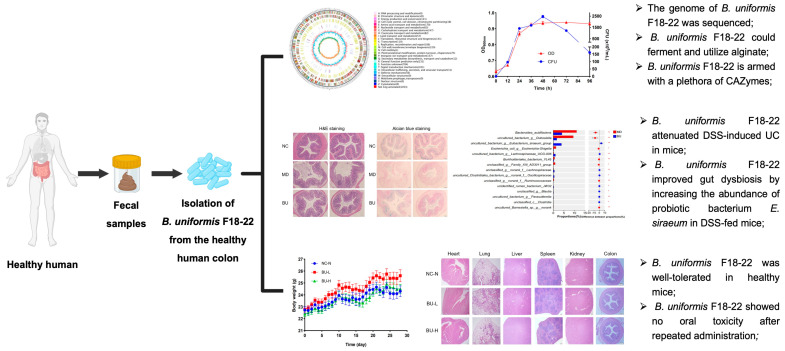
Abstract
Previous studies have demonstrated that the intestinal abundance of Bacteroides uniformis is significantly higher in healthy controls than that in patients with ulcerative colitis (UC). However, what effect B. uniformis has on the development of UC has not been characterized. Here, we show for the first time that B. uniformis F18-22, an alginate-fermenting bacterium isolated from the healthy human colon, protects against dextran-sulfate-sodium (DSS)-induced UC in mice. Specifically, oral intake of B. uniformis F18-22 alleviated colon contraction, improved intestinal bleeding and attenuated mucosal damage in diseased mice. Additionally, B. uniformis F18-22 improved gut dysbiosis in UC mice by increasing the abundance of anti-inflammatory acetate-producing bacterium Eubacterium siraeum and decreasing the amount of pro-inflammatory pathogenetic bacteria Escherichia-Shigella spp. Moreover, B. uniformis F18-22 was well-tolerated in mice and showed no oral toxicity after repeated daily administration for 28 consecutive days. Taken together, our study illustrates that B. uniformis F18-22 is a safe and novel probiotic bacterium for the treatment of UC from the healthy human colon.
Keywords: Bacteroides uniformis, gut microbiota, ulcerative colitis, dextran sulfate sodium, probiotic, alginate, inflammatory bowel disease, Eubacterium siraeum, fermentation, short-chain fatty acids
1. Introduction
Ulcerative colitis (UC) is one type of inflammatory bowel disease (IBD) that is characterized by bacterial dysbiosis in the gut [1,2,3]. Gut dysbiosis contributes significantly to the pathogenesis of UC and new therapeutic techniques, including fecal microbiota transplantation (FMT), and live biotherapeutic products (LBPs) have been developed for the treatment of IBD by targeting the dysbiotic microbiome [4,5,6]. Recently, accumulating evidence has indicated that the intestinal abundance of Bacteroides uniformis is significantly higher in healthy controls than that in patients with UC [7,8], suggesting a potential role of this bacterium in the pathogenesis of colonic diseases. However, up to now, what effect B. uniformis has on the development of UC has not been characterized.
Previous studies have demonstrated that specific strains of B. uniformis are next-generation probiotic bacteria that can be used to improve exercise performance both in mice and humans [9]. Besides, others found that oral administration of B. uniformis could ameliorate the metabolic and immunological dysfunction in mice with high-fat-diet-induced obesity [10]. Additionally, oral intake of B. uniformis was also observed to amplify the metabolic and immune benefits of dietary fiber by targeting gut dysbiosis in obese mice [11]. Collectively, these studies indicate that B. uniformis might be used as a novel probiotic bacterium for the treatment of dysbiosis-associated colonic diseases.
Alginate is a fermentable dietary fiber that could be utilized by specific microbes in the human gut [12,13]. Our previous results indicated that the alginate-fermenting bacterium, Bacteroides xylanisolvens AY11-1, could mediate the beneficial effects of alginate on the colon and oral administration of B. xylanisolvens AY11-1 protects against dextran-sulfate-sodium (DSS)-induced UC in mice [12]. Similar to that of alginate, B. xylanisolvens AY11-1 improved gut dysbiosis and promoted the growth of probiotic bacteria in diseased mice [12]. This study highlights the importance of exploring alginate-fermenting bacteria for the discovery of potential anti-colitis probiotics from the human colon.
In the present study, combined with the observation that the intestinal abundance of B. uniformis is significantly higher in healthy controls than that in IBD patients, we hypothesized that the alginate-fermenting bacterium B. uniformis F18-22 might also be protective against the development of UC [12]. We therefore tested this hypothesis in mice and interestingly, we found that B. uniformis F18-22 could be used as a safe and novel probiotic bacterium for the treatment of UC from the healthy human colon.
Our study consists three parts of experiments. In the first part, we sequenced the whole genome of B. uniformis F18-22 and explored its metabolic potential. In the second part, we investigated the anti-colitis effect of B. uniformis F18-22 and its potential therapeutic mechanisms using a mouse model of DSS-induced colitis. In the third part, we further examined the safety profiles of B. uniformis F18-22 in vivo. All the three parts of the experiments were designed to provide the first evidence for the potential utilization of B. uniformis F18-22 as a next-generation probiotic.
2. Results
2.1. Genomic Analysis of the Alginate-Fermenting Bacterium, B. uniformis F18-22
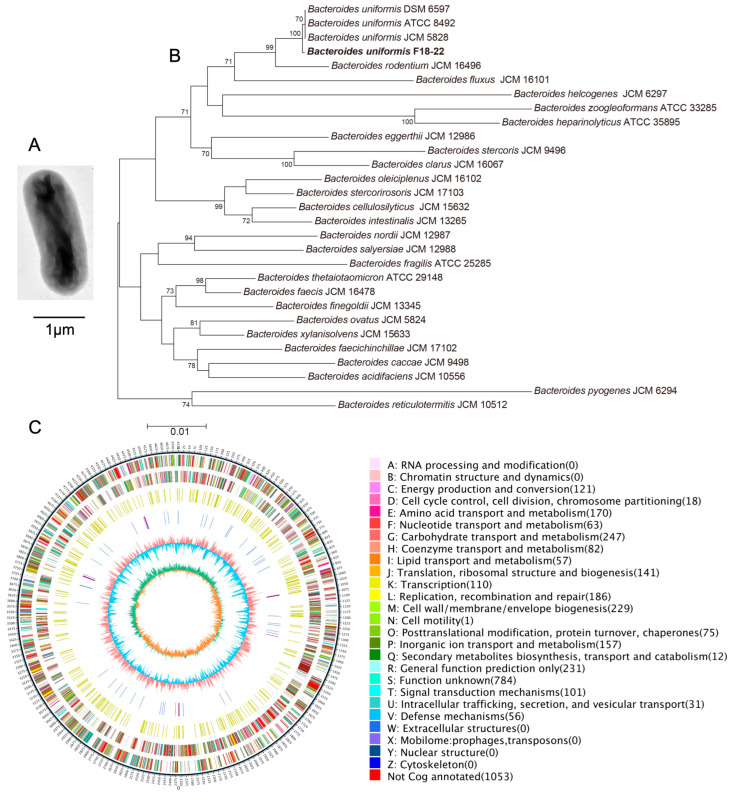
Gut microbiota plays a critical role in the metabolism of nutrients and as a dietary fiber, alginate could be fermented by the human gut microbiota [12,13]. B. uniformis F18-22 is an alginate-fermenting bacterium that has previously been isolated from the healthy human colon [12]. In the present research, we aim to extend our previous study by exploring the metabolic characteristics and the anti-colitis effect of B. uniformis F18-22. A transmission electron microscope (TEM) was first employed to investigate the cell morphology of B. uniformis F18-22. TEM analysis indicated that B. uniformis F18-22 is a rod-shaped bacterium with a cell length of about 2.5 μm (Figure 1A). Phylogenetic tree analysis suggested that B. uniformis F18-22 is closely related to other strains in the same species, including the type strain B. uniformis ATCC 8492 (Figure 1B).
Figure 1.
Genomic analysis of B. uniformis F18-22. TEM analysis of the bacterial cell morphology of B. uniformis F18-22 (A). Phylogenetic tree analysis of B. uniformis F18-22 based on 16S gene sequences (B). COGs function analysis of B. uniformis F18-22 (C).
We next sequenced the whole genome of B. uniformis F18-22 to explore the metabolic potential of this bacterium. The length and the GC content of the genome of B. uniformis F18-22 was identified to be 4,725,126 bp and 46.59%, respectively (Figure 1C). Clusters of Orthologous Group (COG) function analysis indicated that B. uniformis F18-22 has a good capability for carbohydrate transport and metabolism, and a total of 247 identified COGs were annotated in this category (Figure 1C). This indicates that B. uniformis F18-22 might be able to degrade and ferment a wide range of different dietary polysaccharides in the human gut. This could possibly give B. uniformis F18-22 a competitive advantage to survive in the colon when the carbon source is changed between different diets.
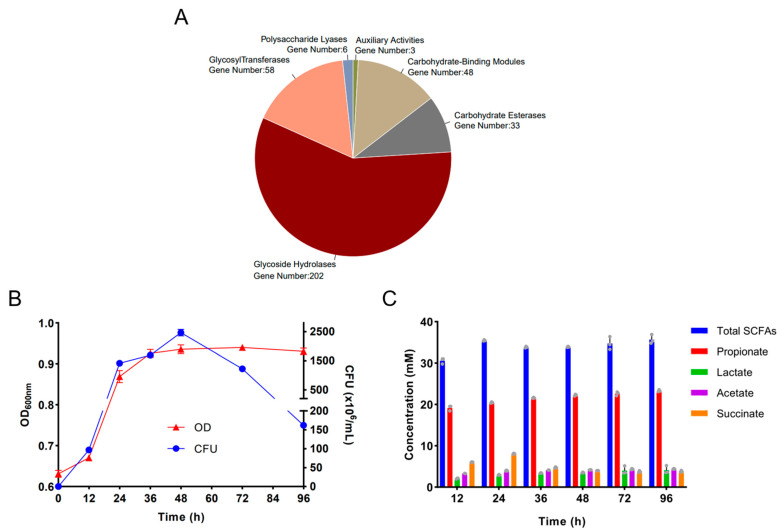
Carbohydrate-active enzymes (CAZymes), including glycoside hydrolases (GHs), glycosyltransferases (GTs), polysaccharide lyases (PLs), carbohydrate esterases (CEs), carbohydrate-binding modules (CBMs), and auxiliary activities (AAs), are the most important enzymes for the metabolism of complex carbohydrates in the human diet. We next investigated the CAZymes in B. uniformis F18-22 and interestingly, a total of 350 genes were identified as responsible for the expression of a different class of CAZymes (Figure 2A). Remarkably, over one half of these genes were identified as responsible for the expression of glycoside hydrolases (GHs) (Figure 2A). In line with previous results [12], further study confirmed that B. uniformis F18-22 could ferment and utilize alginate for its own growth (Figure 2B). B. uniformis F18-22 can grow up to 2.5 × 109 CFUs/mL in about 48 h in the medium containing alginate as a major carbon source (Figure 2B). Moreover, fermentation of alginate by B. uniformis F18-22 produced significant amounts of beneficial short-chain fatty acids (SCFAs), including propionate, lactate, acetate, and succinate (Figure 2C). The majority of the produced SCFAs was propionate, and the minority of the produced SCFAs was lactate, acetate, and succinate (Figure 2C).
Figure 2.
CAZymes analysis of B. uniformis F18-22 and in vitro fermentation of alginate by B. uniformis F18-22. Genomic CAZymes analysis of B. uniformis F18-22 (A). CFUs analysis and growth curve analysis of B. uniformis F18-22 during fermentation of alginate (B). SCFAs analysis of B. uniformis F18-22 produced during fermentation of alginate (C). The gray dots in the panel C represent the three replicates in the experiments.
2.2. B. uniformis F18-22 Attenuated DSS-Induced UC in Mice
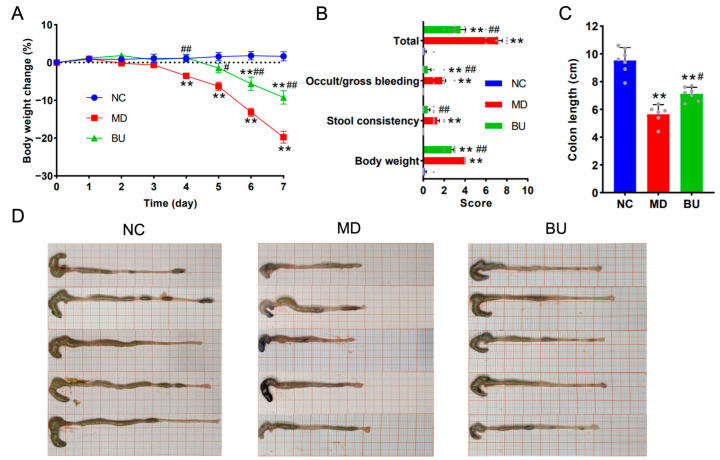
Previous clinical studies have demonstrated that the intestinal abundance of B. uniformis is significantly higher in healthy controls than that in IBD patients [7,8], suggesting a potential role of B. uniformis in protecting against the development of UC in human gut. In this regard, we next investigated the effect of B. uniformis F18-22 on DSS-induced UC in mice. Interestingly, we found that oral intake of B. uniformis F18-22 for seven days significantly retarded the body weight loss, alleviated colon contraction, reduced incidences of intestinal bleeding, and improved stool consistency in diseased mice (Figure 3A–D). Taken together, these results provide the first evidence for the anti-colitis effect of B. uniformis F18-22 in DSS-fed mice.
Figure 3.
Oral intake of B. uniformis F18-22 attenuated DSS-induced UC in mice. Body weight change of the mice (A). Symptom score analysis of the disease (B). Colon length analysis of the mice (C). Representative morphology of the colon (D). Each gray dot in the panel B and C represents one mouse in the experiments. The NC group has 7 mice. The MD group has 6 mice. The BU group has 7 mice. ** p < 0.01 versus NC group; # p < 0.05 versus MD group; ## p < 0.01 versus MD group.
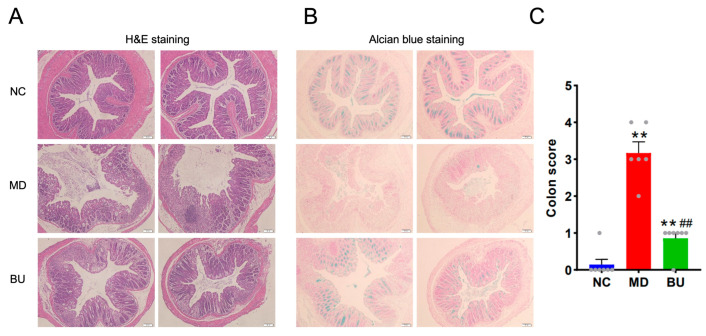
Accumulated evidence has indicated that DSS-induced UC is associated with intestinal mucosal damage in the gut [14]. Therefore, we next performed H&E staining and Alcian blue staining to further explore the anti-colitis effect of B. uniformis F18-22. As expected, oral intake of B. uniformis F18-22 successfully protected the mice from DSS-induced disruption of the colonic epithelial layer (Figure 4A–C). Altogether, dietary intake of B. uniformis F18-22 improved UC and ameliorated intestinal mucosal damage in DSS-fed mice (Figure 3 and Figure 4). Collectively, these results provide new insights into the role of B. uniformis in the development of UC in the human gut.
Figure 4.
Oral administration of B. uniformis F18-22 attenuated DSS-induced mucosal damage. H&E staining of the colonic tissues of the mice (A). Alcian blue staining of the colonic tissues of the mice (B). Colon score analysis based on H&E staining (C). Each gray dot in the panel C represents one mouse in the experiments. The NC group has 7 mice. The MD group has 6 mice. The BU group has 7 mice. ** p < 0.01 versus NC group; ## p < 0.01 versus MD group.
2.3. B. uniformis F18-22 Improved Gut Dysbiosis by Increasing the Abundance of Eubacterium siraeum and Decreasing the Amount of Escherichia-Shigella spp. in DSS-Fed Mice
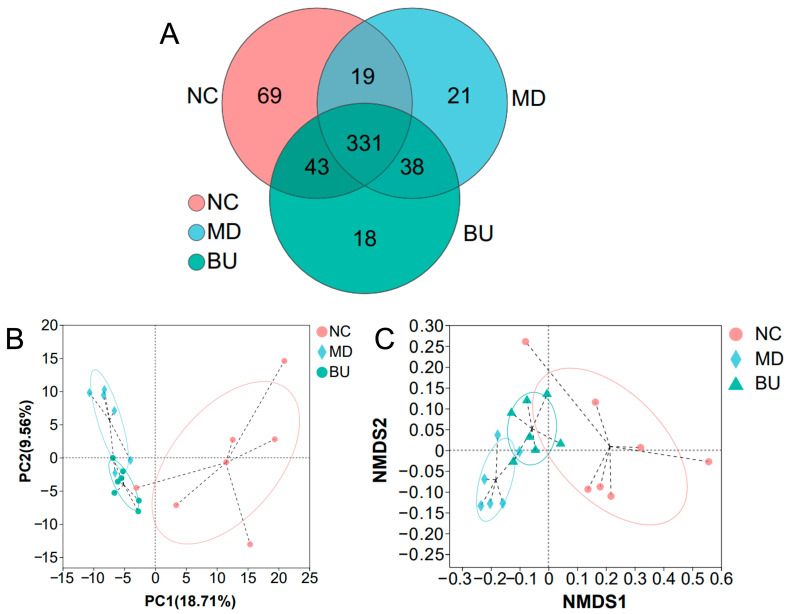
Previous studies have demonstrated that gut microbiota plays a pivotal role in the pathogenesis of UC [4,5,6]. In this sense, we next investigated the effect of B. uniformis F18-22 on the composition of the gut microbiota using 16S high-throughput sequencing. Interestingly, we found that oral administration of B. uniformis F18-22 significantly changed the structure of the gut microbiota in diseased mice (Figure 5A). Specifically, as indicated via PCA and NMDS analyses, B. uniformis F18-22 treatment induced a remarkable shift of the gut microbiota structure in diseased mice towards that in NC mice (Figure 5B,C).
Figure 5.
Oral administration of B. uniformis F18-22 significantly changed the structure of the gut microbiota in DSS-fed mice. Venn diagram analysis of the composition of gut microbiota at the operational taxonomic units (OTUs) level (A). PCA score plot analysis of the gut microbiota (B). NMDS score plot analysis of the gut microbiota (C).
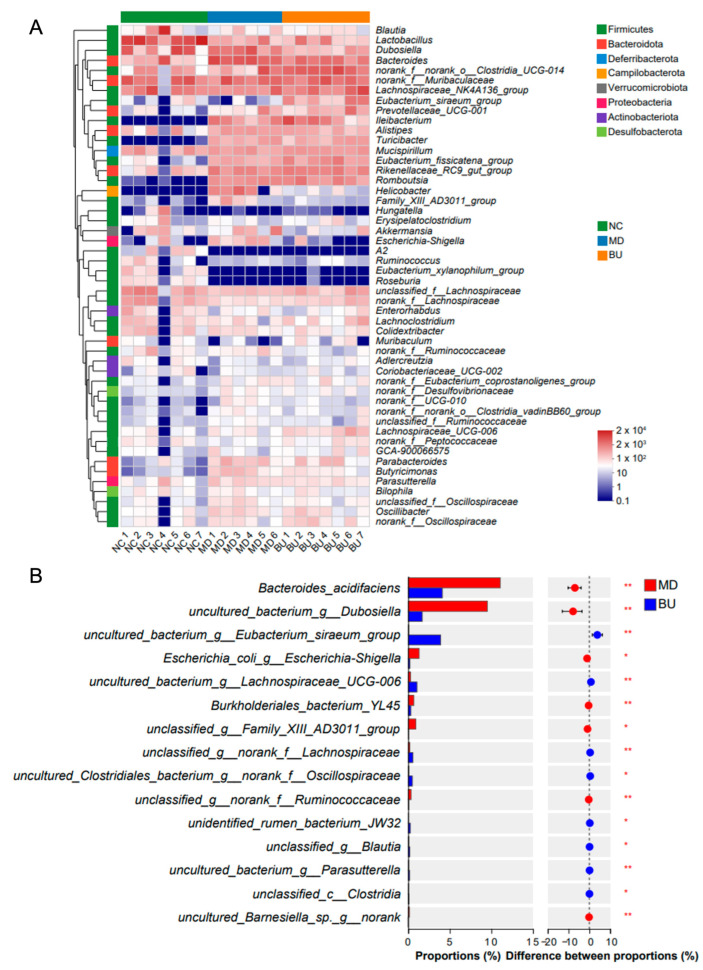
Further analysis indicated that dietary intake of B. uniformis F18-22 modulated the composition of the gut microbiota in DSS-fed mice at both phylum and genus levels (Figure 6A and Figure S1). A Wilcoxon rank-sum test was performed to compare the structural differences of the gut microbiota at the species level between two groups (Figure 6B and Figure S2). Interestingly, B. uniformis F18-22 improved gut dysbiosis in DSS-fed mice by increasing the abundance of anti-inflammatory acetate-producing bacterium Eubacterium siraeum and decreasing the amounts of pro-inflammatory pathogenetic bacteria, Escherichia-Shigella spp., and Bacteroides acidifaciens (Figure 6B).
Figure 6.
Oral administration of B. uniformis F18-22 improved gut dysbiosis in DSS-fed mice. Heatmap analysis of the composition of gut microbiota (A). Wilcoxon rank-sum test analysis (B). * p < 0.05; ** p < 0.01.
2.4. B. uniformis F18-22 Was Well Tolerated in Mice and Showed No Oral Toxicity after Repeated Administration
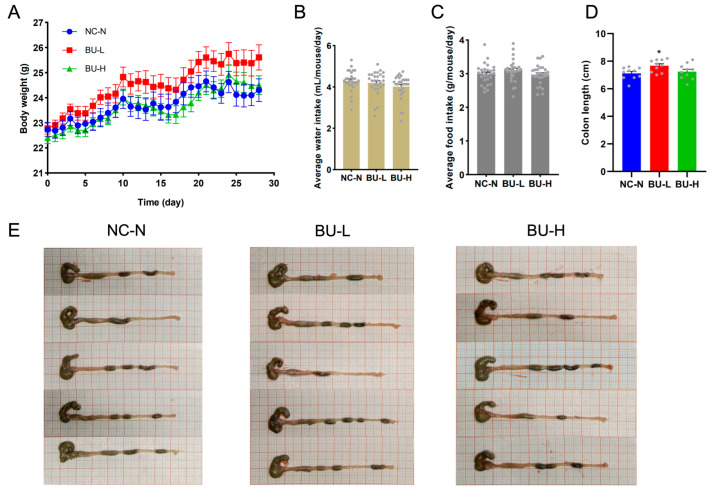
We next investigated the safety profiles of B. uniformis F18-22 in healthy mice. Notably, we found that oral administration of B. uniformis F18-22, both at a low and at a high dosage, exerted no effect on body weight, water and food intake of the mice (Figure 7A–C). Intriguingly, low-dosage treatment of B. uniformis F18-22 slightly increased the colon length whereas high-dosage treatment of B. uniformis F18-22 had no such effect (Figure 7D). However, both low-dosage treatment and high-dosage treatment of B. uniformis F18-22 did not have any effects on stool consistency and fecal pellet morphology (Figure 7E). Collectively, these results indicate that B. uniformis F18-22 is well tolerated in mice even dosed at 10 times the therapeutic concentration.
Figure 7.
B. uniformis F18-22 was well tolerated in mice. Body weight change of the mice (A). Average water intake of the mice (B). Average food intake of the mice (C). Each gray dot in the panel (B,C) represents the value of the daily water intake and daily food intake. The water intake and food intake were monitored for 28 days. Colon length (D). Each gray dot in the panel (D) represents one mouse in the experiments. The NC-N group has 10 mice. The BU-L group has 10 mice. The BU-H group has 10 mice. Representative morphology of the colon (E). * p < 0.05 versus NC-N group.
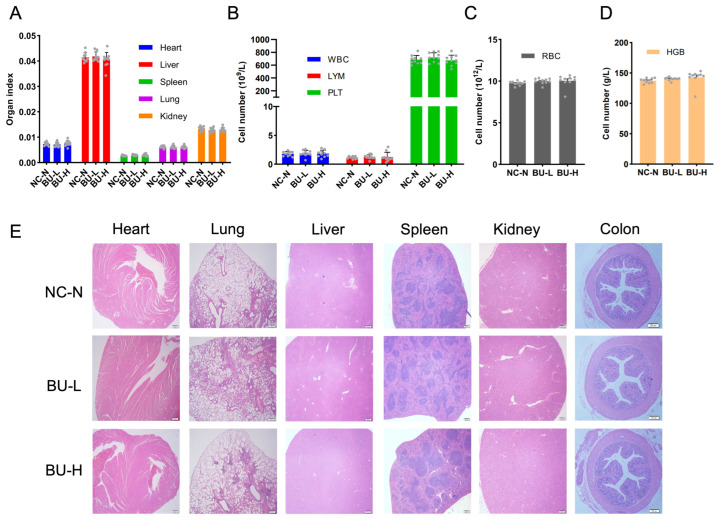
Further analysis indicated that B. uniformis F18-22 had no toxic effect on the major organs of mice including heart, liver, spleen, lung, kidney, and colon (Figure 8A,E). Additionally, both low-dosage treatment and high-dosage treatment of B. uniformis F18-22 treatment did not change the numbers of white blood cells (WBCs), lymphocytes (LYMs), platelets (PLTs) and red blood cells (RBCs) of the mice (Figure 8B,C). Moreover, the hemoglobin (HGB) concentrations in the blood of the mice were also not affected by B. uniformis F18-22 treatment (Figure 8D). Taken together, B. uniformis F18-22 was well tolerated in mice and showed no oral toxicity even after repeated daily administration for 28 consecutive days.
Figure 8.
B. uniformis F18-22 showed no toxic effect on the major organs of mice. Organ index (A). Numbers of the white blood cells, lymphocytes, and platelets (B). Numbers of the red blood cell (C). Hemoglobin concentrations in the blood of the mice (D). Each gray dot in the panel (A–D) represents one mouse in the experiments. The NC-N group has 10 mice. The BU-L group has 10 mice. The BU-H group has 10 mice. H&E staining of the heart, lung, liver, spleen, kidney, and colon (E).
3. Discussion
3.1. Potential Applications of B. uniformis F18-22
Previous clinical studies have demonstrated that the intestinal abundance of B. uniformis is significantly higher in healthy controls than that in UC patients [7,8]. However, what effect B. uniformis has on the development and pathogenesis of UC has not been characterized. Here, we show for the first time that B. uniformis F18-22, an alginate-fermenting bacterium with good safety profiles from the healthy human colon, protects against DSS-induced UC in mice. Our study paves the way for the research and development of B. uniformis F18-22 as a next-generation probiotic bacterium (Figure 9).
Figure 9.
Summary of the main findings of the present study. Part of the figure was created with BioRender.com (accessed on 13 September 2023). * p < 0.05; ** p < 0.01.
3.2. Limitations of the Current Study
Our study has some limitations. First, although we have sequenced and analyzed the whole genome of B. uniformis F18-22, we are still at the very beginning of understanding the metabolic characteristics of this new probiotic bacterium. Many COGs in the genome have not been successfully annotated (Figure 1). Second, B. uniformis F18-22 is armed with a plethora of CAZymes in its genome (Figure 2). It is possible that B. uniformis F18-22 could degrade and ferment a wide range of complex carbohydrates in our daily diet. However, we have only tested alginate in the present study and we do not know if it is the best carbon source for B. uniformis F18-22. Third, based on our results, we could not determine whether or not B. uniformis F18-22 has successfully colonized the gastrointestinal tract in DSS-fed mice. The metabolic fate of B. uniformis F18-22 after oral administration has not been explored.
3.3. Future Directions for the Study of B. uniformis F18-22
Escherichia-Shigella spp. and B. acidifaciens are pathogenic colitis-associated bacteria in the gut [14,15]. In contrast, E. siraeum is an anti-inflammatory acetate-producing probiotic bacterium in the colon [16,17]. Our results suggest that B. uniformis F18-22 could attenuate gut dysbiosis in DSS-fed mice by promoting the growth of E. siraeum and inhibiting the proliferations of Escherichia-Shigella spp., and B. acidifaciens. The present study provides the first evidence for understanding the therapeutic effects of B. uniformis F18-22 on UC from the perspective of dysbiotic gut microbiota. However, more detailed investigations are still needed to fully characterize the anti-colitis mechanisms of this probiotic bacterium.
B. uniformis CECT 7771 is a symbiont bacterium from the colon of healthy infants [10,18]. Previous studies have indicated that oral consumption of B. uniformis CECT 7771 for 90 days is safe in experimental rats [18]. Similarly, we found that B. uniformis F18-22 was well tolerated in mice and showed no oral toxicity after repeated administration for 28 consecutive days even dosed at 10 times the therapeutic concentration. Altogether, these results suggest that the probiotic strains of B. uniformis from healthy individuals generally have a good safety profile in rodents. More studies are therefore warranted to further assess the safety of B. uniformis F18-22 in humans.
Our study indicates that the genome of B. uniformis F18-22 encodes many CAZymes, including glycoside hydrolases (GHs), glycosyltransferases (GTs), polysaccharide lyases (PLs), carbohydrate esterases (CEs), carbohydrate-binding modules (CBMs) and auxiliary activities (AAs). CAZymes are the most important enzymes for the metabolism of complex carbohydrates in the human diet [19,20,21,22]. These results indicate that B. uniformis F18-22 might be able to ferment and utilize a wide range of dietary polysaccharides in the gut. This could possibly give B. uniformis F18-22 a competitive advantage to survive in the colon when the carbon source is changed between different diets. Nonetheless, more intensive examinations are needed to verify this possibility.
4. Materials and Methods
4.1. Chemicals and Reagents
The standard short-chain fatty acids (SCFAs) solutions, including lactate, acetate, propionate, succinate, and butyrate were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Tryptone, peptone, yeast extract, and Tween 80 used for the in vitro anaerobic fermentation experiments were all obtained from Sigma-Aldrich (St. Louis, MO, USA). Dextran sulfate sodium (DSS) was purchased from MP Biomedicals (Solon, OH, USA). DSS was used to induce ulcerative colitis in the colon of C57BL/6J mice.
Hemin, alginate, agar, and L-cysteine hydrochloride were acquired from Sangon Biotech (Shanghai, China). These chemicals were added to the VI medium and were used for the anaerobic culture of the bacterium B. uniformis F18-22. All other chemicals of analytical grade used in the present study were purchased from Sinopharm Chemical (Shanghai, China) unless otherwise specified.
4.2. In Vitro Fermentation
B. uniformis F18-22 is an alginate-fermenting bacterium that has been previously isolated from the healthy human colon [12]. Therefore, the VI culture medium containing alginate as a major carbon source at 8 g/L was applied to investigate the fermentation characteristics of B. uniformis F18-22 as previously described [12,13]. The fermentation experiments were carried out anaerobically (80% N2, 10% H2 and 10% CO2) at 37 °C in an Electrotek AW 500SG anaerobic chamber (Shipley, West Yorkshire, UK).
The SCFAs produced during the in vitro anaerobic fermentation were analyzed and monitored using the well-established high-performance liquid chromatograph (HPLC) (Agilent 1260, Santa Clara, CA, USA) method as previously described [12,13]. Transmission electron microscope (TEM) analysis of the bacterial cell morphology of B. uniformis F18-22 was conducted by Servicebio Technology (Wuhan, China) using the HT7800/HT7700 microscope from Hitachi High-Tech (Shanghai, China). The bacteria used for the TEM analysis were collected at the exponential phase of growth at about 20 h (Figure 2B).
4.3. Animals and Treatment
All the specific-pathogen-free (SPF) animals used in the present study were obtained from Beijing Vital River Laboratory Animal Technology (Beijing, China) (Certificate No. SCXK (Jing) 2016-0011). The animal experiments were approved by the Ethical Committee of Ocean University of China, School of Medicine and Pharmacy (Permission No. OUC-2022-0901-01). The experiments in the present study were performed in good compliance with the Guide for the Care and Use of Laboratory Animals (National Academies Press, 8th edition, 2011) [23].
In the first animal experiment, a total of 20 male 8-week-old C57BL/6J SPF mice were utilized to investigate the therapeutic effects of B. uniformis F18-22 on DSS-induced UC. The mice were randomly divided into 3 different treatment groups: the normal control group (NC, n = 7), the model group (MD, n = 6), and the B. uniformis F18-22 treatment group (BU, n = 7). B. uniformis F18-22 was given daily at a dosage of about 1.00 × 108 colony-forming units (CFUs)/day/mouse via gavage for 7 consecutive days. B. uniformis F18-22 was cultured in the VI culture medium containing alginate as a major carbon source at 8g/L. The bacteria were collected at the exponential phase of growth at about 20 h.
Mice in the MD and BU groups were given free access to 2.0% (w/v) DSS in the daily drinking water for 6 consecutive days. All mice were humanely sacrificed on the 7th day. The colon tissues of the mice were harvested for hematoxylin and eosin (H&E) staining and Alcian blue staining. The cecum tissues were collected for gut microbiota analysis. The symptom scores were calculated based on the stool morphology, intestinal bleeding occurrence and body weight change of the mice at the last day of the experiment. The histopathological colon scores were determined based on H&E staining as previously described [14].
In the second animal experiment, a total of 30 male 8-week-old C57BL/6J SPF mice were applied to further explore the safety profile of B. uniformis F18-22. All mice were randomized into 3 different treatment groups: the normal control group (NC-N, n = 10), the low-dosage treatment group (BU-L, n = 10), and the high-dosage treatment group (BU-H, n = 10). In the BU-L group, B. uniformis F18-22 was given daily at a dosage of about 1.00 × 108 CFUs/day/mouse by gavage for 28 consecutive days, whereas in the BU-H group, B. uniformis F18-22 was given daily at a dosage of about 1.00 × 109 CFUs/day/mouse by gavage for 28 consecutive days. B. uniformis F18-22 was cultured in the VI culture medium containing alginate as a major carbon source at 8 g/L. The bacteria were collected at the exponential phase of growth at about 20 h.
The average water intake and food intake of the mice were monitored every day. All mice were humanely sacrificed on the last day of the experiment. The blood cells of the mice were analyzed using an automatic blood cell analyzer (RT-7600Vet) from Rayto Life and Analytical Sciences (Shenzhen, China). The major organs of the mice were collected and the organ index was calculated and compared between different treatment groups. The H&E staining of the heart, lung, liver, spleen, kidney, and colon was performed as previously described [12,14].
4.4. High-Throughput Sequencing and Bioinformatic Analyses
B. uniformis F18-22 was cultured in the VI culture medium containing alginate as a major carbon source at 8 g/L. The bacteria were collected at the exponential phase of growth at about 20 h. The genomic DNA of B. uniformis F18-22 was extracted and the complete genome was sequenced using the Oxford Nanopore Technologies (ONT) Nanopore PromethION platform from Biomarker Technologies (Beijing, China). The genome analysis was conducted using the online tools from BMKCloud (www.biocloud.net (accessed on 1 August 2023)). Clusters of Orthologous Groups (COGs) function analysis and carbohydrate-active enzymes (CAZymes) analysis of the genome were conducted to explore the metabolic potential of B. uniformis F18-22.
Phylogenetic tree analysis of B. uniformis F18-22 was performed based on the 16S sequences of the bacteria using the Molecular Evolutionary Genetics Analysis (MEGA) software (version 7.0.26) [12,24]. The metagenomic DNAs of the mice’s cecal microbiota were extracted using a Qiagen QIAamp DNA Stool Mini Kit (Hilden, Germany). The 16S V3-V4 hypervariable gene regions were specifically amplified using the well-established universal primers, 338F (ACTCCTACGGGAGGAGCAG) and 806R (GGACTACHVGGGTWTCTAAT), as previously described [12,13,14]. The obtained amplicons were sequenced and analyzed using an Illumina PE300 platform from Majorbio Bio-pharm Biotechnology (Shanghai, China). Bioinformatic analyses of the sequencing data, including Wilcoxon rank-sum test, Venn diagram, non-metric multidimensional scaling (NMDS) score plot, principal components analysis (PCA) and heatmap analysis were all conducted using the online tools from Majorbio Cloud Platform (www.majorbio.com (accessed on 1 August 2023)) [25].
4.5. Statistical Analyses
All results were expressed as the mean ± standard error of mean (SEM). The statistical analyses were performed using Student’s t-test from the GraphPad Prism 8.0.2 software (Boston, MA, USA). The Wilcoxon rank-sum test was performed to compare the structural differences of the gut microbiota between two groups. The results were considered statistically significant at p < 0.05. * p < 0.05; ** p < 0.01.
5. Conclusions
Dietary intake of B. uniformis F18-22, an alginate-fermenting bacterium from the healthy human colon, protects against DSS-induced UC in mice. Specifically, B. uniformis F18-22 retarded body weight loss, alleviated colon contraction, reduced incidences of intestinal bleeding, and improved stool consistency in diseased mice. Additionally, B. uniformis F18-22 improved gut dysbiosis in UC mice by increasing the abundance of anti-inflammatory acetate-producing bacterium, Eubacterium siraeum, and decreasing the amount of pro-inflammatory pathogenetic bacteria, Escherichia-Shigella spp. and B. acidifaciens. Moreover, B. uniformis F18-22 was well tolerated in mice and showed no oral toxicity after repeated daily administration for 28 consecutive days. Taken together, our study illustrates for the first time that B. uniformis F18-22 is a safe and novel probiotic bacterium for the treatment of UC from the healthy human colon.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms241914669/s1.
Author Contributions
Conceptualization, Q.S. and H.Y.; methodology, Q.S. and W.D.; software, W.D., J.Z. (Jiaxue Zhang) and L.C.; validation, Q.S., W.D. and J.Z. (Jiaxue Zhang); formal analysis, W.D., J.Z. (Jiaxue Zhang), L.C., J.Y. and J.Z. (Junyi Zhang); investigation, W.D., J.Z. (Jiaxue Zhang) and L.C.; resources, L.C., J.Y., J.Z. (Junyi Zhang) and G.Y.; data curation, W.D. and J.Z. (Jiaxue Zhang); writing—original draft preparation, Q.S. and W.D.; writing—review and editing, Q.S.; visualization, Q.S., W.D. and J.Z. (Jiaxue Zhang); supervision, Q.S. and H.Y.; project administration, Q.S. and H.Y.; funding acquisition, Q.S. and H.Y. and G.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal experiments were approved by the Ethical Committee of Ocean University of China, School of Medicine and Pharmacy (Permission No. OUC-2022-0901-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded and supported by the National Natural Science Foundation of China (32101032 and 81991522), Taishan Scholars Program (tsqn202306339), Fundamental Research Funds for the Central Universities (3008000/842312003 and 3008000/862201013139), Natural Science Foundation of Shandong Province (ZR2021QC110), Research Project of Qingdao Marine Biomed-ical Research Institute (DR2022002-4), Shandong Provincial Major Science and Technology Project (2021ZDSYS22 and 2020CXGC010601), Major Project of Qingdao National Laboratory for Marine Science and Technology (2022QNLM030004-4), Taishan Scholar Climbing Project (TSPD20210304), and Taishan Industry Leading Talent Project.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhu M., Song Y., Xu Y., Xu H. Manipulating Microbiota in Inflammatory Bowel Disease Treatment: Clinical and Natural Product Interventions Explored. Int. J. Mol. Sci. 2023;24:11004. doi: 10.3390/ijms241311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y., Chen Z., Xu C., Kan S., Chen D. Disturbances of the gut microbiota and microbiota-derived metabolites in inflammatory bowel disease. Nutrients. 2022;14:5140. doi: 10.3390/nu14235140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira E.C.S.D., Quaglio A.E.V., Magro D.O., Di Stasi L.C., Sassaki L.Y. Intestinal Microbiota and miRNA in IBD: A Narrative Review about Discoveries and Perspectives for the Future. Int. J. Mol. Sci. 2023;24:7176. doi: 10.3390/ijms24087176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manichanh C., Borruel N., Casellas F., Guarner F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 5.O’Toole P.W., Marchesi J.R., Hill C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 6.Lavelle A., Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 7.Noor S.O., Ridgway K., Scovell L., Kemsley E.K., Lund E.K., Jamieson C., Johnson I.T., Narbad A. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010;10:134. doi: 10.1186/1471-230X-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nomura K., Ishikawa D., Okahara K., Ito S., Haga K., Takahashi M., Arakawa A., Shibuya T., Osada T., Kuwahara-Arai K., et al. Bacteroidetes species are correlated with disease activity in ulcerative colitis. J. Clin. Med. 2021;10:1749. doi: 10.3390/jcm10081749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita H., Kano C., Ishii C., Kagata N., Ishikawa T., Hirayama A., Uchiyama Y., Hara S., Nakamura T., Fukuda S. Bacteroides uniformis and its preferred substrate, α-cyclodextrin, enhance endurance exercise performance in mice and human males. Sci. Adv. 2023;9:eadd2120. doi: 10.1126/sciadv.add2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauffin Cano P., Santacruz A., Moya Á., Sanz Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE. 2012;7:e41079. doi: 10.1371/journal.pone.0041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Almela I., Romaní-Pérez M., Bullich-Vilarrubias C., Benítez-Páez A., Gómez Del Pulgar E.M., Francés R., Liebisch G., Sanz Y. Bacteroides uniformis combined with fiber amplifies metabolic and immune benefits in obese mice. Gut Microbes. 2021;13:1–20. doi: 10.1080/19490976.2020.1865706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu T., Wang Y., Ma M., Dai W., Pan L., Shang Q., Yu G. Isolation of alginate-degrading bacteria from the human gut microbiota and discovery of Bacteroides xylanisolvens AY11-1 as a novel anti-colitis probiotic bacterium. Nutrients. 2023;15:1352. doi: 10.3390/nu15061352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu T., Pan L., Shang Q., Yu G. Fermentation of alginate and its derivatives by different enterotypes of human gut microbiota: Towards personalized nutrition using enterotype-specific dietary fibers. Int. J. Biol. Macromol. 2021;183:1649–1659. doi: 10.1016/j.ijbiomac.2021.05.135. [DOI] [PubMed] [Google Scholar]
- 14.Pan L., Fu T., Cheng H., Mi J., Shang Q., Yu G. Polysaccharide from edible alga Gloiopeltis furcata attenuates intestinal mucosal damage by therapeutically remodeling the interactions between gut microbiota and mucin O-glycans. Carbohydr. Polym. 2022;278:118921. doi: 10.1016/j.carbpol.2021.118921. [DOI] [PubMed] [Google Scholar]
- 15.Chang C.S., Liao Y.C., Huang C.T., Lin C.M., Cheung C.H.Y., Ruan J.W., Yu W.H., Tsai Y.T., Lin I.J., Huang C.H., et al. Identification of a gut microbiota member that ameliorates DSS-induced colitis in intestinal barrier enhanced Dusp6-deficient mice. Cell Rep. 2021;37:110016. doi: 10.1016/j.celrep.2021.110016. [DOI] [PubMed] [Google Scholar]
- 16.Liu L. Master’s Thesis. Wayne State University; Detroit, MI, USA: 2017. Investigation of Optimal Culture Conditions and Development of a Protective Delivery Method of Eubacterium siraeum as a Potential Probiotic. [Google Scholar]
- 17.Moore W.C., Johnson J.L., Holdeman L.V. Emendation of Bacteroidaceae and Butyrivibrio and descriptions of Desulfomonas gen. nov. and ten new species in the genera Desulfomonas, Butyrivibrio, Eubacterium, Clostridium, and Ruminococcus. Int. J. Syst. Evol. Microbiol. 1976;26:238–252. doi: 10.1099/00207713-26-2-238. [DOI] [Google Scholar]
- 18.Gómez del Pulgar E.M., Benítez-Páez A., Sanz Y. Safety assessment of Bacteroides uniformis CECT 7771, a symbiont of the gut microbiota in infants. Nutrients. 2020;12:551. doi: 10.3390/nu12020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Kaoutari A., Armougom F., Gordon J.I., Raoult D., Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 20.Hehemann J.H., Kelly A.G., Pudlo N.A., Martens E.C., Boraston A.B. Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc. Natl. Acad. Sci. USA. 2012;109:19786–19791. doi: 10.1073/pnas.1211002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hehemann J.H., Correc G., Barbeyron T., Helbert W., Czjzek M., Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 22.Wardman J.F., Bains R.K., Rahfeld P., Withers S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022;20:542–556. doi: 10.1038/s41579-022-00712-1. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press; Washington, DC, USA: 2011. [Google Scholar]
- 24.Tamura K., Stecher G., Kumar S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Y., Yu G., Shi C.P., Liu L.M., Guo Q., Han C., Zhang D., Zhang L., Liu B., Gao H., et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta. 2022;1:e12. doi: 10.1002/imt2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.