Abstract
Changes in bacterial ultrastructure after antibiotic exposure and during the postantibiotic effect (PAE) have been demonstrated by electron microscopy (EM). However, EM is qualitative and subject to individual interpretation. In contrast, flow cytometry gives qualitative and quantitative information. The sizes and nucleic acid contents of Escherichia coli and Pseudomonas aeruginosa were studied during antimicrobial exposure as well as during the PAE period by staining the organisms with propidium iodide and analyzing them with flow cytometry and fluorescence microscopy. The effects of ampicillin, ceftriaxone, ciprofloxacin, gentamicin, and rifampin were studied for E. coli, whereas for P. aeruginosa imipenem and ciprofloxacin were investigated. After exposure of E. coli to ampicillin, ceftriaxone, and ciprofloxacin, filamentous organisms were observed by fluorescence microscopy. These changes in morphology were reflected by increased forward light scatter (FSC) and nucleic acid content as measured by flow cytometry. For the β-lactams the extent of filamentation increased in a dose-dependent manner after drug removal, resulting in formation of distinct subpopulations of bacteria. These changes peaked at 20 to 35 min, and bacteria returned to normal after 90 min after drug removal. In contrast, the subpopulations induced by ciprofloxacin did not return to normal until >180 min after the end of the classically defined PAE. Rifampin resulted in formation of small organisms with low FSC, whereas no distinctive characteristics were noted after gentamicin exposure. For P. aeruginosa an identifiable subpopulation of large globoid cells and increased nucleic acid content was detected after exposure to imipenem. These changes persisted past the PAE, as defined by viability counting. Swollen organisms with increased FSC were detected after ciprofloxacin exposure, even persisting during bacterial growth. In summary, for β-lactam antibiotics and ciprofloxacin, the PAE is characterized by dynamic formation of enlarged cell populations of increased nucleic acid content, whereas rifampin induces a decrease in size and nucleic acid content in the organisms. Flow cytometry is an ideal method for future studies of bacterial phenotypic characteristics during the PAE.
The presence of a temporary inhibition of bacterial growth after previous exposure to antimicrobials, or postantibiotic effect (PAE), was initially described in 1944 (4, 7), but interest in this phenomenon was revitalized more than three decades later (30). The clinical significance of a PAE has been attributed to its impact on antimicrobial dosing, most markedly reflected in the increased use of once-daily aminoglycosides both in normal and in immunocompromised hosts (1, 2, 22, 24, 31). However, despite its potential clinical significance, studies on metabolic events during PAE are limited, and the mechanisms remain poorly understood (3, 9, 14–16, 35). It has been hypothesized that multiple mechanisms may be involved, since patterns of bacterial DNA synthesis and ultrastructure are dependent on the organism-antibiotic combination studied (14, 15). However, most studies on the mechanisms of PAE only measure average values of antibacterial effects or PAEs (3, 5, 9, 14, 16, 17, 33, 35) in organisms which potentially respond in a heterogeneous fashion to antimicrobial agents (15, 27). Therefore, studies which quantitate the potential variability of bacterial populations after exposure to antibiotics are warranted. Flow cytometry is an ideal methodology for study of this phenomenon, since it allows for rapid and sensitive description of physical and biochemical characteristics of individual bacteria or fungi, which already may be of value in antibiotic susceptibility testing (8, 11, 25, 26, 36, 38–41). Although flow cytometry has been used to study several different organisms during continuous exposure to antibiotics, studies on bacteria during the PAE using this technique are lacking. It is therefore of interest to study whether specific physical or biochemical characteristics and variability of bacterial populations during PAE can be detected by flow cytometry. We used this method to make serial measurements of sizes and nucleic acid contents of Escherichia coli and Pseudomonas aeruginosa immediately after antimicrobial exposure and during the PAE after exposure to several commonly used antimicrobial agents.
MATERIALS AND METHODS
Organisms.
E. coli ATCC 25922 and P. aeruginosa ATCC 27853 (American Type Culture Collection, Rockville, Md.) were used in this study.
Antibiotics.
Ampicillin was supplied by Astra (Södertälje, Sweden), ceftriaxone by F. Hoffmann-La Roche Ltd. (Basel, Switzerland), rifampin by Ciba-Geigy (Basel, Switzerland), ciprofloxacin by Bayer AG (Leverkusen, Germany), gentamicin by Roussel Laboratories Ltd. (Uxbridge, United Kingdom), imipenem by Merck Sharp & Dohme International (Rahway, N.J.), and tobramycin by Eli Lilly & Co. (Indianapolis, Ind.). Stock solutions were prepared in sterile saline, except for imipenem and rifampin, for which phosphate-buffered saline and methanol, respectively, were used, and solutions were stored at −20°C until use. MICs were determined by a standard microtiter dilution method (32).
Chemicals and media.
RPMI 1640 (with glutamine), Dulbecco’s minimal medium (DMM), and Hanks’ balanced salt solution were purchased from Gibco (Paisley, United Kingdom). Propidium iodide was from Sigma (St. Louis, Mo.), and Mueller-Hinton agar was from Difco (Detroit, Mich.).
Organism-antibiotic combinations.
Before each experiment, three to four colonies of the test organism were transferred to 5 ml of DMM, diluted serially, and grown overnight at 35.5°C to reach a logarithmic-growth phase. Subsequently, the culture was adjusted to an inoculum of ∼107 CFU/ml by a 0.5 McFarland standard. The bacteria were exposed to the antibiotics for 1 h in prewarmed RPMI 1640 at 37°C. The following combinations were used (concentrations are given in parentheses): E. coli and ampicillin (2, 4, or 8 times the MIC), ceftriaxone (2, 4, or 8 times the MIC), gentamicin (equivalent to or twice the MIC), ciprofloxacin (equivalent to or twice the MIC), or rifampin (equivalent to or twice the MIC), and P. aeruginosa and imipenem (2, 4, or 8 times the MIC) or ciprofloxacin (twice the MIC).
Drug removal.
After 1 h of antibiotic exposure, the antibiotics were removed by spinning the bacterial culture twice for 5 min at 1,500 × g in DMM. The bacteria were subsequently resuspended in prewarmed RPMI 1640.
PAE.
Viable counts were estimated immediately after drug removal and then at 90-min intervals by serial dilution in ice-cold normal saline and plating on Mueller-Hinton agar. The PAE was defined as previously described (7), as the difference in time required for the exposed organisms and unexposed controls to grow 1 log10 unit (in CFU per milliliter). A negative value therefore indicates a growth rate faster than that of control organisms, whereas a positive value indicates a delay in growth.
Fixation and staining for nucleic acids.
Immediately after drug removal, aliquots were taken from the bacterial culture at regular intervals (at 0, 20, 35, 50, 70, and 90 min, and at 90-min intervals thereafter) (14), fixed in formaldehyde buffer (final concentration, 2%), and stored at 4°C. The samples were subsequently centrifuged at 1,500 × g for 12 min. The organisms in the pellet were stained with propidium iodide (final concentration, 200 μg/ml) in 20% ethanol for 4 h in the dark at 37°C. After staining, the organisms were washed twice in Hanks’ balanced salt solution for 5 min, resuspended in normal saline, and analyzed within 2 h.
Flow cytometry and microscopy.
The samples were analyzed in a flow cytometer (FACScan; Becton Dickinson, Sunnyvale, Calif.). Five thousand to 10,000 bacteria were analyzed at each time point with a blue argon laser (488-nm wavelength at 500 mW). The instrument was set at 10-fold linear amplification of narrow-angle forward light scatter (FSC) and at logarithmic amplification of orange (or propidium iodide) fluorescence 2 (FL2). The threshold was adjusted for particles smaller than bacteria. At each time point, the size and nucleic acid content were measured (by FSC and FL2, respectively). Samples were also examined with a fluorescence microscope (Leitz Laborlux D) at selected time points and photographed (with a Nikon FX-35A camera with Kodak Ektachrome 800 ASA film). Each experiment (organism-antibiotic combination) was performed two to five times on separate days, and each combination was analyzed by flow cytometry.
Data analysis and statistics.
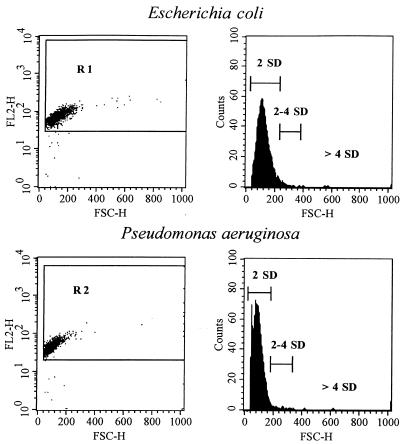
Gating on particles that stained for double-stranded nucleic acids (FL2) was performed with Cell Quest software (Becton Dickinson) as shown in Fig. 2 (left panels). The gates were based on samples from the control culture during the logarithmic-growth phase and adjusted so that the lower limits in FSC were identical to the threshold to avoid the gating out of small organisms. Three size intervals, based on the size distribution of control organisms, were defined as within 2 standard deviations (SDs), between 2 and 4 SDs and >4 SDs, representing the 97.4th, 97.4th to 99.2th, and >99.2th percentiles of the control size distribution, respectively (see Fig. 2, right panels). Comparisons between control organisms and antibiotic-exposed bacteria were based on these predetermined intervals. The Kolmogorov-Smirnov nonparametric test was used for statistical comparisons between control bacteria and organisms in the PAE phase. The level of significance was set at a P value of P <0.01.
FIG. 2.
(Left panels) Dot plots of FSC peak height (shown as FSC-H), indicative of size, and FL2 peak height (shown as FL2-H), indicative of double-stranded nucleic acid content, for the untreated control organisms E. coli ATCC 25922 and P. aeruginosa ATCC 27853 in the logarithmic-growth phase. The gates used are also shown (R1 for E. coli and R2 for P. aeruginosa). (Right panels) Histograms for bacterial size (FSC-H), with three intervals, representing 2 SDs (97.4th percentile), 2 to 4 SDs (97.4th to 99.2th percentile), and >4 SDs (>99.2th percentile) of the control size distribution. These intervals were used for comparisons between controls and antibiotic-exposed organisms.
RESULTS
PAE.
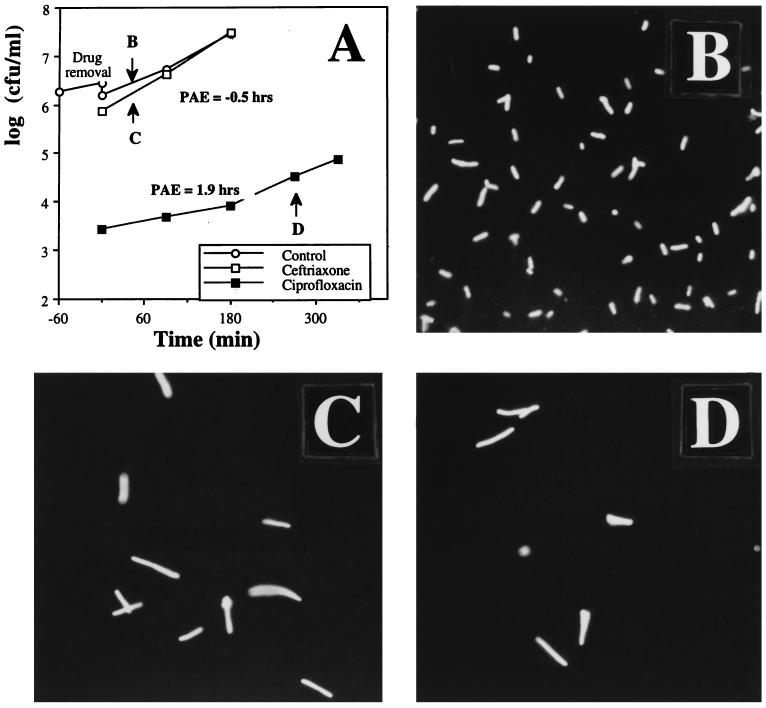
The organism-antimicrobial combinations, with their respective MICs and PAEs, are shown in Table 1. Growth curves from typical PAE experiments with ceftriaxone and ciprofloxacin (each at a concentration equivalent to twice the MIC) against E. coli are shown in Fig. 1A.
TABLE 1.
MICs, multiples of MICs, and duration of PAEs for organism-antibiotic combinations studied by flow cytometry
| Organism | Antibiotic | MIC (μg/ml) | Multiple of MIC | PAE (h) |
|---|---|---|---|---|
| E. coli ATCC 25922 | Ampicillin | 2.0 | 2 | −0.3 |
| 4 | −0.4 | |||
| 8 | −0.3 | |||
| Ceftriaxone | 0.03 | 2 | −0.3 | |
| 4 | −0.2 | |||
| 8 | −0.5 | |||
| Gentamicin | 1.0 | 1 | 0.7 | |
| 2 | 0.6 | |||
| Ciprofloxacin | 0.015 | 1 | 1.5 | |
| 2 | 2.7 | |||
| Rifampin | 16 | 1 | 0.6 | |
| 2 | 1.5 | |||
| P. aeruginosa ATCC 27853 | Imipenem | 2.0 | 2 | 1.3 |
| 4 | 1.9 | |||
| 8 | 2.6 | |||
| Ciprofloxacin | 0.5 | 2 | 0.7 |
FIG. 1.
(A) A typical PAE experiment for E. coli ATCC 25922 after exposure to ceftriaxone or ciprofloxacin (each at a concentration equivalent to twice the MIC), as determined by viability counting. As shown, no PAE was seen after ceftriaxone exposure, but ciprofloxacin induced a PAE of 1.9 h. The organisms were stained with propidium iodide and examined by fluorescence microscopy. (B through D) Photomicrographs of untreated control organisms (B), ceftriaxone-exposed organisms 35 min after drug removal (C), and ciprofloxacin-exposed organisms 270 min after drug removal (D). Both antibiotics induced filamentation, but this morphological form persisted past the classically defined PAE in organisms exposed to ciprofloxacin. Magnification, ×880.
Fluorescence microscopy.
Examples of bacterial morphology as seen through the fluorescence microscope are shown in Fig. 1B through D. Normal-appearing rods are shown in Fig. 1B. The PAE phase was characterized by filament formation after exposure to ceftriaxone and ciprofloxacin. The filaments seen 35 min after ceftriaxone exposure (Fig. 1C) reverted to normal morphology within 90 min, whereas the changes induced by ciprofloxacin, seen 270 min after exposure (Fig. 1D), persisted past resumption of regrowth.
Flow cytometry.
Typical scattergrams showing FSC and FL2 for the untreated control organisms E. coli and P. aeruginosa are shown in Fig. 2 (left panels). FSC is an indicator of size, whereas FL2 represents double-stranded nucleic acid (DNA and RNA) content. As shown, viable organisms in the logarithmic-growth phase had fairly uniform normally distributed size characteristics and nucleic acid contents. Debris outside R1 and R2 was gated out. Three size intervals were defined based on the sizes of the control organisms in the logarithmic-growth phase; these represent the 97.4th, 97.4th to 99.2th, and >99.2th percentile of the control size distribution (Fig. 2, right panels).
Effects of antibiotics.
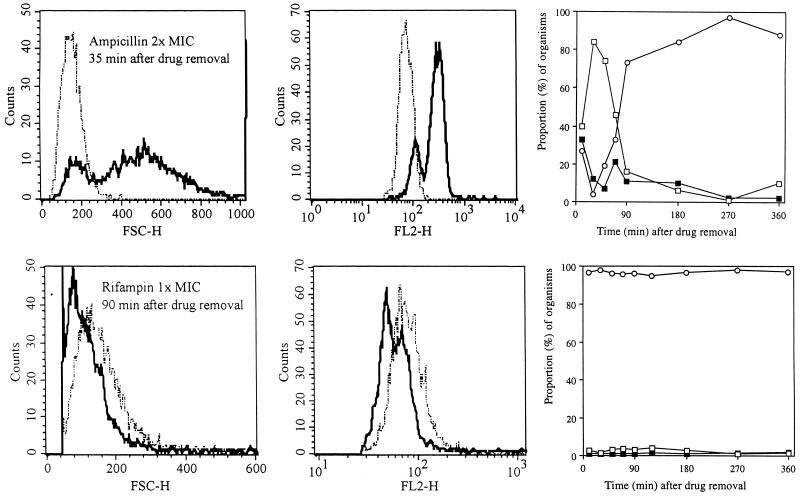
Five antibiotics with different mechanisms of action were tested against E. coli, and the sizes and nucleic acid contents of the organisms were compared to the control distribution. The β-lactams ampicillin and ceftriaxone do not induce a PAE in E. coli, as determined by viability counting (Table 1). Still, a profound effect on bacterial size and nucleic acid content was observed after exposure to these antibiotics. An example of the effects induced by ampicillin is given in Fig. 3 (upper panels). As shown, the bacterial population was characterized by enlarged organisms which continued to increase in size even after drug removal. At 20 to 35 min after drug removal, two populations of bacteria were identified, with large bacteria dominating the culture (Fig. 3, upper left panel), as distinct from the control (P < 0.001). Parallel to the increase in bacterial size, an increase in nucleic acid content occurred (Fig. 3, upper middle panel). Thus, after 35 min, less than 10% of the bacterial population was within 2 SDs of the size of the control population; the remainder showed increased size and increased nucleic acid staining. These changes are summarized in Fig. 3 (upper right graph); rapid convergence towards normal bacterial characteristics was observed during the initial 90 min of the experiment. Similar changes were noted after ceftriaxone exposure (data not shown). For the β-lactams, the extent of the changes in FSC and FL2 was not dependent on the multiple of the MIC tested.
FIG. 3.
Histograms showing a comparison of the size distribution (FSC-H) (left panels) and nucleic acid content (FL2-H) (middle panels) of E. coli during the PAE after exposure to ampicillin at a concentration equivalent to twice the MIC at 35 min after drug removal and after exposure to rifampin at a concentration equivalent to the MIC (lower panels) at 90 min after drug removal. Dotted-and-dashed lines, control organisms; solid lines, organisms previously exposed to the antibiotics. (Upper right graph) Progressive changes in size, compared to sizes of control organisms, as a function of time after previous exposure to ampicillin. (Lower right graph) Summary of the minimal changes in size that were noted after previous exposure to rifampin. The sizes of the antibiotic-treated organisms were compared to three size intervals derived from the control, which are described in text and shown in Fig. 2. Open circles, bacteria in the PAE phase which were within 2 SDs of control size; solid squares, bacteria within 2 to 4 SDs; open squares, organisms >4 SDs from the control distribution.
The PAE phase after rifampin exposure (Fig. 3, lower panels) was characterized by a fairly uniform population of small organisms (lower left panel) with low nucleic acid content (lower middle panel). These growth-suppressed organisms were significantly smaller than control bacteria (P < 0.001). As with ciprofloxacin, these changes outlasted the PAE, as determined by viability counting (270 versus 90 min), and were followed by reversal to normal characteristics during late regrowth.
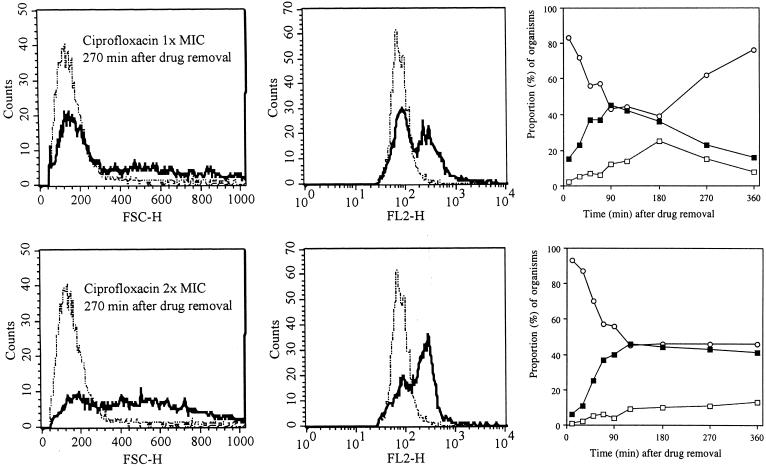
Filamentation was also observed after ciprofloxacin exposure (Fig. 1D). However, in contrast to results with the β-lactams, this morphological alteration did not appear until after drug removal but continued to increase in an apparently dose-dependent manner. An example of these effects, observed 270 min after drug removal, is shown in Fig. 4 (left panels). The sizes of the antibiotic-exposed organisms were clearly different from those of the control population (P < 0.001). In parallel, an increase in nucleic acid content was also seen (Fig. 4, middle panels). The subpopulation of filaments continued to increase for 180 to 360 min after drug removal (Fig. 4, right panels), thus outlasting the classically defined PAE. Similarly, the increased nucleic acid content was still noted at the end of the experiment (data not shown).
FIG. 4.
Histograms showing a comparison of the size distributions (FSC-H; left panels) and nucleic acid contents (FL2-H; middle panels) of E. coli during the PAE after exposure to ciprofloxacin at a concentration equivalent to the MIC and at a concentration equivalent to twice the MIC at 270 min after drug removal. Dotted-and-dashed lines, control organisms; solid lines, bacteria previously exposed to ciprofloxacin. Graphs (right panels) show progressive changes in size compared to sizes of controls. The sizes of antibiotic-treated organisms were compared to three control size intervals described in the text and shown in Fig. 2. Open circles, bacteria within 2 SDs of control size; solid squares, bacteria within 2 to 4 SDs; open squares, organisms >4 SDs from the control distribution.
Gentamicin did not induce any measurable change in bacterial characteristics during PAE (data not shown).
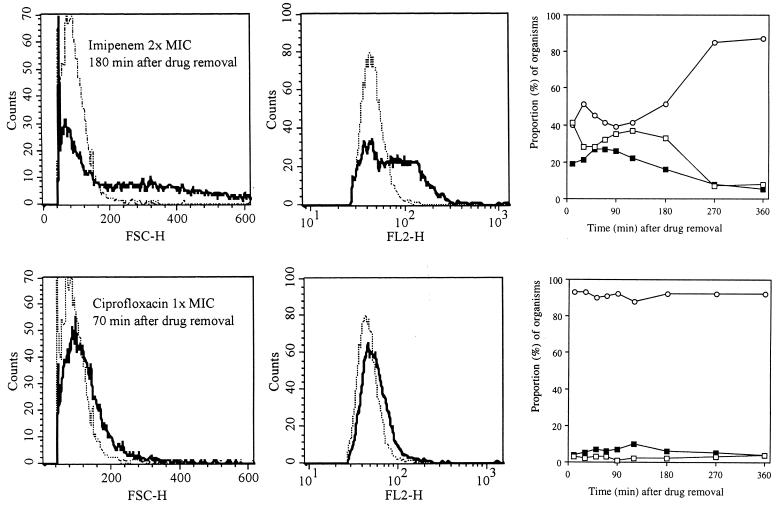
For P. aeruginosa, two antibiotics with different mechanisms of action were tested. Two distinct subpopulations of bacteria were identified during PAE after exposure to imipenem, a β-lactam well known to induce PAE: small organisms and globoid cells of increased size (significantly different from the control; P < 0.001). In contrast to results seen with the E. coli–β-lactam combinations, the large globoid cells persisted for >180 min (Fig. 5, upper left panel). A corresponding increase in nucleic acid content was also seen (Fig. 5, upper middle panel). The subpopulation of globoid organisms persisted past the classically defined PAE (>180 versus 85 min) (Fig. 5, upper right panel).
FIG. 5.
Histograms showing the size distributions (FSC-H; left panels) and nucleic acid contents (FL2-H; middle panels) of P. aeruginosa during the PAE after exposure to imipenem at a concentration equivalent to twice the MIC and to ciprofloxacin at a concentration equivalent to the MIC at 180 and 70 min after drug removal, respectively. Dotted-and-dashed lines, control organisms; solid lines, antibiotic-exposed organisms. The graphs (right panels) show progressive changes in size compared to sizes of controls. The sizes of antibiotic-treated organisms were compared to three control size intervals described in text and shown in Fig. 2. Open circles, bacteria within 2 SDs of control size; solid squares, bacteria within 2 to 4 SDs; open squares, organisms >4 SDs from the control distribution.
Ciprofloxacin produced changes in P. aeruginosa which were characterized by organism swelling (increased diameter and similar length) (Fig. 5, lower left panel) and marginally but consistently increased nucleic acid content (Fig. 5, lower middle panel), but filamentation was not observed at the concentrations tested.
DISCUSSION
In this study we analyzed bacterial characteristics during the PAE by flow cytometry. This method has previously been well described for measurements of antibiotic susceptibility in bacteria and fungi (11, 13, 26, 28, 35, 36, 39–41). It is also ideal for studies on physical and chemical characteristics of bacteria, since it allows for rapid analysis of a large number of individual organisms with high accuracy (8). By performing our sampling at short time intervals, we were able to demonstrate dynamic changes in the organisms after removal of the antibiotics.
We studied the effects of five different antibiotics on flow-cytometric findings for E. coli during PAE: ampicillin, ceftriaxone, ciprofloxacin, rifampin, and gentamicin. Of interest, the β-lactams ampicillin and ceftriaxone, which do not induce a PAE in this species as defined by viability counting, induced progressive bacterial elongation for 20 to 35 min after removal of the antibiotics. This observation was confirmed by fluorescence microscopy (Fig. 1C). It has previously been shown that at low concentrations, ampicillin binds preferentially to penicillin binding protein-3 (PBP-3), causing filament formation (37). Our results thus suggest that the period immediately following drug removal may be characterized by persistent inhibition of PBP-3. Therefore, although it has been claimed that β-lactams do not induce a PAE in gram-negative bacteria (7), with the exception of imipenem (6, 18, 19, 34), this observation suggests persistent intracellular antimicrobial action. Similarly, it has been shown that the PAE in streptococci after penicillin exposure can be induced by inhibition of PBP activity (42).
Rifampin, an inhibitor of DNA-dependent RNA polymerase, caused a uniform and consistent decrease in bacterial size and nucleic acid content which persisted past the duration of the PAE, as determined by viability counting. This antibiotic has previously been shown to result in a profound metabolic suppression in bacteria during the PAE (14). Our current results do not explain why this abnormal morphology was so prevalent in bacteria during the PAE after rifampin exposure. Persistence of the antibiotic, with inhibition in RNA transcription and a subsequent block in protein translation and growth arrest, could potentially account for the presence of these small bacteria. In contrast to the other antimicrobials tested, gentamicin did not induce any discernible changes in bacterial characteristics, as measured by our methodology.
Ciprofloxacin, a quinolone antimicrobial agent which inhibits DNA gyrase and blocks DNA replication, has been well documented to cause filamentation in E. coli (10, 27). In contrast to the relatively short-lived effects of the β-lactams, the effects seen after ciprofloxacin exposure were characterized by a progressive increase in filament formation, lasting past the classically defined PAE (Fig. 4). In fact, at 6 h after drug removal, close to half the bacterial population previously exposed to a concentration of the drug equivalent to twice the MIC was still exhibiting filamentation. These results are in accordance with those of Lorian et al., who observed discrepancy between the PAE as defined by viability counting and the PAE as defined by morphology (27). Due to the progressive increase in filamentation after drug removal, these observations suggest that the aberrant morphology may be a result of persistent intracellular antimicrobial action. We have previously shown that the PAE phase after ciprofloxacin exposure is characterized by a progressive increase in DNA synthesis (14), which could be due to an increase in DNA repair as a result of persistent antimicrobial action during the PAE. Alternatively, the increase in DNA could be due to continued attempts at DNA replication, since DNA polymerase activity is not hampered, but this replication is abortive because the circular DNA can not be separated as a result of gyrase inhibition.
We studied the effects of two antibiotics, imipenem and ciprofloxacin, on PAE in P. aeruginosa. Imipenem primarily inhibits PBP-2 (21). It has previously been shown that this interaction results in the formation of ovoid or globoid cells (15, 20, 37). Imipenem is unique among β-lactams for its ability to induce a PAE in gram-negative bacteria (6, 18, 19, 34). We were able to demonstrate a progressive increase in formation of a subpopulation of globoid cells which persisted past the classically defined PAE. Competitive inhibition of the D2 porin, which facilitates the uptake of basic amino acids and imipenem through the outer membrane (23), has been shown to reduce the duration of PAE after imipenem exposure in P. aeruginosa (5), suggesting that adequate uptake and PBP-2 binding may be prerequisite for PAE induction. Our observations thus suggest that intracellular persistence of this antibiotic could account for its PAE.
At the concentrations tested, ciprofloxacin did not induce filamentation in P. aeruginosa. The size was slightly increased, however, probably as a result of bacterial swelling that has been described previously (15). Similarly, a small but consistent parallel increase in nucleic acid content, which has previously been reported from a study using another methodology (14), was also observed.
In summary, PAE measured by viability counts represents only a mean of the alterations observed for different populations of bacteria. We have shown that the PAE is characterized by formation of bacterial subpopulations of remarkable heterogeneity. Formation of these populations is a dynamic process and can be quantitated by flow cytometry. In some cases, as for β-lactams, the morphological changes induced by continuous exposure to the antibiotics continue to increase for a variable amount of time, whereas ciprofloxacin induces a progressive increase in filamentation which lasts past the classically defined PAE. Future studies could further identify other changes, such as those that measure membrane potential, respiratory activity (29), or lipopolysaccharide surface antigen expression (12).
ACKNOWLEDGMENTS
This study was supported in part by the Icelandic Science Foundation (to S.G.), the University of Iceland Research Fund (to S.G.) and the Icelandic Immunology Society Science Fund (to M.G.).
We thank S. A. Desai and J. R. Perfect for helpful comments on the manuscript.
REFERENCES
- 1.Ali M Z, Goetz M B. A meta-analysis of the relative efficacy and toxicity of single daily dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:796–809. doi: 10.1093/clinids/24.5.796. [DOI] [PubMed] [Google Scholar]
- 2.Bailey T C, Little J R, Littenberg B, Reichley R M, Dunagan W C. A meta-analysis of extended-interval dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:786–795. doi: 10.1093/clinids/24.5.786. [DOI] [PubMed] [Google Scholar]
- 3.Barmada S, Kohlhepp S, Leggett J, Dworkin R, Gilbert D. Correlation of tobramycin-induced inhibition of protein synthesis with postantibiotic effect in Escherichia coli. Antimicrob Agents Chemother. 1993;37:2678–2683. doi: 10.1128/aac.37.12.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigger J W. The bactericidal action of penicillin on Staphylococcus pyogens. Ir J Med Sci. 1944;227:533–568. [Google Scholar]
- 5.Boswell F J, Andrews J M, Gill M J, Wise R. Postantibiotic effect of three carbapenems on Pseudomonas aeruginosa in the presence of lysine. J Antimicrob Chemother. 1995;35:232–233. doi: 10.1093/jac/35.1.232. [DOI] [PubMed] [Google Scholar]
- 6.Bustamante C I, Drusano G L, Tatem B A, Standiford H C. Postantibiotic effect of imipenem on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;26:678–682. doi: 10.1128/aac.26.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig W A, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Williams and Wilkins; 1996. pp. 296–329. [Google Scholar]
- 8.Davey H M, Kell D B. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol Rev. 1996;60:641–696. doi: 10.1128/mr.60.4.641-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson R J, Drobot G R, Karlowsky J A, Zhanel G G, Philips R, Hoban D J. The accumulation of fluoroquinolones in Staphylococcus aureus during the postantibiotic effect. J Antimicrob Chemother. 1994;34:363–370. doi: 10.1093/jac/34.3.363. [DOI] [PubMed] [Google Scholar]
- 10.Diver, J. M., and R. Wise. 1986. Morphological and biochemical changes in Escherichia coli after exposure to ciprofloxacin. J. Antimicrob. Chemother. 18(Suppl. D):31–41. [DOI] [PubMed]
- 11.Durodie J, Coleman K, Simpson I N, Loughborough S H, Winstanley D W. Rapid detection of antimicrobial activity using flow cytometry. Cytometry. 1995;21:374–377. doi: 10.1002/cyto.990210409. [DOI] [PubMed] [Google Scholar]
- 12.Evans M E, Pollack M, Hardegen N J, Koles N L, Guelde G, Chia J K S. Fluorescence-activated cell sorter analysis of binding by lipopolysaccharide-specific monoclonal antibodies to gram-negative bacteria. J Infect Dis. 1990;162:148–155. doi: 10.1093/infdis/162.1.148. [DOI] [PubMed] [Google Scholar]
- 13.Gant V A, Warnes G, Phillips I, Savidge G F. The application of flow cytometry to the study of bacterial responses to antibiotics. J Med Microbiol. 1993;39:147–154. doi: 10.1099/00222615-39-2-147. [DOI] [PubMed] [Google Scholar]
- 14.Gottfredsson M, Erlendsdottir H, Gudmundsson A, Gudmundsson S. Different patterns of bacterial DNA synthesis during postantibiotic effect. Antimicrob Agents Chemother. 1995;39:1314–1319. doi: 10.1128/aac.39.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottfredsson M, Erlendsdottir H, Kolka R, Gudmundsson A, Gudmundsson S. Ultrastructural alterations of bacteria during the postantibiotic effect. Chemotherapy (Basel) 1993;39:153–162. doi: 10.1159/000239120. [DOI] [PubMed] [Google Scholar]
- 16.Guan L, Blumenthal R M, Burnham J C. Analysis of macromolecular biosynthesis to define the quinolone-induced postantibiotic effect in Escherichia coli. Antimicrob Agents Chemother. 1992;36:2118–2124. doi: 10.1128/aac.36.10.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan L, Burnham J C. Postantibiotic effect of CI-960, enoxacin and ciprofloxacin on Escherichia coli: effect on morphology and haemolysin activity. J Antimicrob Chemother. 1992;29:529–538. doi: 10.1093/jac/29.5.529. [DOI] [PubMed] [Google Scholar]
- 18.Gudmundsson, S., B. Vogelman, and W. A. Craig. 1986. The in-vivo postantibiotic effect of imipenem and other new antimicrobials. J. Antimicrob. Chemother. 18(Suppl. E):67–73. [DOI] [PubMed]
- 19.Hanberger H, Nilsson L E, Kihlstrom E, Maller R. Postantibiotic effects of β-lactam antibiotics on Escherichia coli evaluated by bioluminescence assay of bacterial ATP. Antimicrob Agents Chemother. 1990;34:102–106. doi: 10.1128/aac.34.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanberger H, Svensson E, Nilsson M, Nilsson L, Hørnsten E G, Maller R. Effects of imipenem on Escherichia coli studied using bioluminescence, viable counting and microscopy. J Antimicrob Chemother. 1993;31:245–260. doi: 10.1093/jac/31.2.245. [DOI] [PubMed] [Google Scholar]
- 21.Hashizume T, Nakagava J, Tamaki S, Matsuhasi M. Studies of the mechanism of action of imipenem (N-formimidoylthienamycin) in vitro: binding to the penicillin binding proteins (PBPs) in Escherichia coli and Pseudomonas aeruginosa and inhibition of enzyme activities due to the PBPs in E. coli. J Antibiot. 1984;38:394–400. doi: 10.7164/antibiotics.37.394. [DOI] [PubMed] [Google Scholar]
- 22.Hatala R, Dinh T, Cook D J. Once-daily aminoglycoside dosing in immunocompetent adults: a meta-analysis. Ann Intern Med. 1996;124:717–725. doi: 10.7326/0003-4819-124-8-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Hancock R E. The role of specific surface loop regions in determining the function of the imipenem-specific pore protein OprD of Pseudomonas aeruginosa. J Bacteriol. 1996;178:3085–3090. doi: 10.1128/jb.178.11.3085-3090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Efficacy and toxicity of single daily doses of amikacin and ceftriaxone versus multiple daily doses of amikacin and ceftazidime for infection in patients with cancer and granulocytopenia. Ann Intern Med. 1993;119:584–593. doi: 10.7326/0003-4819-119-7_Part_1-199310010-00006. [DOI] [PubMed] [Google Scholar]
- 25.Jernaes M W, Steen H B. Staining of Escherichia coli for flow cytometry: influx and efflux of ethidium bromide. Cytometry. 1994;17:302–309. doi: 10.1002/cyto.990170405. [DOI] [PubMed] [Google Scholar]
- 26.Kirk S M, Callister S M, Lim L C L, Schell R F. Rapid susceptibility testing of Candida albicans by flow cytometry. J Clin Microbiol. 1997;35:358–363. doi: 10.1128/jcm.35.2.358-363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorian V, Ernst J, Amaral L. The postantibiotic effect defined by bacterial morphology. J Antimicrob Chemother. 1989;23:485–491. doi: 10.1093/jac/23.4.485. [DOI] [PubMed] [Google Scholar]
- 28.Mason D J, Allman R, Stark J M, Lloyd D. Rapid estimation of bacterial antibiotic susceptibility with flow cytometry. J Microsc. 1994;176:8–16. doi: 10.1111/j.1365-2818.1994.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 29.Mason D J, Power E G M, Talsania H, Phillips I, Gant V A. Antibacterial action of ciprofloxacin. Antimicrob Agents Chemother. 1995;39:2752–2758. doi: 10.1128/aac.39.12.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald P J, Craig W A, Kunin C M. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977;135:217–223. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- 31.Munckhof W J, Grayson M L, Turnidge J D. A meta-analysis of studies on the safety and efficacy of aminoglycosides given either once daily or as divided doses. J Antimicrob Chemother. 1996;37:645–663. doi: 10.1093/jac/37.4.645. [DOI] [PubMed] [Google Scholar]
- 32.National Committee for Clinical Laboratory Standards. Methods for dilution susceptibility tests for bacteria that grow aerobically. Tentative standard M7-T4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1988. [Google Scholar]
- 33.Odenholt I, Holm S E, Cars O. Effects of benzylpenicillin on Streptococcus pyogenes during the postantibiotic phase in vitro. J Antimicrob Chemother. 1989;24:147–156. doi: 10.1093/jac/24.2.147. [DOI] [PubMed] [Google Scholar]
- 34.Odenholt I, Isaksson B, Nilsson L, Cars O. Postantibiotic and bactericidal effect of imipenem against Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1989;8:136–141. doi: 10.1007/BF01963897. [DOI] [PubMed] [Google Scholar]
- 35.Park M K, Myers R A M, Marzella L. Hyperoxia and prolongation of aminoglycoside-induced postantibiotic effect in Pseudomonas aeruginosa: role of reactive oxygen species. Antimicrob Agents Chemother. 1993;37:120–122. doi: 10.1128/aac.37.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pore R S. Antibiotic susceptibility testing by flow cytometry. J Antimicrob Chemother. 1994;34:613–627. doi: 10.1093/jac/34.5.613. [DOI] [PubMed] [Google Scholar]
- 37.Spratt B G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K-12. Proc Natl Acad Sci USA. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dilla M A, Langlois R G, Pinkel D, Yajko D, Hadley W K. Bacterial characterization by flow cytometry. Science. 1983;220:620–622. doi: 10.1126/science.6188215. [DOI] [PubMed] [Google Scholar]
- 39.Walberg M, Gaustad P, Steen H B. Rapid assessment of ceftazidime, ciprofloxacin, and gentamicin susceptibility in exponentially-growing E. coli cells by means of flow cytometry. Cytometry. 1997;27:169–178. [PubMed] [Google Scholar]
- 40.Walberg M, Gaustad P, Steen H B. Rapid flow cytometric assessment of mecillinam and ampicillin susceptibility. J Antimicrob Chemother. 1996;37:1063–1075. doi: 10.1093/jac/37.6.1063. [DOI] [PubMed] [Google Scholar]
- 41.Wenisch C, Linnau K F, Parschalk B, Zedtwitz-Liebenstein K, Georgopoulos A. Rapid susceptibility testing of fungi by flow cytometry using vital staining. J Clin Microbiol. 1997;35:5–10. doi: 10.1128/jcm.35.1.5-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan S, Bohach G A, Stevens D L. Persistent acylation of high-molecular-weight penicillin-binding proteins by penicillin induces the postantibiotic effect in Streptococcus pyogenes. J Infect Dis. 1994;170:609–614. doi: 10.1093/infdis/170.3.609. [DOI] [PubMed] [Google Scholar]