Abstract
Background:
Hemodynamically unstable high-risk, or massive, pulmonary embolism (PE) has a reported in-hospital mortality of over 25%. Systemic thrombolysis is the guideline-recommended treatment despite limited evidence. The FLAME study (FlowTriever for Acute Massive PE) was designed to generate evidence for interventional treatments in high-risk PE.
Methods:
The FLAME study was a prospective, multicenter, nonrandomized, parallel group, observational study of high-risk PE. Eligible patients were treated with FlowTriever mechanical thrombectomy (FlowTriever Arm) or with other contemporary therapies (Context Arm). The primary end point was an in-hospital composite of all-cause mortality, bailout to an alternate thrombus removal strategy, clinical deterioration, and major bleeding. This was compared in the FlowTriever Arm to a prespecified performance goal derived from a contemporary systematic review and meta-analysis.
Results:
A total of 53 patients were enrolled in the FlowTriever Arm and 61 in the Context Arm. Context Arm patients were primarily treated with systemic thrombolysis (68.9%) or anticoagulation alone (23.0%). The primary end point was reached in 9/53 (17.0%) FlowTriever Arm patients, significantly lower than the 32.0% performance goal (P<0.01). The primary end point was reached in 39/61 (63.9%) Context Arm patients. In-hospital mortality occurred in 1/53 (1.9%) patients in the FlowTriever Arm and in 18/61 (29.5%) patients in the Context Arm.
Conclusions:
Among patients selected for mechanical thrombectomy with the FlowTriever System, a significantly lower associated rate of in-hospital adverse clinical outcomes was observed compared with a prespecified performance goal, primarily driven by low all-cause mortality of 1.9%.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04795167.
Keywords: anticoagulant, pulmonary embolism, thrombosis
WHAT IS KNOWN
Patients with hemodynamically unstable high-risk pulmonary embolism (PE) have reported in-hospital mortality rates over 25%.
Societal guidelines recommend systemic thrombolysis as front-line treatment in high-risk PE despite limited efficacy data and high bleeding risks.
WHAT THE STUDY ADDS
The FLAME study (FlowTriever for Acute Massive PE) is the largest prospective interventional study in high-risk PE, a hemodynamically unstable population that presents enrollment challenges in traditional prospective randomized clinical trials.
In patients selected for mechanical thrombectomy with the FlowTriever System, a lower associated rate of in-hospital adverse outcomes was observed compared with historical literature.
The FLAME study provides evidence to incorporate large-bore mechanical thrombectomy into standardized care pathways for high-risk PE.
See Editorial by Vedantham
High-risk, or massive, pulmonary embolism (PE) is characterized by hemodynamic instability and right ventricular (RV) strain leading to acute right heart failure, hemodynamic collapse, and death, with reported in-hospital mortality over 25%.1–4 Current treatment guidelines recommend prompt initiation of anticoagulation followed by reperfusion treatment with systemic thrombolytic therapy.5,6 These guidelines are based on limited data.7 Additionally, randomized trials of systemic thrombolysis in largely intermediate-risk patients have demonstrated major bleeding and intracranial hemorrhage rates of 9.2% and 1.5%, respectively.8 These bleeding risks are greater in high-risk PE populations.4
Data in high-risk PE patients are generally lacking, as these patients have been largely excluded from randomized trials and single-arm studies of PE treatments.9–12 A retrospective multicenter study of large-bore mechanical thrombectomy (LBMT) in a patient population including high-risk PE and severely ill intermediate-risk PE showed excellent outcomes with 97% of patients surviving through follow-up.13 But with limited high-quality clinical evidence available for interventional treatments in high-risk PE, guidelines recommend catheter-directed treatment only in patients with contraindications to thrombolysis or who fail systemic thrombolytic treatment.5,6
Interventional studies in high-risk PE have inherent randomization challenges related to the low incidence, the emergent presentation, and likely high rates of crossover or combined treatment approaches.14 Given these challenges, nonrandomized studies with prespecified performance goals were suggested by a Scientific Statement from the American Heart Association as an important initial step to generate evidence in this population.14 The FLAME study (FlowTriever for Acute Massive PE) was designed based on this Scientific Statement to evaluate outcomes in consecutive high-risk PE patients treated with LBMT using a prespecified performance goal developed from a contemporary systematic review and meta-analysis of the literature.4
METHODS
The data that support the findings of this study may be made available from the corresponding author upon reasonable request.
Study Design
The FLAME study is a prospective, multicenter, nonrandomized, parallel group, observational study of high-risk PE. Institutional review board approval was obtained at all sites. To capture all presenting patients, a waiver of consent for participation in the study was included to enable unbiased enrollment of patients regardless of mortality outcome. A chart review was performed as an additional measure to ensure the inclusion of all patients and reduce selection bias. Treating physicians obtained procedure-related informed consent per institutional policies.
Adult patients were eligible if the treatment team determined that the PE was the cause of their shock and they met at least 1 of the following criteria: systolic blood pressure (BP) <90 mm Hg or systolic BP decrease of ≥40 mm Hg for >15 minutes, need for vasopressor support, or resuscitation after cardiac arrest with <30 minutes of cardiopulmonary resuscitation and Glasgow Coma scale score >8. Key exclusion criteria were out-of-hospital cardiac arrest with Glasgow Coma Scale score ≤8, witnessed cardiac arrest with ongoing cardiopulmonary resuscitation ≥30 minutes, and history or current evidence of medical conditions or participation in other clinical studies that would preclude enrollment. Full eligibility criteria are specified in Table S1. Investigators were required to have performed a minimum of 10 cases with the FlowTriever System.
Treatment Selection and Follow-Up
The FLAME study protocol did not dictate a specific treatment for PE, so treatment selection was at the discretion of the treating physician. Treatments could include LBMT with the FlowTriever System (Inari Medical, Irvine, CA) or other devices, systemic or catheter-directed thrombolysis, anticoagulation alone, or surgical thrombectomy. Patients were concurrently enrolled in parallel registries depending on the primary treatment strategy selected: the FlowTriever Arm (treated using the FlowTriever System) or Context Arm (treated using other non-FlowTriever therapies). Patients who initially presented with low or intermediate-risk PE and received advanced therapy but progressed to high-risk PE in the same setting were included in a separate registry (Prior Therapy Arm) because their subsequent clinical course could be influenced by the original treatment. As suggested by the American Heart Association Scientific Statement, this parallel registry structure captured relevant information from the full spectrum of clinical scenarios. Importantly, the Context and Prior Therapy Arms were not intended as comparators to the FlowTriever Arm, and no statistical comparisons were planned between these arms. Patients were followed through hospital discharge or 45 days if still hospitalized, whichever was sooner.
End Points
The primary end point was an in-hospital composite of all-cause mortality, bailout to an alternate thrombus removal strategy, clinical deterioration, and major bleeding (Table S2). All potential primary end point events were adjudicated by an independent Clinical Events Committee (CEC; Boston Clinical Research Institute) for the FlowTriever and Context Arms. In the FlowTriever Arm, the primary end point was compared with a prespecified performance goal (see Statistical Analysis).
Secondary safety end points included the primary end point components; ischemic or hemorrhagic stroke; device-related complications, including those related to primary treatment devices, bailout devices, and extracorporeal membrane oxygenation if part of the primary treatment strategy; and injury to a venous or arterial access site utilized for treatment of PE that required intervention to resolve. All secondary safety end points were adjudicated by the CEC. Other secondary end points included utility measures consisting of the length of hospital and intensive care unit stay, use of extracorporeal membrane oxygenation, time to extubation, and discharge location.
Statistical Analysis
Data are presented as numbers (%), mean±SD, or median (interquartile range). Analyses were performed separately for the FlowTriever Arm and the Context Arm. No statistical analyses were performed directly comparing patient characteristics or outcomes between the parallel registries. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R v4.1.2.
The composite primary end point for the FlowTriever Arm was compared with a historical performance goal (32.0%) derived from a subset of 18 published studies (Supplemental Methods and Tables S3 and S4) from a recent systematic review and meta-analysis of high-risk PE outcomes by the FLAME investigators.4 The FLAME study was stopped early after a prespecified interim analysis was performed at enrollment of 50 FlowTriever Arm patients and the primary end point results met established criteria for early stoppage (Supplemental Methods; Table S5).
RESULTS
Patient Characteristics and Primary Treatments
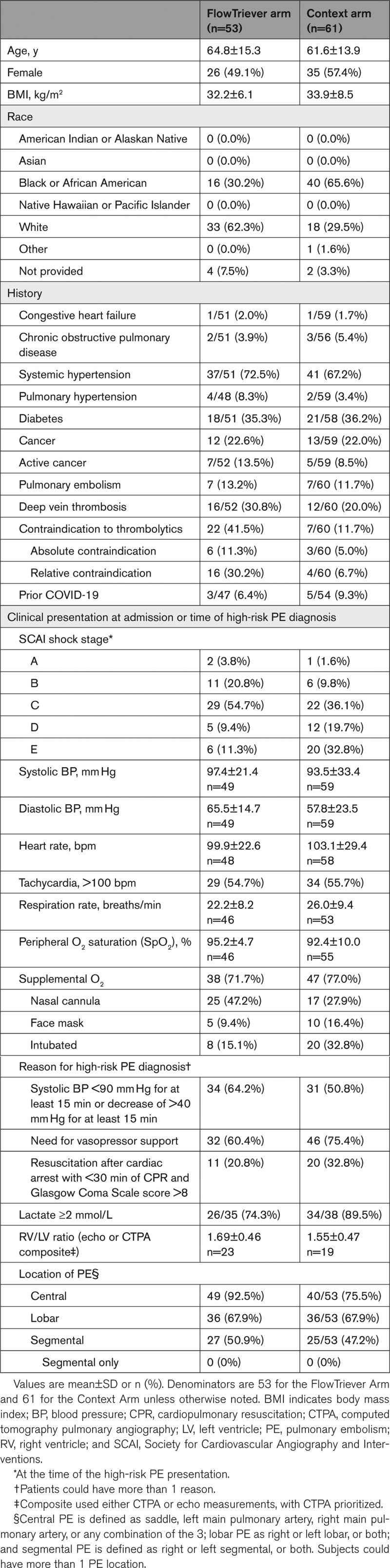
A total of 115 high-risk PE patients were enrolled between March 2021 and November 2022 from 11 sites in the United States: 53 patients in the FlowTriever Arm, 61 patients in the Context Arm, and 1 patient in the Prior Therapy Arm (Figure S1). Baseline characteristics are summarized in Table 1. Patients were most often classified as high-risk PE due to their systolic BP or the need for vasopressor support. A contraindication to thrombolytics was present in 22 (41.5%) FlowTriever Arm patients and 7 (11.7%) Context Arm patients. The FlowTriever Arm and Context Arm each had a large majority of patients in the Society for Cardiovascular Angiography and Interventions shock stage C or higher at the time of presentation, including 11 (20.8%) FlowTriever Arm and 32 (52.5%) Context Arm patients who were in stage D or E.
Table 1.
Demographics, Medical History, and Clinical Presentation
In the FlowTriever Arm, the median estimated blood loss was 100.0 mL (20.0–240.0 mL) overall and 50.0 mL (0.0–200.0 mL) in the 10 procedures that used the FlowSaver device for blood return. In the Context Arm, most patients were treated with systemic thrombolytic therapy as the primary treatment (42/61, 68.9%), followed by anticoagulation alone (14/61, 23.0%), catheter-directed thrombolytic therapy (4/61, 6.6%), and surgical thrombectomy (1/61, 1.6%). No mechanical thrombectomy devices were used as primary treatment in the Context Arm, though non-FlowTriever devices were permitted by the study protocol.
Treatment occurred following PE Response Team activations in 46 (86.8%) FlowTriever Arm patients and 46 (75.4%) Context Arm patients. Off-hours treatments (nights between 18:00 and 7:00 or weekends) occurred in 19 (40.4%) FlowTriever Arm patients and 38 (63.3%) Context Arm patients. The median time to treatment initiation was 6.1 (2.4–27.6) hours in the FlowTriever Arm and 4.1 (1.5–19.6) hours in the Context Arm.
End Points
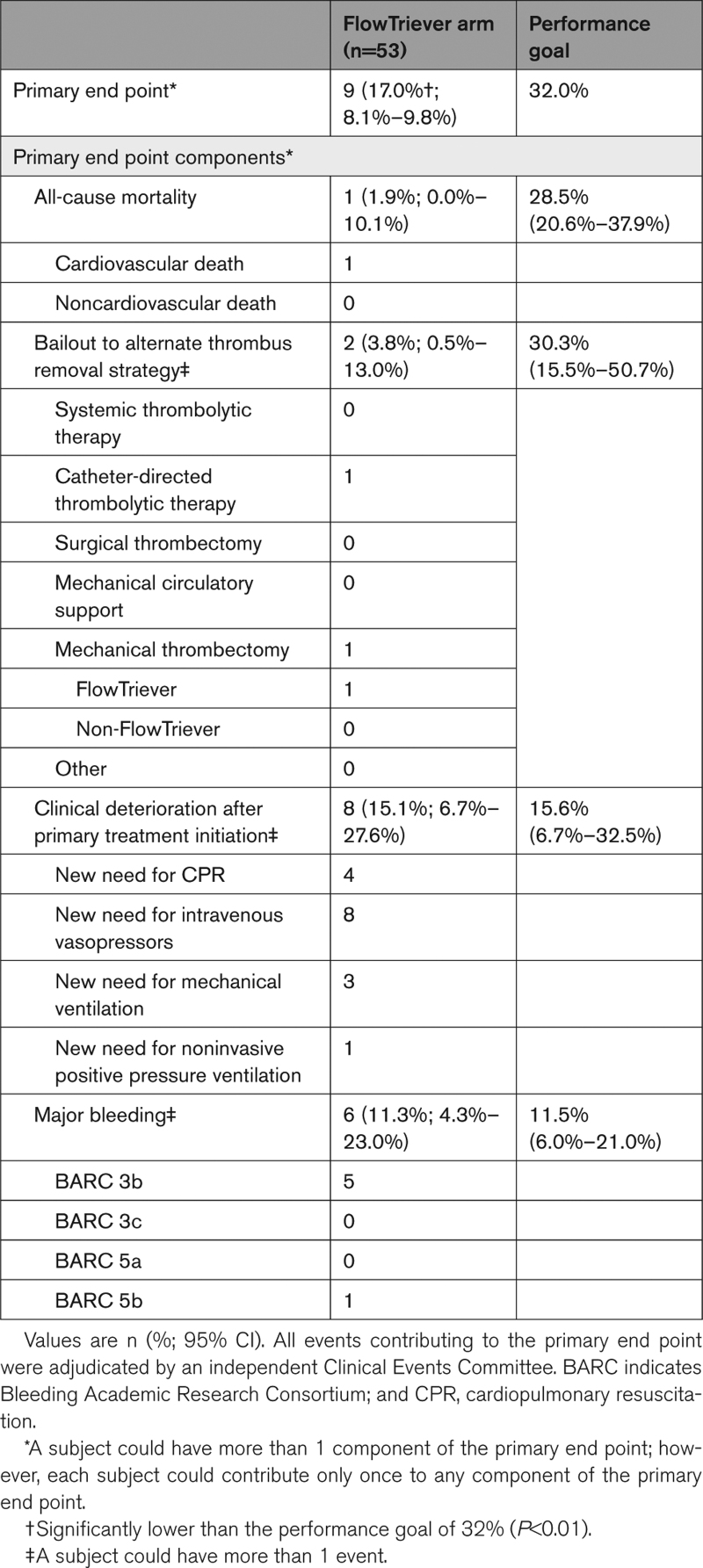
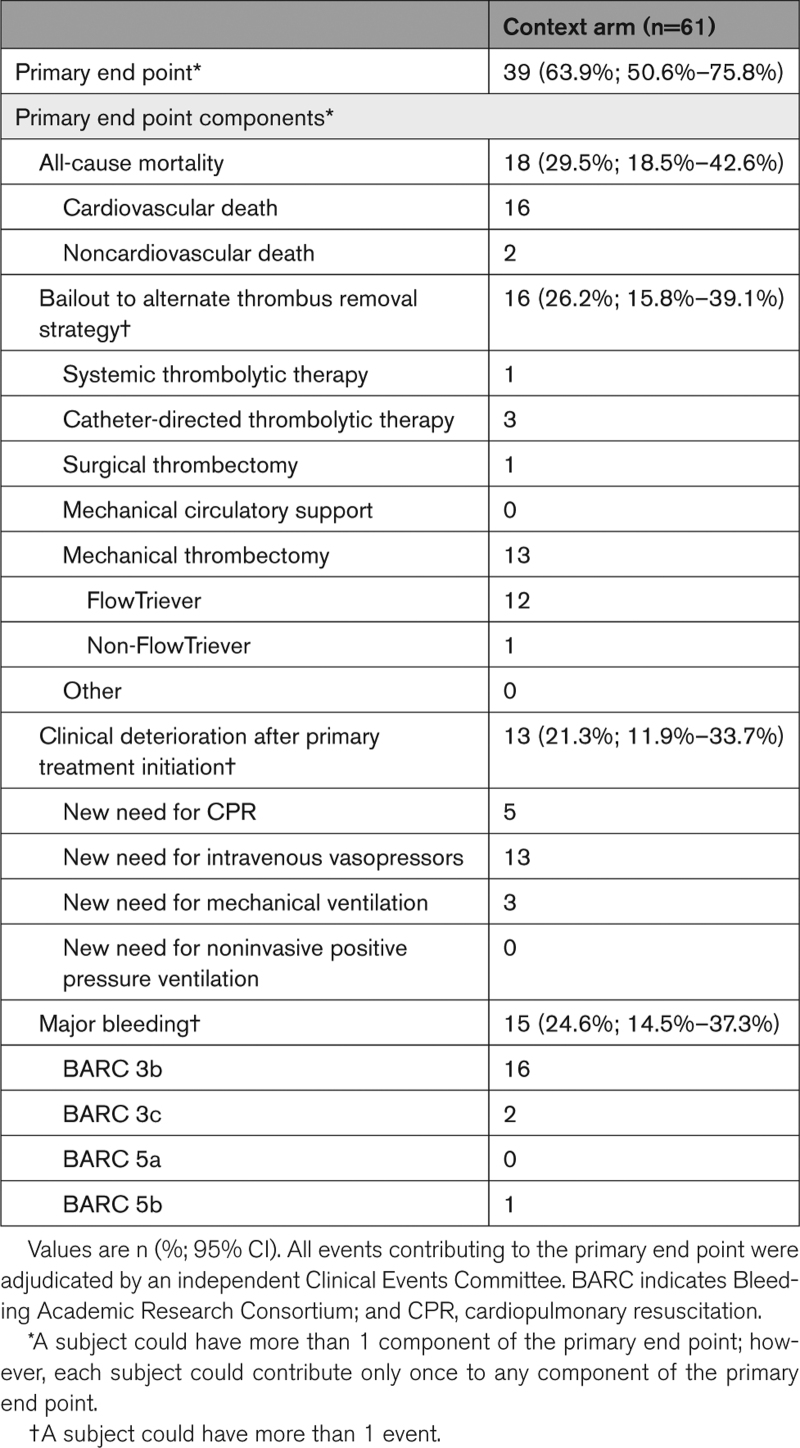
The primary end point was reached in 9/53 (17.0% [95% CI, 8.1%–29.8%]) FlowTriever Arm patients, significantly lower than the performance goal of 32.0% (P<0.01; Table 2). The primary end point was reached in 39/61 (63.9% [95% CI, 50.6%–75.8%]) Context Arm patients (Table 3); this included 28/42 (66.7%) patients treated with systemic thrombolytics and 10/14 (71.4%) patients treated with anticoagulation alone.
Table 2.
Primary End Point in the FlowTriever Arm
Table 3.
Primary End Point in the Context Arm
In-hospital mortality occurred in 1/53 (1.9% [95% CI, 0.0%–10.1%]) FlowTriever Arm patients and 18/61 (29.5%; [95% CI, 18.5%–42.6%]) Context Arm patients. In-hospital mortality stratified by Society for Cardiovascular Angiography and Interventions shock stage is shown in Tables S6 and S7. Bailout occurred in 2/53 (3.8% [95% CI, 0.5%–13.0%]) FlowTriever Arm patients and 16/61 (26.2% [95% CI, 15.8%–39.1%]) Context Arm patients. Clinical deterioration occurred in 8/53 (15.1% [95% CI, 6.7%–27.6%]) FlowTriever Arm patients and in 13/61 (21.3% [95% CI, 11.9%–33.7%]) Context Arm patients. Major bleeding occurred in 6/53 (11.3% [95% CI, 4.3%–23.0%]) FlowTriever Arm patients and in 15/61 (24.6% [95% CI, 14.5%–37.3%]) Context Arm patients. There were no Bleeding Academic Research Consortium 3c (intracranial hemorrhage) major bleeding events in the FlowTriever Arm. There were 2 (3.3%) such events in the Context Arm, both occurring in patients who received systemic thrombolytics (2/42, 4.8%).
Secondary safety end points, serious adverse event incidence, and resource utilization measures are shown in Table S8 (FlowTriever Arm) and Table S9 (Context Arm). Serious adverse events related to the primary treatment device or therapy occurred in 10 (18.9%) FlowTriever Arm patients and 23 (37.7%) Context Arm patients. Device-related complications occurred in 12 (22.6% [95% CI, 12.3%–36.2%]) patients in the FlowTriever Arm, the most common of which were related to hemoglobin decrease or anemia (Table S10) and did not include reports of tricuspid valve injuries, other cardiac injuries, or pulmonary vascular injuries. Device-related complications occurred in 10 (16.4% [95% CI, 8.2%–28.1%]) patients in the Context Arm; the most common are listed in Table S11. Serious adverse event listings are provided in Tables S12 and S13.
DISCUSSION
The FLAME study is the largest prospective study of interventional treatment in high-risk PE. All patients in the FLAME study were critically ill, having met at least one of the European Society of Cardiology criteria for high-risk PE.5 Given the nonrandomized study methodology, FLAME was not designed to enforce the similarity of disease severity or comorbidities across the FlowTriever and Context Arm populations, nor to statistically evaluate differences in patient characteristics or outcomes across these parallel registries. In the FlowTriever Arm, the composite primary end point of clinically relevant adverse outcomes was reached in 17.0% of patients, a significantly lower rate than the performance goal of 32.0% (P<0.01). Importantly, this outcome was driven by a low in-hospital mortality rate of 1.9%. In contrast, the historical mortality rate informing the performance goal was 28.5%. In the Context Arm consisting of all other treatments, the composite primary end point was reached in 63.9% of patients, with an in-hospital mortality rate of 29.5%.
Historically, high-risk PE patients have significant in-hospital mortality of over 25%. Along with their emergent presentation, the low incidence and high rate of crossover treatments make randomization inherently challenging. The difficulty in conducting randomized trials in emergent high-risk PE patients has led to a dearth of quality evidence. To generate evidence in this challenging patient population, the FLAME study was designed based on principles outlined in an American Heart Association Scientific Statement addressing research priorities for interventional studies.14 The FLAME study included a nonrandomized design, parallel registry structure, and an informed consent waiver to enable unbiased enrollment of all high-risk PE patients regardless of mortality outcome. Also, a robust meta-analysis of contemporary treatment strategies in high-risk PE4 was used to establish the prespecified performance goal.
Mechanical thrombectomy with the FlowTriever System was associated with low in-hospital mortality of 1.9%, with only a single in-hospital death. A post hoc analysis stratifying mortality outcomes by disease severity using Society for Cardiovascular Angiography and Interventions shock stages showed that the single death was in a shock stage B patient. All patients treated with the FlowTriever System who were in severely decompensated stages (D/E) survived their hospitalization. The low mortality in the FlowTriever Arm may be due to the rapid effect of thrombus removal, which likely promptly reverses RV strain. Although not collected in FLAME, prior studies have shown an immediate impact on hemodynamics with LBMT. In 800 patients from the FLASH registry (FlowTriever All-Comer Registry for Patient Safety and Hemodynamics), which included 63 (7.9%) high-risk PE patients, significant improvements in hemodynamics were demonstrated immediately after mechanical thrombectomy with the FlowTriever System.15
Importantly, the FLAME study design mimics real-world PE management where treatment selection may be influenced by several factors including PE Response Team consultations, off-hours availability of catheterization laboratory resources, the presence of contraindications to thrombolysis, PE location, whether or not the patient can be both promptly and safely transported to the catheterization laboratory, and the availability of an experienced PE interventionalist. To this end, there were more patients in the Context Arm who were treated off-hours and who had more advanced shock.
The FLAME study results suggest there is a subset of patients with high-risk PE in whom LBMT may be a safe and effective treatment option. Although systemic thrombolytics are the current guideline-recommended treatment and may be the only option for patients too ill to transfer for other treatment, their reflexive utilization in high-risk patients should be reconsidered given known concerns with both safety and efficacy. Outcomes in high-risk PE patients may be positively impacted by the alignment of resources to include LBMT in the standardization of care. This would include immediate transportation of patients who can be stabilized to the cardiac catheterization suite where there is a team accustomed to treating critically ill patients, similar to the care pathways for ST-segment–elevation myocardial infarction and acute ischemic stroke.
Study Limitations
FLAME was not a randomized controlled trial; due to the challenges randomizing critically ill patients, FLAME was designed as a prospective, multicenter, observational study using parallel registries. Several measures were used to capture consecutive patients meeting enrollment criteria at each study site, including informed consent waivers for study participation and hospital-based chart reviews for case capture; despite these efforts, full consecutive enrollment at each site over the enrollment period cannot be guaranteed. Derivation of the meta-analytic performance goal was dependent on largely retrospective high-risk PE literature with variable data missingness and clinical adjudication; as such, we cannot ensure that the historical patient population had a similar acuity to either treatment arm. In addition, as the primary treatment selection was at the discretion of the treating physicians, selection bias was expected to create differences in patient characteristics between the parallel registries, which were not designed to facilitate treatment comparisons. Randomized trials that eliminate selection bias would provide definitive data on the potential benefit of LBMT in high-risk PE.
Conclusions
In hemodynamically unstable high-risk PE patients, patients selected for FlowTriever mechanical thrombectomy incurred a significantly lower associated rate of meaningful in-hospital adverse clinical outcomes compared with a prespecified performance goal, primarily driven by low in-hospital mortality of 1.9%.
ARTICLE INFORMATION
Acknowledgments
The authors thank the participants and staff members of the FLAME study (FlowTriever for Acute Massive PE), and acknowledge writing assistance and editorial review from Jaime Shaw, PhD, Jessica Parsons, PhD, and Craig Markovitz, PhD, and biostatistical support from Caitlin Mills, PhD.
Sources of Funding
Inari Medical sponsored the FLAME study (FlowTriever for Acute Massive PE).
Disclosures
Dr Silver reports consulting fees from Medtronic, Boston Scientific, WL Gore and Associates, Cook Medical, Contego Medical, and Inari Medical; and speakers’ bureau for Bristol Myers Squibb, Astra Zeneca, and Pfizer. Dr Gibson reports consulting fees and research grant support from Inari Medical to Boston Clinical Research Institute and consulting fees from Johnson and Johnson and Bristol Myers Squibb. Dr Giri reports research funds to the institution and advisory boards for Inari Medical, Boston Scientific, Abbott Vascular, Abiomed, Recor Medical, Biosense Webster and equity in Endovascular Engineering. Dr Jaber reports consulting fees from Medtronic and Inari Medical. Dr Toma reports consulting fees from Medtronic, Philips and Neptune Medical. Dr Mina reports consulting fees from Inari Medical. Dr Bowers reports consulting fees from Imperative Vascular and Inari Medical, speakers’ bureau and consulting for Janssen Pharmaceuticals, and equity interest in Imperative Vascular and Biostar Ventures. Dr Greenspon reports research support from Inari Medical and Exponent Inc. Dr Zlotnick reports speakers’ bureau for Abiomed, Angiodynamics, and Inari Medical. Dr Chakravarthy reports equity in Edwards Lifesciences. Dr DuCoffe reports consulting fees from Inari Medical. Dr Butros reports consulting fees from and speakers’ bureau for Inari Medical. Dr Horowitz reports consulting fees from Inari Medical and Penumbra. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Supplemental Results
Figure S1
Tables S1–S13
Supplementary Material
Nonstandard Abbreviations and Acronyms
- FLAME
- FlowTriever for Acute Massive PE
- LBMT
- large-bore mechanical thrombectomy
- PE
- pulmonary embolism
This manuscript was sent to Michael H. Sketch, Jr, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 674 & 675.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.123.013406.
Contributor Information
C. Michael Gibson, Email: charlesmichaelgibson@gmail.com.
Jay Giri, Email: jay.giri@pennmedicine.upenn.edu.
Sameer Khandhar, Email: sameer.khandhar@pennmedicine.upenn.edu.
Wissam Jaber, Email: wissam.jaber@emory.edu.
Catalin Toma, Email: tomacx@upmc.edu.
Bushra Mina, Email: bmina@northwell.edu.
Terry Bowers, Email: terry.bowers@corewellhealth.org.
Lee Greenspon, Email: greensponl@mlhs.org.
Herman Kado, Email: hkado@hotmail.com.
David M. Zlotnick, Email: DZlotnick@KaleidaHealth.org.
Mithun Chakravarthy, Email: mithun.chakravarthy@ahn.org.
Aaron R. DuCoffe, Email: aaron.ducoffe@inova.org.
Paul Butros, Email: rehabutros@gmail.com.
James M. Horowitz, Email: jameshorowitz@gmail.com.
REFERENCES
- 1.Secemsky E, Chang Y, Jain CC, Beckman JA, Giri J, Jaff MR, Rosenfield K, Rosovsky R, Kabrhel C, Weinberg I. Contemporary management and outcomes of patients with massive and submassive pulmonary embolism. Am J Med. 2018;131:1506–1514.e0. doi: 10.1016/j.amjmed.2018.07.035 [DOI] [PubMed] [Google Scholar]
- 2.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113:577–582. doi: 10.1161/CIRCULATIONAHA.105.592592 [DOI] [PubMed] [Google Scholar]
- 3.Sedhom R, Megaly M, Elbadawi A, Elgendy IY, Witzke CF, Kalra S, George JC, Omer M, Banerjee S, Jaber WA, et al. Contemporary national trends and outcomes of pulmonary embolism in the United States. Am J Cardiol. 2022;176:132–138. doi: 10.1016/j.amjcard.2022.03.060 [DOI] [PubMed] [Google Scholar]
- 4.Silver MJ, Giri J, Duffy A, Jaber WA, Khandhar S, Ouriel K, Toma C, Tu T, Horowitz JM. Incidence of mortality and complications in high-risk pulmonary embolism: a systematic review and meta-analysis. J Soc Cardiovasc Angiogr Interv. 2023;2:100548. doi: 10.1016/j.jscai.2022.100548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jimenez D, et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 6.Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, et al. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:2247–2259. doi: 10.1016/j.chest.2021.07.056 [DOI] [PubMed] [Google Scholar]
- 7.Marti C, John G, Konstantinides S, Combescure C, Sanchez O, Lankeit M, Meyer G, Perrier A. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015;36:605–614. doi: 10.1093/eurheartj/ehu218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ, Mukherjee D, Jaff MR, Giri J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311:2414–2421. doi: 10.1001/jama.2014.5990 [DOI] [PubMed] [Google Scholar]
- 9.Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Muller R, Blessing E, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479–486. doi: 10.1161/CIRCULATIONAHA.113.005544 [DOI] [PubMed] [Google Scholar]
- 10.Avgerinos ED, Jaber W, Lacomis J, Markel K, McDaniel M, Rivera-Lebron BN, Ross CB, Sechrist J, Toma C, Chaer R; SUNSET sPE Collaborators. Randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism. JACC Cardiovasc Interv. 2021;14:1364–1373. doi: 10.1016/j.jcin.2021.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, Khandhar S, Amin R, Weinberg M, Engelhardt T, et al. ; FLARE Investigators. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. 2019;12:859–869. doi: 10.1016/j.jcin.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 12.Sista AK, Horowitz JM, Tapson VF, Rosenberg M, Elder MD, Schiro BJ, Dohad S, Amoroso NE, Dexter DJ, Loh CT, et al. ; EXTRACT-PE Investigators. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv. 2021;14:319–329. doi: 10.1016/j.jcin.2020.09.053 [DOI] [PubMed] [Google Scholar]
- 13.Toma C, Khandhar S, Zalewski AM, D’Auria SJ, Tu TM, Jaber WA. Percutaneous thrombectomy in patients with massive and very high-risk submassive acute pulmonary embolism. Catheter Cardiovasc Interv. 2020;96:1465–1470. doi: 10.1002/ccd.29246 [DOI] [PubMed] [Google Scholar]
- 14.Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, Piazza G, Gladwin MT, Chatterjee S, Kobayashi T, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation. 2019;140:e774–e801. doi: 10.1161/CIR.0000000000000707 [DOI] [PubMed] [Google Scholar]
- 15.Toma C, Jaber WA, Weinberg MD, Bunte MC, Khandhar S, Stegman B, Gondi S, Chambers J, Amin R, Leung DA, et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention. 2023;18:1201–1212. doi: 10.4244/EIJ-D-22-00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avgerinos ED, Abou Ali AN, Liang NL, Rivera-Lebron B, Toma C, Maholic R, Makaroun MS, Chaer RA. Catheter-directed interventions compared with systemic thrombolysis achieve improved ventricular function recovery at a potentially lower complication rate for acute pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 2018;6:425–432. doi: 10.1016/j.jvsv.2017.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett NA, Byrne A, Delaney A, Hibbert M, Ramakrishnan N. Management of massive pulmonary embolism: a retrospective single-centre cohort study. Crit Care Resusc. 2010;12:242–247. [PubMed] [Google Scholar]
- 18.Carvalho EM, Macedo FI, Panos AL, Ricci M, Salerno TA. Pulmonary embolectomy: recommendation for early surgical intervention. J Card Surg. 2010;25:261–266. doi: 10.1111/j.1540-8191.2009.00986.x [DOI] [PubMed] [Google Scholar]
- 19.Cho YH, Sung K, Kim WS, Jeong DS, Lee YT, Park PW, Kim DK. Management of acute massive pulmonary embolism: is surgical embolectomy inferior to thrombolysis? Int J Cardiol. 2016;203:579–583. doi: 10.1016/j.ijcard.2015.10.223 [DOI] [PubMed] [Google Scholar]
- 20.de Winter MA, Hart EA, van den Heuvel DAF, Moelker A, Lely RJ, Kaasjager KAH, Stella PR, Chamuleau SAJ, Kraaijeveld AO, Nijkeuter M. Local ultrasound-facilitated thrombolysis in high-risk pulmonary embolism: first Dutch experience. Cardiovasc Intervent Radiol. 2019;42:962–969. doi: 10.1007/s00270-019-02200-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George B, Parazino M, Omar HR, Davis G, Guglin M, Gurley J, Smyth S. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation. 2018;122:1–5. doi: 10.1016/j.resuscitation.2017.11.034 [DOI] [PubMed] [Google Scholar]
- 22.Kuo WT, Banerjee A, Kim PS, DeMarco FJ, Jr, Levy JR, Facchini FR, Unver K, Bertini MJ, Sista AK, Hall MJ, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148:667–673. doi: 10.1378/chest.15-0119 [DOI] [PubMed] [Google Scholar]
- 23.Minakawa M, Fukuda I, Miyata H, Motomura N, Takamoto S, Taniguchi S, Daitoku K, Kondo N; Japan Cardiovascular Surgery Database Organization. Outcomes of pulmonary embolectomy for acute pulmonary embolism. Circ J. 2018;82:2184–2190. doi: 10.1253/circj.CJ-18-0371 [DOI] [PubMed] [Google Scholar]
- 24.Moon D, Lee SN, Yoo KD, Jo MS. Extracorporeal membrane oxygenation improved survival in patients with massive pulmonary embolism. Ann Saudi Med. 2018;38:174–180. doi: 10.5144/0256-4947.2018.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munakata R, Yamamoto T, Hosokawa Y, Tokita Y, Akutsu K, Sato N, Murata S, Tajima H, Mizuno K, Tanaka K. Massive pulmonary embolism requiring extracorporeal life support treated with catheter-based interventions. Int Heart J. 2012;53:370–374. doi: 10.1536/ihj.53.370 [DOI] [PubMed] [Google Scholar]
- 26.Neely RC, Byrne JG, Gosev I, Cohn LH, Javed Q, Rawn JD, Goldhaber SZ, Piazza G, Aranki SF, Shekar PS, et al. Surgical embolectomy for acute massive and submassive pulmonary embolism in a series of 115 patients. Ann Thorac Surg. 2015;100:1245–1251. doi: 10.1016/j.athoracsur.2015.03.111 [DOI] [PubMed] [Google Scholar]
- 27.Pasrija C, Shah A, George P, Kronfli A, Raithel M, Boulos F, Ghoreishi M, Bittle GJ, Mazzeffi MA, Rubinson L, et al. Triage and optimization: a new paradigm in the treatment of massive pulmonary embolism. J Thorac Cardiovasc Surg. 2018;156:672–681. doi: 10.1016/j.jtcvs.2018.02.107 [DOI] [PubMed] [Google Scholar]
- 28.Senturk A, Ucar EY, Berk S, Ozlu T, Altinsoy B, Dabak G, Cakir E, Kadioglu EE, Sen HS, Ozsu S; TUPEG Study Investigators. Should low-molecular-weight heparin be preferred over unfractionated heparin after thrombolysis for severity pulmonary embolism? Clin Appl Thromb Hemost. 2016;22:395–399. doi: 10.1177/1076029614564863 [DOI] [PubMed] [Google Scholar]
- 29.Sharifi M, Berger J, Beeston P, Bay C, Vajo Z, Javadpoor S; “PEAPETT” investigators. Pulseless electrical activity in pulmonary embolism treated with thrombolysis (from the “PEAPETT” study). Am J Emerg Med. 2016;34:1963–1967. doi: 10.1016/j.ajem.2016.06.094 [DOI] [PubMed] [Google Scholar]
- 30.Shiomi D, Kiyama H, Shimizu M, Yamada M, Shimada N, Takahashi A, Kaki N. Surgical embolectomy for high-risk acute pulmonary embolism is standard therapy. Interact Cardiovasc Thorac Surg. 2017;25:297–301. doi: 10.1093/icvts/ivx091 [DOI] [PubMed] [Google Scholar]
- 31.Ucar EY, Araz O, Akgun M, Meral M, Kalkan F, Saglam L, Kaynar H, Gorguner AM. Low-molecular-weight heparin use with thrombolysis: is it effective and safe? Ten years’ clinical experience. Respiration. 2013;86:318–323. doi: 10.1159/000346203 [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Xu Y, Zhang W, Lu W, Chen M, Luo J. Early interventional therapy for acute massive pulmonary embolism guided by minimally invasive hemodynamic monitoring. Int J Clin Exp Med. 2015;8:14011–14017. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.