Abstract
Co-creating patient-facing educational materials (PEMs) can enhance person-centered care by responding to patient priorities and unmet needs. Little data exist on ‘best practices’ for co-creation. We followed the Arksey and O’Malley framework to conduct a systematic literature search of nine databases (MEDLINE, PubMed, EMBASE, CINAHL, PsycINFO, Web of Science, Cochrane Library, Joanna Briggs Institute, TRIP—April, 2022) to identify empirical studies published in English on PEM co-creation to distill ‘best practices’. Following an independent dual review of articles, data were collated into tables, and thematic analysis was employed to synthesize ‘best practices’ that were validated by a patient experienced in co-creating PEMs. Bias was not assessed, given the study heterogeneity. Of 6998 retrieved articles, 44 were included for data extraction/synthesis. Studies utilized heterogeneous methods spanning a range of health conditions/populations. Only 5/45 (11%) studies defined co-creation, 14 (32%) used a guiding framework, and 18 (41%) used validated evaluation tools. Six ‘best practices’ were identified: (1) begin with a review of the literature, (2) utilize a framework to inform the process, (3) involve clinical and patient experts from the beginning, (4) engage diverse perspectives, (5) ensure patients have the final decision, and (6) employ validated evaluation tools. This scoping review highlights the need for clear definitions and validated evaluation measures to guide and assess the co-creation process. Identified ‘best practices’ are relevant for use with diverse patient populations and health issues to enhance person-centered care.
Keywords: co-creation, hypogonadotropic hypogonadism, Kallmann syndrome, patient education, patient participation, value healthcare
1. Introduction
Co-creation is broadly conceptualized as the process of creation through interactions with others [1]. In the context of healthcare, co-creation aims to create value in new goods and/or services by enabling interactions and exchange between diverse stakeholders (e.g., patients/families and healthcare providers). As such, co-creation is a collaborative and democratic approach that recognizes both patients and professionals as equal partners in finding solutions and creating value. Co-creation has been used across a variety of healthcare settings and with diverse patient populations to develop person-centered approaches to care that are responsive to patient-identified needs [2].
A person-centered approach to care considers the whole individual and recognizes patients/families as full, active partners in the design and implementation of healthcare [2]. Key tenets of person-centered care include empathy, respect, engagement, relationship, communication, shared decision making, holistic focus, individualized focus, and coordinated care [3]. Person-centered care is widely acknowledged as an essential component of quality care that can improve a variety of health outcomes, including physical and social well-being, patient knowledge, and satisfaction with care [2].
Co-creation is an aspect of person-centered care representing a low-cost solution to help improve healthcare delivery, including educating and activating patients/families for self-management [4]. Despite the growing use of co-creation in healthcare, there is a paucity of data on co-creation ‘best practices’. The purpose of this scoping review was to address the primary question, “what is known about co-creating patient-facing educational materials (PEM) with patients and/or families?”. We aimed to synthesize findings from the existing literature to identify ‘best practices’ that could serve as a roadmap to improve the co-creation of PEMs.
2. Materials and Methods
We conducted a scoping review guided by the Arksey and O’Malley framework [5]. There is no registered protocol associated with this scoping review. The literature search and review was conducted using Covidence™ 2.0 systematic review software (Veritas Health Innovation, Melbourne, Australia—www.covidence.org, (accessed on 23 December 2022)), and we report study findings using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for the reporting of scoping reviews (PRISMA-ScR).
2.1. Identifying the Research Question
The scoping review process was guided by a single primary question: “what is known about co-creating patient-facing materials with patients and/or families?”
2.2. Identifying the Relevant Literature
With the support of a research Librarian, we conducted literature searches (19 April 2022) in 9 databases (MEDLINE, PubMed, EMBASE, CINAHL, PsycINFO, Web of Science, Cochrane Library, Joanna Briggs Institute, and TRIP). The structured search used the medical subject headings (MeSH) terms and keywords “co-creation” OR “co-production” OR “co-design” OR “co-construction” OR “co-innovation” OR “co-build” OR “codesign” OR “co-establish” OR “collaborative health” OR “patient-directed” OR “patient-centered” OR “patient focused” OR “community based participatory research” OR “patient participation” OR “patient partnership” OR “user-centered design” OR “patient partnerships” OR “patient supported” OR “patient engaged consultative” AND “learning health system” OR “learning healthcare system” OR “patient-facing materials” OR “education materials” OR “patient materials” OR “patient resources” OR “psychoeducational tool” OR “information sheet” OR “patient education materials” OR “patient education” AND “patients” OR “clients” OR “support groups” OR “family members” OR “caregivers” OR “spouses” OR “parents” OR “subjects” OR “participants” OR “patient collaborators”. No language restrictions were placed on retrieving published articles.
2.3. Selecting the Literature
Eligible studies were published in English, had no date restriction, and reported on the process of co-creating patient-facing educational materials (digital or print) involving at least one healthcare professional and at least one patient or family member. Eligible studies could involve any healthcare discipline, disease entity, or research methodology (i.e., quantitative, qualitative, or mixed methods). Studies that only included patients/families as a final validation step were excluded. Articles retrieved from the structured literature search were imported into Covidence™ for screening. After removal of duplicates, all titles and abstracts underwent independent dual review (IRM, ESB, EAW). Subsequently, the remaining articles underwent independent, dual, full-text review (IRM, ESB, EAW). Any discrepancies during the review process were resolved by group discussion (with AAD).
2.4. Charting the Data
Investigators (IRM, ESB, EAW) independently extracted data using a structured form, and findings were cross-checked by another investigator. The structured form developed for this scoping review captured the country the study was conducted in, the topic/health issue, framework/guidelines employed, whether co-creation was operationally defined, outcome measurements/tools, summary of key findings, and author-identified strengths/weaknesses. Risk of bias was not conducted due to the methodological variability of included studies.
2.5. Collating, Summarizing, and Reporting Results
Extracted data from included articles were organized in a master table (Table S1). Findings were reviewed and analyzed using an iterative process to identify thematic elements [6] reflecting aspects that contributed to the study success. Identified themes were collapsed into categorical groups by discussion to identify ‘best practices’.
2.6. Synthesis of Results
Thematic categories of respective strengths (and respective weaknesses) were quantified to identify ‘best practices. The ‘best practices’ were organized along a timeline for the co-creation process to depict the natural sequencing of co-creation best practices.
2.7. Patient and Public Involvement
The final ‘best practices’ were reviewed by a patient leader (NS) who had previously participated in two projects co-creating PEMs. Discussion with the patient leader elicited feedback to refine and validate ‘best practices’.
3. Results
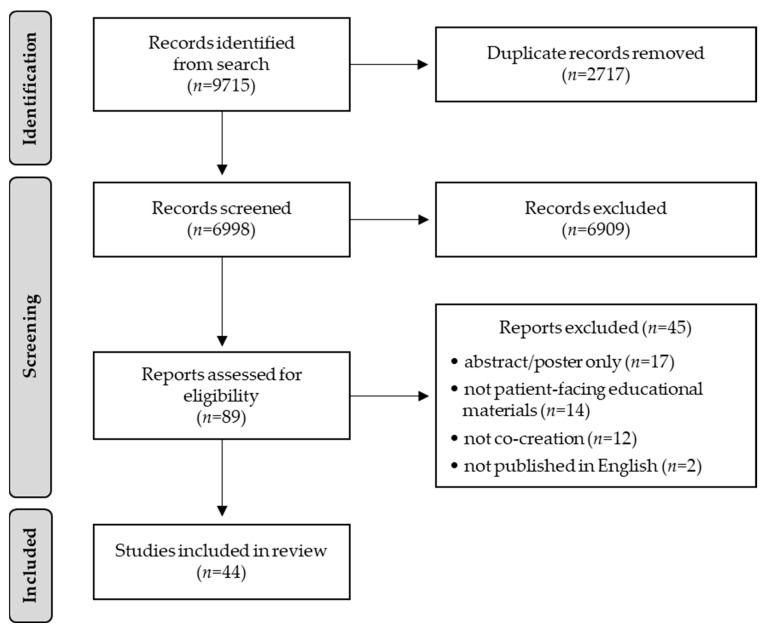
The literature search identified 9715 articles, of which 2717 were duplicates, leaving 6998 articles for eligibility screening (Figure 1). A dual review of the title/abstract identified 6909 not meeting eligibility criteria, leaving 89 articles for full-text review. Dual full-text review removed 44 articles (abstract/poster only (n = 17), not patient-facing educational materials (n = 14), not co-creation (n = 12), not English (n = 2)), leaving 44 articles for data extraction. There was “substantial agreement” [7] in article selection (Cohen’s Kappa = 0.62). The full table of data extraction is provided in Supplemental Material (Table S1) [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51].
Figure 1.
Scoping review PRISMA diagram.
3.1. Characteristics of Identified Studies on Co-Creation of PEMs
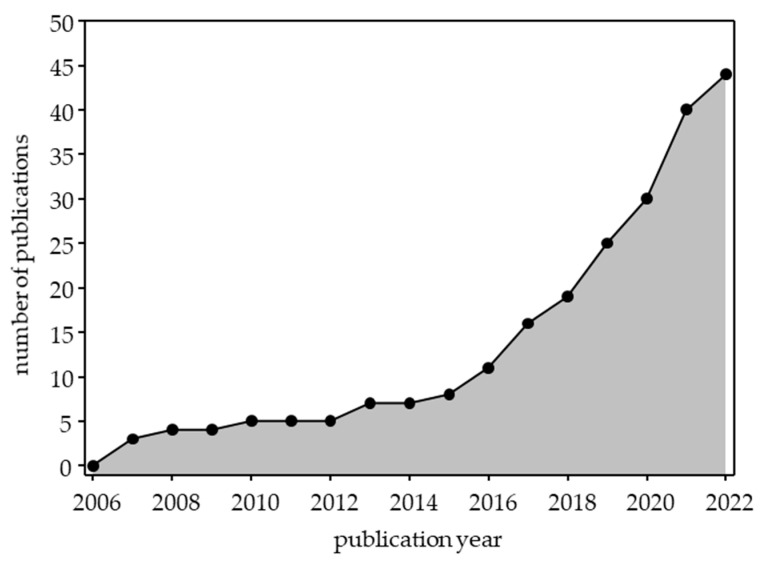
Identified articles were published between 2007 and 2022, and 33/44 (75%) of papers were published since 2017, reflecting that co-creation is a relatively recent adoption in patient education (Figure 2) [9,10,11,12,13,14,15,16,17,18,20,21,23,24,25,26,27,28,31,34,36,37,38,40,41,43,44,45,46,47,48,49,50]. Three-quarters of papers were from anglophone countries—United States: n = 17 [8,9,17,19,20,22,25,27,31,32,33,34,36,42,47,49,50], Canada: n = 7 [10,13,18,28,43,44,51], Australia: n = 4 [14,21,46,48], Ireland: n = 2 [23,35], United Kingdom: n = 1 [39], with four publications from the Netherlands [11,12,15,38], two from Sweden [29,40], and one each from Denmark [45], France [26], Iran [41], Nigeria [30], Norway [37], Spain [16], and Switzerland [24]. In total, 42/44 (95%) articles were reported by groups from high-income countries (not high-income countries: [30,41]). Articles focused on diverse health conditions/patient populations, including specific health conditions, treatments/disease management, informed consent, and health promotion (Table S1). The most common category was cancer/oncology (n = 11) [10,11,14,18,20,31,38,40,41,47,50], followed by chronic health conditions (diabetes [19,23,27,29], cardiovascular disease [17,42,44,45] and chronic kidney disease/renal transplant [12,28,39,48], n = 4 each) and two publications each on asthma [22,32], inflammatory bowel disease [25,49], rare disease (congenital hypogonadotropic hypogonadism) [24,36], and transitional care (i.e., pediatric to adult-oriented care and hospital to home) [35,51]. Only 5/44 (11.3%) studies operationally defined co-creation [12,19,20,25,49].
Figure 2.
Cumulative publications on co-creation of PEMs by year (2006–2022).
3.2. Frameworks Employed to Co-Create PEMs
Most studies employed a framework or approach to guide the co-creation of PEM. However, nearly one-third (14/44, 31.8%) of studies [15,21,26,28,31,33,34,37,38,46,47,48,49,51] did not state a guiding framework. A range of terms were used to describe the approach to the co-creation process (Table S1). The most common approaches were ‘user-centered design/design thinking’ (10/30, 33.3%) [9,14,18,20,25,29,36,41,42,50] and ‘participatory approach’ (i.e., participatory action research, community-based participatory research, learning health system, patient-oriented research, or community engagement [10/30, 33.3%) [8,10,17,22,23,24,27,30,35,40]. One study used a combination of both a user-centered design and a participatory approach [19]. Other heterogeneous terminology was used to describe the approach to co-creation, including constructivist qualitative methodology [13,16,45], social cognitive theory [32], health action process approach [44], plan-do-study-act [39,43], patient empowerment model (interactive learning and action) [11], and an intervention mapping protocol [12]. Almost half (21/44, 47.7%) of studies involved patient partners from the very beginning of the co-creation process [14,17,19,20,21,24,26,28,29,31,34,36,37,38,40,41,46,47,48,50,51].
3.3. Approaches to Evaluate and Measure Effectiveness of Co-Created PEMs
In terms of measuring outcomes of co-creating PEMs, almost a quarter (10/44, 22.7%) of studies [9,11,20,23,25,26,30,39,40,43] did not employ an evaluation method and 4/44 (9.1%) studies [16,17,19,42] only reported on PEM development without reporting outcomes, as they were a component of an ongoing clinical trial. Of the 30 studies reporting outcome measures of co-created PEMs, qualitative methodology (i.e., user interviews, focus groups, open-ended survey responses, diaries, or “think aloud” exercises) was the most common approach, either alone or combined with another instrument (Table 1).
Table 1.
Outcome measures used alone or in combination to evaluate co-created PEMs.
| Measure | Count | Reference(s) |
|---|---|---|
| Qualitative (constructivist) | 15 | [12,13,18,21,28,29,31,32,34,41,44,45,46,50,51] |
| Study-specific questionnaire | 8 | [8,10,12,14,22,35,46,47] |
| Web traffic/usage data | 4 | [34,35,38,49] |
| Validated disease-specific instrument 1 | 4 | [15,27,34,50] |
| Validated readability algorithm 2 | 4 | [24,33,36,48] |
| Validated health literacy instrument 3 | 3 | [12,38,46] |
| Patient Education Materials Assessment Tool (PEMAT) | 3 | [24,36,48] |
| System Usability Scale (SUS) | 2 | [10,37] |
| Mobile Applications Rating Scale (MARS) | 1 | [48] |
| Patient Activation Measure (PAM) | 1 | [38] |
| Decisional Conflict Scale (DCS) | 1 | [10] |
| International Patient Decision Aids Standards (IPDAS) | 1 | [15] |
1 Disease-specific instruments include: Glucose Monitoring Self-Efficacy Scale, Disease Activity Score of 28 Joints (DAS28), Female Self-Advocacy in Cancer Survivorship Scale, PedsQL 4.0, and Adolescent Mediation Barriers Scale Z. 2 Readability algorithms include: Flesch Reading Ease Formula, Flesch Kincaid Grade Level Formula, Gunning Fox Index, Coleman Liau Index, Simple Measure of Gobbledygook (SMOG), Automated Readability Index, and Linsear Write Formula. 3 Health literacy instruments include Health Literacy Questionnaire (HLQ), Health Literacy Assessment Tool for Identifying Facilitating Factors and Barriers to Information, Care, and Services (HLE2), and eHealth Literacy Scales (eHEALS).
3.4. Author-Reported Strengths and Limitations of Co-Creation
Author-reported strengths and weaknesses were captured in the data extraction form (Table S1). Half of the studies (22/44, 50%) cited limited sample size, lack of diversity, and single-center recruitment as a limitation [8,9,10,11,12,13,16,18,20,23,25,27,29,32,34,35,36,41,45,46,47,50]. Additional limitations related to concerns regarding generalizability of findings (7/44, 15.9%) [8,11,15,23,27,30,32], recruitment challenges/possible recruitment bias (5/44, 11.4%) [14,18,24,31,40], and language concerns/English only (4/44, 9.1%) [14,24,28,48]. In relation to relative strengths or promoters of successful co-creation, nearly half of the studies (21/44, 47.7%) [14,17,19,20,21,24,26,28,29,31,34,36,37,38,40,41,46,47,48,50,51] noted including patients and healthcare professionals from the beginning as a promoter of successful co-creation of PEMs. Beginning the process with a literature review to understand the landscape of the issue was identified as a strength by 11/44 (25%) [8,19,20,24,26,28,31,33,37,46,51], and 10/44 (22.7%) [8,9,12,17,22,25,28,32,48,51] highlighted diversity in team members/patients as a strength supporting effective co-creation of PEMs. Three studies underscored that giving patients the “final say” in the co-creation process was important [23,24,35].
3.5. Synthesis of Findings to Identify ‘Best Practices’ for Co-Creating PEMs
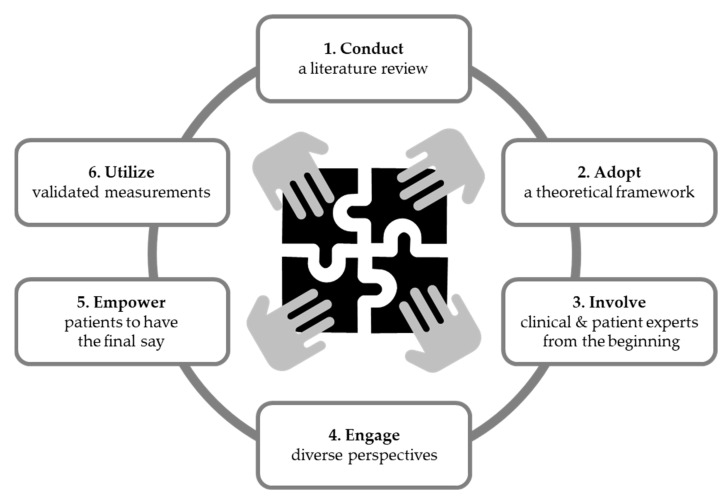
Through iterative discussion, the research team synthesized the findings relating to the use of a guiding theoretical framework, relative strengths/weaknesses of the studies, and outcome measures to identify the salient themes guiding the co-creation process and supporting the development of high-quality PEMs (i.e., understandable, acceptable, and actionable). Six key themes were identified and mapped in a temporal manner to reflect the process from planning through creation to evaluation preceding implementation. The ‘best practices’ include (i) conduct a literature review, (ii) adopt a guiding theoretical framework, (iii) involve patients and healthcare professionals from the beginning, (iv) engage diverse perspectives in the process, (v) empower patients to have the final say, and (vi) utilize validated assessment tools (Figure 3). As a final validation step, we engaged a patient advocate (patient advocate from “I Am HH”) who had previously participated in two co-creation projects [24,36]. The patient leader provided critical feedback and noted that the identified ‘best practices’ are important for engaging patients, and support acceptability of co-created PEMs. Based on the feedback, the ‘best practices’ were deemed acceptable and reflective of practices that support patient-centeredness in the co-creation process.
Figure 3.
The scoping review of the literature identified six ‘best practices’ for co-creating PEMs.
4. Discussion
Our scoping review on co-creation of PEMs identified 44 articles from the systematic review of nine databases. More than half (24/44, 54%) of studies were from groups in North America (United States and Canada). Two-thirds (33/44, 75%) of papers were published since 2017, indicating that co-creation of PEMs is a relatively recent adoption in healthcare, with growing interest. Identified studies spanned a range of health concerns and patient populations, suggesting that co-creation is an adaptable, flexible approach with broad applicability. Co-created PEMs support effective patient education, underpinning patient activation and self-management. As such, using ‘best practices’ for co-creating PEMs holds relevance for an array of stakeholders, including patients/families, healthcare providers, health systems, and payors.
We identified six ‘best practices’ from our scoping review of co-creation of PEMs (Figure 3). First, conducting a literature review provides a deep understanding of the issue under examination, including current evidence and knowledge gaps. Eleven (25%) articles [8,19,20,24,26,28,31,33,37,46,51] reported that the co-creation was based on a literature review. All 11 articles used the literature review to gather available evidence and synthesize existing knowledge to inform PEM development. Notably, there are many types of reviews, and the literature review may be informal or take a more structured approach [52]. Understanding the current state of the science is a critical step for developing PEMs, as end-users may utilize information from PEMs to inform health decisions. Thus, providing accurate, up-to-date information based on the available evidence can support informed decision making. One goal of person-centered care is to engage patients in their healthcare decisions and to support high-quality decisions relating to an individual’s health and care [3]. High-quality decisions are both informed and aligned with one’s values and preferences. Accordingly, summarizing the best available evidence in language that is readily understandable by patients is one key component of supporting patients and families in making high-quality decisions.
Second, co-creating PEMs can be considered a complex intervention. Complex interventions are broadly considered as events that occur within systems (e.g., healthcare systems). Conceptualizing interventions in the context of systems facilitates understanding of the interactions between the interventions and the context in which it is implemented. The United Kingdom Medical Research Council recommends utilizing a guiding theoretical framework when planning and implementing a complex intervention [53]. Thus, utilizing a framework can inform and guide the co-creation process. We observed a range of guiding methodologies among the identified articles. The most frequently employed frameworks were user-centered design/design thinking and participatory approach (i.e., participatory action research, community-based participatory research, learning health system, patient-oriented research, or community engagement). Regardless of the framework employed in the respective articles, authors identified that utilizing a guiding theory or framework provided a dynamic perspective that considered many elements involved in co-creating PMs for the target audience within the specific context. Thus, utilizing a guiding framework can help dissect and address key elements that affect implementation and acceptability for co-created PEMs.
Third, involving both clinical and patient experts from the initial stages ensures that the process adheres to central tenets of patient-centered care (i.e., empathy, respect, engagement, relationship, communication, and shared decision making) [3]. Nearly half (21/44, 48%) of the identified articles included for synthesis involved patients from the beginning of the co-creation process [14,17,19,20,21,24,26,28,29,31,34,36,37,38,40,41,46,47,48,50,51]. The articles cited that including patient perspectives throughout the process helped inform priorities and helped center the final product on patient-identified needs and priorities. Presumably, the ongoing and iterative participation of patients contributes to producing PEMs that are understandable, acceptable, and responsive to patient priorities. Accordingly, involving patients from the beginning of the co-creation process may bolster the creation of person-centered PEMs.
The fourth key factor (engaging diverse perspectives) relates to involving an array of stakeholders with differing perspectives to help create value. Diversity reflects both the type of stakeholder (i.e., healthcare professionals and patients/families) as well as diversity in identity (i.e., race, ethnicity, age, education, etc.). Ten (23%) articles cited the inclusion of diverse perspectives as a strength of the co-creation process [8,9,12,17,22,25,28,32,48,51]. Authors noted that including diverse perspectives enhanced broader applicability and relevance to a wider stakeholder audience. Emphasizing diversity can support end-user acceptability and spur adoption to help overcome translation into practice [54].
Fifth, the notion of empowering patients to have the final say on PEMs additionally supports the patient-centeredness of co-creation. It merits noting that among the identified ‘best practices’, empowering patients to have the final say was only noted as an author-identified strength in 3 of the 44 identified articles. The modest number of supporting articles may be an artifact of our selection process, as 12 studies were excluded (Figure 1) for not meeting our definition of the co-creation process (i.e., involving patients from the beginning). The excluded studies used patients as a final validation step to approve the PEMs created by clinicians/investigators. Thus, excluded studies may provide indirect support for the importance of empowering patients to have the final say. Second, patients are experts in their condition and are in the ideal position to determine whether PEMs are understandable, acceptable, and actionable (i.e., high quality). Valuing patients/families as equal partners in finding solutions by creating space for their perspectives and opinions helps ensure that PEMs are grounded in patient priorities [55]. As noted by the patient advocate involved in this study, partnering with patients/families as equals helps support the relevance and acceptability of co-created PEMs. Involving the patient advocate in this scoping review provided key stakeholder support for including patient empowerment as a ‘best practice’.
Last, utilizing validated instruments represents an important step in improving the rigor of work on co-creating PEMs. Many studies used evaluation methods that were not validated or evidence-based. An important caveat is that validated instruments may not be available for some co-created products (i.e., disease-specific PEMs). Mixed-methods approaches employing validated instruments combined with qualitative methodology can provide in-depth information. However, it is worthwhile to note there are several validated, widely used instruments measuring constructs highly relevant to PEMs. Examples include readability algorithms (e.g., Flesch Reading Ease Formula, Flesch Kincaid Grade Level Formula, Gunning Fox Index, Coleman Liau Index, Simple Measure of Gobbledygook (SMOG), Automated Readability Index, and the Linsear Write Formula), health literacy/numeracy instruments (Short Assessment of Health Literacy (SAHL) [56], Rapid Estimate of Adult Health Literacy in Medicine (REALM) [57], rapid assessment of health literacy [58], Newest Vital Sign (NVS) [59], the System Usability Scale (SUS) [60], and the ‘gold standard’ Patient Education Materials Assessment Tool (PEMAT) from the U.S. Agency for Healthcare Quality and Research [61]. Employing validated tools helps limit bias in evaluation and can provide strong evidence that the materials are acceptable to patients.
This project involved a leader of a patient organization who had experience in co-creating PEMs [24,36] to validate the ‘best practices’ distilled from the literature. The patient advocate had three specific comments. First, in the context of rare disorders, PEMs may serve an important, dual role, as PEMs may also serve to inform clinicians—who may not be familiar with or have specific expertise in a particular rare disorder. Second, PEMs could be written by patients and then validated by HCPs. While no such PEMs were identified in our literature search, it seems plausible that patient organizations could create PEMs that could subsequently be validated by expert clinicians prior to dissemination. Last, the patient advocate noted that co-creation embraces patients as equal partners and thus empowers patients to share their lived experiences. As such, co-creation shifts the approach from a traditional hierarchical paradigm to one that respects and values patient perspectives. Co-creation draws on patient perceptions and experiences of their condition, care, and treatment. The development of patient-reported outcome measures (PROMs) typically utilizes qualitative data from patients to define key constructs and enhance content validity when creating PROMs [62]. As such, future directions could employ co-creation to develop PROMs.
Recent breakthroughs in natural language processing and large language models have helped generate powerful artificial intelligence and machine learning (AI/ML) tools (e.g., ChatGPT4). Such in silico tools have generated significant interest in healthcare. Recent provocative studies suggest that AI/ML chatbots can generate quality, empathetic responses to patient questions [63]. While there is excitement about these emerging technologies, it remains to be seen what role AI/ML may have in creating PEMs. Emerging AI/ML technologies are dependent on the data used to entrain the system, so it remains uncertain whether data sets available on the web could provide similar or equivalent results as involving patients in the co-creation process. Importantly, emotional empathy garnered from sharing one’s experience presents a unique challenge for AI/ML [64]. Sharing experiences is a central aspect of the co-creation process and can create a profound sense of mutual understanding and community.
Strengths and Limitations
As co-creating PEMs is a relatively new phenomenon, we chose to conduct a systematic scoping review, as scoping studies are particularly relevant to areas with emerging evidence [65]. A relative strength of this work is the rigorous, systematic approach to conducting the scoping review that was guided by a well-established methodology. Further, incorporating multiple reviewer perspectives (including a patient with experience co-creating PEMs) increases our confidence in the reliability of the identified ‘best practices’. This study has several limitations. First, our scoping review only identified 44 published articles (2007–2022) reporting on the co-creation of PEMs. We distilled ‘best practices’ across the 44 studies, regardless of health condition or patient population. Having a larger body of published literature to draw from would have strengthened our process in identifying ‘best practices’. While we conducted a structured literature search of nine databases (using numerous terms/keywords), it is possible that not all studies were identified, and we did not conduct an extensive search of the grey literature. Indeed, while the TRIP database includes some grey literature, it should not be considered exhaustive. Additionally, findings may have an anglophone bias, as the majority of studies were published by groups in English-speaking countries. Further, two studies were excluded for not being published in English. As such, caution is merited in extrapolating findings to all cultural/linguistic contexts. Last, we considered co-creation to be a democratic process that involves patients/families as equal partners throughout the project. Accordingly, we excluded studies that involved patients/families at the end of the process as a validation step.
5. Conclusions
There is growing interest in using co-creation as part of forming more person-centered approaches to care. Our synthesis of the existing literature identified six ‘best practices’ for co-creating PEMs: (i) begin by conducting a literature review; (ii) adopt a guiding theoretical framework; (iii) involve patients and healthcare professionals from the beginning; (iv) engage diverse perspectives in the co-creation process; (v) empower patients to have the final say; and (vi) utilize validated assessment tools. Our findings indicate that co-creation is a flexible, broadly applicable approach to enhancing person-centered care that is relevant to wide-ranging health conditions and patient populations. Future directions include clarifying terminology used to describe co-creation, more widespread use of validated and evidence-based evaluation tools, and establishing a structured reporting guideline (i.e., EQUATOR Network) to facilitate comparability of co-creation projects in healthcare.
Acknowledgments
We thank Research Librarian Wanda Anderson for her consultation and assistance with the literature search.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/healthcare11192615/s1, Table S1: Data extraction table summarizing included studies.
Author Contributions
A.A.D. designed the study. I.R.M., E.S.B. and E.A.W. performed the literature search, screened retrieved papers for eligibility, and performed data extraction. All authors (I.R.M., E.S.B., E.A.W., N.S. and A.A.D.) contributed to the data analysis. A.A.D. drafted the first version of the manuscript. All authors (I.R.M., E.S.B., E.A.W., N.S. and A.A.D.) were involved in producing the final version of the manuscript. A.A.D. is the guarantor for this work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The search strategy (including search terms) is delineated in Materials and Methods (Section 2.2), Figure 1 depicts the PRISMA diagram delineating the article selection process, and Supplemental Table S1 provides the data extraction table for all included articles.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (1P50HD104224-01, “Massachusetts General Hospital—Harvard Center for Reproductive Medicine”). Dwyer also receives funding support from the Josiah Macy Jr. Foundation.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ramaswamy V., Ozcan K. What is co-creation? An interactional creation framework and its implications for value creation. J. Bus. Res. 2018;84:196–205. doi: 10.1016/j.jbusres.2017.11.027. [DOI] [Google Scholar]
- 2.NEJM Catalyst What Is Patient-Centered Care? Catal. Carryover. 2017;22:276–282. doi: 10.1056/CAT.17.0559. [DOI] [Google Scholar]
- 3.Hakansson Eklund J., Holmstrom I.K., Kumlin T., Kaminsky E., Skoglund K., Hoglander J., Sundler A.J., Conden E., Summer Meranius M. “Same same or different?” A review of reviews of person-centered and patient-centered care. Patient Educ. Couns. 2019;102:3–11. doi: 10.1016/j.pec.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers S.J., Cramm J.M., Nieboer A.P. The importance of patient-centered care and co-creation of care for satisfaction with care and physical and social well-being of patients with multi-morbidity in the primary care setting. BMC Health Serv. Res. 2019;19:13. doi: 10.1186/s12913-018-3818-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arksey H., O’Malley L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 6.Saldaña J. Coding Manual for Qualitative Researchers. Sage Press; Thousand Oaks, CA, USA: 2009. [Google Scholar]
- 7.McHugh M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sussman A.L., Montoya C., Werder O., Davis S., Wallerstein N., Kong A.S. An Adaptive CBPR Approach to Create Weight Management Materials for a School-Based Health Center Intervention. J. Obes. 2013;2013:978482. doi: 10.1155/2013/978482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudin R.S., Thakore N., Mulligan K.L., Ganguli I. Addressing the Drivers of Medical Test Overuse and Cascades: User-Centered Design to Improve Patient–Doctor Communication. Jt. Comm. J. Qual. Patient Saf. 2022;48:233–240. doi: 10.1016/j.jcjq.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashir N.Y., Moore J.E., Buckland D., Rodrigues M., Tonelli M., Thombs B.D., Bell N.R., Isaranuwatchai W., Peng T., Shilman D.M., et al. Are patient education materials about cancer screening more effective when co-created with patients? A qualitative interview study and randomized controlled trial. Curr. Oncol. 2019;26:124–136. doi: 10.3747/co.26.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petit-Steeghs V., Pittens C., Oosterman J., Broerse J.E.W. Co-creating an empowering health education intervention for urological cancer patients. Health Educ. J. 2021;80:948–960. doi: 10.1177/00178969211035169. [DOI] [Google Scholar]
- 12.Boonstra M.D., Reijneveld S.A., Navis G., Westerhuis R., de Winter A.F. Co-Creation of a Multi-Component Health Literacy Intervention Targeting Both Patients with Mild to Severe Chronic Kidney Disease and Health Care Professionals. Int. J. Environ. Res. Public Health. 2021;18:13354. doi: 10.3390/ijerph182413354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marier-Deschênes P., Gagnon M.-P., Lamontagne M.-E. Co-creation of a post-traumatic brain injury sexuality information toolkit: A patient-oriented project. Disabil. Rehabil. 2021;43:2045–2054. doi: 10.1080/09638288.2019.1686543. [DOI] [PubMed] [Google Scholar]
- 14.Hyatt A., Morkunas B., Davey D., Thai A.A., Trewhella M., Duffy M., Dawson T., Gourlay P., Hutchison J., Milne D. Co-design and development of online video resources about immunotherapy with patients and their family. Patient Educ. Couns. 2020;104:290–297. doi: 10.1016/j.pec.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Spijk-de Jonge M.J., Manders S.H.M., Huis A.M.P., Elwyn G., van de Laar M.A.F.J., van Riel P.L.C.M., Hulscher M.E.J.L. Co-Design of a Disease Activity Based Self-Management Approach for Patients with Rheumatoid Arthritis. Mediterr. J. Rheumatol. 2021;32:21–30. doi: 10.31138/mjr.32.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medina-Perucha L., Galvez-Hernandez P., Garcia-Sangenis A., Moragas A., Cots J.M., Lanau-Roig A., Borras A., Amo I., Barragan N., Monfa R., et al. A Co-Design Process to Elaborate Educational Materials to Promote Appropriate Use of Antibiotics for Acute Lower Respiratory Tract Infections in Primary Healthcare in Catalonia (Spain) Patient Prefer. Adherence. 2021;15:543–548. doi: 10.2147/PPA.S297581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.English A.F., Dickinson L.M., Zittleman L., Nease D.E., Herrick A., Westfall J.M., Simpson M.J., Fernald D.H., Rhyne R.L., Dickinson W.P. A Community Engagement Method to Design Patient Engagement Materials for Cardiovascular Health. Ann. Fam. Med. 2018;16:S58–S64. doi: 10.1370/afm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perlis N., Finelli A., Lovas M., Berlin A., Papadakos J., Ghai S., Bakas V., Alibhai S., Lee O., Badzynski A., et al. Creating patient-centered radiology reports to empower patients undergoing prostate magnetic resonance imaging. Can. Urol. Assoc. J. 2020;15:108–113. doi: 10.5489/cuaj.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslin M., Mullan R.J., Montori V.M. The design of a decision aid about diabetes medications for use during the consultation with patients with type 2 diabetes. Patient Educ. Couns. 2008;73:465–472. doi: 10.1016/j.pec.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 20.McMullen C., Nielsen M., Firemark A., Price P.M., Nakatani D., Tuthill J., McMyn R., Odisho A., Meyers M., Shibata D., et al. Designing for impact: Identifying stakeholder-driven interventions to support recovery after major cancer surgery. Support. Care Cancer. 2018;26:4067–4076. doi: 10.1007/s00520-018-4276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Mahmud A., Long K.A.M., Harrington K.D., Casey K., Bhar S., Curran S., Hunter K., Lim M.H. Developing A Digital Psychoeducational Tool to Reduce Loneliness in Older Adults: A Design Case Study. Int. J. Hum.-Comput. Interact. 2022;38:499–528. doi: 10.1080/10447318.2021.1949854. [DOI] [Google Scholar]
- 22.Garwick A.W., Seppelt A.M. Developing a family-centered participatory action research project. J. Fam. Nurs. 2010;16:269–281. doi: 10.1177/1074840710376175. [DOI] [PubMed] [Google Scholar]
- 23.Pembroke S., Roche E.F., Sleath B., Brenner M., Hilliard C., Cody D., Coyne I. Developing a video intervention to improve youth question-asking and provider education during paediatric diabetes clinic encounters: The Promoting Adolescents Communication and Engagement study. Patient Educ. Couns. 2021;104:2170–2176. doi: 10.1016/j.pec.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 24.COST Action BM1105. Badiu C., Bonomi M., Borshchevsky I., Cools M., Craen M., Ghervan C., Hauschild M., Hershkovitz E., Hrabovszky E., et al. Developing and evaluating rare disease educational materials co-created by expert clinicians and patients: The paradigm of congenital hypogonadotropic hypogonadism. Orphanet J. Rare Dis. 2017;12:57. doi: 10.1186/s13023-017-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalil C., Van Deen W., Dupuy T., Bonthala N., Almario C., Spiegel B. Developing Patient-Centered Inflammatory Bowel Disease-Related Educational Videos Optimized for Social Media: Qualitative Research Study. JMIR Med. Educ. 2020;6:e21639. doi: 10.2196/21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufresne H., de Longcamp A., Compain S., Morice-Picard F., Deladriere E., Bekel L., Godot C., Rateaux M., Godeau M., Jouanne B., et al. Development and co-construction of a therapeutic patient education program for albinism. Ann. Dermatol. Et De Venereol. 2021;148:246–250. doi: 10.1016/j.annder.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 27.McElfish P.A., Rowland B., Riklon S., Aitaoto N., Sinclair K.i.A., Ima S., Kadlubar S.A., Goulden P.A., Hudson J.S., Mamis S., et al. Development and Evaluation of a Blood Glucose Monitoring YouTube Video for Marshallese Patients Using a Community-Based Participatory Research Approach. Policy Politics Nurs. Pract. 2019;20:205–215. doi: 10.1177/1527154419872834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosaasen N., Mainra R., Kukha-Bryson A., Nhin V., Trivedi P., Shoker A., Wilson J., Padmanabh R., Mansell H. Development of a patient-centered video series to improve education before kidney transplantation. Patient Educ. Couns. 2018;101:1624–1629. doi: 10.1016/j.pec.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Nordfeldt S., Hanberger L., Malm F., Ludvigsson J. Development of a PC-based diabetes simulator in collaboration with teenagers with Type 1 diabetes. Diabetes Technol. Ther. 2007;9:17–25. doi: 10.1089/dia.2006.0053. [DOI] [PubMed] [Google Scholar]
- 30.Ajayi I.O., Oladepo O., Falade C.O., Bamgboye E.A., Kale O. The development of a treatment guideline for childhood malaria in rural Southwest Nigeria using participatory approach. Patient Educ. Couns. 2009;75:227–237. doi: 10.1016/j.pec.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Bigelow E.O., Windon M.J., Fakhry C., Kiess A.P., Seiwert T., D’Souza G. Development of a web-based, patient-centered decision aid for oropharyngeal cancer treatment. Oral Oncol. 2021;123:105618. doi: 10.1016/j.oraloncology.2021.105618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleath B., Carpenter D.M., Lee C., Loughlin C.E., Etheridge D., Rivera-Duchesne L., Reuland D.S., Batey K., Duchesne C.I., Garcia N., et al. The development of an educational video to motivate teens with asthma to be more involved during medical visits and to improve medication adherence. J. Asthma Off. J. Assoc. Care Asthma. 2016;53:714–719. doi: 10.3109/02770903.2015.1135945. [DOI] [PubMed] [Google Scholar]
- 33.Ford K., Sankey J., Crisp J. Development of children’s assent documents using a child-centred approach. J. Child. Health Care. 2007;11:19–28. doi: 10.1177/1367493507073058. [DOI] [PubMed] [Google Scholar]
- 34.Hommel K.A., Carmody J., Hershey A.D., Holbein C., Kabbouche-Samaha M., Peugh J., Powers S. Digital Therapeutic Self-Management Intervention in Adolescents With Migraine: Feasibility and Preliminary Efficacy of “Migraine Manager”. Headache. 2020;60:1103–1110. doi: 10.1111/head.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coyne I., Prizeman G., Sheehan A., Malone H., While A.E. An e-health intervention to support the transition of young people with long-term illnesses to adult healthcare services: Design and early use. Patient Educ. Couns. 2016;99:1496–1504. doi: 10.1016/j.pec.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Dwyer A.A., Au M.G., Smith N., Plummer L., Lippincott M.F., Balasubramanian R., Seminara S.B. Evaluating co-created patient-facing materials to increase understanding of genetic test results. J. Genet. Couns. 2021;30:598–605. doi: 10.1002/jgc4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khodambashi S., Haugland D., Ellingsberg A., Kottum H., Sund J.K., Nytrø Ø. An Experimental Comparison of a Co-Design Visualizing Personal Drug Information and Patient Information Leaflets: Usability Aspects; Proceedings of the 16 World Congress of Medical and Health Informatics: Precision Healthcare Through Informatics (MedInfo2017); Hangzhou, China. 21–25 August 2017; pp. 748–752. Studies in Health Technology & Informatics. [DOI] [PubMed] [Google Scholar]
- 38.Ector G.I., Verweij L., Hermens R.P., Blijlevens N.M. Filling the gaps of patient information needs and information perception in chronic myeloid leukemia with the patient-physician co-produced web-based platform CMyLife. Patient Educ. Couns. 2022;105:686–694. doi: 10.1016/j.pec.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Loud F., Jain N., Thomas N. How to develop a patient and carer advisory group in a quality improvement study. J. Ren. Care. 2013;39((Suppl. S2)):2–9. doi: 10.1111/j.1755-6686.2013.12032.x. [DOI] [PubMed] [Google Scholar]
- 40.Grynne A., Browall M., Fristedt S., Ahlberg K., Smith F. Integrating perspectives of patients, healthcare professionals, system developers and academics in the co-design of a digital information tool. PLoS ONE. 2021;16:e0253448. doi: 10.1371/journal.pone.0253448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehdizadeh H., Asadi F., Emami H., Mehrvar A., Nazemi E. An mHealth Self-management System for Support Children with Acute Lymphocytic Leukemia and Their Caregivers: Qualitative Co-design Study. JMIR Form. Res. 2022;6:e36721. doi: 10.2196/36721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyd A.D., Moores K., Shah V., Sadhu E., Shroff A., Groo V., Dickens C., Field J., Baumann M., Welland B., et al. My Interventional Drug-Eluting Stent Educational App (MyIDEA): Patient-Centered Design Methodology. JMIR mHealth uHealth. 2015;3:e74. doi: 10.2196/mhealth.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gollish J.D., Pereira L., MacLeod A.M., Wainwright A., Kennedy D., Robarts S., Dickson P., Clark S. myHip&Knee: Improving Patient Engagement and Self-Management Through Mobile Technology. Healthc. Q. 2019;22:63–67. doi: 10.12927/hcq.2019.25902. [DOI] [PubMed] [Google Scholar]
- 44.Witteman H.O., Presseau J., Nicholas Angl E., Jokhio I., Schwalm J.D., Grimshaw J.M., Bosiak B., Natarajan M.K., Ivers N.M. Negotiating Tensions Between Theory and Design in the Development of Mailings for People Recovering from Acute Coronary Syndrome. JMIR Hum. Factors. 2017;4:e6. doi: 10.2196/humanfactors.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kristiansen A.M., Svanholm J.R., Schjødt I., Mølgaard Jensen K., Silén C., Karlgren K. Patients with heart failure as co-designers of an educational website: Implications for medical education. Int. J. Med. Educ. 2017;8:47–58. doi: 10.5116/ijme.5898.309e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grace S., Horstmanshof L. A realist evaluation of a regional Dementia Health Literacy Project. Health Expect. 2019;22:426–434. doi: 10.1111/hex.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enzinger A.C., Wind J.K., Frank E., McCleary N.J., Porter L., Cushing H., Abbott C., Cronin C., Enzinger P.C., Meropol N.J., et al. A stakeholder-driven approach to improve the informed consent process for palliative chemotherapy. Patient Educ. Couns. 2017;100:1527–1536. doi: 10.1016/j.pec.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muscat D.M., Lambert K., Shepherd H., McCaffery K.J., Zwi S., Liu N., Sud K., Saunders J., O’Lone E., Kim J., et al. Supporting patients to be involved in decisions about their health and care: Development of a best practice health literacy App for Australian adults living with Chronic Kidney Disease. Health Promot. J. Austr. 2021;32((Suppl. S1)):115–127. doi: 10.1002/hpja.416. [DOI] [PubMed] [Google Scholar]
- 49.David J., Tobi C.B., Kennedy S., Jofriet A., Huwe M., Kelekian R., Neihart M., Spotts M., Seid M., Margolis P., et al. Sustainable generation of patient-led resources in a learning health system. Learn. Health Syst. 2021;5:e10260. doi: 10.1002/lrh2.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas T.H., McLaughlin M., Hayden M., Shumaker E., Trybus J., Myers E., Zabiegalski A., Cohen S.M. Teaching patients with advanced cancer to self-advocate: Development and acceptability of the Strong Together™ serious game. Games Health. 2019;8:55–63. doi: 10.1089/g4h.2018.0021. [DOI] [PubMed] [Google Scholar]
- 51.Hahn-Goldberg S., Damba C., Solomon R., Okrainec K., Abrams H., Huynh T. Using co-design methods to create a patient-oriented discharge summary. J. Clin. Outcomes Manag. 2016;23:321–328. [Google Scholar]
- 52.Grant M.J., Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 53.O’Cathain A., Croot L., Duncan E., Rousseau N., Sworn K., Turner K.M., Yardley L., Hoddinott P. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9:e029954. doi: 10.1136/bmjopen-2019-029954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris Z.S., Wooding S., Grant J. The answer is 17 years, what is the question: Understanding time lags in translational research. J. R. Soc. Med. 2011;104:510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grindell C., Coates E., Croot L., O’Cathain A. The use of co-production, co-design and co-creation to mobilise knowledge in the management of health conditions: A systematic review. BMC Health Serv. Res. 2022;22:877. doi: 10.1186/s12913-022-08079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S.Y., Stucky B.D., Lee J.Y., Rozier R.G., Bender D.E. Short Assessment of Health Literacy-Spanish and English: A comparable test of health literacy for Spanish and English speakers. Health Serv. Res. 2010;45:1105–1120. doi: 10.1111/j.1475-6773.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis T.C., Crouch M.A., Long S.W., Jackson R.H., Bates P., George R.B., Bairnsfather L.E. Rapid assessment of literacy levels of adult primary care patients. Fam. Med. 1991;23:433–435. [PubMed] [Google Scholar]
- 58.Chew L.D., Bradley K.A., Boyko E.J. Brief questions to identify patients with inadequate health literacy. Fam. Med. 2004;36:588–594. [PubMed] [Google Scholar]
- 59.Weiss B.D., Mays M.Z., Martz W., Castro K.M., DeWalt D.A., Pignone M.P., Mockbee J., Hale F.A. Quick assessment of literacy in primary care: The newest vital sign. Ann. Fam. Med. 2005;3:514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brooke J. Sus: A ‘quick and dirty’usability. Usability Eval. Ind. 1996;189:189–194. [Google Scholar]
- 61.Shoemaker S.J., Wolf M.S., Brach C. Development of the Patient Education Materials Assessment Tool (PEMAT): A new measure of understandability and actionability for print and audiovisual patient information. Patient Educ. Couns. 2014;96:395–403. doi: 10.1016/j.pec.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothrock N.E., Kaiser K.A., Cella D. Developing a valid patient-reported outcome measure. Clin. Pharmacol. Ther. 2011;90:737–742. doi: 10.1038/clpt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayers J.W., Poliak A., Dredze M., Leas E.C., Zhu Z., Kelley J.B., Faix D.J., Goodman A.M., Longhurst C.A., Hogarth M., et al. Comparing Physician and Artificial Intelligence Chatbot Responses to Patient Questions Posted to a Public Social Media Forum. JAMA Intern. Med. 2023;183:589–596. doi: 10.1001/jamainternmed.2023.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perry A. AI will never convey the essence of human empathy. Nat. Hum. Behav. 2023 doi: 10.1038/s41562-023-01675-w. [DOI] [PubMed] [Google Scholar]
- 65.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The search strategy (including search terms) is delineated in Materials and Methods (Section 2.2), Figure 1 depicts the PRISMA diagram delineating the article selection process, and Supplemental Table S1 provides the data extraction table for all included articles.