Abstract
At a children’s hospital in Riga, Latvia, isolates identified as Salmonella typhimurium were found to be resistant to expanded-spectrum cephalosporins. Two of the resistant strains were analyzed for the mechanism of cephalosporin resistance. Isoelectric focusing revealed a common β-lactamase with a pI of 8.8. In addition, one of the strains produced a pI 7.6 β-lactamase. A transconjugant producing only the pI 7.6 enzyme was susceptible to expanded-spectrum cephalosporins; therefore, this enzyme was most likely SHV-1. Transformants producing only the pI 8.8 β-lactamase were resistant to cefotaxime and aztreonam but were susceptible or intermediate to ceftazidime. A substrate profile determined spectrophotometrically with purified enzyme revealed potent activity against cefotaxime, with a relative kcat value of 95 (benzylpenicillin equal to 100). The enzyme showed lower relative kcat values for ceftazidime (3.3) and aztreonam (9.3). In addition, the enzyme was inhibited by clavulanate, sulbactam and tazobactam, with 50% inhibitory concentrations of 19, 100, and 3.4 nM, respectively. These results indicated the presence of an unusual extended-spectrum β-lactamase. The gene expressing the pI 8.8 β-lactamase was cloned. Nucleotide sequencing revealed a β-lactamase gene that differs from the gene encoding CTX-M-2, which also originated from S. typhimurium, by 11 nucleotides, 4 of which result in amino acid substitutions: Ala27Thr, Val230Gly, Glu254Ala, and Ile278Val. These results indicated the presence of a novel extended-spectrum β-lactamase, designated CTX-M-5, that specifically confers resistance to cefotaxime.
Resistance to expanded-spectrum β-lactam antibiotics in the Enterobacteriaceae is often due to the presence of extended-spectrum β-lactamases (ESBLs), which are most often derivatives of TEM or SHV enzymes (11, 17). However, there is a small but growing family of plasmid-mediated ESBLs, including CTX-M-1 (MEN-1) (2, 4, 5), CTX-M-2 (3, 5), CTX-M-3 (13), CTX-M-4 (12), Toho-1 (15), and Toho-2 (18), that preferentially hydrolyze cefotaxime. These enzymes are not closely related to TEM or SHV β-lactamases (5) but are all members of Ambler’s class A. ESBLs have been found previously in strains of Salmonella spp. including Salmonella typhimurium strains from Turkey (29), Argentina (3, 26), Tunisia (6), Spain (20), and Russia (12). Two of these reports described the CTX-M-2 and the CTX-M-4 β-lactamases (3, 12).
A large outbreak of gastroenteritis caused by Salmonella sp. has occurred among children in Latvia. Over 4,000 cases were reported from 1991 through the first quarter of 1998. Approximately 70% of these patients were under 1 year old, and the illnesses were moderate to severe, with bloody stools and high fever. Some of the cases were complicated by extraintestinal infections. The epidemiology of this outbreak is currently under investigation. In addition to the hospitalized patients, some of the cases occurred among babies residing in orphanages. The clinical isolates of S. typhimurium associated with the majority of these infections were particularly noteworthy because of their increased resistance to expanded-spectrum β-lactam antibiotics, especially cefotaxime. In this study, we report a new member of the CTX-M cefotaxime-hydrolyzing β-lactamase family, designated CTX-M-5.
(This work was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997 [10].)
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains used in this study are listed in Table 1. S. typhimurium strains were cefotaxime-resistant clinical isolates obtained from individual patients at Children’s Hospital, Riga, Latvia. Escherichia coli DH5α and E. coli DH5α/pCLL2300 were used in transformation, transconjugation, and cloning experiments.
TABLE 1.
Bacterial strains used in this study
| Strain | Organism | Plasmid | Characteristics | pI of β-lactamase(s) | Source or reference |
|---|---|---|---|---|---|
| 28 | S. typhimurium | pCLL3425 | Clinical isolate | 8.8 | This study |
| 34 | S. typhimurium | pCLL3422 + pCLL3425 | Clinical isolate | 7.6, 8.8 | This study |
| DH5α | E. coli | E. coli K-12 cloning strain | None | 14 | |
| F φ80dlacZΔM15 Δ(lacZYA-argF) U169 recAI endAI hsdR17 (rK− mK+) supE44 thiI gyrI relAI | |||||
| DH5α | E. coli | pCLL2300 | Kmr cloning vector | None | 24 |
| DH5α | E. coli | pCLL3422 + pCLL2300 | Transconjugant of S. typhimurium 34 | 7.6 | This study |
| DH5α | E. coli | pCLL3425 | Transformant of S. typhimurium 34 | 8.8 | This study |
| DH5α | E. coli | pCLL3417 | 7-kb BamHI fragment containing blaCTX-M-5 in pCLL2300 | 8.8 | This study |
Identification and susceptibility tests.
The isolates were identified initially as S. typhimurium in the microbiology laboratory of the Children’s Hospital Medical Academy of Latvia. This identification was confirmed in the reference laboratory by the API-20E identification system (bioMérieux-Vitek, Hazelwood, Mo.). MICs of various β-lactam antibiotics were determined with broth microdilution tests by standard methods (21). The following antibiotics were used: piperacillin and tazobactam (Lederle Laboratories, Pearl River, N.Y.); ticarcillin and potassium clavulanate (Beecham Laboratories, Bristol, Tenn.); sulbactam (Pfizer Inc., New York, N.Y.); ceftazidime (Glaxo Group Research Ltd., Greenford, England); cefotaxime (Hoechst-Roussel Pharmaceuticals Inc., Somerville, N.J.); imipenem (Merck, Rahway, N.J.); aztreonam, cefepime, and benzylpenicillin (Bristol-Myers Squibb, Princeton, N.J.); cephaloridine (Eli Lilly, Indianapolis, Ind.); cefotetan (Zeneca Pharmaceuticals, Wilmington, Del.); and cefoxitin (Sigma Chemical Company, St. Louis, Mo.). Conditions used for testing β-lactam–β-lactamase inhibitor combinations were those recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (21): ampicillin-sulbactam, 2:1 ratio of drug to inhibitor; ticarcillin-clavulanate, constant concentration of 2 μg/ml of inhibitor; piperacillin-tazobactam, constant concentration of 4 μg/ml of inhibitor. Susceptibility to non-β-lactam antibiotics was determined by a disk diffusion assay (22).
IEF and β-lactamase assays.
Crude preparations of β-lactamases from clinical isolates were obtained by repeated freezing-thawing in 0.02 M sodium acetate, pH 5.2. Isoelectric focusing (IEF) was performed by the method of Matthew et al. (19) by using an LKB Multiphor apparatus with prepared PAGplates, pH 3.5 to 9.5 (Pharmacia LKB, Piscataway, N.J.). The isoelectric point (pI) of each enzyme was confirmed by activity staining with nitrocefin (Becton Dickinson Microbiology Systems, Cockeysville, Md.) following IEF. The pI of CTX-M-5 was calculated by using β-lactamase standards with known isoelectric points.
E. coli DH5α/pCLL3417, which produces the pI 8.8 CTX-M-5 β-lactamase from cefotaxime-resistant S. typhimurium, was used for further enzymatic analysis. CTX-M-5 β-lactamase crude extracts were obtained from 2 liters of log-phase cells in Trypticase soy broth after five cycles of freezing-thawing in 0.02 M sodium acetate followed by 30 min of ultracentrifugation at 100,000 × g using a Beckman L8-M ultracentrifuge. This enzyme extraction was initially eluted from a Sephadex G-75 column equilibrated with 50 mM phosphate buffer, pH 7.0. Fractions were monitored at 280 nm for protein and tested for β-lactamase hydrolytic activity with 50 μg of nitrocefin per ml. Fractions with high enzymatic activity were pooled, concentrated, and subjected to a QAE A50 anion-exchange column equilibrated with 20 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] buffer, pH 8.3. Eluents with enzyme activity were then pooled and concentrated. The purity of the enzyme was checked by using sodium dodecyl sulfate (SDS)–12% polyacrylamide gel electrophoresis (PAGE), as described previously (9).
The enzyme hydrolysis rates of purified CTX-M-5 were determined in 50 mM PO4, pH 7.0, with a Beckman DU7400 spectrophotometer. For each substrate, the initial velocities were determined at six to eight substrate concentrations. The kinetic parameters kcat and Km were expressed as means of at least two independent kinetic evaluations and were calculated by the computer program ENZPACK (Biosoft; Elsevier). The standard deviation of each parameter was less than 20%. For the inhibition study, enzyme and inhibitor were preincubated in a volume of 50 μl for 10 min at 25°C before the addition of 50 μg of nitrocefin per ml (final volume, 1,000 μl). Initial rates of hydrolysis were monitored spectrophotometrically at 495 nm, and 50% inhibitory concentrations (IC50s) were determined graphically.
Nucleic acid techniques.
DNA isolation, restriction enzyme digestions, recombinant DNA manipulations, and transformations of plasmid DNA were performed as described by Sambrook et al. (27). Plasmids conferring ampicillin resistance were transferred from the clinical isolates to an ampicillin-susceptible, kanamycin-resistant E. coli host, strain DH5α/pCLL2300, by filter mating (28). Transconjugants were selected on Luria-Bertani agar plates containing 100 μg of ampicillin per ml and 30 μg of kanamycin per ml. Plasmids conferring cefotaxime resistance were transformed into E. coli DH5α by using CaCl2 (27). SHV-1 β-lactamase was detected by PCR and subsequent NheI digestion, as described previously (23).
To facilitate nucleotide sequencing, a 7-kb BamHI fragment encoding the β-lactamase with a pI of 8.8 from S. typhimurium 34 was cloned from an alkaline lysis plasmid DNA preparation into pCLL2300, a kanamycin resistance-conferring cloning vector (24), and the resulting plasmid was designated pCLL3417. Because the pI 8.8 β-lactamase present in these strains appeared to be phenotypically similar to the CTX-M-1 and CTX-M-2 β-lactamases, sequencing was initiated with 17-bp forward (5′-TCTGACGCTGGGTAAAG-3′) and reverse (5′-CTTTACCCAGCGTCAGA-3′) primers derived from a consensus region of the sequences of these two β-lactamase genes (5). Subsequently, both strands of the entire β-lactamase gene were sequenced with a nested set of custom-synthesized oligonucleotide primers which were specific for the CTX-M-5 β-lactamase gene. Initial DNA sequencing was performed on double-stranded plasmid DNA with a Sequenase kit (United States Biochemical, Cleveland, Ohio) with 35S-dATP label (Amersham, Arlington Heights, Ill.) and completed by dideoxy sequencing with a Taq dye terminator kit (Applied Biosystems, Inc., Foster City, Calif.), according to the respective manufacturers’ instructions.
Nucleotide sequence accession number.
The nucleotide sequence data for blaCTX-M-5 was submitted to the GenBank nucleotide sequence database and assigned accession no. U95364.
RESULTS
Characterization of clinical isolates.
The pIs of the β-lactamases present in crude extracts obtained from the clinical isolates are shown in Table 1. The clinical isolates expressed a β-lactamase with a pI of approximately 8.8. In addition, strain 34 produced a second enzyme with a pI of 7.6, which correlated with SHV-1. The presence of a non-ESBL SHV-type (most likely SHV-1) enzyme in the clinical isolates was confirmed in this strain by PCR with SHV-1 specific oligonucleotides and subsequent digestion with NheI (data not shown).
Plasmid profiles of strains 28 and 34 revealed multiple small plasmids ranging in size from 2 to 10 kb. In addition, strain 34 had one large plasmid (>50 kb). Mating experiments transferred the single large plasmid from S. typhimurium 34. This transconjugant (DH5α/pCLL3422 + pCLL2300) expressed only the pI 7.6 β-lactamase. Transforming plasmid DNA from strain 34 resulted in transformant DH5α/pCLL3425, which contained a single 10-kb plasmid and expressed only the pI 8.8 β-lactamase.
Susceptibility.
MICs for the clinical isolates, transformant, transconjugant, and clone are listed in Table 2. With NCCLS criteria for intermediate and resistant used to define clinical resistance, the clinical isolates of S. typhimurium were resistant to the penicillins, amoxicillin-clavulanate, ampicillin-sulbactam, ticarcillin-clavulanate, cefotaxime, and aztreonam. The clinical isolates were susceptible to imipenem, cefoxitin, and ceftazidime, although the MICs of the latter were increased. S. typhimurium 28, which lacks the pI 7.6 β-lactamase, was susceptible to piperacillin-tazobactam. In addition, the clinical isolates of S. typhimurium were also resistant to chloramphenicol and trimethoprim-sulfamethoxazole but were susceptible to gentamicin, kanamycin, and ciprofloxacin (data not shown).
TABLE 2.
MICs of β-lactam drugs for S. typhimurium clinical isolates and E. coli derivatives
| Strain | MIC (μg/ml)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | AMC | SAM | TIC | TIM | PIP | PTZ | CAZ | CTX | ATM | IPM | FOX | |
| S. typhimurium strains | ||||||||||||
| 28 | >128 | 16 | 64 | >128 | 128 | >128 | 2 | 8 | 128 | 32 | 0.25 | 2 |
| 34 | >128 | 32 | 64 | >128 | >128 | >128 | 64 | 8 | 128 | 32 | 0.25 | 2 |
| E. coli strains | ||||||||||||
| DH5α | 8 | 8 | 8 | 4 | 1 | 1 | 0.25 | 0.25 | ≤0.12 | ≤0.12 | 0.25 | 8 |
| DH5α/pCLL2300 | 8 | 8 | 8 | 4 | 1 | 1 | 0.25 | 0.25 | ≤0.12 | ≤0.12 | 0.25 | 8 |
| DH5α/pCLL3422 + pCLL2300 | >128 | 64 | 32 | >128 | 128 | 128 | 8 | 1 | 0.5 | 0.5 | 0.5 | 8 |
| DH5α/pCLL3425 | >128 | 32 | 32 | >128 | 128 | >128 | 1 | 16 | >128 | 128 | 0.5 | 16 |
| DH5α/pCLL3417 | >128 | 32 | 64 | >128 | 128 | >128 | 0.5 | 16 | >128 | 128 | 0.5 | 8 |
MICs were determined in broth microdilution tests (21). AMP, ampicillin; AMC, amoxicillin-clavulanate; SAM, ampicillin-sulbactam; TIC, ticarcillin; TIM, ticarcillin-clavulanate; PIP, piperacillin; PTZ, piperacillin-tazobactam; CAZ, ceftazidime; CTX, cefotaxime; ATM, aztreonam; IPM, imipenem; FOX, cefoxitin.
The pI 7.6 β-lactamase plasmid present in transconjugant strain DH5α/pCLL3422 + pCLL2300 conferred resistance to the penicillins, amoxicillin-clavulanate, ampicillin-sulbactam, and ticarcillin-clavulanate. The pI 8.8 β-lactamase in strains DH5α/pCLL3425 and DH5α/pCLL3417 conferred resistance to cefotaxime and aztreonam, the penicillins, and the β-lactamase inhibitor combinations except for piperacillin-tazobactam. Although MICs of ceftazidime were increased, they were not increased to the level that would be considered resistant clinically (21).
β-Lactamase characterization.
After two steps of column chromatography, the purified CTX-M-5 enzyme yielded a major single band (95% homogeneity) in both IEF and SDS–12% PAGE. From SDS-PAGE, the molecular weight of the CTX-M-5 β-lactamase was estimated to be 29,000 based upon Bio-Rad low-molecular-weight markers. The isoelectric point of homogeneously purified CTX-M-5 was 8.8, consistent with the enzyme produced from the wild-type strain.
The substrate and inhibition profiles are shown in Table 3. CTX-M-5 β-lactamase showed broad-spectrum hydrolytic activity against penicillin, cephalosporins, and aztreonam. Cefotaxime was a good substrate for CTX-M-5, with comparable hydrolytic activity (kcat, 210 s−1) and affinity (Km, 77 μM) to those of benzylpenicillin. Cephaloridine was also a good substrate, with higher hydrolytic activity but lower affinity. Ceftazidime was readily hydrolyzed by the CTX-M-5 enzyme but had a lower rate (<5%) and lower affinity (higher Km) than those of cefotaxime or benzylpenicillin. The physiological efficiency (kcat/Km) of CTX-M-5 for ceftazidime was only 6% of that of cefotaxime. Aztreonam was also hydrolyzed by CTX-M-5 with a lower rate and lower affinity than cefotaxime. Cefoxitin and imipenem were poor substrates for CTX-M-5, with relative hydrolysis rates of <0.1% of that of benzylpenicillin. Clavulanic acid, tazobactam, and sulbactam were all effective β-lactamase inhibitors for the CTX-M-5 β-lactamase, with IC50s of 19, 100, and 3.4 nM respectively. CTX-M-5 is a cefotaxime-hydrolyzing β-lactamase which is inhibited by clavulanate and is therefore classified in functional group 2be.
TABLE 3.
Substrate and inhibitor profiles of purified CTX-M-5 β-lactamase
| Substrate or inhibitor | kcat (s−1) | Relative kcat | Km (μM) | kcat/Km (mM−1s−1) | IC50 (nM) |
|---|---|---|---|---|---|
| Substrates | |||||
| Benzylpenicillin | 230 | 100 | 50 | 4,600 | |
| Cephaloridine | 1,500 | 680 | 350 | 4,400 | |
| Cefotaxime | 210 | 95 | 77 | 2,800 | |
| Ceftazidime | 7.4 | 3.3 | 440 | 17 | |
| Aztreonam | 21 | 9.3 | 730 | 29 | |
| Cefoxitin | 0.41 | 0.18 | NCa | NC | |
| Imipenem | 0.0027 | 0.0012 | NC | NC | |
| Inhibitors | |||||
| Clavulanic Acid | 19 | ||||
| Sulbactam | 100 | ||||
| Tazobactam | 3.3 |
NC, not calculated (rates were too low to obtain reliable values).
Nucleotide sequencing.
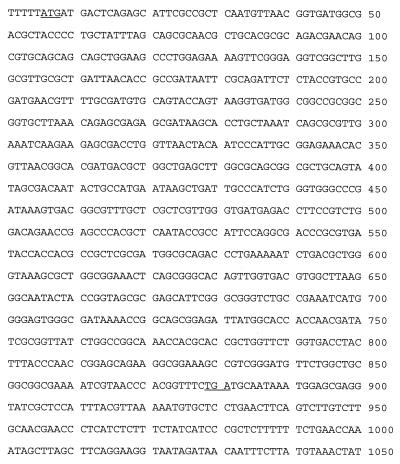
The nucleotide sequence of blaCTX-M-5 is given in Fig. 1, and the derived amino acid sequence is given in Fig. 2. The blaCTX-M-5 β-lactamase gene is 879 bp long, and the encoded protein consists of 293 amino acids. It is 63.2% identical to the CTX-M-1 β-lactamase gene and 97.5% identical to the CTX-M-2 β-lactamase gene (5). There is similar homology on the protein level. The nucleotide sequence of blaCTX-M-5 differs from blaCTX-M-2 by 11 nucleotides, 4 of which give rise to amino acid changes: threonine for alanine 27, glycine for valine 230, alanine for glutamate 254, and valine for isoleucine 278.
FIG. 1.
Nucleotide sequence of the blaCTX-M-5 β-lactamase gene. Underlined nucleotides are start and stop codons.
FIG. 2.
Derived amino acid sequence of the CTX-M-5 β-lactamase compared to the cefotaxime-hydrolyzing β-lactamases CTX-M-1, CTX-M-2 (5), CTX-M-3 (13), CTX-M-4 (12), Toho-1 (15), and Toho-2 (18). Dots indicate identical amino acids; underlining indicates the 70SXXK73, 130SDN132, E-166, and 234KTG236 conserved sequences that are typical of class A serine β-lactamases. Amino acid numbering is according to the scheme outlined by Ambler et al. (1).
DISCUSSION
A serious outbreak of S. typhimurium in Latvia was further complicated by the fact that the outbreak strain was resistant to multiple antibiotics, including ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole, and cefotaxime, which are commonly used to treat serious infections caused by Salmonella spp. These strains were uniformly susceptible to ciprofloxacin; however, the extremely young ages of most of the patients prevented its use in treatment.
The isolates described in this report were resistant to cefotaxime due to the presence of a novel extended-spectrum β-lactamase, CTX-M-5. It is interesting that in this small family of cefotaxime-hydrolyzing β-lactamase, CTX-M-2, which is the enzyme most closely related to CTX-M-5, and CTX-M-4 also originated in S. typhimurium (3, 12). The strains of S. typhimurium described by Bauernfeind et al. from which CTX-M-2 was detected were isolated from an outbreak of severe infections in Buenos Aires, Argentina, in 1990 (3). Therefore, it is unlikely that the Argentinean strains are related to the Latvian strains described in this study. Further observations of E. coli, Klebsiella pneumoniae, and Proteus mirabilis strains expressing CTX-M-2 were made in Germany, Israel, and Paraguay (5). The other members of the CTX-M family of β-lactamases were found in E. coli and Citrobacter freundii strains isolated in Germany, Italy, Japan, Poland, and Russia (2, 4, 12, 13, 15). The wide geographical distribution of occurrences of these β-lactamases implies that they represent independent genetic events; however, it is interesting that CTX-M-3, CTX-M-4, and CTX-M-5 were all found in isolates from Eastern European countries (12, 13). To date, all of the strains expressing CTX-M-type enzymes have carried the β-lactamase on a plasmid. The origin of the enzymes remains unknown, although they are most closely related to the chromosomally mediated β-lactamase from Klebsiella oxytoca (5, 25).
The CTX-M family of ESBLs is interesting in that it has uniquely potent hydrolytic activity against cefotaxime. Like the cefotaxime hydrolyzing β-lactamases CTX-M-1, CTX-M-2, and Toho-1 (3, 4, 15), CTX-M-5 has more potent activity against cefotaxime than against ceftazidime. This finding confirms the analysis of the relationship between compound structure and enzymatic stability by Bauerfeind et al., who concluded that the carboxy-isopropoximino group of ceftazidime protects the compound from attack by the CTX-M-type enzymes, whereas the methoximino group of cefotaxime provides the compound better affinity to the enzyme (4). The enzyme kinetic data for CTX-M-2 (3), which is most closely related to CTX-M-5, indicated a hydrolytic pattern similar to that of CTX-M-5, in that cephaloridine is a better substrate than benzylpenicillin or cefotaxime. Toho-1 (15), which differs from CTX-M-2 by only two amino acid residues, hydrolyzed cephaloridine and cefotaxime much faster (10- to 16-fold) than benzylpenicillin. These differences in substrate profiles might also represent experimental differences between various investigators’ laboratories.
The combination of CTX-M-5 and SHV-1 in three of the clinical isolates studied conferred resistance to piperacillin-tazobactam. However, clinical isolates or laboratory constructs producing only one of these enzymes were susceptible to this β-lactam–β-lactamase inhibitor combination. It appears that the combination of SHV-1 plus an ESBL can confer resistance to piperacillin-tazobactam, whereas strains expressing the ESBL alone are often susceptible (7, 8, 16, 30). The resistance to piperacillin-tazobactam in these strains expressing multiple β-lactamases is most likely attributable to the SHV-1 enzyme, which is not inhibited as well by β-lactamase inhibitors as most other class A enzymes (11).
The prevalence of this cefotaxime-hydrolyzing β-lactamase among S. typhimurium strains in Latvia may be related to the increased usage of expanded-spectrum cephalosporins in recent years; however, it is difficult to assess whether or not this resistance developed as a direct result of cephalosporin therapy. With the recognition of the high prevalence of resistance, efforts have been made to curtail antibiotic use in patients with salmonellosis, and efforts to control the spread of S. typhimurium are under way. The detection of a new member of the CTX-M family in Latvia raises important questions about whether such enzymes arise de novo in multiple geographic locations or, alternatively, are transmitted across national borders. The importance of these questions emphasizes the need for global surveillance of antibiotic resistance so that appropriate control strategies can be designed and implemented.
ACKNOWLEDGMENTS
We thank Ian Fingerman and Xiang Ma for technical assistance in the cloning and sequencing of blaCTX-M-5 and the microbiology laboratory staffs of the Children’s Hospital Medical Academy, Riga, Latvia; St. Louis Children’s Hospital, St. Louis, Mo.; and the Missouri State Health Department Laboratory, Jefferson City, Mo.
The partnership between the Children’s Hospital Medical Academy of Latvia and St. Louis Children’s Hospital was sponsored by the American International Health Alliance, Washington, D.C.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frére J-M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthélémy M, Péduzzi J, Bernard H, Tancrède C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 3.Bauernfeind A, Casellas J M, Goldberg M, Holley M, Jungwirth R, Mangold P, Röhnisch T, Schweighart S, Wilhelm R. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection. 1992;20:158–163. doi: 10.1007/BF01704610. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18:294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben Hassen A, Bejaoui M, Lakhoua M R, Redjeb S B. Profil épidémiologique de la résistance de 153 souches de Salmonella (S. typhi excludes) isolées en milieu pédiatrique Tunisien de 1985 à 1990. Pathol Biol. 1993;41:706–712. [PubMed] [Google Scholar]
- 7.Bradford P A, Cherubin C E, Idemyor V, Rasmussen B A, Bush K. Multiply resistant Klebsiella pneumoniae strains from two Chicago hospitals: identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing β-lactamases in a single isolate. Antimicrob Agents Chemother. 1994;38:761–766. doi: 10.1128/aac.38.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford P A, Jacobus N V, Bhachech N S, Bush K. TEM-28 from an Escherichia coli clinical isolate is a member of the His-164 family of TEM-1 extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1996;40:260–262. doi: 10.1128/aac.40.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford P A, Urban C, Mariano N, Projan S J, Rahal J J, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997;41:563–569. doi: 10.1128/aac.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford P A, Yang Y, Sahm D, Grope I, Gardovska D, Storch G. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. CTX-M-3, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of S. typhimurium in Latvia, abstr. C-186; p. 78. [Google Scholar]
- 11.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazouli M, Tzelepi E, Sidorenko S V, Tzouvelekis L S. Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolyzing class A β-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob Agents Chemother. 1998;42:1259–1262. doi: 10.1128/aac.42.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gniadkowski M, Schneider I, Pałucha A, Jungwirth R, Mikiewicz B, Bauernfeind A. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother. 1998;42:827–832. doi: 10.1128/aac.42.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 15.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobus N V, Labthavikul P, Spengler M D, Bradford P A, Bush K, Testa R T. Abstracts of the 95th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1995. Characterization of β-lactamases in three collections of E. coli and K. pneumoniae as related to susceptibility to piperacillin/tazobactam and other extended spectrum beta lactams, abstr. A-73; p. 156. [Google Scholar]
- 17.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma L, Ishii Y, Ishiguro M, Matsuzawa H, Yamaguchi K. Cloning and sequencing of the gene encoding Toho-2, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob Agents Chemother. 1998;42:1181–1186. doi: 10.1128/aac.42.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthew M A, Harris A M, Marshall M J, Ross G W. The use of isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 20.Morosini M I, Canton R, Martinez-Beltran J, Negri M C, Perez-Diaz J C, Baquero F, Blazquez J. New extended-spectrum TEM-type β-lactamase from Salmonella enterica subsp. enterica isolated in a nosocomial outbreak. Antimicrob Agents Chemother. 1995;39:458–461. doi: 10.1128/aac.39.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A2. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 23.Nüesch-Inderbinen M T, Kayser F H, Hächler H. Survey and molecular genetics of SHV β-lactamases in Enterobacteriaceae in Switzerland: two novel enzymes, SHV-11 and SHV-12. Antimicrob Agents Chemother. 1997;41:943–949. doi: 10.1128/aac.41.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen B A, Gluzman Y, Tally F P. Cloning and sequencing of the class B β-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990;34:1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynaud A, Pédyzzu J, Barthélémy M, Labia R. Cefotaxime-hydrolyzing activity of the β-lactamase of Klebsiella oxytoca D488 could be related to a threonine residue at position 140. FEMS Microbiol Lett. 1991;81:185–192. doi: 10.1016/0378-1097(91)90301-p. [DOI] [PubMed] [Google Scholar]
- 26.Rossi A, Lopardo H, Woloj M, Picandet A M, Mariño M, Galds M, Radice M, Gutkind G. Non-typhoid Salmonella spp. resistant to cefotaxime. J Antimicrob Chemother. 1995;36:697–702. doi: 10.1093/jac/36.4.697. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Trieu-Cuot P, Courvalin P. Transposition behavior of IS15 and its progenitor IS15-D: are cointegrates exclusive end products? Plasmid. 1985;14:80–89. doi: 10.1016/0147-619x(85)90034-4. [DOI] [PubMed] [Google Scholar]
- 29.Vahaboglu H, Hall L M C, Mulazimoglu L, Dodanli S, Yildirim I, Livermore D M. Resistance to extended-spectrum cephalosporins, caused by PER-1 β-lactamase, in Salmonella typhimurium from Istanbul, Turkey. J Med Microbiol. 1995;43:294–299. doi: 10.1099/00222615-43-4-294. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Bhachech N, Bradford P A, Jett B D, Sahm D F, Bush K. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli isolates producing TEM-10 and TEM-43 β-lactamases from St. Louis, Missouri. Antimicrob Agents Chemother. 1998;42:1671–1676. doi: 10.1128/aac.42.7.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]