Abstract
We report on the cloning and sequencing of the vanA gene cluster present in the glycopeptide-resistant clinical isolate Bacillus circulans VR0709 (R. Fontana, M. Ligozzi, C. Pedrotti, E. M. Padovani, and G. Cornaglia, Eur. J. Clin. Microbiol. Infect. Dis. 16:473–474, 1997). The presence of a vanA-related gene in VR0709 was demonstrated in a PCR assay which permitted the specific amplification of an internal segment of vanA. Southern blotting suggested that the vanA gene was located in the chromosome in a 7.6-kb EcoRI fragment. DNA sequence analysis revealed the presence of all seven genes of the vanA cluster (vanR, vanS, vanH, vanA, vanX, vanY, and vanZ). The degree of identity between homologous proteins encoded by Tn1546 and the chromosome of B. circulans VR0709 ranged from 87 to 95%. Neither PCR nor Southern blotting with specific primers and probes, respectively, showed the presence of open reading frames (ORFs) 1 and 2 which encode the transposase and the resolvase of Tn1546, respectively, the transposon found to carry the vanA gene cluster in enterococci. Determination of the sequences of the flanking regions of the van gene cluster of B. circulans revealed perfect inverted repeats of 10 bp which delineated a 9.2-kb region containing the van gene cluster and an ORF which encoded a putative protein (178 residues) which displayed a low level of identity (28%) to the resolvase of Tn1546. These results suggest that glycopeptide resistance in B. circulans VR0709 is associated with the acquisition of a vanA gene cluster which shows a high degree of homology with that of enterococci. In B. circulans, however, the cluster is not carried by Tn1546 and is borne by the chromosome.
Most gram-positive bacteria are naturally susceptible to glycopeptide antibiotics (vancomycin and teicoplanin). Resistance to these compounds is an intrinsic property of some occasional human pathogens such as lactobacilli, leuconostoc, pediococci, Erysipelothrix rhusiopathiae, Enterococcus gallinarum, Enterococcus casseliflavus, and Enterococcus flavescens (22). Acquisition of glycopeptide resistance was first described in enterococci in 1988 (9, 20) and is mediated by three classes of related gene clusters that confer inducible resistance to high levels of vancomycin and teicoplanin (vanA), inducible resistance to various levels of vancomycin (vanB), or resistance to vancomycin and low levels of teicoplanin (vanD) (1, 4, 12, 14, 21).
VanA enterococci harbor a Tn3-like transposon (the prototype was designated Tn1546) which carries a cluster of seven genes (vanR, vanS, vanH, vanA, vanX, vanY, and vanZ) required for the phenotypic expression of resistance and which is generally located on large plasmids (1). The vanB cluster is carried by large conjugative elements and less frequently by plasmids (17). Transfer of genetic elements carrying vanA and vanB genes to susceptible enterococcal strains and, in the case of vanA, to strains of nonenterococcal species such as Streptococcus sanguis, Streptococcus lactis, Streptococcus pyogenes, Bacillus thuringiensis, Staphylococcus aureus, and listeriae of different species has been achieved under laboratory conditions (2, 3, 8, 10, 13). However, the natural spread of vancomycin resistance determinants among gram-positive bacteria other than enterococci has seldom been reported. To date, this phenomenon has been documented by the isolation from clinical specimens of one vancomycin-resistant strain of Oerskovia turbata and one strain of Arcanobacterium (Corynebacterium) haemolyticum, both of which harbor the vanA gene, and of one Streptococcus bovis strain that harbors the vanB gene (15, 16).
Recently, we described a glycopeptide-resistant strain of Bacillus circulans (a species naturally susceptible to these antibiotics) isolated from a catheter tip as part of routine activities at the Clinical Microbiology Laboratory of the Verona University Hospital (6). Since the natural dissemination of vancomycin resistance determinants among gram-positive bacteria is a matter of serious public health concern, we studied the mechanism of vancomycin resistance in our clinical isolate of B. circulans. We found that this strain harbored a vanA-like gene cluster with a high degree of homology with that of enterococci but was borne by the chromosome and was apparently carried by a genetic element other than Tn1546.
MATERIALS AND METHODS
Bacterial strains.
B. circulans VR0709 is a motile, catalase-positive, aerobic, spore-forming, gram-positive rod and was identified by the API CH and API 20E System biochemical profiles (bioMèrieux, Marcy l’Etoile, France). B. circulans ATCC 4513 was obtained from the American Type Culture Collection, Rockville, Md. Enterococcus faecium BM4147 carrying Tn1546 in plasmid pIP816 was used as the reference strain. Escherichia coli DH5α was purchased from Stratagene (Cambridge, United Kingdom). Bacteria were routinely grown in brain heart infusion (BHI) broth and agar (Difco, Detroit, Mich.) at 37°C.
Susceptibility test.
The MICs of vancomycin and teicoplanin were determined on Mueller-Hinton broth (MHB) or Mueller-Hinton agar (MHA) (Difco) by standard dilution techniques (11).
Inducibility of vancomycin resistance.
Cultures of strain VR0709 were grown overnight in MHB at 37°C in the presence of subinhibitory concentrations of vancomycin (8 μg/ml) and were diluted to an A640 of 0.1 in fresh MHB with and without the same concentrations of vancomycin. The cultures were grown at 37°C with shaking, and A640 values were measured at regular intervals on a Pharmacia spectrophotometer.
Primers and digoxigenin-labeled probes.
Primers A1 and A2 (vanA), B1 and B2 (vanB), C1 and C2 (vanC1), and D1 and D2 (vanC2, vanC3), selected by Dutka-Malen et al. (5), were used to specifically amplify the van genes. The primer sets used for amplification of ORFs 1 and 2 and the van genes of Tn1546 were as described previously (7). When necessary, primers were also derived from the B. circulans DNA by sequencing. The primers were synthesized by Boehringer Mannheim, Mannheim, Germany. To be used as hybridization probes, the PCR products were labeled directly with digoxigenin (DIG)-dUTP by using a PCR DIG-labeling mix (Boehringer Mannheim). The reaction was run in a 100-μl volume under the PCR conditions described below.
Preparation of DNA.
Plasmid DNA was prepared by a rapid alkaline lysis method (18). For preparation of total DNA, 3 ml of bacterial culture in BHI broth (37°C, 18 to 48 h) was centrifuged (14,000 × g, 10 min). After washing with 0.85% sterile NaCl, the bacteria were suspended in 400 μl of sucrose solution (6.7%), 25 μl of lysozyme (10 mg/ml) was added, and the mixture was incubated for 30 min at 37°C. Fifty microliters of 20% sodium dodecyl sulfate (SDS) was then added, and the mixture was incubated for 30 min and digested with 5 μl of proteinase K solution (20 mg/ml) at 37°C for 30 min. Genomic DNA was extracted twice with 1 volume of phenol-chloroform-isoamyl alcohol. After adding 0.1 volume of 3 M sodium acetate, DNA was precipitated in ethanol, air dried, and dissolved in 80 μl of sterile water.
PCR amplification.
PCR was performed on a DNA thermal cycler (MiniCycler; GENENCO, Florence, Italy) in a final volume of 50 μl containing 250 ng of DNA as template, 0.5 μM (each) primer, 200 μM (each) deoxynucleoside triphosphate, and 2 U of Taq high-fidelity DNA polymerase (Boehringer Mannheim) in a 1× amplification buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2). The PCR mixtures were denatured (2 min at 94°C) and were then subjected to 35 cycles of amplification (1 min of annealing at 55°C, 1 min of elongation at 72°C, and 1 min of denaturation at 94°C) and a final elongation step of 72°C for 10 min. PCR products were resolved by electrophoresis in a 1% agarose gel stained with ethidium bromide.
DNA-DNA hybridization.
Genomic and plasmid DNAs were digested with restriction enzymes as recommended by the manufacturer (Boehringer Mannheim) and were electrophoresed through a 0.8% agarose gel. The DNA restriction fragments were electroblotted onto a Hybond-N+ nylon membrane (Amersham International, Amersham, United Kingdom) and were hybridized with the DIG-dUTP-labeled PCR products. Hybridizations were performed as follows: prehybridization and hybridization were carried out for 1 and 18 h, respectively, at 68°C in 5× SSC (1× SSC is 0.15 M sodium chloride plus 0.015 M sodium citrate)–0.02% SDS–1% (wt/vol) blocking reagent for nucleic acid hybridization (Boehringer Mannheim)–0.1% N-lauryl-sarcosine, followed by two washings in 0.2× SSC–0.1% SDS at 65°C for 15 min.
DNA cloning and sequencing.
The sequence from vanR to vanY of the B. circulans chromosome was determined on cloned PCR fragments obtained by amplification of B. circulans DNA with primers derived from the sequence of Tn1546 or based on the sequence obtained from fragments of B. circulans DNA. The region upstream from vanR was sequenced by cloning and sequencing first the 1.5-kb HindIII fragment containing vanR (Fig. 1) and then the fragment produced by inverse PCR carried out with a primer (3′ to 5′) based on the 5′ sequence of the 1.5-kb HindIII fragment and a primer (5′ to 3′) based on the 3′ end sequence of the 7.6-kb EcoRI fragment (Fig. 1). The sequence downstream from vanZ of B. circulans was determined by cloning and sequencing the 1.7-kb HindIII fragment containing vanZ. The amplification products were blunt ended and phosphorylated by standard procedures (18) and were then ligated into SmaI-digested, dephosphorylated SK plasmid. Fragments derived from enzymatic digestion were cloned into pUC18. M13 universal and reverse primers were used to sequence both strands of the inserts. Sequencing was performed with three independent PCR clones by the dideoxynucleotide termination method (19) with an ALF Sequencer and fluorescent dATP (Pharmacia, St. Albans, United Kingdom).
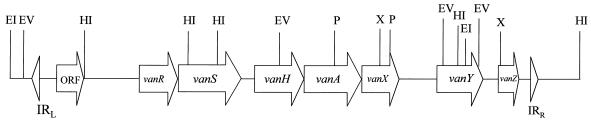
FIG. 1.
Restriction map of the 9.2-kb EcoRI-HindIII fragment of B. circulans VR0709 containing the vanAbc cluster. EI, EcoRI; EV, EcoRV; HI, HindIII; P, PstI; X, XbaI.
Nucleotide sequence accession numbers.
The nucleotide sequences of the genes of the B. circulans vanA (vanAbc) cluster have been assigned EMBL accession no. Y15704 (vanAbc), Y15705 (vanHbc), Y15706 (vanRbc), Y15707 (vanSbc), Y15708 (vanXbc), Y17303 (vanYbc), Y17304 (vanZbc), and Y17305 (ORF).
RESULTS AND DISCUSSION
B. circulans VR0709 properties.
B. circulans VR0709 is a motile, catalase-positive, aerobic, spore-forming, gram-positive rod identified as B. circulans by conventional biochemical methods. On BHI agar or MHA it formed ovoid colonies which swarmed after only a few days of incubation. Morphologically, the isolate showed the characteristic shape of aerobic bacilli. It formed rods (0.8 to 1 μm in width and 2 to 5 μm in length) that mostly contained a central or subterminal spore. Interestingly, the spore-forming ability was greatly reduced for bacteria grown in the presence of vancomycin at sub-MICs.
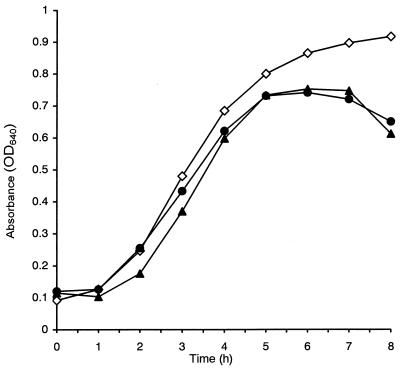
Strain VR0709 is the first natural clinical isolate of a vancomycin-resistant Bacillus described so far. It expressed resistance to glycopeptides in both liquid and solid media. In MHB, the MICs of vancomycin and teicoplanin were 64 and 32 μg/ml, respectively; in MHA the MICs were 256 and 32 μg/ml, respectively. The MICs of both antibiotics for strain ATCC 4513 were 0.5 μg/ml. Inducibility experiments showed that the rates of growth of B. circulans VR0709 were unaltered by exposure to vancomycin at sub-MICs, suggesting that expression of resistance to glycopeptides was constitutive in this organism (Fig. 2).
FIG. 2.
Growth of B. circulans VR0709 in MHB in the absence of vancomycin (diamonds) and in the presence of 8 μg of vancomycin per ml after pregrowth in MHB with (black circles) and without (black triangles) the antibiotic. OD640, optical density at 640 nm.
Identification and localization of vancomycin resistance determinant of B. circulans VR0709.
The presence of the van genes was investigated in VR0709 by PCR carried out with primers from the vanA, vanB, and vanC genes of enterococci (5). A positive reaction was obtained with vanA primers only, which gave the expected 800-bp amplification product. No amplification of B. circulans ATCC 4513 DNA was obtained with the same sets of primers.
In enterococci the vanA gene cluster is found to reside on transposons that are similar or related to Tn1546 and that are located either on plasmids or on the chromosome. B. circulans VR0709 was found to harbor a small (7.28-kb) plasmid designated pVR0709; after EcoRI digestion the plasmid gave four fragments of 2.91, 2.15, 1.54, and 0.82 kb, respectively. None of these fragments hybridized with DIG-labeled amplicons of the enterococcal vanA gene, whereas the same probe reacted with a 7.6-kb EcoRI fragment of total DNA, suggesting a chromosomal location of the gene. In contrast, the vanA gene of Tn1546 was confirmed to reside in a 4.1-kb EcoRI fragment of E. faecium BM4147 plasmid and total DNA.
Characterization of the van gene cluster of B. circulans VR0709.
The presence of other genes of the vanA cluster in strain VR0709 was investigated by PCR performed with primers from ORFs 1 and 2 and from all the seven genes of the enterococcal cluster. If amplification products of the expected size were not obtained, the presence of a gene was investigated by probing the total DNA with DIG-labeled amplicons of the corresponding enterococcal gene. By using this strategy the presence of sequences homologous to all seven genes of the cluster but not to ORFs 1 and 2 was demonstrated.
To evaluate the homology of the van cluster of VR0709 with that of enterococci, the 9.2-kb EcoRI-HindIII fragment of B. circulans DNA (Fig. 1) was sequenced. It was found to contain sequences homologous to the vanR, vanS, vanH, vanA, vanX, vanY, and vanZ genes, in that order. The analysis of the nucleotide and the derived amino acid sequences showed a high degree of identity (93 to 96%) between homologous genes of Tn1546 and B. circulans vanA gene clusters as well as the encoded proteins (87 to 95%) (Table 1). However, the degree of identity between the genes and the intergenic regions was less than would be expected if there had been the direct transfer of the gene cluster from enterococci, suggesting that these genes might have been acquired from a source different from that from which the enterococcal genes were acquired. No EcoRI sites were found in B. circulans vanSbc, which explained the difference in the length of the EcoRI fragment which hybridized with the vanA probe. The genes of VR0709 were designated vanRbc, vanSbc, vanHbc, vanAbc, vanXbc, vanYbc, and vanZbc and the entire cluster was designated vanAbc.
TABLE 1.
Comparison between the vanAbc and vanA gene clusters
| B. circulans gene or sequence | Length (bp) | % Nucleotide identity | % Amino acid identity |
|---|---|---|---|
| IRL-ORFa | 195 | 33 | |
| ORFb | 534 | 54 | 28 |
| ORF-vanRc | 737 | 53 | |
| vanR | 696 | 91 | 89 |
| vanS | 1,140 | 93 | 92 |
| vanS-vanH | 213 | 89 | |
| vanH | 969 | 91 | 89 |
| vanA | 1,032 | 94 | 95 |
| vanA-vanX | 5 | 100 | |
| vanX | 609 | 95 | 94 |
| vanX-vanY | 434 | 73 | |
| vanY | 894 | 93 | 89 |
| vanY-vanZ | 154 | 76 | |
| vanZ | 486 | 91 | 87 |
| vanZ-IRRd | 138 | 44 |
The nucleotide sequence was matched with the left terminal IR (IRL)-ORF1 intervening sequence of Tn1546.
The nucleotide and amino acid sequences were matched with ORF2 of Tn1546.
The nucleotide identity was calculated by matching a 211-bp region upstream from vanRbc with ORF2-vanR intergenic sequence of Tn1546.
The nucleotide sequence was matched with vanZ-IRR intervening sequence of Tn1546.
The sequence of the vanbc cluster significantly diverged from that of Tn1546 6 bp upstream from the vanRbc start codon and immediately downstream from the vanZbc stop codon (Fig. 3). A search for stop codons of each DNA strand in the nucleotide sequence 1.8 kb upstream from the translated initiation codon of vanRbc identified one open reading frame (ORF) transcribed in the same direction as the vanbc genes. The putative coding region in the ORF was assigned to positions 612 to 1146 of the 9.2-kb EcoRI-HindIII fragment. This was based on the presence of an in-frame ATG translation initiation codon preceded by a ribosome-binding site-like sequence. The corresponding 534-bp sequence could code for a protein of 178 amino acids and with a molecular mass of 21.564 Da. This protein showed a 28% amino acid sequence identity with the resolvase encoded by Tn1546 ORF2 (Table 1). The ORF-vanRbc intergenic region was 731 bp long and did not show significant homology with the ORF2-vanR region of Tn1546 up to the 6-bp sequence immediately upstream from the start codon of vanRbc.
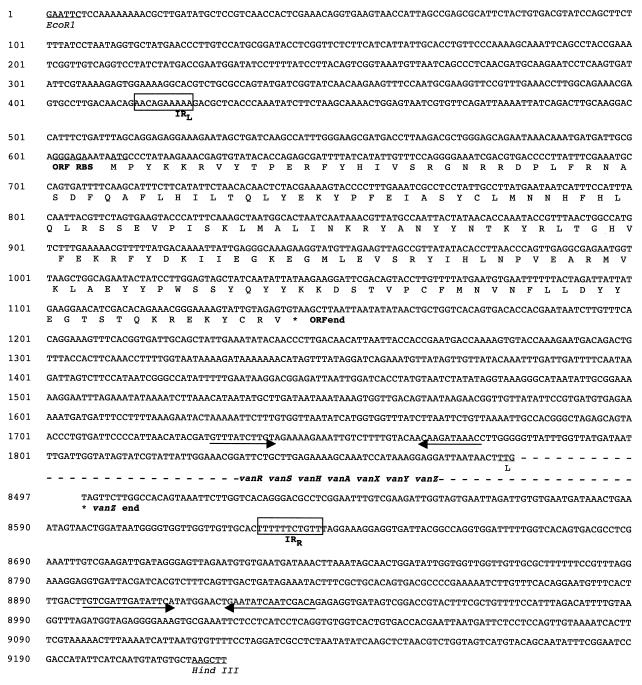
FIG. 3.
Nucleotide sequence of the regions flanking the vanA gene cluster contained in the 9.2-kb EcoRI-HindIII fragment of B. circulans DNA. The left (IRL) and right (IRR) terminal IRs of the hypothetical transposon-like element containing the vanAbc cluster are boxed. The deduced amino acid sequence of the ORF is shown below the nucleotide sequence. Ribosome-binding sites (RBS) and translation initiation codons are underlined. The IRs in the ORF-vanRbc intergenic region and at the left extremity of the fragment are indicated by arrows.
Sequence analysis of the region downstream from the vanZ stop codon did not identify any ORF. Perfect 10-bp inverted repeats (IRs; positions 416 to 425 and 8628 to 8637) that were not related to the IRs of Tn1546 but that might delineate an 8,221-bp transposon-like element were found. No direct repeats suggestive of target sequence duplication were found.
These results suggest that Tn1546-like transposons were not components of the element responsible for the transfer of the vanA gene cluster in B. circulans. Heterogeneity of the vanA cluster was recently observed in clinical isolates of enterococci from the northeastern United States, but this was explained by insertion of IS1251 in the vanS-vanH intergenic region of Tn1546-like transposons or by truncation of this element (7). The possibility that B. circulans VR0709 contained a truncated Tn1546 lacking ORFs 1 and 2 was excluded by the very low level of identity in the sequence downstream from vanZbc and the lack of the right terminal IR (IRR) which ends Tn1546-like transposons. To the best of our knowledge this is the first report of the presence of the vanA cluster in a genetic element other than Tn1546.
In spite of the increasing number of nonenterococcal species found to be able to acquire the van cluster under laboratory or natural conditions, the clinical relevance of the resistance encoded by these genes still remains confined to enterococci (22). No serious infections or nosocomial outbreaks have been reported as being caused by nonenterococcal strains carrying van genes. In particular, it is surprising that clinical isolates of staphylococci carrying van genes have never been isolated, even though these microorganisms naturally exchange antibiotic resistance determinants with enterococci and the transfer of the vanA gene by conjugation from E. faecalis to S. aureus has been obtained under laboratory conditions (13). It is possible that the expression of the van genes in certain nonenterococcal species is not compatible with survival in natural environments (i.e., host biological fluids or tissues). The recent isolation of a clinical S. aureus isolate in which a reduced level of vancomycin susceptibility was associated with an increased level of synthesis of peptidoglycan precursors and PBPs 2 and 2′ (13) provides indirect support for this hypothesis and should predict that, in this species, clinically relevant resistance will be acquired by mechanisms other than those encoded by van gene clusters.
ACKNOWLEDGMENTS
We thank P. Courvalin for the gift of E. faecium BM4147 and Marco Aldegheri for invaluable technical assistance.
This work was supported by the Consiglio Nazionale delle Ricerche, grant 96.03074.CT04, and by a grant from the University of Verona.
REFERENCES
- 1.Arthur M, Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993;37:1563–1571. doi: 10.1128/aac.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biavasco F, Giovanetti E, Miele A, Vignaroli C, Faccinelli B, Varaldo P E. In vitro conjugative transfer of Van A vancomycin resistance between clinical enterococci and listeriae of different species. Eur J Clin Microbiol Infect Dis. 1996;15:50–59. doi: 10.1007/BF01586185. [DOI] [PubMed] [Google Scholar]
- 3.Brisson-Noel A, Dutka-Malen S, Molinas C, Leclercq R, Courvalin P. Cloning and heterospecific expression of the resistance determinant vanA encoding high-level resistance to glycopeptides in Enterococcus faecium BM4147. Antimicrob Agents Chemother. 1990;34:924–927. doi: 10.1128/aac.34.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugg T D H, Dutka-Malen S, Arthur M, Courvalin P, Walsh C T. Identification of vancomycin resistance protein VanA as a d-alanine ligase of altered substrate specificity. Biochemistry. 1991;30:2017–2021. doi: 10.1021/bi00222a002. [DOI] [PubMed] [Google Scholar]
- 5.Dukta-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–27. doi: 10.1128/jcm.33.1.24-27.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana R, Ligozzi M, Pedrotti C, Padovani E M, Cornaglia G C. Vancomycin-resistant Bacillus circulans carrying the vanA gene responsible for vancomycin resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1997;16:473–474. doi: 10.1007/BF02471915. [DOI] [PubMed] [Google Scholar]
- 7.Handwerger, S., J. Skoble, L. F. Discotto, and M. Pucci. Heterogeneity of the vanA gene cluster in clinical isolates of enterococci from the northeastern United States. Antimicrob. Agents Chemother. 39:362–368. [DOI] [PMC free article] [PubMed]
- 8.Hiramatsu K, Hanagi H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 9.Leclerq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319:157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 10.Leclercq R, Derlot E, Weber M, Duval J, Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989;33:10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. M100-S6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 12.Nicas T I, Wu C Y, Hobbs J N, Preston D A, Allen N E. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother. 1989;33:1121–1124. doi: 10.1128/aac.33.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble W C, Virani Z, Cree R G A. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;93:159–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 14.Perichon B, Reynolds P, Courvalin P. VanD-type glycopeptide-resistant Enterococcus faecium BM4339. Antimicrob Agents Chemother. 1997;41:2016–2118. doi: 10.1128/aac.41.9.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Power G M, Abdulla Y H, Talsania H G, Spice W, Aathithan S, French G L. vanA genes in vancomycin-resistant clinical isolates of Oerskovia turbata and Arcanobacterium (Corynebacterium) haemolyticum. J Antimicrob Chemother. 1995;36:595–606. doi: 10.1093/jac/36.4.595. [DOI] [PubMed] [Google Scholar]
- 16.Poyart C, Pierre C, Quesne G, Pron B, Berche P, Trieu-Cuot P. Emergence of vancomycin resistance in the genus Streptococcus: characterization of a vanB transferable determinant in Streptococcus bovis. Antimicrob Agents Chemother. 1997;41:24–29. doi: 10.1128/aac.41.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintiliani R, Jr, Courvalin P. Conjugal transfer of the vancomycin resistance determinant vanB between enterococci involves the movement of large genetic elements from chromosome to chromosome. FEMS Microbiol Lett. 1994;119:359–364. doi: 10.1111/j.1574-6968.1994.tb06913.x. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uttley A H C, Collins C H, Naidoo J, George R C. Vancomycin-resistant enterococci. Lancet. 1988;i:57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 21.Woodford N, Johnson A P, Morrison D, Chin A T, Stephenson J R. Two distinct forms of vancomycin resistance amongst enterococci. Lancet. 1990;335:226. doi: 10.1016/0140-6736(90)90317-x. [DOI] [PubMed] [Google Scholar]
- 22.Woodford N, Johnson A P, Morrison D, Speller D C E. Current perspective on glycopeptide resistance. Clin Microbiol Rev. 1995;8:585–615. doi: 10.1128/cmr.8.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]