Abstract
Gram-negative rods (GNR) carrying the transferable carbapenem resistance gene blaIMP, including Pseudomonas aeruginosa and Serratia marcescens, have been isolated from more than 20 hospitals in Japan. Although the emergence of such multiple-drug-resistant bacteria is of utmost clinical concern, little information in regard to the distribution of blaIMP-positive GNR in hospitals and the clinical characteristics of infected patients is available. To address this, a system for the rapid detection of the blaIMP gene with a simple DNA preparation and by enzymatic detection of PCR products was developed. A total of 933 ceftazidime-resistant strains of GNR isolated between 1991 and 1996 at Nagasaki University Hospital, Nagasaki, Japan, were screened for the blaIMP gene; 80 isolates were positive, including 53 P. aeruginosa isolates, 13 other glucose-nonfermenting bacteria, 13 S. marcescens isolates, and 1 Citrobacter freundii isolate. Most of the patients from whom blaIMP-positive organisms were isolated had malignant diseases (53.8%). The organisms caused urinary tract infections, pneumonia, or other infections in 46.3% of the patients, while they were just colonizing the other patients evaluated. It was possible that blaIMP-positive P. aeruginosa strains contributed to the death of four patients, while the other infections caused by GNR carrying blaIMP were not lethal. DNA fingerprinting analysis by pulsed-field gel electrophoresis suggested the cross transmission of strains within the hospital. The isolates were ceftazidime resistant and were frequently resistant to other antibiotics. Although no particular means of pathogenesis of blaIMP-positive GNR is evident at present, the rapid detection of such strains is necessary to help with infection control practices for the prevention of their dissemination and the transmission of the resistance gene to other pathogenic bacteria.
Carbapenems are the most potent agents used for chemotherapy of infectious diseases caused by gram-negative rods (GNR) due to their high affinity for PBP 2, stability to most β-lactamases including extended-spectrum β-lactamases, and excellent permeation across bacterial outer membranes (3). β-Lactamases hydrolyzing carbapenems identified as metallo-β-lactamases have been found in a limited number of species, such as Bacillus cereus (9, 10), Aeromonas hydrophila (11, 19, 22), Flavobacterium odoratum (17), Legionella gormanii (5), Bacteroides fragilis (6, 15, 25, 28), and Stenotrophomonas (Xanthomonas) maltophilia (16). Recently, however, a novel plasmid-mediated metallo-β-lactamase, IMP-1, which is produced in the presence of the blaIMP gene, was found in Pseudomonas aeruginosa (20, 21, 27), Serratia marcescens (1, 8), and other members of the family Enterobacteriaceae (21), which frequently cause nosocomial outbreaks. Several S. marcescens and P. aeruginosa strains carrying the blaIMP gene have been isolated multifocally in more than 20 hospitals in Japan, including our own hospital (20, 21).
The emergence of the multidrug-resistant bacteria is a phenomenon of concern to the clinician and the pharmaceutical industry, because the multidrug resistance is a major cause of treatment failure in the treatment of infectious disease (4). IMP-1 belongs to β-lactamase subgroup 3a in Bush’s functional group and is able to hydrolyze a wide variety of β-lactams including penicillins, cephems, and carbapenems (14), so that blaIMP-positive GNR are also a threat. In particular, the dissemination of these types of bacteria and nosocomial outbreaks are of utmost clinical concern. However, the spread of strains of GNR carrying the blaIMP gene in each hospital has not been reported. Also, the clinical characteristics of and the outcomes for patients infected with blaIMP-positive GNR have not been clarified.
To address these points, we first developed a rapid and simple method for the detection of the blaIMP gene in which we used a combination of a simple DNA preparation method and the enzymatic detection of the PCR products. We applied this method to the screening of clinical isolates recovered between 1991 and 1996 in our hospital. We then reviewed retrospectively the clinical charts of the patients infected with GNR carrying the blaIMP gene to clarify their clinical characteristics and significance. Genomic DNA fingerprinting profiles were also established by pulsed-field gel electrophoresis (PFGE) to examine how these resistant strains disseminated in the hospital.
(This work was presented in part at the 97th General Meeting of the American Society for Microbiology, Miami, Fla., 4 to 8 May 1997 [7].)
MATERIALS AND METHODS
Bacterial strains.
The study was conducted at Nagasaki University Hospital, an 829-bed hospital in Nagasaki, Japan. Freeze-dried, stock clinical strains isolated between 1991 and 1996 were used in the study. The isolates were identified with Vitek Gram-Negative Identification cards (bioMerieux-Vitek, Inc., Hazelwood, Mo.). The MIC of ceftazidime (CAZ) for all GNR carrying the blaIMP gene has been reported to be greater than 128 μg/ml (20, 21). Therefore, isolates judged to be CAZ resistant (CAZ MIC, ≥64 μg/ml) by broth microdilution MIC testing were selected for detection of the blaIMP gene. Over the 6-year study period, a total of 933 CAZ-resistant isolates of GNR were available for evaluation. A total of 1,325 clinical isolates were screened for the blaIMP gene since an additional 210 CAZ-susceptible and imipenem (IPM)-resistant and 182 CAZ-susceptible and IPM-susceptible P. aeruginosa isolates were included as controls (Table 1).
TABLE 1.
Isolates of GNR screened for blaIMP gene
| Organism | Phenotypea | No. of isolates tested | No. (%) of isolates positive for blaIMP |
|---|---|---|---|
| Pseudomonas aeruginosa | CAZ-R | 208 | 53 (25.5) |
| Pseudomonas aeruginosa | CAZ-S, IMP-R | 210 | 0 (0) |
| Pseudomonas aeruginosa | CAZ-S, IMP-S | 182 | 0 (0) |
| Stenotrophomonas maltophilia | CAZ-R | 167 | 0 (0) |
| Other GNF bacteria | CAZ-R | 41 | 13 (31.7) |
| Escherichia coli | CAZ-R | 10 | 0 (0) |
| Enterobacter species | CAZ-R | 298 | 0 (0) |
| Citrobacter species | CAZ-R | 125 | 1 (0.8) |
| Serratia marcescens | CAZ-R | 82 | 13 (15.9) |
| Proteus species | CAZ-R | 2 | 0 (0) |
R, resistant (MIC, ≥64 μg/ml); S, susceptible (MIC, ≤4 μg/ml).
Eight strains of P. aeruginosa carrying the blaIMP gene and isolated in 1992 and 1993 (20) were used as positive controls for the detection of the blaIMP gene. P. aeruginosa ATCC 27853 was used as a negative control and for quality control of the antimicrobial susceptibility test.
Preparation of samples and DNA amplification.
Template DNA for PCR was prepared by heat lysis of the bacteria in a PCR mixture as described by Clark et al. (2), with some modification. One or two bacterial colonies from a plate that had been incubated overnight were suspended in 100 μl of sterile water and were then diluted to a concentration of approximately 106 CFU/ml in a sterile 96-well microplate. The suspension was used as the template for amplification by PCR. PCRs were carried out in 50-μl volumes containing 2.5 U of recombinant Taq DNA polymerase (Takara Shuzo Co., Ltd., Shiga, Japan), 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dTTP), primers at 0.5 μM each, and 20 μl of diluted bacterial suspension. The oligonucleotide primers were selected from published sequences (1, 21) and were as follows: sense, 5′-CTA CCG CAG CAG AGT CTT TG-3′; antisense, 5′-AAC CAG TTT TGC CTT ACC AT-3′. For enzymatic detection, the sense and antisense primers were labeled at their 5′ ends with biotin and digoxigenin, respectively. Amplification was conducted with a Perkin-Elmer Cetus model 9600 DNA thermal cycler programmed as follows: bacterial lysis and release of DNA for 10 min at 95°C and 35 cycles of 30 s of denaturation at 94°C, 30 s of annealing at 58°C, and 30 s of extension at 72°C; the products were held at 4°C until analysis.
ED-PCR.
Enzymatic detection of PCR products (ED-PCR) was performed as described by Ubukata et al. (26), with some modification. A 96-well immunoplate (Nunc, Roskilde, Denmark) was coated with 10 μg of streptavidin (Southern Biotechnology Associates, Inc., Birmingham, Ala.) per ml overnight at 4°C and was blocked with 0.1% gelatin (Bio-Rad Laboratories, Hercules, Calif.) for 3 h at 37°C. A total of 20 μl of the amplified mixture and 100 μl of anti-digoxigenin antibody conjugated with alkaline phosphatase (300 mU/ml; Boehringer Mannheim, Mannheim, Germany) were inoculated into the wells. The plate was incubated at 37°C for 60 min and was washed three times, and each well was inoculated with 100 μl of p-nitrophenylphosphate in diethanolamine buffer (1 mg/ml; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). After a 30-min incubation at 37°C, the optical density (OD) at 410 nm (OD410) was measured with a microplate reader.
Review of hospital charts.
The hospital charts of the patients from whom blaIMP-positive GNR were isolated were reviewed, and the following information was recorded: age, sex, ward, underlying diseases, use of antibiotics before and after the isolation, and the results of serial bacterial examinations. Patients infected or colonized with blaIMP-positive GNR were divided into three categories on the basis of clinical significance: (i) infection, if the patients showed both an acute systemic inflammatory response (e.g., fever and elevation of C-reactive protein levels) and local infectious signs or symptoms (e.g., purulent sputum and pyuria); (ii) colonization, if the patients displayed no local infectious signs or symptoms, even though they had a systemic inflammatory response (e.g., an isolate in the urine of a patient without pyuria); and (iii) unknown for patients for whom insufficient information was available.
DNA fingerprinting analysis by PFGE.
Total DNA was prepared from each strain and was digested with the restriction enzymes SpeI and XbaI for P. aeruginosa and S. marcescens, respectively, by using GenePath group reagent kits (Bio-Rad Laboratories) according to the manufacturer’s instructions. The samples were electrophoresed with the Gene Navigator System (Pharmacia LKB Biotechnology, Uppsala, Sweden) at 180 V for 22 h with pulse times ranging from 10 to 45 s (20) and at 170 V for 22 h with pulse times ranging from 5 to 50 s for P. aeruginosa and S. marcescens, respectively. A bacteriophage lambda DNA ladder (Bio-Rad) was used as a molecular size marker.
The DNA restriction patterns for P. aeruginosa produced by PFGE were subjected to computer-assisted analysis with Dendron software (Soltech, Inc., Oakdale, Calif.) (18). The Dendron package calculated the similarity value (SAB) for each pair of fingerprints on the basis of band position. SAB ranges from 0 to 1.0, where 0 indicates that the fingerprints have no bands in common and 1.0 indicates that the fingerprints are identical. In the evaluation of the DNA restriction patterns for S. marcescens, if the numbers of fragment differences were six or fewer, the fragment patterns were considered derivatives of a common ancestor (24).
Antibiotics and antimicrobial susceptibility tests.
Antimicrobial susceptibility tests for blaIMP-positive GNR were performed by the microdilution method with cation-adjusted Mueller-Hinton broth (BBL Microbiology Systems, Cockeysville, Md.) according to the recommendations of the National Committee for Clinical Laboratory Standards (12). The MICs of potent antibiotics for P. aeruginosa, including piperacillin (Toyama Chemical Co., Ltd., Toyama, Japan), cefozopran (Takeda Chemical Industries, Ltd., Osaka, Japan), CAZ (Japan Glaxo Co., Tokyo, Japan), aztreonam (Eizai Co., Ltd., Tokyo, Japan), IPM (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan), meropenem (Sumitomo Pharmaceutical Ltd., Osaka, Japan), gentamicin (Schering-Plough K.K., Osaka, Japan), and ciprofloxacin (Bayer Yakuhin, Ltd., Osaka, Japan), were determined.
RESULTS
Isolation of blaIMP-positive GNR by ED-PCR.
By using positive controls, blaIMP-positive P. aeruginosa strains could be detected with the naked eye as a change in the color of the wells to yellow (the OD410 ranged between 0.611 and 1.411) without electrophoresis and with equivalent sensitivity to ethidium bromide staining. CAZ-susceptible and IMP-resistant P. aeruginosa isolates (210 strains) and those susceptible to both CAZ and IPM (182 strains) were all negative for the blaIMP gene by ED-PCR (OD410, <0.104).
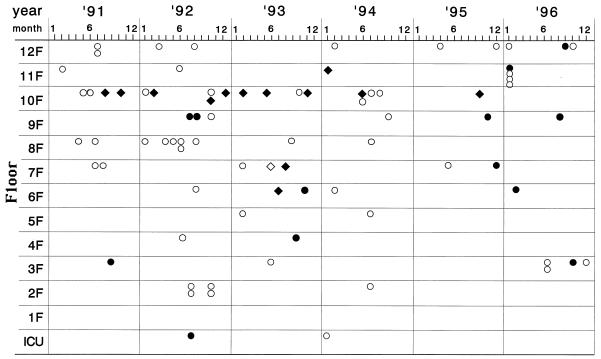
During the 6-year study period, 80 of 933 CAZ-resistant strains of GNR were positive for the blaIMP gene by ED-PCR; 53 P. aeruginosa isolates, 13 other glucose nonfermenting (GNF) bacteria (the other GNF bacteria included 11 Pseudomonas putida isolates, 1 Pseudomonas stutzeri isolate, and 1 Alcaligenes xylosoxidans isolate), 13 S. marcescens isolates, and 1 Citrobacter freundii isolate (Table 1). Figure 1 demonstrates the isolation time of each of the GNR and clinical ward from which each of the blaIMP-positive GNR were isolated. The greatest numbers of blaIMP-positive P. aeruginosa isolates (18 of 53 patients; 34.0%) were obtained in 1992. P. aeruginosa and other GNF bacteria were distributed among many floors and wards, while most S. marcescens strains (10 of 13) were isolated from a specific floor. The isolation of blaIMP-positive S. marcescens strains peaked in 1993, and then the rate of isolation decreased and the organisms disappeared. No significant annual increase in the rate of isolation of whole blaIMP-positive isolates was observed.
FIG. 1.
Distribution of blaIMP-positive GNR detected by a combination of heat lysis of bacteria and ED-PCR in Nagasaki University Hospital between 1991 and 1996. ○, P. aeruginosa; •, other GNF bacteria; ⧫, S. marcescens; ◊, C. freundii; ICU, intensive care unit.
Characteristics of the patients infected or colonized with blaIMP-positive GNR.
The age range of the patients from whom blaIMP-positive P. aeruginosa was isolated was wide (0 to 87 years) compared with that of the patients from whom other GNR were isolated (41 to 85 years). The predominant source of isolation was the urinary tract (40.0%), followed by the respiratory tract (18.8%) and abscesses, pus, and wounds (15.0%) for P. aeruginosa. In addition to the urinary tracts, P. putida and S. marcescens were also frequently isolated from bile (5 and 6 of 13 isolates, respectively); all patients had an indwelling percutaneous bile catheter.
Most of the patients had malignant diseases (53.8%). Systemic administration of antineoplastic agents was noted only for the patients infected with P. aeruginosa.
In total, blaIMP-positive GNR including P. aeruginosa were thought to have caused infection in 46.3% of patients. blaIMP-positive P. aeruginosa strains were thought to have caused infection in 50.9% of the patients. Urinary tract infections (40.7%) and pneumonia (22.2%) were the predominant infections caused by P. aeruginosa. In contrast, among the patients infected or colonized with S. marcescens isolates, most (61.5%) were thought to be only colonized. For 16 patients (20.0%), no antibiotics had been administered before the isolation of blaIMP-positive GNR, while carbapenems and cephems had been administered to 12 (15.0%) and 31 (38.8%) patients, respectively.
A C. freundii isolate carrying the blaIMP gene was isolated from the midstream urine of an 85-year-old male patient under rehabilitation after acute myocardial infarction. His urinary catheter had been removed a month prior to the isolation of the organism, and he had not received any antibiotics during that month. The organism caused cystitis and was eliminated by the administration of ofloxacin with improvement of clinical symptoms and inflammatory signs.
Outcomes for the patients infected with blaIMP-positive GNR.
Serial bacterial examination was not performed for 43 patients (53.8%). Of the 53 patients infected or colonized with blaIMP-positive P. aeruginosa, for only 7 patients (13.2%) the bacteria were eliminated by subsequent antibiotic treatment (4 patients) or by removal of the urinary catheter without the administration of antibiotics (3 patients). In contrast, the isolation of organisms persisted for 16 patients (20.0%), despite treatment with several antibiotics. In the patients infected or colonized with blaIMP-positive GNR other than P. aeruginosa, rates of elimination were higher than the rate of elimination of P. aeruginosa (46.5% for both S. marcescens and other GNR). In total, elimination of blaIMP-positive GNR was confirmed for 20 patients (25.0%) following antibiotic therapy (16 patients), removal of the urinary catheter (3 patients), or no treatment (1 patient), while isolations persisted for 17 patients (21.3%).
Of the 13 patients who died after becoming infected with blaIMP-positive P. aeruginosa, infection was thought to have been the possible cause of death in 4 patients: pneumonia in a patient with adult T-cell leukemia during therapy with antineoplastic agents, clinical sepsis in a patient with chronic renal failure, and terminal pneumonia in two patients with esophageal cancer. No patients died of infection due to blaIMP-positive GNR other than P. aeruginosa.
DNA fingerprinting analysis by PFGE.
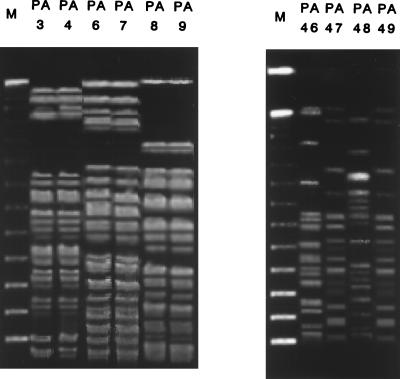
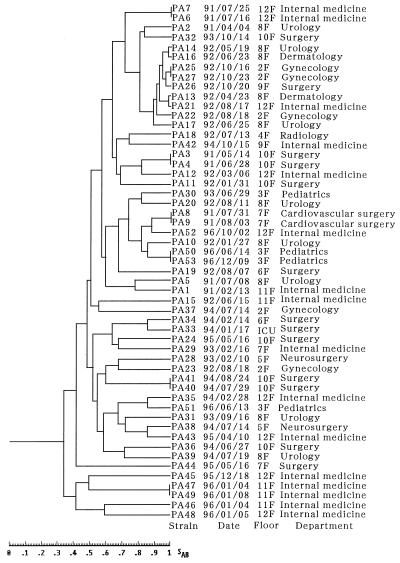
Figure 2 presents some of the profiles of the DNA of P. aeruginosa isolates obtained by fingerprinting by PFGE and SpeI digestion. PFGE patterns could not be distinguished in some lanes (lanes PA3 and PA4, PA6 and PA7, and PA8 and PA9 in Fig. 2A and PA47 and PA49 in Fig. 2B), suggesting nosocomial spread. On the other hand, two strains isolated on the same ward and on the same day, such as the isolates in lanes PA46 and PA47 (Fig. 2B), had different PFGE profiles. The dendrogram based on the computer-assisted comparison of the PFGE profiles of all strains tested is shown in Fig. 3. The date, floor, and department of isolation are also included. The PFGE profiles were divided into 28 groups when the cutoff of the SAB was set at 0.75. When the cutoff of the SAB was set at 0.90, the strains could be divided into 40 groups. The spread of blaIMP-positive P. aeruginosa by clusters of related strains in 1992 (strains PA13, PA14, PA16, PA17, PA21, PA22, PA25, PA26, and PA27) was suggested, while a great diversity was found previously and subsequently.
FIG. 2.
DNA fingerprinting profiles for clinical isolates of blaIMP-positive P. aeruginosa. Total DNA was prepared for each clinical isolate, digested with SpeI, and then subjected to PFGE. Lanes M, DNA molecular size marker. The strains suggesting typical nosocomial spread (A) and whose profiles are discrepant (B) are shown. (A) Three pairs of isolates showing indistinguishable PFGE profiles isolated on the same ward during a short period in 1991. Four strains isolated within 5 days in 1996. The PFGE profiles could not be distinguished for the strains in lanes PA47 and PA49, while those profiles that were different from each other were found for the strains in lanes PA46 and PA47 isolated on the same day.
FIG. 3.
Dendrogram based on computer-assisted comparison of PFGE profiles of 53 isolates of blaIMP-positive P. aeruginosa and their isolation date, floor, and department.
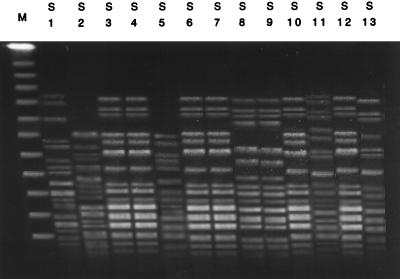
Figure 4 presents the profiles of the DNA of S. marcescens isolates obtained by fingerprinting by PFGE and XbaI digestion. The samples were also ordered by date of isolation. The PFGE patterns were varied and divided the strains into six groups. Genetically related strains, such as those in lanes 3, 4, 6, 7, 10, and 12, were isolated on the same ward, suggesting the nosocomial spread of the organism.
FIG. 4.
DNA fingerprinting profiles for the clinical isolates of blaIMP-positive S. marcescens. Total DNA was prepared for each clinical isolate, digested with XbaI, and then subjected to PFGE. Lanes M, DNA molecular size marker; S1 to S13, blaIMP-positive S. marcescens ordered by date of isolation in the 6-year study period. The PFGE profiles of the lanes 3, 4, 6, 7, 10, and 12 suggest that these isolates are genetically related to each other.
Antimicrobial susceptibility tests.
The distributions of the MICs of the antibiotics tested are listed in Table 2. All strains tested were resistant to CAZ (MIC, ≥64 μg/ml), while their levels of resistance to carbapenems varied. Notably, the IMP MIC at which 50% strains are inhibited for blaIMP-positive P. aeruginosa was 8 μg/ml. Ciprofloxacin was relatively potent against the entire group of blaIMP-positive GNR. Aztreonam also had potent activity against P. aeruginosa, S. marcescens, and C. freundii isolates. Moreover, some P. aeruginosa, S. marcescens, and C. freundii isolates, particularly S. marcescens, were susceptible to gentamicin.
TABLE 2.
Antimicrobial susceptibilities of isolates of GNR positive for blaIMP gene
| Drug | MIC (μg/ml)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
P. aeruginosa (n = 53)
|
Other GNF bacteria (n = 13)
|
S. marcescens (n = 13)
|
|||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | |
| Piperacillin | 16–>128 | 64 | >128 | 32–>128 | >128 | >128 | 16–>128 | 16 | >128 |
| CAZ | 64–>128 | >128 | >128 | 64–>128 | 128 | >128 | 64–>128 | >128 | >128 |
| Aztreonam | 1–>128 | 8 | 128 | 16–>128 | 128 | >128 | 1–>128 | 2 | 16 |
| Cefozopran | 128–>128 | >128 | >128 | 128–>128 | >128 | >128 | 128–>128 | >128 | >128 |
| IPM | 2–>128 | 8 | >128 | 8–>128 | 128 | >128 | 16–>128 | >128 | >128 |
| Meropenem | 4–>128 | 16 | >128 | 8–>128 | >128 | >128 | 128–>128 | >128 | >128 |
| Gentamicin | 0.25–>128 | >128 | >128 | 16–>128 | >128 | >128 | 2–>128 | 4 | 32 |
| Ciprofloxacin | 0.06–32 | 4 | 32 | 0.125–32 | 16 | 32 | 0.5–>128 | 2 | 4 |
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
DISCUSSION
P. aeruginosa and other GNR are opportunistic pathogens and frequently cause severe infections in immunocompromised hosts. Since antibiotics are frequently ineffective against carbapenem-resistant GNR since they are often multidrug resistant, early recognition and an understanding of how these resistant organisms spread are important. Carbapenem resistance in GNR caused by metallo-β-lactamases is of particular importance because it is plasmid mediated and is transferable to other GNR (1, 8, 20, 27). Thus, we applied the combination of heat lysis of the bacteria and ED-PCR for the rapid detection of the blaIMP gene from stored clinical isolates. Recently, Senda et al. (21) reported on the detection of the blaIMP gene by PCR. Since our method is simpler, uses 96-well immunoplates, and eliminates the agarose gel electrophoresis step, several hundred bacterial isolates could be screened at once without using ethidium bromide, a possible carcinogen.
In this study, it was shown that 80 clinical strains of GNR carrying the blaIMP gene were isolated in our hospital during the 6-year study period. P. aeruginosa was identified in 66.7% of blaIMP-positive isolates of GNR, followed by S. marcescens and P. putida. This is the first report indicating that clinical isolates of C. freundii and P. stutzeri carry the blaIMP gene. A total of 4,679 P. aeruginosa strains including 266 CAZ-resistant strains were isolated during the study period in our hospital. Of 266 CAZ-resistant isolates, 208 could be tested in this study. P. aeruginosa was the predominant blaIMP-positive GNR at the end of 1992. Subsequently, S. marcescens was frequently isolated in 1993. Although the blaIMP gene was detected in many isolates of both P. aeruginosa and S. marcescens in our hospital, it could not be proven whether the blaIMP gene was transferred from one species to another in nature or whether they acquired the resistance gene independently.
The fact that most patients had malignant diseases may suggest that malignancy is a possible risk factor for the isolation of blaIMP-positive GNR. Three patients were thought to have died of pneumonia due to blaIMP-positive P. aeruginosa. However, such pneumonia was found in the patients with severe underlying diseases only, so it is unclear whether the presence of the blaIMP gene contributed to their deaths. Other GNF bacteria and S. marcescens frequently colonize the bile ducts of patients with indwelling percutaneous bile catheters. The virulence of these bacteria did not seem to be significant in this study. Prior use of carbapenems was confirmed for only 15.0% of the patients, indicating that selective pressure from carbapenems is not always required for the isolation of blaIMP-positive GNR, while 38.8% of patients were administered cephems prior to the isolation of blaIMP-positive GNR. blaIMP-positive GNR were also isolated from antibiotic-free patients, suggesting that the resistant organisms can spread without the use of antibiotics, possibly as hospital infections.
The MIC data indicated that most isolates were resistant to several classes of antibiotics. Aztreonam and ciprofloxacin had relatively potent antimicrobial activities. Some strains had a carbapenem-sensitive phenotype, even though they carried the blaIMP gene. Probable reasons for this are as follows. (i) A secondary regulatory system, in addition to the structural gene, may exist so that blaIMP genes are sometimes silent, and (ii) the rate at which the carbapenems are hydrolyzed may be low due to a gene dosage effect related to the plasmid copy number.
GNR carrying the blaIMP gene were eliminated from some patients following antibiotic administration. The antibiotics used and their potencies against the organisms varied. Therefore, it is unclear whether the antibiotics contributed to the elimination of the blaIMP-positive GNR.
In conclusion, the clinical implications of this study, that multidrug-resistant GNR carrying the blaIMP gene will spread and cause further hospital outbreaks, are of concern. Since P. aeruginosa is one of the most frequently isolated organisms in large hospitals, the increase in the number of blaIMP-positive P. aeruginosa isolates may promote the transmission of the resistance gene to other virulent GNR such as Escherichia coli and Klebsiella pneumoniae. Although most blaIMP-positive GNR detected in our study were thought to have low levels of virulence, we must be cautious about the trend toward the subsequent isolation of such resistant bacteria. Prospective screening for blaIMP-positive GNR and evaluation of the clinical characteristics of patients infected or colonized with such isolates are thought to be useful. The discovery and design of inhibitors of metallo-β-lactamases are also necessary to overcome the threat of these multidrug-resistant bacteria (13, 23).
ACKNOWLEDGMENTS
We are grateful to Chikako Mochida, Fumiaki Iori, Yumi Ozaki, and Kazuyuki Sugahara for technical expertise. We also thank Noriko Kajitani for assistance with the preparation of the manuscript.
This study was supported in part by a grant from the Alumni Association of Nagasaki University School of Medicine in 1996 (to Y. Hirakata), a grant for the Study of Drug-Resistant Bacteria funded by the Japanese Ministry of Health and Welfare in 1996 (to Y. Hirakata), a Grant-in-Aid for Scientific Research (09770186) from the Japanese Ministry of Education, Culture, Sports, and Science in 1997 (to Y. Hirakata), and a grant from the Kurozumi Medical Foundation in 1997 (to Y. Hirakata).
REFERENCES
- 1.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark N C, Cooksey R C, Hill B C, Swenson J M, Tenover F C. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993;37:2311–2317. doi: 10.1128/aac.37.11.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornaglia G, Russell K, Satta G, Fontana R. Relative importances of outer membrane permeability and group 1 β-lactamase as determinants of meropenem and imipenem activities against Enterobacter cloacae. Antimicrob Agents Chemother. 1995;39:350–355. doi: 10.1128/aac.39.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies J. Inactivation of antibiotics and the dissemination of resistant genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 5.Fujii T, Sato K, Miyata M, Mitsuhashi S. Biochemical properties of β-lactamase produced by Legionella gormanii. Antimicrob Agents Chemother. 1986;29:925–926. doi: 10.1128/aac.29.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herberg M, Edlund C, Lindqvist L, Rylander M, Nord C E. Purification and characterization of an imipenem hydrolyzing metallo β-lactamase from Bacteroides fragilis. Antimicrob Agents Chemother. 1992;29:105–113. doi: 10.1093/jac/29.2.105. [DOI] [PubMed] [Google Scholar]
- 7.Hirakata Y, Izumikawa K, Yamaguchi T, Matsuda J, Tomono K, Koga H, Yamada Y, Kohno S, Kamihara S. Abstracts of 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Distribution and clinical background of Pseudomonas aeruginosa carrying the metallo-β-lactamase gene blaIMP, abstr. L17; p. 376. [Google Scholar]
- 8.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwabara S, Lloyd P H. Protein and carbohydrate moieties of a preparation of β-lactamase II. Biochem J. 1971;124:215–220. doi: 10.1042/bj1240215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim H M, Pene J J, Shaw R M. Cloning, nucleotide sequence, and expression of the Bacillus cereus 5/B/6 β-lactamase II structural gene. J Bacteriol. 1988;172:2873–2878. doi: 10.1128/jb.170.6.2873-2878.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massida O, Rossolimi G M, Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among class B metallo-β-lactamases. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically. 2nd ed. Approved standard. NCCLS document M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 13.Payne D J, Bateson J H, Gasson B C, Proctor D, Khushi T, Farmer T H, Tolson D A, Bell D, Skett P W, Marshall A C, Reid R, Ghosez L, Combret Y, Marchand-Brynaert J. Inhibition of metallo-β-lactamases by a series of mercaptoacetic acid thiol ester derivatives. Antimicrob Agents Chemother. 1997;41:135–140. doi: 10.1128/aac.41.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen B A, Gluzman Y, Tally F P. Cloning and sequencing of the class B β-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990;34:1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saino Y, Kobayashi F, Inoue M, Mitsuhashi S. Purification and properties of inducible penicillin β-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982;22:564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato K, Fujii T, Okamoto R, Inoue M, Mitsuhashi S. Biochemical properties of β-lactamase produced by Flavobacterium odoratum. Antimicrob Agents Chemother. 1985;27:612–614. doi: 10.1128/aac.27.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid J, Voss E, Soll D R. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J Clin Microbiol. 1990;28:1236–1243. doi: 10.1128/jcm.28.6.1236-1243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segatore B, Massida O, Satta G, Setacci D, Amicosante G. High specificity of cphA-encoded metallo-β-lactamase from Aeromonas hydrophilia AE036 for carbapenems and its contribution to β-lactam resistance. Antimicrob Agents Chemother. 1993;37:1324–1328. doi: 10.1128/aac.37.6.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, Ohta M. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senda K, Arakawa Y, Ichiyama S, Nakashima K, Ito H, Ohsuka S, Shimokata K, Kato N, Ohta M. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams. J Clin Microbiol. 1996;34:2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon K, King A, Phillips I. β-Lactamases with high activity against imipenem and Sch 34343 from Aeromonas hydrophilia. J Antimicrob Chemother. 1986;17:45–50. doi: 10.1093/jac/17.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Strynadka N C, Jensen S E, Johns K, Blanrhard H, Page M, Matagne A, Frere J M, James M N. Structural and kinetic characterization of a β-lactamase-inhibitor protein. Nature. 1994;368:657–660. doi: 10.1038/368657a0. [DOI] [PubMed] [Google Scholar]
- 24.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson J S, Malamy M H. Sequencing the gene for an imipenem-cefoxitin-hydrolyzing enzyme (CfiA) from Bacteroides fragilis TAL2480 reveals strong similarity between CfiA and Bacillus cereus β-lactamase II. J Bacteriol. 1990;172:2584–2593. doi: 10.1128/jb.172.5.2584-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ubukata K, Nakagami S, Nitta A, Yamane A, Kawakami S, Sugiura M, Konno M. Rapid detection of mecA gene in methicillin-resistant staphylococci by enzymatic detection of polymerase chain reaction products. J Clin Microbiol. 1992;30:1728–1733. doi: 10.1128/jcm.30.7.1728-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yotsuji A, Minami S, Inoue M, Mitsuhashi S. Properties of novel β-lactamase produced by Bacteroides fragilis. Antimicrob Agents Chemother. 1983;24:925–929. doi: 10.1128/aac.24.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]