Abstract
The β-lactam susceptibilities of 65 strains of Streptococcus pneumoniae for which penicillin MICs covered a broad range were assessed. The order of potency was amoxicillin (AMX) = amoxicillin-clavulanate (AMC) > penicillin G > cefpodoxime (CPO) > cefuroxime (CXM) > cefprozil > cefaclor > loracarbef > cefixime. No decrease in susceptibility was seen following repeated subculture of two penicillin-susceptible strains of S. pneumoniae in AMX, AMC, cefaclor, or loracarbef, whereas repeated exposure to CPO and CXM resulted in 4- to 32-fold decreases in susceptibility for both strains. When one of these strains was exposed to concentrations of CPO, CXM, AMX, and AMC achieved in the serum of humans following the administration of an oral dose, all agents were rapidly bactericidal, with no decrease in susceptibility up to 72 h. This was consistent with antibiotic concentrations exceeding the MICs for 100% of the dosing interval. For a penicillin-resistant strain, MICs were exceeded for 29% of the 12-h dosing interval for 500 mg of AMX, 42% of the interval for AMC with 875 mg of AMX and 125 mg of clavulanate (875/125 mg of AMC) 21% of the interval for 500 mg of CXM, and 0% of the interval for 200 mg of CPO. Consequently, only 875/125 mg of AMC produced a sustained bactericidal effect. A four- to eightfold reduction in susceptibility to CPO and CXM and cross-resistance with cefotaxime, but not penicillin or AMC, were selected following exposure to simulated serum CPO and CXM concentrations. In addition, AMX and AMC were the only agents which consistently produced a >99% reduction in bacterial numbers in time-kill studies using concentrations of antibiotic achieved in middle ear fluid for all three strains of penicillin-resistant S. pneumoniae tested.
The incidence of penicillin resistance is increasing among clinical isolates of Streptococcus pneumoniae. Penicillin-resistant pneumococci have been present at high frequencies (44 to 59%) in South Africa (23), Hungary (28), and Spain (13) for some time, but rates of >40% have now also been reported in France, Argentina, Uruguay, Mexico, Israel, Saudi Arabia, Nigeria, Kenya, Japan, and Korea (24). Penicillin-resistant pneumococci are often also resistant to a number of other classes of antibiotics, such as tetracyclines, chloramphenicol, and trimethoprim-sulfamethoxazole (1, 13), making the choice of therapy difficult. The increasing incidence of macrolide resistance (13, 15), which confers a high level of resistance to all currently available agents in that class, also means that agents such as erythromycin, clarithromycin, and azithromycin will frequently be less effective. It is hoped that the development of quinolone antibiotics with improved activity against gram-positive bacteria will overcome doubts as to the usefulness of these agents in treating pneumococcal infections (27). The use of quinolones is contraindicated in children and infants, however, and many have further toxicity problems which restrict their use. These agents also have a higher potential than, for example, β-lactams and macrolides to select for mutational resistance, and there are documented cases of clinical failures that can be directly related to the emergence of bacterial resistance following quinolone therapy (31, 32).
Resistance to other antibiotic classes means that orally administered β-lactam antibiotics may still be the first choice for empiric therapy of community-acquired respiratory tract infections, even in countries with a high incidence of penicillin-resistant pneumococci, because the variable or nonabsolute nature of this resistance means that some β-lactam antibiotics remain efficacious (20). It is therefore of great importance to choose the most potent β-lactam antibiotic against the key respiratory tract pathogens (S. pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis), to use it at a dose high enough to be effective against penicillin-resistant strains of S. pneumoniae, and to choose the compound least likely to select a higher level of β-lactam resistance.
In the studies described here, the in vitro potencies of a number of orally administered β-lactam antibiotics against S. pneumoniae were compared, as were their potential to select for resistance. The effectiveness of the antibiotic concentrations achieved in middle ear fluid (MEF) against penicillin-resistant pneumococci was examined, and for the most potent agents, the concentration-time profiles achieved in the serum of humans following the administration of a conventional oral dose were simulated in an in vitro pharmacodynamic model. This was used to assess bactericidal activities and the potential to select for resistance among penicillin-susceptible and penicillin-resistant pneumococci.
MATERIALS AND METHODS
Compounds.
Amoxicillin trihydrate and potassium clavulanate were supplied as laboratory reference standards by SmithKline Beecham Pharmaceuticals (Worthing, United Kingdom). Cefuroxime was used as the commercially available injectable preparation (Zinacef; Glaxo); cefaclor (Distaclor; Dista Labs) and cefixime (Cefspan; Fujisawa) were extracted from the commercially available capsules; and cefpodoxime (Roussel), cefprozil (Bristol Myers Squibb), and loracarbef (Eli Lilly & Co.) were all kindly supplied as soluble powders by the manufacturers.
Bacterial strains.
The MICs for a number of clinical isolates of S. pneumoniae from France, Hungary, South Africa, and the United Kingdom with a range of susceptibilities to penicillin were determined by the agar dilution method. A penicillin-susceptible type strain, S. pneumoniae ATCC 6303, and a penicillin-susceptible clinical isolate from Hungary, S. pneumoniae 5303, were used in the serial passage experiments. Three penicillin-resistant strains of S. pneumoniae (one from South Africa [strain N1387] and two S. pneumoniae strains kindly supplied by Dr. Ridgeway, Prescot, United Kingdom [strains R1 and R2]) which had different patterns of susceptibility to the test β-lactam antibiotics (Table 1) were chosen for the time-kill studies. A type strain, S. pneumoniae ATCC 6303, and a penicillin-resistant clinical isolate, S. pneumoniae 1320b, supplied by Jenny Dahl Knudsen (Copenhagen, Denmark), were used in studies with the in vitro pharmacodynamic model. S. pneumoniae R6, a susceptible control strain, and S. pneumoniae R61a2x, a laboratory-derived variant of R6 with alterations in penicillin-binding proteins (PBPs) 1a and 2x (6), were both kindly provided by Chris Dowson (Sussex University, Brighton, United Kingdom).
TABLE 1.
β-Lactam MICs for strains of S. pneumoniae used in time-kill studies with concentrations of antibiotics achievable in MEF
| Compound | MIC (μg/ml)
|
Human data
|
||||
|---|---|---|---|---|---|---|
| N1387 | R1 | R2 | Peak concn (μg/ml) in MEF | Dose | Refer- ence | |
| Penicillin G | 4 | 2 | 4 | |||
| Amoxicillin | 2 | 2 | 2 | 2.5 | 13.3 mg/kg | 22 |
| Amoxicillin-clavulanatea | 2 | 2 | 2 | 2.5/0.6 | 250/62.5 mg | 14 |
| Cefuroxime | 2 | 4 | 8 | 2.5 | 250 mg | 17 |
| Cefprozil | 2 | 8 | 16 | 2.0 | 15 mg/kg | 21 |
| Cefaclor | 8 | 128 | 128 | 3.8 | 15 mg/kg | 25 |
| Loracarbef | 8 | 128 | 128 | 3.9 | 15 mg/kg | 26 |
| Cefixime | 8 | 32 | 32 | 0.8 | 8 mg/kg | 3 |
Tested at a 2:1 ratio of amoxicillin:clavulanate; MICs are expressed as the concentration of amoxicillin.
MIC determinations.
Serial twofold dilutions of antibiotic were prepared in Mueller-Hinton agar (BBL) supplemented with 5% (vol/vol) sterile defibrinated horse blood. The agar was inoculated with each test organism at 105 CFU/spot, and the plates were incubated for 18 to 24 h at 37°C. The MIC was the lowest concentration of antibiotic that completely inhibited visible bacterial growth.
Serial passage experiments.
A series of twofold dilutions of twice the final required concentrations of antibiotic were prepared in 1-ml volumes of Todd-Hewitt broth (Oxoid) supplemented with 5% (vol/vol) heat-inactivated horse serum. Overnight broth cultures of the test organisms were diluted to the turbidity of a 0.5 McFarland barium sulfate standard, and a further 10-fold dilution was made in Todd-Hewitt broth plus 5% serum. Each antibiotic concentration was inoculated with 1 ml of the test culture to give approximately 5 × 106 CFU/ml. The MIC of each antibiotic was determined following 24 h of incubation at 37°C, and the culture containing the highest antibiotic concentration allowing visible growth was adjusted to the turbidity of a 0.5 McFarland standard. Following a 10-fold dilution, this culture was used to inoculate a fresh series of antibiotic dilutions, as described above. This process was repeated for 5 days. The final MICs were noted, and the end-of-passage isolates were tested for stability of resistance and cross-resistance with other agents by a conventional agar dilution MIC determination procedure as described above.
Time-kill studies.
Solutions containing the antibiotics at the peak concentrations reported to be achieved in the MEF of children (Table 1) were prepared in 20-ml volumes of Todd-Hewitt broth (Oxoid) supplemented with 5% (vol/vol) heat-inactivated horse serum. The media were inoculated to give 105 to 106 CFU/ml and were incubated at 37°C on an orbital shaker. Samples were taken for assessment of the numbers of viable bacteria at 0, 1, 3, 5, 7, and 9 h. Serial 10-fold dilutions of the samples were prepared in Todd-Hewitt broth, and four dilutions were plated in triplicate onto nutrient agar (Lab M) supplemented with 5% (vol/vol) sterile horse blood and 0.4% β-lactamase (Penase; Difco) to inactivate the antibiotic that was carried over. The numbers of CFU were determined following 24 h of incubation at 37°C, with the limit of detection being 1.67 × 101 CFU/ml.
In vitro pharmacodynamic model.
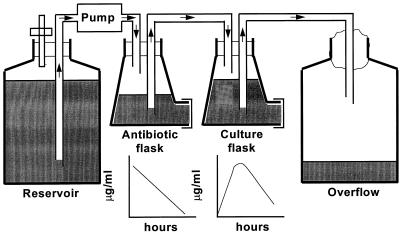
The open, one-compartment model used was based on the biexponential model originally described by Grasso et al. (16) in 1978, and is shown in Fig. 1. The flow rate of the pump and the volumes in the flasks were set to simulate the elimination rate of the antibiotics with the shortest half-life (t1/2; i.e., t1/2 = 1 h for amoxicillin, cefuroxime, and clavulanate), whereas cefpodoxime (t1/2 = 2 h) was added at regular intervals to simulate its slower elimination from humans. The dilution rate of the bacterial cultures in the open system was therefore the same for all of the test antibiotics. Repeated doses of antibiotic were administered automatically every 12 h with a pump (Watson Marlow) interfaced to a microcomputer (Commodore). Mueller-Hinton broth (Difco) supplemented with 5% (vol/vol) sterile, heat-treated horse serum was used. Samples were removed from the culture flask at regular time points for determination of the concentration of antibiotic and the number of viable bacteria present. Viable bacterial counts were determined as described above for the time-kill studies, and the antibiotic concentrations were assayed microbiologically. Colonies from the viable count plates were tested for β-lactam susceptibility by determination of the MIC by the agar dilution method to detect any selection of resistance.
FIG. 1.
Diagrammatic representation of the in vitro pharmacodynamic model.
Microbiological assays.
Amoxicillin was assayed with a commercially available Bacillus subtilis NCTC 6633 spore suspension (Difco) in nutrient agar (Lab M), and clavulanate was assayed with Klebsiella pneumoniae NCTC 11228 in nutrient agar supplemented with 60 μg of benzylpenicillin/ml (19). Cefuroxime and cefpodoxime were assayed with Escherichia coli ESS (an extrasensitive permeability mutant) in nutrient agar.
RESULTS
MIC determinations.
The penicillins amoxicillin (alone and in the presence of clavulanate) and penicillin G were the most active β-lactams against penicillin-susceptible (Pens) and penicillin-intermediate (Peni) pneumococci. Amoxicillin was also the most potent agent against penicillin-resistant (Penr) S. pneumoniae, whereas cefpodoxime was more active than penicillin G against some strains (Table 2). The most active cephalosporins tested were cefpodoxime and cefuroxime, which are both available as oral ester formulations. Cefprozil was two- to fourfold less active than cefuroxime overall, and cefaclor was less active than cefprozil, although cefaclor was slightly more active than loracarbef. Interestingly, cefaclor and loracarbef were more active than cefixime against some strains of S. pneumoniae but were less active against others.
TABLE 2.
Comparative activities of 10 orally available β-lactam compounds against S. pneumoniaea
| Compound | Pens (n = 23)
|
Peni (n = 22)
|
Penr (n = 20)
|
|||
|---|---|---|---|---|---|---|
| MIC range (μg/ml) | MIC90 (μg/ml) | MIC range (μg/ml) | MIC90 (μg/ml) | MIC range (μg/ml) | MIC90 (μg/ml) | |
| Penicillin G | 0.008–0.06 | 0.03 | 0.12–1.0 | 1.0 | 2–8 | 8 |
| Amoxicillin | 0.008–0.03 | 0.03 | 0.015–1.0 | 1.0 | 0.5–2 | 2 |
| Amoxicillinclavulanateb | 0.008–0.03 | 0.03 | 0.008–1.0 | 1.0 | 0.5–2 | 2 |
| Cefpodoxime | 0.008–2 | 0.12 | 0.12–4 | 2 | 2–8 | 4 |
| Cefuroxime | 0.008–0.5 | 0.25 | 0.25–4 | 2 | 1.0–16 | 8 |
| Cefprozil | 0.03–1.0 | 0.25 | 0.12–16 | 8 | 1.0–16 | 16 |
| Cefaclor | 0.03–1.0 | 1.0 | 0.5–64 | 64 | 0.5–128 | 128 |
| Loracarbef | 0.03–4 | 2 | 1.0–128 | 64 | 4–128 | 128 |
| Cefixime | 0.008–4 | 4 | 1.0–32 | 32 | 8–32 | 32 |
| Clavulanate | 1.0–256 | 16 | 8–256 | 256 | 64–1,024 | 1,024 |
The susceptibility of S. pneumoniae was classified on the basis of its susceptibility to penicillin G.
Tested at a 2:1 ratio of amoxicillin:clavulanate and expressed as the concentration of amoxicillin.
Clavulanate was the least active compound tested and is not available for clinical use alone but is available as a β-lactamase inhibitor in combination with amoxicillin. It was included as a reference for the amoxicillin-clavulanate studies, and because it binds selectively to PBP 3 in S. pneumoniae (33), it was of interest to see whether the activity of clavulanate was affected in the same way as the activities of the other agents by altered PBPs in Penr pneumococci. In fact, the MIC at which 90% of strains are inhibited (MIC90) for clavulanate increased 64-fold between the Pens and Penr strains, which was similar to the effect seen for the other agents tested (32- to 128-fold increases) except penicillin G, the MIC90 of which increased 256-fold, and cefixime, the MIC90 of which increased only 8-fold.
Serial passage experiments.
No decrease in susceptibility was seen following exposure of S. pneumoniae ATCC 6303 and S. pneumoniae 5303 to amoxicillin, amoxicillin-clavulanate, cefaclor, or loracarbef for five subcultures. Following exposure to cefpodoxime, however, S. pneumoniae ATCC 6303 showed a fourfold decrease in susceptibility to this compound (Table 3), whereas the culture passaged in cefuroxime not only was fourfold less susceptible to cefuroxime but was also fourfold less susceptible to cefpodoxime and cefixime.
TABLE 3.
Agar dilution MICs of test β-lactams for isolates of S. pneumoniae ATCC 6303 and S. pneumoniae 5303 following five serial passages through increasing antibiotic concentrations
| S. pneumoniae strain and sourcea | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| Amoxicillin | Amoxicillin-clavulanateb | Cefuroxime | Cefpodoxime | Cefaclor | Cefixime | Loracarbef | |
| ATCC 6303 (parent) | 0.03 | 0.03 | 0.03 | 0.03 | 2 | 0.25 | 1.0 |
| Amoxicillin (0.015) | 0.03 | 0.03 | 0.03 | 0.03 | 2 | 0.25 | 1.0 |
| Amoxicillin-clavulanateb (0.015) | 0.015 | 0.03 | 0.015 | 0.03 | 0.5 | 0.25 | 0.5 |
| Cefpodoxime (0.12) | 0.015 | 0.03 | 0.015 | 0.12c | 0.5 | 0.25 | 0.5 |
| Cefuroxime (0.12) | 0.03 | 0.03 | 0.12 | 0.12 | 2 | 1.0 | 1.0 |
| Cefaclor (2) | 0.015 | 0.015 | 0.03 | 0.03 | 1.0 | 0.25 | 1.0 |
| Loracarbef (0.5) | 0.03 | 0.03 | 0.03 | 0.03 | 2 | 0.25 | 1.0 |
| Cefixime (0.5) | 0.008 | 0.015 | 0.03 | 0.25 | 0.5 | 0.25 | 0.5 |
| 5303 (parent) | 0.008 | 0.008 | 0.015 | 0.015 | 0.5 | 0.12 | 0.5 |
| Amoxicillin (0.015) | 0.008 | 0.015 | 0.03 | 0.015 | 0.5 | 0.12 | 0.5 |
| Amoxicillin-clavulanateb (0.002) | 0.008 | 0.015 | 0.015 | 0.015 | 1.0 | 0.12 | 0.5 |
| Cefpodoxime (0.03) | 0.015 | 0.015 | 0.12 | 0.06 | 0.5 | 0.5 | 2 |
| Cefuroxime (0.5) | 0.008 | 0.008 | 0.5 | 0.12 | 2 | 2 | 4 |
| Cefaclor (1.0) | 0.008 | 0.015 | 0.015 | 0.015 | 0.5 | 0.12 | 0.5 |
| Loracarbef (0.5) | 0.008 | 0.015 | 0.015 | 0.03 | 0.5 | 0.25 | 1.0 |
| Cefixime (0.25) | 0.015 | 0.03 | 0.03 | 0.03 | 1.0 | 0.25 | 1.0 |
Expressed as the antibiotic and concentration (in micrograms per milliliter) from which it was isolated.
Tested at a 2:1 ratio and expressed as the concentration of amoxicillin.
Boldface values indicate a fourfold or greater increase in the MIC compared with that for the parent strain.
Following repeated subculture in the presence of cefixime, S. pneumoniae 5303 became fourfold less susceptible to amoxicillin-clavulanate, although the susceptibility to the cephalosporins was unchanged. This strain became fourfold less susceptible to cefpodoxime, cefixime, and loracarbef and eightfold less susceptible to cefuroxime following 5 days of exposure to increasing concentrations of cefpodoxime. In addition, when S. pneumoniae 5303 was passaged in cefuroxime, it became 4-fold less susceptible to cefaclor, 8-fold less susceptible to cefpodoxime and loracarbef, 16-fold less susceptible to cefixime, and 32-fold less susceptible to cefuroxime, whereas the susceptibility of this culture to amoxicillin and amoxicillin-clavulanate was unchanged (Table 3).
Time-kill studies with concentrations achievable in MEF.
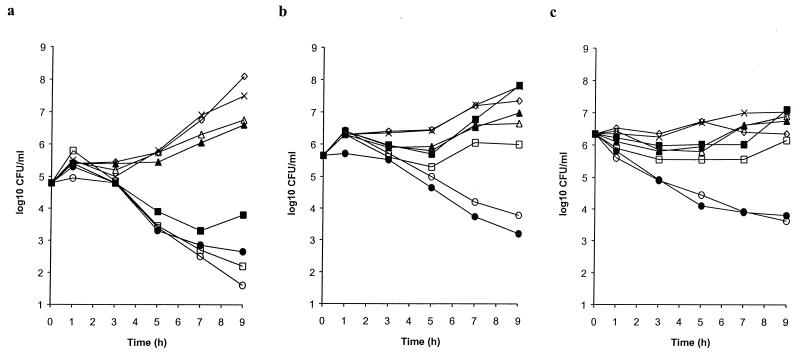
When tested at peak concentrations measured in the MEF of children following the administration of a conventional oral dose (Table 1), cefixime was inactive against all three strains of Penr S. pneumoniae tested and cefaclor and loracarbef only marginally inhibited growth (Fig. 2). Cefprozil had activity similar to those of cefaclor and loracarbef against S. pneumoniae R1 and S. pneumoniae R2 (MICs, 8 and 16 μg/ml, respectively) but had an antibacterial effect against S. pneumoniae N1387, which was more susceptible to cefprozil by agar dilution MIC determinations (MIC, 2 μg/ml). Cefuroxime was marginally more active than cefprozil against S. pneumoniae R1 and S. pneumoniae R2 (MICs, 4 and 8 μg/ml, respectively) and was effective against S. pneumoniae N1387 (MIC, 2 μg/ml). In contrast, the use of amoxicillin and amoxicillin-clavulanate resulted in 2- to 3-log decreases in viable bacterial numbers for all three strains of Penr S. pneumoniae (MICs, 2 μg/ml) over the 9-h test period.
FIG. 2.
Bactericidal activities of amoxicillin-clavulanate (•), amoxicillin (○), cefuroxime (□), cefprozil (■), cefaclor (▴), loracarbef (▵), and cefixime (◊) at concentrations achieved in MEF. The growth in the untreated control culture (×) was used for comparison. (a) S. pneumoniae N1387. (b) S. pneumoniae R1. (c) S. pneumoniae R2.
In vitro pharmacodynamic model.
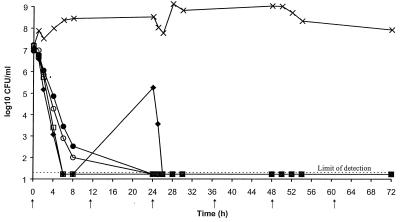
The MIC data indicated that amoxicillin, amoxicillin-clavulanate, cefpodoxime, and cefuroxime are the most potent oral β-lactam antibiotics against S. pneumoniae, including penicillin-resistant strains. These four antibiotics were therefore chosen to be tested in the in vitro pharmacodynamic model. Twice-daily doses of amoxicillin (500 mg), amoxicillin-clavulanate (500 plus 125 mg, respectively), cefpodoxime (200 mg), and cefuroxime (250 mg) were simulated against the typically susceptible strain S. pneumoniae ATCC 6303 (Fig. 3). All were rapidly bactericidal, with the numbers of viable bacteria being reduced to the limit of detection (1.67 × 101 CFU/ml) by 6 h in the cultures treated with cefpodoxime and cefuroxime and by 24 h in the cultures treated with amoxicillin and amoxicillin-clavulanate. Some regrowth was seen by 24 h in the cefpodoxime-treated culture, but the viable bacterial count had fallen below the limit of detection by 26 h and no further regrowth was seen. No selection of resistance was seen in this study.
FIG. 3.
Bactericidal activities of simulated concentrations achieved in the serum of humans following the administration of oral doses of amoxicillin-clavulanate at 500 plus 125 mg, respectively, twice daily (•), 500 mg of amoxicillin twice daily (○), 200 mg of cefpodoxime twice daily (⧫), and 250 mg of cefuroxime twice daily (□) against S. pneumoniae ATCC 6303. The growth of an untreated control culture (×) was used for comparison. Arrows represent the times at which the antibiotic doses were administered.
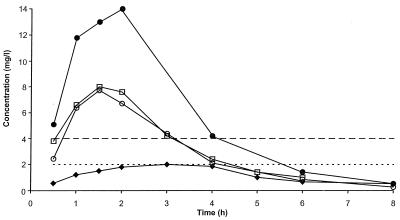
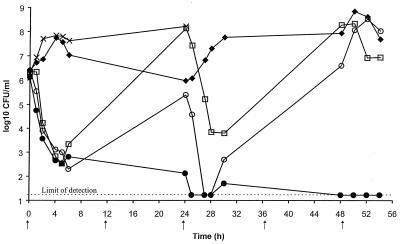
Higher dosages of cefuroxime (500 mg twice daily) and amoxicillin-clavulanate (875 plus 125 mg, respectively, twice daily) were simulated against S. pneumoniae 1320b, a penicillin-resistant strain, since higher doses of oral β-lactam antibiotics are recommended in some countries for respiratory tract infections. Cefpodoxime was again tested at 200 mg twice daily because a higher dose of this antibiotic is not available. The concentrations of antibiotic achieved in the serum of humans (18, 19, 34a, 34a, 35) are presented in Fig. 4. These were simulated in the in vitro pharmacodynamic model, and microbiological assay results confirmed that the antibiotic concentrations obtained in the culture flasks were close to those achieved in humans.
FIG. 4.
Concentrations achieved in the serum of humans following the administration of oral doses of 875 mg of amoxicillin (•) (34a), 500 mg of amoxicillin (○) (19), 200 mg of cefpodoxime (⧫) (35), and 500 mg of cefuroxime (□) (18). The MICs of cefpodoxime and cefuroxime (–––) and amoxicillin and amoxicillin-clavulanate (....) for S. pneumoniae 1320b are also indicated.
Simulated concentrations of cefpodoxime achieved in the serum of humans following the administration of an oral dose of 200 mg were essentially ineffective, with the growth being similar to that in the untreated control culture (Fig. 5). This result was not unexpected, because the MIC of cefpodoxime for S. pneumoniae 1320b is 4 μg/ml, whereas the peak concentration achievable in serum following the administration of a 200-mg oral dose is only 2.1 μg/ml (Fig. 4). The MIC of cefuroxime for this strain is also 4 μg/ml, but following the administration of an oral dose of 500 mg of cefuroxime, a peak concentration of 8 μg/ml is achieved in the serum of humans. Consequently, cefuroxime showed initial bactericidal activity, but the culture regrew fully between the doses, which is consistent with the concentration in serum falling below 4 μg/ml between 3 and 4 h after dosing (Fig. 4).
FIG. 5.
Bactericidal activities of simulated concentrations achieved in the serum of humans following the administration of oral dosages of amoxicillin-clavulanate at 875 plus 125 mg, respectively, twice daily, (•), 500 mg of amoxicillin twice daily (○), 200 mg of cefpodoxime twice daily (⧫), and 500 mg of cefuroxime twice daily (□) against S. pneumoniae 1320b. The growth of an untreated control culture (×) was used for comparison. Arrows represent the times at which the antibiotic doses were administered.
Amoxicillin and amoxicillin-clavulanate showed similar bactericidal activities up to 6 h, after which the culture treated with the lower dose of amoxicillin (500 mg) showed some regrowth by 24 h. The bacterial numbers were reduced to the limit of detection by 26 h, 2 h after administration of the 24-h dose, but the amoxicillin-treated culture fully regrew by 54 h. Amoxicillin-clavulanate (875 and 125 mg, respectively) was rapidly bactericidal and reduced the numbers of viable bacteria to the limit of detection (1.67 × 101 CFU/ml) by 25 h, with no regrowth by the end of the experiment (54 h) (Fig. 5).
On testing of the organisms isolated at the end of the study for β-lactam susceptibility, cultures of S. pneumoniae 1320b treated with simulated serum cefpodoxime and cefuroxime concentrations were shown to contain from 48 h onward isolates that were four- to eightfold less susceptible to cefpodoxime and eightfold less susceptible to cefuroxime than isolates in the untreated control culture (Table 4). These isolates showed cross-resistance with another cephalosporin, cefotaxime, but no cross-resistance with the penicillins penicillin G and amoxicillin-clavulanate. The organisms isolated following 54 h of exposure to simulated serum amoxicillin concentrations and 24 h of exposure to amoxicillin-clavulanate showed no decrease in susceptibility to penicillins or cephalosporins compared with the susceptibility of the untreated control culture (Table 4).
TABLE 4.
MICs for S. pneumoniae 1320b following treatment with simulated concentrations of amoxicillin, amoxicillin-clavulanate, cefuroxime, and cefpodoxime achieved in the serum of humans
| Source of S. pneumoniae 1320ba | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Penicil- lin G | Amoxicillinclavulanateb | Cefpo- doxime | Cefu- roxime | Cefo- taxime | |
| Untreated control, 6 h | 4 | 2 | 4 | 4 | 1 |
| Amoxicillin at 500 mg, 54 h | 2 | 2 | 4 | 4 | 1 |
| Amoxicillin-clavulanateb at 875/125 mg, 24 h | 4 | 1 | 4 | 4 | 1 |
| Cefpodoxime at 200 mg, 24 h | 2 | 2 | 8 | 8 | 1 |
| Cefpodoxime at 200 mg, 54 h | 4 | 2 | 16c | 32 | 4 |
| Cefuroxime at 500 mg, 24 h | 2 | 2 | 4 | 4 | 2 |
| Cefuroxime at 500 mg, 48 h | 2 | 2 | 16 | 32 | 4 |
| Cefuroxime at 500 mg, 54 h | 2 | 1 | 32 | 32 | 8 |
| S. pneumoniae R6 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 |
| S. pneumoniae R61a2x | ≤0.03 | ≤0.03 | 1 | 4 | 0.5 |
Expressed as the dose of antibiotic from which it was isolated and the time of isolation.
Tested at a 2:1 ratio and expressed as the concentration of amoxicillin.
Boldface values indicate fourfold or greater increases in the MIC compared with that for the parent strain.
DISCUSSION
A number of Peni and Penr strains of S. pneumoniae were fully susceptible to amoxicillin and amoxicillin-clavulanate in agar dilution MIC determinations according to the current breakpoints of the National Committee for Clinical Laboratory Standards (MICs, ≤0.5 μg/ml), whereas some Pens strains were resistant (MICs, 0.5 to 4 μg/ml) to the cephalosporins.
The most potent cephalosporins tested were cefpodoxime and cefuroxime, but these were also the compounds with which less susceptible isolates of Pens S. pneumoniae were selected following repeated subculture. The 4- to 32-fold decreases in susceptibility to cefpodoxime, cefuroxime, and cefixime seen following exposure to cefpodoxime and cefuroxime, the lack of cross-resistance with amoxicillin and amoxicillin-clavulanate, and the fact that repeated subculture in amoxicillin or amoxicillin-clavulanate did not lead to the selection of less susceptible isolates were all consistent with data reported by Sifaoui et al. (34). Those investigators isolated one-step mutants from two Pens strains of S. pneumoniae following growth in the presence of cefuroxime, cefpodoxime, cefixime, cefotaxime, and ceftriaxone at frequencies ranging from 1 in 108 to 1 in 106, and the strains were 2- to 16-fold less susceptible to these agents (34). In the same study, the frequencies of mutation following exposure to ampicillin, amoxicillin, amoxicillin-clavulanate, ampicillin-sulbactam, cefaclor, and loracarbef were up to 10-fold lower, and at most, only a 2-fold increase in the MIC was observed. Moreover, one class of cephalosporin-resistant mutants was more susceptible to the penicillins than the parent cultures (34).
Otitis media is an important community-acquired respiratory tract infection that is often caused by Penr S. pneumoniae. Recent studies (4, 7, 10) have shown that β-lactam resistance has resulted in the clinical and bacteriological failure of oral cephalosporins in the treatment of otitis media, particularly when the MICs for S. pneumoniae exceed 0.5 μg/ml. In our studies, only amoxicillin and amoxicillin-clavulanate produced a decrease in the numbers of viable bacteria (99 to 99.9%) for all three strains of S. pneumoniae tested; this was not surprising since only these agents achieved peak concentrations in MEF which exceeded the MICs for all three strains. Cefpodoxime was not tested because data on its concentration in MEF were not available at the time, although a recent report (9) has shown that the concentrations of this compound in MEF (0.2 μg/ml) are at least 10-fold lower than the MICs for Penr S. pneumoniae. In addition to the concentrations in MEF, the concentrations of β-lactams in serum have been found to be important in the bacteriological eradication of the two key pathogens associated with otitis media, S. pneumoniae and H. influenzae (9), with time above the MIC being the most significant pharmacokinetic parameter. A time above the MIC in serum of at least 40% of the dosing interval is required for β-lactams to produce maximal (≥80%) bacteriological cure rates for patients with otitis media and maximal survival rates (≥90%) in animal models of infection (8).
The relevance of the time above the MIC in serum was demonstrated in the in vitro pharmacodynamic model with the four most potent agents, amoxicillin, amoxicillin-clavulanate, cefpodoxime, and cefuroxime, against Pens and Penr strains of S. pneumoniae. The model simulates the concentrations of antibiotic achieved in the serum of humans, considered by many investigators to be predictive of outcome in the treatment of respiratory tract infections caused by pathogens, such as S. pneumoniae and H. influenzae, which are not intracellular (5, 12, 30). The rapid bactericidal activity and lack of selection of resistance seen against S. pneumoniae ATCC 6303 were most likely because of the long period of time above the MICs of the antibiotics tested (100% of the dosing interval). The lack of selection of resistance seen with this strain in the pharmacodynamic model was consistent with the fact that the development of β-lactam resistance by Pens S. pneumoniae in the clinic is considered to have originally arisen via acquisition of genetic material from other streptococcal species rather than by mutation within S. pneumoniae (11).
It has been suggested, however, that once the mosaic genes encoding less susceptible PBPs have been acquired by S. pneumoniae, they may mutate further to give even higher levels of β-lactam resistance (2, 6). This was examined in the in vitro model, in which penicillin-resistant strain S. pneumoniae 1320b was also exposed to amoxicillin, amoxicillin-clavulanate, cefpodoxime, and cefuroxime at concentrations achieved in the serum of humans. Although the concentrations of amoxicillin and cefuroxime obtained following the administration of an oral dose of 500 mg are similar, the lower MIC of amoxicillin meant that the time above the MIC was longer for this antibiotic (7 h in 24 h for amoxicillin, 5 h in 24 h for cefuroxime). Even so, regrowth of S. pneumoniae was seen between doses for both amoxicillin and cefuroxime but not for mg amoxicillin-clavulanate at 875 and 125 mg, respectively. This was probably due to the increased time above the MIC for the amoxicillin component, which was 10 of 24 h (42%) for the dosage of 875 mg twice daily.
As seen in the passage experiments, isolates with reduced cephalosporin susceptibility emerged following exposure of S. pneumoniae 1320b to simulated serum cefpodoxime and cefuroxime concentrations. This was unexpected in the case of cefpodoxime, because there appeared to be no selection pressure for a higher level of resistance, since the MIC for the parent culture of S. pneumoniae 1320b (4 μg of cefpodoxime/ml) already exceeded the maximum concentration in serum (2.1 μg of cefpodoxime/ml). The finding was consistent, however, with data reported by Negri et al. (29) in 1994. They showed that when populations of Peni and Penr S. pneumoniae were mixed, the Penr strain was selected as prevalent by all the β-lactams tested, even at concentrations below the MIC for the Peni strain. In our study, the isolates selected from cultures treated with simulated serum cefpodoxime and cefuroxime concentrations were four- to eightfold less susceptible to cefpodoxime, cefuroxime, and cefotaxime but were not cross-resistant with the penicillins tested. This is consistent with mutations in PBPs 1a and 2x, which have been shown to be necessary for cephalosporin resistance in S. pneumoniae, and was exemplified by a characterized variant, S. pneumoniae R61a2x (6). Mutation in PBP 2b is also required to confer resistance to penicillins. The decreased susceptibility to cefotaxime observed in the present study (MICs of up to 8 μg/ml) could have severe consequences in the treatment of more serious infections caused by S. pneumoniae, such as meningitis, for which parenteral cephalosporins are often recommended when Penr pneumococci are suspected.
In conclusion, cross-resistance between β-lactam antibiotics cannot be assumed for S. pneumoniae, and in the clinical situation, it is important to determine the susceptibility of the strain to the specific agent being considered for use in treatment. Because this is not likely to be known when empirical therapy of community-acquired infections is required, epidemiological data and local resistance patterns must be relied upon and the susceptibilities of other important respiratory pathogens, such as H. influenzae and M. catarrhalis, which may produce β-lactamases, should also be considered. It is important to choose an agent that is unlikely to lead to the selection of a higher level of resistance and to administer it at a dose high enough to ensure optimal coverage against penicillin-resistant pneumococci. In these studies, amoxicillin and amoxicillin-clavulanate were the most effective oral β-lactams against penicillin-susceptible and penicillin-resistant pneumococci and, unlike some of the cephalosporins, did not lead to the selection of resistance.
REFERENCES
- 1.Baquero, F., J. Martínez Beltrán, and E. Loza. 1991. A review of antibiotic resistance patterns of Streptococcus pneumoniae in Europe. J. Antimicrob. Chemother. 28(Suppl. C):31–38. [DOI] [PubMed]
- 2.Baquero, F., and M. C. Negri. 1997. Strategies to minimize the development of resistance. J. Chemother. 9(Suppl. 3):29–37. [PubMed]
- 3.Barry B, Gehanno P, Blume N. Clinical outcome of otitis media caused by pneumococci with decreased susceptibility to penicillin. Scand J Infect Dis. 1994;26:446–452. doi: 10.3109/00365549409008618. [DOI] [PubMed] [Google Scholar]
- 4.Beque P, Garabedian E N, Denoyel F, Broussin B. Program and abstracts of the 11th Interdisciplinary Meeting of Anti-Infectious Chemotherapy. 1991. Diffusion du cefixime dans le liquide auriculare chez l’enfant, abstr. 102/C7. [Google Scholar]
- 5.Cars O. Efficacy of beta-lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagn Microbiol Infect Dis. 1997;27:29–33. doi: 10.1016/s0732-8893(97)00020-5. [DOI] [PubMed] [Google Scholar]
- 6.Coffey T J, Daniels M, McDougal L K, Dowson C G, Tenover F C, Spratt B G. Genetic analysis of clinical isolates of Streptococcus pneumoniae with high-level resistance to expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 1995;39:1306–1313. doi: 10.1128/aac.39.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, R., F. De La Rocque, M. Boucherat, C. Doit, E. Bingen, and P. Geslin. 1994. Treatment failure in otitis media: an analysis. J. Chemother. 6(Suppl. 4):17–24. [PubMed]
- 8.Craig W A. Antimicrobial resistance issues of the future. Diagn Microbiol Infect Dis. 1996;25:213–217. doi: 10.1016/s0732-8893(96)00162-9. [DOI] [PubMed] [Google Scholar]
- 9.Craig W A, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Dagan R, Abramson O, Leibovitz E. Impaired bacteriologic response to oral cephalosporins in acute otitis media caused by pneumococci with intermediate resistance to penicillin. Pediatr Infect Dis J. 1996;15:980–985. doi: 10.1097/00006454-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Dowson C G, Coffey T J, Kell C, Whiley R A. Evolution of penicillin resistance in Streptococcus pneumoniae: the role of Streptococcus mitis in the formation of a low affinity PBP 2B in S. pneumoniae. Mol Microbiol. 1993;9:635–643. doi: 10.1111/j.1365-2958.1993.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 12.Drusano, G. L., and W. A. Craig. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J. Chemother. 9(Suppl. 3):38–44. [PubMed]
- 13.Fenoll A, Marton Bourgon C, Munoz R, Vicioso D, Casal J. Serotype, distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain. Rev Infect Dis. 1991;13:56–60. doi: 10.1093/clinids/13.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Geslin P, Fremaux A, Sissia G. Streptococcus pneumoniae: état actuel de la sensibilité aux beta-lactamamines en France. Med Mal Infect. 1991;21:3–11. [Google Scholar]
- 15.Geslin P, Fremaux A, Sissia G, Spicq C, Aberrane S. Epidemiologie de la resistance aux antibiotiques de Streptococcus pneumoniae en France. Med Mal Infect. 1994;24:948–961. [Google Scholar]
- 16.Grasso S, Meinardi G, DeCarneri I, Tamassia V. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob Agents Chemother. 1978;13:570–576. doi: 10.1128/aac.13.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad J, Isaacson G, Respler D. Concentration of cefuroxime in serum and middle ear effusion after single dose treatment with cefuroxime axetil. Pediatr Infect Dis J. 1991;10:294–298. doi: 10.1097/00006454-199104000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Harding S M, Williams P E O, Ayrton J. Pharmacology of cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob Agents Chemother. 1984;25:78–82. doi: 10.1128/aac.25.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson D, Cooper D L, Horton R, Langley P F, Staniforth D H, Sutton J A. Absorption, pharmacokinetic and metabolic studies with Augmentin. In: Croydon E A P, Michel M F, editors. Augmentin: clavulanate-potentiated amoxicillin. Proceedings of the European Symposium. Amsterdam, The Netherlands: Excerpta Medica; 1983. pp. 83–101. [Google Scholar]
- 20.Jacobs, M. 1997. Respiratory tract infections: epidemiology and surveillance. J. Chemother. 9(Suppl. 3):10–17. [PubMed]
- 21.Kafetzis D A, Carabinos C, Bairamis T, Apostolopoulos N. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Diffusion of four oral cephalosporins into the middle ear exudate (MEE) of children suffering from acute otitis media (AOM), abstr. 941; p. 291. [Google Scholar]
- 22.Kim H K, Cantekin E I, Bluestone C D, Reilly J S. Pharmacokinetic study of the concentration of amoxicillin in middle ear effusions of children with chronic otitis media with effusion. Ann Otol Rhinol Laryngol. 1983;96:42–44. [Google Scholar]
- 23.Klugman K. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klugman, K., F. Goldstein, S. Kohno, and F. Baquero. 1997. The role of 4th generation cephalosporins in the treatment of infections caused by penicillin-resistant streptococci. Clin. Microbiol. Infect. 3(Suppl. 1):S48–S60.
- 25.Krause P J. Penetration of amoxicillin, cefaclor, erythromycin-sulfisoxazole and trimethoprim-sulfamethoxazole into the middle ear fluid of patients with chronic serous otitis media. J Infect Dis. 1982;145:815–821. doi: 10.1093/infdis/145.6.815. [DOI] [PubMed] [Google Scholar]
- 26.Kusmiesz H, Shelton S, Brown O, Manning S, Nelson J. Loracarbef concentrations in middle ear fluid. Antimicrob Agents Chemother. 1990;34:2030–2031. doi: 10.1128/aac.34.10.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B L, Padula A M, Kimbrough R C, Jones S R, Chaisson R E, Mills J, Sande M A. Infectious complications with respiratory pathogens despite ciprofloxacin therapy. N Engl J Med. 1991;325:520–521. doi: 10.1056/nejm199108153250719. [DOI] [PubMed] [Google Scholar]
- 28.Marton A. Pneumococcal antibiotic resistance: the problem in Hungary. Clin Infect Dis. 1992;15:106–111. doi: 10.1093/clinids/15.1.106. [DOI] [PubMed] [Google Scholar]
- 29.Negri M C, Morosini M L, Loza E, Baquero F. In vitro selective antibiotic concentrations of β-lactams for penicillin-resistant Streptococcus pneumoniae populations. Antimicrob Agents Chemother. 1994;38:122–125. doi: 10.1128/aac.38.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nix D E, Goodwin S D, Peloquin C A, Rotella D L, Schentag J J. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection. Antimicrob Agents Chemother. 1991;35:1953–1959. doi: 10.1128/aac.35.10.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Trallero E, Garcia-Arenzana J M, Jimenez J A, Peris A. Therapeutic failure and selection of resistance to quinolones in a case of pneumococcal pneumonia treated with ciprofloxacin. Eur J Clin Microbiol Infect Dis. 1990;9:905–906. doi: 10.1007/BF01967510. [DOI] [PubMed] [Google Scholar]
- 32.Peterson L R, Quick J N, Jensen B, Homann S, Johnson S, Tenquist J, Shanholtzer C, Petzel R A, Sinn L, Gerding D N. Emergence of ciprofloxacin-resistance in nosocomial methicillin-resistant Staphylococcus aureus (MRSA) isolates during ciprofloxacin plus rifampicin therapy for MRSA colonization. Arch Intern Med. 1990;150:2151–2155. [PubMed] [Google Scholar]
- 33.Severin A, Severina E, Tomasz A. Abnormal physiological properties and altered cell wall composition in Streptococcus pneumoniae grown in the presence of clavulanic acid. Antimicrob Agents Chemother. 1997;41:504–510. doi: 10.1128/aac.41.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sifaoui F, Kitzis M D, Gutmann L. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Selection of one step resistant mutants of Streptococcus pneumoniae by different oral β-lactam antibiotics, abstr. C7; p. 72. [Google Scholar]
- 34a.Smithkline Beecham Pharmaceuticals. Data on file.
- 35.Wise, R. 1990. The pharmacokinetics of the oral cephalosporins—a review. J. Antimicrob. Chemother. 26(Suppl. E):13–20. [DOI] [PubMed]