Abstract
Physiological differentiation (including antibiotic production) in microorganisms usually starts when cells encounter adverse environmental conditions and is frequently accompanied by an increase in the accumulation of intracellular ppGpp. We have found that the acquisition of certain streptomycin-resistant (str) mutations enables cells to overproduce antibiotics, demonstrating an increase in productivity 5- to 50-fold greater than that of wild-type strains. The frequency of such antibiotic-overproducing strains among the str mutants was shown to range from 3 to 46%, as examined with several strains of the genera Streptomyces, Bacillus, and Pseudomonas. Analysis of str mutants from Bacillus subtilis Marburg 168 revealed that a point mutation occurred within the rpsL gene, which encodes the ribosomal protein S12, changing Lys-56 (corresponding to Lys-43 in Escherichia coli) to Asn, Arg, Thr, or Gln. Antibiotic productivity increased in a hierarchical manner depending upon which amino acid residue replaced Lys at this position. The strA1 mutation, a genetic marker frequently used for mapping, had no effect on antibiotic productivity even though it was found to result in an amino acid alteration of Lys-56 to Ile. Gene replacement experiments with the str alleles demonstrated unambiguously that the str mutation is responsible for the antibiotic overproductivity observed. These results offer a rational approach for improving the production of antibiotic (secondary metabolism) from microorganisms.
One of the significant bacterial regulatory systems is the stringent response which is initiated by nutrient limitation and causes an immediate cessation of RNA accumulation and other cellular reactions. The guanine nucleotides ppGpp (guanosine 5′-diphosphate 3′-diphosphate) and pppGpp (guanosine 5′-triphosphate 3′-diphosphate) are believed to be responsible for this stringent response. Mutants with mutations in the relA or relC (=rplK) genes (which code for the ppGpp synthetase and the ribosomal L11 protein, respectively) fail to synthesize (p)ppGpp (for reviews, see references 3 and 14). Recently, through working with Streptomyces coelicolor A3(2), an unambiguous correlation was established between ppGpp synthesis and antibiotic production as demonstrated from the results of three independent laboratories using various approaches at the molecular level (4, 28, 35). We have found (35, 43) that the impairment in antibiotic production resulting from a relA or relC mutation in S. coelicolor A3(2) could be completely restored by introducing mutations conferring resistance to streptomycin (str). These mutations result in the alteration of the Lys-88 amino acid in ribosomal protein S12 (rpsL gene product) to Glu or Arg (35, 43). No accompanying restoration of ppGpp synthesis was detected in these relA str or relC str mutants. It is therefore apparent that acquisition of certain str mutations allows antibiotic production to be initiated without the requirement for ppGpp. This offers a possible strategy for improving the antibiotic productivity of wild-type prokaryotic microorganisms.
The initiation of antibiotic biosynthesis (so-called secondary metabolism) usually occurs at the transition between vegetative growth and morphological development, such as the sporulation stage of the organism, and considerable effort has been directed towards gaining a detailed understanding of the mechanism involved (reviewed in references 5–7, 22, 26, 38, and 58). Members of the genera Streptomyces, Bacillus, and Pseudomonas are soil bacteria that produce a high proportion of agriculturally and medically important antibiotics. The development of rational approaches to improve the production of antibiotics from these organisms is therefore of considerable industrial and economic importance. This paper describes the effect of the introduction of str mutations on antibiotic production in these organisms.
MATERIALS AND METHODS
Bacterial strains and preparations of mutants.
The strains of Streptomyces spp. and other bacteria used in this study are listed in Table 1. Bacillus subtilis 168 is a standard strain frequently used for studying sporulation. Strains 60009, 61884, 61883, and 61953 are derivatives of strain 168. The spontaneous streptomycin-resistant mutants of each organism were obtained as colonies that grew within 7 to 14 days (for Streptomyces spp.) or within 3 to 7 days (for other bacteria) after spores or cells were spread on glucose-yeast extract-malt extract (GYM) agar containing streptomycin at a stated concentration (see below). The mutants obtained were used for subsequent study after single-colony isolation was done.
TABLE 1.
Antibiotic-producing microorganisms used in this study
| Strain | Antibiotic produced | Description | Source or reference |
|---|---|---|---|
| S. antibioticus 3720 | Actinomycin | Prototrophic wild type | 31 |
| S. chattanoogensis ISP5002 | Fredericamycin | Prototrophic wild type | JCM 4299 (= ATCC 19739) |
| S. lavendulae MA406 A-1 | Formycin | Prototrophic wild type | 30 |
| B. subtilis 168 | NIa | trpC2 | Standard strain for studying sporulation |
| B. subtilis 60009 | NI | strA1 | From E. Freese; originally isolated by Goldthwaite et al. as str-1 (15) and now designated rpsL (39) |
| B. subtilis 61884 | NI | aspB66 trpC2 | 33 |
| B. subtilis 61883 | Impaired antibiotic production | aspB66 trpC2 relA1 | Isogenic relA mutant to 61884 (33) |
| B. subtilis 61953 | Impaired antibiotic production | thr-5 trpC2 relC | 34 |
| B. cereus 2045 | FR900493 | Prototrophic wild type | 29a |
| P. pyrrocinia 2327 | Pyrrolnitrin | Prototrophic wild type | 25 |
NI, not identified, but inhibits growth of S. aureus.
Media and growth conditions.
GYM medium, nutrient sporulation medium, and synthetic medium (S7 medium) were described previously (32, 33). NG medium, a medium developed for antibiotic production by Bacillus subtilis, contained (per liter) 10 g of nutrient broth (Difco), 10 g of glucose, 2 g of NaCl, 5 mg of CuSO4 · 5H2O, 7.5 mg of FeSO4 · 7H2O, 3.6 mg of MnSO4 · 5H2O, 15 mg of CaCl2 · 2H2O, and 9 mg of ZnSO4 · 7H2O (adjusted to pH 7.2 with NaOH). All strains were stocked after growing on GYM agar at 30°C.
Production and determination of antibiotics.
All strains, including B. subtilis, were incubated at 30°C with shaking. The culture conditions for the production of each antibiotic were optimized as described below.
(i) Fredericamycin.
Streptomyces chattanoogensis ISP 5002 was precultured in galactose-glycerol-corn steep liquor medium for 2 days. Then, cells were inoculated into the production medium followed by 4 days of cultivation. The production medium consisted of (per liter) 5 g of l-phenylalanine, 1.5 g of ammonium sulfate, 0.5 g of K2HPO4, 0.5 g of KH2PO4, and 2 g of CaCO3 (adjusted to pH 7.2 with NaOH). Concentration of fredericamycin produced was determined by high-performance liquid chromatography analysis.
(ii) Actinomycin.
Streptomyces antibioticus 3720 (= ATCC 14888) was grown in peptone (NZ-amine; type A) medium for 30 h. Cells were inoculated into a production medium (galactose-glucose-glutamate medium) and cultivated for 4 days as described previously (31). The concentration of actinomycin produced was determined spectrometrically at 443 nm after extraction with ethyl acetate.
(iii) Formycin.
Streptomyces lavendulae MA406 A-1 was cultivated in maltose-polypeptone-yeast extract (MPY) medium for 2 days as described previously (30). Concentrations of formycin were determined by bioassay using Xanthomonas oryzae as a test organism.
(iv) Pyrrolnitrin.
Pseudomonas pyrrocinia 2327 (=ATCC 15958) was precultured in glucose-polypeptone-meat extract medium (2) for 2 days. Cells (0.1 ml) were then inoculated into the same medium (5 ml) and cultivated for 3 days. Concentrations of pyrrolnitrin were determined by a bioassay using Candida albicans as a test organism.
(v) FR900493.
Bacillus cereus 2045 (=FERM BP-1791) was precultured in bouillon medium for 10 h. Cells (0.1 ml) were inoculated into 5 ml of production medium consisting of (per liter) 20 g of polypeptone, 20 g of corn steep liquor, and 5 g of NaCl (adjusted to pH 7.5 with NaOH). Concentrations of FR900493 {3-[(5-aminomethyl-3,4-dihydroxytetrahydrofuran-2-yl)oxy]-2-[(3-aminopropyl) methylamino]-3-[5-(3,4-dihydro-2,4-dioxo-2H-pyrimidin-1-yl)-3,4-dihydroxytet- rahydrofuran-2-yl]propionic acid} were determined by a bioassay using Staphylococcus aureus 209P.
(vi) B. subtilis antibiotic.
Strains of B. subtilis were grown in NG medium (see above) plus requirements (Trp, Thr, or Asp [each at 50 μg/ml]) by direct inoculation of spores into the medium. Cultivation was carried out at 30°C for 30 h. Antibiotic production was determined by a bioassay using S. aureus 209P as described previously (34). Antibiotic activity is expressed as units per milliliter. One unit per milliliter produced a 1.5-mm-wide halo (diameter of clear area, 11 mm). Test solutions were diluted appropriately to produce haloes of this diameter.
Sporulation conditions for B. subtilis.
The cells were inoculated at an optical density at 600 nm (OD600) of 0.01 to 0.02 into flasks containing sporulation medium (33) plus requirements (Trp, Thr, or Asp [each at 50 μg/ml]) and were incubated at 37°C for the indicated times with vigorous shaking. The spore titer was measured by first heating the culture for 20 min at 75°C and then plating after appropriate dilution.
Nutritional shift-down and assay of ppGpp.
B. subtilis cells were grown in 20 ml of synthetic medium (33) plus 1% (wt/vol) vitamin-free Casamino Acids (Difco) and requirements (Trp, Thr, or Asp [each at 50 μg/ml]). When cells had grown to mid-exponential phase (OD600 = 2 to 2.5), they were collected on a membrane filter (0.45-μm pore size), immediately washed with 10 ml of synthetic medium, and the filters with cells were quickly transferred to 20 ml of the synthetic medium without Casamino Acids. After incubation for 15 min, cells were rapidly collected as just described, and the filters were then laid upside down on 10 ml of formic acid (1 M) in a plastic petri dish. After a 60-min incubation at 4°C, cells were removed by centrifugation. The supernatant was filtered through a syringe and vacuum evaporated. The residue was dissolved in 200 μl of water and used for the determination of ppGpp.
ppGpp was determined by high-performance liquid chromatography as described previously (32). Intracellular ppGpp concentrations were expressed in picomoles per AM unit (1 AM unit [or 1 AM600] is the amount of cells which produced an OD600 of 1 when suspended in 1 ml). One AM unit was equivalent to 0.425 mg of cells (dry weight).
Detection of the rpsL gene mutation in B. subtilis.
The rpsL gene of B. subtilis (as shown in the sequence obtained by H. Yoshikawa [DDBJ accession no. D64127]) was amplified from B. subtilis genomic DNA by PCR using synthetic oligonucleotide primers P1 (5′-CCACCTGGGTATGTGGGTT-3′) and P2 (5′-GCATCTGAATATCCTCCCT-3′). The PCR products were directly sequenced manually with α-35S-dCTP or automatically by using the ABI PRISM 310 Genetic Analyzer. The chosen primers amplify the whole rpsL gene so that we can evaluate whether not finding a mutation in a particular gene indicates that the defect must be in another gene.
Replacement of the rpsL gene.
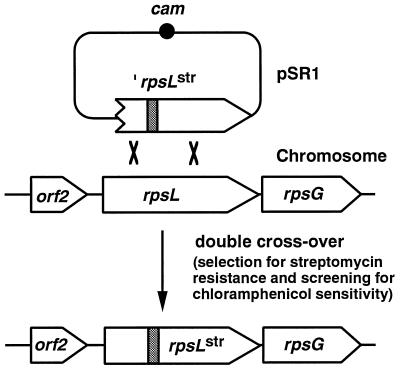
Mutant rpsL genes were amplified by PCR with mutant genomic DNA (str-5, str-9, or str-10) as a template. The primers used were P3 (5′-CCTTGAATTCGCCTACAATTAATCAGCTAA-3′, corresponding to positions 3 to 22 of the rpsL open reading frame [underlining indicates the cleavage site for EcoRI]) and P4 (5′-CCTTAAGCTTGCATCTGAATATCCTCCCT-3′, corresponding to positions 27 to 47 of the gene outside the C-terminal end of the rpsL open reading frame [underlining indicates the cleavage site for HindIII). The resulting 479 bp of PCR product were digested with EcoRI and HindIII and then cloned into the corresponding sites of pAG58 (25). pAG58 is an expression vector which can integrate into the B. subtilis chromosome if the plasmid contains a DNA fragment homologous to part of the chromosome. This plasmid contains the chloramphenicol resistance gene (cam) as a selective marker in B. subtilis. The resulting plasmids, named pSR1, pSR150, and pSR87, were used to transform wild-type B. subtilis 168, and the transformants were first selected for streptomycin resistance. The concentration of streptomycin used for the selection of transformants was 100 μg/ml. The resulting str transformants were then screened for chloramphenicol sensitivity to obtain the clones with the mutant alleles, in which the gene replacement occurred by a double-crossover event (see Fig. 2).
FIG. 2.
Replacement of the rpsL locus by double-crossover recombination between chromosome and pSR1 containing a mutated rpsL allele. rpsLstr indicates the mutated rpsL gene (Lys-56 to Arg). The shaded area represents the mutation site. cam and rpsG indicate the chloramphenicol resistance gene and the ribosomal protein S7-encoding gene, respectively.
RESULTS
Effect of str mutation on Streptomyces spp.
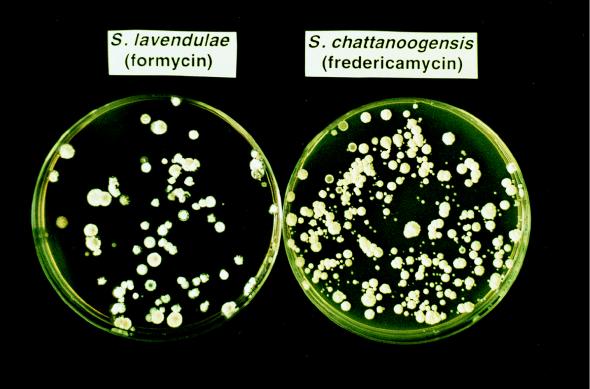
We first examined the effect of a str mutation on antibiotic production in three Streptomyces spp., S. chattanoogensis (which produces fredericamycin), S. antibioticus (which produces actinomycin), and S. lavendulae (which produces formycin). When the spores of these Streptomyces spp. were spread and incubated on GYM agar containing 5 or 30 μg of streptomycin per ml, streptomycin-resistant (str) mutants developed after 7 to 14 days at a frequency of 10−6 to 10−8. These spontaneous str mutants were characterized from a wide variety of colonies by size, morphology, and pigment formation. S. lavendulae and S. chattanoogensis are shown as examples (Fig. 1). We examined antibiotic production using each of 50 to 100 str mutants that were selected randomly. Strikingly, in S. chattanoogensis nearly half of the str mutants tested exhibited a significantly increased ability (greater than fivefold) to produce fredericamycin. The highest productivity detected was 26-fold higher than that of the wild-type strain (Table 2). The level of streptomycin resistance of the high-level-antibiotic-producing (high-producing) strains ranged from 50 to 200 μg/ml. Similarly, strains producing high levels of actinomycin and formycin could be detected at a relatively high frequency (3 to 4%) among str mutants of S. antibioticus and S. lavendulae, respectively (Table 2). Thus, like actinorhodin production by S. coelicolor A3(2) (19), introduction of mutations conferring resistance to streptomycin was shown to be effective for improving the antibiotic productivity of the Streptomyces spp. tested.
FIG. 1.
Spontaneously generated streptomycin-resistant colonies of S. lavendulae and S. chattanoogensis. Spores (ca. 109) of wild-type S. lavendulae and S. chattanoogensis were spread on GYM agar plates containing 5 or 30 μg of streptomycin per ml, respectively, and incubated at 30°C for 10 days.
TABLE 2.
Antibiotic productivity of bacterial streptomycin-resistant mutants
| Microorganism | MIC (μg/ml) | Concn of streptomycin used for selecting str mutants (μg/ml) | Antibiotic (production [μg/ml]) in parental (wild-type) strain | Frequency (%) of str mutants producing increased antibioticc | Highest productivity detected (μg/ml) |
|---|---|---|---|---|---|
| S. chattanoogensis | 1 | 30 | Fredericamycin (10) | 46 (46/100) | 260 |
| S. antibioticus | 1 | 5 | Actinomycin (12) | 4 (2/50) | 63 |
| S. lavendulae | 1 | 5 | Formycin (25) | 3 (2/60) | 130 |
| P. pyrrocinia | 100 | 300 | Pyrrolnitrin (1.5) | 30 (30/100) | 15 |
| B. cereus | 1 | 3 | FR900493 (76) | 7 (9/70) | 550 |
| B. subtilisa | 1 | 5 | B. subtilis antibiotic (8)b | 3 (7/240) | 380b |
| 1 | 400 | B. subtilis antibiotic (8)b | 19 (37/194) | 80b |
Strain 168 was used.
Expressed in units per milliliter.
Mutants producing more than fivefold more antibiotic than the wild type. Numbers in parentheses are number of mutants producing increased antibiotic/number of mutants tested.
Effect of str mutations on Bacillus and Pseudomonas.
Introduction of the str mutation also improved antibiotic productivity of bacteria such as Bacillus spp. and Pseudomonas spp. (Table 2). In B. cereus (which produces the nucleoside antibiotic FR900493) and P. pyrrocinia (which produces pyrrolnitrin), the frequency of antibiotic overproducing strains among str mutants ranged from 7 to 30%.
B. subtilis 168 and its derivatives produce an antibiotic (23, 34). str mutants of the organism developed on GYM agar containing either a low (5 μg/ml) or a high (400 μg/ml) concentration of streptomycin were examined. As shown in Table 2, antibiotic-overproducing strains were detected at a higher frequency among str mutants selected at a high concentration, rather than a low concentration, of streptomycin (19 versus 3%). Antibiotic production was found to be 50-fold greater than that of the wild-type strain in one case (380 U/ml for the str mutant compared with 8 U/ml for strain 168). These str mutants exhibit only a slight decrease in growth rate compared to the wild-type strain.
We examined whether acquisition of resistance to drugs other than streptomycin can also give rise to an increase in antibiotic-producing ability in B. subtilis 168. None of the mutants (we tested 60 mutants for each drug resistance) resistant to chloramphenicol, tetracycline, lincomycin, or spectinomycin exhibited increased antibiotic production.
Characterization of str mutants derived from B. subtilis.
We focused on B. subtilis for further investigation, since this organism offers a feasible system for genetic analysis at the molecular level.
str mutants generated on GYM agar containing a high concentration (400 μg/ml) of streptomycin (we examined 11 representative strains) were all found to have a mutation within the rpsL gene, as was the case for strain 60009, which was previously known to contain the str mutation strA1 (Table 3). It should be emphasized that the altered amino acid positions detected were all located at Lys-56 (corresponding to Lys-43 in Escherichia coli) of the ribosomal protein S12 encoded by the rpsL gene. These str mutants are classified in three groups according to the ability to produce antibiotic. The first group (KO-263 to KO-266), which alters Lys-56 to Asn, is characterized by a lack of increase in antibiotic-producing ability. The second group (KO-267 to KO-271), which alters Lys-56 to Arg or Thr, is characterized by antibiotic production 10-fold greater than that of the parent strain. The third group (KO-272 and KO-273) alters Lys-56 to Gln and produced a four- to fivefold increase of antibiotic. Thus, the ability to produce antibiotic correlates tightly with which amino acid species is altered at position 56, although these mutations all confer a high level of resistance to streptomycin (400 to 2,000 μg/ml). The strA1 mutation (39), a frequently used genetic marker for B. subtilis genetics, was found to alter Lys-56 to Ile. This mutation had no effect on antibiotic productivity (Table 3). The str mutants with a high level of resistance sporulated as well as, or somewhat less than, the parent strain (Table 3).
TABLE 3.
Summary of mutations on the B. subtilis rpsL gene resulting in amino acid exchange in the ribosomal protein S12
| B. subtilis strain | Amt of antibiotic produced (U/ml)a | Spores/mlb in sporulation medium
|
Position of mutation in rpsL genec | Amino acid position (exchange) | Resistance to streptomycin (μg/ml)d | |
|---|---|---|---|---|---|---|
| Alone | Plus 0.5% glucose | |||||
| 168 (parent strain) | 8 | 1.5 × 109 | 2.5 × 108 | 1 | ||
| 60009 (strA1) | 6 | 3 × 108 | 5 × 107 | A-167→T | 56 (Lys→Ile) | 1,000 |
| KO-263 (str-1) | 8 | 4 × 108 | 2 × 107 | A-168→T | 56 (Lys→Asn) | 500 |
| KO-264 (str-2) | 6 | 6 × 108 | 3 × 108 | A-168→T | 56 (Lys→Asn) | 500 |
| KO-265 (str-3) | 6 | 8 × 108 | 3 × 108 | A-168→T | 56 (Lys→Asn) | 500 |
| KO-266 (str-4) | 6 | 9 × 108 | 2 × 108 | A-168→T | 56 (Lys→Asn) | 500 |
| KO-267 (str-5) | 64 | 1.2 × 109 | 1 × 108 | A-167→G | 56 (Lys→Arg) | 2,000 |
| KO-268 (str-6) | 64 | 1.5 × 109 | 3 × 107 | A-167→G | 56 (Lys→Arg) | 1,000 |
| KO-269 (str-7) | 80 | 1 × 109 | 4 × 107 | A-167→G | 56 (Lys→Arg) | 1,000 |
| KO-270 (str-8)e | 80 | 8 × 108 | 1 × 108 | C-64→T | 22 (Pro→Ser) | 1,000 |
| A-167→G | 56 (Lys→Arg) | |||||
| KO-271 (str-9) | 80 | 1.5 × 109 | 1 × 107 | A-167→C | 56 (Lys→Thr) | 1,000 |
| KO-272 (str-10) | 42 | 1 × 108 | 1 × 107 | A-166→C | 56 (Lys→Gln) | 400 |
| KO-273 (str-11) | 30 | 1 × 108 | 1 × 107 | A-166→C | 56 (Lys→Gln) | 400 |
| KO-274 (str-12) | 380 | 3 × 109 | 3 × 109 | Nonef | 20 | |
| KO-275 (str-13) | 240 | 2.5 × 109 | 1.5 × 109 | None | 5 | |
| KO-276 (str-14) | 80 | 8 × 108 | 1 × 108 | None | 20 | |
| KO-277 (str-15) | 64 | 2 × 109 | 2 × 108 | None | 5 | |
| KO-278 (str-16) | 80 | 8 × 108 | 3 × 108 | None | 5 | |
Determined after 2 days of culture at 30°C in NG medium.
Determined after 20 h of culture at 37°C in sporulation medium with or without glucose as indicated.
Numbering originates from the start codon (ATG) of the open reading frame.
Determined after 24 h of incubation at 37°C on NG agar.
This mutant revealed double mutation (C-64 and A-167) within the rpsL gene.
Mutations were not detected within the rpsL gene.
We next analyzed str mutants with the ability to resist a low level of streptomycin (5 to 20 μg/ml). Mutant colonies were initially generated on GYM agar containing 5 μg of streptomycin per ml. Five str mutants (KO-274 to KO-278) were capable of an antibiotic productivity boost of 10- to 50-fold. As summarized in Table 3, no mutation was detected within the rpsL gene from any of these str mutants, even though certain mutations within the rpsL gene are known to result in low-level resistance to streptomycin (18). Strikingly, strains KO-274 and KO-275 showed an increased ability to produce antibiotic accompanied with a greater sporulation ability than the parent strain, especially when cultured in sporulation medium plus 0.5% glucose (Table 3). We therefore conclude that the str-12 and str-13 mutations affect positively both the production of antibiotic and the formation of spores.
As determined by nutritional shift-down experiments, the str mutants accumulated 29 to 114% of the maximum amount of ppGpp accumulated by parent strain 168 (47, 125, and 32 pmol/AM600 for mutants KO-267, KO-271, and KO-274 respectively, compared with 110 pmol/AM600 for strain 168). The str mutants KO-274 and KO-275, which exhibit low-level resistance to streptomycin, revealed a fourfold-increased sensitivity to thiostrepton, tetracycline, and spectinomycin.
Gene replacement between str and str+ alleles.
In order to clearly demonstrate that the str mutations discussed above are responsible for the observed phenotype, i.e., the increased antibiotic-producing ability, gene replacement experiments were performed (see Materials and Methods). The plasmids pSR1, pSR150, and pSR87, which contain the EcoRI-HindIII fragment of the mutant rpsL gene from either strain KO-267 (str-5), KO-271 (str-9), or KO-272 (str-10), respectively, was used to transform the parent strain 168. Our strategy for gene replacement of rpsL with mutant alleles by a double-crossover event is illustrated in Fig. 2. Streptomycin-resistant but chloramphenicol-sensitive recombinants all overproduced antibiotic as much as the original mutant strains. Gene replacement of the mutant rpsL genes was confirmed by directly sequencing the PCR products. Thus, we concluded that str-5, str-9, and str-10 mutations (altering Lys-56 to Arg, Thr, and Gln, respectively) are responsible for the observed increase in antibiotic productivity.
DISCUSSION
Our principal finding in this study was that the introduction of a specified str mutation into any bacterial genera gives rise to a marked increase in antibiotic productivity, thus expanding the previous preliminary work with S. coelicolor A3(2) (19). This novel breeding approach not only results in yielding high-producing strains but also makes it possible to generate these overproducing strains at a surprisingly high frequency (3 to 50% in general). Transferring this trait into other procaryotic microorganisms should therefore offer a convenient and effective method for improving antibiotic productivity. Indeed, as reported previously (43), introduction of a certain str mutation (altering Lys-88 to Glu in ribosomal protein S12) was effective even for activating the silent gene(s) in Streptomyces lividans, which is involved in production of the blue-colored antibiotic actinorhodin in this organism.
Elucidating the mechanism of initiation of so-called secondary metabolism has been the subject of a large number of publications, but very few have focused on ribosomal function or on the translational machinery. It is important to point out that among the ribosome-binding drugs tested so far, only streptomycin has been shown to be effective for improving antibiotic productivity (reference 19 and this study). The action of streptomycin on bacterial ribosomes has been studied in great detail (reviewed by Wallace et al. [54] and Cundliffe [8]), and among the numerous effects attributed to this drug, the misreading of mRNA codons is the best known. Positions in S12 affected by amino acid alterations which have previously been reported to confer streptomycin resistance in E. coli and Mycobacterium tuberculosis are Lys-43, Lys-88, and Pro-91 (11, 12, 21, 29, 53, 56). In S. coelicolor the alteration in Arg-86 also confers resistance (albeit low-level resistance) to streptomycin (43). The alteration in Lys-56 (corresponding to Lys-43 in E. coli and S. coelicolor) identified in B. subtilis in the present study therefore corresponds to one of these recognized positions (Table 3). Unlike those in E. coli and M. tuberculosis, the str mutations detected within the rpsL gene in B. subtilis were all represented by changes at the Lys-56 position (Table 3). As reported previously (43), mutation at Lys-43 in S. coelicolor does not result in an increase in antibiotic productivity, although it elicits a high level of resistance to streptomycin. Of particular interest is the fact that antibiotic productivity of B. subtilis increased in a hierarchical manner depending upon which species of amino acid replaced Lys at position 56 (Table 3). The set of mutants described in this paper therefore may offer a feasible system for studying the regulation of bacterial secondary metabolism at the translational level, together with the bldA (encoding leucine-tRNA) mutant of S. coelicolor as studied by Guthrie and Chater (17). We have thus demonstrated that an altered ribosomal protein S12, resulting from specific mutations in the rpsL gene which confer high level resistance to streptomycin, elicits the ability to overproduce antibiotics not only in streptomycetes but also in members of the family Bacillaceae. Although DNA sequencing was not attempted in this study for the gram-negative bacterium P. pyrrocinia, it is possible that the pyrrolnitrin overproducing str mutants (Table 2) also have a mutation in the rpsL gene.
In B. subtilis, in addition to the relA and relC mutations, a number of other mutations affecting the ability to produce antibiotic have been reported. These include abrA (which may be same as rev-4) and abrB (which is probably the same as absA and absB) (summarized by Piggot and Hoch [39]). sco mutations (scoA, scoB, scoC, and scoD) affecting sporulation control give rise to various degrees of overproduction of alkaline proteases (9). Mutations abrA, abrB, absA, and absB were all originally found by their ability to resist an antibiotic produced by the wild-type sporulating B. subtilis strain 168 (16, 24, 52). Some of these mutations compensate for the detrimental pleiotropic effects resulting from a spo0A mutation by restoring antibiotic and protease production, competence for transformation, and polymyxin resistance (51). The abrB locus has been cloned and sequenced (37). Mutations in this gene affect the transcription of a variety of genes, including aprE (which encodes subtilisin) (10), tycA (which encodes tyrocidine synthetase) (13, 27, 40), and spo0E and spoVG (57). Recent studies have demonstrated that the abrB gene encodes a 10.5-kDa protein which functions as either an activator or a repressor of the expression of a variety of genes by binding to the promoter regions of these genes (46, 55; reviewed briefly by O’Reilly and Devine [36]). Thus, the abrB locus appears to be the major locus responsible for regulating transition stage gene expression. Interestingly, all of the abrB mutants studied so far have been found to have alterations (missing or changed in polarity) in one or more of several different 50S ribosomal proteins (42, 50, 51). However, the causal relationship between the abrB mutation and the observed alteration in ribosomal proteins still remains obscure. The mutation rev-4 (which may be the same as abrA) has been reported to restore the suppressed sporulation caused by other mutations in the RNA polymerase, ribosomal proteins, and the protein synthesis factor EF-G (41). The rev-4 mutation has been located in orfR of the spo0F region (49). It is possible that the streptomycin-resistant mutants identified in the present study that do not have a mutation in the S12 protein (e.g., KO-274 − KO-278 [Table 3]) harbor a mutation in a ribosomal protein other than S12 (or in an rRNA component). This notion may be supported by the fact that those str mutants demonstrated an increased sensitivity to certain ribosome-binding antibiotics such as thiostrepton, and moreover str mutants (as examined with KO-274) revealed an impaired ability to accumulate ppGpp (see Results). In relation to this argument Staal and Hoch (44) reported a new class of str mutation, called strB, which confers conditional streptomycin resistance and is distinct from the classical strA (= rpsL) locus.
There are detailed studies from several laboratories that could shed light on the relationship of various phenotypes to ppGpp levels. On the basis of the isolation and analysis of relaxed (relC) mutants of several Streptomyces spp., ppGpp has been shown to play a central role in triggering the onset of antibiotic production (30–32). In agreement with our results, ppGpp accumulation was noted to coincide with transcription of the pathway-specific activation genes for undecylprodigiosin (redD) and actinorhodin (actII-ORF4) in S. coelicolor (45, 47, 48), and for bialaphos (brpA) in Streptomyces hygroscopicus (20). Antibiotic production by B. subtilis is also abolished by mutations such as relA and relC, which cause a deficiency in ppGpp synthetase and ribosomal L11 protein, respectively (34). As will be reported in detail elsewhere, acquisition of certain str mutations by B. subtilis relA and relC mutants (61883 and 61953, respectively) restores the antibiotic productivity lost in these mutants, without any accompanying ppGpp accumulation. The dependence of B. subtilis on ppGpp for the initiation of antibiotic production is therefore apparently bypassed by certain str mutations, as was the case in S. coelicolor A3(2) (35, 43). The molecular basis for the observed role of the altered ribosomal protein S12 (and another putative ribosomal protein) is unclear. It is, however, conceivable that the specified ribosomal mutations led to a change in ribosomal structure which gave rise to the initiation of the secondary metabolism (by an entirely unknown mechanism), without the requirement of ppGpp. In fact, as reported by Allen and Noller (1), mutations in ribosomal proteins S12 and S4 may influence the higher-order structure of 16S rRNA. However, we cannot rule out the possibility that what we are observing is a novel regulation system, mediated by ribosomes, and not directly related with translation. We are now attempting to clarify, by analyzing protein and RNA formation and by studying mRNA and ppGpp levels, (i) whether certain rpsL alleles induce a stress response or not and (ii) how the combination of rpsL and rel affects these parameters.
ACKNOWLEDGMENTS
This study was supported partly by grants from the Basic Research Core System and the Joint Basic Research Cooperation of the Science and Technology Agency of Japan.
Footnotes
Dedicated to the late Edward Katz for his pioneering work regarding the implication of antibiotics in microbial secondary metabolism.
REFERENCES
- 1.Allen P N, Noller H F. Mutations in ribosomal proteins S4 and S12 influence the high order structure of 16S ribosomal RNA. J Mol Biol. 1989;208:457–468. doi: 10.1016/0022-2836(89)90509-3. [DOI] [PubMed] [Google Scholar]
- 2.Arima K, Imanaka H, Kousaka M, Fukuda A, Tamura G. Studies of pyrrolnitrin, a new antibiotic. I. Isolation and properties of pyrrolnitrin. J Antibiot (Tokyo) Ser A. 1965;18:201–204. [PubMed] [Google Scholar]
- 3.Cashel M, Gentry D R, Hernandez V J, Vinella D. The stringent response. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1458–1496. [Google Scholar]
- 4.Chakraburtty R, Bibb M J. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J Bacteriol. 1997;179:5854–5861. doi: 10.1128/jb.179.18.5854-5861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champness W C, Chater K F. Regulation and integration of antibiotic production and morphological differentiation in Streptomyces spp. In: Piggot P, Moran C P, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C: American Society for Microbiology; 1994. pp. 61–93. [Google Scholar]
- 6.Chater K F, Bibb M J. Regulation of bacterial antibiotic production. In: Kleinkauf H, von Dohren H, editors. Products of secondary metabolism. Bio/Technology. Vol. 6. Weinheim, Germany: VCH; 1997. pp. 57–105. [Google Scholar]
- 7.Chater, K. F., and R. Losick. The mycelial life-style of Streptomyces coelicolor A3(2) and its relatives. In H. Shapiro and M. Dworkin (ed.), Bacteria as multicellular organisms, in press. Oxford University Press, New York, N.Y.
- 8.Cundliffe E. Recognition sites for antibiotics within rRNA. In: Hill W E, Dahlberg A, Garrett R A, Moore P B, Schlessinger D, Warner J R, editors. The ribosome: structure, function, and evolution. Washington, D.C: American Society for Microbiology; 1990. pp. 479–490. [Google Scholar]
- 9.Dod B, Balassa G, Raulet E, Jeannoda V. Spore control (sco) mutations in Bacillus subtilis. II. Sporulation and the production of extracellular proteases and α-amylase by sco mutants. Mol Gen Genet. 1978;163:45–56. [Google Scholar]
- 10.Ferrari E, Henner D J, Perego M, Hoch J A. Transcription of Bacillus subtilis subtilisin and expression of subtilisin in sporulation mutants. J Bacteriol. 1988;170:289–295. doi: 10.1128/jb.170.1.289-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finken M, Kirschner P, Meier A, Wrede A, Böttger E C. Molecular basis of streptomycin resistance in Mycobacterium tuberculosis: alterations of the ribosomal protein S12 gene and point mutations within a functional 16S ribosomal RNA pseudoknot. Mol Microbiol. 1993;9:1239–1246. doi: 10.1111/j.1365-2958.1993.tb01253.x. [DOI] [PubMed] [Google Scholar]
- 12.Funatsu G, Wittmann H G. Ribosomal proteins. XXXIII. Location of amino-acid replacements in protein S12 isolated from Escherichia coli mutants resistant to streptomycin. J Mol Biol. 1972;68:547–550. doi: 10.1016/0022-2836(72)90108-8. [DOI] [PubMed] [Google Scholar]
- 13.Furbaß R, Marahiel M A. Mutant analysis of interaction of Bacillus subtilis transcription regulator AbrB with the antibiotic biosynthesis gene tycA. FEBS Lett. 1991;287:153–156. doi: 10.1016/0014-5793(91)80038-5. [DOI] [PubMed] [Google Scholar]
- 14.Gallant J A. Stringent control in E. coli. Annu Rev Microbiol. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- 15.Goldthwaite C, Dubnau D, Smith I. Genetic mapping of antibiotic resistance markers in Bacillus subtilis. Proc Natl Acad Sci USA. 1970;65:96–103. doi: 10.1073/pnas.65.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guespin-Michel J F. Phenotypic reversion in some early blocked sporulation mutants of Bacillus subtilis: genetic study of polymyxin resistant partial revertants. Mol Gen Genet. 1971;112:243–254. [PubMed] [Google Scholar]
- 17.Guthrie E P, Chater K F. The level of a transcript required for production of a Streptomyces coelicolor antibiotic is conditionally dependent on a tRNA gene. J Bacteriol. 1990;172:6189–6193. doi: 10.1128/jb.172.11.6189-6193.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkin T M, Chambliss G H. Genetic analysis of a streptomycin-resistant oligosporogenous Bacillus subtilis mutant. J Bacteriol. 1984;157:202–210. doi: 10.1128/jb.157.1.202-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hesketh A, Ochi K. A novel method for improving Streptomyces coelicolor A3(2) for production of actinorhodin by introduction of rpsL (encoding ribosomal protein S12) mutations conferring resistance to streptomycin. J Antibiot. 1997;50:532–535. doi: 10.7164/antibiotics.50.532. [DOI] [PubMed] [Google Scholar]
- 20.Holt T G, Chang C, Laurentwinter C, Murakami T, Garres J I, Davies J E, Thompson C J. Global changes in gene expression related to antibiotic synthesis in Streptomyces hygroscopicus. Mol Microbiol. 1992;6:969–980. doi: 10.1111/j.1365-2958.1992.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 21.Honoré N, Cole S T. Streptomycin resistance in mycobacteria. Antimicrob Agents Chemother. 1994;38:238–242. doi: 10.1128/aac.38.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopwood D A, Chater K F, Bibb M J. Genetics and antibiotic production in Streptomyces coelicolor A3(2) In: Vining L, Stuttard C, editors. Genetics and biochemistry of antibiotic production. Newton, Mass: Butterworth-Heinemann; 1995. pp. 65–102. [DOI] [PubMed] [Google Scholar]
- 23.Huh J-W, Shima J, Ochi K. ADP-ribosylation of proteins in Bacillus subtilis and its possible importance in sporulation. J Bacteriol. 1996;178:4935–4941. doi: 10.1128/jb.178.16.4935-4941.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito J, Mildner G, Spizizen J. Early blocked asporogenous mutants of Bacillus subtilis 168. I. Isolation and characterization of mutants resistant to antibiotic(s) produced by sporulating Bacillus subtilis 168. Mol Gen Genet. 1971;112:104–109. doi: 10.1007/BF00267488. [DOI] [PubMed] [Google Scholar]
- 25.Jaacks K J, Healy J, Losick R, Grossman A D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989;171:4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz E, Demain A L. The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev. 1977;41:449–474. doi: 10.1128/br.41.2.449-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marahiel M A, Zuber P, Czekay G, Losick R. Identification of the promoter for peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation in Bacillus subtilis. J Bacteriol. 1987;169:2215–2222. doi: 10.1128/jb.169.5.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Costa O H, Arias P, Romero N M, Parro V, Mellado R P, Malpartida F. A relA/spoT homologous gene from Streptomyces coelicolor A3(2) controls antibiotic biosynthetic genes. J Biol Chem. 1996;271:10627–10634. doi: 10.1074/jbc.271.18.10627. [DOI] [PubMed] [Google Scholar]
- 29.Meier A, Kirschner P, Bange F-C, Vogel U, Böttger E C. Genetic alterations in streptomycin-resistant Mycobacterium tuberculosis: Mapping of mutations conferring resistance. Antimicrob Agents Chemother. 1994;38:228–233. doi: 10.1128/aac.38.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Ochi, K., M. Ezaki, M. Iwami, T. Komori, and M. Kousaka. 1989. Japanese patent 1-296992.
- 30.Ochi K. Occurrence of the stringent response in Streptomyces sp. and its significance for the initiation of morphological and physiological differentiation. J Gen Microbiol. 1986;132:2621–2631. doi: 10.1099/00221287-132-9-2621. [DOI] [PubMed] [Google Scholar]
- 31.Ochi K. A rel mutation abolishes the enzyme induction needed for actinomycin synthesis by Streptomyces antibioticus. Agric Biol Chem. 1987;51:829–835. [Google Scholar]
- 32.Ochi K. Metabolic initiation of differentiation and secondary metabolism by Streptomyces griseus: significance of the stringent response (ppGpp) and GTP content in relation to A factor. J Bacteriol. 1987;169:3608–3616. doi: 10.1128/jb.169.8.3608-3616.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochi K, Kandala J C, Freese E. Initiation of Bacillus subtilis sporulation by the stringent response to partial amino acid deprivation. J Biol Chem. 1981;256:6866–6875. [PubMed] [Google Scholar]
- 34.Ochi K, Ohsawa S. Initiation of antibiotic production by the stringent response of Bacillus subtilis Marburg. J Gen Microbiol. 1984;130:2473–2482. doi: 10.1099/00221287-130-10-2473. [DOI] [PubMed] [Google Scholar]
- 35.Ochi K, Zhang D, Kawamoto S, Hesketh A. Molecular and functional analysis of the ribosomal L11 and S12 protein genes (rplK and rpsL) of Streptomyces coelicolor A3(2) Mol Gen Genet. 1997;256:488–498. doi: 10.1007/pl00008614. [DOI] [PubMed] [Google Scholar]
- 36.O’Reilly M, Devine K. Expression of AbrB, a transition state regulator from Bacillus subtilis, is growth phase dependent in a manner resembling that of Fis, the nucleoid binding protein from Escherichia coli. J Bacteriol. 1997;179:522–529. doi: 10.1128/jb.179.2.522-529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perego M, Spiegelman G B, Hoch J A. Structure for the gene for the transition state regulator abrB: regulator synthesis is controlled by the spoOA sporulation gene in Bacillus subtilis. Mol Microbiol. 1988;2:689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 38.Piepersberg W. Streptomycin and related aminoglycosides. In: Vining L C, Stuttard C, editors. Genetics and biochemistry of antibiotic production. Newton, Mass: Butterworth-Heinemann; 1995. pp. 531–570. [DOI] [PubMed] [Google Scholar]
- 39.Piggot P J, Hoch J A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985;49:158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson J B, Gocht M, Marahiel M A, Zuber P. AbrB, a regulator of gene expression in Bacillus, interacts with the transcription initiation regions of a sporulation gene and an antibiotic biosynthesis gene. Proc Natl Acad Sci USA. 1989;86:8457–8461. doi: 10.1073/pnas.86.21.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharrock R A, Leighton T. Suppression of defective-sporulation phenotypes by the Bacillus subtilis mutation rev4. Mol Gen Genet. 1982;186:432–438. [Google Scholar]
- 42.Shiflett M A, Hoch J A. Alterations of ribosomal proteins causing changes in the phenotype of spo0A mutants of Bacillus subtilis. In: Chambliss G, Vary J C, editors. Spores VII. Washington, D.C: American Society for Microbiology; 1978. pp. 136–138. [Google Scholar]
- 43.Shima J, Hesketh A, Okamoto S, Kawamoto S, Ochi K. Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2) J Bacteriol. 1996;178:7276–7284. doi: 10.1128/jb.178.24.7276-7284.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staal S P, Hoch J A. Conditional dihydrostreptomycin resistance in Bacillus subtilis. J Bacteriol. 1972;110:202–207. doi: 10.1128/jb.110.1.202-207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strauch E, Takano E, Baylis H A, Bibb M J. The stringent response in Streptomyces coelicolor A3(2) Mol Microbiol. 1991;5:289–298. doi: 10.1111/j.1365-2958.1991.tb02109.x. [DOI] [PubMed] [Google Scholar]
- 46.Strauch M A, Spiegelman G B, Perego M, Johnson W C, Burbulys D, Hoch J A. The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein. EMBO J. 1989;8:1615–1621. doi: 10.1002/j.1460-2075.1989.tb03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takano E, Bibb M J. The stringent response, ppGpp and antibiotic production in Streptomyces coelicolor A3(2) Actinomycetologica. 1994;8:1–10. [Google Scholar]
- 48.Takano E, Gramajo H C, Strauch E, Andres N, White J, Bibb M J. Transcriptional regulation of the redD transcriptional activator gene accounts for growth phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2) Mol Microbiol. 1992;6:2797–2804. doi: 10.1111/j.1365-2958.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 49.Trach K, Chapman J W, Piggot P, LeCoq D, Hoch J A. Complete sequence and transcriptional analysis of the spo0F region of the Bacillus subtilis chromosome. J Bacteriol. 1988;170:4194–4208. doi: 10.1128/jb.170.9.4194-4208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trowsdale J, Shiflett M, Hoch J A. New cluster of ribosomal genes in Bacillus subtilis with regulatory role in sporulation. Nature. 1978;272:179–181. doi: 10.1038/272179a0. [DOI] [PubMed] [Google Scholar]
- 51.Trowsdale J, Chen S M H, Hoch J A. Genetic analysis of phenotypic revertants of spo0A mutants in Bacillus subtilis: a new cluster of ribosomal genes. In: Chambliss G, Vary J C, editors. Spores VII. Washington, D.C: American Society for Microbiology; 1978. pp. 131–135. [Google Scholar]
- 52.Trowsdale J, Chen S M H, Hoch J A. Genetic analysis of a class of polymyxin resistant partial revertants of stage 0 sporulation mutants of Bacillus subtilis: map of the chromosome region near the origin of replication. Mol Gen Genet. 1979;8:61–70. doi: 10.1007/BF00267691. [DOI] [PubMed] [Google Scholar]
- 53.van Acken U. Protein chemical studies on ribosomal proteins S4 and S12 from ram (ribosomal ambiguity) mutants of Escherichia coli. Mol Gen Genet. 1975;140:61–68. doi: 10.1007/BF00268989. [DOI] [PubMed] [Google Scholar]
- 54.Wallace B J, Tai P-C, Davis B D. Streptomycin and related antibiotics. In: Hahn F E, editor. Antibiotics V—mechanism of action of antibacterial agents. New York, N.Y: Springer-Verlag; 1979. pp. 272–303. [Google Scholar]
- 55.Xu K, Strauch M A. In vitro selection of optimal AbrB-binding sites: comparison to known in vivo sites indicates flexibility in AbrB binding and recognition of three-dimensional DNA structures. Mol Microbiol. 1996;19:145–158. doi: 10.1046/j.1365-2958.1996.358882.x. [DOI] [PubMed] [Google Scholar]
- 56.Yaguchi M, Wittmann H G. Cooperative control of translational fidelity by ribosomal proteins in Escherichia coli. II. Localization of amino acid replacements in proteins S5 and S12 altered in double mutants resistant to neamine. Mol Gen Genet. 1975;142:35–43. doi: 10.1007/BF00268753. [DOI] [PubMed] [Google Scholar]
- 57.Zuber P, Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuber P, Nakano M M, Marahiel M A. Peptide antibiotics. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C: American Society for Microbiology; 1993. pp. 897–916. [Google Scholar]