Abstract
Vitamin D is one significant prohormone substance in human organ systems. It is a steroidal hormone produced in the skin upon exposure to UVB rays. This paper presents a systematic review of the utilization of topical vitamin D, specifically cholecalciferol, calcipotriol, and tacalcitol, in the treatment of vitiligo. It considers the role of vitamin D in stimulating the synthesis of melanin and melanogenesis, which can help with the process of repigmentation. The inclusion of calcipotriol or tacalcitol in Narrowband Ultraviolet Phototherapy (NB-UVB) has shown the potential to enhance therapeutic outcomes for vitiligo. However, their effectiveness in combination with Psoralens Long Wave Ultraviolet Radiation (PUVA) and Monochromatic Excimer Light (MEL) treatment for vitiligo is limited. In contrast, combining topical corticosteroids with vitamin D analogues has demonstrated superior efficacy in treating vitiligo compared to using vitamin D analogues alone, while also providing the added benefit of reducing corticosteroid-related adverse effects. In addition, treating stable vitiligo with topical cholecalciferol and microneedling has shown success. Future studies are needed to ascertain an efficient method of administering vitamin D topically as an anti-vitiligo agent.
Keywords: vitiligo, analogue, repigmentation, microneedling, single-blinded
1. Introduction
Vitamin D is an essential fat-soluble steroidal hormone that can either be obtained from dietary sources, such as mushrooms or fish, or synthesized within the epidermal skin layers upon exposure to UVB rays [1,2]. Epidemiological research indicates that a significant proportion of the world’s population lacks adequate levels of vitamin D3, the active form of vitamin D [3,4]. Vitamin D deficiency affects over a billion individuals worldwide, making it increasingly prevalent [5,6,7]. Low blood vitamin D levels have been associated with an elevated risk of chronic diseases including cancer, hypertension, inflammation, and diabetes [8,9]. Furthermore, children born to pregnant women with low vitamin D levels have been found to exhibit autistic symptoms at the age of six, highlighting the potential benefit of vitamin D supplementation during pregnancy as a fundamental solution to this issue [10,11].
Vitiligo, an autoimmune pigmentary disorder characterized by the loss of functional melanocytes in the epidermis and infundibulum of the hair, manifests as clearly defined depigmented patches or macules [12,13]. Research [14,15] has shown that vitiligo impacts individuals of all skin colors and genders. Clinically, vitiligo can present in three different types: (a) unclassified vitiligo, which affects the majority or entirety of the body surface area, (b) segmental vitiligo, characterized by lesions following a dermatomal pattern, and (c) generalized vitiligo, the most common type, which displays bilateral and symmetrical distribution of the lesions [16,17,18]. Notably, individuals with vitiligo and other autoimmune diseases often exhibit low levels of vitamin D [19,20]. Vitamin D exerts a significant influence on the activity of keratinocytes and melanocytes through various mechanisms [21,22]. Firstly, it promotes the differentiation and maturation of keratinocytes, leading to the development of a well-structured epidermal barrier. This helps in maintaining the integrity of the skin and facilitates the repigmentation process in vitiligo [23]. Additionally, vitamin D has immunomodulatory effects, suppressing excessive immune responses that can contribute to melanocyte destruction in vitiligo. Furthermore, it enhances the production of melanin pigment within melanocytes, aiding in the repigmentation of depigmented skin patches [14]. Vitamin D also influences the release of various growth factors and cytokines that promote the survival and proliferation of melanocytes [24]. Elevated levels of thioredoxin have been associated with abnormal calcium uptake in the keratinocytes and melanocytes of vitiligo-affected skin, which can potentially inhibit melanogenesis by reducing tyrosinase activity [25,26,27]. While it is crucial for many people to consume foods fortified with vitamin D and obtaining a little sun exposure is important for establishing a healthy vitamin D level, certain populations, such as the elderly, obese individuals, dark-skinned populations, and breastfed newborns, may require dietary supplements to meet their vitamin D requirements [28,29,30]. Vitamin D and its metabolites play a vital role in various physiological functions [21]. For instance, they are essential in maintaining calcium homeostasis, promoting bone mineralization, and as a treatment option for a range of skin disorders, including psoriasis and vitiligo [8,14].
Several studies [14,31,32] have shown the therapeutic benefits of vitamin D supplementation in various experimental animal models, including those for allergic encephalomyelitis, collagen-induced arthritis, type 1 diabetes, inflammatory bowel disease, autoimmune thyroiditis, and systemic lupus erythematosus. Consequently, vitamin D supplements represent a promising treatment option for autoimmune conditions such as vitiligo [12]. Treatments for vitiligo usually include phototherapy as well as topical and oral immunomodulators such as corticosteroids and calcineurin inhibitors. The first-line therapies for vitiligo include topical corticosteroids (TCS) of moderate to high potency and calcineurin inhibitors (TCI), both of which suppress the cellular immune response [33,34]. Topical corticosteroids, commonly used for vitiligo, can lead to skin thinning and atrophy, especially with prolonged use. Some topical treatments, including calcineurin inhibitors, may cause skin irritation, burning, or itching, which can be uncomfortable for the patient. While phototherapy is effective, it may cause side effects such as redness, itching, and dry skin. Narrow-band UVB treatment, while safer than PUVA, can still lead to phototoxic reactions in some individuals [12,35]. These strategies have some efficacy in inducing repigmentation, although they do have certain drawbacks. This makes vitamin D therapy an alternative option for treating vitiligo.
The vitamin D family encompasses five molecules, with the two most prominent ones being vitamins D3 (cholecalciferol) and D2 (ergocalciferol) (Figure 1a and Figure 1b, respectively). Although both have beneficial effects on human health, they are obtained through different sources. Vitamin D3 is acquired from animal sources, such as fatty fish, liver, and eggs, whereas dietary vitamin D2 is commonly derived from plants, particularly mushrooms and yeast [36,37,38]. Both vitamin D2 and vitamin D3 exhibit the same affinity for the vitamin D receptor, indicating that neither form binds more strongly to the receptor [36,39]. Numerous studies [40,41] have demonstrated a significant difference in the effect on blood levels of circulating vitamin D between vitamin D2 and D3 supplementation. Vitamin D3 has been found to be more effective than vitamin D2 in increasing the body’s vitamin D and calcium levels, confirming that cholecalciferol supplementation is more efficient than ergocalciferol in improving vitamin D status [21]. Both vitamins D2 and D3 are inactive until they reach the liver, which modifies their chemical composition to create calcidiol, a molecule that the body uses to store vitamin D. In the kidneys, the active metabolite of the hormone, calcitriol (Figure 1c), is then formed from calcidiol (Figure 2) [36,42]. In dermatology, various derivatives (metabolites) of vitamin D, such as calcipotriol, calcitriol, tacalcitol, maxacalcitol, and hexafluoro-1,25 dihydroxy vitamin D3, are utilized [21,43]. Calcipotriol (Figure 1d), also known as calcipotriene, is commercially available under brand names, such as Dovonex® and Diavonex® (Leo Pharmaceutical Products, Ballerup, Denmark) It exhibits comparable efficacy to calcitriol due to its identical affinity for vitamin D3 receptors while causing less hypercalcemia than calcitriol [44,45]. Calcipotriol also exerts a significant impact on inflammatory mediators, the immune systems, and melanocytes, and it may enhance melanin formation by activating keratinocytes and melanocytes [46,47].
Figure 1.
Chemical structures for types of vitamin D and its analogous [1].
Figure 2.
The relation between vitamin D and melanin. Abbreviations: CYP2R1, Cytochrome P450 Family 2 Subfamily R Member 1; CYP27B1, Cytochrome P450 Family 27 Subfamily B Member 1; KITLG, KIT-ligand; SLC24A5, solute carrier family 24 member 5; MATP, Membrane-associated transporter protein; OCA2, Oculocutaneous albinism type 2.
Tacalcitol (Figure 1e) is a synthetic vitamin D3 analogue that is comparable to calcitriol in terms of its affinity for vitamin D receptors and subsequent effects [44,48]. In a prospective open-label, left-right trial, the efficacy of two different vitamin D3 equivalents, calcipotriol and tacalcitol, in combination with NB-UVB phototherapy, was evaluated in patients with chronic stable plaque psoriasis. Thirty patients received NB-UVB phototherapy three times/week, along with daily application of tacalcitol to the target lesion on the left side and twice-daily application of calcipotriol to the right side. Both vitamin D3 analogues were deemed safe and effective, but calcipotriol exhibited greater efficacy, better absorption, faster onset of action, and more consistent treatment response [49]. Maxacalcitol (Figure 1f) shares the same topical mechanism as other vitamin D3 analogues, but it was reported to be 10 times more effective than calcipotriol and tacalcitol in reducing keratinocyte proliferation while being 60 times less calcemic than calcitriol [50,51].
Formulations are developed for active compounds like calcipotriol and tacalcitol to address their solubility and partitioning and to ensure appropriate dose delivery [52]. These analogues undergo specific modifications to enhance their affinity for vitamin D receptors while minimizing systemic side effects. Delivery enhancers play a crucial role in improving the skin permeability, solubility, and partitioning of the drug [53]. These enhancers include compounds that promote stratum corneum hydration and chemical penetration enhancers [54]. Strategies such as complexation, microencapsulation, or inclusion into certain delivery systems may be employed to enhance the solubility, bioavailability, and skin penetration of these compounds [55,56,57,58]. Micronutrients are commonly delivered using enhancers such as gums, oral tablets, buccal sprays, creams, gels, and ointments [59,60]. When designing topical skin formulations, it is essential to consider factors such as dosage calculations, delivery vehicles, drug distribution, and absorption control [61,62]. In medical facilities, ointments containing activated vitamin D3 derivatives are frequently employed for the treatment of psoriasis vulgaris, ichthyosis, vitiligo, and palmoplantar keratosis [44,63].
2. Melanin and Vitamin D Relation
Previtamin D3 is synthesized by solar exposure in the stratum Malpighii, which is composed of the stratum Basale and stratum Spinosum of the epidermis [64]. This region is principally where the epidermal 7-dehydrocholesterol reservoir is located (Figure 2) [65]. This thermally unstable previtamin undergoes temperature-dependent isomerization over the course of three days after formation to become vitamin D3 [66]. Plasma vitamin D binding protein preferentially transports vitamin D3 from the skin into circulation [67]. Increases in latitude or skin melanin levels inhibit the synthesis of previtamin D3 in the epidermis. Following a single complete body exposure to three minimum erythemal doses of UV radiation, serum vitamin D3 levels increased by almost 10 times, and serum 25-hydroxyvitamin-D levels doubled [68]. The most common cell types that make up the epidermis are keratinocytes and melanocytes. Melanocytes produce the pigment melanin, the skin color that distinguishes racial groups, mostly determined by the amount of melanin in the epidermis [69]. Most melanocytes are in the stratum Basale, the deepest layer of the epidermis, where the tyrosinase enzyme uses tyrosine to produce melanin [70]. Additionally, the long cytoplasmic processes of the melanocyte cells convey the melanin-containing pigment granules to other neighboring epidermal cells that are ascending towards the surface; therefore, each of the five epidermal strata contains melanin. The unique mechanism for the epidermal synthesis, storage, and continuous release of vitamin D into circulation prompted research into the potential therapeutic benefits of using the skin as a site for synthesizing and absorbing vitamin D3 metabolites [22,71,72]. Multiple studies have repeatedly shown that vitamin D3 can accelerate repigmentation by increasing tyrosinase activity and melanogenesis [14,24,73]. Moreover, vitamin D protects the epidermal melanin layer and restores melanocyte stability, firstly by regulating melanocyte activation, proliferation, migration, and pigmentation pathways and secondly by moderating T cell activation, which is probably connected with melanocyte disappearance in vitiligo [14]. Because melanin, the pigment that gives skin its color, filters UV rays, it is recognized that patients with hypovitaminosis D are at risk for developing skin pigmentation [14,74].
The existing research on the utilization of topical vitamin D, whether as a monotherapy or in combination, for the treatment of vitiligo is currently limited and inadequate [75,76,77]. Consequently, a pivotal research inquiry arises: “What is the efficacy and comparative effectiveness of topical vitamin D, encompassing various forms, such as calcipotriol and tacalcitol, in the management of vitiligo?”.
Aim/Hypothesis: This literature aims to conduct a comprehensive review of scholarly sources investigating the use of topical vitamin D in the treatment of vitiligo. The review will encompass various study designs, including randomized controlled trials and prospective comparative studies, to provide a thorough analysis of the current knowledge in this area. Additionally, studies examining different forms of topical vitamin D, such as calcipotriol and tacalcitol, will be included to evaluate their individual efficacy and comparative effectiveness. The ultimate goal of this systematic review is to gain a deeper understanding of the role of topical vitamin D in vitiligo treatment and offer valuable insights for clinicians, researchers, and patients seeking evidence-based approaches to managing vitiligo.
3. Methods
3.1. Search Strategy
The evaluation of vitamin D’s efficacy in topical treatment for vitiligo involved a literature search primarily conducted in February 2023. PubMed and Scopus databases were utilized for this purpose. The search keywords employed were “vitiligo” AND “vitamin D” OR “calciferol” AND “cholecalciferol” OR “ergocalciferol” OR “tacalcitol” OR “maxacalcitol” OR “calcipotriol” AND “cream” OR “ointment” OR “paste” OR “gel” OR “lotion” OR “solution” OR “foam” OR “suspension” OR “spray”. The search encompassed the period from 2003 to 2023 and included articles, books and book chapters, clinical trials, and randomized controlled trials. Additional filters were applied to narrow down the search results to the English language and exclude reviews or newspaper articles.
3.2. Study Screening and Data Extraction
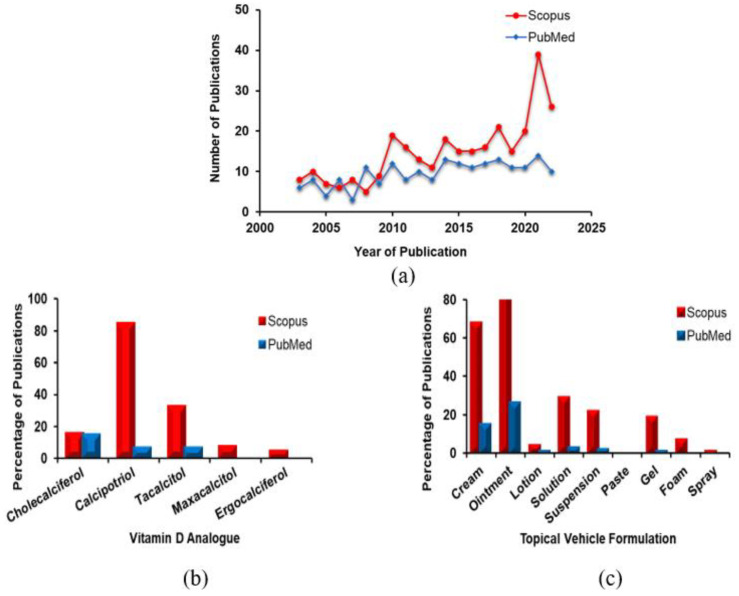
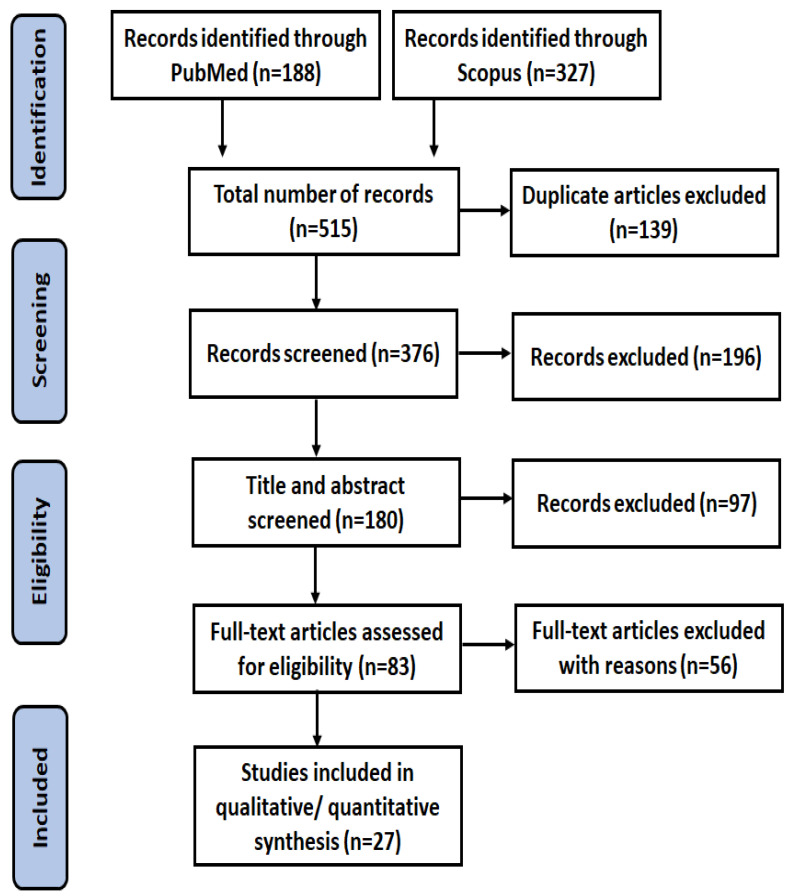
The data obtained from the literature search were imported into EndNote 20 for organization and management. To determine the eligibility of the articles, two reviewers independently assessed the titles, abstracts, and full texts. A total of 327 articles were found on Scopus and 188 articles on PubMed, as shown in (Figure 3a). Both search engines yielded a similar distribution of articles. The included articles were evaluated based on the vitamin D analogues and types of topical vehicle formulation, as depicted in (Figure 3d,c). The titles and abstracts of the articles were screened for relevance, resulting in a total of 83 publications. These publications underwent a detailed review, and ultimately 27 studies were selected and summarized in (Table 1).
Figure 3.
Analysis of search results. (a) The total number of journal articles published between 2003 and 2023 in Scopus (n = 327) and PubMed (n = 188) on the topic of vitamin D’s involvement in the treatment of vitiligo; (b) the subjective data indicated the proportions of journal articles published between 2003 and 2023 in Scopus and PubMed on vitamin D derivatives; and (c) types of topical vehicle formulations. Accessed database on 6 February 2023.
Table 1.
Characteristics of included studies.
| Source | Study Design | Types of Vitiligo |
No. of Patients |
Subtype | Treatment Duration | Intervention | Fitzpatrick Skin Type | Types of Topical Vit-D |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Abdel 2015 [78] | Randomized blinded comparative study | Non-segmental | 44 | Localized and stable | 3 months |
|
NR | Ointment |
|
| Ada 2005 [79] | Right-left comparison prospective single-blinded study | Non-segmental | 20 | Generalized vitiligo | 12 months |
|
NR | Cream |
|
| Akdeniz [80] 2014 | Randomized double-blind comparative study | Non-segmental | 45 | NR | 6 months |
|
NR | Ointment |
|
| Arca 2006 [81] | Comparative prospective study | Non-segmental | 40 | Stable | 4 months |
|
NR | Ointment |
|
| Bakr 2021 [82] | Prospective randomized comparative study | Non-segmental | 30 | Stable | 3 months |
|
NR | Ointment |
|
| Baysal 2003 [47] | Prospective right/left comparative, open study | Non-segmental | 22 | Generalized | 9 months |
|
NR | Cream |
|
| Cherif 2003 [83] | Prospective study | Non-segmental | 23 | Bilateral symmetrical | 15 weeks |
|
IV, V | Ointment |
|
| Gargoom 2004 [84] | Prospective right/left comparison study | Segmental and non-segmental | 18 | Focal/mucosal | 4–6 months |
|
NR | Cream/Ointment |
|
| Goktas 2006 [75] | Prospective right/left comparison study | Non-segmental | 24 | Generalized | 6 months |
|
II, III | Cream |
|
| Goldinger 2007 [85] | Prospective right/left comparative single-blinded trial | Non-segmental | 10 | Bilateral symmetrical | 15 months |
|
II, III, IV | Ointment |
|
| Hartmann 2005 [86] | Prospective right/left comparative study | Non-segmental | 10 | Symmetrical (vulgaris/acrofacial /localized) | 12 months |
|
II, III | Ointment |
|
| Ibrahim 2019 [87] | Prospective right/left comparative study | Segmental and non-segmental | 25 | Stable and symmetrical, focal and generalized | 6 months |
|
III, IV | Ointment | 60% showed excellent improvement for the calcipotriol combination compared to 32% for tacrolimus |
| Juntongjin 2021 [88] | Prospective randomized double-blind comparative study | Non-segmental | 13 | Acral vitiligo | 24 weeks |
|
IV | Ointment |
|
| Katayama 2003 [89] | Prospective uncontrolled open trial | Segmental and non-segmental | 15 | Vulgaris vitiligo | 3 months |
|
NR | Ointment | 40% of patients responded in a good to excellent way clinically |
| Khullar 2015 [90] | Prospective right-left comparative study | Non-segmental | 27 | Generalized vitiligo (vulgaris and acrofacial | 24 weeks |
|
III, IV, V | Ointment | 51.4% reduction in the lesions for NB and 49% for combination (good repigmentation) |
| Kullavanija-ya 2004 [91] | Prospective open bilateral comparison study | Non-segmental | 20 | Symmetrical vitiligo | 15 months |
|
NR | Ointment |
|
| Kumaran 2006 [92] | Randomized trial | Non-segmental | 49 | Localized vitiligo | 3 months |
|
NR | Ointment |
|
| Leone 2006 [93] | Prospective randomized open-label study | Non-segmental | 32 | Generalized and symmetrical | 12 months |
|
II, III, IV | Ointment |
|
| Lotti 2008 [94] | Prospective open study | Segmental and non-segmental | 470 | Vulgaris vitiligo | 6 months |
|
I, II, III, IV | Ointment |
|
| Lu-Yan 2006 [95] | Randomized single-blind within a patient-controlled trial | Segmental and non-segmental | 38 | Symmetrical, localized, vulgaris | 8 weeks |
|
NR | Cream | 25.7% excellent repigmentation in the combination (early pigmentation with lower total dosage) to 5.7% for excimer monotherapy |
| Oh 2011 [96] | Prospective randomized single-blinded paired comparative study | Non-segmental | 20 | Localized | 16 weeks |
|
NR | Ointment |
|
| Onita 2004 [97] | Prospective open trial | Segmental and non-segmental | 27 | Vulgaris vitiligo | NR |
|
NR | Ointment | 48% of patients improved > 30%(moderate) |
| Rodriguez 2009 [98] | Randomized double-blind placebo-controlled study | Non-segmental | 80 | NR | 4 months |
|
NR | Ointment | There was no reduction of the size of the lesion < 25% (poor repigmentation) |
| Sahu 2016 [99] | Prospective open label right/left intraindividual trial | Non-segmental | 30 | Symmetrical vitiligo | 24 weeks |
|
NR | Ointment | 16.6% had moderate. 53.3% good 30% excellent repigmentation at the end of therapy |
| Salem 2023 [100] | Prospective comparative study | Segmental and non-segmental | 25 | Stable vitiligo | 19 months |
|
II, III, IV | Solution | 52% excellent to good response in the combination |
| Vazquez 2003 [101] | Prospective open pilot study | Non-segmental | 10 | NR | NR |
|
NR | Ointment |
|
| Xing 2012 [102] | Prospective open uncontrolled trial | Segmental and non-segmental | 31 | Focal or generalized | 12 weeks |
|
NR | Ointment |
|
Abbreviations: NR, Not Reported; PUVA, Psoralens Long Wave Ultraviolet Radiation; NB-UVB, Narrowband Ultraviolet Phototherapy; MEL, Monochromatic excimer light; BM, Betamethasone.
3.3. Inclusion and Exclusion Criteria
The selection of studies was carried out based on the following inclusion criteria: (1) study design: prospective or randomized controlled trials; (2) interventions: vitamin D derivatives, including cholecalciferol, ergocalciferol, calcipotriol, tacalcitol, and maxacalcitol; (3) topical vehicle formulation: cream, ointment, lotion, foam, solution, suspension, gel, spray, paste; (4) outcomes: evaluation of results based on the total number of vitiligo lesions on each participant’s body or each patch, as well as the degree of repigmentation on a quartile scale (<25% (poor), ≥25% (moderate), ≥50% (good), and ≥75% (excellent)). Exclusion criteria were (1) duplicate publications; (2) studies published outside the specified time frame (between 2003 and 2023); (3) studies that were not randomized controlled trials or prospective trials; (4) studies involving oral or injectable forms of vitamin D (this study focuses on topical administration); (5) studies without full-text availability. The selection process for inclusion and exclusion of articles is illustrated in the PRISMA flow diagram shown in (Figure 4).
Figure 4.
PRISMA diagram of the study identification.
4. Results
Based on the classification in (Figure 3b), calcipotriol had the highest proportion of publications (85%) in the Scopus search engine, followed by tacalcitol (33%) and cholecalciferol (16%). In the PubMed search engine, cholecalciferol (vitamin D3) had the largest proportion of publications (15%), followed by calcipotriol and tacalcitol, both with a percentage of 7%. Maxacalcitol and ergocalciferol had lower percentages (less than 10% in Scopus) or were not reported in publications in PubMed. In terms of topical vehicle formulation, ointment had the highest proportion of publications in both Scopus (83%) and PubMed (26%), which aligns with the lipophilic nature of vitamin D. Cream had the second highest proportion of publications for topical vehicle formulation (68% in Scopus and 15% in PubMed). Other formulations, such as solutions, suspensions, and gels, had lower proportions, but they were more popular in Scopus compared to PubMed, as shown in (Figure 3c). The findings from the 27 trials (n = 1198) summarized in (Table 1) revealed that calcipotriol accounted for nearly 70% of the topically used vitamin D analogues for vitiligo treatment. Tacalcitol and cholecalciferol contributed to 22% and 8% of the total, respectively. Ointment represented 77.5% of the vehicles utilized for the topical formulation, while cream and solution vehicles constituted 18.5% and 4% of the total, respectively. In terms of patient outcomes, a good response was achieved in 40% of the cases, while an excellent and poor response was observed in 26% each. A moderate response was reported in 18% of the cases.
5. Discussion
Several studies [84,90,103] have demonstrated the efficacy of calcipotriol in repigmenting vitiligo lesions, whether used alone or in combination with Psoralens Long Wave Ultraviolet Radiation (PUVA) or Narrowband Ultraviolet Phototherapy (NB-UVB). However, there have also been publications [104,105] that reported hyperpigmentation in psoriasis patients treated with phototherapy and calcipotriol. Baysal et al. [47] conducted an open prospective right-left comparative study involving 22 patients with generalized vitiligo of up to 9 months duration. Calcipotriol cream was applied twice a day on one side, while both sides received PUVA radiation twice weekly. The study reported a good response of 53.3% for the combination group and 53.11% for the PUVA alone group, with no statistically significant difference between the two groups (p = 0.980). In another study by Cherif et al. [83], a prospective right-left comparative study was performed to evaluate the efficacy of the combination of calcipotriol and PUVA in treating vitiligo. One side of the body received a twice-daily application of 0.005% calcipotriol ointment, while the other side remained untreated. PUVA radiation was administered three times per week on each side. The combination group achieved 69% poor to moderate repigmentation, compared to 52% for the PUVA alone group (p = 0.015), with a higher level of repigmentation observed on the calcipotriol-treated side. Parsad et al. [103] described the use of a topical ointment containing calcipotriol 50 µg/g in combination with PUVA for the treatment of vitiligo. They found that combining calcipotriol with PUVA therapy reduced the treatment duration by up to 18 months while maintaining high effectiveness and faster response. Vazquez et al. [101] conducted a prospective open study involving 10 patients with non-segmental vitiligo, using a combination of PUVA radiation and calcipotriene ointment. Their study reported a lower degree of repigmentation compared to the findings of Parsad et al. [103]. Overall, the combination therapy of calcipotriol and PUVA shows promise in the treatment of vitiligo, although the reported efficacy varies among different studies. Further research is needed to establish the optimal protocol, determine the long-term effectiveness, and assess the safety of this combination therapy in vitiligo treatment.
NB-UVB has been demonstrated to be an effective and safe treatment option for vitiligo [75]. Several prospective comparative studies have investigated the use of 0.005% calcipotriol ointment (apart from Arca et al., who used 0.05% calcipotriol) with or without NB-UVB on non-segmental symmetrical vitiligo patients. Kullavanijaya [91], Hartmann et al. [86], Arca et al. [81], and Khullar et al. [90] reported that (only) calcipotriol had no significant effect on the overall response rate, while NB-UVB monotherapy was found to be significantly effective in treating vitiligo (p < 0.05). However, conflicting results were observed in prospective comparison trials conducted by Ada et al. [79] and Goktas et al. [75]. Both studies treated NB-UVB to both sides of the body and applied calcipotriol cream twice daily to one side for non-segmental vitiligo lesions. Ada et al. [79] found no significant difference (p > 0.05) with the use of calcipotriol in combination with NB-UVB, while Goktas et al. [75] demonstrated that the combination resulted in a 51% higher early pigmentation and a good response compared to 39% with NB-UVB alone (p = 0.0006). In a study by Lotti et al. [94], 470 patients with segmental and non-segmental vitiligo and Fitzpatrick skin types I, II, III, and IV were enrolled in a 6-month treatment course. The effects of calcipotriol with NB-UVB were compared to those of tacrolimus, topical betamethasone (BM), and L-phenylalanine with NB-UVB. The combination treated with calcipotriol ointment 50 µg/g achieved good repigmentation with no side effects in 75.6% of cases, whereas 90.2% of the BM combination group experienced cutaneous atrophy as a side effect. In conclusion, NB-UVB therapy is effective as a monotherapy for vitiligo treatment. However, the role of calcipotriol in combination with NB-UVB remains debatable and may yield varying results. Further research is necessary to determine the optimal treatment approach and evaluate the potential side effects associated with different treatment combinations.
The xenon chloride excimer laser emits a precise 308 nm light wavelength in a focused form and is also available as an incoherent version called the excimer lamp [106]. In the treatment of vitiligo, Monochromatic Excimer Light (MEL) delivered through both lasers and lamps has demonstrated superior efficacy compared to NB-UVB and induces more cellular alteration than traditional UVB modalities [107]. Goldinger et al. [85] conducted a comparative right-left single-blinded trial with 10 individuals having non-segmental bilateral symmetrical lesions and Fitzpatrick skin types II and III to investigate the effect of calcipotriol on the effectiveness of MEL in treating vitiligo. One side received an application of 0.005% calcipotriol ointment, while MEL with a 308 nm light wavelength was administered to both sides. The study found no significant improvement in the efficacy of MEL after 15 months with the addition of calcipotriol (p > 0.05). In a recent study by Juntongjin [88], acral vitiligo was treated with MEL and calcipotriol, followed by monotherapy with either 0.005% calcipotriol ointment or clobetasol ointment. The study revealed no significant difference between the two treatments (p > 0.05), with partial repigmentation observed in 85% of lesions treated with calcipotriol and 77% of lesions treated with clobetasol.
The combination of topical calcipotriol and topical steroids has shown effectiveness in treating psoriasis while reducing the adverse effects of steroids. Kumaran et al. [92], Xing and Xu [102], Abdel and Ibrahim [78], and Ibrahim et al. [87] conducted studies on stable vitiligo lesions using a combination of BM 0.5 mg/g and calcipotriol ointment 50 g/g (equivalent to 0.05 mg/g and 0.005% concentration). These trials consistently reported successful repigmentation and a reduction in the adverse effects associated with BM. In a study by Akdeniz et al. [80], the effects of calcipotriol in combination with BM and NB-UVB were compared to calcipotriol combined with NB-UVB or NB-UVB alone on non-segmental vitiligo lesions. The researchers found that the combination therapy of calcipotriol and BM resulted in a repigmentation rate of 63.33%, compared to 60.67% for calcipotriol and 46.67% for NB-UVB alone. The only statistically significant difference was observed between the BM group and NB-UVB monotherapy (p = 0.0048). These findings indicate that the combination of calcipotriol and topical steroids, such as BM, can be effective in achieving repigmentation and minimizing adverse effects in the treatment of vitiligo lesions.
Fractionated lasers have emerged as an innovative technology for skin repair, creating microscopic therapeutic zones that facilitate the penetration of externally administered agents, thus improving efficacy without causing epidermal damage [108,109]. In a prospective, randomized, and comparative trial, Bakr et al. [82] treated 30 patients with stable non-segmental vitiligo using a CO2 laser, followed by three months of therapy with either calcipotriol 0.05% ointment, tacrolimus, or NB-UVB. Remarkable results were observed with all three treatments: 10% calcipotriol, 30% tacrolimus, and 40% NB-UVB phototherapy. Moreover, all combinations were found to be effective and safe, with NB-UVB therapy being the most efficacious. Following laser treatment, 70% of the patients experienced minor transient adverse effects such as pain, erythema, and crustations, which resolved spontaneously within a few days. In a comparative trial conducted by Gargoom et al. [84], the effects of 50 µg/g calcipotriol cream and ointment as monotherapy were examined in 18 patients with segmental and non-segmental vitiligo lesions for up to 6 months. The study revealed that ointment was more effective than cream, with 77.8% of patients showing improvement and 22.2% showing no response. Among those who exhibited improvement, 21.4% achieved excellent results, and 28.6% achieved a good response. These findings indicate the potential of fractionated lasers for enhancing the efficacy of externally administered agents in vitiligo treatment. The combination therapies involving calcipotriol, tacrolimus, and NB-UVB have demonstrated positive outcomes, while the use of calcipotriol ointment as monotherapy has also shown effectiveness in improving vitiligo lesions.
Several studies have indicated that formulations containing the same drug at the same concentration can exhibit varying rates of drug delivery through the skin due to the physicochemical properties of the vehicle used. Moreover, formulations employing the same ingredients can also result in different drug delivery rates based on the droplet size of the emulsion. In vitro, drug release tests provide valuable insights into evaluating the impact of formulation factors such as dose formulas, vehicle composition, and drug solubility in vehicles. These considerations are crucial when formulating topical preparations [110,111].
Formulation components have two primary effects on skin permeation: Firstly, they can modify the lamellar structure of intercellular lipids in the stratum corneum. These components have the potential to enhance or impede the permeation of the drug through the skin by altering the organization and packing of the intercellular lipids. This, in turn, influences the pathway and rate at which drug molecules can traverse the stratum corneum. Secondly, formulation components can impact the solubility properties of lipids. The solubility of both the drug and the vehicle in these lipids can have implications for drug permeation through the skin. The lipid composition of the stratum corneum is a significant determinant of skin permeability. The composition of lipids in the stratum corneum plays a vital role in determining skin permeability. Formulation components can modulate the solubility of the drug in these lipids, consequently influencing the drug’s ability to penetrate the skin [112,113,114].
Recent findings suggest that tacalcitol is more effective than calcipotriol when combined with NB-UVB phototherapy for the treatment of vitiligo [115]. In the study conducted by Katayama et al. [89], which was the first to report on the effectiveness of tacalcitol in treating vitiligo, 15 individuals with segmental and non-segmental vulgaris vitiligo were treated with tacalcitol ointment and sunlight exposure for up to three months. The study revealed a clinically good to excellent response in 40% of patients (p < 0.05). In contrast, Rodriguez et al. [98] conducted a randomized double-blind placebo-controlled investigation involving 80 patients with non-segmental vitiligo over a duration of 4 months. They applied topical tacalcitol ointment (4 µg/g) once a day at night and performed daily sunlight exposure for 30 min. The study found no significant reduction in lesion size or response of less than 25% response (p > 0.05), suggesting that the influence of tacalcitol on repigmentation is limited. To compare the effectiveness of NB-UVB phototherapy alone and in combination with tacalcitol for vitiligo treatment, Leone et al. [93] enrolled 32 adults with symmetrical and generalized vitiligo lesions and Fitzpatrick skin types II, III, and IV over 12 months. Patients were randomly selected on one side to apply tacalcitol ointment (4 µg/g) daily and received NB-UVB phototherapy twice a week on both sides. The combination treatment demonstrated a significantly shorter therapy duration (p = 0.0005) and higher repigmentation scores compared to the side treated by NB-UVB alone. Tacalcitol was found to enhance the effectiveness of treatment and accelerate the repigmentation process. In a study by Sahu et al. [99], the therapy duration was reduced to six months, and NB-UVB was administered three times a week. The combined group, treated with 4 µg/g tacalcitol ointment and NB-UVB, achieved a moderate response in 16.6% of cases, a good response in 53.3%, and excellent repigmentation in 30% of cases at the end of the treatment period (p < 0.001). These findings highlight the superior efficacy and accelerated repigmentation associated with the combination of tacalcitol and NB-UVB phototherapy in the treatment of vitiligo compared to monotherapy approaches.
In a randomized single-blind controlled experiment conducted by Lu-Yan et al. [95], 38 adults with symmetrical or close lesions were enrolled in an eight-week study. They used topical tacalcitol cream (2 µg/g) twice daily, in combination with 308-nm MEL once a week for each lesion. The combination treatment resulted in a significantly higher excellent repigmentation rate of 25.7% compared to 5.7% for excimer monotherapy (p < 0.05). The combination approach demonstrated early pigmentation with a lower total dosage. In a randomized single-blinded paired comparative study by Oh et al. [96], 20 patients with localized non-segmental vitiligo were treated for 16 weeks. Daily application of the ointment tacalcitol (20 µg/g) and twice-weekly administration of the excimer laser were used either as monotherapy or in combination. The study found no significant differences (p > 0.05) in repigmentation between the treatments. Tacalcitol, whether administered alone or in combination with MEL, had limited effects on repigmentation.
VD3, previously used as an oral treatment for psoriasis, gradually fell out of favor due to its associated hypercalcemic adverse effects. Consequently, limited research has been conducted on the topical delivery of VD3. In a prospective open trial by Onita [97], 27 adult patients with vulgaris vitiligo who had a poor clinical response to previous treatments such as topical corticosteroids and PUVA were treated with vitamin D3 ointment and PUVA phototherapy. The study demonstrated that approximately 48% of patients achieved moderate improvements of more than 30%. This combination therapy shows promise as an alternative treatment for vitiligo. In a recent prospective comparison study conducted by Salem et al. [100], 25 patients with stable segmental and non-segmental vitiligo (Fitzpatrick skin types II, III, and IV) were enrolled. Each patient received treatment for a minimum of two patches, with one patch treated using microneedle alone and the other patch treated with a combination of microneedle and topical cholecalciferol (0.5–2 mL solution). After 19 months of treatment, a 52% excellent to good response rate was observed with the combined therapy. Although there were no statistically significant differences in treatment response between the two types of vitiligo in the treated patches (p > 0.05), there was a difference in the pattern of pigmentation between the two groups (p = 0.013). These findings suggest that topical vitamin D3, when used in combination with microneedling, can be effective in treating stable vitiligo.
6. Conclusions
Vitamin D and its analogues, such as calcipotriol and tacalcitol, are commonly used topically for the treatment of pigmentation disorders. When used in conjunction with NB-UVB therapy, the addition of these derivatives has the potential to enhance the therapeutic outcomes for vitiligo. Tacalcitol has been shown to have stronger efficacy compared to calcipotriol in this regard. However, there is limited evidence supporting the use of vitamin D analogues to enhance the effectiveness of PUVA and MEL treatments for vitiligo, although some positive effects have been observed with the use of calcipotriol in a few studies. The combination of topical corticosteroids with vitamin D analogues has demonstrated greater efficacy in the treatment of vitiligo compared to using vitamin D analogues alone. Furthermore, incorporating vitamin D analogues in the treatment regimen can help mitigate the adverse effects associated with corticosteroids. However, additional research is needed to explore the optimal methods of topically administering vitamin D analogues as an anti-vitiligo agent. Further studies are necessary to investigate the specific protocols, dosages, and treatment durations to maximize the therapeutic benefits of vitamin D analogues in the management of vitiligo.
Author Contributions
K.A.-S. and Y.M., conceptualization; K.A.-S., writing—preparation of the original draft; K.A.-S. and M.A., drawing figures; M.A. and S.E.A., reviewing and assessing the articles’ eligibility; V.R.L.-S., M.A., X.J., S.E.A. and M.I., review and editing; Y.M., supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Norman A. Vitamin D. Elsevier; Amsterdam, The Netherlands: 2012. pp. 1–101. [Google Scholar]
- 2.Agostini D., Donati Zeppa S. Vitamin D, Diet and Musculoskeletal Health. Nutrients. 2023;15:2902. doi: 10.3390/nu15132902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostafa W.Z., Hegazy R.A. Vitamin D and the skin: Focus on a complex relationship: A review. J. Adv. Res. 2015;6:793–804. doi: 10.1016/j.jare.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Illescas-Montes R., Melguizo-Rodríguez L., Ruiz C., Costela-Ruiz V.J. Vitamin D and autoimmune diseases. Life Sci. 2019;233:116744. doi: 10.1016/j.lfs.2019.116744. [DOI] [PubMed] [Google Scholar]
- 5.Holick M.F., Chen T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 6.Charoenngam N., Shirvani A., Holick M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma. 2019;10:1082–1093. doi: 10.1016/j.jcot.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narvaez J., Maldonado G., Guerrero R., Messina O.D., Rios C. Vitamin D megadose: Definition, efficacy in bone metabolism, risk of falls and fractures. Open Access Rheumatol. Res. Rev. 2020;12:105–115. doi: 10.2147/OARRR.S252245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawarkar S., Ashtekar A. Transdermal vitamin D supplementation—A potential vitamin D deficiency treatment. J. Cosmet. Dermatol. 2020;19:28–32. doi: 10.1111/jocd.13085. [DOI] [PubMed] [Google Scholar]
- 9.Alsaqr A., Rasoully M., Musteata F.M. Investigating transdermal delivery of vitamin D3. AAPS PharmSciTech. 2015;16:963–972. doi: 10.1208/s12249-015-0291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamagishi N., Namioka T., Okura N., Sato S., Kim D., Furuhama K., Naito Y. Application of a reservoir-type calcitriol transdermal patch in dairy cattle. J. Vet. Med. Sci. 2009;71:845–848. doi: 10.1292/jvms.71.845. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z., Ding R., Wang J. The association between vitamin D status and autism spectrum disorder (ASD): A systematic review and meta-analysis. Nutrients. 2020;13:86. doi: 10.3390/nu13010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-smadi K., Imran M., Leite-Silva V.R., Mohammed Y. Vitiligo: A Review of Aetiology, Pathogenesis, Treatment, and Psychosocial Impact. Cosmetics. 2023;10:84. doi: 10.3390/cosmetics10030084. [DOI] [Google Scholar]
- 13.Sharquie K.E., Sharquie I.K., Al Hamza A.N. Psoriasis, pityriasis alba, and vitiligo (PPV) are a triad of one disease: New observation. Our Dermatol. Online/Nasza Dermatol. Online. 2021;12:314–323. [Google Scholar]
- 14.AlGhamdi K., Kumar A., Moussa N. The role of vitamin D in melanogenesis with an emphasis on vitiligo. Indian J. Dermatol. Venereol. Leprol. 2013;79:750. doi: 10.4103/0378-6323.120720. [DOI] [PubMed] [Google Scholar]
- 15.Okoro U., Usatine R.P., Heath C.R. DX across the Skin Color Spectrum. Volume 111. Quadrant Healthcom Inc.; Parsippany, NJ, USA: 2023. pp. 106–107. [Google Scholar]
- 16.Wolff K., Goldsmith L.A., Katz S.I., Gilchrest B.A., Paller A.S., Leffell D.J. Fitzpatrick’s Dermatology in General Medicine. McGraw-Hill; New York, NY, USA: 2008. [Google Scholar]
- 17.Ortonne J.-P., Passeron T. Vitiligo and other disorders of hypopigmentation. Dermatology. 2008;1:4–5. [Google Scholar]
- 18.Boniface K., Seneschal J., Picardo M., Taïeb A. Vitiligo: Focus on clinical aspects, immunopathogenesis, and therapy. Clin. Rev. Allergy Immunol. 2018;54:52–67. doi: 10.1007/s12016-017-8622-7. [DOI] [PubMed] [Google Scholar]
- 19.Saniee S., Zare A.G., Radmehr A. Zinc, vitamin D, and TSH levels in patients with vitiligo. Erciyes Med. J. 2019;41:148–152. doi: 10.14744/etd.2019.40316. [DOI] [Google Scholar]
- 20.Hassan I., Bhat Y.J., Majid S., Sajad P., Rasool F., Malik R.A., Haq I.U. Association of vitamin D receptor gene polymorphisms and serum 25-hydroxy vitamin D levels in vitiligo–A case-control study. Indian Dermatol. Online J. 2019;10:131. doi: 10.4103/idoj.IDOJ_97_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saputra M.A.R., Purwoko I.H., Toruan T.L. The Role of Topical Vitamin D in Vitiligo: A Narrative Literature Review. Biosci. Med. J. Biomed. Transl. Res. 2022;6:2516–2526. [Google Scholar]
- 22.Naik P.P., Farrukh S.N. Influence of ethnicities and skin color variations in different populations: A review. Ski. Pharmacol. Physiol. 2022;35:65–76. doi: 10.1159/000518826. [DOI] [PubMed] [Google Scholar]
- 23.Hawker N.P., Pennypacker S.D., Chang S.M., Bikle D.D. Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J. Investig. Dermatol. 2007;127:874–880. doi: 10.1038/sj.jid.5700624. [DOI] [PubMed] [Google Scholar]
- 24.Birlea S.A., Costin G.E., Norris D.A. Cellular and molecular mechanisms involved in the action of vitamin D analogs targeting vitiligo depigmentation. Curr. Drug Targets. 2008;9:345–359. doi: 10.2174/138945008783954970. [DOI] [PubMed] [Google Scholar]
- 25.Lotti T., Gori A., Zanieri F., Colucci R., Moretti S. Vitiligo: New and emerging treatments. Dermatol. Ther. 2008;21:110–117. doi: 10.1111/j.1529-8019.2008.00178.x. [DOI] [PubMed] [Google Scholar]
- 26.Travis L.B., Silverberg N.B. Calcipotriene and corticosteroid combination therapy for vitiligo. Pediatr. Dermatol. 2004;21:495–498. doi: 10.1111/j.0736-8046.2004.21418.x. [DOI] [PubMed] [Google Scholar]
- 27.Mohammed Y.H., Yamada M., Lin L.L., Grice J.E., Roberts M.S., Raphael A.P., Benson H.A.E., Prow T.W. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE. 2014;9:e101956. doi: 10.1371/journal.pone.0101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitamin D–Fact Sheet for Health Professionals. [(accessed on 21 September 2023)]. Available online: https://manoxblog.com/2018/05/01/vitamin-d-fact-sheet-for-health-professionals/
- 29.Buttriss J.L., Lanham-New S.A. Is a vitamin D fortification strategy needed? Nutr. Bull. 2020;45:115. doi: 10.1111/nbu.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itkonen S.T., Andersen R., Björk A.K., Brugård Konde Å., Eneroth H., Erkkola M., Holvik K., Madar A.A., Meyer H.E., Tetens I. Vitamin D status and current policies to achieve adequate vitamin D intake in the Nordic countries. Scand. J. Public Health. 2021;49:616–627. doi: 10.1177/1403494819896878. [DOI] [PubMed] [Google Scholar]
- 31.Wessels I., Rink L. Micronutrients in autoimmune diseases: Possible therapeutic benefits of zinc and vitamin D. J. Nutr. Biochem. 2020;77:108240. doi: 10.1016/j.jnutbio.2019.108240. [DOI] [PubMed] [Google Scholar]
- 32.Murdaca G., Greco M., Borro M., Gangemi S. Hygiene hypothesis and autoimmune diseases: A narrative review of clinical evidences and mechanisms. Autoimmun. Rev. 2021;20:102845. doi: 10.1016/j.autrev.2021.102845. [DOI] [PubMed] [Google Scholar]
- 33.Garg B.J., Saraswat A., Bhatia A., Katare O.P. Topical treatment in vitiligo and the potential uses of new drug delivery systems. Indian J. Dermatol. Venereol. Leprol. 2010;76:231. doi: 10.4103/0378-6323.62961. [DOI] [PubMed] [Google Scholar]
- 34.Bilal A., Anwar I. Guidelines for the management of vitiligo. J. Pak. Assoc. Dermatol. 2014;24:68–78. [Google Scholar]
- 35.Bouceiro Mendes R., Alpalhão M., Filipe P. UVB phototherapy in the treatment of vitiligo: State of the art and clinical perspectives. Photodermatol. Photoimmunol. Photomed. 2022;38:215–223. doi: 10.1111/phpp.12740. [DOI] [PubMed] [Google Scholar]
- 36.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnarumma D., Arena A., Trovato E., Rigano F., Zoccali M., Mondello L. A miniaturized comprehensive approach for total lipidome analysis and vitamin D metabolite quantification in human serum. Anal. Bioanal. Chem. 2023;415:4579–4590. doi: 10.1007/s00216-023-04756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makris K., Bhattoa H.P., Cavalier E., Phinney K., Sempos C.T., Ulmer C.Z., Vasikaran S.D., Vesper H., Heijboer A.C. Recommendations on the measurement and the clinical use of vitamin D metabolites and vitamin D binding protein–A position paper from the IFCC Committee on bone metabolism. Clin. Chim. Acta. 2021;517:171–197. doi: 10.1016/j.cca.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janoušek J., Pilařová V., Macáková K., Nomura A., Veiga-Matos J., Silva D.D.d., Remiao F., Saso L., Malá-Ládová K., Malý J. Vitamin D: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022;59:517–554. doi: 10.1080/10408363.2022.2070595. [DOI] [PubMed] [Google Scholar]
- 40.Joseph M. Vitamin D2 vs. D3: An Evidence-Based Comparison. [(accessed on 29 December 2020)]. Available online: https://www.nutritionadvance.com/vitamin-d2-vs-d3/
- 41.Shieh A., Chun R.F., Ma C., Witzel S., Meyer B., Rafison B., Swinkels L., Huijs T., Pepkowitz S., Holmquist B. Effects of high-dose vitamin D2 versus D3 on total and free 25-hydroxyvitamin D and markers of calcium balance. J. Clin. Endocrinol. Metab. 2016;101:3070–3078. doi: 10.1210/jc.2016-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olmos-Ortiz A., Avila E., Durand-Carbajal M., Díaz L. Regulation of calcitriol biosynthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients. 2015;7:443–480. doi: 10.3390/nu7010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brożyna A.A., Slominski R.M., Nedoszytko B., Zmijewski M.A., Slominski A.T. Vitamin D signaling in psoriasis: Pathogenesis and therapy. Int. J. Mol. Sci. 2022;23:8575. doi: 10.3390/ijms23158575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wat H., Dytoc M. Off-label uses of topical vitamin D in dermatology: A systematic review. J. Cutan. Med. Surg. 2014;18:91–108. doi: 10.2310/7750.2013.13109. [DOI] [PubMed] [Google Scholar]
- 45.De Rie M.A., De Hoop D., Jönsson L., Bakkers E.J.M., Sørensen M. Pharmacoeconomic evaluation of calcipotriol (Daivonex®/Dovonex®) and UVB phototherapy in the treatment of psoriasis: A Markov model for the Netherlands. Dermatology. 2001;202:38–43. doi: 10.1159/000051583. [DOI] [PubMed] [Google Scholar]
- 46.Yalçin B., Şahin S., Bükülmez G., Karaduman A., Atakan N., Akan T., Kölemen F. Experience with calcipotriol as adjunctive treatment for vitiligo in patients who do not respond to PUVA alone: A preliminary study. J. Am. Acad. Dermatol. 2001;44:634–637. doi: 10.1067/mjd.2001.112357. [DOI] [PubMed] [Google Scholar]
- 47.Baysal V., Yildirim M., Erel A., Kesici D. Is the combination of calcipotriol and PUVA effective in vitiligo? J. Eur. Acad. Dermatol. Venereol. 2003;17:299–302. doi: 10.1046/j.1468-3083.2003.00795.x. [DOI] [PubMed] [Google Scholar]
- 48.Glowka E., Stasiak J., Lulek J. Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics. 2019;11:347. doi: 10.3390/pharmaceutics11070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dua I., Aggarwal K., Jain V.K. Comparative evaluation of efficacy and safety of calcipotriol versus tacalcitol ointment, both in combination with NBUVB phototherapy in the treatment of stable plaque psoriasis. Photodermatol. Photoimmunol. Photomed. 2017;33:275–281. doi: 10.1111/phpp.12324. [DOI] [PubMed] [Google Scholar]
- 50.Nagpal S., Lu J., Boehm M.F. Vitamin D analogs: Mechanism of action and therapeutic applications. Curr. Med. Chem. 2001;8:1661–1679. doi: 10.2174/0929867013371950. [DOI] [PubMed] [Google Scholar]
- 51.Trémezaygues L., Reichrath J. Vitamin D analogs in the treatment of psoriasis: Where are we standing and where will we be going? Derm. Endocrinol. 2011;3:180–186. doi: 10.4161/derm.17534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tapfumaneyi P., Imran M., Alavi S.E., Mohammed Y. Science of, and insights into, thermodynamic principles for dermal formulations. Drug Discov. Today. 2023;6:103521. doi: 10.1016/j.drudis.2023.103521. [DOI] [PubMed] [Google Scholar]
- 53.Yousef S.A., Mohammed Y.H., Namjoshi S., Grice J.E., Benson H.A.E., Sakran W., Roberts M.S. Mechanistic evaluation of enhanced curcumin delivery through human skin in vitro from optimised nanoemulsion formulations fabricated with different penetration enhancers. Pharmaceutics. 2019;11:639. doi: 10.3390/pharmaceutics11120639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leite-Silva V.R., Grice J.E., Mohammed Y., Moghimi H.R., Roberts M.S. Percutaneous Penetration Enhancers Drug Penetration into/through the Skin: Methodology and General Considerations. Springer; Berlin/Heidelberg, Germany: 2017. The Influence of emollients on dermal and transdermal drug delivery; pp. 77–93. [Google Scholar]
- 55.Prausnitz M.R., Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalluri H., Banga A.K. Transdermal delivery of proteins. Aaps Pharmscitech. 2011;12:431–441. doi: 10.1208/s12249-011-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin X., Imran M., Mohammed Y. Topical Semisolid Products—Understanding the Impact of Metamorphosis on Skin Penetration and Physicochemical Properties. Pharmaceutics. 2022;14:2487. doi: 10.3390/pharmaceutics14112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boshrouyeh R., Amari S., Boshrouyeh Ghandashtani M., Alavi S.E., Ebrahimi Shahmabadi H. A topical gel nanoformulation of amphotericin B (AmB) for the treatment of cutaneous leishmaniasis (CL) J. Sol-Gel Sci. Technol. 2023;105:768–780. doi: 10.1007/s10971-023-06041-w. [DOI] [Google Scholar]
- 59.Grammatikopoulou M.G., Gkiouras K., Nigdelis M.P., Bogdanos D.P., Goulis D.G. Efficacy of vitamin D3 buccal spray supplementation compared to other delivery methods: A systematic review of superiority randomized controlled trials. Nutrients. 2020;12:691. doi: 10.3390/nu12030691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin X., Alavi S.E., Shafiee A., Leite-Silva V.R., Khosrotehrani K., Mohammed Y. Metamorphosis of Topical Semisolid Products—Understanding the Role of Rheological Properties in Drug Permeation under the “in Use” Condition. Pharmaceutics. 2023;15:1707. doi: 10.3390/pharmaceutics15061707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adepu S., Ramakrishna S. Controlled drug delivery systems: Current status and future directions. Molecules. 2021;26:5905. doi: 10.3390/molecules26195905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kompella U.B., Lee V.H.L. Delivery systems for penetration enhancement of peptide and protein drugs: Design considerations. Adv. Drug Deliv. Rev. 2001;46:211–245. doi: 10.1016/S0169-409X(00)00137-X. [DOI] [PubMed] [Google Scholar]
- 63.Parish J.L. Topical vitamin D3 analogues: Unapproved uses, dosages, or indications. Clin. Dermatol. 2002;20:558–562. doi: 10.1016/S0738-081X(02)00269-9. [DOI] [PubMed] [Google Scholar]
- 64.Akgül Z. Theory and Research in Health Sciences. Serüven Publishing; Güzelbahçe, Turkey: 2022. Biological properties of vitamin D and its effect on the pathogenesis of periodontal. [Google Scholar]
- 65.Hanel A., Carlberg C. Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 2020;173:113595. doi: 10.1016/j.bcp.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 66.Wan M. Ph.D. Thesis. King’s College London; London, UK: 2021. What Dose of Vitamin D Is Required to Achieve Target Serum 25-Hydroxyvitamin D Levels in Children? [Google Scholar]
- 67.Chen T.C., Lu Z., Holick M.F. Vitamin D: Physiology, Molecular Biology, and Clinical Applications. Springer Science & Business Media; Berlin, Germany: 2010. Photobiology of vitamin D; pp. 35–60. [Google Scholar]
- 68.Milliken S.V.I., Wassall H., Lewis B.J., Logie J., Barker R.N., Macdonald H., Vickers M.A., Ormerod A.D. Effects of ultraviolet light on human serum 25-hydroxyvitamin D and systemic immune function. J. Allergy Clin. Immunol. 2012;129:1554–1561. doi: 10.1016/j.jaci.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Cichorek M., Wachulska M., Stasiewicz A., Tymińska A. Skin melanocytes: Biology and development. Adv. Dermatol. Allergol. 2013;30:30–41. doi: 10.5114/pdia.2013.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yousef H., Alhajj M., Sharma S. Anatomy, Skin (Integument), Epidermis. StatPearls Publishing; Treasure Island, FL, USA: 2017. [PubMed] [Google Scholar]
- 71.Costin G.-E., Hearing V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 72.Bhattacharya B., Chauhan D., Singh A.K., Chatterjee M. Skin Cancer: Pathogenesis and Diagnosis. Springer; Singapore: 2021. Melanin based classification of skin types and their susceptibility to UV-induced cancer; pp. 41–67. [Google Scholar]
- 73.Karagün E., Ergin C., Baysak S., Erden G., Aktaş H., Ekiz Ö. The role of serum Vitamin D levels in vitiligo. Postep. Dermatol. Alergol. 2016;33:300–302. doi: 10.5114/pdia.2016.59507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imran M., Jin X., Ali M., Tapfumaneyi P., Lelasseur P., Carlo L., Jude A., Bourg A.L., Panchal B., Dick A. The Pandemic and Your Skin—Direct and Indirect Impact of COVID-19. Cosmetics. 2023;10:34. doi: 10.3390/cosmetics10010034. [DOI] [Google Scholar]
- 75.Goktas E.O., Aydin F., Senturk N., Canturk M.T., Turanli A.Y. Combination of narrow band UVB and topical calcipotriol for the treatment of vitiligo. J. Eur. Acad. Dermatol. Venereol. 2006;20:553–557. doi: 10.1111/j.1468-3083.2006.01546.x. [DOI] [PubMed] [Google Scholar]
- 76.Forschner T., Buchholtz S., Stockfleth E. Current state of vitiligo therapy–evidence-based analysis of the literature. JDDG J. Der Dtsch. Dermatol. Ges. 2007;5:467–475. doi: 10.1111/j.1610-0387.2007.06280.x. [DOI] [PubMed] [Google Scholar]
- 77.Chen J., Wan Y., Lin Y., Jiang H. Current art of combination therapy with autologous platelet-rich plasma for stable vitiligo: A meta-analysis. Int. Wound J. 2021;18:251–260. doi: 10.1111/iwj.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdel Latif A.A., Ibrahim S.M. Monochromatic excimer light versus combination of topical steroid with vitamin D3 analogue in the treatment of nonsegmental vitiligo: A randomized blinded comparative study. Dermatol. Ther. 2015;28:383–389. doi: 10.1111/dth.12289. [DOI] [PubMed] [Google Scholar]
- 79.Ada S., Sahin S., Boztepe G., Karaduman A., Kölemen F. No additional effect of topical calcipotriol on narrow-band UVB phototherapy in patients with generalized vitiligo. Photodermatol. Photoimmunol. Photomed. 2005;21:79–83. doi: 10.1111/j.1600-0781.2005.00139.x. [DOI] [PubMed] [Google Scholar]
- 80.Akdeniz N., Yavuz I.H., Gunes Bilgili S., Ozaydın Yavuz G., Calka O. Comparison of efficacy of narrow band UVB therapies with UVB alone, in combination with calcipotriol, and with betamethasone and calcipotriol in vitiligo. J. Dermatol. Treat. 2014;25:196–199. doi: 10.3109/09546634.2013.777381. [DOI] [PubMed] [Google Scholar]
- 81.Arca E., Taştan H.B., Erbil A.H., Sezer E., Koç E., Kurumlu Z. Narrow-band ultraviolet B as monotherapy and in combination with topical calcipotriol in the treatment of vitiligo. J. Dermatol. 2006;33:338–343. doi: 10.1111/j.1346-8138.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 82.Bakr R.M., Abdel-Gaber R.M., Tawfik Y.M. A comparative study on the use of fractional CO2 laser with tacrolimus or calcipotriol or narrow band ultraviolet-B in treatment of stable nonsegmental vitiligo. Dermatol. Ther. 2021;34:e14604. doi: 10.1111/dth.14604. [DOI] [PubMed] [Google Scholar]
- 83.Cherif F., Azaiz M.I., Ben Hamida A., Ben O., Dhari A. Calcipotriol and PUVA as treatment for vitiligo. Dermatol. Online J. 2003;9:4. doi: 10.5070/D32FK376FR. [DOI] [PubMed] [Google Scholar]
- 84.Gargoom A.M., Duweb G.A., Elzorghany A.H., Benghazil M., Bugrein O.O. Calcipotriol in the treatment of childhood vitiligo. Int. J. Clin. Pharmacol. Res. 2004;24:11–14. [PubMed] [Google Scholar]
- 85.Goldinger S.M., Dummer R., Schmid P., Burg G., Seifert B., Läuchli S. Combination of 308-nm xenon chloride excimer laser and topical calcipotriol in vitiligo. J. Eur. Acad. Dermatol. Venereol. 2007;21:504–508. doi: 10.1111/j.1468-3083.2006.02016.x. [DOI] [PubMed] [Google Scholar]
- 86.Hartmann A., Lurz C., Hamm H., Bröcker E.B., Hofmann U.B. Narrow-band UVB311 nm vs. broad-band UVB therapy in combination with topical calcipotriol vs. placebo in vitiligo. Int. J. Dermatol. 2005;44:736–742. doi: 10.1111/j.1365-4632.2004.02154.x. [DOI] [PubMed] [Google Scholar]
- 87.Ibrahim Z.A., Hassan G.F., Elgendy H.Y., Al-Shenawy H.A. Evaluation of the efficacy of transdermal drug delivery of calcipotriol plus betamethasone versus tacrolimus in the treatment of vitiligo. J. Cosmet. Dermatol. 2019;18:581–588. doi: 10.1111/jocd.12704. [DOI] [PubMed] [Google Scholar]
- 88.Juntongjin P., Sangganjanavanich P. Efficacy of the combined excimer light and topical calcipotriol for acral vitiligo: A randomized double-blind comparative study. Dermatol. Ther. 2021;34:e14886. doi: 10.1111/dth.14886. [DOI] [PubMed] [Google Scholar]
- 89.Katayama I., Ashida M., Maeda A., Eishi K., Murota H., Bae S.J. Open trial of topical tacalcitol [1 alpha 24(OH)2D3] and solar irradiation for vitiligo vulgaris: Upregulation of c-Kit mRNA by cultured melanocytes. Eur. J. Dermatol. 2003;13:372–376. [PubMed] [Google Scholar]
- 90.Khullar G., Kanwar A.J., Singh S., Parsad D. Comparison of efficacy and safety profile of topical calcipotriol ointment in combination with NB-UVB vs. NB-UVB alone in the treatment of vitiligo: A 24-week prospective right-left comparative clinical trial. J. Eur. Acad. Dermatol. Venereol. 2015;29:925–932. doi: 10.1111/jdv.12726. [DOI] [PubMed] [Google Scholar]
- 91.Kullavanijaya P., Lim H.W. Topical calcipotriene and narrowband ultraviolet B in the treatment of vitiligo. Photodermatol. Photoimmunol. Photomed. 2004;20:248–251. doi: 10.1111/j.1600-0781.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- 92.Kumaran M.S., Kaur I., Kumar B. Effect of topical calcipotriol, betamethasone dipropionate and their combination in the treatment of localized vitiligo. J. Eur. Acad. Dermatol. Venereol. 2006;20:269–273. doi: 10.1111/j.1468-3083.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- 93.Leone G., Pacifico A., Iacovelli P., Paro Vidolin A., Picardo M. Tacalcitol and narrow-band phototherapy in patients with vitiligo. Clin. Exp. Dermatol. 2006;31:200–205. doi: 10.1111/j.1365-2230.2005.02037.x. [DOI] [PubMed] [Google Scholar]
- 94.Lotti T., Buggiani G., Troiano M., Assad G.B., Delescluse J., De Giorgi V., Hercogova J. Targeted and combination treatments for vitiligo. Comparative evaluation of different current modalities in 458 subjects. Dermatol. Ther. 2008;21((Suppl. S1)):S20–S26. doi: 10.1111/j.1529-8019.2008.00198.x. [DOI] [PubMed] [Google Scholar]
- 95.Lu-Yan T., Wen-Wen F., Lei-Hong X., Yi J., Zhi-Zhong Z. Topical tacalcitol and 308-nm monochromatic excimer light: A synergistic combination for the treatment of vitiligo. Photodermatol. Photoimmunol. Photomed. 2006;22:310–314. doi: 10.1111/j.1600-0781.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 96.Oh S.H., Kim T., Jee H., Do J.E., Lee J.H. Combination treatment of non-segmental vitiligo with a 308-nm xenon chloride excimer laser and topical high-concentration tacalcitol: A prospective, single-blinded, paired, comparative study. J. Am. Acad. Dermatol. 2011;65:428–430. doi: 10.1016/j.jaad.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 97.Onita A. New aspects on vitamin D3 ointment: Vitamin D3 therapy for vitiligo. Clin. Calcium. 2004;14:137–140. [PubMed] [Google Scholar]
- 98.Rodríguez-Martín M., García Bustínduy M., Sáez Rodríguez M., Noda Cabrera A. Randomized, double-blind clinical trial to evaluate the efficacy of topical tacalcitol and sunlight exposure in the treatment of adult nonsegmental vitiligo. Br. J. Dermatol. 2009;160:409–414. doi: 10.1111/j.1365-2133.2008.08906.x. [DOI] [PubMed] [Google Scholar]
- 99.Sahu P., Jain V.K., Aggarwal K., Kaur S., Dayal S. Tacalcitol: A useful adjunct to narrow-band ultraviolet-B phototherapy in vitiligo. Photodermatol. Photoimmunol. Photomed. 2016;32:262–268. doi: 10.1111/phpp.12265. [DOI] [PubMed] [Google Scholar]
- 100.Salem M.W., Adel Abd El Azim A., Galal S.A. Efficacy of topical vitamin D combined with microneedling in the treatment of vitiligo: A comparative study. J. Cosmet. Dermatol. 2023;22:1521–1527. doi: 10.1111/jocd.15621. [DOI] [PubMed] [Google Scholar]
- 101.Vázquez-López F., López-Escobar M., Pérez-Oliva N. Calcipotriene and Vitiligo. Arch. Dermatol. 2003;139:1656–1657. doi: 10.1001/archderm.139.12.1656. [DOI] [PubMed] [Google Scholar]
- 102.Xing C., Xu A. The effect of combined calcipotriol and betamethasone dipropionate ointment in the treatment of vitiligo: An open, uncontrolled trial. J. Drugs Dermatol. 2012;11:e52–e54. [PubMed] [Google Scholar]
- 103.Parsad D., Saini R., Verma N. Combination of PUVAsol and topical calcipotriol in vitiligo. Dermatology. 1998;197:167–170. doi: 10.1159/000017991. [DOI] [PubMed] [Google Scholar]
- 104.Rütter A., Schwarz T. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete. Volume 51. Springer; Berlin/Heidelberg, Germany: 2000. Market hyperpigmentation in psoriatic plaque as a sequelae of combination therapy with UVB-311 and calcipotriol; pp. 431–433. [DOI] [PubMed] [Google Scholar]
- 105.Schiener R., Behrens-Williams S.C., Pillekamp H., Kaskel P., Peter R.U., Kerscher M. Calcipotriol vs. tazarotene as combination therapy with narrowband ultraviolet B (311 nm): Efficacy in patients with severe psoriasis. Br. J. Dermatol. 2000;143:1275–1278. doi: 10.1046/j.1365-2133.2000.03900.x. [DOI] [PubMed] [Google Scholar]
- 106.Pacifico A., Leone G. Photo(chemo)therapy for vitiligo. Photodermatol. Photoimmunol. Photomed. 2011;27:261–277. doi: 10.1111/j.1600-0781.2011.00606.x. [DOI] [PubMed] [Google Scholar]
- 107.Novák Z., Bónis B., Baltás E., Ocsovszki I., Ignácz F., Dobozy A., Kemény L. Xenon chloride ultraviolet B laser is more effective in treating psoriasis and in inducing T cell apoptosis than narrow-band ultraviolet B. J. Photochem. Photobiol. B Biol. 2002;67:32–38. doi: 10.1016/S1011-1344(02)00280-4. [DOI] [PubMed] [Google Scholar]
- 108.Manstein D., Herron G.S., Sink R.K., Tanner H., Anderson R.R. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2004;34:426–438. doi: 10.1002/lsm.20048. [DOI] [PubMed] [Google Scholar]
- 109.Bogdan Allemann I., Kaufman J. Fractional photothermolysis—An update. Lasers Med. Sci. 2010;25:137–144. doi: 10.1007/s10103-009-0734-8. [DOI] [PubMed] [Google Scholar]
- 110.Lu G.W., Gao P. Handbook of Non-Invasive Drug Delivery Systems. Elsevier; Amsterdam, The Netherlands: 2010. Emulsions and microemulsions for topical and transdermal drug delivery; pp. 59–94. [DOI] [Google Scholar]
- 111.Karadzovska D., Brooks J.D., Monteiro-Riviere N.A., Riviere J.E. Predicting skin permeability from complex vehicles. Adv. Drug Deliv. Rev. 2013;65:265–277. doi: 10.1016/j.addr.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 112.Kim B., Cho H.-E., Moon S.H., Ahn H.-J., Bae S., Cho H.-D., An S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020;4:1–12. doi: 10.1186/s41702-019-0053-z. [DOI] [Google Scholar]
- 113.Haque T., Talukder M.M.U. Chemical enhancer: A simplistic way to modulate barrier function of the stratum corneum. Adv. Pharm. Bull. 2018;8:169. doi: 10.15171/apb.2018.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manconi M., Caddeo C., Sinico C., Valenti D., Mostallino M.C., Lampis S., Monduzzi M., Fadda A.M. Penetration enhancer-containing vesicles: Composition dependence of structural features and skin penetration ability. Eur. J. Pharm. Biopharm. 2012;82:352–359. doi: 10.1016/j.ejpb.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 115.Liu X., Yao Z., Wang Y., Chai L., Zhou X. Vitamin D analogs combined with different types of phototherapy in the treatment of vitiligo: A systematic review of randomized trials and within-patient studies. Int. Immunopharmacol. 2022;109:108789. doi: 10.1016/j.intimp.2022.108789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.