Abstract
Background:
Oxygen is the commonest intervention provided to critically ill patients requiring mechanical ventilation. Despite this, it is unclear how much oxygen should be administered to patients in order to promote the best clinical outcomes and it has been suggested that a strategy of conservative oxygen therapy (COT) may be advantageous. We therefore sought to answer the question of whether COT versus usual or liberal oxygen therapy was beneficial to adult patients receiving mechanical ventilation on an intensive care unit (ICU) by performing a systematic review and meta-analysis.
Methods:
Studies were included if they were randomised controlled trials comparing COT to liberal or usual oxygen therapy strategies in acutely ill adults (aged ⩾18 years) admitted to an ICU, and reported an outcome of interest. Studies were excluded if they were limited to a specific single disease diagnosis. The review was registered on PROSPERO (CRD42022308436). Risk of bias was assessed using a modified Cochrane Risk of Bias assessment tool. Effect estimates were pooled using a random effects model with the between study variance estimated using restricted maximum likelihood and standard errors calculated using the method of Hartung-Knapp/Sidik-Jonkman. Between study heterogeneity was quantified using the I2 statistic. The certainty in the body of evidence was assessed using GRADE criteria.
Results:
Nine eligible studies with 5727 participants fulfilled all eligibility criteria. Trials varied in their definitions of COT and liberal or usual oxygen therapy. The pooled estimate of risk ratio for 90 day mortality for COT versus comparator was 0.99 (95% confidence interval 0.88–1.12, 95% prediction interval 0.82–1.21). There was low heterogeneity among studies (I2 = 22.4%). The finding that mortality was similar for patients managed with COT or usual/liberal oxygen therapy was graded as moderate certainty.
Conclusions:
In critically ill adults admitted to an ICU, COT is neither beneficial nor harmful when compared to usual or liberal oxygen therapy. Trials to date have been inconsistent in defining both COT and liberal or usual oxygen therapy, which may have had an impact on the results of this meta-analysis. Future research should focus on unifying definitions and outcome measures.
Keywords: Oxygen, critical illness, systematic review, meta-analysis
Introduction
The titration of supplemental oxygen to achieve a minimum threshold or specific range of arterial oxygenation, either in terms of peripheral oxygen saturation (SpO2) or arterial partial pressure of oxygen (PaO2) is one of the commonest interventions for patients on an intensive care unit (ICU). This practice has traditionally focused on maintaining adequate arterial oxygenation in order to prevent the potential harm that may occur secondary to tissue hypoxia. However, evidence exists to suggest that an excessively high fraction of inspired oxygen (FIO2) and/or PaO2 may be detrimental to lung function and lead to excessive mortality, commonly termed oxygen toxicity.1,2 It is also plausible that patients receiving mechanical ventilation are at greater risk of harm from the toxic effects of oxygen through exacerbation of pathophysiology related to ventilator-induced lung injury. 3 As a result, a strategy of ‘permissive hypoxaemia’ was proposed for the management of patients requiring mechanical ventilation with the hypothesis that lowering the FIO2 would reduce the impact of oxygen toxicity and therefore improve survival.4 –6 Following a number of conflicting retrospective database analyses7 –9 an era of randomised controlled trials (RCTs) began, to evaluate what is now termed ‘conservative oxygen therapy’ (COT). These trials have sought to determine whether administering less oxygen to patients, by targeting lower levels of arterial oxygenation results in improved survival from critical illness. Trials of this nature are now being published in rapid succession, so it is important to update the process of data synthesis in order to understand the overall picture of benefit versus harm for COT.
The aim of this systematic review and meta-analysis was to provide an up-to-date synopsis of currently published data related to the evaluation of COT in a mixed (heterogenous) cohort of critically ill adults requiring mechanical ventilation on an ICU.
Methods
This review was registered on PROSPERO (CRD42022308436) on May 4th 2022; no formal protocol manuscript was prepared. The review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines. 10
Search strategy
Searches were conducted on September 6th 2022 of the following databases:
MEDLINE
Embase
Cochrane Central Register of Controlled Trials (CENTRAL)
VHL Regional Portal (LILICS, IBECS, BBO Dentistry, CUMED and Index Psychology)
The WHO International Clinical Trials Registry Portal (ICTRP) was searched on September 6th 2022. Full details of the search strategy, which was based on a previously published systematic review of COT in hospitalised patients, 11 are provided in the Supplemental Data. Reference lists of review articles identified by the search were also screened to identify any additional relevant studies.
Selection criteria
Studies were included if they met the inclusion of being an RCT comparing liberal (or usual) oxygenation to conservative oxygenation in acutely ill adults (aged ⩾18 years) admitted to an ICU, and reported an outcome of interest. Studies were excluded if they were limited to: patients undergoing elective surgery, patients with chronic respiratory illness, extracorporeal life support, hyperbaric oxygen therapy, comparison between different oxygen delivery modalities, pregnant patients or patients with psychiatric illness. We restricted this further to identify studies of general critically ill patients, including heterogeneous syndromes such as sepsis or acute respiratory distress syndrome (ARDS) but excluding specific groups such as patients admitted to critical care following cardiac arrest or stroke.
Two reviewers (DM and HM) independently screened titles and abstracts. The full text of all studies that either reviewer identified as potentially eligible were retrieved and the same two reviewers independently screened these against the inclusion and exclusion criteria, with any disagreements resolved through consensus.
Analysis
Two reviewers (DM and DH) independently extracted data for eligible studies using a standardised pro forma. The outcome of interest was mortality at 90 days following randomisation. Where this was not available, either the closest available time point prior to 90 days (e.g. 30 or 60 days) or mortality at hospital discharge was used as a substitute. Where data on more than one population were reported, data from the full intention-to-treat population were extracted. Risk of bias was assessed independently by two reviewers (DM and DH) using the Cochrane Risk of Bias assessment tool version 1, 12 modified to remove assessment of risk of bias from lack of blinding of participants and personnel as this was not deemed possible. A study was classified as at high risk of bias overall if one or more domains were high risk. The effect estimate was the log risk ratio. Effect estimates were pooled using a random effects model with the between study variance estimated using restricted maximum likelihood (REML), 13 and standard errors calculated using the method of Hartung-Knapp/Sidik-Jonkman. 14 Between study heterogeneity was quantified using the I2 statistic. A 95% prediction interval for the overall result (providing a plausible range for the effect size in a future study) was presented in addition to the 95% confidence interval. The primary analysis included all eligible studies, with a sensitivity analysis restricted to studies judged to be at low risk of bias. A contour-enhanced funnel plot was used to assess the risk of bias due to missing results. 15 The certainty in the body of evidence for the effect of conservative versus liberal oxygen therapy on mortality in critically ill adults was assessed independently by two reviewers (DM and DH) using GRADE criteria. 16 The initial quality of the body of evidence was assessed as high, due to the study design of randomised trials. This was subsequently downgraded for the presence of risk of bias, inconsistency, indirectness, imprecision and/or publication bias.
We also conducted a post-hoc sensitivity analysis to include only trials that used an intended COT target of (or approximately equivalent to an SpO2 of 88%–92%) to exclude trials where the COT target might be considered by some to be insufficiently conservative.
All statistical analyses were conducted in Stata/MP version 16.1 (StataCorp LP, College Station, TX).
Results
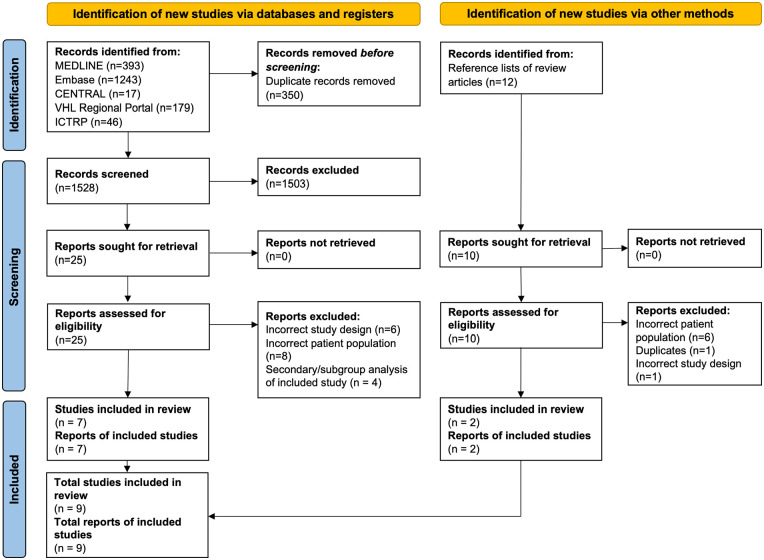
The search identified 1878 records; once duplicates were removed, 1528 unique records were screened for eligibility (Figure 1). Full texts were obtained for 25 publications, which yielded seven eligible studies.17 –23 Screening of reference lists from review articles identified a further 10 publications, two of which were eligible studies 24,25; resulting in a total of nine eligible studies that were included in the review. A list of studies excluded at the full text examination stage, along with the reasons for exclusion, is provided in the Supplemental Data section. Trial registry searches identified five additional studies likely to be eligible, detailed in the Supplemental Data. Four of these studies are currently recruiting, with the recruitment status of the other study unclear.
Figure 1.
PRISMA flow diagram.
Source: For more information, visit: http://www.prisma-statement.org/.
Study characteristics
There were nine eligible trials with a total of 5727 participants; the included studies are summarised in Table 1. Trial size ranged from 34 18 to 2888 25 participants. Whilst intervention and comparator could be classified into either lower or higher oxygenation targets for all studies, there was considerable variation in how these were defined and subsequently there was overlap of targets between trials. Trials also differed in their use of SpO218,20,21,23,24 or PaO217,25 as the chosen target, whilst some used a combination of both.19,22 The comparator to COT included liberal blood oxygenation targets,17,22 approximately normal blood oxygenation targets,18,19,23,25 and an FIO2 greater than 0.21.20 –22,24 The duration of the interventions also varied, with the shortest being 24 h 21 and longest being up to 90 days. 25 Trials took place in Europe,17 –19,21,22,25 Australia and New Zealand20,23 and China 24 ; with all studies including more males than females.
Table 1.
Description of included trials.
| Study | Patient population | Initial mode of oxygen delivery | Country/region | Interventions | Participants | Baseline oxygenation a | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liberal | Conservative | Duration | Sample size | Age a | Male (%) | Liberal | Conservative | ||||
| Gelissen et al. 17 | SIRS | MV, NC, mask or HFNO | Netherlands | PaO2 14–18 kPa | PaO2 8–12 kPa | Up to 14 days (while in ICU) | 400 | 68 | 65 | PaO2 12.3 kPa FIO2 0.46 | PaO2 11.6 kPa FIO2 0.45 |
| Martin et al. 18 | MV for respiratory failure | MV only | UK | SpO2 ⩾ 96% b | SpO2 88%–92% | Until extubation, discharge or death | 34 | 66 | 65 | SpO2 97% PaO2 11.7 kPa FIO2 0.45 | SpO2 94% PaO2 11.0 kPa FIO2 0.40 |
| Schjørring et al. 25 | Receiving supplemental O2 in ICU for respiratory failure | MV, NIV, open system | Europe | PaO2 12 kPa | PaO2 8 kPa | Up to 90 days (while in ICU) | 2888 | 70 | 64 | SaO2 95% PaO2 10.3 kPa FIO2 0.70 | SaO2 94% PaO2 10.3 kPa FIO2 0.70 |
| Barrot et al. 19 | Mechanical ventilation for ARDS | MV only | France | PaO2 12–14 kPa SpO2 ⩾ 96% | PaO27.3–9.3 kPa SpO2 88%–92% | Up to 7 days (while intubated) | 201 | 63 c | 64 | PaO2 12.3 kPa c FIO2 0.80 c | PaO2 12.0 kPa c FIO2 0.80 c |
| Mackle et al. 20 | Mechanical ventilation | MV only | Australia & New Zealand | SpO2 ⩾ 91% FIO2 ⩾ 0.3 | SpO2 91%–96% | Up to 28 days (while in ICU) | 965 | 58 c | 63 | SpO2 96.7% c PaO2 14.9 kPa | SpO2 97.1% c PaO2 14.7 kPa |
| Yang et al. 24 | Admissions with expected ICU stay ⩾72 h | MV and other modalities | China | SpO2 ⩾ 96%, FIO2 ⩾ 0.3 | SpO2 90%–95% | Not reported | 224 | 60 versus 56 d | 67 | Not reported | Not reported |
| Asfar et al. 21 | Mechanical ventilation with septic shock | MV only | France | FIO2 1.0 e | SaO2 88%–95% e | 24 h | 434 | 68 | 64 | SaO2 99% PaO2 16.3 kPa | SaO2 97% PaO2 16.9 kPa |
| Girardis et al. 22 | Admissions with expected ICU stay ⩾72 h | MV and other modalities | Italy | SpO2 97%–100%, PaO2⩽ 20 kPa, FIO2 ⩾ 0.4 | SpO2 94%–98% or PaO2 9.3–13.3 kPa | Not reported | 478 | 65 versus 63d,f | 57 f | Not reported | Not reported |
| Panwar et al. 23 | Mechanical ventilation | MV only | Australia, New Zealand, France | SpO2 ⩾ 96% | SpO2 88%–92% | While receiving mechanical ventilation | 103 | 62 c | 63 | SpO2 96% c PaO2 10.9 kPa FIO2 0.44 c | SpO2 95% c PaO2 10.8 kPa FIO2 0.44 c |
SIRS: systemic inflammatory response syndrome.
Median unless indicated.
Changed to usual care during the study.
Mean.
Liberal versus conservative.
2 × 2 factorial design, also evaluated hypertonic versus isotonic saline.
Only reported for modified ITT population (length of stay at least 72 h and at least one arterial blood gas per day).
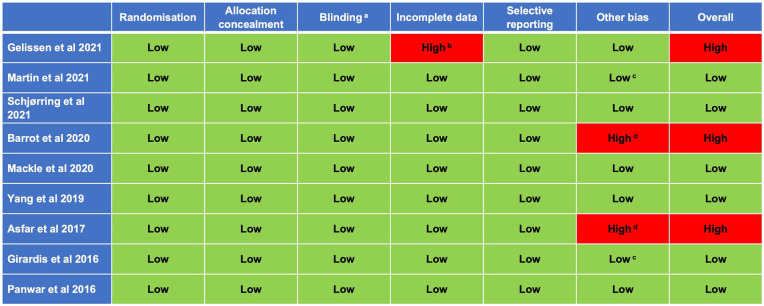
Risk of bias
Of the nine included studies, risk of bias was assessed as low in all domains for six studies (Figure 2), although it was noted that blinding of participants and personnel was not possible in any study. Of the three studies assessed as high risk of bias,17,19,21 two studies were stopped early; one due to possible harm from both interventions (this was a two-by-two factorial trial also evaluating the effect of hypertonic saline) 21 and the other for a potential increased risk of serious adverse events and futility, 19 resulting in potentially biased estimates of treatment effect. In addition, two further trials failed to reach the target number of participants due to poor recruitment, but this was not felt to have led to bias.18,22 One trial was assessed as having a high risk of bias as over 30% of participants were excluded post-randomisation due to a failure to obtain written deferred consent from the patient or their legal representative within 24 h following randomisation. 17
Figure 2.
Risk of bias assessment.
aOnly blinding of outcome assessment evaluated; impossible to blind participants or personnel.
bDowngraded due to post-randomisation exclusion of almost one-third of patients for lack of informed consent.
cTerminated early due to slow recruitment, not data-driven.
dDowngraded due to data-driven early termination for either apparent benefit (Girardis et al. 22 ) or harm (Barrot et al. 19 ).
Primary outcome
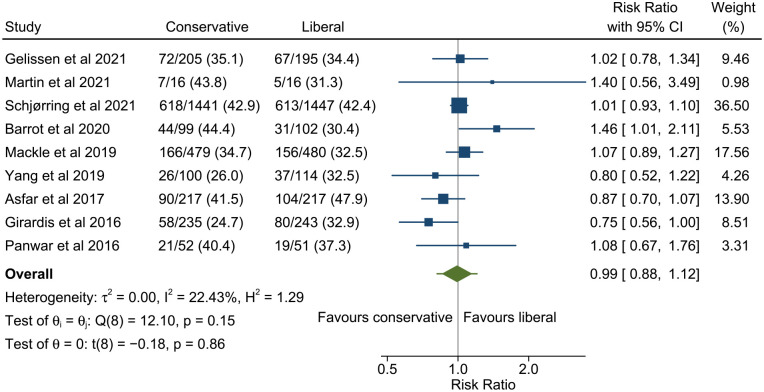
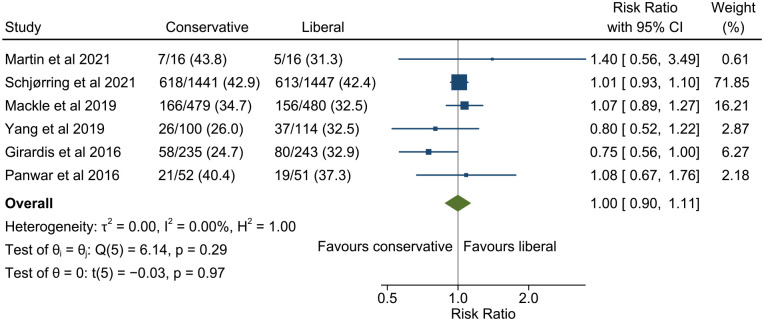
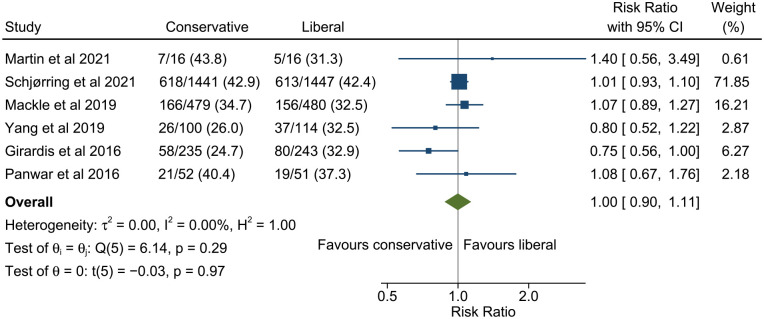
All studies reported 90 day mortality except Girardis et al. 22 in which mortality was only reported to hospital discharge. The pooled estimate of risk ratio for 90 day mortality for conservative versus liberal oxygenation was 0.99 (95% confidence interval 0.88–1.12, 95% prediction interval 0.82–1.21; Figure 3). There was low heterogeneity among studies (I2 = 22.4%). Results were similar when restricted only to studies with low risk of bias (Figure 4) but heterogeneity was further reduced (risk ratio 1.00, 95% confidence interval 0.90–1.11, 95% prediction interval 0.89–1.12, I2 = 0.0%). There was no evidence of funnel plot asymmetry (Figure 5).
Figure 3.
Meta-analysis of the effect of conservative versus liberal oxygen therapy on 90 day mortality for critically ill patients.
The diamond around the overall result shows the 95% confidence interval and the horizontal line the 95% prediction interval for a future study.
Figure 4.
Meta-analysis of the effect of conservative versus liberal oxygen therapy on 90 day mortality for critically ill patients in studies at low risk of bias.
The diamond around the overall result shows the 95% confidence interval and the horizontal line the 95% prediction interval for a future study.
Figure 5.
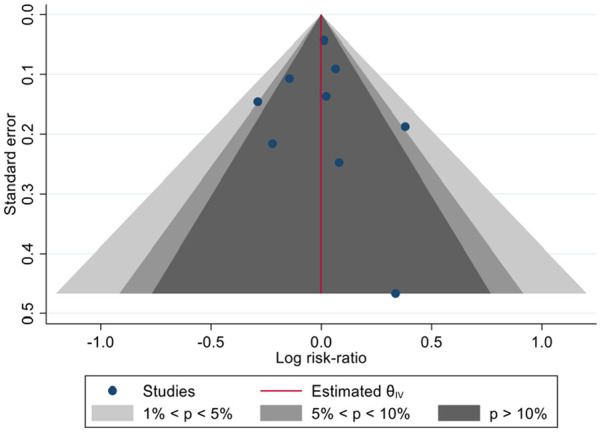
Contour-enhanced funnel plot to assess risk of bias due to missing results.
The finding that mortality was similar for patients managed with conservative or liberal oxygen therapy was graded as moderate certainty. The evidence was downgraded one step for imprecision, as the 95% confidence interval did not rule out a potentially important difference in outcome favouring either conservative or liberal oxygen therapy.
Post hoc sensitivity analysis
A sensitivity analysis was performed which only included trials with a COT SpO2 target of 88%–92% (or an approximately equivalent PaO2). Four trials fulfilled that criteria, contributing a total of 3224 patients to the analysis.18,19,23,25 The pooled estimate of risk ratio for 90 day mortality for conservative versus liberal oxygenation was 1.13 (95% confidence interval 0.85–1.51, 95% prediction interval 0.55–2.34; Figure 6).
Figure 6.
Post hoc sensitivity analysis showing a meta-analysis of the effect of conservative oxygen therapy defined as an SpO2 of between 88% and 92% versus liberal oxygen therapy on 90 day mortality.
The diamond around the overall result shows the 95% confidence interval and the horizontal line the 95% prediction interval for a future study.
Discussion
This systematic review and meta-analysis of nine trials that included 5727 adult patients demonstrated that in a mixed population of critically ill patients, a lower (COT) versus higher (liberal or usual oxygen therapy) oxygenation target did not result in a difference in 90 day mortality. This finding is consistent with previous meta-analyses that were performed prior to the inclusion of more recent studies.26 –28 Overall, no meta-analysis of COT in this cohort of patients has shown the intervention to be either beneficial or harmful in terms of survival. In only one meta-analysis that separated trials into normoxaemia versus hyperoxaemia and relative hypoxaemia versus normoxaemia were the highest oxygen therapy targets associated with the highest mortality. 27 Our post hoc sensitivity analysis which only included trials which implemented a COT target of approximately 88%–92% also found no difference in mortality between the intervention and comparator groups (Figure 6). The addition of new trials to previous analyses has not altered the overall picture of COT being neither harmful nor beneficial in critically ill patients. Whether COT effects specific sub-groups of patients differently remains to be seen.
Schjørring et al.’s trial contributed 50% of the patients included in this analysis so should be considered in more detail as it will have had the greatest influence on the overall results of this review. 25 The trial enrolled adults with acute hypoxaemic respiratory failure admitted to an ICU who were receiving at least 10 L per minute of oxygen via an open system (e.g. face mask) or 50% oxygen via a closed system (e.g. invasive or non-invasive ventilation). The COT group had a target of a PaO2 of 8 kPa whilst in the comparator group it was 12 kPa. The majority of patients had a diagnosis of pneumonia and were receiving mechanical ventilation at the time of enrolment. One of the key issues with the trial was that a median PaO2 of only 9.4 kP (IQR 8.9–10.2) was achieved in the COT groups, compared to a median of 12.4 (IQR 11.6–13.2) in the comparator group (Table 2). So, whilst there was reasonable separation of oxygenation between the groups it was not as much as the investigators had hoped for. None the less, this was a well-conducted trial which has good external validity.
Table 2.
Intended versus achieved oxygen targets of included trials.
| Study | Intervention | Achieved SpO2 (%) | Achieved PaO2 (kPa) | Achieved FIO2 | ||||
|---|---|---|---|---|---|---|---|---|
| Liberal | Conservative | Liberal | Cons | Liberal | Cons | Liberal | Cons | |
| Difference | Difference | Difference | ||||||
| Gelissen et al. 17 | PaO2 14–18 kPa | PaO2 8–12 kPa | 97.2 | 95.8 | 12.8 | 10.8 | 0.51 | 0.4 |
| 1.4 | 2.0 | 0.11 | ||||||
| Martin et al. 18 | SpO2 ⩾ 96% | SpO2 88%–92% | 97 | 91 | 11.8 | 8.6 | 0.37 | 0.35 |
| 6 | 3.2 | 0.02 | ||||||
| Schørring et al. 25 | PaO2 12 kPa | PaO2 8 kPa | 96 | 93 | 12.4 | 9.4 | 0.56 | 0.43 |
| 3 | 3.0 | 0.13 | ||||||
| Barrot et al. 19 | PaO2 12–14 kPa | PaO2 7.3–9.3 kPa | 97 | 93 | 13.6 | 9.9 | 0.4 | 0.5 |
| SpO2 ⩾ 96% | SpO2 88%–92% | 3.80 | 3.7 | 0.16 | ||||
| Mackle et al. 20 | SpO2 ⩾ 91% | SpO2 91%–96% | / | / | / | / | / | / |
| FIO2 ⩾ 0.3 | / | *1.6 | *0.05 | |||||
| Yang et al. 24 | SpO2 ⩾ 96% FIO2 ⩾ 0.3 | SpO2 90%–95% | 98.2 | 95.7 | 13.1 | 11.2 | 0.42 | 0.33 |
| 2.5 | 1.9 | 0.09 | ||||||
| Asfar et al. 21 | FIO2 1.0 | SaO2 88%–95% | 99 | 97 | / | / | / | / |
| 2 | / | / | ||||||
| Girardis et al. 22 | SpO2 97%–100% PaO2 ⩽ 20 kPa FIO2 ⩾ 0.4 | SpO2 94%–98% PaO2 9.3–13.3 kPa | / | / | 13.6 | 11.6 | 0.39 | 0.36 |
| / | 2.0 | 0.03 | ||||||
| Panwar et al. 23 | SpO2 ⩾ 96% | SpO2 88%–92% | 97.0 | 93.4 | 12.3 | 9.3 | 0.36 | 0.26 |
| 4 | 3.0 | 0.1 | ||||||
Data in the achieved columns are a mixture of different point estimates, many of which have been estimated from the Supplemental Data provided alongside primary manuscripts.
One of the most important factors to consider when interpreting data from studies in this field is the way in which investigators defined the intervention (COT) and the comparator (usual or liberal oxygen use) in their trial. There are no agreed oxygenation parameters for COT which means that different research groups have chosen different levels of hypoxaemia as their COT targets. This makes comparing the effects of interventions challenging. To complicate matters further, comparator groups include ‘usual’ oxygenation (as defined by the investigators) or hyperoxaemia (loosely defined as ‘liberal’ oxygenation). As outlined by other authors, this has led to a situation where the COT parameters of some studies overlap with the comparator parameters of others. 27 This situation is comparable to the ‘wet versus dry’ discussions that occurred over the last two decades in the field of perioperative fluid therapy research. 29 These difference in design between the trials weakens the overall message when their data are combined in a meta-analysis and must be considered when interpreting the overall result. The study by Asfar et al. requires careful consideration as it was designed to evaluate the efficacy and safety of hyperoxia (100% oxygen) but the comparator group was within the range that would be classified as COT (an SpO2 of 88%–95%). 21 By comparison Mackle et al.′s trial, which was designed to test the hypothesis that COT would result in more ventilator-free days than usual oxygen therapy, had a COT SpO2 target of 91%–96% 20 ; higher (less conservative) than that of Asfar et al.’s study. 21 This example emphasises the difficulty with terminology in these studies and how in Asfar et al.′s study, COT was compared to extreme hyperoxia, making the comparator to COT very different to the other studies in this review.
A further factor to consider in the design and conduct of trials evaluating COT is that the intervention (usually titration of supplemental oxygen to maintain a target SpO2 or PaO2) may not achieve its desired effect. For example, as outlined above, in the HOT-ICU study the achieved level of oxygenation in the COT groups was higher than intended. 25 Failing to achieve the intended COT target appears to be common, which means that in some cases the intended oxygenation range has not been truly evaluated. The actual oxygenation achieved within trials should be used to determine whether adequate separation of oxygenation has occurred between each group in the trial. The separation of oxygenation indices between groups can be considered for each of the available measures (SpO2, PaO2 and FIO2) to determine if there were clinically meaningful differences between groups (Table 2). Which of these measures is of most importance from a causative perspective is unknown and will depend upon the underlying mechanism of oxygen-related harm in critically ill patients. Presently it is thought that high concentrations of oxygen lead to pulmonary injury, so it is plausible that a clinically meaningful reduction in FIO2 in patients receiving COT group could be the principal way to quantify the effective reduction of harm in these trials. There was wide variation in FIO2 separation between groups in the trials included in this study (0.02–0.16), which may have contributed to the overall finding of COT having no effect. This inherent limitation in the design of COT trials not only complicates the interpretation of each trial but has a compounding effect when data is pooled in the form of meta-analysis. Independent patient data analysis may go some way to address this. If FIO2 is the most important factor in oxygenation, it remains unclear what the minimum FIO2 separation should be between groups for the intervention to really be considered as being ‘conservative’.
Another factor that may affect the interpretation of meta-analyses of COT trials is the change in usual practice that has occurred during the period that these trials have been conducted. A greater awareness of the potential harm of high concentration oxygen appears to have driven a drift towards COT in everyday practice,30 –32 which makes formally evaluating it harder as time goes on. For COT, the apparent shift towards a more conservative approach to oxygenation in usual practice may have made it difficult to distinguish from COT protocols in some of these trials. This may be the reason for most trials to date selecting an arbitrary level of minimum oxygenation or specific ‘normal’ arterial oxygenation target in the comparator (‘liberal’) group. Whilst this will help to separate the two groups in terms of oxygenation, there is a risk that this approach compares COT to non-standard, non-individualised practices. The two largest trials of COT currently recruiting patients have adopted subtly different approaches to the design of their comparator groups. The UK-ROX trial (https://fundingawards.nihr.ac.uk/award/NIHR130508) has a usual care comparator group with no restrictions of the administration of oxygen; the Mega-ROX trial 33 has implemented a minimum FIO2 of 0.30 in the comparator group. This point, highlights the importance of considering comparator groups in the interpretation of clinical trials.
As with any systematic review and meta-analysis, one of the key limitations is the design of the search strategy and the process of selecting eligible articles. In this study, we focused on studies that included critically ill adults with mixed pathologies and chose to exclude studies that were limited to only recruiting specific cohorts of patients, such as post cardiac arrest. The reason for this was to ensure the findings would be generalisable to a broad range of patients admitted to ICUs for mechanical ventilation. It is possible that by excluding single pathology studies we excluded findings that were potentially relevant to our original question but this had to be balanced against producing findings dominated by particular pathologies. Broader eligibility criteria would have included more trials but potentially reduced the applicability of the findings. Finally, it is possible that combining studies with markedly different intervention and comparator groups may have impacted on our ability to detect any signal of benefit or harm with COT.
The findings of this systematic review and meta-analysis rule out the potential of a large treatment effect (>10%) in either direction in 90 day mortality in adult patients admitted to ICU for mechanical ventilation, but do not exclude the possibility of smaller, important treatment effects. With such a widely used intervention, smaller treatment effects are still likely to provide important patient benefit, therefore much larger trials, powered to detect smaller treatment effects are required. The UK-ROX and Mega-ROX trials are both aiming to recruit substantially larger numbers of participants than the total number included in this study (16,500 and 40,000 respectively) and therefore have the potential to answer this question effectively, facilitate meaningful sub-group analyses and could be combined as an independent patient data meta-analysis. The findings of this study also demonstrate a position of reasonable equipoise and therefore provide further support for the need to fully evaluate COT in adult critically ill patients. Such equipoise is essential for this research to be successful. 34
Conclusion
In a heterogenous cohort of adult patients admitted to ICUs, any signal of benefit or harm from COT was insufficient to be significant when currently available trials data was combined in a meta-analysis. Trials in this field have defined COT and liberal or usual oxygen therapy differently, which may have influenced this finding.
Supplemental Material
Supplemental material, sj-docx-1-inc-10.1177_17511437231192385 for The effect of conservative oxygen therapy on mortality in adult critically ill patients: A systematic review and meta-analysis of randomised controlled trials by Daniel S Martin, Helen T Mckenna, Kathryn M Rowan, Doug W Gould, Paul R Mouncey, Michael PW Grocott and David A Harrison in Journal of the Intensive Care Society
Acknowledgments
We would like to thank Dr Ronan O’Driscoll for his invaluable input to this manuscript.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DM is the Editor in Chief of the Journal of the Intensive Care Society. DM and MG were investigators for one of the trials in the review. DM, KR DG, PM, MG and DH are all members of the investigator team for the UK-ROX trial (NIHR130508), which is currently recruiting patients: https://www.isrctn.com/ISRCTN13384956.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Daniel S Martin  https://orcid.org/0000-0001-6220-8235
https://orcid.org/0000-0001-6220-8235
Doug W Gould  https://orcid.org/0000-0003-4148-3312
https://orcid.org/0000-0003-4148-3312
David A Harrison  https://orcid.org/0000-0002-9002-9098
https://orcid.org/0000-0002-9002-9098
References
- 1. Hochberg CH, Semler MW, Brower RG. Oxygen toxicity in critically ill adults. Am J Respir Crit Care Med 2021; 204: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pannu SR. Too much oxygen: hyperoxia and oxygen management in mechanically ventilated patients. Semin Respir Crit Care Med 2016; 37: 16–22. [DOI] [PubMed] [Google Scholar]
- 3. Sinclair SE, Altemeier WA, Matute-Bello G, et al. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med 2004; 32: 2496–2501. [DOI] [PubMed] [Google Scholar]
- 4. Martin DS, Grocott MP. Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit Care Med 2013; 41: 423–432. [DOI] [PubMed] [Google Scholar]
- 5. Abdelsalam M, Cheifetz IM. Goal-directed therapy for severely hypoxic patients with acute respiratory distress syndrome: permissive hypoxemia. Respir Care 2010; 55: 1483–1490. [PubMed] [Google Scholar]
- 6. Abdelsalam M. Permissive hypoxemia: is it time to change our approach? Chest 2006; 129: 210–211. [DOI] [PubMed] [Google Scholar]
- 7. de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care 2008; 12: R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eastwood G, Bellomo R, Bailey M, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med 2012; 38: 91–98. [DOI] [PubMed] [Google Scholar]
- 9. Helmerhorst HJ, Arts DL, Schultz MJ, et al. Metrics of arterial hyperoxia and associated outcomes in critical care. Crit Care Med 2017; 45: 187–195. [DOI] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021; 10: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 2018; 391: 1693–1705. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper H, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis. Manhattan, NY: Russell Sage Foundation, 2019. [Google Scholar]
- 14. Veroniki AA, Jackson D, Bender R, et al. Methods to calculate uncertainty in the estimated overall effect size from a random-effects meta-analysis. Res Synth Methods 2019; 10: 23–43. [DOI] [PubMed] [Google Scholar]
- 15. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 16. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 17. Gelissen H, de Grooth H-J, Smulders Y, et al. Effect of low-normal vs high-normal oxygenation targets on organ dysfunction in critically ill patients: a randomized clinical trial. JAMA 2021; 326: 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin DS, McNeil M, Brew-Graves C, et al. A feasibility randomised controlled trial of targeted oxygen therapy in mechanically ventilated critically ill patients. J Intensive Care Soc 2021; 22: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrot L, Asfar P, Mauny F, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med 2020; 382: 999–1008. [DOI] [PubMed] [Google Scholar]
- 20. ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group, Mackle D, Bellomo R, Bailey M, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med 2020; 382: 989–998. [DOI] [PubMed] [Google Scholar]
- 21. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med 2017; 5: 180–190. [DOI] [PubMed] [Google Scholar]
- 22. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA 2016; 316: 1583–1589. [DOI] [PubMed] [Google Scholar]
- 23. Panwar R, Hardie M, Bellomo R, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med 2016; 193: 43–51. [DOI] [PubMed] [Google Scholar]
- 24. Yang X, Shang Y, Yuan S. Low versus high pulse oxygen saturation directed oxygen therapy in critically ill patients: a randomized controlled pilot study. J Thorac Dis 2019; 11: 4234–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schjørring OL, Klitgaard TL, Perner A, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med 2021; 384: 1301–1311. [DOI] [PubMed] [Google Scholar]
- 26. Chen X-L, Zhang B-L, Meng C, et al. Conservative oxygen therapy for critically ill patients: a meta-analysis of randomized controlled trials. J Intensive Care Med 2021; 9: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cumpstey AF, Oldman AH, Martin DS, et al. Oxygen targets during mechanical ventilation in the ICU: a systematic review and meta-analysis. Crit Care Explor 2022; 4: e0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu L, Tian Y. Liberal or conservative oxygen therapy for ventilated patients in the ICU: a meta-analysis of randomized controlled trials. J Cardiothorac Surg 2021;16: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellamy MC. Wet, dry or something else? Br J Anaesth 2006; 97: 755–757. [DOI] [PubMed] [Google Scholar]
- 30. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 2010; 303: 2165–2171. [DOI] [PubMed] [Google Scholar]
- 31. Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care 2014; 4: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Post B, Palmer E, Harris S, et al. Oxygenation of the critically ill in selected intensive care units in the UK: are we usual? Br J Anaesth 2020; 125: e277–e279. [DOI] [PubMed] [Google Scholar]
- 33. Young PJ, Arabi YM, Bagshaw SM, et al. Protocol and statistical analysis plan for the mega randomised registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU (Mega-ROX). Crit Care Resusc 2022; 24: 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin D, Harrison D, Mouncey P, et al. Past, present and future of conservative oxygen therapy in critical care. Thorax 2022; 77: 431–432. [DOI] [PubMed] [Google Scholar]
- 35. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-inc-10.1177_17511437231192385 for The effect of conservative oxygen therapy on mortality in adult critically ill patients: A systematic review and meta-analysis of randomised controlled trials by Daniel S Martin, Helen T Mckenna, Kathryn M Rowan, Doug W Gould, Paul R Mouncey, Michael PW Grocott and David A Harrison in Journal of the Intensive Care Society