Abstract
The amyloid cascade model of the pathogenesis of Alzheimer disease (AD) is well supported in observational studies. Its therapeutic corollary asserts that removal of amyloid-β peptide (“amyloid”) would provide clinical benefits. After 2 decades of pursuing the strategy of amyloid removal without success, clinical trials of the antiamyloid monoclonal antibody (AAMA) donanemab and a phase 3 clinical trial of lecanemab have reported clinical benefits linked to amyloid removal. Lecanemab (trade name, Leqembi) is the first with published phase 3 trial results. When administered through IV every 2 weeks to patients with elevated brain amyloid and mild cognitive impairment or mild dementia, lecanemab delayed cognitive and functional worsening by approximately 5 months in an 18-month double-blind, placebo-controlled trial. The trial was well conducted, and the results favoring lecanemab were internally consistent. The demonstration that lecanemab treatment delayed clinical progression in persons with mild symptoms due to AD is a major conceptual achievement, but a better appreciation of the magnitude and durability of benefits for individual patients will require extended observations from clinical practice settings. Amyloid-related imaging abnormalities (ARIA) that were largely asymptomatic occurred in approximately 20%, slightly more than half of which were attributable to treatment and the rest to underlying AD-related amyloid angiopathy. Persons who were homozygous for the APOE ε4 allele had greater ARIA risks. Hemorrhagic complications with longer-term lecanemab use need to be better understood. Administration of lecanemab will place unprecedented pressures on dementia care personnel and infrastructure, both of which need to grow exponentially to meet the challenge.
Background
Alzheimer Disease and the Amyloid Cascade Model
Alzheimer disease (AD), defined neuropathologically as a disorder of amyloid-containing plaques and tau-containing neurofibrillary tangles,1 is the most common cause of later-life cognitive impairment and dementia. The onset of cognitive impairment is typically insidious and gradual, and it is preceded by a long prodrome that may be asymptomatic or associated with subjective cognitive complaints. Its early overt symptomatic manifestations typically involve impaired learning of new information resulting in complaints of forgetfulness, repetition of questions, and misplacing of personal items, but these initial changes do not necessarily interfere with independent living. In younger patients, executive, anomic, or visual impairments may dominate the initial presentations. Eventually, the cognitive deficits lead to loss of independence in daily activities, at which point the diagnosis of dementia applies. While there have been great advances in the development of fluid and imaging biomarkers to establish the presence or absence of the core biology of AD, all stakeholders must never forget that the diagnosis of cognitive impairment depends on an unhurried face-to-face assessment and clinical acumen of a skilled clinician.
After the discovery of disease-associated sequence variations in the amyloid precursor protein (APP) gene,2 the amyloid cascade hypothesis for AD was formulated by Hardy et al.3 (Figure 1). The model was grounded on the neuropathologic observation of high burdens of aggregated amyloid-β peptide (hereafter referred to as “amyloid”) in affected individuals. The critical role for APP or amyloid was subsequently supported by the discovery of disease-associated sequence variants in 2 other genes (PSEN1 and PSEN2) that are also involved in the proteolytic cleavage of APP and a mutation in the APP gene that is protective against AD.4 Allelic variations in the APOE gene have a major impact on the development of AD and supported the amyloid cascade hypothesis because ApoE protein has many actions that involve interactions with amyloid.5 With the development of amyloid tracers for PET imaging and its application in both cognitively unimpaired and impaired persons, it has become clear that the burden of brain amyloid is a reliable predictor of the development of progressive cognitive decline in AD.6 The downstream neurodegeneration including neurofibrillary tangle formation and neuron loss that is the proximate driver of cognitive impairment is contingent on the prior presence of elevated isocortical amyloid. Tau PET imaging has clarified that there is a 10+ year lag between the widespread cortical accumulation of amyloid and the subsequent acceleration of pathologic tau accumulation inside and outside of the medial temporal lobe.7 The spread of tauopathy involves functional networks that anticipate the appearance of amnestic and nonamnestic symptoms of AD dementia.8 By the time that overt cognitive impairment appears, deceleration of amyloid accumulation is occurring9 and substantial neurodegeneration and expansion of tauopathy outside of the medial temporal lobe is present. Persons who have elevated amyloid and substantial tauopathy have a much higher probability of experiencing near-term cognitive decline than those who have elevated amyloid without substantial tauopathy.10 Therapeutic expectations for antiamyloid agents are contingent on the evolving, stage-specific influences of amyloidosis on downstream neurodegeneration.
Figure 1. Amyloid Cascade Hypothesis of Alzheimer Disease Pathogenesis and Its Related Therapeutic Conjectures.
The model posits that elevations of aggregated amyloid-β peptide occur asymptomatically and induce downstream consequences including tauopathy and other neurodegenerative changes, eventually culminating in cognitive impairment. Blue arrows indicate clinically covert pathologic changes, the purple arrow indicates pathologic changes with early symptomatic consequences, and the red arrows indicate changes with overt clinical consequences. Green arrows indicate therapeutic intervention and hypothesized alterations in downstream pathologic and clinical consequences. The orange arrow indicates the influence of other cerebrovascular and non-Alzheimer neurodegenerative pathologic processes that modify the clinical expression of Alzheimer pathology.
The conceptualization of AD as a disorder of amyloidosis and tauopathy is useful, but it creates an unrealistically simple view when applied to therapeutics. The histopathologic heterogeneity of AD is considerable,1 and the molecular biology of amyloidosis involves more complexity than can be addressed with a narrowly targeted antibody.11 Furthermore, other non-AD pathologies co-occur with AD,12 such as cerebrovascular disease, α-synuclein, and TDP-43 pathology. In the setting of multietiology disease, AD pathology may not necessarily be the dominant or sole driver of cognitive decline.
Bottom line: Genetics, imaging, and neuropathology data indicate a relationship between amyloid accumulation and the cognitive disorder of AD, but evidence of therapeutic benefit of amyloid removal in clinical trials is necessary to establish that amyloid is causal in the AD pathway. Detection of a clinical benefit is made challenging by the clinical and pathologic heterogeneities of AD and by the frequent co-occurrence of AD with other brain diseases.
Quest for Antiamyloid Treatments
The therapeutic conjecture of the amyloid cascade hypothesis is this: amyloid-lowering therapies should interrupt neurodegeneration and cognitive decline to an appreciable degree (Figure 1). For the past 20 years, amyloid reduction approaches have been directed at the earliest stages of the disease, when the model postulates the greatest therapeutic effect.
The first attempt at an antiamyloid treatment involved active immunization with AN-1792.13 In a mouse model, a synthetic amyloid peptide induced an immune reaction that successfully cleared amyloid plaques. When the same approach was attempted in humans, an unexpected serious complication arose in the form of an immune-mediated meningoencephalitis that led to early termination of the trial.14 Subsequent autopsy studies in a few AN-1792 patients showed that while amyloid was cleared from the brain, the neurodegenerative disease and its clinical manifestation of dementia progressed nonetheless.15 A few years later in 2005, the first attempt at passive immunization was initiated with an antiamyloid monoclonal antibody (AAMA), bapineuzumab. That agent ultimately failed to show benefits.16 Since then, and up to 2021, there had never been a successful trial of one of these antibodies (see several reviews of these earlier agents for more details.17)
A drug-induced inflammatory lesion not as dramatic as the meningoencephalitis seen with AN-1792 that was dubbed amyloid-related imaging abnormality–edema (ARIA-e) has been seen with all of the AAMAs to date (the topic of ARIA will be discussed in detail further). The key point regarding ARIA-e and its hemorrhagic mate ARIA-h, an increased likelihood of cerebral microbleeds (CMB) or superficial siderosis, is that these adverse events were not sufficiently dangerous or threatening as to halt further efforts to refine the antiamyloid antibody strategy. The occurrence of ARIA led to overly cautious approaches to dosing of the AAMAs, however.
Other approaches to amyloid lowering have included small molecule interventions and inhibition of 1 of the 2 enzymes that cleave APP, namely beta secretase and gamma secretase. The beta secretase inhibitor verubecestat dramatically reduced brain amyloid production but lowered brain amyloid burden only to a small degree,18 but all beta secretase inhibitor trials were unsuccessful.18-23 Most of the beta secretase and gamma secretase inhibitors caused cognitive decline that exceeded that of the placebo group. These trials are reviewed elsewhere.17
In retrospect, a deficiency of the early AAMA trials and the secretase inhibitor trials was that they did not sufficiently lower brain amyloid. That changed in 2015 when a phase 1b trial reported that aducanumab substantially lowered brain amyloid levels24 and led to a pair of phase 3 trials of aducanumab in persons with MCI and mild dementia due to AD that became the focus of an intense controversy. In the retrospective analyses25 following the declaration of futility, 1 of the 2 trials showed that high-dose aducanumab was superior to placebo on clinical outcomes, while the other trial, which had been conducted identically but had achieved slightly lower groupwise reductions in amyloid levels, failed to do so (Figure 2). In June 2021, the US FDA issued an accelerated approval for aducanumab based on its ability to reduce brain amyloid and acknowledged that the clinical benefits had not been convincingly demonstrated.26 The uncertainty of clinical benefits contributed to a very muted acceptance of the drug by providers, payors, caregivers, and patients.
Figure 2. Two Views of the Amyloid-β Removal Results of 4 AAMAs.
(A) Groupwise adjusted mean declines in amyloid PET levels (y-axis) at different time points (x-axis) in centiloid values and (B) Percent of participants who achieved “complete” amyloid removal (y-axis) at different time points (x-axis). “Complete” removal levels were specified differently by each sponsor. Data were obtained from publications or presentations for aducanumab25 “Aduc” using 18F-florbetapir in ENGAGE and EMERGE trials, donanemab using 18F-florbetapir,27 gantenerumab using 18F-florbetapir30 “gant” GRADUATE I and II, and lecanemab using 18F-florbetaben, 18-F-florbetapir, or 18F-flutemetamol.36 Because each study used slightly different PET imaging methodologies and lecanemab allowed any of 3 tracers, centiloid scale values are difficult to compare precisely across different studies. Placebo group adjusted mean values, which in all trials showed small increases over time, are not shown. Percent of those exhibiting “complete removal” of amyloid in placebo groups also not shown (see text for lecanemab placebo group data). “●”—indicates a time point at which PET scan was performed. (Note: Donanemab trial scan was performed at week 24 but depicted here as week 26 for illustrative purposes). Aducanumab published SUVR data were transformed into centiloid values using the equation CL = 100*(SUVR – 1.0124)/0.4339.25
In March 2021, a phase 2 trial was reported in which patients with MCI or mild dementia due to AD received monthly IV infusions of the AAMA donanemab.27 Donanemab attacks a pyroglutamate posttranslationally modified form of amyloid28 (which is a very different target compared with aducanumab or any of the other AAMAs). Donanemab proved to be very efficient at clearing brain amyloid (Figure 2A) and did so “completely” in two-thirds of patients (Figure 2B). “Complete” removal meant that measured amyloid PET signal receded to levels reflecting background measurement variation. Dosing was discontinued in patients who achieved complete amyloid clearance. The phase 2 trial produced evidence of a modest but clear-cut clinical benefit, a reduction in cognitive decline that occurred over the course of the 18-month trial on the primary outcome measure, a cognitive and functional composite. The donanemab trial was the first unequivocal demonstration that prompt extensive amyloid clearance could produce some clinical benefits, thereby falsifying the assertion that “amyloid lowering never causes clinical benefits.” In addition, in post hoc analyses, donanemab seemed to slow brain tau accumulation by PET imaging.29 The results of the phase 3 trial of donanemab were reported in summary form in a press release on May 3, 2023 (see below).
Another AAMA, gantenerumab, failed to demonstrate clinical benefits in a pair of large phase 3 trials in MCI and mild dementia due to AD reported at the Clinical Trials in Alzheimer Disease (CTAD) conference on November 30, 2022.30 The degree of amyloid lowering and the proportion showing complete amyloid clearance with subcutaneous dosing regimen of gantenerumab was much lower than the sponsor had expected based on preliminary work31 (Figure 2). Few persons treated with subcutaneous gantenerumab achieved substantially complete clearance of amyloid after 2 years of treatment. The gantenerumab results show that the statement “any amyloid lowering in beneficial” is false.
Secondary prevention studies with AAMAs that lacked potent amyloid-lowering properties have also been conducted in the past several years. A trial of gantenerumab and solanezumab failed to show benefits in a cohort of at-risk and very mildly impaired persons with dominantly inherited AD.32 The AAMA crenezumab failed in a secondary prevention trial in persons with genetic AD in a community in Colombia.33 In cognitively unimpaired older persons with elevated brain amyloid,34 solanezumab did not reduce either cognitive decline or the risk of progression to symptomatic disease.35 Nor did solanezumab remove brain amyloid.
Bottom line: Before 2021, no AAMA or agents blocking the production of amyloid had succeeded in producing convincing clinical benefit. The demonstration of clinical benefits with donanemab in 2021 in a phase 2 study showed that clearance of plaque-associated amyloid produced a clinical signal.
Lecanemab Phase 3 Clinical Trial and Beyond
Cognitive Outcomes and Amyloid Removal
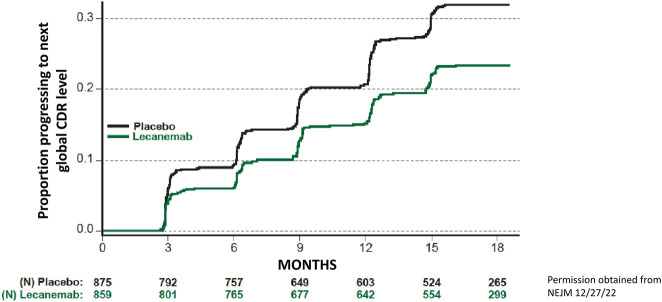
Lecanemab is an AAMA raised against a pathological sequence variant within the amyloid sequence in APP that binds to soluble amyloid protofibrils.36 A phase 2 trial of lecanemab (first reported on July 25, 2018, under the drug name of BAN2401) showed that the drug avidly lowered brain amyloid and clarified the optimal dosing but did not lead to a definitive statement about clinical benefits because of the limitations imposed by its dose-finding adaptive design and restrictions on dosing in APOE ε4 carriers.37 On November 29, 2022, the phase 3 trial results were published.36 The lecanemab trial included persons with MCI and mild dementia who had elevated brain amyloid. In a 1795-person, randomized, placebo-controlled, parallel group design using a single dose of lecanemab at 10 mg/kg administered through IV every 2 weeks, the group receiving lecanemab showed significantly less decline on the primary outcome measure, the Clinical Dementia Rating sum of boxes (CDRsb). Lecanemab treatment resulted in a 27% (0.45 rating points) reduction in decline on the CDRsb, which translates to approximately 5 months of reduction in decline over 18 months compared with the placebo group. The magnitude of the effect was similar to what was seen in the lecanemab phase 2 trial.37 In another analysis measuring survival without a decline in a global CDR decline also favored lecanemab with 32% of placebo group reaching that endpoint after 18 months compared with 23% of lecanemab-treated patients (Figure 3). In addition, all the secondary cognitive and functional outcome measures significantly favored lecanemab treatment compared with placebo. The fact that two-thirds of placebo-treated patients had not declined 1 global CDR step illustrates the challenges for interpreting the benefits of any intervention in the slow-moving progression of mildly symptomatic cognitive impairment due to AD.
Figure 3. Cumulative Survival Analysis for Time to Decline 1 Global CDR Point, From Phase 3 Lecanemab Trial36.
Time in months is on the x-axis, and proportion of participants worsening by 1 global CDR point is on the y-axis for placebo group (black line) and lecanemab-treated group (green line). The numbers of at-risk participants are given for each group at each time point, below the x-axis. A decline of 1 global CDR represents either a change from 0.5 to global CDR 1 or higher or from a global CDR 1 to a global CDR of 2 or higher. Because changes in disease severity across global CDRs are not equal and because the data in the figure include a mix of persons who started at global CDR of 0.5 (comprising approximately 81% of participants in each treatment group) and some who started at global CDR of 1 (comprising 19% of participants in each treatment group), the difference in curves might be more applicable to persons starting with a global CDR of 0.5.
Post hoc subgroup analyses that did not control for covariates such as age and sex generally showed consistency of benefits across MCI and mild dementia and APOE ε4 noncarriers and APOE ε4 heterozygotes. There were some anomalies, however, in other subgroup analyses. For example, APOE ε4 homozygotes showed a point estimate that favored placebo, while Black participants, women, and patients younger than 65 years showed point estimates that favored lecanemab but with CIs that included zero. The subanalysis for Black participants was clearly underpowered, although the trial succeeded in recruiting 44 Black participants, far more and had been recruited for trials of aducanumab. Further detailed analyses by the sponsor are needed to interpret the subgroup analyses in a meaningful way. Similar to any post hoc analyses, the subgroup findings must be viewed as exploratory and of uncertain reliability for predicting future outcomes.
In the phase 3 donanemab trial, the drug reduced decline on the CDRsb by 36%, and all the secondary outcomes were reported as positive (Lilly press release 5-3-23). More detailed information is not currently available.
Trials are currently underway with both lecanemab38 and donanemab examining asymptomatic persons in the AD pathway. Those trials will not report their results for several years.
Amyloid Removal and Other Biomarkers in AAMA Trials
In lecanemab's PET scan substudy involving 698 patients, brain amyloid reduction was substantial36 (Figure 2). After 12 months, 54% of patients had experienced “complete” removal of amyloid; after 18 months, 68% of lecanemab-treated patients exhibited “complete” amyloid removal.39 Dosing was continued in patients achieving complete clearance. Almost all the plasma and CSF biomarkers in the phase 3 lecanemab trial showed differences in a direction of improvement compared with untreated patients. The rate of accumulation of tau in the temporal lobe by PET imaging was also slowed in treated patients.39 Lecanemab was not associated with loss of hippocampal volume, but ventricular enlargement and reductions in cortical thickness occurred in the treated group,39 findings of uncertain significance.40
The donanemab phase 2 trial,29 the donanemab phase 3 trial (Lilly press release, 5-3-23), and the lecanemab phase 3 trial36 imaging results (Figure 2) support a conjecture41 that clinical success of the AAMAs is contingent on the thoroughness of amyloid removal as expressed by the percentage of treated patients who experience complete amyloid removal by PET imaging. Lesser degrees of amyloid removal, as was seen with aducanumab in the ENGAGE trial25 and gantenerumab,30 were not associated with clinical benefit. Because donanemab and lecanemab have different molecular targets—a pyroglutamate modification vs soluble amyloid protofibrils—claims about benefits for one agent vs another based on uniqueness of therapeutic mechanisms may be premature. No individual-level data on clinical outcomes in relation to amyloid removal are available from any of the 4 AAMAs.
AAMAs are intended for patients with elevated brain amyloid, a status that requires biomarker proof. Amyloid PET imaging plays a pivotal role in the selection of patients for AAMA therapies because it offers a quantitative and topographic view of brain amyloid. However, the inaccessibility of amyloid PET means that CSF assays will be a more common way to detect elevated brain amyloid in routine practice.
Bottom line: The phase 3 results of lecanemab in MCI and mild dementia showed a convincing, albeit modest, benefit at 18 months on the primary outcome measure and all secondary outcomes including cognitive, functional, and biomarker measures. Lecanemab rapidly and thoroughly reduced brain amyloid levels in more than two-thirds of treated patients. Donanemab, in its phase 3 trial, produced similar but numerically slightly larger clinical benefits and extensively removed brain amyloid.
Amyloid-Related Imaging Abnormalities of Lecanemab and the AAMAs
ARIA is extensively discussed in a recent review.42 The rate of ARIA-e or ARIA-h with lecanemab was 21%, compared with 9.5% seen in the placebo group.36 There were 13 deaths during the double-blind phase of the study, and these were evenly distributed between treated and placebo groups. In the open-label extension phase of the lecanemab trial, 2 deaths have occurred, both having a relationship to the concomitant use of anticoagulants. A third death was reported in a lecanemab-treated patient who received tissue plasminogen activator (TPA) for an acute stroke.43 Timely reporting of serious adverse events and deaths in the ongoing open-label extension study of lecanemab will be needed.
In the donanemab phase 3 trial, ARIA-e occurred in 24% and ARIA-h in 31.4% of donanemab treated patients, roughly twice the rate seen with lecanemab (Lilly press release, 5-3-23).
The ARIA complications of lecanemab and donanemab are manageable with diligent and close follow-up of patients who are started on an AAMA. Serious consequences of ARIA, especially macrohemorrhages, are rare.36,43,44 CMBs and the underlying pathologic entity of cerebral amyloid angiopathy (CAA) occur in persons with elevated brain amyloid.45 One way to minimize AAMA-induced ARIA risks is to avoid treating persons with existing CAA who have >4 CMBs because the presence of some CMBs increases the likelihood that more will occur.45
Persons who must be on anticoagulant therapy should not receive an AAMA because of the increased risk of macrohemorrhage. In the lecanemab phase 3 trial and its open-label extension, the rate of macrohemorrhage was 3.6% (5/140) in lecanemab-treated persons on anticoagulants vs 0.3% (5/1471) in lecanemab-treated persons not on anticoagulants.46 In addition, because of known complications of TPA therapy for acute stroke, patients considering the use of an AAMA should be warned that they may not be able to receive TPA for acute stroke once they initiate AAMA therapy.
ARIA-e detection requires frequent monitoring with MRI over the first year of treatment and prompt suspension of treatment if ARIA-e appears. After the first 6 to 12 months, the risk of new ARIA-e diminishes,44,47 and surveillance for ARIA-e can eventually be relaxed, though monitoring for incident ARIA-h should continue at a frequency not yet established. The key challenge in managing ARIA-e is its timely recognition. This is not a trivial matter because the radiographic appearance is subtle. It may be difficult for those radiologists without experience to detect it.
Knowledge of APOE genotype was highly relevant to the risk of ARIA because carriage of 1 e4 allele approximately doubles the risk of ARIA from lecanemab36 and donanemab.27 Risks are nearly 4 times higher for APOE ε4 homozygotes compared with noncarriers.
Bottom line: ARIA-e and ARIA-h are risks of AAMA treatment that require frequent monitoring. If conservative exclusion criteria are followed and ongoing monitoring is diligently conducted, ARIA poses a small risk of serious permanent complications.
Expected Impact of Lecanemab on Clinical Practice
Regulatory and Coverage Matters
The US FDA issued an accelerated approval for lecanemab on January 6, 2023, and indicated that a decision on regular approval would be issued by July 6, 2023. On June 9, 2023 an Advisory Committee to the FDA votes 6-0 in favor of a regular approval of lecanemab. Consistent with the CMS decision Memo of April 7, 2022, CMS reiterated in a memo of February 22, 2023,48 that Medicare would cover lecanemab under the auspices of a Coverage with Evidence Development (CED) framework, even if the US FDA granted regular approval to lecanemab. The required infrastructure to conduct a single arm trial of lecanemab that met CMS’ requirements under a CED does not currently exist. Thus, access to lecanemab by Medicare patients may be restricted until such a framework can be organized. It is unclear how private insurers will approach coverage of lecanemab. The cost of lecanemab was set by the sponsor at $26,500 per year.
The US FDA declined to issue an accelerated approval of donanemab, citing insufficient numbers of patients treated for longer than a year in the phase 2 study,49 but more favorable decisions are likely in the future, given the phase 3 trial results.
Limitations on Who Has Access to AAMAs
The indication for treatment with an AAMA is likely to be MCI and mild dementia due to AD. The numbers of individuals with those diagnoses in the 50–90 years age range in the United States is large.50 Clinical trials for patients with very mild cognitive complaints (subjective cognitive impairment) and those with more advanced disease are either underway or are likely to be developed. Until evidence of benefit emerges from those milder or more advanced groups of patients, therapy with lecanemab should be restricted to the severity range of the patients who were studied in the lecanemab phase 3 trial.
The presence of medical and neurologic comorbidities may make AAMA therapy unattractive to many patients. The consequences of active medical disease, active psychiatric disease, and alternative neurocognitive diagnoses may overwhelm potential benefits from an AAMA. Severe visual or auditory impairments may obscure any beneficial effects of AAMA treatment. Potential AAMA recipients will need to be able and willing to undergo multiple MR scans. In 1 analysis of Medicare data, application of the inclusion and exclusion criteria for the aducanumab trials25 eliminated 85% of persons with MCI and 92% with a dementia diagnosis from potential treatment.51
The state of dementia care in the United States and elsewhere is inadequate to handle the potential volume of patients who might seek an approved labor-intensive, parenterally administered AAMA therapy.52 There are insufficient numbers of behavioral neurologists, general neurologists with experience in dementia care, and geriatric psychiatry and geriatric colleagues with similar expertise. There are inadequate numbers of neuropsychologists with expertise in dementia diagnosis to assist the physicians in making accurate estimations of the severity of cognitive impairment. In addition, there is a gap in the neuroradiologic expertise for diagnosing ARIA. Access to dementia diagnostic facilities is limited in both urban and rural areas of the United States because of the scarcity of those with the necessary training.53 It may be challenging to provide intravenous AAMA therapy in geographically remote regions of the United States. In urban areas and elsewhere, access to dementia diagnostic services have been more difficult for Black individuals.54
Bottom line: The numbers of patients eligible for lecanemab will be limited by disease severity criteria, the presence of comorbidities, financial considerations, and logistical barriers.
Clinical Meaningfulness and Understanding the Benefit vs Risk Calculation
With the observation that not 1 but 2 AAMAs have produced statistically significant results in well-done phase 3 trials moves the focus of attention to the magnitude of the clinical benefit and its clinical meaningfulness for patients.
Neither lecanemab nor donanemab produced clinical improvement or sustained clinical stability. Yet, those are unrealistic to expect.55 The challenge for patients, families, and clinicians is how much delay in worsening is meaningful to them.
The delay in decline between the lecanemab-treated and donanemab-treated patients over 18 months may not be apparent to patients and family members. While the magnitude of the effect of both AAMAs exceeds the 95% CI of random variation,56 many treated patients will inevitably exhibit some decline in cognition or function (Figure 3). We know from experience with cholinesterase inhibitors that neither patients, family members, nor treating physicians can recognize a quantitative slowing of clinical worsening of this magnitude. Instead, all parties entering the therapeutic partnership for lecanemab therapy will have to accept that the groupwise clinical trial results alone are the basis for expectations for an individual patient.
In the setting of the slow deterioration in cognition that occurs with MCI and mild dementia due to AD, 18 months is too short a time interval to achieve or appreciate maximal benefits. The open-label long-term extension observations from the lecanemab and donanemab trials will be critical to understanding the benefits as they appear at 3 or 4 years after initiation of therapy. The outcomes from a small group of patients who had participated in the open-label extension of the lecanemab phase 2 trial57 provide a view of the benefits of therapy beyond 18 months. After a gap in treatment during which brain amyloid levels rose only minimally but plasma markers sensitive to brain amyloid rebounded, the rate of decline in cognitive functioning in the lecanemab-treated group did not continue to decelerate but neither did it catch up to the group that had been on placebo during the double-blind portion of the trial. While these observations are consistent with a disease-modifying effect of lecanemab, they do not indicate further expansion of treatment benefits over time. These results must be viewed with caution because of the small numbers of patients involved and the attrition of nearly two-thirds of those completing the double-blind phase. It will take some time for long-term extension data from a much larger group of patients from the phase 3 lecanemab trial to become available. In the meantime, it is unknown whether the delay in worsening by lecanemab treatment will grow larger over time compared with the expected decline of the placebo group, whether the approximate 5-month treatment difference will remain the same, or whether the benefit will shrink. Along with magnitude of benefit, the durability of the drug effect is the real measure of clinical meaningfulness.
The benefits of lecanemab and donanemab must be weighed against the risks of ARIA, the need for genetic testing and counseling because of the APOE genotype-specific risks for ARIA, the inconvenience of every 2-week (for lecanemab) or every 4-week (for donanemab) IV infusions, the need for several MRI scans over the first year of therapy, the need for some type of monitoring of brain amyloid levels, and, of course, the out-of-pocket costs of the entire package of tests and activities for individual families.
Bottom line: The clinical meaningfulness of the benefits of lecanemab and donanemab as seen after 18 months of treatment is encouraging but subject to different impressions of meaningfulness. Neither appears to delay disease progression nor bring about sustained stabilization nor improvement. Some stakeholders may view the current evidence of benefit as sufficiently strong to justify treatment; others may disagree. The subsequent trajectory of those treated with AAMAs beyond 18 months will be critical to establishing whether AAMAs can bend the downward trajectory of AD in a clinically valuable way.
What This Means to the Practice of Neurology, Geriatrics, and Geriatric Psychiatry
The consequences of the introduction of lecanemab therapy into the clinic for dementia care specialists may be substantial. For neurologists with specialty interests outside of dementia care, a patient seeking potential treatment with an AAMA might be best referred to a behavioral neurology subspecialist. On the contrary, for adult neurologists, geriatricians, or psychiatrists who wish to become involved in dementia care, a brief refresher course for proper patient selection and AAMA-specific management principles may be necessary but would also have to be accompanied by investing in additional practice infrastructure. Creating the care team and facilities to deliver lecanemab treatment safely and efficiently is necessary and will probably require buy-in and support from the health system(s) within which the clinician practices. The combination of a potentially large number of patients, the extensive hands-on work needed for administering the AAMAs, and the potential ARIA events means that several clinicians may need to share the responsibilities.
Appropriate use recommendations have been formulated for lecanemab58 (where a reader can obtain more details). From a logistical and safety perspective, the issues with both lecanemab and donanemab are virtually the same. The logistical challenges of selecting the right patients for one of the AAMAs involve several steps and the input of dementia care physicians in consultation with several other specialists (Figure 4). A clinical diagnosis of MCI or mild dementia due to AD will require an initial visit with the clinician and would benefit from an in-depth evaluation by a neuropsychologist skilled in aging and dementia. An MRI scan for basic diagnosis and for evidence of both arteriosclerotic cerebrovascular disease and CAA is essential. A CT scan is not an acceptable substitute because of the need to detect CMBs before, and ARIA during, treatment. Confirmation of elevated brain amyloid is required, preferably by PET scanning, or if unavailable, with CSF studies of Aβ42 and tau peptide levels. For the purposes of predicting the risk of ARIA, APOE genotyping (together with genetic counseling) is necessary.
Figure 4. Flow Diagram for Some of the Activities Involved in the Initial Screening of Patients for Suitability to Receive an Approved AAMA and Activities Needed to Initiate Treatment With an AAMA.

Clinical expertise beyond dementia care neurology includes neuropsychologists, neuroradiologists, genetics counselors, and primary care physicians.
Once a patient is cleared to receive an AAMA, the logistics of IV administration must be coordinated with an infusion center or centers. Some of the key issues include insuring that orders are transmitted in a timely manner and that the treating clinician is available if infusion-related reactions occur. The clinician will also need to arrange for follow-up MRI scans on a conservative schedule (roughly every 3 months for the first year after initiation of treatment) or in the case of incident ARIA.58 The timing of infusions every 2 weeks needs to include a provision to ensure that the MRI is read and reviewed before the next infusion. For safety reasons, the many steps and interactions here will require a dedicated staff person in ready communication with the treating clinician.
Impact on Clinical Research
Other approaches to treating AD, such as with anti-tau antibodies or nonamyloid-directed, nontau-directed therapies have not yielded success to date and therefore will not be part of the dementia clinical care ecosystem in 2023. If anything, the modest effect size seen with lecanemab highlights the need to seek non-amyloid approaches to AD therapeutics and to consider therapeutic efforts directed at non-AD etiologies. Trials of novel agents need to move forward vigorously and will have to account for the presence of lecanemab or donanemab in the marketplace. Although neither may immediately gain the informal designation of standard of care, penetration of AAMA treatments into the community will affect recruitment and retention of persons into clinical trials of novel agents.59 Design of clinical trials for the AAMA era will also require new approaches, but those are issues beyond the scope of this essay.
Bottom Line: Managing lecanemab or donanemab therapy will be challenging and will require many modifications to current approaches to dementia care and clinical research. Accounting for, and treating, elevated brain amyloid in the context of combination therapy trials may make it possible to identify more clearly the benefits of nonamyloid approaches.
Is AAMA Therapy Right for My Patient?
Clinicians, patients, and families should approach the decision about AAMA therapy with a fresh mindset not influenced by past disappointments and controversies. Based on the demonstrated benefits of lecanemab,36 and the similar findings for donanemab, the issue for treating clinicians, patients, family members and other stakeholders will be whether the magnitude of delay of decline is considered potentially meaningful. Careful attention to making a correct diagnosis of a mild cognitive disorder deemed likely to be due to Alzheimer pathology must come from leadership from dementia care specialists. They must also provide leadership on diagnostic matters that bear on safety. Stakeholders must weigh the promise of the magnitude of the clinical benefit in their particular situation against the costs, burdens, risks and logistical challenges of administering an AAMA (Table). There are definite risks associated with AAMA therapy mainly relating to brain macrohemorrhage that can be mitigated by excluding persons at higher risk, including those who are APOE ε4 homozygotes or those with existing CMBs. In presenting the case for the use of the drug to patients, an unhurried, realistic, and thoughtful consideration of therapeutic goals should be conducted.
Table.
Key Points in Putting AAMA Therapy in Perspective
Glossary
- AAMA
antiamyloid monoclonal antibody
- AD
Alzheimer disease
- APP
amyloid precursor protein
- APOE
apolipoprotein E
- ARIA
amyloid-related imaging abnormalities
- CAA
cerebral amyloid angiopathy
- CMB
cerebral microbleeds
- CDRsb
Clinical Dementia Rating sum of boxes
- CTAD
Clinical Trials in Alzheimer disease
- US FDA
US Food and Drug Administration
- MCI
mild cognitive impairment
- TPA
tissue plasminogen activator
Appendix. Authors

Study Funding
The authors report no targeted funding.
Disclosure
D.S. Knopman serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network Treatment Unit study. He served on a Data Safety monitoring Board for a tau therapeutic for Biogen (until 2021) but received no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He has served as a consultant for Roche, Samus Therapeutics, Magellan Health, Biovie, and Alzeca Biosciences but receives no personal compensation. He attended an Eisai advisory board meeting for lecanemab on December 2, 2022, but received no compensation directly or indirectly. He receives funding from the NIH. L. Hershey serves as an Associate Editor for the journal Neurology. She prepares annual updates on memory loss, pre-MCI, vascular cognitive impairment and other topics for MedLink Neurology. Go to Neurology.org/N for full disclosures.
References
- 1.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1-13. doi: 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349(6311):704-706. doi: 10.1038/349704a0 [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353-356. doi: 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- 4.Cacace R, Sleegers K, Van Broeckhoven C. Molecular genetics of early-onset Alzheimer's disease revisited. Alzheimers Dement. 2016;12(6):733-748. doi: 10.1016/j.jalz.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 5.Huynh TV, Davis AA, Ulrich JD, Holtzman DM. Apolipoprotein E and Alzheimer's disease: the influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. J Lipid Res. 2017;58(5):824-836. doi: 10.1194/jlr.r075481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts RO, Aakre JA, Kremers WK, et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 2018;75(8):970-979. doi: 10.1001/jamaneurol.2018.0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therneau TM, Knopman DS, Lowe VJ, et al. Relationships between β-amyloid and tau in an elderly population: an accelerated failure time model. Neuroimage. 2021;242:118440. doi: 10.1016/j.neuroimage.2021.118440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzmeier N, Dewenter A, Frontzkowski L, et al. Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer's disease. Sci Adv. 2020;6(48):eabd1327. doi: 10.1126/sciadv.abd1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagust WJ, Landau SM. Temporal Dynamics of β-amyloid accumulation in aging and Alzheimer disease. Neurology. 2021;96(9):e1347–e1357. doi: 10.1212/wnl.0000000000011524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ossenkoppele R, Pichet Binette A, Groot C, et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat Med. 2022;28(11):2381-2387. doi: 10.1038/s41591-022-02049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Kim C, Haldiman T, et al. Distinct conformers of amyloid beta accumulate in the neocortex of patients with rapidly progressive Alzheimer's disease. J Biol Chem. 2021;297(5):101267. doi: 10.1016/j.jbc.2021.101267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karanth S, Nelson PT, Katsumata Y, et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 2020;77(10):1299-1307. doi: 10.1001/jamaneurol.2020.1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173-177. doi: 10.1038/22124 [DOI] [PubMed] [Google Scholar]
- 14.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46-54. doi: 10.1212/01.wnl.0000073623.84147.a8 [DOI] [PubMed] [Google Scholar]
- 15.Nicoll JAR, Buckland GR, Harrison CH, et al. Persistent neuropathological effects 14 years following amyloid-beta immunization in Alzheimer's disease. Brain. 2019;142(7):2113-2126. doi: 10.1093/brain/awz142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of Bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370(4):322-333. doi: 10.1056/nejmoa1304839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackley SF, Zimmerman SC, Brenowitz WD, et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: instrumental variable meta-analysis. BMJ. 2021;372:n156. doi: 10.1136/bmj.n156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan MF, Kost J, Voss T, et al. Randomized trial of verubecestat for prodromal Alzheimer's disease. N Engl J Med. 2019;380(15):1408-1420. doi: 10.1056/nejmoa1812840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wessels AM, Tariot PN, Zimmer JA, et al. Efficacy and safety of lanabecestat for treatment of early and mild Alzheimer disease: the AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol. 2020;77(2):199-209. doi: 10.1001/jamaneurol.2019.3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer's disease. N Engl J Med. 2018;378(18):1691-1703. doi: 10.1056/nejmoa1706441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henley D, Aisen P, Romano G. Preliminary analyses of data from an ongoing trial of atabecestat in preclinical Alzheimer's disease. N Engl J Med. 2019;380:1483-1485. [DOI] [PubMed] [Google Scholar]
- 22.Doody RS, Raman R, Farlow M, et al. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369(4):341-350. doi: 10.1056/nejmoa1210951 [DOI] [PubMed] [Google Scholar]
- 23.Coric V, Salloway S, van Dyck CH, et al. Targeting prodromal Alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 2015;72(11):1324-1333. doi: 10.1001/jamaneurol.2015.0607 [DOI] [PubMed] [Google Scholar]
- 24.Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537(7618):50-56. doi: 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
- 25.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197-210. doi: 10.14283/jpad.2022.30 [DOI] [PubMed] [Google Scholar]
- 26.Dunn B, Stein P, Temple R, Cavazzoni P. An appropriate use of accelerated approval—aducanumab for Alzheimer's disease. N Engl J Med. 2021;385(9):856-857. doi: 10.1056/nejmc2111960 [DOI] [PubMed] [Google Scholar]
- 27.Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691-1704. doi: 10.1056/nejmoa2100708 [DOI] [PubMed] [Google Scholar]
- 28.Bayer TA. Pyroglutamate Aβ cascade as drug target in Alzheimer's disease. Mol Psychiatry. 2022;27(4):1880-1885. doi: 10.1038/s41380-021-01409-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shcherbinin S, Evans CD, Lu M, et al. Association of amyloid reduction after donanemab treatment with tau pathology and clinical outcomes: the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(10):1015-1024. doi: 10.1001/jamaneurol.2022.2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bateman RJ. Topline results of phase III GRADUATE I & II pivotal trials with subcutaneous gantenerumab. (Abstract) J Prev Alzheimers Dis, Volume 9, supplement issue 1, December 2022, p 51-248. [Google Scholar]
- 31.Klein G, Delmar P, Voyle N, et al. Gantenerumab reduces amyloid-beta plaques in patients with prodromal to moderate Alzheimer's disease: a PET substudy interim analysis. Alzheimers Res Ther. 2019;11(1):101. doi: 10.1186/s13195-019-0559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salloway S, Farlow M, McDade E, et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease. Nat Med. 2021;27(7):1187-1196. doi: 10.1038/s41591-021-01369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostrowitzki S, Bittner T, Sink KM, et al. Evaluating the safety and efficacy of crenezumab vs placebo in adults with early Alzheimer disease: two phase 3 randomized placebo-controlled trials. JAMA Neurol. 2022;79(11):1113-1121. doi: 10.1001/jamaneurol.2022.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Insel PS, Donohue MC, Sperling R, Hansson O, Mattsson‐Carlgren N. The A4 study: β-amyloid and cognition in 4432 cognitively unimpaired adults. Ann Clin Transl Neurol. 2020;7(5):776-785. doi: 10.1002/acn3.51048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lilly Investors. Accessed March 8, 2023. investor.lilly.com/news-releases/news-release-details/lilly-provides-update-a4-study-solanezumab-preclinical
- 36.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9-21. doi: 10.1056/nejmoa2212948 [DOI] [PubMed] [Google Scholar]
- 37.Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. doi: 10.1186/s13195-021-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafii MS, Sperling RA, Donohue MC, et al. The AHEAD 3-45 Study: design of a prevention trial for Alzheimer's disease. Alzheimers Dement. 2023;19(4):1227-1233. doi: 10.1002/alz.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bateman RJ. Clarity AD: a phase 3 placebo-controlled, double-blind, parallel-group, 18-month study evaluating lecanemab in early Alzheimer's disease: imaging, plasma, and CSF biomarkers assessments from clarity AD. (Abstract) J Prev Alzheimers Dis, Volume 9, supplement issue 1, December 2022, p 51-248 [Google Scholar]
- 40.Barkhof F, Knopman D. Brain shrinkage in anti-amyloid-β Alzheimer trials: neurodegeneration or pseudo-atrophy? Neurology. 2023;100(20):941-942. doi: 10.1212/wnl.0000000000207268 [DOI] [PubMed] [Google Scholar]
- 41.Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21(4):306-318. doi: 10.1038/s41573-022-00391-w [DOI] [PubMed] [Google Scholar]
- 42.Cogswell PM, Barakos JA, Barkhof F, et al. Amyloid-related imaging abnormalities with emerging alzheimer disease therapeutics: detection and reporting recommendations for clinical practice. AJNR Am J Neuroradiol. 2022;43(9):E19–e35. doi: 10.3174/ajnr.a7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reish NJ, Jamshidi P, Stamm B, et al. Multiple cerebral hemorrhages in a patient receiving lecanemab and treated with t-PA for stroke. N Engl J Med. 2023;388(5):478-479. doi: 10.1056/nejmc2215148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salloway S, Chalkias S, Barkhof F, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early alzheimer disease. JAMA Neurol. 2022;79(1):13-20. doi: 10.1001/jamaneurol.2021.4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graff-Radford J, Lesnick T, Rabinstein AA, et al. Cerebral microbleed incidence, relationship to amyloid burden: the Mayo Clinic Study of Aging. Neurology. 2020;94(2):e190-e199. doi: 10.1212/wnl.0000000000008735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabbagh M, van Dyck CH. Response to: multiple cerebral hemorrhages in a patient receiving lecanemab and treated with t-PA for stroke. N Engl J Med. 2023;388(5):480. doi: 10.1056/NEJMc2215907 [DOI] [PubMed] [Google Scholar]
- 47.Sabbagh M. Clarity AD: a phase 3 placebo-controlled, double-blind, parallel-group, 18-month study evaluating lecanemab in early Alzheimer's disease: safety Profile of lecanemab in early Alzheimer's disease. (Abstract) J Prev Alzheimers Dis, Volume 9, supplement issue 1, December 2022, p 51-248 [Google Scholar]
- 48.CMS.gov. Accessed February 8, 2023. https://www.cms.gov/newsroom/press-releases/cms-statement-response-alzheimers-associations-request-reconsider-final-national-coverage
- 49.Accessed January 19, 2023. investor.lilly.com/news-releases/news-release-details/us-food-and-drug-administration-issues-complete-response-0.
- 50.Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. 2022;79(12):1242-1249. doi: 10.1001/jamaneurol.2022.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson TS, Ayanian JZ, Souza J, Landon BE. Representativeness of participants eligible to be enrolled in clinical trials of aducanumab for Alzheimer disease compared with medicare beneficiaries with Alzheimer disease and mild cognitive impairment. JAMA. 2021;326(16):1627-1629. doi: 10.1001/jama.2021.15286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hlavka JP, Mattke S, Liu JL. Assessing the preparedness of the health care system infrastructure in six European countries for an Alzheimer's treatment. Rand Health Q. 2019;8(3):2. [PMC free article] [PubMed] [Google Scholar]
- 53.Xu WY, Jung J, Retchin SM, Li Y, Roy S. Rural-urban disparities in diagnosis of early-onset dementia. JAMA Netw Open. 2022;5(8):e2225805. doi: 10.1001/jamanetworkopen.2022.25805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark PC, Kutner NG, Goldstein FC, et al. Impediments to timely diagnosis of Alzheimer's disease in African Americans. J Am Geriatr Soc. 2005;53(11):2012-2017. doi: 10.1111/j.1532-5415.2005.53569.x [DOI] [PubMed] [Google Scholar]
- 55.Petersen RC, Aisen PS, Andrews JS, et al. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimers Dement. 2023. doi: 10.1002/alz.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jutten RJ, Sikkes SAM, Van der Flier WM, Scheltens P, Visser PJ, Tijms BM. Finding treatment effects in Alzheimer trials in the face of disease progression heterogeneity. Neurology. 2021;96(22):e2673-e2684. doi: 10.1212/wnl.0000000000012022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer's disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi: 10.1186/s13195-022-01124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cummings J, Apostolova LF, Rabinovici GD, et al. Lecanemab: Appropriate Use Recommendations. Prev Alz Dis 2023; Published online March 27, 2023, 10.14283/jpad.2023.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grill JD, Karlawish J. Implications of FDA approval of a first disease-modifying therapy for a neurodegenerative disease on the design of subsequent clinical trials. Neurology. 2021;97(10):496-500. doi: 10.1212/wnl.0000000000012329 [DOI] [PMC free article] [PubMed] [Google Scholar]