 1017–1031 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that deletion of DISC1, a risk factor gene for neuropsychiatric disorders, in mice impairs GSK3β function and alters neural progenitor division and differentiation.
- 155.Singh KK et al. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron 67, 33–48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Katsu T et al. The human frizzled-3 (FZD3) gene on chromosome 8p21, a receptor gene for Wnt ligands, is associated with the susceptibility to schizophrenia. Neurosci. Lett 353, 53–56 (2003). [DOI] [PubMed] [Google Scholar]
- 157.Yoon K-J et al. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell 15, 79–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the capacity to study cellular alterations associated with human neuropsychiatric disorders by using induced PSC models and identifies disruption to radial glial polarity and complex expression in cells with a 15q11.2 mutation.
- 158.Barnat M et al. Huntington’s disease alters human neurodevelopment. Science 369, 787–793 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies differences in progenitor cell polarity in primary human cortical tissue carrying the Huntington mutation during neurogenesis.
- 159.Mehta SR et al. Human Huntington’s disease iPSC-derived cortical neurons display altered transcriptomics, morphology, and maturation. Cell Rep. 25, 1081–1096 (2018). [DOI] [PubMed] [Google Scholar]
- 160.Nagano T, Morikubo S & Sato M Filamin A and FILIP (filamin A-interacting protein) regulate cell polarity and motility in neocortical subventricular and intermediate zones during radial migration. J. Neurosci 24, 9648–9657 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sarkisian MR et al. MEKK4 signaling regulates filamin expression and neuronal migration. Neuron 52, 789–801 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rakic P Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 33, 471–476 (1971). [DOI] [PubMed] [Google Scholar]
- 163.Miyata T, Kawaguchi A, Okano H & Ogawa M Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727–741 (2001). [DOI] [PubMed] [Google Scholar]
- 164.McConnell SK Migration and differentiation of cerebral cortical neurons after transplantation into the brains of ferrets. Science 229, 1268–1271 (1985). [DOI] [PubMed] [Google Scholar]
- 165.Gorski JA et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci 22, 6309–6314 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tkachenko LA, Zykin PA, Nasyrov RA & Krasnoshchekova EI Distinctive features of the human marginal zone and Cajal–Retzius cells: comparison of morphological and immunocytochemical features at midgestation. Front. Neuroanat 10, 26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rakic P Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol 145, 61–83 (1972). [DOI] [PubMed] [Google Scholar]
- 168.Kosodo Y et al. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 23, 2314–2324 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Qian X et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


