Abstract
Cassava (Manihot esculenta Crantz) is considered one of the essential tuber crops, serving as a dietary staple food for various populations. This systematic review provides a comprehensive summary of the nutritional and therapeutic properties of cassava, which is an important dietary staple and traditional medicine. The review aims to evaluate and summarize the phytochemical components of cassava and their association with pharmacological activities, traditional uses, and nutritional importance in global food crises. To collect all relevant information, electronic databases; Cochrane Library, PubMed, Scopus, Web of Science, Google Scholar, and Preprint Platforms were searched for studies on cassava from inception until October 2022. A total of 1582 studies were screened, while only 34 were included in this review. The results of the review indicate that cassava has diverse pharmacological activities, including anti-bacterial, anti-cancer, anti-diabetic, anti-diarrheal, anti-inflammatory, hypocholesterolemic effects, and wound healing properties. However, more studies that aim to isolate the phytochemicals in cassava extracts and evaluate their pharmacological property are necessary to further validate their medical and nutritional values.
Keywords: global food security, cassava, Manihot esculenta, terpenes, cyanogenic Glycosides, anti-diarrhea, anti-cancer, anti-bacterial, anti-diabetic, anti-inflammatory, hypocholesterolemic, wound healing
Introduction
Cassava, Manihot esculenta Crantz, is recognized as one of the most important tuber crops cultivated in tropical and subtropic regions, providing a major source of food to more than 800 million people worldwide. 1 The cassava plant is a perennial woody shrub that can grow up to three meters in tropical regions. The plant is believed to have originated in Latin America, where the native Indians discovered it more than 4000 years ago. 2 The crop was introduced to Africa by Portuguese traders in 1558 and brought to Asia by Europeans from South America between the late eighteenth century and early nineteenth century. 3 It is referred to as the primary ingredient of the cuisines in underdeveloped nations and was named by FAO in 2003 as Africa's most crucial root crop and source of nutritional calories. The New Partnership for Africa Development adopted the “Cassava: A Powerful Poverty Fighter in Africa” slogan to improve cassava verities and germplasm and increase its nutritional values in African countries.
It is described as a dicotyledonous plant belonging to a genus of Euphorbiaceae family called Manihot, and it is grown in areas between 30°N and 30°S from the equator, where it is distributed throughout Africa, Asia, Central and South America.4–6 Given that cassava is considered a dietary staple food in many countries, including the Democratic Republic of Congo, Ghana, Brazil, and Indonesia, Nigeria is recognized as the world's largest cassava producer, producing over 60 million tonnes in 2020. 7 Moreover, cassava is among Thailand's main economic crops, which was established as the largest dried cassava, flour, and starch exporter in 2020. 8 Despite having this nutritional and economic importance in numerous countries, cassava crops are underutilized in some countries due to higher demand for cereal crops, lack of accessibility to grow cassava on a large scale in addition to farmers’ lack of knowledge on the cultivation of cassava varieties with better properties. In South Africa, the crop is primarily cultivated by small-scale farmers in rural areas for food, while Pietersburg is the only area with a starch manufacturing factory for commercial farming of cassava.9,10
A wide range of cassava varieties was tested for producing high-quality cassava flour. β-carotene enriched cassava varieties have illustrated high viscosity values suitable for products requiring high gel strength and elasticity. 11 Furthermore, enriching flour produced from low- and medium-cyanide cassava varieties with nutrients was more efficient with certain micro-fungi such as Saccharomyces cerevisiae. 12 The most diverse cassava germplasm collection is preserved by the International Center for Tropical Agriculture (CIAT), and over 43 000 samples of cassava germplasm samples have been distributed to 84 countries since 1979. For instance, Naitaima-31 is a cassava variety released by CIAT and their partners, which has been developed by crossing two germplasms to possess the following properties: large harvest, good cooking qualities, and resistance against whiteflies. In contrast to the farmer's varieties, Naitama-31 has indeed produced higher yields without the need to use pesticides and is now cultivated for commercial purposes in Brazil and Colombia. 13 The variety of yellow cassava was exploited in the beverages industry, as its use as a raw material for beer production has been found to be beneficial not only nutritionally but also in terms of cutting overhead costs of production. A study has shown that beer produced from blending sorghum with yellow cassava could be a rich source of nutrients and vitamins. 14
Additionally, cassava contains various chemical components. These components include balanophonin, scopoletin, and tannins which have been studied to exhibit anti-oxidant activity, anti-proliferative and anti-inflammatory properties.15,16 Besides these beneficial phytochemicals, toxic chemical compounds – linamarin and lotaustralin are cyanogenic glycosides that can also be found in cassava leaves and roots.17,18 These components are known to be detrimental to human health as they may contribute to developing neurological disorders, especially when consuming raw cassava or long-term consumption of incorrectly processed tubers. 18 Hence, cassava should be processed appropriately before consumption as various effective detoxification methods are available for cassava to be eaten safely, such as drying and boiling.19,20
Despite the numerous studies and reviews on cassava, recent reviews have primarily emphasized its potential to meet future food demand, as well as its applications in biocomposites and bioenergy. Some of these reviews have also delved into specific topics such as the extraction of starch and antinutrients from the plant. However, a comprehensive systematic review that provides an overview of the potential benefits of cassava is still lacking. In order to bridge this gap, the present review objectives are to assess and summarize the phytochemical constituents of cassava and their corresponding pharmacological activities, which may arise due to the existence of bioactive compounds generated by the cassava plant. In addition, the botanical characteristics and traditional uses of different parts of the cassava plant are summarized. Principally, this review assesses and discusses the pharmacological activities of cassava extracts from human clinical trials, in vivo, and in vitro studies and compares them with other extracts from different natural sources. The present study delved into the therapeutic potential of various phytochemicals found in cassava's leaves, stems, roots, and tubers, with a particular focus on their anti-cancer, anti-inflammatory, anti-diarrheal, hypocholesterolemic, anti-bacterial, anti-diabetic, and wound healing activities.
Methods
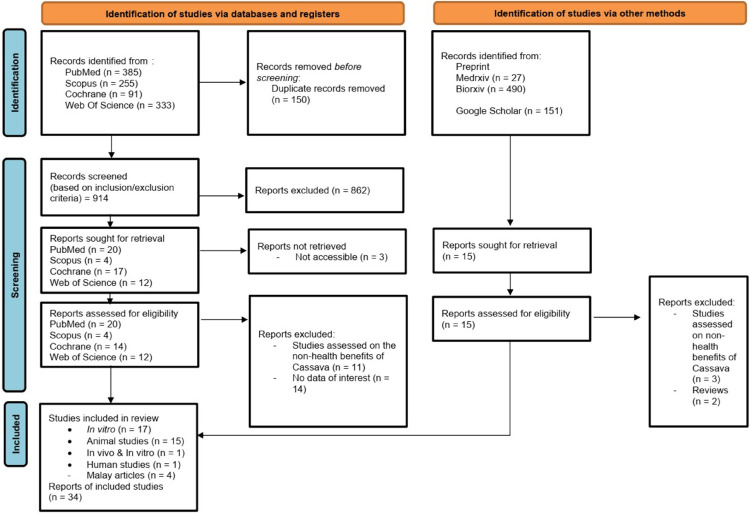
All relevant information on cassava was collected from the following electronic databases: Cochrane Library, PubMed, Scopus, Web of Science, Google Scholar, and Preprint Platforms (Medxriv and Biorxiv), from inception until October 2022. The search results are shown in Figure 1 (PRISMA flow chart). The search was conducted using the following terms: “Manihot esculenta Crantz”, “Manihot esculenta”, cassava, tapioca, ubi kayu, singkong, phytochemistry, flavonoid, alkaloid, pharmacology, antidiarrhea, anti-oxidant, benefit, clinical effect, nutraceutical, ethnobotany, ethnomedicine, health benefit, medicine, phytotherapy. The terms “ubi kayu” and “singkong” were only included in the search for articles from Google Scholar. The search terms used for the preprint platforms and Cochrane Library were limited to “Manihot esculenta Crantz” and cassava to ensure the search results were restricted to the topic of interest. The search strategies for the combination of the terms include the use of Boolean operators “AND” and “OR” and the use of truncation symbols, including question mark (?), asterisk (*), and quotation mark (“”). The reporting complies with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement.
Figure 1.
PRISMA flow chart.
Two researchers independently screened and reviewed the articles and reports. Any discrepancy was resolved by discussion with a third reviewer. The following inclusion criteria were used to assess the eligibility of the studies to be included in the review: articles including original research, reports, technical papers, and conference proceedings. Besides, studies reported in the English and Malay language were included, as well as studies that assess the pharmacological activities and health benefits of cassava's leaves, leaf extract, rhizomes (shoot), and roots. The type of studies includes human, in vivo, animal, and in vitro laboratory studies with no limitation to the year of publication of the studies were all covered. In order to ensure the relevance and appropriateness of the studies included in the review, a set of exclusion criteria was applied, which encompassed various types of literature such as reviews, short surveys, editorials, notes, letters, and books, as well as studies that focused on non-pharmacological aspects of cassava or investigated the association between cassava residual toxicity and human diseases, agronomic aspects, or the wastewater of cassava.
Results
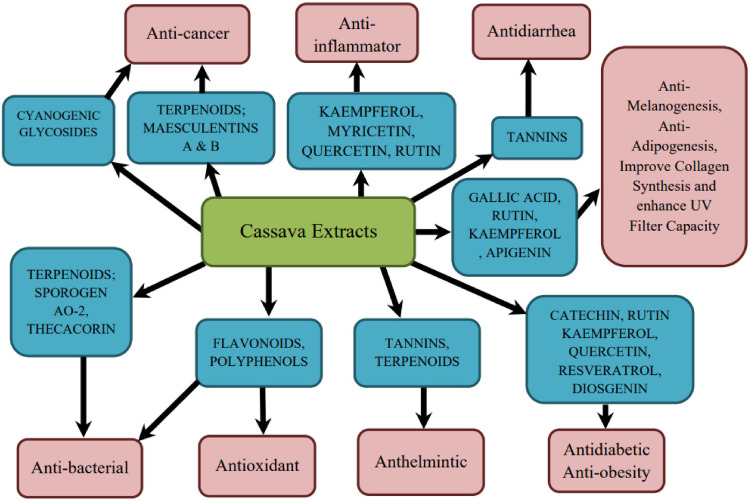
Based on previous studies, cassava has demonstrated multiple pharmacological activities, including its effect as an anti-oxidant which provides hepaprotective and nephroprotective effects, anti-inflammatory, analgesic, anti-cancer, anti-bacterial, anti-diabetic, anti-hypercholesterolemic, collagen and fibroblast synthesis enhancement in wound healing, anti-diarrheal and anthelmintic activity as illustrated in Figure 2. The PRISMA flow diagram depicted in Figure 1 shows the search strategy used and its search results; 1582 studies were screened, and 150 studies were excluded from this review after deduplication. A total of 1548 studies were further excluded for not meeting the determined inclusion criteria, thus leaving only 34 studies to be retrieved and included in this review. The number of studies retrieved includes fifteen animal studies, seventeen in vitro studies, one mixed in vivo and in vitro study, and one clinical study. The outcome of these studies, plant components of cassava, extraction techniques, dosing regimens, isolated phytochemicals, method of isolation, research subjects, and their corresponding pharmacological effects are summarized in Table 1.
Figure 2.
Pharmacological activities of Manihot esculenta (cassava) based on its phytochemical constituents.
Table 1.
An Inclusive Summary of literature-Based Investigations Presenting the Plant Components of Cassava, Extraction Techniques, Dosing Regimens, Isolated Phytochemicals, Method of Isolation, Research Subjects, and their Corresponding Pharmacological Effects and an Outcome Summary.
| Plant part | Extraction method/ duration/ yield | Concentrations/Dosages used | Phytochemicals characterized | Isolation method | Experimental subject | Pharmacological effect. (Summary of outcomes) | Reference |
|---|---|---|---|---|---|---|---|
| Young leaves | Cassava powder was stirred in pure water until filtration and lyophilization for 24 h. The extraction yield is 7.4%. | 10, 50, and 100 µg/mL | Flavonoids (rutin and two other unidentified compounds) | HPLC-UV/DAD | Human promyelocytic leukemia cells (HL-60) | Antioxidant, antiradical. The extract reduced advanced lipid peroxidation markers and inhibited free radical formation. It also reduced ROS production in cellular models without toxicity. | 21 |
| Young leaves (3 months to 15 months after planting) | Oven-dried leaves powder was prepared differently for each in vitro assay | 5 to 100 μg/mL | Flavonoids (catechin, rutin, kaempferol, quercetin), tannins (tannic acid), stilbenes (resveratrol), saponins (diosgenin) | HPLC-DAD | In vitro study | Antidiabetic, Anti-obesity. Bound phenolics displayed greater lipase inhibitory activity than their free counterparts. Although phenolic contents declined post in vitro digestion, they exhibited significant antidiabetic and anti-obesity inhibitory activities | 22 |
| Leaves, 5-month- old | Maceration in 95% ethanol for 24 h | Anti-oxidant: 0-500 µg/mL Anti-melanogenesis: 250-500 µg/mL Anti-adipogenesis: 500-1000 µg/mL Enhance collagen synthesis: 125-250 µg/mL UV filter capability: 62.5-1000 µg/mL |
Rutin, kaempferol, apigenin, gallic acid | HPLC UV-DAD | In vitro studies | Anti-oxidant, anti-melanogenesis, anti-adipogenesis, enhanced collagen synthesis, UV filter capability. | 23 |
| Leaves, 6-month-old | Leaves were macerated in methanol for 24 h with 5% sulfuric acid. | The IC50 value of 156.03 ppm | Qualitative phytochemical screening test indicated the presence of: Flavonoids, phenols | N/D | In vitro study | Anti-oxidant. Cassava leaf methanol extract has flavonoids and phenolic groups, weak antioxidant capacity in metal ion reduction, and an IC50 value of 156.03 ppm. | 24 |
| Leaves (6, 9, 12, and 15 months after planting) | Cassava powder is mixed with methanol overnight at room temperature. | 300 mg | Polyphenols (p-Hydroxybenzoic acid, protocatechiuc acid, syringic acid, gallic acid, vanillic acid, gentisic acid, benzoic acid, salicylic acid) | HPLC/DAD | In vitro study | Anti-oxidant. DPPH method showed decreased antioxidant activity after in vitro digestion, but FRAP method showed no difference between bioaccessible and methanolic polyphenols. | 25 |
| Leaves | The powder was extracted with 90% ethanol using Soxhlet apparatus at 50 °C-55 °C for 3 days. 50 g powder yielded 10 g of extract after drying and concentrating. | 50, 100, or 200 mg/kg | N/D | N/D | Adult Wistar rats | Anti-oxidant. The extract lacked antioxidant activity on MDA levels but boosted antioxidant enzymes; SOD, GSH, and CAT | 26 |
| Leaves | Cassava powders were extracted with ethanol-water (1:1, v/v) solution three times within four hours | 100, 200 and 400 mg/kg bw | Flavonoids | Macroporous resin column chromatography | Male ICR mice (weighting 18-22 g) | Anti-oxidant, Hepatoprotective. Cassava leaf flavonoid extract reduced ALT and AST in mouse serum and improved CCl4-induced liver damage, but did not restore normal levels. | 27 |
| Leaves | N/D | 150, 300, and 450 mg/kg b.w. | N/D | N/D | Mus musculus mice, 2-3 months (200 grams), gentamicin-induced hepatotoxicity | Hepatoprotective, anti-oxidant. Cassava leaf extract at 450 mg/kg improved liver histopathology and protected against gentamicin-induced hepatotoxicity. | 28 |
| Leaves | Maceration of cassava powdered leaves for 24 h with methanol/acetone | 200, 100, and 50 mg/kg bw | Qualitative phytochemical screening tests indicated the presence of the following: Flavonoids, alkaloids, saponins, anthocyanins, tannins, and triterpene | N/D | Male Albino Wistar rats | Anti-oxidant, Nephroprotective. The extracts prevented acetaminophen-induced damage in a dose-dependent manner and had antioxidant activity similar to silymarin. | 29 |
| Leaves | Maceration of cassava powdered leaves in 96% ethanol for three days. The powder yielded 20 g after concentrating. | 179.2 mg/kg BB | N/D | N/D | Female Sprague Dawley rats, 2-3 months (± 200 grams) | Anti-oxidant, Anti-inflammatory. Cassava leaf extract reduces MMP-8 expression in gingival fibroblasts of rats with ovarian dysfunction and P. gingivalis-induced periodontitis. | 30 |
| Leaves | Wild (WMEE) and micropropagated (MMEE) cassava shoots were powdered and extracted using ethanol by cold extraction | 1000 µg/ml showed anti-oxidant, and 250 µg/ml showed cytotoxicity effects | Alkaloids, flavonoids, tannins, steroids (N-Hexadecanoic acid, octadecanoic acid, pentadecanoic acid, dodecanoic acid, tetradecanoic acid, hexamethyl-cyclotrisiloxane 1H,15H-Hexadecamethy loctasiloxane), triterpenoids (Trans-squalene squalene, lupeol) | FITR, Gas Chromatography/Mass Spectrometry analysis | In vitro studies | Anti-oxidant, cytotoxicity. WMEE and MMEE extracts had significant hydrogen peroxide scavenging activity. WMEE extract showed potential cytotoxicity in colon carcinoma cells compared to MMEE extract. | 31 |
| Leaves | Dried leaves were extracted with hydroethanol (20:80), followed by ultra-sound for one hour. Aqueous phases were then concentrated and lyophilized. | 500 µg/mL | Polyphenols, flavonoids | HPLC/DAD, UV-Vis & FTIR analysis | In vitro study | Anti-oxidant, Anti-bacterial effect against P. aeruginosa meropenem-resistant species | 32 |
| Leaves | Maceration of leaves in 96% ethanol for 24 h. The yield of cassava ethanol extract was 21.873%. | MIC values of ethyl acetate fraction against: -S. epidermidis: 2.5%-5.0% (w/v) - P. acnes: 1.25%-2.5% (w/v). MBC value of ethyl acetate fraction against: - S. epidermidis was: 5% (w/v) - P. acnes: 2.5% (w/v) |
Qualitative phytochemical screening tests indicated the presence of: Flavonoids, polyphenols, quinone, saponin | N/D | In vitro study | Anti-bacterial. The ethanol extract of cassava leaves has antibacterial activity against clinical isolates of Staphylococcus epidermidis and Propionibacterium acnes. The ethyl acetate fraction displayed the highest activity against both bacteria. | 33 |
| Leaves | Maceration of ground powder leaves in ethanol for 3 days | 100, 250, 500 mg/kg | Qualitative phytochemical screening tests indicated the presence of: Terpenoids, tannins, flavonoids, carotenoids | N/D | In Vivo, rat models | Anti-inflammatory, Analgesic, Antipyretic. carrageenan and histamine-induced oedema, acetic acid-induced writhing, and yeast-induced pyrexia in rats | 34 |
| Leaves | Crude leaf extract | 12.5%, 25% cassava leaf extract | N/D | N/D | In vitro study | Anti-inflammatory. Cassava leaf extract can inhibit the COX-2 expression in neutrophils in a dose-dependent manner. | 35 |
| Leaves | The leaves were boiled in distilled water for 30 min before being filtered and evaporated to dryness at 40 °C. The yield of the extract is 8.33%. | 100, 200 or 400 mg/kg, p.o. and 1-4% w/w in petroleum jelly, topically | N/D | N/D | Young adults Sprawley-Dawley rats (150-200 g) and mice (18-22 g) | Anti-inflammatory, analgesic. The extract (oral and topical) significantly inhibited carrageenan-induced rat paw edema and xylene-induced ear swelling in mice. Extract had higher anti-inflammatory activity than indomethacin (10 mg/kg, s.c. or 1% w/w in petroleum jelly). For analgesic effects, extract significantly inhibited acetic acid- and acetylcholine-induced mouse writhing tests, similar to aspirin (100 mg/kg, i.p.). Extract had lower analgesic activity than aspirin, except for the topically administered extract on acetylcholine-induced pain. | 36 |
| Leaves | The dry cassava leaf powder was macerated in 70% ethanol for 24 h. The yield of the extract is about 10 g in weight. | 12.8, 25.6, 51.3, and 102.6 mg/kg BW | N/D | N/D | Balb/c mice aged 23 months and weighing 20-30 g | Analgesic, anti-inflammatory. All groups tested did not exhibit significant results compared to the positive control (paracetamol). | 37 |
| Leaves | Distillation | 100, 200, and 400 ppm | Hydrogen cyanide from cassava cyanide extract (CCE) | Gas chromatography isolation | Alveolar epithelial cells (A549 cell line) | Anti-proliferative. Nitrile radicals were reduced with bioactive compound concentration. Bioactive principles concentration negatively correlated with mitochondrial/lysosomal functions. CCE showed necrotic cell death at 400 ppm | 38 |
| Leaves | Maceration in deionized water for 24 h | 200 µg/mL | N/D | N/D | In vitro study | Enhanced wound healing by 96.10% in combination with therapeutic ultrasound. | 39 |
| Leaves | Maceration in 95% ethanol for 48 h | 10 g of each extract was reconstituted in 100 ml of DMSO and used for topical application; administered topically twice daily for 21 days | Tannins, flavonoids (anthocyanins), saponins, alkaloids | Qualitative screening by Folin-ciocalteu's method (phenolic acids) and pH differential method (anthocyanin) | Wistar rats (120- 180 g) | Enhanced collagen synthesis in wound healing in type I diabetic rat. | 40 |
| Leaves | Maceration in 70% ethanol | 0.5%, 1%, 2%, 4% | N/D | N/D | In vitro study | Enhanced fibroblast proliferation in wound healing at 0.5% with increased fibroblast proliferation | 41 |
| Leaves | Maceration with dichloromethane /methanol/water for 3 h | 2400, 1200, 600, 300, and 150 µg/ml | phenols, catechic tannins: proanthocyanidols (condensed tannins), flavonoids, flavans, coumarins, triterpenes, and sterols | Thin layer chromatography | In vitro egg hatch assay, larval development, L3 migration inhibition, and adult worm motility assays | Anthelmintic activity against Haemonchus contortus. The methanolic extract of cassava leaf against larval development (57.6%), with a dose-dependent effect. | 42 |
| Leaves | The leaf powder was extracted by hot distilled water and dried, followed by the percolation method. | 25%, 50%, and 75% | N/D | N/D | In vitro study | Anthelmintic activity against Haemonchus contortus. The concentration of 75% of cassava leaf extract showed the highest anthelmintic activity. | 43 |
| Leaves | Soxhlet extraction with 90% ethanol at 50 °C-55 °C for 3 days | 50, 100 and 200 mg/kg | N/D | N/D | Adult Wistar rats | Anti-diarrheal. The extracts exhibited a dose-dependent reduction in intestinal fluid volume, and significantly inhibited gastrointestinal motility by 100 and 200 mg/kg | 44 |
| Leaves and stalks | Dry powder was macerated in ethyl acetate for 48 h. | 50 mg/kg BW | Qualitative phytochemical screening tests indicated the presence of: Tannins, flavonoids, saponins, alkaloids | N/D | Wistar rats (120-180 g) induced hyperglycemia | Analgesic, Anti-inflammatory. The ethyl acetate extract of cassava leaf and stalk had peripheral analgesic activities of 69.02% and 60.73% respectively as compared to Paracetamol (57.32%). | 45 |
| Leaves and stem | Maceration in 96% alcohol for 7 days | 2 and 4 mg/kg | N/D | N/D | Albino rats sp. Rattus novergicus (app. 150 g) | Anti-inflammatory. The ethanolic extract at 4 mg/kg has reduced plantar edema by 37.7% in this acute inflammation animal model after 3 h of administration. | 46 |
| Leaf stalk | Cassava leaf stalks were extracted with methanol/ acidified methanol/acetone, followed by centrifugation for 10 min at 10 000 rpm. | 0.1 g extract | Flavonoid (anthocyanin), phenolic acids | Estimation technique by Folin-ciocalteu's method (phenolic acids) and aluminum chloride colorimetric method (flavonoids) | In vitro study | Anti-oxidant. | 15 |
| Shoots (soft branches of the aerial parts) | The air-dried powder of the cassava shoots was refluxed with distilled water at 90 °C for 30 min, and the extraction was repeated three times. The aqueous extract yield is 41.0 g in weight. | 50, 100 mg/kg bwt/day orally | quercetin 3-O-rutinoside (rutin), kaempferol 3-O-rutinoside, myricetin 3-Orutinoside, quercetin triglycoside, kaempferol triglycoside | HPLC-PDA | Male Albino rats (180-200 g) | Anti-oxidant, Hepatoprotective. The extract ameliorated liver enzymes, malondialdehyde, and homocysteine, while increased paraoxonase-1. It also improved histopathological and histochemical parameters | 47 |
| Stems | Fresh, milled stems were extracted with 95% ethanol thrice within three weeks. The extract yield is 1.8 kg in 75 kg weight. | Four fractions were isolated: Ethyl-acetate (66 gm), n-Butanol (78 gm), Petroleum ether (245 gm), Aqueous (1298 gm). | Phenolic compounds; Coniferaldehyde, isovanillin, 6-deoxyjacareubin, scopoletin, syringaldehyde, pinoresinol, p-coumaric acid, ficusol, balanophonin, and ethamivan | Fractionation and column chromatography | In vitro study | Anti-oxidant. Ethyl acetate and n-butanol fractions showed the highest DPPH˙ and ABTS·+ scavenging activities | 48 |
| Roots | Cassava roots were homogenized in boiling methanol (99.5%) | 2.5 to 300 μg/ml. Thirty-two minutes of contact time was required for 60 g of extract, yielding 1.7 g of purified product. |
Linamarin | Adsorption using activated carbon and ultrafiltration | - human breast cancer (MCF-7) cell line - human colon adenocarcinoma (HT-29) - acute myelogenous leukemia line (HL-60) |
Anti-cancer; cytotoxic effect. Linamarin's cytotoxic effects on MCF-7, HT-29, and HL-60 cells were determined via the MTT assay. Linamarase significantly increased cytotoxicity, with a 10-fold decrease in HL-60 cells’ IC50 values. | 49 |
| Root (Tapioca starch) | Commercial source of resistant tapioca starch (RS3-tapioca) and pre-gelatinized cornstarch (CS) | 150 g/kg retrograded tapioca starch and 473.2 gelatinized CS in RS3-tapioca diet, while CS contains 623.2 g/kg CS for 28 days | N/D | N/D | Six-month-old, retired breeder Wistar female rats | Lower cholesterol levels. RS3-tapioca may prevent the ovarian hormone deficiency-induced elevation of plasma cholesterol concentration through increased fecal excretion of bile acid and an augmented intestinal pool of bile acid. | 50 |
| Roots (tubers) | Cassava flour was mixed with water and stirred at 4 °C overnight, followed by centrifugation for 20 min. | 0, 0.1, 0.2, and 0.5 mg/ml of diet | Mucopolysaccharide | Fractionation of the crude extract | In vivo study; Wistar rats (weighing 200-250 g) and in vitro study; 3T3-L1, rat glioma C6 cell lines, and HepG2 human hepatoma | Hepatoprotective. Sweet cassava polysaccharides diet increased GSTYa1 and UGT1A6 mRNA levels, decreased CYP1A1 mRNA levels, and inhibited CYP1A2, while its ethanol extracts scavenged hydroxyl radical and superoxide in a dose-dependent manner. | 51 |
| Tubers | 50 g of gari (a creamy-white granular flour prepared from cassava tubers) soaked in 1 liter of cold water are 5.0 and 2.8 mmol/1 of sodium and potassium, respectively | 50, 100 ml/kg | N/D | N/D | Male patients aged 4-24 months diagnosed with acute diarrhea | Anti-diarrheal. | 52 |
| Cassava cross-linked octenyl succinic maltodextrin | Prepared in the laboratory | 20 (CCOMD-L) and 40 (CCOMD-H) g/kg | Resistant starch such as maltodextrin | N/D | Male ICR mice (diabetic and non-diabetic) | Lower blood glucose, hypocholesterolemic. CCOMD diet improved body weight recovery (14.9% CCOMD-L; 18.5% CCOMD-H), blood glucose and insulin levels, plasma total cholesterol (8.1-9.1%), and LDL cholesterol (28.9-39.4%) levels. CCOMD diet also enhanced the short-chain fatty acid content in the intestine. | 53 |
Botanical Description and Taxonomy
Cassava is known as “gbaguda” or “imidaka” in Nigeria; “eyabya” in Tanzania, “kamoteng kahoy” or “balanghoy” in the Phillippines; “singkong”, “ketela pohon” or “ubi kayu” in Indonesia; “yuca” or “manioc” in America.3,54–57 The taxonomical classification of cassava is shown in Table 2. Cassava is generally propagated by stem cuttings. 58 The leaves consist of a palm-shaped leaf blade with 3 to 9 lobes. The color of the leaf may vary with age as purple, dark, or light green in mature leaves, while young leaves have purple buds on their leaves, which eventually turn green. The leaf also consists of a petiole, which varies from green to purple. 59 The stem has sparing branches where the branchlets have light green to reddish color. 60 The stem's diameter and color are influenced by plant variety and age. Furthermore, the root, which is the essential part for dietary purposes, grows between 8 and 15 inches long, and the diameter of the root ranges from 1 to 4 inches, 59 which contains a large amount of starch, and a thin reddish brown fibrous bark is covering the root. They can grow in groups of 4-8 at the base of the stem. 60 Less important and rarely formed is the fruit of cassava. The fruit takes about three months to mature, and it is a trilocular dehiscent capsule with a globate to ovoidal shape. The epicarp and mesocarp of the fruit dry up once the seed matures, and the endocarp proceeds to split open, causing the seeds to disperse. It must be noted that cassava trees are cultivated by planting the cut stem, and it takes ten months or longer to harvest the cassava roots. Cassava plant parts, including its leaves, stems/stalk, rhizome, and roots, are depicted in Figure 3.
Table 2.
Taxonomical Classification of M. Esculenta Crantz. 61
| Taxonomical hierarchy of Manihot Esculenta Crantz | |
|---|---|
| Common names | tapioca, gbaguda, imidaka, eyabya, kamoteng kahoy, balanghoy, singkong, ketela pohon, ubi kayu, yuca, manioc |
| Kingdom | Plantae |
| Subkingdom | Viridiplantae |
| Superdivision | Embryophyta |
| Division | Tracheophyta |
| Subdivision | Spermatophytina |
| Class | Magnoliopsida |
| Superorder | Rosanae |
| Order | Malpighiales |
| Family | Euphorbiaceae |
| Genus | Manihot |
| Species | Manihot esculenta Crantz |
Figure 3.
Cassava plant parts in different growth stages. (A) Cassava leaves. (B) Cassava stems/stalk. (C) Cassava rhizome and roots.
Nutritional Values
Cassava has various features that make it eligible to be exploited commercially due to its high nutritional value for humans and animals. Withstanding harsh environmental conditions such as drought, acidic soil, and the flexible cropping calendar are among many factors that made it industrially attractive. Indeed, cassava is highly tolerant to drought due to its leaf-drooping mechanism as well as rapid stomatal closure in water-scarce conditions.62,63 Additionally, it tolerates highly acidic soil and soil rich in aluminum and manganese. 64 Although it is not advisable to consume cassava that grows in soil with highly contaminated inorganic pollutants such as nickel, zinc, and cadmium, it can still be utilized to produce bioenergy.65,66 Cassava's roots and leaves are the most consumed parts by humans as well as animals. Particularly, cassava roots are enriched with carbohydrates, thus supplying a vital energy source that may range between 110-149 kcal per 100 g, whereas the leaves provide 91 kcal energy per 100 g. Although the leaves provide lower energy than the roots, they are known to be rich in vitamins and minerals. 62 Cassava can be exploited for a broad range of potential economic and industrial applications such as animal feeds, fuel production, and as a raw material for manufacturing industries. 67
Cassava is a significant source of carbohydrates for around 500 million individuals, primarily due to its higher energy yield per unit area in comparison to other major crops. 68 The nutritive components of the mature cassava plant are mainly concentrated in its roots and leaves, which respectively account for 50% and 6% of the plant. 69 Cassava root is an efficient energy source that produces about 250 000 calories per hectare per day. The root is rich in carbohydrates, mainly starch, with some sucrose, glucose, fructose, and maltose. Cassava has both bitter and sweet varieties, with the latter containing up to 17% sucrose. The fiber content is low, while the lipid content ranges from 0.1% to 0.3%. The protein content is also low, with some essential amino acids in very low amounts, but an abundance of glutamic acid, aspartic acid, and arginine. Bitter varieties contain cyanogenic compounds, which can be reduced through processing. Overall, cassava roots are rich in calories but low in protein, fat, and some minerals and vitamins, making their nutritional value lower than other crops like cereals and legumes. 70
Cassava leaves are an abundant source of protein, minerals, and vitamins B1, B2, and C, as well as carotenoids. In addition, the leaves contain higher levels of vitamins A, B, and K, and a variety of essential minerals, including calcium, copper, magnesium, iron, manganese, potassium, sodium, and zinc. 71 In comparison to legumes and leafy vegetables, cassava leaves have a higher crude protein content, crude fat, and mineral content, except for soybeans. The protein content of cassava leaves ranges from 14% to 40% of DM in various varieties, with an amino acid composition comparable to fresh egg protein and higher than soybean protein. Cassava leaves contain mostly starch, and their mineral content varies depending on the variety and age of the plant. The vitamin content of cassava leaves, including thiamin, riboflavin, niacin, vitamin A, and vitamin C, is also considerable. Cassava leaf meal has similar mineral and vitamin content to other leaves, and in some cases, it surpasses that of legumes, leafy legumes, cereals, eggs, milk, and cheese. However, iron from plant sources is less bioavailable than that from animal sources. Cassava leaves are high in fiber, with a content range of 1 to 10 g/100 g FW, compared to the fiber content of legumes and leafy vegetables reported in Table 3. 70
Table 3.
| Factors | Parts of cassava (Proximate composition of 100 g) | |||
|---|---|---|---|---|
| Roots | Leaves | |||
| Food energy (kcal) | 110-149 | 91 | ||
| Carbohydrate (mg) | 25 300-35 700 | 7000-18 300 | ||
| Lipid (mg) | 30-50 | 200-2900 | ||
| Protein (mg) | 300-3500 | 1000-10 000 | ||
| Dietary fiber (mg) | 100-3700 | 500-10 000 | ||
| Essential Minerals | Calcium (mg) | 19-176 | 34-708 | |
| Iron (mg) | 0.3-14 | 0.4-8.3 | ||
| Phosphorus (mg) | 6-152 | 27-211 | ||
| Magnesium (mg) | 30-80 | 120-420 | ||
| Potassium (mg) | 250-720 | 350-1230 | ||
| Copper (mg) | 0.2-0.6 | 0.3-1.2 | ||
| Manganese (mg) | 0.3-0.1 | 7.2-25.2 | ||
| Sodium (mg) | 7.6-21.3 | 5.1-17.7 | ||
| Zinc (mg) | 1.4-4.1 | 7.1-24.9 | ||
| Vitamins | A (mg) | 0.005-0.035 | 8.3-11.8 | |
| B (mg) | Niacin | 0.6-1.09 | 1.3-2.8 | |
| Riboflavin | 0.03-0.06 | 0.21-0.74 | ||
| Thiamine | 0.03-0.28 | 0.06-0.31 | ||
| C (mg) | Ascorbic acid | 14.9-50 | 60-370 | |
Traditional Applications of Cassava in Ethnomedicine and Culinary Practices
Several indigenous groups have utilized cassava for its medicinal properties, and the consumption of this plant is safe when appropriately extracted and processed. Cassava has been used in traditional medicine for various ailments, including abscesses, ringworms, sores, and wounds in Nigeria and the Guianas, although there is insufficient scientific evidence to support these uses.73,74 For example, the starch extracted from cassava roots is combined with rum and applied topically to children to treat abscesses and skin eruptions. Additionally, grated cassava roots are mixed with aloe vera juice and cattle tallow for external application to treat ringworm and acute dermatitis, while the grated root is applied directly to cuts and sores. Cassava leaves are also used for medicinal purposes, such as hemostatic plasters to treat wounds. 74 In rural areas of Tanzania, cassava leaves and stems are commonly used to induce abortion in unwanted pregnancies, as they have been found to have a strong contractive effect on the uterus. 57 Moreover, Paraguayan migrants in Argentina use the root of wild cassava as one of the many ingredients required in a decoction to treat urinary infections. 75
In Borneo Island, located in South East Asia, some ethnic groups such as Bidayuh, Selako, Melanau, Iban, and Kayan folks also use cassava as traditional medicine. Some people from Bidayuh, Selako, and Melanau groups use cassava to alleviate headaches by preparing a paste from the pulp to be applied topically as a poultice. The Ibans consume cassava to relieve constipation by drinking a concoction made with a combination of honey and juices from the leaves. Besides, the Kayan folks believe that fresh juice prepared from cassava leaves can heal hematemesis. 76 Remarkably, the juice of grated cassava roots is used as a treatment for constipation and dyspepsia, yet the aqueous leaves’ soak is applied to treat fever in the south pacific area. Notably, cassava has also been used in Fiji to treat glaucoma by folding the stem or the heated leaves and rubbing them onto the affected eyes. 77 In China, cassava leaf extract has been used as a traditional Chinese medicine to control cancer, although insufficient scientific proof exists. 49 Cassava is believed to be useful in treating snake or scorpion bites due to its bioactive chemical contents in different cultures, such as India and Papua New Guinea. 78 It is also consumed as a wheat alternative in Western cuisine for patients diagnosed with celiac disease. 79
Various methods of cassava utilization for food are adopted in South America and West Africa, including grating and pounding cassava tubers into a pulp, shaping them into pies or cakes, cooking in various ways, and making soups and drinks. Cassava is also used to make Farina, Ampesi, and Macaroni. In Indonesia, cassava is used to make Tiwul, Oyek, and Gatot. The juice extracted from the preparation of Farina is used to make Cassareep and Tucupay sauces. Cassava pudding is also made by grating cassava roots and mixing with coconut or banana. In several African countries, cassava is prepared in diverse methods. For instance, Gari, a significant food in Ghana, Nigeria, Guinea, Benin, and Togo, is made by fermenting cassava pulp with Corynebacterium manihot and Geotrichum candidum. After fermentation, the partially dried pulp is sieved, heated, and stirred until it becomes light and crisp. Good quality gari should be creamy yellow with uniform grains. Other cassava-based foods in west African countries include Meduame-M-bong, Attieke, Chick-wangue, Kapok pogari, and Lafun, each prepared through specific processes involving soaking, pounding, fermentation, drying, and sieving, and consumed with various accompaniments.80,81 Lactic acid bacteria addition to cassava products involves the use of bacterial strains, such as Lactobacillus, Leuconostoc, and Pediococcus, to ferment cassava-based foods and improve their nutritional and sensory properties. This process results in the conversion of carbohydrates into lactic acid, which lowers the pH of the product, making it more acidic with sour flavor and inhibiting the growth of harmful bacteria. Lactic acid bacteria also produce various beneficial compounds, such as vitamins, amino acids, and enzymes, that enhance the nutritional quality and digestibility of cassava products. The addition of lactic acid bacteria to cassava-based foods is a common practice in many traditional food fermentation processes, such as Nigerian gari, fufu, and gari Foto. 82
Phytochemistry
The Activities of Phenolic Compounds in Cassava
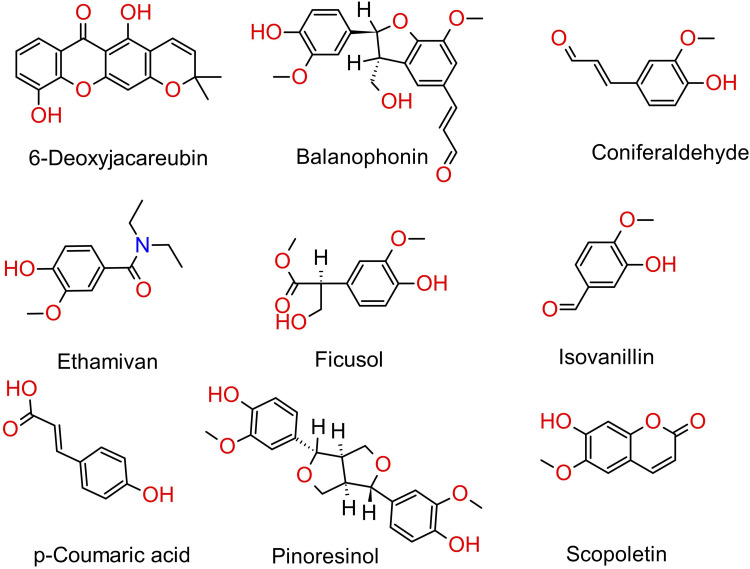
The cassava plant has been reported to contain different flavonoids, polyphenols, starch, terpenoids, reducing sugars, alkaloids, steroids, carotenoids, fatty acids, and benzoic acid derivatives.48,83,84 The presence of phenolic compounds and flavonoids in cassava's stem and leaf extracts exerts an anti-oxidant effect on the plant, as observed from the significant DPPH anti-radical and free radical scavenging activities.84,85 The extraction of milled cassava stems with ethanol solvent has led to the isolation of diverse phenolic compounds: 6-deoxyjacareubin, balanophonin, coniferaldehyde, ethamivan, ficusol, isovanillin, p-coumaric acid, pinoresinol, scopoletin, and syringaldehyde, each possesses a different strength of anti-oxidant activity.48,86 Moreover, a further study has also identified eight more phenolic compounds, p-hydroxybenzoic acid, protocatechuic acid, syringic acid, gallic acid, vanillic acid, gentisic acid, benzoic acid and salicylic acid from methanolic extract of cassava leaves which are further isolated and studied to contribute to the anti-oxidant activity of cassava plant. 25 Jampa et al (2022) reported that the maceration of leaves in the ethanol solvent identified gallic acid, rutin, kaempferol, and apigenin. 23 These four compounds exhibit potent anti-oxidant activities, which contribute to the anti-melanogenesis effect, anti-adipogenesis activity, the enhancement of collagen synthesis as well as UV filtering properties. 23 Furthermore, polyphenol glycosides such as; quercetin 3-O-rutinoside (rutin), kaempferol 3-O-rutinoside (nicotiflorin), myricetin 3-O-rutinoside, quercetin triglycoside, and kaempferol triglycoside were identified from cassava shoot extract which demonstrated anti-inflammatory activity. Polyphenols and flavonoids were also detected from the hydroethanolic extract of dried cassava leaves, where this extract has demonstrated an anti-bacterial effect. 32 Figure 4 illustrates some of the phenolic compounds extracted from cassava.
Figure 4.
Structures of some phenolic compounds isolated from cassava.
The Activities of Tannins in Cassava
Tannin, a phenolic compound found in cassava leaves, has been characterized and extracted in various studies, either as tannic acid 22 or proanthocyanidin (condensed tannins). 42 The isolation process involved macerating the leaves with dichloromethane, methanol, or water, leading to the extraction of condensed tannins along with other chemical compounds such as unspecified flavonoids, coumarins, triterpenes, and sterols. Thin layer chromatography was employed for identification, and revealing the presence of tannins and terpenoids believed to be responsible for the observed anthelmintic activity against Haemonchus contortus parasites. 42 Furthermore, in addition to tannic acid, the HPLC-DAD method was utilized to isolate various flavonoids, including catechin, rutin, kaempferol, quercetin, resveratrol, and diosgenin, from oven-dried cassava leaf powder. This extract has exhibited inhibitory activities against α-amylase, α-glucosidase, and lipase enzymes, showcasing its potential as an anti-diabetic and anti-obesity agent. 22
The Activities of Terpenes and Cyanogenic Glycosides in Cassava
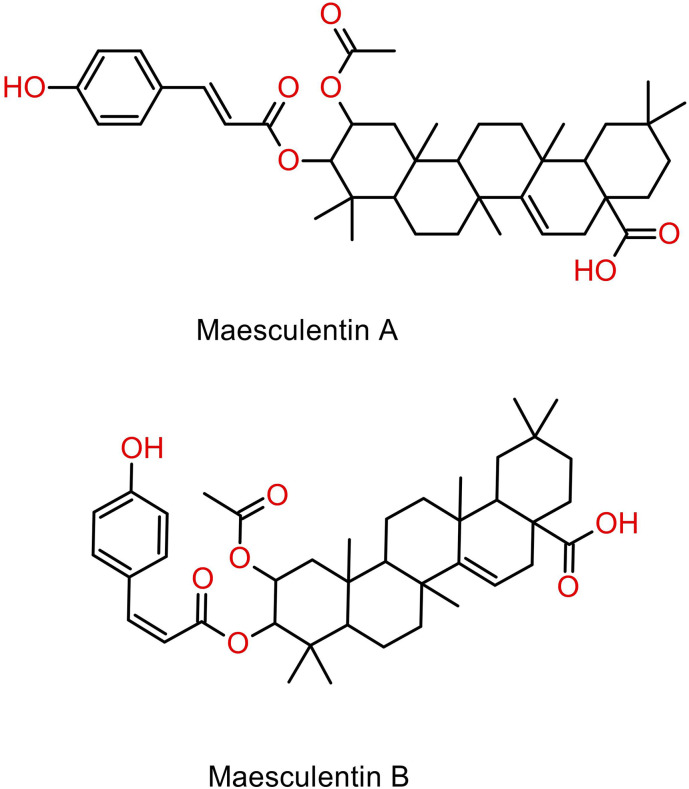
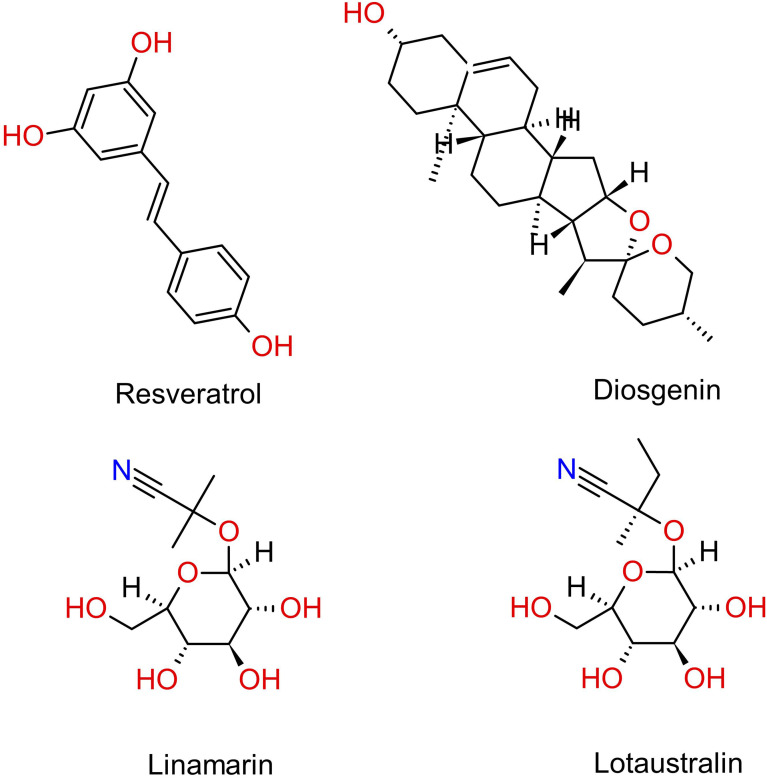
Cassava was found to contain a group of terpenoids comprising tetraterpenoids (carotenoids), triterpenes, triterpenoids, and triterpene glycosides (saponins), through maceration with ethanol, methanol, or acetone.29,34 Recently, a silica gel column was employed to isolate a new diterpene and a novel sesquiterpene from the ethanol extract of cassava stems. The newly discovered diterpene was designated as yucalexin P-23, alongside three other known compounds, namely yucalexin P-15, protocatechuic acid, and catalpinic acid, where the latter two demonstrated antimicrobial activity. 87 Similarly, in another study, the same method was used to isolate two new triterpenoids from a combined ethanolic stem extract, which were identified as maesculentins A and B, as depicted in Figure 5. Worthy of note that both compounds are cis-trans isomers of each other, which have demonstrated moderate anti-cancer activity where the configuration of the double bond may play a significant role in its cytotoxic activity. 88 Recently, silica gel column was employed to isolate and identify two new terpenoids from ethanolic cassava stem extract, namely sporogen AO-2, a sesquiterpene, and thecacorin C, a diterpene. Both compounds were found to display modest anti-bacterial activity upon examination. 89 Table 4 illustrates the chemical components of cassava categorized according to their respective phytochemical classes, the plant parts from which they were extracted, and the extraction method employed. Cassava also contains toxic compounds, namely linamarin, and lotaustralin, which are cyanogenic glycosides, as illustrated in Figure 6. Linamarin was isolated and has been shown to exhibit potent cytotoxic activity upon homogenization of cassava roots in boiling methanol. 49 Similarly, cytotoxic activity was also demonstrated by hydrogen cyanide derived from the cyanogenic glycosides isolated using gas chromatography from distillation cassava leaf extract. 38 Figure 6 shows the structures of stilbene derivative (resveratrol), saponin (diosgenin), and cyanogenic glycosides (linamarin and lotaustralin) isolated from cassava.
Figure 5.
Structures of terpenoids (Maesculentins A and B) isolated from cassava.
Table 4.
| Phytochemical groups | Components | Plant part | Extraction methods/solvent used | References |
|---|---|---|---|---|
| Flavonoids |
|
Aerial parts (leaves and stem), roots |
|
23,33,34,47,95 |
| Phenolic acids |
|
Leaves |
|
34,40,48 |
| Terpenoids |
|
Leaves, stem, twigs |
|
31,88,89,98 |
| Fatty acids |
|
Leaves |
|
31 |
| Saponin (steroid) |
|
Leaves |
|
22,45 |
| Cyanogenic glycosides |
|
Leaves, roots |
|
38,49 |
| Benzoic acid derivatives |
|
Leaves |
|
25,88 |
| Carotenoids |
|
Leaves, roots |
|
19,95–97 |
| Carbohydrate |
|
Leaves, roots |
|
53,93 |
Figure 6.
Structures of stilbene compound (resveratrol), saponin (diosgenin), and cyanogenic glycosides (linamarin and lotaustralin) isolated from cassava.
The Effect of Variety, Cultivar, Age, and Extraction Solvent and Methods on the Isolated Compounds
Carotenoids comprising β-carotene are identified using HPLC in the acetone extraction of cassava leaves, where the carotenoid composition in cassava was shown to be influenced by the age of the plant, the variety of cassava plant, and cultivar. 19 In contrast to the carotene content of other herbs and vegetables analyzed by Chaiareekitwat et al (2000), the β-carotene content of cassava leaves was recorded to be the highest after six months of planting. 19 Extractions may also be influenced by the variety of the plant, particle size, and plant parts. 90 For instance, the phenolic content was 681.5 and 442.4 GAE mg/100 g in the peel and the stem from the yellow-fleshed cassava variety with a particle size of 0.2 m, respectively. 90 In their study, Gnamien et al (2022) evaluated the energy values, carbohydrate, and starch contents of cassava roots to identify the most favorable harvest and maturity stage that would yield cassava roots with the highest nutritional value. They found that the cassava roots harvested in the twelfth month had the highest energy values (389.21 Kcal/100 g of DM), with corresponding carbohydrate and starch levels of 94.65 and 83.54 g/100 g of DM, respectively. 91 Furthermore, cultivar type, geographical area, and altitude may also influence the extraction yield of the plant. To illustrate, tannin contents were analyzed from the leaves of six Malaysian cassava cultivars, and the highest tannin content was 10.05 mg/100 g in a cultivar named Sri Pontian (a sweet variety), while the lowest content was 5.74 mg/100 g in the cultivar Sri Kanji 2 (a bitter variety). 92 Furthermore, cassava plants cultivated at two locations in Sabah, Borneo Island part of Malaysia, have shown different total extracted starch yields where the total starch content is 61.21 g/100 g and 51.77 g/100 g in Semporna and Tawau, respectively. 93 Noerwijati and Budiono (2015) conducted a study to investigate the effect of altitudes on starch yield in different cassava clones. They compared the starch yield of cassava clones grown at low (80 m), middle (530 m), and high (800 m) altitudes. The study found that the highest starch yield was obtained at low altitudes with a mean of 11.65 t ha−1, while the lowest starch yield was obtained at high altitudes with a par of 1.18 t ha−1. 94
Discussion
Anti-Cancer Effects
Due to their cytotoxic and anti-proliferative activity, cassava leaf extracts have been utilized as a traditional medicine in China for cancer treatment. In vitro cytotoxic activity of cassava was examined by Chinnadurai et al (2019) on human colon adenocarcinoma cells (HT-29) using wild and micropropagated cassava ethanol extracts (WMEE and MMEE respectively). 31 Both WMEE and MMEE extracts showed decreasing cell viability with increasing concentration, with IC50 values of 23.77 μg/ml and 133.45 μg/ml, respectively. Phytochemical screening indicated the presence of alkaloids, flavonoids, steroids, tannins, and triterpenoids, while GC-MS analysis identified trans-Squalene, squalene, and lupeol in WMEE, and N-Hexadecanoic acid, octadecanoic acid, pentadecanoic acid, dodecanoic acid, tetradecanoic acid, cyclotrisiloxane, and 1H,15H-hexadecamethyloctasiloxane in MMEE. 31 Cassava's anti-cancer properties may be attributed to linamarin and hydrogen cyanides. 49 Linamarin, hydrolyzed by linamarase, has been found to inhibit growth signals, and proto-oncogenes such as c-Myc gene, and increase p53 protein expression which results in the inhibition of G1 phase cell division.99,100 Purified linamarin is more potent than crude linamarin due to other unidentified phytochemical components in the crude extract. Cassava cyanide extract (CCE) also demonstrated significant anti-proliferative activity in adenocarcinoma human alveolar basal epithelial cells (A549) at a concentration of 400 ppm using MTT and neutral red uptake (NRU) assay. 38 Uniquely, Maesculentins A and B were isolated from the stems of cassava extract in a study conducted by Pan et al (2015), and both components demonstrated moderate cytotoxic activity against the HGC-27 tumor cell line. Although there is a lack of research on their mechanism of action, the study proposed that the unsaturation of the chemical structure may significantly contribute to their anti-cancer property. 88
Anti-Inflammatory Activities
The traditional use of cassava for alleviating pain and treating topical skin sores, redness, and febrifuge is supported by several studies demonstrating its anti-inflammatory activity.30,34–37,45–47 Lungit et al (2020) reported that cassava leaf extract fed to female Sprague-Dawley rats with P. gingivalis-induced periodontitis and ovarian dysfunction has significantly reduced MMP-8 expression. 30 MMP-8 is stimulated by the pro-inflammatory cytokine TNF-α to contribute to the development of periodontitis. The anti-inflammatory action of cassava leaf extract involved a reduction in the expression of TNF-α, which subsequently decreased collagen degradation. 30 Furthermore, cassava leaf extract might exhibit anti-inflammatory activity in Albino rats with plantar edema at a dose of 4 mg/kg, as edema was decreased in 37.67% of the treatment group. The extract's proposed mechanism of action may involve the inhibition of eicosanoids that consequently inhibit nitric oxide synthase, which is synthesized during the late phase of local acute inflammation. 46 Another reported that ethanolic cassava leaf extract has significantly reduced carrageenan-induced paw edema, comparable to the positive control used in the study. It has also shown a significant reduction in the acetic acid-induced writhing assay, and this result might be due to the blockage of calcium influx or cyclooxygenase inhibition that subsequently reduces prostaglandin and thromboxane production. 34 Ajayi et al (2015) found that the ethyl acetate extract of cassava leaves or stalks reduced acetic acid-induced writhing in Wistar rats at 50 mg/kg BW. The extract's analgesic effect was significantly higher than that of paracetamol, the positive control. The extract's phytochemical screening tests indicated the presence of tannins, flavonoids, saponins, and alkaloids that may contribute to its analgesic and anti-inflammatory effects. 45
Elshamy et al (2021) found that aqueous cassava shoot extract reduced paracetamol-induced liver injury, as evidenced by improved histopathological and histochemical tests and reduced levels of homocysteine, malondialdehyde, and liver enzymes. The extract contains quercetin 3-O-rutinoside (rutin), kaempferol 3-O-rutinoside, myricetin 3-O-rutinoside, quercetin triglycoside, and kaempferol triglycoside that may contribute to its anti-oxidant and anti-inflammatory activity. 47 Additionally, Meilawaty et al (2019) showed that cassava leaves have anti-inflammatory effects on human neutrophil cell culture. Two groups were treated with 12.5% or 25% cassava leaf extracts, while two control groups were included. The study measured COX-2 expression, which is necessary for producing prostaglandins, and found that 25% extract had stronger inhibition than 12.5% extract. However, the study noted that the administration time of cassava extract was too short for optimal anti-inflammatory action. 35 Adeyemi et al (2008) found that cassava extract exhibited significant and dose-dependent anti-inflammatory activity in rats and mice. When compared to the control (indomethacin), cassava extract showed superior effects at a topical dose of 4% and an oral dose of 400 mg/kg. The extract may act by inhibiting early 5-hydroxytryptamine, middle-phase kinins and prostaglandins, and late-phase autacoids release. It may also inhibit the cyclooxygenase enzyme, similar to traditional anti-inflammatory drugs. 36
Anti-Diarrheal Activities
Despite the traditional use of cassava in diverse gastrointestinal-related disorders, there is limited scientific research exploring its potential efficacy in this regard. Bahekar and Kale (2015) conducted a study demonstrating the significant anti-diarrheal activity of cassava ethanolic leaf extract in Wistar rats. The effectiveness of the extract was found to be dosage-dependent, with higher concentrations (200 mg/ kg) showing greater inhibition of gastrointestinal motility and reduction in intestinal fluid accumulation. 44 The anti-diarrheal mechanism of cassava leaf extract involves the inhibition of prostaglandin synthesis. Prostaglandins play a role in regulating gastrointestinal motility and intestinal fluid transport through the modulation of cAMP levels. 101 In particular, prostaglandin E2 and its receptors (EP1 and EP3) promote muscle contraction, while EP2 and EP4 receptors induce muscle relaxation via cAMP upregulation. 102 Inhibiting prostaglandin synthesis hinders these processes, leading to decreased gastrointestinal motility and intestinal fluid accumulation. While the study did not identify the specific phytochemical constituents, tannins and flavonoids were suggested as potential contributors to the anti-diarrheal properties of the extract. 44 In multiple studies, tannins have demonstrated their anti-diarrheal effect by reducing diarrhea duration and inhibiting the CFTR chloride channel.103–105 Certain flavonoids, including quercetin and kaempferol, have shown inhibition of prostaglandin synthesis through cyclooxygenase-2 or PGE synthase enzymes. 106 Furthermore, in different studies, quercetin has also demonstrated its capability to reduce fluid accumulation and intestinal motility and inhibit muscle contraction.107,108 Grange (1994) conducted a study on 66 children with acute diarrhea to evaluate the efficacy and safety of cassava salt suspension (CSS) in comparison to the standard oral rehydration solution (WHO-ORS). The study found that CSS was as effective as WHO-ORS with only four children experiencing therapy failure. CSS can be used as a substrate for homemade oral rehydration solution, but it may not be suitable for patients with significant electrolyte imbalance due to low sodium and potassium content. 52
Hypocholesterolemic Effects
Liu et al (2006) investigated the cholesterol-lowering effects of retrograded tapioca starch (RS3-tapioca) in the context of hypercholesterolemia due to ovarian hormone deficiency. The study found that RS3-tapioca significantly decreased total plasma cholesterol concentration in rats undergoing ovariectomy specifically in conditions that lead to increased blood cholesterol, such as ovariectomy and diabetes Additionally, rats fed with RS3-tapioca exhibited significantly higher secretion and excretion of bile acids compared to rats fed with gelatinized corn starch (CS). This suggests that the cholesterol-lowering mechanism of RS3-tapioca may involve increased bile acid secretion and fecal bile acid excretion. Furthermore, the rats fed with RS3-tapioca showed significantly lower levels of (VLDL + LDL)-cholesterol, indicating that a reduction in VLDL secretion may contribute to the hypocholesterolemic effects observed. 50 Wang et al (2014) conducted a study to investigate the impact of cassava cross-linked octenyl succinic maltodextrin (CCOMD) on the blood cholesterol and glucose levels of diabetic and non-diabetic mice. The results showed that CCOMD reduced total blood cholesterol and LDL cholesterol levels in both low (20 g/kg) and high doses (40 g/kg), and also improved blood HDL cholesterol levels. Therefore, CCOMD could be used to treat and prevent hyperlipidemia in diabetes. 53
Anti-Bacterial Activities
Lima et al (2017) assessed the antibacterial properties of hydroethanolic cassava leaf extract against Meropenem-resistant Pseudomonas aeruginosa and Methicillin-resistant Staphylococcus aureus. The extract exhibited satisfactory inhibitory activity against P. aeruginosa at 500 and 1000 µg/mL doses but was ineffective against S. aureus at the same dosages. HPLC/UV-VIS analysis identified phenolic compounds, including flavonoids, which may contribute to the extract's antibacterial activity. 32 The ethyl acetate fraction of cassava ethanol extract was found to possess antibacterial properties against Staphylococcus epidermidis and Propionibacterium acnes, with minimum inhibitory concentration (MIC) values ranging from 2.5% to 5.0% and 1.25% to 2.5%, respectively. The minimum bactericidal concentration (MBC) value of this fraction was 5% and 2.5% against S. epidermidis and P. acnes, respectively. Phytochemical screening revealed the presence of flavonoids, polyphenols, quinones, and saponins in the extract, which may account for its antibacterial activity. 33 Flavonoids can inhibit bacterial growth through multiple mechanisms, including porin inhibition, membrane damage, and inhibition of energy metabolism and nucleic acid synthesis via topoisomerase and dihydrofolate reductase enzymes. 109 Cassava extract contains flavonoids like kaempferol, quercetin, and apigenin, which can prevent bacterial adhesion and invasion. Specifically, kaempferol inhibits the sortase A enzyme, thereby preventing Gram-positive bacterial adhesion. 110 Moreover, quercetin and apigenin demonstrated their anti-bacterial action by inhibiting DNA gyrase, preventing further bacterial growth.111,112 In addition, apigenin was shown to exhibit its anti-bacterial effect by multiple possible mechanisms, including cytoplasmic membrane damage, inhibition of energy metabolism, peptidoglycan synthesis, and β-lactamase activity. 113 Saponins have demonstrated their anti-bacterial action by increasing bacterial cell wall permeability through the reduction of surface tension, leading to cell leakage and the release of intracellular components, 114 while quinones exhibit their anti-bacterial activity by inhibiting catalase enzyme and mitochondrial electron transport. 115
Anti-Diabetic Effects
Laya, et al (2022) investigated the anti-diabetic properties of cassava leaves by assessing their inhibitory effects on α-amylase and α-glucosidase enzymes. These enzymes contribute to the rise of postprandial blood glucose levels by breaking down carbohydrates into glucose. Inhibiting these enzymes can help prevent hyperglycemia and mitigate diabetes-related complications. HPLC-DAD analysis of cassava leaf identified the presence of flavonoids (quercetin, kaempferol, catechin, rutin), tannins (tannic acid), stilbenes (resveratrol), and saponins (diosgenin).116,117 The study demonstrated that these phytochemicals exhibit potent inhibitory effects on α-amylase and α-glucosidase, both before and after gastrointestinal digestion. 22 Notably, quercetin, as well as the positive control, acarbose, have shown a competitive pattern for the α-glucosidase inhibitory activity; thus, it helps to delay the absorption of glucose and is also capable of inhibiting α-amylase enzyme.118,119 Quercetin and kaempferol have been found to preserve pancreatic β-cell survival and improve insulin secretion by inhibiting apoptotic signals and stimulating survival pathways, thereby exhibiting anti-diabetic properties. 120 Additionally, catechin and tannic acid have demonstrated potent α-glucosidase and α-amylase inhibitory activities, respectively, in a more potent manner than acarbose, according to two separate studies.121,122 Shen et al (2017) investigated the anti-diabetic activities of resveratrol, a type of stilbenes found in cassava, which inhibited α-amylase and α-glucosidase enzymes at IC50 of 3.62 μg/L and 17.54 μg/L respectively. 123 Rutin has also shown anti-diabetic properties by reducing glucose absorption and improving insulin resistance by increasing PPARγ expression. 124 Furthermore, rutin reduces the activities of G6Pase and GP enzymes, leading to a reduction in gluconeogenesis activity. 125 Diosgenin, a saponin extracted from cassava leaves, also exhibited anti-diabetic effects by inhibiting α-amylase, α-glucosidase, and SGLT-1 activity while improving insulin secretion by stimulating the regeneration of pancreatic β-cells. 126 In addition, Wang et al (2014) examined the effect of cassava cross-linked octenyl succinic maltodextrin (CCOMD) on blood glucose and cholesterol levels in diabetic and non-diabetic mice. The result indicated that the blood glucose and insulin levels of mice fed with low and high doses of CCOMD improved after 12 weeks compared to the rats in non-diabetic and model control groups. The study concluded that CCOMD might lower blood glucose levels by enhancing glucose metabolism. 53
Wound Healing Activities
Cassava has been traditionally used for wound healing in Nigeria and The Guianas. Riliani et al (2021) investigated the ethanol extract of cassava leaves for its wound-healing properties. They found that 0.5% cassava leaf extract significantly stimulated fibroblast proliferation and preserved fibroblast morphology for up to 48 h. However, the fibroblast migration rate did not show a significant difference compared to the control group. It is noteworthy that the study did not conduct a bioassay-guided fractionation of the ethanol extract to elucidate the specific phytochemical compound(s) responsible for the observed activity. 41 Cassava extract contains antioxidants such as flavonoids, saponins, tannins, triterpenoids, and gallic acid, which may contribute to its wound-healing activity. Gallic acid may enhance wound healing by protecting cells from oxidative stress, increasing anti-oxidant gene expression, and promoting cell migration through the activation of various kinases. 127 Moreover, saponins help heal wounds by inhibiting early-phase inflammation, promoting re-epithelialization, and increasing matrix synthesis. 128 Tannins, on the other hand, promote wound healing through their anti-oxidant properties. 129 Despite those healing actions, toxic cyanogenic glycosides, especially linamarin, may also reduce cassava's wound-healing ability. It has been found that linamarin exerts an anti-proliferative effect which may disrupt the fibroblast proliferation process; thus, this may explain the extract's slow rate of wound healing. Anwar and Bohari (2019) examined the in vitro wound healing activity of aqueous cassava leaf extract, both with and without therapeutic ultrasound, on the HSF-1184 cell line. The study found that all dosages (100, 200, and 300 µg/mL) of cassava extract led to wound closure. However, the addition of therapeutic ultrasound to the extract resulted in a faster and more consistent rate of cell migration. Notably, the dosage of 200 µg/mL of cassava extract without any further addition demonstrated the highest wound closure at all time intervals (4, 6, 8, and 10 h). 39 Ajayi et al (2016) compared the ethanolic extracts of cassava leaves and N. latifolia leaves on wounded diabetic rats. Both extracts contained alkaloids, flavonoids, saponins, and tannins. However, the total phenolic and anthocyanin contents were lower in cassava leaf extract compared to N. latifolia. After 21 days, the percentage of wound closure was 69.54% with cassava leaf extract and 98.75% with N. latifolia leaf extract, with the latter showing enhanced recovery when compared to the positive control; povidone-iodine. 40 Therefore, it is suggested that the total phenolic and anthocyanin contents affect the potency of each extract's wound healing capability, and the four characterized phytochemicals may involve the wound healing mechanism. In addition, a longer time and a higher dose are suggested to be employed to observe a possibly better wound-healing activity of cassava leaf extract since the recovery effect was already observed at the selected dosage.
Future Directions
This review illustrates the need for more in vivo and clinical studies to establish the safety and efficacy of cassava leaf extracts for cancer treatment. It also highlights the incomplete identification and quantification of bioactive compounds in cassava leaf extracts that may contribute to their anti-cancer activity. Studies limited to specific cancer cell lines may not be generalizable to other types of cancer, indicating the need for more studies on a wider range of cancer types. The anti-inflammatory and antioxidant effects of cassava extracts and their potential mechanisms of action are also discussed through diverse research shown here, but further studies are required to understand their optimal administration time, dose, and safety in treating inflammation and pain. Additionally, cassava has been traditionally used for gastrointestinal disorders, but there is limited scientific research on its potential efficacy, with few studies demonstrating its anti-diarrheal activity. Tannins and flavonoids were suggested as potential contributors to the extract's anti-diarrheal properties, which have been demonstrated in multiple studies in different contexts to reduce diarrhea duration and inhibit prostaglandin synthesis which was not established in tannins extracted from cassava. Cassava salt suspension was also found to be as effective as WHO-ORS in treating acute diarrhea in children, with no safety studies or an analysis of the electrolytes available which is necessary for this age category. This review also highlights the scientific gaps in the research on “resistant starch-tapioca” and “cassava cross-linked octenyl maltodextrin”, including the lack of studies exploring their efficacy and safety in humans and the need for further investigation into their specific mechanisms of action. Moreover, the need for more research on the antibacterial properties of cassava leaf extract against other types of bacteria besides Pseudomonas aeruginosa, Staphylococcus epidermidis, and Propionibacterium acnes is mentioned.
Furthermore, Laya et al (2022) suggest potential anti-diabetic effects of cassava leaves and their phytochemicals, but the study lacks mechanistic information and assesses only in vitro α-amylase and α-glucosidase inhibition. Thus, extrapolation to in vivo or human models is unclear. In vivo experimentation is necessary to confirm these properties. Furthermore, there is no literature on cassava extract's effect on PPARγ expression, a vital factor in glucose and lipid metabolism. Therefore, research must further examine cassava leaves and their phytochemicals’ anti-diabetic potential and underlying mechanisms. There are still some gaps that need to be addressed to fully understand the potential of cassava extract as a wound-healing agent, including the need to determine the optimal dosages and duration of cassava extract application, investigate the potential negative effects of toxic cyanogenic glycosides on its efficacy, and conduct more clinical trials to establish its efficacy and safety for human use. Finally, a greater focus on industrial and agricultural patterns, which have invested more in exploiting cassava in recent times, can help reduce global food crises and make use of vast non-arable lands.
Limitations of the Study
The present study exhibits several limitations that warrant consideration when interpreting the findings. Firstly, a notable limitation lies in the omission of investigations about the correlation between cassava residual toxicity and human diseases. Deliberately excluding such research may hinder a comprehensive understanding of potential adverse effects or toxicological aspects associated with cassava consumption. Additionally, a limitation arises from the exclusion of studies focusing on non-pharmacological aspects of cassava. The study's substantial scope was confined solely to the examination of cassava's pharmacological activities, thereby overlooking valuable research on agronomic aspects and cassava wastewater, which could contribute to a more holistic comprehension of its impact on human health.
Moreover, the study's inclusion criteria limited articles and reports to those published in English and Malay languages, leading to a language bias that might have excluded relevant studies published in other languages, such as Spanish, thus potentially compromising the review's comprehensiveness. Furthermore, the exclusion of certain types of literature, such as reviews, short surveys, editorials, notes, letters, and books, could have resulted in overlooking valuable insights and perspectives on the topic, consequently limiting the breadth of information considered.
Lastly, it is pertinent to acknowledge that some of the included studies examining cassava's pharmacological properties may not have met the same rigorous standards as the rest of the studies. However, their inclusion was necessitated by the scarcity of alternative studies validating these pharmacological properties. This aspect introduces a potential source of bias in the interpretation of the overall findings.
Despite these limitations, the study serves as an essential stepping stone toward understanding the pharmacological activities of cassava. However, future research endeavors should strive to address these limitations to enhance the robustness and applicability of the scientific knowledge in this domain. Moreover, the “Future Trends” subsection offers researchers an additional perspective, encouraging further investigations to expand the breadth of research possibilities concerning cassava.
Conclusion
In conclusion, cassava represents a highly promising crop with considerable commercial potential owing to its exceptional nutritional profile and adaptability to challenging environmental conditions. The roots and leaves of cassava offer essential carbohydrates, vitamins, and minerals, rendering it a crucial food source for millions of people. Moreover, cassava possesses significant medicinal value, as its stems and leaves contain diverse phytochemical compounds with antioxidant, anthelmintic, and anti-diabetic properties. Furthermore, terpenes present in cassava demonstrate antimicrobial and anti-cancer activities. The plant exhibits therapeutic potential across various health conditions, encompassing cancer, inflammation, diarrhea, cholesterol management, and diabetes. The widespread culinary applications of cassava worldwide underscore its importance in global food systems. Nonetheless, comprehensive identification of bioactive compounds remains insufficient, necessitating broader studies to optimize administration for inflammation and pain. Furthermore, directing attention towards industrial and agricultural practices can contribute to mitigating food crises and fully exploiting cassava's potential.
Acknowledgements
Authors highly appreciate Dr Hui Poh Goh and Dr Nurolaini Kifli from the department of pharmacy, PAPRSB Institute of Health Sciences, for their participation in the discussions to choose the topic and the main points in this paper.
Abbreviation
- ABTS·+
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation
- ALT
Alanine Aminotransferase
- AST
Aspartate Aminotransferase
- BW
body weight
- CAT
Catalase
- CCOMD
Cassava Cross-linked Octenyl Maltodextrin
- CCE
cassava cyanide extract
- CIAT
International Center for Tropical Agriculture
- CS
Cassava Starch
- DPPH˙
2,2-Diphenyl-1-picrylhydrazyl radical
- DM
Dry Mass
- FAO
Food and Agriculture Organization
- G6Pase
glucose-6-phosphatase
- GAE
Gallic Acid Equivalence
- GC-MS
gas chromatography-mass spectrometry
- GSH
Glutathione
- IC50
Half maximal inhibitory concentration
- MMP-8
matrix metalloproteinase-8
- MMEE
micropropagated cassava ethanol extracts
- PARγ
peroxisome proliferator-activated receptor gamma
- ROS
Reactive Oxygen Species
- RS3-tapioca
Resistant-Starch-tapioca
- SGLT-1
sodium-glucose transport protein 1
- SOD
Superoxide Dismutase
- TNF-α
tumor necrosis factor-alpha
- WMEE
wild cassava micropropagated ethanol extracts.
Footnotes
Author Contributions: Long Chiau Ming, Said Moshawih: Conceptualization. Long Chiau Ming, Mohd Ikmal Asmuni: Methodology. Siti Raudhah Noor Shifa Putri Mohidin, Said Moshawih: Formal analysis. Siti Raudhah Noor Shifa Putri Mohidin, Said Moshawih: Writing – original draft. Siti Raudhah Noor Shifa Putri Mohidin, Said Moshawih, Andi Hermansyah, Naeem Shafqat: Writing – review & editing. Long Chiau Ming, Naeem Shafqat: Supervision. All authors have read and agreed to the published version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This research contains no human or animal objects and needs no ethical approval.
Informed Consent: Informed consent is not applicable to this study.
ORCID iDs: Siti Raudhah Noor Shifa Putri Mohidin https://orcid.org/0000-0002-4695-1472
Said Moshawih https://orcid.org/0000-0003-4840-0460
Long Chiau Ming https://orcid.org/0000-0002-6971-1383
References
- 1.McCallum EJ, Anjanappa RB, Gruissem W. Tackling agriculturally relevant diseases in the staple crop cassava (Manihot esculenta). Curr Opin Plant Biol. 2017;38(8):50‐58. [DOI] [PubMed] [Google Scholar]
- 2.Akinpelu A, Amamgbo L, Olojede A, Oyekale A. Health implications of cassava production and consumption. J Agric Soc Res (JASR). 2011;11(1):118-125. [Google Scholar]
- 3.Malik AI, Kongsil P, Nguyễn VA, et al. Cassava breeding and agronomy in Asia: 50 years of history and future directions. Breed Sci. 2020;70(2):18180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassar N, Ortiz R. Cassava improvement: challenges and impacts. J Agric Sci. 2007;145(2):163‐171. [Google Scholar]
- 5.Souza LS, Alves AAC, de Oliveira EJ. Phenological diversity of flowering and fruiting in cassava germplasm. Sci Hortic (Amsterdam). 2020;265(4):109253. [Google Scholar]
- 6.Feyisa AS. Micropropagation of Cassava (Manihot esculenta Crantz). Extensive Rev. 2021;1(1):49‐57. [Google Scholar]
- 7.FAOSTAT. Production; Cassava; all Countries. Food and Agriculture Organization of the United Nations. 2020.
- 8.FAOSTAT. Production; dried Cassava; all Countries Food and Agriculture Organization of the United Nations. 2020.
- 9.Li X, Yadav R, Siddique KH. Neglected and underutilized crop species: the key to improving dietary diversity and fighting hunger and malnutrition in Asia and the Pacific. Front Nutr. 2020;7(11):593711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afrol. Cassava – many uses, mostly underutilised. http://www.afrol.com/archive/cassava.htm. Accessed2022.
- 11.Maziya-Dixon B, Adebowale A, Onabanjo O, Dixon A. Effect of variety and drying methods on physico-chemical properties of high quality cassava flour from yellow cassava roots. Paper presented at: African Crop Science Conference Proceedings. 2005.
- 12.Oboh G, Oladunmoye M. Biochemical changes in micro-fungi fermented cassava flour produced from low-and medium-cyanide variety of cassava tubers. Nutr Health. 2007;18(4):355‐367. [DOI] [PubMed] [Google Scholar]
- 13.(CIAT) ICfTA. Cassava diversity. https://ciat.cgiar.org/what-we-do/crop-conservation-and-use/cassava-diversity/. Published 2019. Accessed 10/10, 2022.
- 14.Akpoghelie PO, Edo GI, Akhayere E. Proximate and nutritional composition of beer produced from malted sorghum blended with yellow cassava. Biocatal Agric Biotechnol. 2022;45(10):102535. [Google Scholar]
- 15.Suresh R, Saravanakumar M, Suganyadevi P. Anthocyanins from Indian cassava (Manihot esculenta Crantz) and its antioxidant properties. Int J Pharm Sci Res. 2011;2(7):1819. [Google Scholar]
- 16.Yuan C, Wang M-H, Wang F, et al. Network pharmacology and molecular docking reveal the mechanism of Scopoletin against non-small cell lung cancer. Life Sci. 2021;270(4):119105. [DOI] [PubMed] [Google Scholar]
- 17.Ferraro V, Piccirillo C, Tomlins K, Pintado ME. Cassava (Manihot esculenta Crantz) and yam (Dioscorea spp.) crops and their derived foodstuffs: safety, security and nutritional value. Crit Rev Food Sci Nutr. 2016;56(16):2714‐2727. [DOI] [PubMed] [Google Scholar]
- 18.Rivadeneyra-Domínguez E, Rodríguez-Landa J. Preclinical and clinical research on the toxic and neurological effects of cassava (Manihot esculenta Crantz) consumption. Metab Brain Dis. 2020;35(1):65‐74. [DOI] [PubMed] [Google Scholar]
- 19.Chaiareekitwat S, Latif S, Mahayothee B, et al. Protein composition, chlorophyll, carotenoids, and cyanide content of cassava leaves (Manihot esculenta Crantz) as influenced by cultivar, plant age, and leaf position. Food Chem. 2022;372(3):131173. [DOI] [PubMed] [Google Scholar]
- 20.Junior ENM, Chisté RC, da Silva Pena R. Oven drying and hot water cooking processes decrease HCN contents of cassava leaves. Food Res Int. 2019;119(5):517‐523. [DOI] [PubMed] [Google Scholar]
- 21.Tsumbu CN, Deby-Dupont G, Tits M, et al. Antioxidant and antiradical activities of Manihot esculenta Crantz (Euphorbiaceae) leaves and other selected tropical green vegetables investigated on lipoperoxidation and phorbol-12-myristate-13-acetate (PMA) activated monocytes. Nutrients. 2011;3(9):818‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laya A, Koubala BB, Negi PS. Antidiabetic (α-amylase and α-glucosidase) and anti-obesity (lipase) inhibitory activities of edible cassava (Manihot esculenta Crantz) as measured by in vitro gastrointestinal digestion: effects of phenolics and harvested time. Int J Food Prop. 2022;25(1):492‐508. [Google Scholar]
- 23.Jampa M, Sutthanut K, Weerapreeyakul N, Tukummee W, Wattanathorn J, Muchimapura S. Multiple bioactivities of Manihot esculenta leaves: UV filter, anti-oxidation, anti-melanogenesis, collagen synthesis enhancement, and anti-adipogenesis. Molecules. 2022;27(5):1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayakti PI, Anisa N, Ramadhan H. Antioxidant activity of methanol extract of cassava leaves (Manihot esculenta Crantz) using CUPRAC method. Jurnal Ilmiah Farmasi. 2022:97‐106. [Google Scholar]
- 25.Laya A, Koubala BB. Polyphenols in cassava leaves (Manihot esculenta Crantz) and their stability in antioxidant potential after in vitro gastrointestinal digestion. Heliyon. 2020;6(3):e03567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahekar SE, Kale RS. Evaluation of antioxidant activity of Manihot esculenta Crantz in wistar rats. J Pharm Bioallied Sci. 2016;8(2):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao H-T, Qiu B, Du F-L, et al. The protective effects of cassava (Manihot esculenta Crantz) leaf flavonoid extracts on liver damage of carbon tetrachloride injured mice. Afr J Tradi Complement Altern Med. 2015;12(1):52‐56. [Google Scholar]
- 28.Dewi R, Normasari R. Protective effect of cassava leaf extract on gentamicin-induced hepatotoxity in mice. J Agromedicine Med Sci. 2019;5(3):177‐182. [Google Scholar]
- 29.Okoro IO, Kadiri HE, Aganbi E. Comparative phytochemical screening, in vivo antioxidant and nephroprotective effects of extracts of cassava leaves on paracetamol-intoxicated rats. J Rep Pharm Sci. 2019;8(2):188. [Google Scholar]
- 30.Lungit Kuncaraningtyas P. Potensi Ekstrak Daun Singkong (Manihot Esculenta Crantz) Terhadap Ekspresi Mmp-8 Fibroblas Gingiva Pada Model Tikus Disfungsi Ovarium Dan Periodontitis, Fakultas Kedokteran Gigi Universitas Jember. 2020.
- 31.Chinnadurai V, Viswanathan P, Kalimuthu K, Vanitha A, Ranjitha V, Pugazhendhi A. Comparative studies of phytochemical analysis and pharmacological activities of wild and micropropagated plant ethanol extracts of Manihot esculenta. Biocatal Agric Biotechnol. 2019;19(5):101166. [Google Scholar]
- 32.Lima ZM, da Trindade LS, Santana GC, et al. Effect of Tamarindus indica L. and Manihot esculenta extracts on antibiotic-resistant bacteria. Pharmacognosy Res. 2017;9(2):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustarichie R, Sulistyaningsih S, Runadi D. Antibacterial activity test of extracts and fractions of cassava leaves (Manihot esculenta Crantz) against clinical isolates of Staphylococcus epidermidis and Propionibacterium acnes causing acne. Int J Microbiol. 2020;2020(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bokanisereme UFY, Okechukwu PN. Anti-inflammatory, analgesic and anti-pyretic activity of cassava leaves extract. Asian J Pharm Clin Res. 2013;6(4):89‐92. [Google Scholar]
- 35.Meilawaty Z, Dharmayanti AWS, Prafitasari D. The effect of cassava (Manihot esculenta) leaf extract on COX-2 expression in the neutrophil cell culture exposed to the lipopolysaccharide of Escherichia coli (in-vitro study). Padjadjaran J Dent. 2019;31(1):60‐66. [Google Scholar]
- 36.Adeyemi OO, Yemitan OK, Afolabi L. Inhibition of chemically induced inflammation and pain by orally and topically administered leaf extract of Manihot esculenta Crantz in rodents. J Ethnopharmacol. 2008;119(1):6‐11. [DOI] [PubMed] [Google Scholar]
- 37.Miladiyah I. Analgesic activity of ethanolic extract of Manihot esculenta Crantz leaves in mice. Universa Medicina. 2011;30(1):3‐10. [Google Scholar]
- 38.Joseph T, Sreejith S, Joseph X, et al. Effect of cyanide ions (CN-) extracted from cassava (Manihotesculenta Crantz) on alveolar epithelial cells (A549 cells). Toxicology. 2021;464(12):153019. [DOI] [PubMed] [Google Scholar]
- 39.Anwar U, Bohari SPM. Effect of Manihot Esculenta Aqueous extract and therapeutic ultrasound in accelerating the wound healing process in vitro. Jurnal Teknologi. 2019;81(4):13-20. [Google Scholar]
- 40.Ajayi EIO, Popoola G, Ojediran E. Wound healing potential of Nuclea latifolia and Manihot esculenta leaf extracts in type 1 diabetic rats. Afr J Tradi Complement Altern Med. 2016;13(1):1‐5. [Google Scholar]
- 41.Riliani M, Kusuma I, Halim A, Muhammad A, Fitrianto A, Narendra IBE. The Role of Fibroblast Proliferation in Wound Healing by Different Plants: An Experimental Study. 2021.
- 42.Marie-Magdeleine C, Udino L, Philibert L, Bocage B, Archimède H. In vitro effects of Cassava (Manihot esculenta) leaf extracts on four development stages of Haemonchus contortus. Vet Parasitol. 2010;173(1-2):85‐92. [DOI] [PubMed] [Google Scholar]
- 43.Ariyanti E. Anthelmintic effects of Cassava (Manihot esculenta) leaf extracts in the Haemonchus contortus in vitro. Jurnal Sain Veteriner. 2012;30(2):71-78. [Google Scholar]
- 44.Bahekar SE, Kale RS. Antidiarrheal activity of ethanolic extract of Manihot esculenta Crantz leaves in Wistar rats. J Ayurveda Integr Med. 2015;6(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ajayi E, Ogungbuji E, Ganiyu N. Analgesic potential of certain traditional African herbal extracts in high fat diet-manipulated hyperglycaemic rats. Ife Journal of Science. 2015;17(2):511‐517. [Google Scholar]
- 46.Amado-Cornejo ND, aldine Atusparia-Flores G, Huamán-Cabrera MV, et al. Anti-inflammatory activity of the ethnolic extract of the leaves of Manihot esculenta crantz (YUCA) in an experimental model of acute inflammation. Rev Fac Med Hum Enero. 2020;20(1):94‐98. [Google Scholar]
- 47.Elshamy AI, El Gendy AE-NG, Farrag ARH, et al. Shoot aqueous extract of Manihot esculenta Crantz (cassava) acts as a protective agent against paracetamol-induced liver injury. Nat Prod Res. 2021;35(22):4724‐4728. [DOI] [PubMed] [Google Scholar]
- 48.Yi B, Hu L, Mei W, et al. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules. 2011;16(12):10157‐10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Idibie CA, Davids H, Iyuke SE. Cytotoxicity of purified cassava linamarin to a selected cancer cell lines. Bioprocess Biosyst Eng. 2007;30(4):261‐269. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Sawauchi H, Ogawa H, Kishida T, Ebihara K. Retrograded tapioca starch prevents ovarian hormone deficiency-induced hypercholesterolemia. J Nutr Sci Vitaminol. 2006;52(2):134‐141. [DOI] [PubMed] [Google Scholar]
- 51.Charles AL, Huang T-C. Sweet cassava polysaccharide extracts protects against CCl4 liver injury in Wistar rats. Food Hydrocolloids. 2009;23(6):1494‐1500. [Google Scholar]
- 52.Grange AO. Evaluation of cassava-salt suspension in the management of acute diarrhoea in infants and children. J Diarrhoeal Dis Res. 1994;12(1):55‐58. [PubMed] [Google Scholar]
- 53.Wang L, Zheng M, Wang Y, et al. Anti-diabetic activity of cassava cross-linked octenyl succinic maltodextrin in STZ-induced diabetic mice. Int J Biol Macromol. 2014;64(3):247‐251. [DOI] [PubMed] [Google Scholar]
- 54.Iwuagwu O. The spread of cassava (manioc) in Igboland, south-east Nigeria: a reappraisal of the evidence. Agric Hist Rev. 2012;60(1):60‐76. [Google Scholar]
- 55.Looc MKP, Baco KJP, Jaictin TL, Tumapon NL, Ogoc LJM. Cassava (Manihot esculenta) As A Main Component In Making Natural Homemade Paste Academia. 2018.
- 56.Herminingrum S. The genealogy of traditional Javanese cassava-based foods. J Ethn Foods. 2019;6(1):1‐16. [Google Scholar]
- 57.Rasch V, Sørensen PH, Wang AR, Tibazarwa F, Jäger AK. Unsafe abortion in rural Tanzania–the use of traditional medicine from a patient and a provider perspective. BMC Pregnancy Childbirth. 2014;14(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fathima AA, Sanitha M, Tripathi L, Muiruri S. Cassava (Manihot esculenta) dual use for food and bioenergy: a review. Food Energy Secur. 2022;12(1):e380. [Google Scholar]
- 59.Ceballos H, Cruz AGAdl. Cassava taxonomy and morphology. 2012.
- 60.USDA. Cassava Manihot Esculenta Crantz. United States Department of Agriculture Natural Resources Conservation Service 2003.
- 61.Kamphayae S, Kumagai H, Butcha P, Ritruechai V, Udchachon S. Yeast mixture of liquid beer and cassava pulp with rice straw for the growth of dairy heifers. Trop Anim Health Prod. 2017;49(3):491‐496. [DOI] [PubMed] [Google Scholar]
- 62.Pushpalatha R, Gangadharan B. Is cassava (Manihot esculenta Crantz) a climate “smart” crop? A review in the context of bridging future food demand gap. Trop Plant Biol. 2020;13(3):201‐211. [Google Scholar]
- 63.Muiruri SK, Ntui VO, Tripathi L, Tripathi JN. Mechanisms and approaches towards enhanced drought tolerance in cassava (Manihot esculenta). Curr Plant Biol. 2021;28(12):100227. [Google Scholar]
- 64.Cock J, Howeler R. The ability of cassava to grow on poor soils. Crop Tolerance Suboptimal Land Conditions. 1978;32(1):145‐154. [Google Scholar]
- 65.Shen S, Chen J, Chang J, Xia B. Using bioenergy crop cassava (Manihot esculenta) for reclamation of heavily metal-contaminated land. Int J Phytoremediation. 2020;22(12):1313‐1320. [DOI] [PubMed] [Google Scholar]
- 66.Harrison U, Osu S, Ekanem J. Heavy metals accumulation in leaves and tubers of cassava (Manihot esculenta Crantz) grown in crude oil contaminated soil at Ikot Ada Udo, Nigeria. J Appl Sci Environ Manag. 2018;22(6):845‐851. [Google Scholar]
- 67.Li M, Zi X, Tang J, Xu T, Gu L, Zhou H. Effects of cassava foliage on feed digestion, meat quality, and antioxidative status of geese. Poult Sci. 2020;99(1):423‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Sharkawy MA. Cassava biology and physiology. Plant Mol Biol. 2004;56(4):481‐501. [DOI] [PubMed] [Google Scholar]
- 69.Tewe OO, Lutaladio N. Cassava for livestock feed in sub-Saharan Africa. FAO. 2004.
- 70.Montagnac JA, Davis CR, Tanumihardjo SA. Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr Rev Food Sci Food Saf. 2009;8(3):181‐194. [DOI] [PubMed] [Google Scholar]
- 71.SEBİOMO A, BANJO F. The phytochemical, proximate and mineral contents of cassava leaves and nutritive values of associated arthropod pests. J Turkish Chem Soc Section A: Chem. 2020;7(3):675‐690. [Google Scholar]
- 72.Bradbury JH, Holloway WD. Chemistry of tropical root crops: significance for nutrition and agriculture in the Pacific . 1988.
- 73.Afolabi L, Adeyemi O, Yemitan O. Cassava leaves have anti-inflammatory and analgesic principles, which justify its use in traditional African medicine. J Ethnopharmacol. 2008;119(1):6‐11. [DOI] [PubMed] [Google Scholar]
- 74.DeFilipps RA, Maina SL, Crepin J. Medicinal plants of the Guianas (Guyana, Surinam, French Guiana). Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana). 2004.
- 75.Kujawska M, Schmeda-Hirschmann G. The use of medicinal plants by Paraguayan migrants in the Atlantic Forest of Misiones, Argentina, is based on Guaraní tradition, colonial and current plant knowledge. J Ethnopharmacol. 2022;283(1):114702. [DOI] [PubMed] [Google Scholar]
- 76.Chai PP. Medicinal Plants of Sarawak. Paul Chai PK; 2006. [Google Scholar]
- 77.Organization WH. Medicinal Plants in the South Pacific: Information on 102 Commonly Used Medicinal Plants in the South Pacific. WHO Regional Office for the Western Pacific; 1998. [Google Scholar]
- 78.Nwose EU, Onodu BC, Anyasodor AE, Sedowo MO, Okuzor JN, Culas RJ. Ethnopharmacological values of cassava and its potential for diabetes and dyslipidemia management: knowledge survey and critical review of report. J Intercult Ethnopharmacol. 2017;6(3):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuete V. Physical, hematological, and histopathological signs of toxicity induced by African medicinal plants. In: Kuete V ed. Toxicological Survey of African Medicinal Plants. Elsevier; 2014:635‐657. [Google Scholar]
- 80.Balagopalan C. Cassava utilization in food, feed and industry. In: Hillocks RJ, Thresh JM, Bellotti AC. eds. Cassava: Biology, Production and Utilization. CABI Wallingford UK; 2001:301‐318. [Google Scholar]
- 81.Panghal A, Munezero C, Sharma P, Chhikara N. Cassava toxicity, detoxification and its food applications: a review. Toxin Reviews. 2019.
- 82.Steinkraus KH. Lactic acid fermentations. In: Foods. NRCUPotAoBtTF, ed. Applications of Biotechnology to Fermented Foods. National Academies Press; 1992:1‐16. [Google Scholar]
- 83.Okoro IO. Effects of extraction solvents on the antioxidant and phytochemical activities of Manihot esculenta leaves. Iran J Toxicol. 2020;14(1):51‐58. [Google Scholar]
- 84.Linn KZ, Myint PP. Estimation of nutritive value, total phenolic content and in vitro antioxidant activity of Manihot esculenta Crantz.(Cassava) leaf. J Med Plants. 2018;6(6):73‐78. [Google Scholar]
- 85.Mehran MJ, Zendehbad H, Malla S. Free radical scavenging and antioxidant potential activity of cassava plants. Asian J Pharm Clin Res. 2014;7(1):66‐70. [Google Scholar]
- 86.Moshawih S, Cheema MS, Ibraheem ZO, Tailan ND, Hakim MN. Cosmos caudatus extract/fractions reduce smooth muscle cells migration and invasion in vitro: a potential benefit of suppressing atherosclerosis. Porto Biomed J. 2017;2(6):293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li S-S, Hu L-F, Zhao Y-X, et al. A new diterpene from the stems of Manihot esculenta. J Asian Nat Prod Res. 2011;13(10):961‐964. [DOI] [PubMed] [Google Scholar]
- 88.Pan Y-M, Zou T, Chen Y-J, et al. Two new pentacyclic triterpenoids from the stems of Manihot esculenta. Phytochem Lett. 2015;12(6):273‐276. [Google Scholar]
- 89.Zeng Y-B, Li S-S, Mei W-L, Dong W-H, Li K-M, Dai H-F. Two new terpenoids from the stems of Manihot esculenta. J Asian Nat Prod Res. 2015;17(3):280‐284. [DOI] [PubMed] [Google Scholar]
- 90.Ekeledo E, Latif S, Abass A, Müller J. Antioxidant potential of extracts from peels and stems of yellow-fleshed and white cassava varieties. Int J Food Sci Technol. 2021;56(3):1333‐1342. [Google Scholar]
- 91.Gnamien ACA. Evolution of the Physicochemical Characteristics of Cassava Roots of the Yacé Variety According to the Stage of Harvest. 2022.
- 92.Jamil SS, Bujang A. Nutrient and antinutrient composition of different variety of cassava (Manihot esculenta Crantz) leaves. Jurnal Teknologi. 2016;78(6):59-63. [Google Scholar]
- 93.Hasmadi M, Harlina L, Lee J, Mansoor A, Jahurul M, Zainol M. Extraction and characterization of cassava starch cultivated in different locations in Sabah, Malaysia. Food Res. 2021;5(3):44‐52. [Google Scholar]
- 94.Noerwijati K, Budiono R. Yield and yield components evaluation of Cassava (Manihot esculenta Crantz) clones in different altitudes. Energy Procedia. 2015;65(1):155‐161. [Google Scholar]
- 95.Tao H, Cui B, Zhang H, Bekhit AE-D, Lu F. Identification and characterization of flavonoids compounds in cassava leaves (Manihot esculenta Crantz) by HPLC/FTICR-MS. Int J Food Prop. 2019;22(1):1134‐1145. [Google Scholar]
- 96.Oliveira RA, De Carvalho ML, Nutti RM, De Carvalho LJ, Fukuda WG. Assessment and degradation study of total carotenoid and β-carotene in bitter yellow cassava (Manihot esculenta Crantz) varieties. Afr J Food Sci. 2010;4(4):148‐155. [Google Scholar]
- 97.Udoh LI, Agogbua JU, Keyagha ER, Nkanga II. Carotenoids in Cassava (Manihot esculenta Crantz). 2022.
- 98.Pan Y-M, Zhang Y, Wang X-N, et al. Chemical constituents from the stems of Manihot esculenta. Nat Prod Bioprospect. 2015;5(1):55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al Fourjani WA. In Vitro Anticancer Properties of Linamarin Controlled Release From Biodegradable Poly-Lactic Co-Glycolic Acid Nanoparticle. Universiti Putra Malaysia; 2005. [Google Scholar]
- 100.Wuryanto MA, Susanto HS, Hariyadi S, Mustofa M. Anticancer Activity of Linamarin From Cassave Leaves (Manihot Esculenta Cranz) on Raji Cells. 2020.
- 101.Sanders KM. Role of prostaglandins in regulating gastric motility. Am J Physiol-Gastrointest Liver Physiol. 1984;247(2):G117‐G126. [DOI] [PubMed] [Google Scholar]
- 102.Heeney A, Rogers A, Mohan H, Mc Dermott F, Baird A, Winter D. Prostaglandin E2 receptors and their role in gastrointestinal motility–potential therapeutic targets. Prostaglandins Other Lipid Mediators. 2021;152(2):106499. [DOI] [PubMed] [Google Scholar]
- 103.Bonelli F, Turini L, Sarri G, Serra A, Buccioni A, Mele M. Oral administration of chestnut tannins to reduce the duration of neonatal calf diarrhea. BMC Vet Res. 2018;14(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wongsamitkul N, Sirianant L, Muanprasat C, Chatsudthipong V. A plant-derived hydrolysable tannin inhibits CFTR chloride channel: a potential treatment of diarrhea. Pharm Res. 2010;27(3):490‐497. [DOI] [PubMed] [Google Scholar]
- 105.Girard M, Thanner S, Pradervand N, Hu D, Ollagnier C, Bee G. Hydrolysable chestnut tannins for reduction of postweaning diarrhea: efficacy on an experimental ETEC F4 model. PLoS One. 2018;13(5):e0197878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hämäläinen M, Nieminen R, Asmawi MZ, Vuorela P, Vapaatalo H, Moilanen E. Effects of flavonoids on prostaglandin E2 production and on COX-2 and mPGES-1 expressions in activated macrophages. Planta Med. 2011;77(13):1504‐1511. [DOI] [PubMed] [Google Scholar]
- 107.Huang W, Ouyang S, Lin Y, Zhang H. Effect of quercetin on gastrointestinal motility and its mechanism. World Chin J Digestol. 2009;17(18):1815‐1820. [Google Scholar]
- 108.Carlo GD, Mascolo N, Izzo AA, Capasso F, Autore G. Effects of quercetin on the gastrointestinal tract in rats and mice. Phytother Res. 1994;8(1):42‐45. [Google Scholar]
- 109.Farhadi F, Khameneh B, Iranshahi M, Iranshahy M. Antibacterial activity of flavonoids and their structure–activity relationship: an update review. Phytother Res. 2019;33(1):13‐40. [DOI] [PubMed] [Google Scholar]
- 110.Yang W-Y, Won TH, Ahn C-H, et al. Streptococcus mutans sortase A inhibitory metabolites from the flowers of Sophora japonica. Bioorg Med Chem Lett. 2015;25(7):1394‐1397. [DOI] [PubMed] [Google Scholar]
- 111.Plaper A, Golob M, Hafner I, Oblak M, Šolmajer T, Jerala R. Characterization of quercetin binding site on DNA gyrase. Biochem Biophys Res Commun. 2003;306(2):530‐536. [DOI] [PubMed] [Google Scholar]
- 112.Morimoto Y, Baba T, Sasaki T, Hiramatsu K. Apigenin as an anti-quinolone-resistance antibiotic. Int J Antimicrob Agents. 2015;46(6):666‐673. [DOI] [PubMed] [Google Scholar]
- 113.Eumkeb G, Chukrathok S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant Enterobacter cloacae. Phytomedicine. 2013;20(3-4):262‐269. [DOI] [PubMed] [Google Scholar]
- 114.Mandal P, Babu SS, Mandal N. Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia. 2005;76(5):462‐465. [DOI] [PubMed] [Google Scholar]
- 115.Kurban S, Deniz NG, Sayil C, et al. Synthesis, antimicrobial properties, and inhibition of catalase activity of 1, 4-naphtho-and benzoquinone derivatives containing N-, S-, O-substituted. Heteroat Chem. 2019;2019(1):1-12. [Google Scholar]
- 116.Hossain U, Das AK, Ghosh S, Sil PC. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem Toxicol. 2020;145(11):111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaur N, Kumar V, Nayak SK, Wadhwa P, Kaur P, Sahu SK. Alpha-amylase as molecular target for treatment of diabetes mellitus: a comprehensive review. Chem Biol Drug Des. 2021;98(4):539‐560. [DOI] [PubMed] [Google Scholar]
- 118.Martinez-Gonzalez AI, Díaz-Sánchez Á, De La Rosa L, Bustos-Jaimes I, Alvarez-Parrilla E. Inhibition of α-amylase by flavonoids: structure activity relationship (SAR). Spectrochim Acta, Part A. 2019;206(1):437‐447. [DOI] [PubMed] [Google Scholar]
- 119.Proença C, Freitas M, Ribeiro D, et al. α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure–activity relationship study. J Enzyme Inhib Med Chem. 2017;32(1):1216‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ghorbani A, Rashidi R, Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: a mechanistic review. Biomed Pharmacother. 2019;111(3):947‐957. [DOI] [PubMed] [Google Scholar]
- 121.Mrabti HN, Jaradat N, Fichtali I, et al. Separation, identification, and antidiabetic activity of catechin isolated from Arbutus unedo L. Plant roots. Plants. 2018;7(2):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lou W, Chen Y, Ma H, Liang G, Liu B. Antioxidant and α-amylase inhibitory activities of tannic acid. J Food Sci Technol. 2018;55(9):3640‐3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen Y, Xu Z, Sheng Z. Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity, and to conjugate methylglyoxal. Food Chem. 2017;216(2):153‐160. [DOI] [PubMed] [Google Scholar]
- 124.Dubey S, Ganeshpurkar A, Ganeshpurkar A, Bansal D, Dubey N. Glycolytic enzyme inhibitory and antiglycation potential of rutin. Future J Pharm Sci. 2017;3(2):158‐162. [Google Scholar]
- 125.Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed Pharmacother. 2017;96(12):305‐312. [DOI] [PubMed] [Google Scholar]
- 126.Gan Q, Wang J, Hu J, et al. The role of diosgenin in diabetes and diabetic complications. J Steroid Biochem Mol Biol. 2020;198(4):105575. [DOI] [PubMed] [Google Scholar]
- 127.Yang DJ, Moh SH, Son DH, et al. Gallic acid promotes wound healing in normal and hyperglucidic conditions. Molecules. 2016;21(7):899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim YS, Cho I-H, Jeong M-J, et al. Therapeutic effect of total ginseng saponin on skin wound healing. J Ginseng Res. 2011;35(3):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bakondi E, Bai P, Erdélyi K, Szabó C, Gergely P, Virág L. Cytoprotective effect of gallotannin in oxidatively stressed HaCaT keratinocytes: the role of poly (ADP-ribose) metabolism. Exp Dermatol. 2004;13(3):170‐178. [DOI] [PubMed] [Google Scholar]