Abstract
In addition to its potent efficacy in animal models against Candida sp., Aspergillus fumigatus, and Histoplasma capsulatum, the clinical candidate pneumocandin MK-991 (formerly L-743,872) was also extremely potent against Pneumocystis carinii in models of immune-compromised animals. MK-991 was approximately 14 times more potent than the original natural product lead, pneumocandin B0. The 90% effective dose (ED90) of MK-991 for cyst clearance in the rat model for pneumocystis was 0.011 mg/kg of body weight when delivered parenterally for 4 days twice a day (b.i.d.). In a mouse model, under the same experimental parameters, the ED90 was 0.02 mg/kg. MK-991 was also effective orally, with an ED90 for cyst clearance of 2.2 mg/kg against acute infection in rats (b.i.d. for 4 days). Complete prevention of P. carinii development was achieved in immunocompromised mice at a daily oral dose of 2.25 mg/kg. As reported previously for other pneumocandins and echinocandins, MK-991 selectively prevented the development of P. carinii cysts. When used as a prophylactic agent, neither stage of the organism appeared in the lungs of animals. In response to an acute infection, cysts were eliminated rapidly, while trophozoite forms persisted. Despite good efficacy as an oral agent in murine models, the low oral absorption of this class may limit the use of MK-991 to parenteral therapy.
Pneumocystis carinii is one of the most common and serious opportunistic infections in patients with AIDS, as well as in those receiving immunosuppressive therapy as a result of organ transplantation or cancer chemotherapy. The most widely used agent for the treatment and prevention of P. carinii pneumonia (PCP), trimethoprim-sulfamethoxazole (TMP-SMZ), has a high incidence of adverse reactions in AIDS patients (2). Rapid oral desensitization to TMP-SMZ has been attempted (5, 8, 10), and while initial results are positive, studies have been limited to small numbers of patients, and there is clearly a need for a new class of compounds to treat PCP.
A novel natural product, pneumocandin B0, originally isolated from a fermentation of Glarea lozoyensis, was found to have activity in an experimental rat model for PCP (13). Synthetic chemical modifications of the parent structure have resulted in more potent and soluble compounds (12). The pneumocandins act by inhibiting the synthesis of β- 1,3-glucan, a major component of the cyst wall of P. carinii (7). The lack of a mammalian β-1,3-glucan synthase counterpart should provide a superior therapeutic window over standard PCP treatments, such as TMP-SMZ and pentamidine.
While β-1,3-glucan represents a major portion of the wall of the cyst, it is not found in great abundance in the trophozoite form of the organism. From previous studies (13), we know that short-term treatment (4 days) with pneumocandin analogs does not have a significant effect on trophozoites. Treatment over a period of 2 weeks (the standard length of treatment for known PCP agents) results in no significant increase or decrease in the level of trophozoites. This result suggests that inhibition of cyst proliferation has an effect on the ability of the trophozoites to multiply. Finally, when prophylactic effects of the pneumocandins were evaluated, inhibition of the cyst form of the organism was found to result in inhibition of the trophozoite form as well, implying that the cyst stage is required for trophozoite proliferation (11).
The efficacy of one of the most potent semisynthetic pneumocandins, MK-991, against PCP is evaluated here. This compound was evaluated orally (p.o.) and parenterally in murine models for PCP. Short-term (4 day) and long-term (as much as 21 days) uses, as well as prophylactic use, were evaluated. In addition, the effects of the drug on the organization of the cyst and localization of the drug in the cyst wall after 2 days of drug treatment were evaluated.
MATERIALS AND METHODS
Compound.
The MK-991 compound used in these studies was synthesized by the Merck Synthetic Chemical Research Group from natural products produced and isolated by the Merck Natural Product Drug Discovery Group. MK-991 is water soluble (>20 mg/ml), and all experiments were performed with the compound in sterile water.
Animals.
The dexamethasone-immunosuppressed animal model used in these studies has been described elsewhere (4, 12). For these studies, C3Heb/FeJ mice were obtained from Jackson Laboratories (Bar Harbor, Maine) and Sprague-Dawley rats were obtained from Taconic Labs (Germantown, N.Y.). All animals were VAF inbred strains raised under barrier condi- ions and housed in microisolator cages. The mice received 4 mg of dexamethasone (Butler, Columbus, Ohio) per liter in their drinking water to cause immunosuppression, while the rats received 1.5 to 2.0 mg/liter. For treatment of acute disease, the animals were immunosuppressed 6 to 7 weeks prior to the start of treatment, whereas for the prophylactic study, treatment with MK-991 began on the day on which immunosuppressive therapy was initiated. All animals also received 1 g of tetracycline per liter in their drinking water for prevention of bacterial infections. Food (23% protein rodent chow; Purina, St. Louis, Mo.) and water were supplied ad libitum.
Efficacy studies.
For acute therapy studies, animals were treated with MK-991 subcutaneously (s.c.) twice a day (b.i.d.) for as much as 21 days or were gavaged for p.o. treatment. Prophylactic treatment was administered once a day (q.d.) for 6 weeks by gavage, and treatment was initiated at the start of immunosuppression. In studies in which TMP-SMZ was used for comparison, the drug was delivered in the drinking water at a dose of 200 mg of TMP–1,000 mg of SMZ per liter of water. The dose levels for individual experiments are indicated in the figure legends. The 90% effective dose (ED90) was defined as the effective dose for 90% cyst clearance relative to vehicle controls. All data are the mean numbers (log10) of cysts ± standard errors (indicated by error bars).
Evaluation of lung tissue.
Excised lungs were homogenized in 5.0 ml of phosphate-buffered saline (Sigma) with a Brinkmann homogenizer (Brinkmann Instruments, Westbury, N.Y.) set at speed no. 3 for approximately 5 s. Homogenates were centrifuged (Beckman GPR centrifuge) at 1,700 × g for 10 min. The erythrocytes were lysed by resuspension of the pellet in 5.0 ml of a 0.85% ammonium chloride (Mallinckrodt, Paris, Ky.) solution and incubated for 5 min in a 37°C water bath and were then centrifuged at 1,700 × g (10 min). The pellets were washed once in saline, and the final pellets were resuspended in 100 μl of saline for mice and 2 ml of saline for rats. A 5.0-μl aliquot of each sample was evenly applied and air dried onto Teflon-coated microscope slides with a fixed surface area (11-mm circles; Carlson Scientific, Peotone, Ill.). The extent of disease for each animal was determined by microscopic analysis of stained slides. The total numbers of cysts per animal lung were determined by quantitating the numbers of cysts in 20 microscope fields (×1,000) of homogenized lung tissue on slides fixed with ether-sulfuric acid and were stained with Toluidine Blue O (14). The total number of cysts per animal lung was determined as a function of the surface area on the slide, the volume of the applied sample, and the total volume of the processed homogenate. The limit of detection for cyst counts was a log mean of 3.36.
Quantitation of P. carinii trophozoites (nuclei) was achieved by hybridization with two P. carinii-specific genomic DNA probes essentially as described previously (6). Total genomic DNA was prepared from lung tissue of individual mice, quantitated, and immobilized on a nylon membrane. The hybridization probes (clones B13-2 and A2-1) were single-copy genomic clones isolated from a rat P. carinii isolate. Both of these clones cross-hybridize to genomic DNA prepared from a murine P. carinii isolate but do not hybridize to genomic DNA prepared from murine tissues, including heart, intestine, and kidney tissues. The probes also do not hybridize to genomic DNAs prepared from the following organisms: Trypanosoma brucei, Eimeria tenella, Toxoplasma gondii, Saccharomyces cerevisiae, Candida albicans, Cryptococcus neoformans, Aspergillus nidulans, Pseudomonas aeruginosa, Streptococcus pneumoniae, Aspergillus fumigatus, and cytomegalovirus-infected human foreskin fibroblasts. Hybridization signals were quantitated with a PhosphorImager (Molecular Dynamics, Inc.).
Lung tissue prepared for electron microscopy was fixed according to the procedure described by Nollstadt et al. (9).
RESULTS
Parenteral treatment with MK-991 in rats and mice.
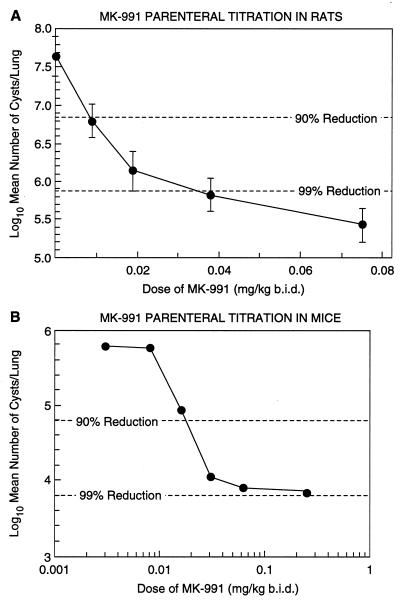
Parenteral titrations of MK-991 in rats (Fig. 1A) and mice (Fig. 1B) after 4 days of treatment resulted in ED90s for cyst clearance of 0.011 and 0.02 mg/kg of body weight, respectively. This represents at least a 14-fold improvement in the ED90 for rats compared with that for the parent compound pneumocandin B0, which is 0.15 mg/kg (12).
FIG. 1.
(A) Rats with acute P. carinii infections were treated s.c. b.i.d. for 4 days with 0.075, 0.038, 0.019, or 0.009 mg of MK-991 per kg. There were five rats in each group. The ED90 was estimated to be 0.011 mg/kg. (The limit of detection was 4.66.) (B) Mice with acute P. carinii infections were treated s.c. b.i.d. for 4 days with various doses of MK-991. There were five mice in each group. The ED90 was determined to be 0.02 mg/kg. (The limit of detection was 3.36.)
P. carinii cyst clearance in rats and mice.
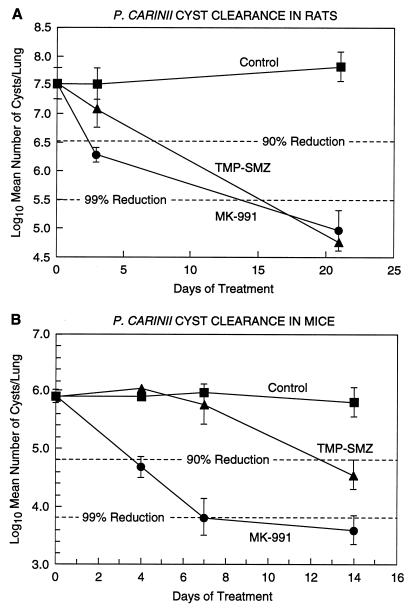
A 90% reduction in the number of cysts relative to controls was seen in rats after 4 days of s.c. treatment with 1.0 mg of MK-991 per kg b.i.d. (Fig. 2A) and in mice after 4 days of treatment with 0.03 mg/kg b.i.d. (Fig. 2B). In comparison, mice treated with TMP-SMZ did not reach a 90% reduction in the level of cysts until almost 2 weeks of treatment (Fig. 2B). This is similar to a result obtained previously with TMP-SMZ (11).
FIG. 2.
(A) Rats with acute P. carinii infections were treated for 21 days with 1 mg of MK-991 per kg b.i.d. or were treated with TMP-SMZ (Bactrim) via their drinking water (200 mg of TMP–1,000 mg of SMZ/liter of water). A 90% reduction in the number of cysts was seen with MK-991 after 4 days of treatment. In contrast, the Bactrim-treated group did not show a 90% reduction until after >1 week of treatment. (The limit of detection, scored as one cyst per 20 fields, was 4.66.) (B) Mice with acute P. carinii infections were treated with 0.31-mg/kg MK-991 b.i.d. or Bactrim in water for 14 days. There were 15 mice per group. While a 90% reduction in cysts was seen at 4 days with MK-991, the Bactrim-treated group did not reach 90% reduction until almost 2 weeks of treatment. (The limit of detection for this experiment was 3.36.)
P. carinii trophozoite clearance in rats.
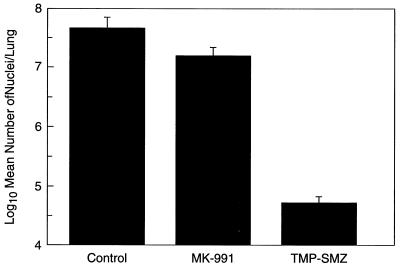
When lung tissue from the previous experiment was evaluated for nuclei after 21 days of treatment, rats in the MK-991-treated group showed a 66.3% reduction in the level of nuclei compared to controls, while rats in the TMP-SMZ-treated group showed a 99.9% reduction in nuclei (Fig. 3).
FIG. 3.
Trophozoite clearance in rats. Rats with acute P. carinii infections treated for 21 days with 1 mg of MK-991 per kg b.i.d. or Bactrim in water were evaluated for trophozoites with a P. carinii-specific DNA probe. Rats in the MK-991-treated group showed a 66.3% reduction in the level of trophozoites, while rats in the Bactrim-treated group showed a 99.9% reduction in the number of trophozoites.
Oral treatment with MK-991 in rats.
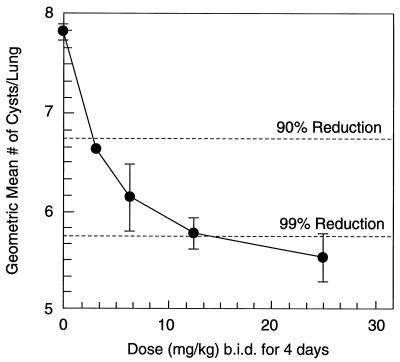
MK-991 was gavaged b.i.d. in a dose titration of 0.78 to 6.25 mg/kg for 4 days. The ED90 for cyst clearance in rats was 2.2 mg/kg (Fig. 4).
FIG. 4.
Oral efficacy in rats. Rats with acute P. carinii infections were gavaged b.i.d. for 4 days with 0.78, 0.163, 3.13, or 6.25 mg of MK-991 per kg. Each group contained five rats. The ED90 was estimated to be 2.2 mg/kg. (The limit of detection was 4.66.)
Oral prophylaxis of MK-991 in mice.
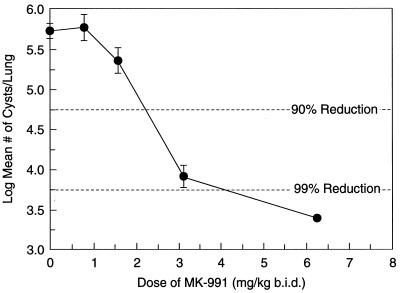
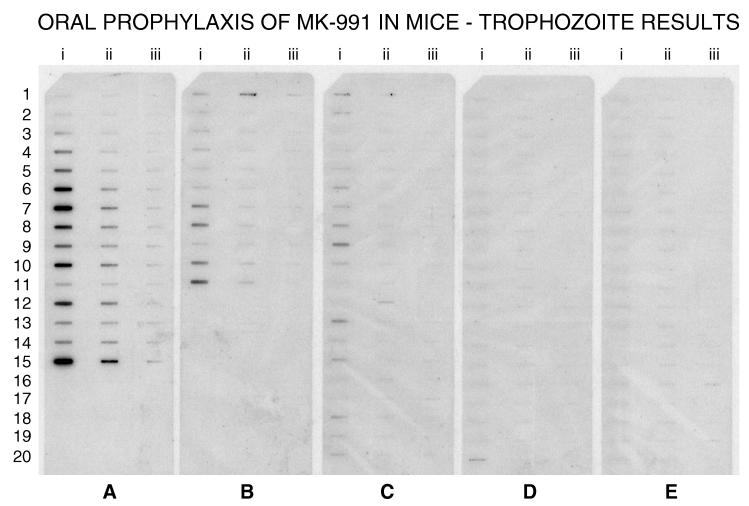
In a prophylaxis study, mice were treated q.i.d. for 8 weeks with MK-991. Immunosuppression with dexamethasone coincided with the start of drug treatment. The ED90 for cyst reduction was 2.25 mg/kg (Fig. 5). When nuclei were quantitated with a P. carinii-specific DNA probe, a 90% difference in the number of nuclei compared to controls, was seen with 1.1 mg of MK-991 per kg (Fig. 6).
FIG. 5.
Oral prophylaxis of MK-991 in mice. Mice with latent P. carinii infections were treated with MK-991 once a day for 8 weeks at doses of 6.25, 3.13, 1.56, or 0.78 mg/kg. Treatment began at the start of immunosuppression. The ED90 for cyst reduction was estimated to be 2.25 mg/kg. (The limit of detection was 3.36.)
FIG. 6.
Efficacy of daily oral prophylactic use of MK-991 as measured by hybridization with a P. carinii-specific DNA probe. Total genomic DNA prepared from lungs of individual animals was immobilized on a nylon membrane in three dilutions, 25 i.e., (column i), 2.5 (column ii), and 0.25 (column iii) μg. (A) P. carinii-specific hybridization in nonmedicated, infected control mice (n = 15); (B, C, D, and E) results from mice treated with 0.78 (n = 11), 1.56 (n = 20), 3.13 (n = 20), and 6.25 (n = 20) mg of MK-991/kg, respectively. The ED90 for prevention of P. carinii trophozoites was estimated to be 1.1 mg/kg.
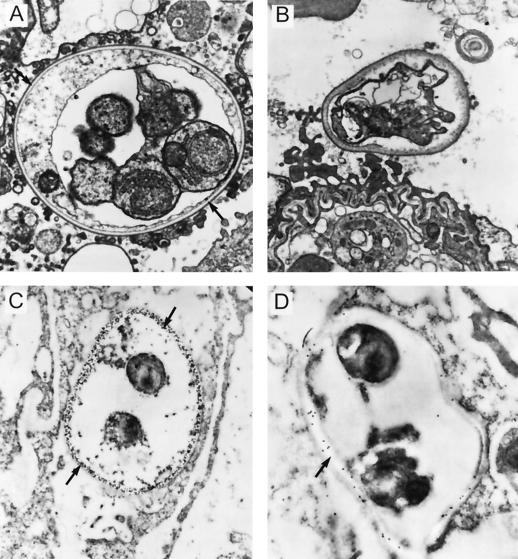
Localization of drug in the cyst wall of P. carinii.
Lung tissue from infected rats was fixed and prepared for electron microscopy. The wall of a normal cyst contains an electron-dense layer and an electron-lucent layer. The proposed location of β-1,3-glucan is the electron-translucent layer (Fig. 7A). When a portion of the same sample was fixed for immunogold electron microscopy and incubated with antisera specific for β-1,3-glucan, the β-1,3-glucan antibody reacted only with the translucent layer of the cell wall (Fig. 7C). No reactivity was seen with the surrounding lung tissue or the trophozoites. In cysts from rats treated for 2 days with another pneumocandin, L-733,560 (12), the electron-lucent layer was absent, the electron-dense layer was thickened, and the wall of the cyst lost the typical rigid shape (Fig. 7B). In treated lung tissue fixed with immunogold and incubated with antibody to pneumocandin (Fig. 7D), the antibody was specific to the area corresponding to the remnants of the translucent layer, strongly suggesting that the pneumocandins localize in the cyst wall.
FIG. 7.
(A) Mature P. carinii cyst containing trophozoites in rat lung tissue. The electron-lucent layer of the cyst wall is the proposed location of β-1,3-glucan. (B) Cyst from a rat with acute PCP treated for 2 days with the pneumocandin L-733,560. The electron-lucent layer is absent, while the electron-dense layer has thickened and the wall has lost its rigid shape. (C) Section of the lung sample in panel A fixed for immunogold electron microscopy. The section was incubated with antiserum specific for β-1,3-glucan and labelled with immunogold. The β-1,3-glucan antibody reacts only with the translucent layer of the cyst wall. There is no reactivity seen with the surrounding lung tissue or with the trophozoites. (D) Section of the lung sample shown in panel B. The section was incubated with antibody made against L-733,560 and then labelled with immunogold. The antibody specifically targets what is left of the translucent layer, demonstrating that the pneumocandins localize in the cyst wall upon treatment of the animal.
DISCUSSION
Synthetic chemical modification of the natural product pneumocandin B0, including reduction of glutamine and substitution of the hemiaminal with ethylene diamine, results in the structure of MK-991. These modifications have led to significant improvement in the activity against P. carinii in murine models. The parenteral activity of MK-991 in the rat model for PCP is approximately 14-fold greater than that of the natural product, with an ED90 of 0.011 versus 0.15 mg/kg for pneumocandin B0. For oral treatment of acute disease, a dose of 2.2 mg/kg is effective at eliminating 90% of the cysts b.i.d. in 4 days. Since the β-1,3-glucan component of P. carinii is specific for the cyst stage of the organism, treatment of acute disease has little effect on the trophozoite stage. However, in studies in which MK-991 was given prophylactically, development of both stages was prevented at a daily oral dose of 2.25 mg/kg.
These results, combined with the efficacy of MK-991 against other pathogenic fungi, including C. albicans and A. fumigatus (1, 3), demonstrate the potential for the use of this compound as a broad-spectrum agent in the immunocompromised patient. The lack of a mammalian β-1,3-glucan counterpart implies that this class of compounds would provide a safer alternative to amphotericin B, the most effective drug currently used for the treatment of systemic fungal infections, which is highly toxic and not efficacious against PCP. Currently, MK-991 is in development as a broad-spectrum antifungal agent for parenteral treatment of systemic infections. Due to the specificity of MK-991 for the cyst stage of P. carinii, the benefits of treatment with MK-991 for acute PCP are not known. The more likely scenario would be the use of MK-991 for prophylaxis of PCP. Since parenteral dosing would have limited applications for prophylaxis and aerosol delivery for prevention of PCP has proven effective, delivery of MK-991 via aerosol might prove useful. In a previous study with aerosolized L-693,989 (11), a related pneumocandin, prevention of P. carinii cysts and trophozoites was achieved at a daily dose of 7 μg/lung or a weekly dose of 77.9 μg/lung. Since the parenteral ED90 for cyst clearance with L-693,989 is 0.15 mg/kg (4) versus 0.11 (rats) to 0.2 (mice) mg/kg for MK-991, the delivery dose necessary for aerosol prophylaxis with MK-991 should be less than the doses described for L-693,989.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of echinocandin antifungal MK-0991 (L-743,872) efficacy in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Absar N, Daneshvar H, Beall G. Desensitization to trimethoprim/sulfamethoxazole in HIV-infected patients. J Allergy Clin Immunol. 1994;93:1001–1005. doi: 10.1016/s0091-6749(94)70048-6. [DOI] [PubMed] [Google Scholar]
- 3.Bartizal K, Gill C J, Abruzzo G K, Flattery A M, Kong L, Scott P M, Smith J G, Leighton C E, Bouffard A, Dropinski J F, Balkovec J. In vitro preclinical evaluation studies with echinocandin antifungal MK-0991 (L-743,872) Antimicrob Agents Chemother. 1997;41:2326–2332. doi: 10.1128/aac.41.11.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frenkel J, Good J, Schultz J. Latent pneumocystic infections in rats, relapse and chemotherapy. Lab Invest. 1966;15:1559–1577. [PubMed] [Google Scholar]
- 5.Gluckstein D, Ruskin J. Rapid oral desensitization to trimethoprim-sulfamethoxazole (TMP-SMZ): use in prophylaxis for Pneumocystis carinii pneumonia in patients with AIDS who were previously intolerant to TMP-SMZ. Clin Infect Dis. 1995;20:849–853. doi: 10.1093/clinids/20.4.849. [DOI] [PubMed] [Google Scholar]
- 6.Liberator P A, Anderson J W, Powles M, Pittarelli L A, Worley M, Becker-Hapak M, Graves D C, Schmatz D M. Comparative study of antipneumocystis agents in rats by using a Pneumocystis carinii-specific DNA probe to quantitate infection. J Clin Microbiol. 1992;30:2968–2974. doi: 10.1128/jcm.30.11.2968-2974.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto Y, Matsuda S, Tegoshi T. Yeast glucan in the cyst wall of Pneumocystis carinii. J Protozool. 1989;36:21S–22S. doi: 10.1111/j.1550-7408.1989.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen M, Weiss P J, Wallace M R. Two-day oral desensitization to trimethoprim-sulfamethoxazole in HIV infected patients. AIDS. 1995;9:573–575. doi: 10.1097/00002030-199506000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Nollstadt K H, Powles M A, Fujioka H, Aikawa M, Schmatz D M. Use of β-1,3-glucan-specific antibody to study the cyst wall of Pneumocystis carinii and effects of pneumocandin B0 analog L-733,560. Antimicrob Agents Chemother. 1994;38:2258–2265. doi: 10.1128/aac.38.10.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palusci V J, Kaul A, Lawrence R M, Haines K A, Kwitten P L. Rapid oral desensitization to trimethoprim-sulfamethoxazole in infants and children. Pediatr Infect Dis J. 1996;15:456–460. doi: 10.1097/00006454-199605000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Powles M A, McFadden D C, Liberator P A, Anderson J W, Vadas E B, Meisner D, Schmatz D M. Aerosolized L-693,989 for Pneumocystis carinii prophylaxis in rats. Antimicrob Agents Chemother. 1994;38:1397–1401. doi: 10.1128/aac.38.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmatz D M, Powles M A, McFadden D M, Nollstadt K, Bouffard F A, Dropinski J F, Liberator P, Anderson J. New semisynthetic pneumocandins with improved efficacies against Pneumocystis carinii in the rat. Antimicrob Agents Chemother. 1995;39:1320–1323. doi: 10.1128/aac.39.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmatz D M, Powles M A, McFadden D C, Pittarelli L, Balkovec J, Hammond M, Zambias R, Liberator P, Anderson J. Antipneumocystis activity of water-soluble lipopeptide L-693,989 in rats. Antimicrob Agents Chemother. 1992;36:1964–1970. doi: 10.1128/aac.36.9.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmatz D M, Romancheck M A, Pittarelli L A, Schwartz R E, Fromtling R A, Nollstadt K H, Vanmiddlesworth F L, Wilson K E, Turner M J. Treatment of Pneumocystis carinii pneumonia with 1,3, β-glucan synthesis inhibitors. Proc Natl Acad Sci USA. 1990;87:5950–5954. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]