Abstract
Rationale:
Stimulant drugs like methamphetamine (MA) activate brain reward circuitry, which is linked to the development of problematic drug use. It is not clear how drugs like MA alter neural response to a non-drug reward.
Objectives:
We examined how acute MA impacts neural response to receipt of a monetary reward relative to a loss in healthy adults. We hypothesized that MA (vs. placebo) would increase mesolimbic neural activation to reward, relative to loss.
Methods:
In a within-subject, randomized, cross-over, double-blind, placebo-controlled design, 41 healthy adults completed the Doors monetary reward task during fMRI after ingestion of placebo or 20 mg MA. We examined drug effects on neural response to reward receipt (Win vs. Loss) using a priori anatomical striatal regions of interest (nucleus accumbens (NAcc), caudate, putamen).
Results:
MA decreased NAcc BOLD activation to reward vs loss compared to placebo (p=.007) without altering caudate or putamen BOLD activation. Similar effects for reward vs. loss were obtained using whole brain analysis. Additional exploratory ROI analysis comparing reward and loss activation relative to a neutral ‘fixation’ period indicated that MA increased NAcc BOLD activation during loss trials, without decreasing activation during win trials.
Conclusions:
This preliminary evidence suggests that MA increases NAcc neural response to the receipt of monetary loss. Additional studies are needed to replicate our findings and clarify the mechanisms contributing to altered mesolimbic neural response to reward and loss receipt during stimulant intoxication.
Introduction
Use of stimulant drugs like methamphetamine (MA) has significantly increased over the past decade (Guerin and Kim 2021). Overdose deaths involving stimulant drugs are estimated to have increased by 46% from 2018 to 2020 (Han et al. 2021). In addition, individuals who use stimulants like MA often suffer tremendous physical, psychological, and psychosocial costs (NIDA 2017; 2019), including increased risk of suicidal behavior (Marshall et al. 2011). Thus, it is crucial that we identify risk factors for problematic substance use, including problematic MA use, to inform prevention and intervention efforts.
Brain reward circuitry is one risk factor that is linked to subjective drug reward and the development of substance use disorders (SUD). Stimulant drugs like MA activate brain reward circuitry, especially dopaminergic mesolimbic reward circuitry (Koob and Volkow 2016). Indeed a single dose of a stimulant is associated with greater dopamine release in the ventral striatum, putamen, and caudate (Oswald et al. 2005), and with greater dopamine cell activity, levels of D2 receptors, and level of D2/D3 receptor binding (Abi-Dargham et al. 2003; Drevets et al. 2001; Leyton et al. 2002; Martinez et al. 2003; Volkow et al. 2002). This activation of dopaminergic mesolimbic circuitry is linked to subjective drug reward (Webber et al. 2021) and thought to play an important role in continued substance use and SUD. In addition to producing direct feelings of wellbeing, stimulant drugs may also enhance responses to non-drug rewards (Robbins et al. 1989).
Non-drug rewards activate the same reward regions as single doses of rewarding drugs, indicating that neural response to drug-related rewards and non-drug rewards are similar (Haber and Knutson 2010). This is likely due to dopamine neurotransmission in brain reward circuitry that is linked to motivation to seek and acquire drug-related and non-drug rewards. Tasks that probe brain reward circuitry during functional magnetic resonance imaging (fMRI) can be used to determine individuals’ neural response to monetary rewards (Luijten et al. 2017), that may be related to risk for SUD. Initial brain responses upon receipt of monetary rewards are thought to reflect learning processes and signaling the salience of stimuli (Miller et al. 2014; Redish 2004), making this an important construct implicated in the development of SUD. Accumulating evidence from longitudinal and cross-sectional studies demonstrate that adolescents at risk for SUD show hyperactive brain reward circuitry to rewards (e.g., Bjork et al. 2010; Heitzeg et al. 2015; Ivanov et al. 2012; Stice and Yokum 2014; Stice et al. 2013), suggesting that this may be a risk factor for the development of SUD. In previous fMRI studies, we have shown that among healthy young adults, greater neural response to anticipation and receipt of non-drug rewards (in the drug-free state) is related to rewarding effects of drugs: brain reward signals were greater in individuals who reported greater subjective euphoric response to d-amphetamine and to greater motivation (i.e., wanting) to consume more alcohol after a single moderate dose of alcohol (Crane et al. 2018; Langenecker et al. 2020; Radoman et al. 2021). However, it is not clear how a stimulant may acutely impact neural reward responsivity.
To our knowledge, only two prior studies have examined the neural acute effects of stimulants on reward receipt utilizing fMRI. In the first study, 8 healthy adult volunteers received 1 dose of oral amphetamine (0.25 mg/kg) or placebo in a double-blind, within-subject, counterbalanced design, about 150-165 minutes prior to completing a monetary reward task during fMRI (Knutson et al. 2004). The authors found that amphetamine did not significantly alter neural activation to reward receipt compared to placebo (Knutson et al. 2004), although the sample size of this study was small, which may have contributed to the null findings. In the second study, 22 male volunteers received 4 doses of 20 mg dexamphetamine or placebo. Participants received the first 3 doses within 48-hours without imaging, and the 4th dose after a two-week washout period. After the 4th administration of drug or placebo, subjects completed a rewarded gambling task during a fMRI scan (O'Daly et al. 2014). Participants who received 4 doses of dexamphetamine exhibited less BOLD activation during reward receipt in the bilateral amygdala compared to those who received placebo on all sessions (O'Daly et al. 2014). The design of the study does not separate effects of the single dose on the day of the scan from the consequences of the 3 previous doses. Further, the study included a relatively small sample of males (only) and used a between-subject design, limiting the generalizability of the findings.
The goal of the current study was to examine the effects of an acute dose of MA on neural response to receipt of monetary reward in healthy young adults using a within-subject, randomized, cross-over, double-blind, placebo-controlled design. Participants completed the Doors monetary reward task (Carlson et al. 2011; Foti and Hajcak 2009; Hajcak et al. 2006) during fMRI. This task captures neural activation reward and loss, but does not include neutral trials. Thus the primary outcome measure, Win vs Loss, does not distinguish between activations during reward or loss separately. We hypothesized that MA would increase mesolimbic neural activation to reward compared to loss, relative to placebo.
Materials and Methods
Design
In a within-subject, randomized, cross-over, double-blind, placebo-controlled design, healthy adults completed fMRI approximately 70 minutes after ingestion of MA (20 mg oral) or placebo. During the scan they completed a monetary reward task, and the primary outcome measure was brain activation during receipt of reward (Win vs. Loss). As part of a larger study, participants also completed two drug administration visits, receiving 1 dose of MA (20 mg oral) and placebo, on average ten days (range 3-35 days) prior to their fMRI drug administration sessions—data from these sessions is not included in the current study. The Institutional Review Board at University of Chicago (UC) approved the study and written informed consent was obtained.
Participants
Participants were right-handed, healthy young adults aged 18-35 who reported occasional nonmedical substance use and were recruited from nearby college campuses and surrounding communities through online and printed advertisements. Of the 50 individuals who completed both drug administration sessions with Doors fMRI data, three participants were excluded for poor task engagement (as described below) and six participants were excluded from analyses for significant movement (as described below). This left a final sample size of 41 (see Table 1). Inclusion criteria included body mass index between 19-26, normal electrocardiogram, at least a high school education, and English fluency. Exclusion criteria were 1) history of psychosis, severe post-traumatic stress disorder (PTSD), or major depressive disorder (MDD); 2) current SUD; 3) serious medical conditions; 4) night shift work; 5) pregnant, lactating, or planning to become pregnant in the next 3 months (for females); 6) heavy alcohol or caffeine use (>4 drinks/day); 7) medication use (other than birth control); and 8) contraindication for fMRI. Females not on hormonal contraception completed drug administration sessions in the follicular phase of their menstrual cycle (White et al. 2002).
Table 1.
Participant Characteristics
| Healthy Young Adults (N = 41) |
|
|---|---|
| Age | 24.8 (4.7) |
| Sex (% Female) | 51% |
| Ethnicity (% Hispanic) | 22% |
| Race | |
| % Caucasian | 65% |
| % More than 1 Race | 15% |
| % Black | 5% |
| % Asian | 15% |
| BMI | 23.9 (2.6) |
| Current substance use | |
| Alcohol (drinks/week) | 1.4 (1.1) |
| Cannabis (times/month) | 4.5 (7.5) |
| Caffeine (cups/day) | 1.1 (1.0) |
| % Used cigarettes (cigarettes/day) | 5% (n= 2, 0.1 ± 0.8) |
| Lifetime drug use (at least once) | |
| % Used stimulants (# times used) | 27% (n= 11, 2.5 ± 3.1) |
Note. All values are mean (SD) or percentage
Measures
Drug Effects Questionnaire (DEQ; Johanson and Uhlenhuth 1980)
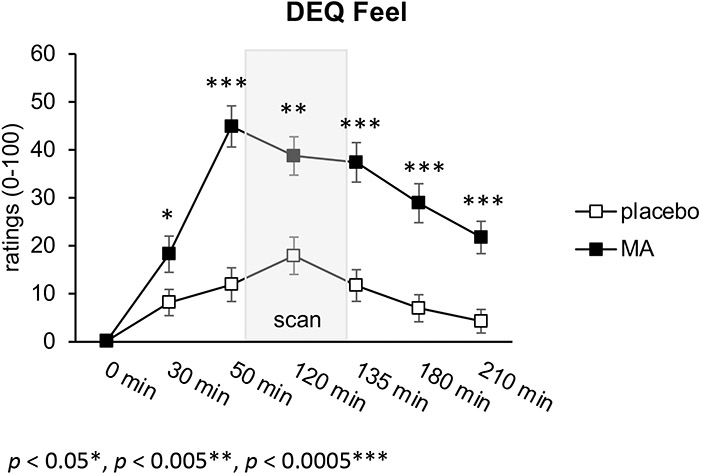
To assess subjective drug responses, participants completed the DEQ throughout the drug administration visit, including at baseline (0 minutes; prior to drug administration), 30 minutes, 50 minutes (prior to the fMRI scan), 120 minutes (during the fMRI scan), 135 minutes (after the fMRI scan), 180 minutes, and 210 minutes post-drug administration. As part of the DEQ, participants were asked to rate their response to the question “Do you feel any drug effects?” (rated from “none at all”=0 to “a lot”=100 on an analog scale). MA significantly increased ratings of feeling drug effects compared to placebo: drug x time interaction (F(6,228)= 9.0, p< 0.001), at 30, 50, 120, 135, 180, and 210 min (see Figure 1).
Figure 1.
Subjective Drug Response Over Time. MA significantly increased ratings of feeling drug effects compared to placebo: drug x time interaction (F(6,228)= 9.0, p< 0.001), at 30, 50, 120, 135, 180, and 210 min.
Addiction Research Center Inventory (ARCI; Martin et al. 1971)
To assess subjective response to MA, participants completed the Addiction Research Center Inventory (ARCI; Martin et al, 1971) at baseline and five subsequent timepoints. The Morphine–Benzedrine Group (MBG, euphoric effects) scale was used, as this scale has been shown to represent the positive, rewarding effects of amphetamine (e.g., de Wit and Phillips 2012; Fischman and Foltin 1991). MA significantly increased ratings of euphoria compared to placebo: drug x time interaction (F(5,200)=12.2, p<0.0001), at 30, 50, 135, 180, and 210 min.
Doors Task
During the scan participants completed a reward-guessing game, the ‘Doors’ task which provides an index of reactivity to monetary rewards and losses. Participants were told that behind one of the doors there was a monetary prize of $0.50 (‘↑’) while behind the other door there was a loss of $0.25 (‘↓’) and that they should use a button box to choose one of the two doors to either win or lose money for each trial (Supplemental Fig. 1). They were told they had a chance of winning between $0 and $15.00 at the end of the task depending on their performance. However, unbeknownst to participants, the task was rigged, so task behavior had no impact on actual outcomes. The task consisted of 30 predetermined Wins and 30 Losses presented in a pseudorandom order over two runs. The task lasted for 15-min and is based on a task used in previous studies (Carlson et al. 2011; Foti and Hajcak 2009; Hajcak et al. 2006). To ensure that participants were engaged in the task, we examined responses to win and loss trials and excluded three participants who responded to <75% of trials across the two runs. The contrast of interest in the imaging data was Win vs. Loss during the feedback phase. During the placebo condition, the task robustly activated brain reward circuitry during Win vs. Loss, including the striatum (see Supplemental Fig. 2). This contrast between Win and Loss has been shown to be more a sensitive indicator of neural activation during reward processing than examining Win or Loss individually (Carlson et al. 2011; Forbes et al. 2014; Foti et al. 2011; Proudfit 2015) and striatal activation has been shown to increase during Win events and decrease during Loss events (Delgado et al. 2000). It is important to note that the task does not provide a measure of ‘neutral’ feedback.
Study Procedure
Participants completed an initial screening and orientation visit during which they provided informed consent and were familiarized with laboratory procedures and study protocol. Eligible participants then completed two drug administration sessions with fMRI visits in a within-subject, randomized, double-blind, placebo-controlled design. Participants were asked to abstain from drugs for at least 48 hours, and alcohol, for 24 hours prior to each visit, which was verified by self-report, breath alcohol, and urine screens.
During the two drug administration sessions participants received MA (20 mg) and placebo in alternating order. Sessions took place from 09:00 to 13:00 hours in comfortable, living-room-like rooms, and were separated by at least 72 h. Participants were tested individually. Participants were instructed not to eat for at least eight hours before each session and were given a light snack after drug administration. To minimize drug expectancies they were told they could receive one of the following: stimulant, sedative, or placebo. On each session subjects first completed pre-drug DEQ, and had blood pressure and heart rate measured. At 09:30 hours, MA (20 mg oral; Desoxyn tablets with dextrose filler in size 00 opaque capsules) or placebo (dextrose only) was administered under double blind conditions. Participants completed the DEQ at six timepoints following capsule administration. Approximately 70 minutes after drug administration, participants completed the Doors task during the fMRI scan. Sessions ended at 13:00 hours, after confirmation that blood pressure and heart rate had returned to within twenty percent of baseline. After completing all sessions, participants were debriefed and compensated for their participation.
fMRI Data Acquisition
Functional MRI data was collected using a 3T Philips Achieva scanner with a 32-channel headcoil and a gradient-echo echo-planar imaging sequence with the following acquisition parameters: TR=2000; TE=28; 39 3mm thick axial slices aligned to the AC-PC line, 0.6mm slice gap; 20×20cm FOV; SENSE factor = 2, Flip angle = 77°. Four images were acquired and discarded just prior to task start. A high resolution T1-weighted image (MPRAGE sequence) was also acquired to assess for incidental findings, and for alignment and spatial standardization of the functional data. Data was collected with reversed phase-encode blips, resulting in pairs of images with distortions going in opposite directions. From these pairs the susceptibility-induced off-resonance field was estimated using a method similar to that described in (Andersson et al. 2003), as implemented in FSL (Smith et al. 2004) and the two images were combined into a single corrected one. Participant head motion was minimized with foam packing around the head. Stimuli were viewed via projection onto a mirror mounted on the headcoil.
fMRI Data Analyses
Motion outliers were identified based on framewise displacement (FD; >0.5 mm) using FSL’s motion outlier tool and then censored from analyses. In addition, imaging data were inspected and individuals with >2mm displacement in any direction for at least one of the two fMRI sessions were not included in the analysis (n=6). The remaining subjects met criteria for high quality and scan stability. There were no significant drug condition difference in peak movement (PBO: M= 0.68 mm, SD= 0.68 mm; MA: M= 0.52 mm, SD= 0.58 mm), mean movement, or variability (p-values>.05).
Preprocessing of fMRI data was conducted using Statistical Parametric Mapping software (SPM12, Wellcome Department of Imaging Neuro-Science, London, UK). Images were spatially realigned, slice-time corrected, warped to Montreal Neurological Institute (MNI) space using the participant’s mean functional image, resampled to 2 mm3 voxels, and smoothed (5 mm3 kernel). The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Condition effects were modeled with event-related regressors representing the occurrence of receipt for Win or Loss. Effects were estimated at each voxel, and for each subject. Individual contrast maps for Win trials (vs. Loss trials) were created for each person. Individual motion parameter files were included in the first levels models as regressors-of-no-interest.
To test our hypotheses, contrast maps for Win vs. Loss were entered into a paired t-test in SPM. To confirm that the task successfully activated reward-related regions during Win vs. Loss trials, we examined task activation during the placebo session. Reward receipt relative to loss (Win vs. Loss) significantly activated a large contiguous cluster of frontal and mesolimbic reward regions, including bilateral nucleus accumbens (NAcc), caudate, and putamen (see Supplemental Figure 2). For the primary a priori ROI analysis, we identified bilateral striatal anatomical regions (NAcc, caudate, putamen) defined via the AAL3 atlas (Rolls et al. 2020). The parameter estimates/β-weights were extracted for each participant for each session (PBO, MA) from ROIs representing BOLD signal response (parameter estimates, arbitrary units [a.u.]) averaged across all voxels within each anatomical region during Win vs. Loss. Then, repeated-measure one-way (drug) ANOVAs were conducted with the extracted bilateral ROI BOLD activity as the dependent variables (SPSS version 28; SPSS Inc, Chicago IL) and age as a covariate.
For exploratory whole-brain analyses, neural activity from the paired t-test second level model with the Win vs. Loss contrast was considered significant if it exceeded cluster-based significance thresholding adjustment for multiple comparisons across a functional reward uniformity mask downloaded from Neurosynth in June 2022 from a meta-analysis of 922 studies by searching for the term “reward” (https://neurosynth.org/analyses/terms/reward/), as our hypotheses were specific to reward-related regions. Based on simulations performed using 3dFWHMx and 3dClustSim with the auto-correlation function (acf) (10,000 iterations; updated and ‘bug-free’ on June 2022; [https://afni-nimh-nihgov.proxy.cc.uic.edu/pub/dist/doc/program_help/3dClustSim.html]; Cox, 1996), correction at α < 0.05 is achieved with a voxel threshold of p < 0.001 and a cluster size of at least 34 contiguous voxels (volume= 272 mm3).
Results
Effect of MA on Striatal ROIs During Reward
Participants demonstrated less NAcc BOLD activation during Win vs. Loss after MA compared to placebo, F(1,40)= 8.10, p= .007 (see Figure 2A). Age was not a significant covariate (p= .93). The results remained significant after controlling for lifetime frequency of recreational and prescription stimulant use (n=11), average servings of caffeine per day during the past month, average number of cigarettes per day during the past month (n=2), frequency of past month alcohol use, and frequency of past month cannabis use, F(1,36)= 4.38, p= .04 (all substance use covariates in this model were non-significant, p-values >.05). The drug did not significantly alter caudate or putamen BOLD activation during Win vs. Loss (p-values > .05).
Figure 2.
Drug Condition Differences in Region-of-Interest (ROI) BOLD Striatal Reward Reactivity (Win vs. Loss). Panel A shows less NAcc extracted response (in β-weights/parameter estimates of activation) during Win vs. Loss to MA compared to placebo (p< 0.007). Panel B demonstrates caudate extracted response (in β-weights/parameter estimates of activation) during Win vs. Loss to MA compared to placebo (ns). Panel C shows putamen extracted response (in β-weights/parameter estimates of activation) during Win vs. Loss to MA compared to placebo (ns).
There were no significant main effects of sex or interaction of drug and sex for NAcc or caudate BOLD activation (p-values > .05). There was a significant main effect of sex for putamen BOLD activation, such that females had greater putamen BOLD activation than males (F(1,39)= 5.88, p= .02, but no significant interaction of sex and drug on putamen BOLD activation, F(1,39)= 1.61, p= .11.
Exploratory Whole Brain Analyses
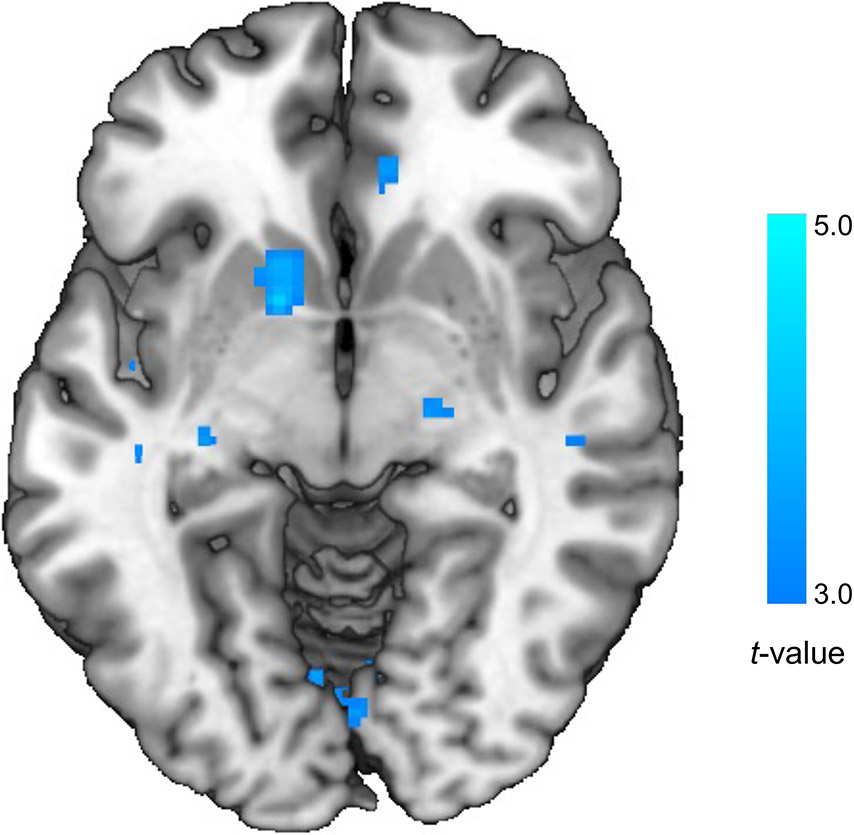
Exploratory whole brain analysis revealed that MA decreased BOLD activation during Win vs. Loss in the striatum, including the NAcc, caudate, and putamen, compared to placebo (MNI peak [−14, 8, −6], k= 36 voxels, Z= 3.86, p < 0.05, corrected; see Figure 3).
Figure 3.
Exploratory Whole Brain Analysis: MA Decreased Striatal BOLD Activation Compared to Placebo. Exploratory whole brain analysis revealed that MA decreased BOLD activation during Win vs. Loss in the striatum, including the NAcc, caudate, and putamen, compared to placebo (MNI peak [−14, 8, −6], k= 36 voxels, Z= 3.86, p < 0.05, corrected).
Exploratory Analyses of Win Vs. Loss Events
To better understand whether Win events or Loss events contributed to the drug effect during Win vs. Loss receipt, we examined Win and Loss trials relative to a Fixation period (Win vs. Fixation and Loss vs. Fixation contrasts). The parameter estimates/β-weights were extracted for each participant for each session (PBO, MA) from ROIs representing BOLD signal response (parameter estimates, arbitrary units [a.u.]) averaged across all voxels within each anatomical region during Win vs. Fixation and Loss vs. Fixation contrasts. Then, repeated-measure one-way (drug) ANOVAs were conducted with the extracted bilateral ROI BOLD activity as the dependent variables (SPSS version 28; SPSS Inc, Chicago IL). Exploratory analyses found that participants demonstrated less putamen BOLD activation during Win vs. Fixation to MA compared to placebo, F(1,40)= 14.22, p< .001 (see Figure 4C). The drug did not significantly alter NAcc or caudate BOLD activation during Win vs. Fixation (p-values >.05; see Figure 4).
Figure 4.
Exploratory Region-of-Interest (ROI) BOLD Analyses of Win vs. Loss Events. Panel A shows NAcc extracted response (in β-weights/parameter estimates of activation) during Win vs. Fixation to MA compared to placebo (ns). Panel B demonstrates caudate extracted response (in β-weights/parameter estimates of activation) during Win vs. Fixation to MA compared to placebo (ns). Panel C shows less putamen extracted response (in β-weights/parameter estimates of activation) during Win vs. Fixation to MA compared to placebo (p< .001). Panel D shows greater NAcc extracted response (in β-weights/parameter estimates of activation) during Loss vs. Fixation to MA compared to placebo (p= .02). Panel E demonstrates caudate extracted response (in β-weights/parameter estimates of activation) during Loss vs. Fixation to MA compared to placebo (ns). Panel F shows less putamen extracted response (in β-weights/parameter estimates of activation) during Loss vs. Fixation to MA compared to placebo (p= .04). Note: the activation for win and loss events is negative- this is likely due to the fact that win and loss condition are contrasted with a non-task condition and does not necessarily mean that “true” striatal activation decreased during win and loss events.
In addition, exploratory analyses revealed that participants demonstrated greater NAcc BOLD activation during Loss vs. Fixation to MA compared to placebo, F(1,40)= 6.17, p= .02 (see Figure 4D). However, participants demonstrated less putamen BOLD activation during Loss vs. Fixation to MA compared to placebo, F(1,40)= 4.59, p= .04 (see Figure 4F). The drug did not significantly alter caudate BOLD activation during Loss vs. Fixation (p= .24).
There were no significant main effects of sex or interaction of drug and sex for NAcc, putamen, or caudate BOLD activation during Win vs. Fixation (p-values > .05). There were no significant main effects of sex or interaction of drug and sex for NAcc BOLD activation during Loss vs. Fixation (p-values > .05). There was a significant main effect of sex for putamen BOLD activation, such that females had less putamen BOLD activation than males (F(1,39)= 6.99, p= .01), but no significant interaction of sex and drug on putamen BOLD activation, F(1,39)= 0.04, p= .84. There was also a significant main effect of sex for caudate BOLD activation during Loss vs. Fixation, such that females had less caudate BOLD activation than males (F(1,39)= 6.65, p= .01), but no significant interaction of sex and drug on caudate BOLD activation, F(1,39)= 0.04, p= .85.
Exploratory Analyses of Relationships with Subjective Euphoric Response
The peak change difference scores for ARCI-MBG (average MA peak change score minus average placebo peak change score; Mayo and de Wit 2015) were used for analyses. Striatal Win vs. Loss ROIs, Win vs. Fixation ROIs, or Loss vs. Fixation ROIs were not correlated with subjective euphoric response (ARCI-MBG) to MA (vs. placebo) (p-values >.05).
Discussion
This is one of the first studies to examine the effects of an acute dose of MA on neural response to receipt of monetary reward in healthy young adults. We found that MA resulted in less NAcc BOLD activation during reward vs loss receipt compared to placebo, even after controlling for lifetime frequency of recreational and prescription stimulant use and recent substance use. The drug did not significantly alter caudate or putamen ROI BOLD activation during reward vs loss receipt. Exploratory whole brain analysis revealed that MA decreased BOLD activation during reward vs loss receipt in the striatum, including regions in the NAcc, caudate, and putamen, compared to placebo. It is important to note that regions of the caudate and putamen were significant in whole brain analysis, but did not reach statistical significance in ROI analyses (although they showed a similar pattern as the NAcc findings). Additional exploratory analyses to examine if our findings were driven by neural response to win or to loss suggested that our NAcc ROI findings were driven by increased neural activation during loss trials after MA. We also found preliminary evidence that MA decreased putamen neural activation during win and loss trials. We found no effect of MA on caudate neural activation during win and loss trials, and neural responses to reward or loss were unrelated to subjective feelings of euphoria. Together, our findings suggest that stimulant drugs like MA that are known to activate brain reward circuitry may acutely increase neural response to the receipt of non-drug, monetary loss in the NAcc. However, MA may decrease neural activation to reward and loss in other striatal regions like the putamen.
These findings provide novel information about how stimulant drugs like MA acutely alter immediate neural response to non-drug reward and loss. Previous studies have found that stimulant drugs like MA activate dopaminergic mesolimbic circuitry (Abi-Dargham et al. 2003; Drevets et al. 2001; Leyton et al. 2002; Martinez et al. 2003; Oswald et al. 2005; Volkow et al. 2002). In addition, receipt of non-drug rewards and loss activate mesolimbic circuitry, suggesting that drug rewards and non-drug rewards activate similar neural circuits (Haber and Knutson 2010). Importantly, both drug-related and non-drug reward activation of mesolimbic brain reward circuitry are linked to the development of SUD. Contrary to our hypothesis, we found MA resulted in decreased mesolimbic neural response to the receipt of non-drug, monetary rewards relative to loss. Exploratory analyses suggested that increased NAcc neural response to loss, but decreased putamen neural response to reward and loss. It is possible that amphetamine selectively enhances affective responses to loss stimuli, and therefore increases activity in the NAcc. Using the Monetary Incentive Delay (MID) task, Knutson et al (2004) also found that dextroamphetamine increased ventral striatal activation during anticipation of losses but not gains, and this effect was associated with increased positive arousal ratings for loss incentive cues (Knutson et al. 2004). Because the Doors task does not provide a measure of ‘neutral’ feedback, we conducted an exploratory analysis comparing win and loss trials to a fixation period. However, these exploratory analyses comparing task activation to non-task activation should be interpreted with caution. It is also possible that MA dampened the neural signal associated with reward and loss in the putamen, perhaps due to an increase in basal dopaminergic tone in the putamen. Thus, the increased tone may have reduced response to the transient external signal of reward receipt. It is not clear what might be contributing to decreased putamen neural activation to loss. Future studies will need to replicate these findings using a task that can better separate neural activation to reward and loss by comparing these events to a neutral event (e.g., MID task) to inform whether MA increases neural activation to loss or decreases neural activation to reward (or both) across striatal regions and examine the mechanisms underlying these BOLD responses during MA intoxication.
To our knowledge, this is the first study with a relatively large sample size to examine how a single dose of a stimulant drug impacts neural response to a non-drug reward and loss. As noted in the introduction, Knutson et al (2004) found no significant effect of a single dose of amphetamine (0.25 mg/kg) compared to placebo on neural reward receipt among 8 healthy adult volunteers. In addition, O’Daly et al (2014) reported that d-amphetamine (20 mg, after 3 earlier doses) reduced amygdala BOLD activation during receipt of reward although it did not alter striatal BOLD activation. It is notable that the stimulant drug in O’Daly et al. (2014) and the present study dampened neural response to reward, albeit in different regions than the putamen.
It is important to note that females in our study exhibited greater putamen activation to reward vs. loss than males, but this was not related to drug effects. In addition, exploratory analyses suggested that this effect was driven by females demonstrating less activation to loss events (vs. fixation). Previous studies have found no sex differences in neural response to reward or loss receipt using a the MID task (Brislin et al. 2022; Cao et al. 2019) and positron emission tomography (PET) studies find no sex differences in striatal dopamine D2/3 receptor availability or density among healthy adults (Woodcock et al. 2020). However, a recent PET study found females have greater NAcc dopamine release to methylphenidate than males (a similar, non-significant pattern was seen in the dorsal striatum) (Manza et al. 2022). Participants in the aforementioned PET study did not complete a reward task and completed PET, not fMRI, which may in part explain why we found sex differences in a different region. Future studies will examine this question with larger sample sizes to better understand potential sex differences, especially in how stimulant drugs impact neural reward and loss processing.
The current study had both strengths and limitations. It included a relatively large sample of males and females, and a within-subject design. However, the analysis fMRI BOLD activation during a task does not allow us to determine if the reduction in reward vs loss signal was related to increased MA-induced dopaminergic mesolimbic tone. Future preclinical studies or studies combining fMRI with PET or single-photon emission computerized tomography (SPECT) studies could examine the mechanisms contributing to our findings. Another limitation was that the Doors task only captures one aspect of reward processing, reward vs loss receipt. Ongoing studies will examine if stimulant drugs acutely impact responses to reward vs loss separately, or other aspects of reward processing, including reward anticipation vs receipt. In addition, future studies with larger sample sizes will examine how neural responses to reward may be related to individual differences in subjective response to MA. Finally, our study included a homogeneous sample of healthy young adults, most of whom had never used stimulant drugs before participating in the study. Whereas the homogeneity of the sample increased power by minimizing variability, it also limits generalizability to a more heterogeneous population. It remains to be determined whether these findings will hold true with other samples, such as individuals with psychiatric conditions, or chronic stimulant or other substance use.
In conclusion, our findings provide novel preliminary evidence that MA, which activates brain reward circuitry, increased NAcc response to loss, but did not affect response to wins. On the other hand, MA may decrease putamen response to reward and to loss. The findings add to a growing literature suggesting that stimulants not only produce direct feelings of wellbeing, but also alter mesolimbic circuitry with non-drug rewards and losses, using monetary reward tasks. Either of these effects could contribute to the abuse liability of stimulant drugs. Additional studies are needed to replicate our findings and clarify the mechanisms contributing to altered mesolimbic neural response to reward and loss receipt during stimulant intoxication.
Supplementary Material
Acknowledgments
This publication was funded by the National Institute on Drug Abuse (NIDA) (R01DA002812, PI: HdW). NAC was supported by NIDA (K23DA048132, PI: NAC). HM was supported by the National Insitute of General Medical Sciences (NIGMS) (T32GM07019). The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of NIDA, NIGMS, or the National Institutes of Health.
Footnotes
Dr. de Wit has served on Scientific Advisory committees for Gilgamesh Pharmaceuticals, Awakn Life Sciences and MIND Foundation, and she is on the Board of Directors of PharmAla Biotech. None of these were related to the research reported here. Drs. Crane and Molla declare no conflicts of interest.
References
- Abi-Dargham A, Kegeles LS, Martinez D, Innis RB, Laruelle M (2003) Dopamine mediation of positive reinforcing effects of amphetamine in stimulant naive healthy volunteers: results from a large cohort. Eur Neuropsychopharmacol 13: 459–68. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20: 870–88. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW (2010) Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. Journal of child psychology and psychiatry, and allied disciplines 51: 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brislin SJ, Weigard AS, Hardee JE, Cope LM, Martz ME, Zucker RA, Heitzeg MM (2022) Sex Moderates Reward- and Loss-Related Neural Correlates of Triarchic-Model Traits and Antisocial Behavior. Clin Psychol Sci 10: 700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Bennett M, Orr C, Icke I, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, Buchel C, Quinlan EB, Desrivieres S, Flor H, Frouin V, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot JL, Nees F, Orfanos DP, Paus T, Poustka L, Hohmann S, Frohner JH, Smolka MN, Walter H, Schumann G, Whelan R, Consortium I (2019) Mapping adolescent reward anticipation, receipt, and prediction error during the monetary incentive delay task. Hum Brain Mapp 40: 262–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G (2011) Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage 57: 1608–16. [DOI] [PubMed] [Google Scholar]
- Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, Phan KL (2018) Neural activation to monetary reward is associated with amphetamine reward sensitivity. Neuropsychopharmacology 43: 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ (2012) Do initial responses to drugs predict future use or abuse? Neuroscience and biobehavioral reviews 36: 1565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA (2000) Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84: 3072–7. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA (2001) Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological psychiatry 49: 81–96. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW (1991) Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict 86: 1563–70. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R (2014) Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS One 9: e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G (2009) Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological psychology 81: 1–8. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G (2011) Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp 32: 2207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin AA, Kim JH (2021) Age of Onset and Its Related Factors in Cocaine or Methamphetamine Use in Adults from the United States: Results from NHANES 2005-2018. Int J Environ Res Public Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF (2006) The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological psychology 71: 148–54. [DOI] [PubMed] [Google Scholar]
- Han B, Compton WM, Jones CM, Einstein EB, Volkow ND (2021) Methamphetamine Use, Methamphetamine Use Disorder, and Associated Overdose Deaths Among US Adults. JAMA Psychiatry 78: 1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, Hardee JE (2015) Neuroimaging Risk Markers for Substance Abuse: Recent Findings on Inhibitory Control and Reward System Functioning. Curr Addict Rep 2: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Liu X, Shulz K, Fan J, London E, Friston K, Halperin JM, Newcorn JH (2012) Parental substance abuse and function of the motivation and behavioral inhibition systems in drug-naive youth. Psychiatry research 201: 128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH (1980) Drug preference and mood in humans: d-amphetamine. Psychopharmacology 71: 275–9. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bjork JM, Fong GW, Hommer D, Mattay VS, Weinberger DR (2004) Amphetamine modulates human incentive processing. Neuron 43: 261–9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3: 760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Kling LR, Crane NA, Gorka SM, Nusslock R, Damme KSF, Weafer J, de Wit H, Phan KL (2020) Anticipation of monetary reward in amygdala, insula, caudate are predictors of pleasure sensitivity to d-Amphetamine administration. Drug and alcohol dependence 206: 107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A (2002) Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology 27: 1027–35. [DOI] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kuhn S, Machielse MW, Sescousse G (2017) Disruption of Reward Processing in Addiction : An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry. [DOI] [PubMed] [Google Scholar]
- Manza P, Shokri-Kojori E, Wiers CE, Kroll D, Feldman D, McPherson K, Biesecker E, Dennis E, Johnson A, Kelleher A, Qu S, Tomasi D, Wang GJ, Volkow ND (2022) Sex differences in methylphenidate-induced dopamine increases in ventral striatum. Mol Psychiatry 27: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BD, Galea S, Wood E, Kerr T (2011) Injection methamphetamine use is associated with an increased risk of attempted suicide: a prospective cohort study. Drug and alcohol dependence 119: 134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR (1971) Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12: 245–58. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M (2003) Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23: 285–300. [DOI] [PubMed] [Google Scholar]
- Mayo LM, de Wit H (2015) Acquisition of responses to a methamphetamine-associated cue in healthy humans: self-report, behavioral, and psychophysiological measures. Neuropsychopharmacology 40: 1734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EM, Shankar MU, Knutson B, McClure SM (2014) Dissociating motivation from reward in human striatal activity. J Cogn Neurosci 26: 1075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA (2017) Trends & Statistics [Online]. Available: https://www.drugabuse.gov/related-topics/trends-statistics
- NIDA (2019) Methamphetamine Research Report [Online]. Available: https://nida.nih.gov/publications/research-reports/methamphetamine/what-are-long-term-effects-methamphetamine-misuse.
- O'Daly OG, Joyce D, Tracy DK, Azim A, Stephan KE, Murray RM, Shergill SS (2014) Amphetamine sensitization alters reward processing in the human striatum and amygdala. PLoS One 9: e93955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS (2005) Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology 30: 821–32. [DOI] [PubMed] [Google Scholar]
- Proudfit GH (2015) The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology 52: 449–59. [DOI] [PubMed] [Google Scholar]
- Radoman M, Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, Phan KL (2021) Striatal activation to monetary reward is associated with alcohol reward sensitivity. Neuropsychopharmacology 46: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD (2004) Addiction as a computational process gone awry. Science 306: 1944–7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ (1989) Limbic-striatal interactions in reward-related processes. Neuroscience and biobehavioral reviews 13: 155–62. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Huang CC, Lin CP, Feng J, Joliot M (2020) Automated anatomical labelling atlas 3. Neuroimage 206: 116189. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1: S208–19. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S (2014) Brain reward region responsivity of adolescents with and without parental substance use disorders. Psychology of addictive behaviors 28: 805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS (2013) Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biological psychiatry 73: 869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ (2002) Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol 12: 557–66. [DOI] [PubMed] [Google Scholar]
- Webber HE, Lopez-Gamundi P, Stamatovich SN, de Wit H, Wardle MC (2021) Using pharmacological manipulations to study the role of dopamine in human reward functioning: A review of studies in healthy adults. Neuroscience and biobehavioral reviews 120: 123–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H (2002) Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacology, biochemistry, and behavior 73: 729–41. [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Zakiniaeiz Y, Morris ED, Cosgrove KP (2020) Sex and the dopaminergic system: Insights from addiction studies. Handb Clin Neurol 175: 141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.