Abstract
Nitazoxanide (NTZ), a drug currently being tested in human clinical trials for efficacy against chronic cryptosporidiosis, was assessed in cell culture and in two animal models. The inhibitory activity of NTZ was compared with that of paromomycin (PRM), a drug that is partially effective against Cryptosporidium parvum. A concentration of 10 μg of NTZ/ml (32 μM) consistently reduced parasite growth in cell culture by more than 90% with little evidence of drug-associated cytotoxicity, in contrast to an 80% reduction produced by PRM at 2,000 μg/ml (3.2 mM). In contrast to its efficacy in vitro, NTZ at either 100 or 200 mg/kg of body weight/day for 10 days was ineffective at reducing the parasite burden in C. parvum-infected, anti-gamma-interferon-conditioned SCID mice. Combined treatment with NTZ and PRM was no more effective than treatment with PRM alone. Finally, NTZ was partially effective at reducing the parasite burden in a gnotobiotic piglet diarrhea model when given orally for 11 days at 250 mg/kg/day but not at 125 mg/kg/day. However, the higher dose of NTZ induced a drug-related diarrhea in piglets that might have influenced its therapeutic efficacy. As we have previously reported, PRM was effective at markedly reducing the parasite burden in piglets at a dosage of 500 mg/kg/day. Our results indicate that of all of the models tested, the piglet diarrhea model most closely mimics the partial response to NTZ treatment reported to occur in patients with chronic cryptosporidiosis.
Cryptosporidium parvum, an enteric protozoan, is estimated to be one of the top three or four causes of self-limiting diarrhea in humans and in several animal species (14). In immunodeficient individuals, such as people with AIDS, cryptosporidiosis may lead to chronic diarrhea and wasting (12). Of the many chemotherapeutic and immunotherapeutic agents tried in recent years, none were consistently effective against this infection in laboratory animals or in patients with chronic diarrhea (1).
Nitazoxanide (NTZ) is a nitrothiazole benzamide compound that has a wide range of antimicrobial activity against helminthic parasites, particularly cestodes, and bacterial pathogens (2, 6, 8, 15). Clinical trials are currently under way to determine the efficacy of NTZ against C. parvum infection in individuals with AIDS. To date, the results from these trials have been varied. In a study of 15 patients in Mexico, doses of 1 or 2 g/day resulted in parasite clearance in 100% of the patients with AIDS and cryptosporidiosis (8a). A clinical trial conducted in the United States by Davis et al. (4) involved 22 patients with AIDS and cryptosporidiosis who were treated orally with 0.5 to 2 g of NTZ/day for 4 weeks. In contrast to the study in Mexico, results from this trial revealed that the parasite burden improved in nine patients, with only four individuals displaying a significant reduction in or absence of oocysts in their feces. When doses of 1 g/day or higher were used, a reduction in bowel movement frequency was observed in 15 patients, with a complete resolution of diarrhea in 4 individuals. Since no apparent toxicity was observed with any of the doses used, the authors hypothesized that increasing the frequency of treatment and/or the dose might improve the anticryptosporidial efficacy of NTZ. A third study (5) involved 12 patients with AIDS and cryptosporidiosis who received 1 g of NTZ/day for 7 days. The results indicated that seven patients had a marked reduction in fecal parasite counts and four had complete resolution of diarrhea. Transient episodes of vomiting were observed in some of these individuals. In this study, NTZ was also found to be effective against other protozoans, including Isospora belli, Entamoeba histolytica, and Giardia lamblia.
In 1996, NTZ was approved by the Food and Drug Administration for limited compassionate use in patients. Additional controlled clinical trials are under way to determine the true efficacy and benefit of treatment with NTZ for chronic cryptosporidiosis. Due to the current use of NTZ in humans, we performed extensive preclinical studies to determine the in vitro and in vivo efficacy of NTZ against C. parvum in a cell culture system and two animal models of infection (16, 17).
MATERIALS AND METHODS
C. parvum.
The C. parvum GCH1 isolate was used for all of the studies presented here. This isolate was originally obtained from a patient with AIDS and has been maintained in our laboratory by repeated passage through calves (16, 17). Prior to use, the oocysts were purified from fecal material and treated with 1% sodium hypochloride as previously described (3).
Antiserum preparation.
An antiserum reactive against the sporozoite stage of C. parvum was prepared by immunizing a rabbit with 108 purified, intact sporozoites mixed with Freund’s incomplete adjuvant (Sigma, St. Louis, Mo.). All injections were intramuscular, with the first booster given 2 weeks after the initial immunization and four additional boosters given weekly thereafter. The rabbit was bled 4 days after the final booster, and the serum was harvested. This antiserum was also found to recognize other parasite forms of C. parvum.
NTZ activity in cell culture.
MDBK cells selectively cloned for susceptibility to C. parvum infection (11) were plated in 96-well microliter plates (Falcon, Franklin Lakes, N.J.). To determine the dose response to NTZ (molecular weight, 307.2; Romark Laboratories, Tampa, Fla.), 3.0 × 104 C. parvum oocysts were added with or without drugs to each well 72 h later, when the cells were confluent. Paromomycin sulfate (PRM) (molecular weight, 615.6; Parke-Davis, N.J.) was used as a positive control drug. All drug dilutions were made in Dulbecco’s minimum essential medium (GIBCO BRL, Grand Island, N.Y.) supplemented with 5% fetal bovine serum (GIBCO), 500 U of penicillin, 500 μg of streptomycin/ml (GIBCO), 1 mM sodium pyruvate (GIBCO), 2 mM l-glutamine (GIBCO), and 0.2% dimethyl sulfoxide (DMSO) (Sigma) (culture medium). Culture medium was added to wells containing MDBK cells infected with C. parvum oocysts as a negative control. All drug concentrations and controls were tested in quadruplicate. Following an incubation for 48 h (37°C, 8% CO2) the monolayers were methanol fixed and reacted in an indirect immunofluorescence assay to determine the intensity of infection. Fixed wells were rehydrated for 15 min with phosphate-buffered saline (PBS) containing 1% normal goat serum (NGS) (GIBCO). Following rehydration, the parasite-reactive rabbit antiserum was diluted 1:1,000 in PBS containing 1% NGS, added to the wells, and incubated for 1 h at room temperature. All wells were washed three times with PBS, and bound antibody was detected with a fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G antibody (Cappel, Durham, N.C.) diluted 1:100 in PBS containing 1% NGS. Following a 1-h incubation at room temperature, the wells were washed three times with PBS and dried. The extent of C. parvum infection was quantitated, under UV light microscopy, with a microcomputer video imaging device (Imaging Research Inc., St. Catharines, Ontario, Canada) specifically designed for this purpose (18). The percent inhibition of infection was calculated as follows: 1 − (mean number of parasites in wells with drug/mean number of parasites in control wells) × 100. All results were assigned inhibition scores of 0, 1, 2, 3, and 4 for inhibition of 0 to 30%, 31 to 55%, 56 to 70%, 71 to 90%, and 91 to 100%, respectively.
Cytotoxicity assay.
The cytotoxicities of NTZ and PRM to MDBK cells were determined by the CellTiter 96 AQueous non-radioactive cell proliferation assay (Promega Corp., Madison, Wis.). Controls for each cytotoxicity assay included (i) uninfected cells incubated in culture medium, (ii) infected cells incubated in culture medium, and (iii) cells exposed to a freeze-thaw lysate containing 3.0 × 104 oocyst equivalents in culture medium. The percent cytotoxicity was calculated from the optical density (OD) as follows: [(mean OD of uninfected cells − mean OD of infected cells)/mean OD of uninfected cells] × 100. All results were assigned cytotoxicity scores of 0, 1, 2, 3, and 4 for percent cytotoxicity of 0 to 5%, 6 to 25%, 26 to 50%, 51 to 75%, and 76 to 100%, respectively. Cytotoxicity scores of 0, 1, and 2 are considered to indicate nontoxicity, mild toxicity, and moderate toxicity, respectively, and scores of 3 and 4 are regarded as indicating severe toxicity for MDBK cells. A negative percent toxicity results when the OD of the infected cells is greater than the OD of the uninfected cells.
NTZ activity in SCID mice.
The anti-gamma-interferon (IFN-γ)-conditioned SCID mouse model has been described previously (17). Briefly, newly weaned (3- to 4-week-old) male inbred C.B-17 SCID mice were purchased from Jackson Laboratories, Bar Harbor, Maine. All mice were housed in microisolator cages in IACUC-approved facilities and were handled according to National Institutes of Health guidelines. Prior to the initiation of a drug trial, the animals were randomized into seven groups of seven mice each. Each mouse was primed with an intraperitoneal injection of 1 mg of XMG1.2, an IFN-γ-neutralizing monoclonal antibody (kindly provided by R. Coffman, DNAX Research Institute, Palo Alto, Calif.). Two hours later, each mouse in six of the seven groups received an oral inoculation of 107 oocysts. Drug treatment was initiated on day 6 of infection, coinciding with the onset of oocyst excretion in the feces. Treatment schedules were as follows: group 1, 200 mg of NTZ/kg of body weight/day; group 2, 100 mg of NTZ/kg/day; group 3, 200 mg of NTZ/kg/day combined with 2,500 mg of PRM/kg/day; group 4, 100 mg of NTZ/kg/day combined with 2,500 mg of PRM/kg/day, and group 5, 2,500 mg of PRM/kg/day. NTZ was dissolved in 100% DMSO and administered orally in two divided doses of 30 μl each per day. PRM was dissolved in the drinking water to a concentration of 10 mg/ml (16.2 mM), resulting in a dose of 2,500 mg/kg/day based on the daily water consumption. Group 6 consisted of uninfected mice treated with 200 mg of NTZ/kg/day combined with 2,500 mg of PRM/kg/day (drug toxicity control group). Group 7 consisted of seven mice treated orally with 30 μl of DMSO twice per day (the placebo control group). All mice were treated for 10 days and maintained for an additional 5 days after the end of treatment. The level of oocyst shedding was determined three times per week throughout the study by microscopic observation of 30 high-power fields of a modified acid-fast stained fecal smear (17) from each infected animal. Results are presented as the mean log oocysts shed per group ± the 95% confidence intervals. Body weights were determined one time per week throughout the study. Results are presented as the mean body weight per group ± the 95% confidence intervals. At necropsy, sections were taken from the pyloric region of the stomach, mid-small intestine, ileum, cecum, and proximal colon for histologic analysis to determine the extent of mucosal infection. Each site was assigned a score depending on the extent of infection, as follows: 0, no infection; 1, very difficult-to-find parasite forms; 2, sparse but easily found parasite forms; 3, abundant parasite forms but focally distributed; 4, extensive presence of parasite forms covering most mucosal surfaces; and 5, extensive presence of parasite forms covering the entire mucosal surface. The data are presented as the mean total score of the five sites ± 95% confidence intervals.
NTZ activity in the piglet diarrhea model.
Thirty-one gnotobiotic piglets derived by cesarean section from four litters were maintained inside sterile isolators for the duration of the experiment as described previously (16). Twenty-six of the 31 piglets were challenged with oocysts 24 h after derivation. Because these experiments were not performed simultaneously, the infecting dose of 5 × 106 excysting oocysts was calculated based on the percent oocyst excystation in vitro (rate of excystation). The rate of in vitro excystation was determined following incubation of the oocysts in 0.75% taurocholic acid for 45 min at 37°C. Once the in vitro excystation rate was determined, the inocula were adjusted accordingly so that 13 piglets received 2 × 107 oocysts and 13 piglets received 7 × 106 oocysts.
Piglets were observed two or three times daily for signs of diarrhea, depression, and anorexia and for overall appearance. Diarrhea was defined as a twofold increase in the frequency, volume, and water content of the fecal discharge of a piglet compared to those in uninfected control piglets. Body weights and fecal samples were obtained daily. Within 3 days after challenge, piglets were assigned to groups based on a combination of body weight, onset of oocyst shedding, and diarrhea status. The piglets were then started on a daily treatment schedule of either 250 mg of NTZ/kg (five piglets), 125 mg of NTZ/kg (six piglets), 500 mg of PRM/kg (five piglets), or a placebo (milk; nine piglets). Five uninfected control animals served as drug toxicity controls; three piglets received NTZ at 250 mg/kg/day, and two piglets received 125 mg/kg/day. One of the 26 infected piglets was euthanized because of severe illness and was excluded from the study. All drugs were administered via the milk diet in two divided doses daily for 11 days. The number of oocysts present in an entire modified-acid-fast-stained fecal smear was determined daily for each piglet. Since piglets develop diarrhea as a consequence of C. parvum infection, determination of the number of oocysts shed must account for any variability in fecal consistency that we observe. In particular, the presence of watery diarrhea will influence the number of oocysts detected in a fecal smear due to the effective dilution of the fecal material by the increased fluid content. Because of this, we have devised a scoring system that accounts for both the qualitative nature of the fecal material and the number of oocysts detected (17). Scores are assigned in this system as follows: 0, no oocysts detected; 1, ≤10 oocysts; 2, ≤25 oocysts; 3, ≤50 oocysts; 4, ≤100 oocysts; and 5, >100 oocysts. Results are presented as the mean oocyst shedding score for each treatment group ± standard error of the mean. Surviving piglets were euthanized 11 days after the onset of treatment, and six gut sections (the pyloric region of the stomach, three equally spaced small intestinal sites, the cecum, and the colon) were removed for histologic analysis of the extent of mucosal infection. Each site was assigned a score depending on the extent of infection by the system described above for the anti-IFN-γ-conditioned SCID mouse. Results are presented as the total score of the six sites for individual piglets.
Statistical analysis.
Data were analyzed using the Statistical Package for Social Sciences versions 6.1 through 7.5 for Windows in either an OS/2 or Windows NT environment. Analyses of variance were conducted for all comparisons in experiments with multiple comparison groups, and if groups showed differences at the P level of <0.05 then further analysis was undertaken. When parametric tests were not appropriate, standard nonparametric tests (details are given below) were used.
Only differences where the two-tailed P value was ≤0.05 are reported as significant. Since data for oocyst shedding are not normally distributed, the number of oocysts shed was log transformed for the purpose of normalizing the data. So as to avoid taking the log of zero when no oocysts were detected, we used the common statistical practice of adding 0.5 to all oocyst shedding values prior to transformation.
RESULTS
Studies with cell culture.
NTZ was highly effective against C. parvum in the in vitro system. Table 1 presents the results of one of six combined NTZ and PRM assays performed over the last 3 years. In this experiment, medium containing the drug and the oocysts was maintained on the cell monolayers for the entire 48-h incubation period. At a concentration of 10 μg/ml (32 μM), NTZ was significantly more inhibitory against C. parvum (93%) than a 2,000-μg/ml (3.2 mM) concentration of PRM (82%), the positive control drug. Combined treatment with NTZ and PRM was no more effective against C. parvum than either drug administered alone, indicating that neither a synergistic nor an additive effect occurred in this system. The cytotoxicity levels were low (0 to 25%) for all controls and at all concentrations of drug tested with the exception of 100 μg/ml (325 μM) of NTZ.
TABLE 1.
Dose responses for inhibition by PRM and NTZ of C. parvum forms in cell culturea
| Medium or drug(s) | Concn | Parasite countb | Growth
inhibition
|
Toxicityc
|
||
|---|---|---|---|---|---|---|
| % | Score | % | Score | |||
| Medium | 1,416.4 ± 302 | NAd | NA | 0.0 | 0 | |
| Medium + DMSO | 1,231.7 ± 281 | NA | NA | 1.06 | 0 | |
| PRM | 3.2 mM (2 mg/ml) | 256.4 ± 64.8 | 81.9 | 3 | −1.7 | 0 |
| 1.6 mM (1 mg/ml) | 293.6 ± 96.7 | 79.3 | 3 | 7.7 | 1 | |
| 0.8 mM (0.5 mg/ml) | 398.3 ± 87.1 | 71.9 | 3 | 2.3 | 0 | |
| 0.4 mM (0.25 mg/ml) | 453.9 ± 75 | 68 | 2 | −7.5 | 0 | |
| NTZ | 325 μM (100 μg/ml) | NDe | ND | ND | 74.1 | 3 |
| 32.5 μM (10 μg/ml) | 87.3 ± 20.1 | 93 | 4 | −25 | 0 | |
| 3.25 μM (1 μg/ml) | 695 ± 173 | 44 | 1 | 11.3 | 1 | |
| 0.325 μM (0.1 μg/ml) | 1,105 ± 127 | 10.3 | 0 | 18 | 1 | |
| PRM-NTZf | 0.4 mM/3.25 μM | 422.4 ± 65 | 70 | 2 | 16 | 1 |
| 0.2 mM/3.25 μM | 619 ± 158 | 47 | 1 | 12.2 | 1 | |
| Mediumg | ND | NA | NA | 20 | 1 | |
| Lysateh | ND | NA | NA | 17 | 1 | |
The dose responses were evaluated after all parasites and treatments were applied to the MDBK cells and incubated for 48 h.
Mean number of parasites per field ± standard deviation. Values were determined by counting parasites in 16 fields per well for a total of 4 wells per treatment.
Toxicity values were determined with uninfected cells.
NA, not applicable.
ND, not determined.
Combined treatment with PRM and NTZ.
Toxicity values were determined for cells infected with 3 × 104 oocysts/well.
Toxicity values were determined for cells exposed to 3 × 104 oocyst equivalents per well.
Studies with mice.
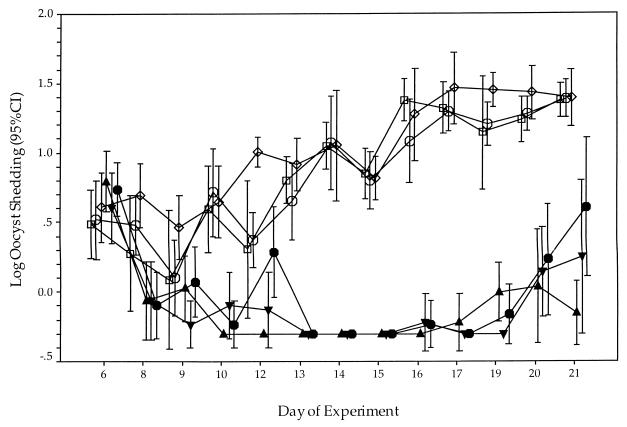
The efficacy of NTZ, either alone or in combination with PRM, was tested in the anti-IFN-γ-conditioned SCID mouse model of acute cryptosporidiosis. While an initial study showed that a partial reduction in oocyst shedding (data not shown) was produced by NTZ, we were unable to replicate this result in several subsequent experiments. The following are the combined results of two independent trials that failed to show efficacy in this model. No difference in the log oocyst shedding between any of the groups treated with NTZ alone and the placebo control group was observed (Fig. 1). In contrast, all mice treated with PRM shed oocysts at significantly lower levels than mice treated with either NTZ alone or the placebo (P < 0.001). Coadministration of NTZ and PRM was no more effective at reducing the level of oocyst shedding than treatment with PRM alone. In general, no significant differences in mean body weight were observed between any of the groups of mice (data not shown).
FIG. 1.
Log oocyst shedding from C. parvum-infected, anti-IFN-γ-conditioned SCID mice. Three-week-old male SCID mice received a single injection of 1 mg of XMG1.2 2 h prior to oral inoculation with 107 GCH1 oocysts. The level of oocyst shedding in the feces of each mouse was assessed three times per week. Treatment began after day 6 and ended on day 15. Results are presented as the log of the mean number of oocysts shed per group ± the 95% confidence interval (CI) (bars). Each group had seven mice. Symbols: , 100 mg of NTZ/kg/day and 2,500 mg of PRM/kg/day; ▾, 200 mg of NTZ/kg/day and 2,500 mg of PRM/kg/day; ▴, 2,500 mg of PRM/kg/day; , 100 mg of NTZ/kg/day; □, 200 mg of NTZ/kg/day; and ◊, placebo.
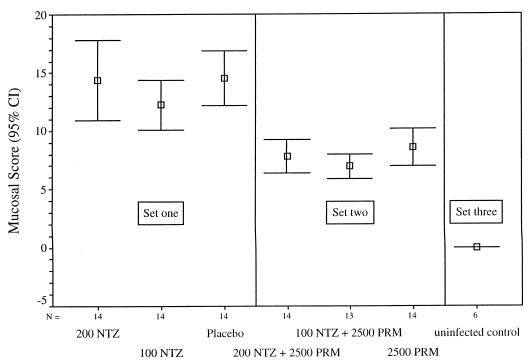
Student-Newman-Keuls analysis of variance revealed that the extent of mucosal infection was significantly greater in mice treated with either NTZ alone or the placebo than in animals treated with PRM (Fig. 2; P < 0.001). Coadministration of NTZ and PRM did not significantly alter the extent of mucosal infection compared to that in mice that received PRM alone.
FIG. 2.
Mucosal infection scores for treatment groups of C. parvum-infected, anti-IFN-γ-conditioned SCID mice. At necropsy, sections were taken from the pyloric region of the stomach, mid-small intestine, ileum, cecum, and proximal colon for histological analysis to determine the extent of mucosal infection. Each site was assigned a score depending on the extent of infection ranging from 0 (no infection) to 5 (extensively infected mucosa). Results are presented as the mean total score of the five sites for each group ± 95% confidence intervals (CI) (bars). When they were tested by Student-Newman-Keuls analysis of variance (α = 0.05), the mucosal scores fell into three sets, with significant differences among them (6 df; F = 18.167; P < 0.001). Mice treated with placebo or with NTZ alone (at 100 or 200 mg/kg/day) formed one distinguishable statistical set (set one), mice treated with 2,500 mg of PRM/kg/day with or without NTZ (100 or 200 mg/kg/day) formed a second distinguishable set (set two), and the uninfected controls formed a third statistical set (set three). The mucosal infection scores for the mice treated with PRM (n = 41) were significantly lower than those for the mice not treated with PRM (n = 42) (7.78 ± 0.38 versus 13.69 ± 0.73, respectively; P < 0.001). In contrast, the analysis of the effects on the mucosal infection score of the presence and absence of NTZ treatment did not show any differences between groups (data not shown).
Studies with piglets.
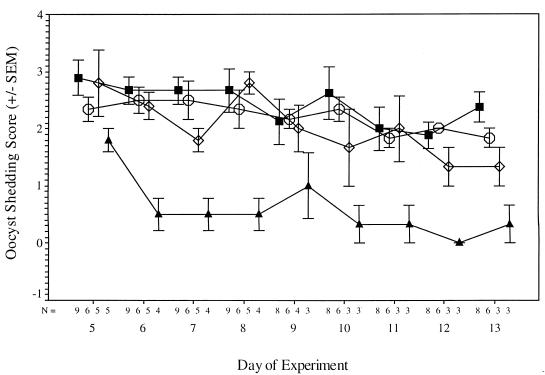
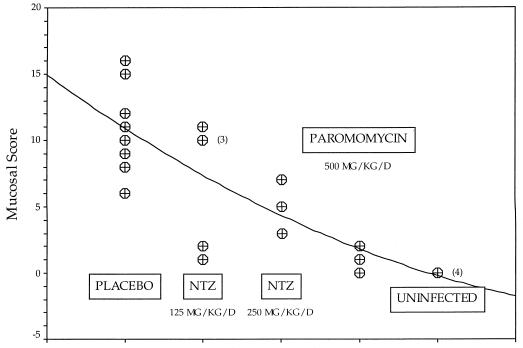
The piglet model offers an added advantage in that the piglets develop diarrhea as a consequence of infection. In addition to the piglet euthanized before treatment began, 4 of the 25 animals challenged with C. parvum were euthanized due to poor health associated with diarrhea, including 1 piglet from the placebo group (on day 5 after challenge), 2 from the group receiving 250 mg of NTZ/kg (on days 8 and 9 after challenge), and 1 from the PRM-treated group (on day 5 after challenge). Three additional piglets, 1 placebo-treated, 1 PRM-treated, and 1 uninfected control piglet, were euthanized on day 8 after challenge for a comparative analysis of the extent of mucosal infection. The analysis of both the level of oocyst shedding and extent of mucosal infection revealed that NTZ at 250 mg/kg/day significantly reduced the extent of mucosal infection in these piglets, but it was not as effective as PRM at 500 mg/kg/day (Fig. 3 and 4).
FIG. 3.
Fecal oocyst excretion scores of infected piglets treated with NTZ at either 125 ( ) or 250 (◊) mg/kg/day, placebo (■), or PRM at 500 mg/kg/day (▴). In multiple regression analysis, the oocyst excretion score was found to be significantly related to treatment group, with the highest scores being observed in the placebo group, followed by the lower-dose NTZ group and then the higher-dose NTZ group, and the lowest scores being observed in the PRM group (F = 42.507; P < 0.001). Further comparison revealed that, during days 7 through 13, the scores for the piglets treated with NTZ at 250 mg/kg/day were significantly lower than those for the placebo-treated piglets (Z = −3.258; P = 0.001, two-tailed Wilcoxon signed rank test). In contrast, subgroup comparison of the infected placebo-treated control piglets and piglets treated with NTZ at 125 mg/kg/day did not reveal any significant difference. Oocyst shedding was significantly less marked in the PRM-treated group than in any of the other groups (P < 0.001). Values are means ± standard errors of the means (SEM).
FIG. 4.
Intestinal mucosal scores for 24 piglets euthanized on day 13 of the experiment. The line represents a quadratic least-squares fit of the data (R2 = 0.64849; P < 0.001). Analysis of variance revealed that the mucosal score varied significantly from group to group (F = 22.21; P < 0.0001). Comparisons made by using the Mann-Whitney U test and Wilcoxon rank sum test revealed that the scores were significantly different for the following pairs of groups: NTZ at 250 mg/kg/day and PRM (P = 0.050), infected controls and NTZ at 250 mg/kg/day (P = 0.025), infected controls and PRM (P = 0.014), and infected controls and uninfected controls (P = 0.014). The score for the group treated with NTZ at 125 mg/kg/day was not significantly different from that for the infected control group (P = 0.282). 3 and 4 denote that three and four piglets, respectively, had the same scores.
With the exception of those in the PRM-treated group, all infected piglets manifested diarrhea of various degrees within 56 h after challenge. Diarrhea persisted until the end of the experiment, 13 days later (11 days after the start of treatment). Table 2 provides a cumulative analysis of the number of days of diarrhea observed for each of the treatment groups. A chi-square analysis of these data revealed very significant differences among the treatment groups (overall Pearson’s chi-square value of 88.096 with 5 df; P < 0.001). Uninfected piglets given NTZ at a dose of 125 mg/kg/day did not have diarrhea (0 of 24 days of observation). In contrast, on 22 of 36 observation days, uninfected piglets given NTZ at a dose of 250 mg/kg/day had significant drug-induced diarrhea (P ≤ 0.001 for both Pearson’s chi-square and Fisher’s exact test [two-tailed]). Among the infected groups, only the PRM-treated group had less-frequent diarrhea than the infected placebo control group. The percentage of days of observation that the piglets in the PRM-treated group showed diarrhea was significantly lower than that in any of the other infected groups (chi-square P < 0.001, and Fisher’s exact two-tailed test, P < 0.001, for comparison with the infected placebo control group, the group receiving NTZ at 125 mg/kg/day, and the group receiving NTZ at 250 mg/kg/day).
TABLE 2.
Numbers of days that piglets had diarrhea
| Group | No. of
daysa
|
%b | ||
|---|---|---|---|---|
| + | − | Total | ||
| Infected | ||||
| Placebo | 97 | 23 | 120 | 80 |
| NTZ (250 mg/kg) | 49 | 11 | 60 | 81 |
| NTZ (125 mg/kg) | 62 | 10 | 72 | 86 |
| PRM | 30 | 30 | 60 | 50 |
| Uninfected | ||||
| NTZ (250 mg/kg) | 22 | 14 | 36 | 61 |
| NTZ (125 mg/kg) | 0 | 24 | 24 | 0 |
+, number of observation days that piglets had diarrhea; −, number of days that piglets did not have diarrhea.
%, percentage of observation days that piglets had diarrhea.
DISCUSSION
In testing drugs for their efficacy against C. parvum, our laboratory utilizes an in vitro system for the initial identification of potentially active compounds. With this in vitro system, NTZ proved to be highly effective in inhibiting C. parvum growth in cell culture when used at a concentration of 10 μg/ml (32 μM). The in vitro system described in this communication has been extensively used by us over the last 3 years to screen well over 300 compounds for activity against C. parvum. It is highly standardized, and results are reproducible and consistent when the inhibitory activity of a drug is 50% or greater. This is evident with the positive control drug, PRM, which consistently displays a 75 to 85% inhibitory activity against C. parvum at a concentration of 2,000 μg/ml (3.2 mM). Moreover, we observe little or no cytotoxic effect on our in vitro system when oocysts or oocyst lysates are left on the cell monolayers for the entire 48-h incubation period. This is in contrast to a system described in another report, where a cytotoxic effect caused by oocyst fluid or debris was encountered when [3H]uracil incorporation was used to measure parasite growth (7).
One caveat regarding the in vitro system is that it is less sensitive and displays greater variability when the inhibitory activity of a drug is below 40%. In our hands, this reduction in sensitivity is independent of the cell line, growth conditions, or infectious dose used (data not shown). As a consequence, investigations of drugs with less than a 40 to 50% inhibitory activity against C. parvum are normally not pursued further.
Several in vitro systems for drug screening that have been described in the literature use a variety of different procedures and cell lines. The cell lines used include A-549 (10), T84 (9), Caco-2 (13), and HCT-8 (19) cells. These procedures can vary with respect to the timing of the start of drug treatment in relation to infection with oocysts or sporozoites, the number of days of incubation, the methods of fixation, and the quantitation, analysis, and presentation of the data (18). As a consequence of this, it is often difficult to compare results generated by different laboratories. In addition, obtaining similar results with different in vitro systems can be a major problem, which in part may be attributed to the inherent variability associated with the oocysts used. In particular, oocyst infectivity and/or excystation rate may vary with the origin, age, and methods of storage and purification of the oocysts. While these variables are often difficult to control or predict, they underscore the need for a standardized system for the in vitro evaluation of drugs for anticryptosporidial activity.
Drugs which are found to have an in vitro inhibitory activity against C. parvum of 50% or greater are subsequently evaluated in our anti-IFN-γ-conditioned SCID mouse model (17). This is perhaps the most convenient model in terms of size, availability, maintenance, standardization, and manipulation. In addition, this model allows the establishment of rapid acute or chronic infections that can be readily monitored. While at 10 μg/ml (32 μM) NTZ proved to be highly effective in inhibiting C. parvum growth in cell culture, it did not reduce the extent of infection in the anti-IFN-γ-conditioned SCID mouse model at doses of 100 and 200 mg/kg/day. Since the mechanism of action and the therapeutic targets of NTZ are unknown, any number of biochemical and pharmacological factors could affect the drug’s efficacy in vivo.
As with the in vitro system, all rodent models of cryptosporidiosis have limitations, the key one being the absence of diarrhea (18). As a consequence, it is our belief that rodent models of cryptosporidiosis function merely as in vivo correlates of the in vitro screening system. As such, only drugs that are highly effective against C. parvum are identified in these models. It has been our experience with rodent models of cryptosporidiosis that it is difficult to detect the activities of mildly effective drugs at concentrations that are not toxic. PRM is our drug of choice for the positive control because it can be used at very high concentrations without toxic side effects in mice. However, even high concentrations of PRM do not completely eliminate the infectious organism from the gastrointestinal tracts of mice.
As a result of the lower sensitivity and the absence of diarrhea in rodent models, we have developed the piglet diarrhea model for drug evaluation. We feel that this is an extremely valuable model since the presence of diarrhea is a significant factor in determining the efficacy of orally administered drugs (16). Our data indicate that at a dose of 250 mg/kg NTZ resulted in a statistically significant reduction in the extent of both oocyst shedding and mucosal infection towards the end of the treatment schedule. However, NTZ was less effective than PRM in reducing the parasite burden. Moreover, only the treatment with PRM also caused a reduction in the severity of diarrhea. This may in part be due to a diarrheagenic effect of NTZ. The results of treatment of uninfected piglets with NTZ indicated that this drug can be diarrheagenic when administered at moderate to high doses. It is therefore conceivable that the anticryptosporidial activity of NTZ may be more effective if the drug were less diarrheagenic. Moreover, our results indicate that, compared to that in the SCID mouse model, the effect in the piglet model more closely mimicks the partial benefit of NTZ treatment observed in some patients with chronic cryptosporidiosis.
In summary, we found the anticryptosporidial activity of NTZ to be highly effective in cell culture, partially effective in the piglet diarrhea model, and ineffective in the anti-IFN-γ-conditioned SCID mouse model. The cell culture system remains in our view a useful predictor of potentially effective anticryptosporidial drugs. While demonstration of the activity of a drug in vitro does not guarantee its function in vivo, it has been our experience that drugs are unlikely to inhibit C. parvum in vivo without inhibiting it in vitro. Despite its limitations, our SCID mouse model of cryptosporidiosis remains a viable model for identifying drugs that are highly active against C. parvum in vivo. However, the piglet diarrhea model remains our model of choice for the detection of partially effective drugs, and it is clearly the most accurate predictor of the efficacy of drugs in humans with chronic cryptosporidiosis.
ACKNOWLEDGMENTS
We are grateful to Melissa Paris for expert technical assistance.
This work was supported by NIH contract NOI-AI-25143.
REFERENCES
- 1.Blagburn B L, Soave R. Prophylaxis and chemotherapy: human and animal. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis—1997. Boca Raton, Fla: CRC Press; 1997. pp. 111–128. [Google Scholar]
- 2.Cavier R, Rossignol J F. Pharmacological study of various antihelminthic combinations. Rev Med Vet. 1982;133:779–783. [Google Scholar]
- 3.Current W L. Techniques and laboratory maintenance of Cryptosporidium. In: Pubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals. Boston, Mass: CRC Press; 1990. pp. 31–49. [Google Scholar]
- 4.Davis L J, Soave R, Dudley R E, Fessel J W, Faulkner S, Mamakos J P. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Nitazoxanide (NTZ) for AIDS-related cryptosporidial diarrhea (CD): an open-label safety, efficacy and pharmacokinetic study, abstr. LM50; p. 289. [Google Scholar]
- 5.Doumbo O, Rossignol J F, Pichard E, et al. Nitazoxanide in treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am J Trop Med Hyg. 1997;56:637–639. doi: 10.4269/ajtmh.1997.56.637. [DOI] [PubMed] [Google Scholar]
- 6.Dubreuil L, Houcke I, Mouton Y, Rossignol J-F. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob Agents Chemother. 1996;40:2266–2270. doi: 10.1128/aac.40.10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggleston M T, Tilley M, Upton S J. Enhanced development of Cryptosporidium parvumin vitro by removal of oocyst toxins from infected cell monolayers. J Helminthol Soc Wash. 1994;61:122–125. [Google Scholar]
- 8.Euzeby J, Prom-Tep S, Rossignol J F. Study of the antihelminthic properties of nitazoxanide in dog, cat and sheep. Rev Med Vet. 1980;131:687–696. [Google Scholar]
- 8a.Feregrino G M, Higuera R F, Rossignol J F, et al. Abstracts from the XIth International Conference on AIDS, Vancouver, Canada. 1996. Extraordinary potency of the nitazoxanida. A new antiparisitary against the Cryptosporidium parvuminfections in advanced AIDS. [Google Scholar]
- 9.Flanigan T, Marshall R, Redman D, Kaetzel C, Ungar B. In vitro screening of therapeutic agents against Cryptosporidium: hyperimmune cow colostrum is highly inhibitory. J Protozool. 1991;38:225S–227S. [PubMed] [Google Scholar]
- 10.Giacometti A, Cirioni O, Scalise G. In vitro activity of macrolides alone and in combination with artemisin, atovaquone, dapsone, minocycline or pyrimethamine against Cryptosporidium parvum. J Antimicrob Chemother. 1996;38:399–408. doi: 10.1093/jac/38.3.399. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths J K, Moore R, Dooley S, Keusch G T, Tzipori S. Cryptosporidium parvuminfection of Caco-2 cell monolayers induces an apical monolayer defect, selectively increases transmonolayer permeability, and causes epithelial cell death. Infect Immun. 1994;62:4506–4514. doi: 10.1128/iai.62.10.4506-4514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffiths J K. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment and diagnosis. Adv Parasitol. 1998;40:37–85. doi: 10.1016/s0065-308x(08)60117-7. [DOI] [PubMed] [Google Scholar]
- 13.McDonald V, Stables R, Warhurst D C, Barer M R, Blewett D A, Chapman H D, Connolly G M, Chiodini P L, McAdam K P W J. In vitro cultivation of Cryptosporidium parvumand screening for anticryptosporidial drugs. Antimicrob Agents Chemother. 1990;34:1498–1500. doi: 10.1128/aac.34.8.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donoghue P J. Cryptosporidiumand cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 15.Rossignol J F, Maisonneuve H. Nitazoxanide in the treatment of Taenia saginata and Hymenolepis nanainfections. Am J Trop Med Hyg. 1984;33:511–512. doi: 10.4269/ajtmh.1984.33.511. [DOI] [PubMed] [Google Scholar]
- 16.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzipori S, Rand W, Theodos C. Evaluation of a two-phase scid mouse model preconditioned with anti-interferon-γ monoclonal antibody for drug testing against Cryptosporidium parvum. J Infect Dis. 1995;172:1160–1164. doi: 10.1093/infdis/172.4.1160. [DOI] [PubMed] [Google Scholar]
- 18.Tzipori S. Cryptosporidium parvum: laboratory investigations and chemotherapy. Adv Parasitol. 1998;40:188–221. doi: 10.1016/s0065-308x(08)60121-9. [DOI] [PubMed] [Google Scholar]
- 19.Woods K M, Nesterenko M V, Upton S J. Efficacy of 101 antimicrobials and other agents in the development of Cryptosporidium parvum in vitro. Ann Trop Med Parasitol. 1996;60:603–615. doi: 10.1080/00034983.1996.11813090. [DOI] [PubMed] [Google Scholar]