Abstract
Purpose
The purpose of this study was to present the determination of inter- and intra-day variations in tear flow rate, and tear fluid protein concentration, as well as protein composition regarding their impact for future biomarker studies.
Methods
Tear fluid was collected noninvasively from 18 healthy subjects by performing Schirmer tests at 4 different time points repetitive in a period of 2 days. The tear flow rate on the Schirmer test strips was measured. Proteins were extracted from strips and quantified using amino acid analysis. Protein composition was analyzed by the strips data-independent (DIA) based mass spectrometry. To exclude any impairments to health, volunteers underwent a detailed neurological as well as an ophthalmological examination.
Results
Whether tear fluid was collected from oculus sinister or oculus dexter did not affect the tear flow rate (P ≈ 0.63) or protein concentration (P ≈ 0.97) of individual subjects. Moreover, protein concentration was independent from the tear volume, so that a change in volume may only influence the total protein amount. When the examination days were compared, investigation of tear flow rate (P ≈ 0.001) and protein concentration (P ≈ 0.0003) indicated significant differences. Further, mass spectrometric analysis of tear fluid revealed 11 differentially regulated proteins when comparing both examination days.
Conclusions
Our findings provide evidence of inter-day variation in tear flow rate, tear proteome concentration, and composition in healthy subjects, suggesting that inter-day variation needs to be taken into consideration in biomarker research of tear fluid. Identified proteins were assigned to functions in the immune response, oxidative and reducing processes, as well as mannose metabolism.
Keywords: mass spectrometry, protein concentration, Schirmer test, tear film, tear flow rate, tear volume
Tears have a variety of functions, and protection against pathogens is provided not only by its rinsing action, but also by various antimicrobial substances it contains.1 Although tear fluid appears quite inconspicuous, the diversity of its functionality makes it an interesting object of research. The analysis of tear fluid offers several advantages. Collection is simple and minimally to noninvasive, for example, using a glass capillary or Schirmer strips.2,3 In addition, it contains fewer microbes than other mucosal surfaces1 and has a lower complexity of the proteome compared to, for example, plasma.4 Because differences in low-complexity samples can be detected easier than in highly complex ones, the search for biomarkers is simpler.4 Specific tear biomarkers were identified for eye diseases, such as dry eye, Sjogren's syndrome, glaucoma, and age-related macular degeneration.5 In addition, tear fluid biomarkers are discussed in neurodegenerative diseases.3,6–9
Currently, only a limited number of biomarkers found in tear fluid have been validated in independent study groups, as shown also for other body fluids.10,11 We hypothesize that high variability of protein abundance within tears, between as well as within individuals, might be a reason for that. Hence, we investigated the inter- and intra-individual variability of the tear fluid proteome in a time course. We also analyzed tear fluid rate as well as protein concentration.
Methods
Study Group
This longitudinal study design comprises 18 subjects. Ethics approval was obtained from the ethics committee of the Ruhr-University Bochum (approval number 4905-14), the study was conducted in accordance with the Declaration of Helsinki. The participants were informed about the procedure and gave written consent. The sequence of sample collection up to clinical (neurological as well as ophthalmologic) examinations was the same for each subject.
Ocular and Neurological Assessments
All participants underwent a patient health (Patient Health Questionnaire 4-item [PHQ-4])12 and an adapted Michigan Neuropathy Screening Instrument (MNSI)13,14 evaluation. PHQ-4 scores of 6 or greater are considered “yellow flags” and PHQ-4 scores of 9 or greater are “red flags” for the presence of a depressive or an anxiety disorder.15 The MNSI included 2 assessments: a 15-item self-administered questionnaire and a lower extremity examination that includes inspection and assessment of vibratory sensation and ankle reflexes. A score of ≥ 7 is considered abnormal. With the MNSI we wanted to exclude neuropathies that could affect the corneal nerves and possibly the tear film. Then, the following dry eye examinations were performed: Schirmer tests, the Ocular Surface Disease Index (OSDI) evaluation,16 tear break-up-time (TBUT), and corneal fluorescein staining.17 The 12 items of the OSDI questionnaire were graded and the OSDI score was calculated. For TBUT, 5 mL of 2% sodium fluorescein solution was instilled and the average time until the appearance of the first break in the tear film was calculated from 3 measurements using cobalt blue illumination at the slit-lamp. Corneal staining was evaluated under cobalt blue illumination 2.5 to 3.0 minutes after fluorescein instillation, staining levels were graded according to the National Eye Institute (NEI)/Industry Workshop scale.18
Assessment of Tear Flow Rate
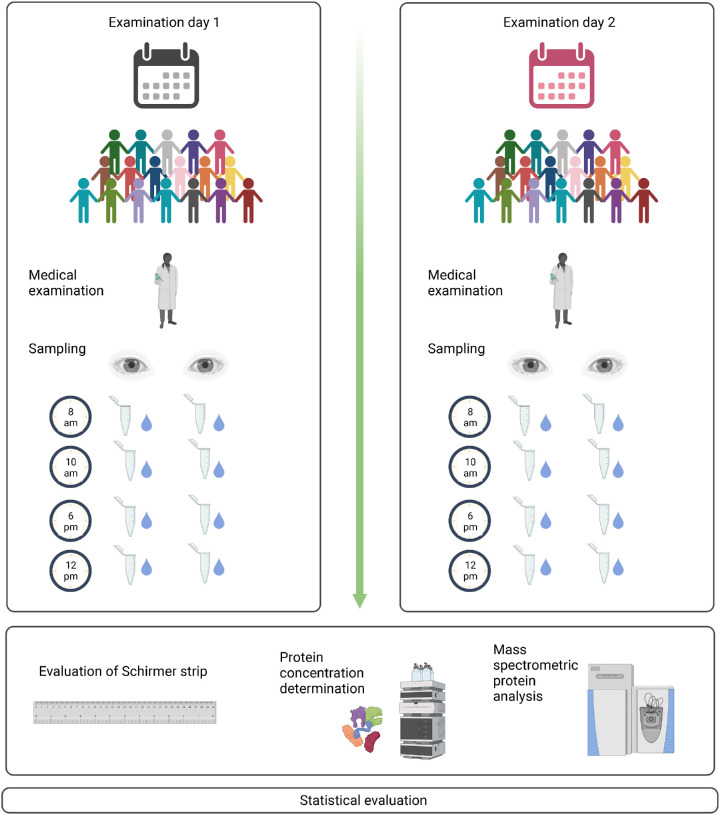
For the assessment of tear flow rate, a Schirmer test strip (Haag-Streit UK LtdA, Bishop's Stortford, Hertfordshire, England, UK) was placed in the lower lid of each eye simultaneously, without anesthesia (inter-eye variability analysis). After 5 minutes, the strips were removed and the tear flow was measured with a ruler. The tear flow rate was defined as mm tear flow (with a maximum of 30 mm) within 5 minutes. The strips where transferred in a reaction tube before being stored at −80°C for later analysis. To assess intra-eye variability during the day, the sampling process was repeated four times throughout the day (8 AM, 12 AM, 6 PM, and 10 PM). To evaluate intra-individual variability between days, the collecting was repeated 2 days later at the same time points (Fig. 1).
Figure 1.
Sampling process. Tear fluid was collected from 18 healthy subjects at 8 AM, 12 AM, 6 PM, AND 10 PM as well as 2 days later at the same time points. There were 72 samples from the left eyes as well as from the right eyes that were obtained per day, 288 in total. The proteins were eluted by extraction buffer and quantified using quantitative amino acid analysis. Differences in the proteome were analyzed by mass spectrometry (created with BioRender.com).
Tear Fluid Protein Elution
Proteins were eluted from the Schirmer tear test strips by adding 500 µL PBS solution (Thermo Fisher Scientific Inc., USA) containing 1% Triton X-100 (AppliChem GmbH, Germany) as well as protease inhibitor (complete EDTA-free Protease Inhibitor Tablets; Roche Diagnostics GmbH, Germany). After incubation overnight at 4°C, the resulting sample solution was transferred to a new reaction tube and aliquoted prior to storing at −80°C. The remaining strips were discarded.
Analysis of Tear Protein Concentration
A volume of 4 µL tear fluid was used for performing protein concentration determination by amino acid analysis according to Guntermann et al.19 For a detailed method description, see May et al.20 To avoid contamination, glass vials were first incubated in a muffle furnace (Carbolite CWF 1100, USA) at 400°C for 4 hours. After that 4 µL of eluted tear fluid was transferred to the glass vial, dried in a vacuum concentrator (RVC2-25CD plus), and then placed in an evacuation vessel. Thereafter 400 µL of 6 M hydrochloric acid and a phenol crystal were added. The samples were evacuated alternately four times and aerated with argon. Acid gas phase hydrolysis was performed at 150°C for 1 hour followed by evacuation. Derivatization was performed by adding 30 µL AccQ-Fluor borate buffer with internal standard norleucine and 10 µL AccQ-Fluor reagent (10 mM 6-aminoquinolyl-N-hydroxysuccinimidylcarbamate in acetonitrile), followed by incubation at 55°C for 10 minutes. The primary and secondary amines were converted to stable derivatives. The stable amine derivatives were separated on a C18 reversed-phase column (2.1 mm 100 mm length; Waters GmbH, Germany) by dissolving in 10 µL of 20 mM hydrochloric acid. A two-solvent gradient system was used for separation (solvent A: AccQ-Tag Ultra Eluent A, and solvent B: AccQ-Tag Ultra Eluent B). The flow rate was set at 0.7 µL per minute with a column temperature of 55°C and increasing solvent A in the gradient. Amino acid derivatives were detected at an emission wavelength of 260 nm (Waters GmbH, Germany). Each sample was run in duplicate. Different concentrations of an internal amino acid standard were used for quantification. Taking into account the volume and molar mass of each amino acid, the protein concentration of the tear sample was calculated.
Sample Preparation for Mass Spectrometric Analysis
Tear samples from 10 female subjects were selected for additional mass spectrometric analysis, to reveal variations in the tear proteome. A detailed description of the mass spectrometric analysis is given in the Supplementary File S1. Data files generated as part of the described workflow will be hosted in the public repository PRIDE under the identifier PXD037811.
Statistical Analysis
Coefficient of variation - The relative measure of dispersion can be expressed by the coefficient of variation (CV). This indicates the ratio between the standard deviation (SD) and the mean value () in percent (formula 1).
| (1) |
In order to draw conclusions about the degree of dispersion of tear protein concentrations within subjects, an intra-individual CV was calculated for both days (CVi). An inter-subject CV (CVs) was calculated to determine the measure of dispersion between all subjects. It was formed from the median of all CVis. Furthermore, the measure of dispersion within each time point and within a day was calculated independently of oculus sinister (OS) and oculus dexter (OD) and referred to as intra-time point CV (CVt) and intra-day CV (CVd), respectively.
Functional Pathway Analysis
Functional proteomic analysis was performed using Reactome, a curated database for the visualization, interpretation, and analysis of pathway knowledge.21 The implemented pathway overview visualization ReacFoam is based on Voronoi tessellation.
Results
Tear secretion and protein concentration were analyzed, followed by protein identification. First, this was determined within a single subject at each of the different time points (intra-individual). Second, the differences of the tear fluid between the subjects were calculated (inter-subject). In addition, the measured values were compared at defined time points (intra-time point), during two single examination days (intra-day), and finally between the respective days (inter-day).
All study subjects (mean age 26.83 ± 4.00 years; 7 men and 11 women) were healthy volunteers with no previous eye diseases, surgeries, or injuries. None of the volunteers suffered from diabetes or rheumatism, two were taking medication for hypothyroidism, and two had a history of migraine. Mean PHQ-4 score was 0.99 ± 1.44 for all participants. Hence, there was no indication of a depressive or an anxiety disorder. In addition, MNSI score was unremarkable for all subjects (0.22 ± 0.43).
All dry eye paraments were unremarkable, corneal fluorescein staining was 0.06 ± 0.24 for both eyes from all subjects, mean OSDI score was 4.78 ± 6.27 for all subjects, and tear break-up time was 10.72 ± 2.22 seconds for OD and for 10.44 ± 2.66 seconds for OS (Table 1). Thus, according to the examination results of the clinical tear film parameters, study subjects had a healthy ocular surface.
Table 1.
Study Group Characteristics and Dry Eye Results
| OD (Mean ± SD) | OS (Mean ± SD) | |
|---|---|---|
| Age, y | 26.83 ± 4.00 | |
| Gender (M/F) | 7/11 | |
| Corneal fluorescein staining (points) | 0.06 ± 0.24 | 0.06 ± 0.24 |
| OSDI score (points) | 4.78 ± 6.27 | |
| Tear break-up time (s) | 10.72 ± 2.22 | 10.44 ± 2.66 |
Tear Flow Rate Shows no Significant Differences Between Eyes, But Between Days
The tear flow rate (mm tear flow with a maximum of 30 mm within 5 min) at both days of OS and OD was examined (Fig. 2). The averaged tear flow rate of all 18 healthy subjects on day 1 for OS was 21.15 ± 8.47 mm and for OD 22.46 ± 9.1 mm. On day 2, 18.94 ± 9.58 mm was obtained for OS and 18.64 ± 9.75 mm for OD. Tear flow rate of OS and OD was not significantly different on the respective days (P > 0.05). However, a higher tear flow rate was observed on day 1 compared to day 2 (P < 0.001).
Figure 2.
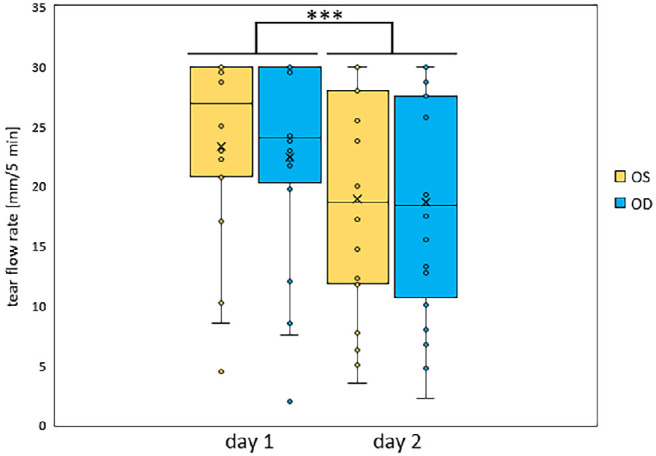
Averaged tear flow rate of OS and OD on day 1 and 2. Mean tear flow rate from the left (OS; yellow) and right eye (OD; blue) of all 18 subjects for day 1 and 2 were plotted, which was significantly lower on day 2. Values are median ± second/third quartiles ± first/fourth quartiles. *** P < 0.001.
Tear Secretion Underlies Individual Biological Variations
The intra-individual CVi values of tear flow rate of each subject on day 1 and day 2, as well as for OS (see Supplementary File S1 Table S1A) and OD (see Supplementary File S1 Table S1B) were determined. Next, the inter-subject CVs values were calculated from the mean value of the respective CVi. Here, the dispersion of the tear flow rate on day 1 was 11.11% for OS and 21.61% for OD. On day 2, a mean dispersion of 30.59% for OS and 27.77% for OD was calculated. To support this finding, CVt was determined. This CVt gives the measure of dispersion of the tear flow rate of all subjects independent of OS or OD at one time point. The median of all four time points, called CVd, of day 1 was 43.38% and that of day 2 was 59.29%. That the tear flow rate varied more in the morning than in the evening or vice versa could not be detected (see Supplementary File S1 Table S2). With regard to OS and OD, tear flow rates on both days were subject to similar variation (P > 0.05). When comparing day 1 and day 2, the tear flow rate varied significantly more on day 2 than on day 1 (P ≈ 0.019).
Protein Concentration Shows no Significant Differences Between Left and Right Eye, But Between Days 1 and 2
Tear secretion of a single subject varies over the day. This raises the question whether the protein concentration also varies depending on the time of day. Hence, the protein concentrations of the individual time points of each subject were averaged and plotted separately for OS and OD as well as day 1 and day 2 (Fig. 3). On day 1, the averaged protein concentration in the tear fluid was 0.52 ± 0.31 µg/µL of OS and 0.55 ± 0.34 µg/µL of OD. The mean protein concentration of the tear fluid on day 2 was 0.42 ± 0.22 µg/µL for OS and 0.39 ± 0.19 µg/µL for OD. Similar protein concentrations were measured in the tear fluid of OS and OD on the respective days. In summary, no significant difference was detected between OS and OD (P ≈ 0.97). In contrast, the protein concentration on day 1 was significantly higher in both eyes than on day 2 (P ≈ 0.0003).
Figure 3.
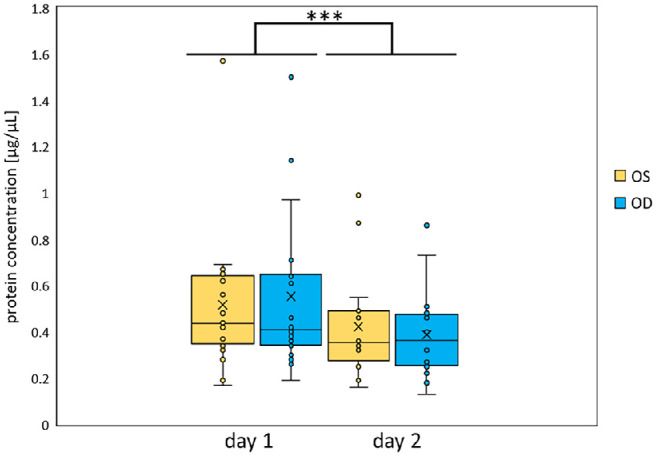
Averaged protein concentrations of the tear fluid of OS and OD on day 1 and 2. Mean protein concentrations in µg/µL of tear fluid from all 18 subjects were plotted for both day 1 and day 2 for OS (yellow) and OD (blue). It was significantly lower on day 2. Values are median ± second/third quartiles ± first/fourth quartiles. ***P < 0.001.
As for the tear flow rate, the intra-individual dispersion measure was calculated (CVi) to show the variance of the protein concentration in the tear fluid within the individual subjects. Direct comparison of CVi values revealed no significant differences for OS and OD (P ≈ 0.31). When comparing the CVi values of day 1 and day 2, there were significant differences (P ≈ 0.0018). Furthermore, the inter-subject coefficient of variation was calculated (CVs), based on the median of all CVi. The CVs had a value of 19.15% for OS and 15.58% for OD on day 1. On day 2, the CVs was 25.23% for OS (see Supplementary File S1 Table S3A) and 24.16% for OD (see Supplementary File S1 Table S3B). The variance of the protein concentration between both eyes on the respective days was comparable. Compared to day 1, the variance of the protein concentration on day 2 was higher. Comparisons of inter-subject variance was carried out using determination of the CVt for each time point (see Supplementary File S1 Table S4). These varied on day 1 from 69.58% to 94.09%. On day 2, variations in the variances ranged from 51.42% to 85.51%. Moreover, the variance between the time points of both days was calculated (CVd). This variance was 73.36% on day 1 and 63.59% on day 2.
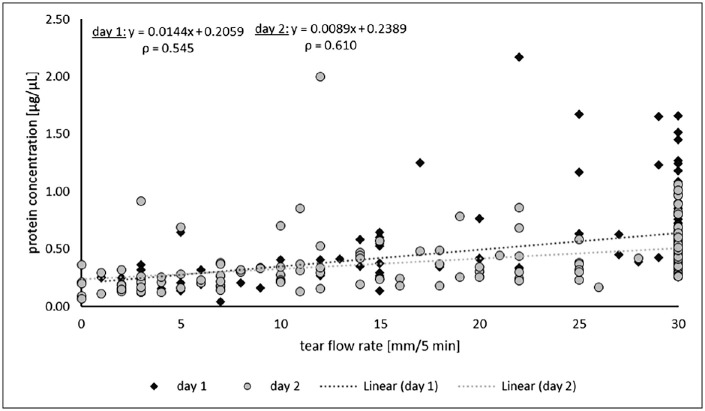
Moderate Correlation Between Tear Flow Rate and Protein Concentration
Because both tear flow rate and protein concentration varied in the subjects, it is reasonable to assume that there exists a dependence between these parameters. As with the tear flow rate, higher values were detected for protein concentration on day 1 compared to day 2. Accordingly, the tear fluid volume potentially correlates with the protein concentration. To check a possible dependency, the protein concentration was plotted as a function of the tear flow rate (Fig. 4). Spearman's rank correlation coefficient ρ revealed a value of 0.545 for day 1 and 0.610 for day 2, indicating a moderate positive correlation between the tear flow rate and the protein concentration, also over both examination days (ρ = 0.598).22
Figure 4.
Relationship between tear flow rate and protein concentration on day 1 and day 2. Protein concentrations of the tear fluid were plotted against the tear flow rates from every subject. No distinction was made between OS and OD. Values for day 1 are represented by circles, and for day 2 by squares. The respective regression lines were shown by dotted lines in the respective color of the days, as well as their associated functions and Spearman's rank correlation coefficient ρ. A moderate positive correlation between the tear flow rate and the protein concentration, also over both examination days, was noted.
Tear Fluid Proteins With Meaningful Function in Immune-Metabolic Homeostasis
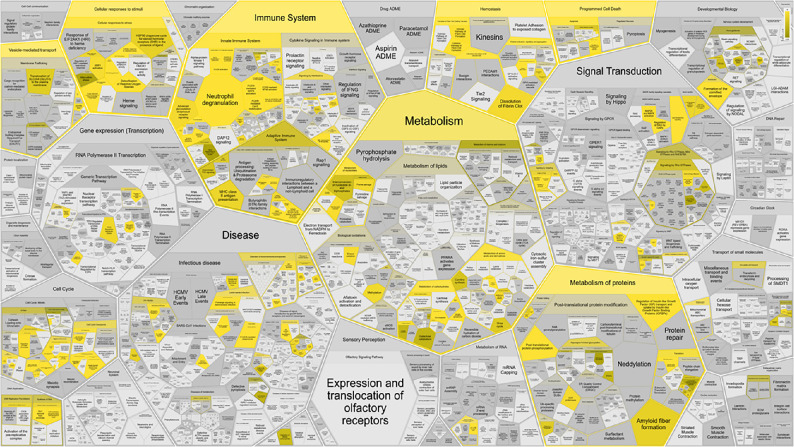
Proteins within the tear fluid of 10 female subjects were further evaluated by data-independent (DIA) mass spectrometry. For data analysis, a spectral library was created from an already published dataset.23 In total, 2362 unique peptides were identified and assigned to 595 different proteins within our samples (see Supplementary File S2 Sheet 1). These proteins were further investigated to determine their biological context using Reactome pathway database. There were 475 out of the 595 proteins that were found, whereby 183 pathways were hit by at least one of them.23 Seven major pathway elements comprising “immune system”, “metabolism”, especially “metabolism of proteins”, “homeostasis”, “cellular response to stimuli”, “vesicle-mediated transport”, as well as “programmed cell death” were visualized with ReacFoam (Fig. 5).
Figure 5.
High level pathway overview of human tear fluid proteins. There were 475 proteins (out of 595 proteins) contained in the spectral library that were assigned to 183 different biological pathways. Significant pathway elements are shown with a gradient from copper (P ≤ 0.05) to yellow (P closer to 0) based on the Benjamini–Hochberg procedure.
Significant Differences of the Tear Fluid of Both Test Days
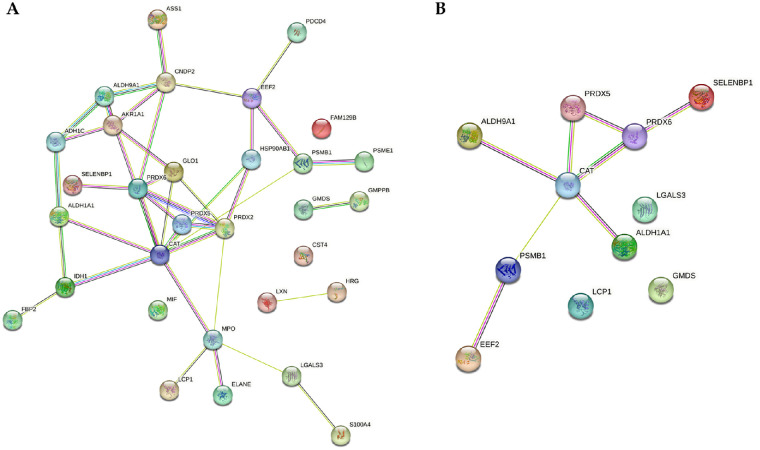
Two-sample t-test between OS and OD (P < 0.01) revealed no significant differences between OS and OD at both, peptide as well as protein levels. Differentially regulated peptides assigned to 31 various proteins (P < 0.01) were observed between day 1 and day 2 (Fig. 6A), independent of OS or OD. Further, only proteins to which at least two peptides could be assigned were then considered. After applying these search criteria, 11 proteins remained (Table 2, see Supplementary File S2 Sheet 2). Two of these proteins were upregulated. Interestingly, the identified proteins show a strong interaction according to pathway analysis (Fig. 6B).
Figure 6.
Protein interaction analysis using STRING. Database analysis showing the interaction between the differential and highly significant proteins in tear fluid. Comparison of tear fluid from 2 examination days resulted in 31 differential proteins (A), of which eleven proteins were significantly regulated with at least 2 peptides (B).
Table 2.
Overview of Significant Differential Proteins
| Protein ID | Protein Name | Regulation on Day 2 |
|---|---|---|
| P30041 | Peroxiredoxin-6 (PRDX6) | ↓ |
| P13639 | Elongation factor 2 (EEF2) | ↑ |
| P04040 | Catalase (CAT) | ↓ |
| P49189 | 4-trimethylaminobutyraldehyde dehydrogenase (ALDH9A1) | ↓ |
| P00352 | Retinal dehydrogenase 1 (ALDH1A1) | ↓ |
| Q13228 | Methanethiol oxidase (SELENBP1) | ↓ |
| P13796 | Plastin-2 (LCP1) | ↑ |
| P17931 | Galectin-3 (LGALS3) | ↓ |
| O60547 | GDP-mannose 4,6 dehydratase (GMDS) | ↓ |
| P20618 | Proteasome subunit beta type-1 (PSMB1) | ↓ |
| P30044 | Peroxiredoxin-5 (mitochondrial) (PRDX5) | ↓ |
Protein regulation at day 2 relative to day 1 is indicated by an arrow. Arrow down means proteins are less expressed at day 2 than at day 1, and arrow up means proteins are more expressed at day 2 than at day 1.
Discussion
To date, studies have not focused on day-dependent tear fluid variation in healthy subjects comparable to ours. Diurnal analysis on tear stability, tear film osmolarity, cytokine, and chemokine concentrations were already investigated in the past by other groups.24–27 In addition, protein variations of small proteins with a molecular weight under 20 kDa within the tear fluid were described.28 A comprehensive differential analysis of the whole proteome of the tear fluid over the course of the day and between days in a clinically well-characterized as well as statistically powerful study population of healthy subjects has not yet been performed. Supplemented by simultaneous analysis of tear secretion and tear protein concentration of the same study group, our results extend the knowledge of tear film variation during and between days and confirm published findings.
Tear fluid analysis offers a noninvasive and promising method for predictive, preventive, and personalized medicine.29 Thus, diseases directly related to the ocular surface can be diagnosed at an early stage.30 Furthermore, diseases of other organs can be detected, it can be used for external drug screening, as well as for pharmacokinetic studies.30,31 Because the eye is in direct contact with the outside world and thus exposed to stimuli, including dirt particles, light, wind, and also pathogens,32 it is reasonable to assume that the tear fluid concentration, rate, as well as proteome is subject to diurnal changes. Therefore, the timing and method of tear fluid collection should be considered. Consequently, this work aimed to analyze human tear fluid over the course of the day.
Variations in the Tear Flow Rate of Healthy Individuals
Due to constant contact with the environment, tear fluid is subject to diurnal fluctuations.33 Some studies state that tear fluid osmolarity is not subject to intra- or inter-day variations,34 whereas others suggest it is somewhat influenced by the time of day.35 Garcia et al. used the tear fluorescein clearance-test.34 Therefore, depending on the type of intended tear fluid analysis, it is essential to consider the timing of the study. To learn more about possible time-dependent variations, protein concentration and content were examined over time.
In the present study, the tear flow rate was first investigated with regard to its variance. The individual tear flow rate revealed no significant differences between OS and OD on both days. In addition, the corresponding intra-individual dispersion measures (CVi) showed no significant differences. In summary, in regard to healthy subjects, it makes no difference to both the average and variance of the flow rate whether tear fluid is sampled from OS or OD. By contrast, Bachhuber et al. found significantly lower tear flow rates in the second eye.36 However, this could be due to a slight temporal offset when inserting the Schirmer tear test strip in their study as a comparable effect was not observed in the same study using glass capillaries.
Examination of individual tear flow rate between the two experimental days revealed a significantly shorter rate on day 2 (P < 0.001). Comparing the variances of the CVi between the two experimental days also revealed slightly significant differences (P ≈ 0.019). To make clearer statements about the different variances in tear formation, the method of determining tear formation needs to be adapted. One solution for better comparability would be to calculate the tear flow rate per minute.37 In conclusion, considering the obtained data, a prompt repeated collection of tear fluid should be avoided. The reason for this is that lower tear secretion could result in the total amount of protein in the tear fluid being too low for further proteomic analysis. Furthermore, the lower variance of tear flow rate on day 1 argues for more accurate and reproducible results.
Variations in the Tear Fluid Protein Concentration
When comparing OS with OD, neither a significant difference between the individual protein concentration nor between the individual variance of the subjects could be detected. With this, it is irrelevant for the protein concentration whether tear fluid is collected from OS or OD.
When looking at the individual protein concentrations of the subjects, significant differences (P < 0.001) were detected when comparing the examination days. The variance of protein concentration was higher within subjects on day 2 than on day 1, and likewise for the tear flow rate. A comparison of the variances of protein concentrations also revealed significant differences between days (P ≈ 0.0018). This result allows the interpretation that for better reproducibility of analyses, tear collection has to be performed on one day. Further studies need to show whether tear flow rate and protein concentration have a normal biological variance or whether relatively timely repeated collection carries such an effect.
Correlation Between Tear Flow Rate and Protein Concentration
Many diseases can affect tear secretion.38,39 Similarly, external conditions, including low humidity, can lead to decreased tear secretion.40 Tear flow could have an effect on protein concentration. Hence, we investigated the correlation between tear flow rate and protein concentration on day 1 and 2. Here, the protein concentration was moderately dependent on the tear volume and a change in volume therefore only influences the total protein quantity. For further studies, this means that if tear production is too low, the total protein amount might not be sufficient for mass spectrometric analyses. Furthermore, tear samples do not need to be matched, which greatly simplifies sample generation and preparation for mass spectrometric analyses.
Protein Regulation of Tear Fluid
Mass spectrometry-based proteomic analysis of body fluids is widely used to identify potential disease biomarkers.41 If these biomarkers exhibit a high natural variance between individuals, their specificity is highly questionable.10 False conclusions regarding an individual's disease status could be the result.42,43 Hence, to make statements about possible disease-specific biomarkers, it must first be ensured that the comparison group provides constant results in protein abundance. Proteins that are already highly differentially expressed in healthy individuals must be present and significantly up- or downregulated in diseased individuals, to truly function as biomarkers. It is postulated that this variance can lead to false positive results.10 The high biological protein variance described for cerebrospinal fluid could also apply to tear fluid. Consequently, this protein variance should be included in the assessment of appropriate biomarker candidates. Furthermore, tear studies also have to consider how much difference there is in the variance of the results between eyes. No significantly differentially regulated proteins between OS and OD could be identified in this work. However, 11 proteins were significantly differentially regulated between day 1 and day 2. According to the STRING database, six proteins could be associated with leukocyte activation, six proteins with oxidation and reduction processes, respectively, with two proteins playing a role in one as well as in the other processes.44 Interestingly, one protein could be linked exclusively to mannose metabolism and showed no direct interactions with the other proteins. LGALS3, downregulated on day 2, is a galactose-specific lectin that binds to IgE and in this way plays a key component in the defense against microbes, such as Streptococcus pneumoniae.45 Uchino et al. found a significantly higher concentration of LGALS3 in the tear fluid of patients with dry eye disease compared to healthy individuals.46 Whether the study results of Uchino et al. are true hits or the result of a naturally high variance of this protein should be analyzed in subsequent studies with larger as well as equally sized study groups. ALDH1A1, downregulated on day 2, has a protective function against cytotoxicity induced by oxidative stress in human lens epithelial cells.47 Furthermore, ALDH1A1 oxidizes retinaldehyde to retinoic acid. Both are derivatives of vitamin A and serve as crystallins to maintain tear transparency.48,49 The presence of vitamin A and its derivatives is essential for maintaining ocular surface integrity.50 PRDX5 was upregulated in patients with dermatochalasis, a conjunctival disease.51 However, only small study groups were analyzed and the fold changes found were small (1.92). SELENBP1 was upregulated in the tear fluid of hard contact lens wearers compared to soft contact lens wearers.52 Contact lenses might also have affected the altered regulation of SELENBP1 observed in our study.
Already in 2012, the group of Gonzales et al. reported subtle differences when comparing tear fluid samples by MALDI-TOF mass spectrometry from 6 individuals over 7 days. In this study, the focus was on proteins with a molecular weight below 20 kDa.28 These results are further extended and underlined by the results of the DIA-based mass spectrometric method used in our study. Using this approach, all ions in a predefined m/z range (or time window) are fragmented. This results in a more complete map of the fragment ion spectra. Thus, allowing for more reliable quantification and greater coverage of the whole proteome for quantification.
Summarized, our results support the hypothesis that the natural variability strongly impacts tear secretion, concentration, as well as protein content. All these can affect project results. Therefore, when planning a study or evaluating results with tear fluid, investigations should be given a high emphasis toward comparable sampling conditions, a biometric design, as well as a sufficiently large study group due to natural variation.
Strengths and Weaknesses
In our study, we analyzed the variability of tear fluid in terms of protein concentration, tear secretion, and protein composition both during the day and across days. Healthy subjects who underwent both neurologic and ophthalmologic examinations were selected to obtain a well-characterized study population. The study population presented here is intended to allow other researchers to compare it extensively with their own. This is particularly important when comparing the here described study group to corresponding patient ones. Environmental conditions and their effects on the tear fluid are not insignificant, as has been described extensively in the literature.40,53,54 In our study, we deliberately chose not to use controlled conditions in terms of temperature, humidity, or controlled airflow chambers because we wanted to have the most realistic clinical conditions possible. In everyday clinical practice, these tests are rarely performed under these standardized conditions and we wanted the results to be comparable with other studies. This also applies to potential protein biomarkers for disease. They must have high sensitivity and specificity, even if the environment is not standardized. The listed proteins were variable under normal conditions in healthy subjects. Therefore, if these proteins turn out to be disease biomarkers in studies, they should be considered with caution, as has already been shown for other body fluids, such as cerebrospinal fluid.55 We strongly recommend that future studies investigate the variance in tear proteome, tear protein concentration, and tear secretion in regions with, for example, different climatic conditions or replicate them under different standardized conditions. These results will form the basis for robust biomarker candidates or clinical parameters related to tear flow rate and concentration for ophthalmologic, neurologic, or other diseases. In addition, it would be interesting to study larger time intervals to further assess the natural variations of the tear fluid in healthy subjects.
Supplementary Material
Acknowledgments
The authors thank all co-workers involved in the conduct of the study for their commitment.
Supported by the Deutsche Parkinson Gesellschaft, the Medical Faculty at Ruhr-University Bochum (FoRUM), the American European Congress of Ophthalmic Surgery (AECOS), the HUPO Brain Proteome Project (HBPP), PURE, a project of North Rhine-Westphalia, a federal German state, ProDi Zentrum für Proteindiagnostik, and by de.NBI, a project of the German Federal Ministry of Education and Research (FKZ 031 A 534A).
The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Disclosure: A. Guntermann, None; O. Fatoba, None; M. Kronenberg, None; S. Reinehr, None; P. Grotegut, None; M. Schargus, None; T. Tsai, None; S. Ivanova, None; B. Serschnitzki, None; N. Kumowski, None; C. Maier, None; K. Marcus, None; H.B. Dick, None; S.C. Joachim, None; C. May, None
References
- 1. McDermott AM. Antimicrobial compounds in tears. Exp Eye Res. 2013; 117: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Posa A, Bräuer L, Schicht M, Garreis F, Beileke S, Paulsen F.. Schirmer strip versus capillary tube method: non-invasive methods of obtaining proteins from tear fluid. Ann Anat. 2013; 195: 137–142. [DOI] [PubMed] [Google Scholar]
- 3. Ponzini E, Santambrogio C, De Palma A, Mauri P, Tavazzi S, Grandori R.. Mass spectrometry-based tear proteomics for noninvasive biomarker discovery. Mass Spectrom Rev. 2022; 41(5): 842–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hagan S, Martin E, Enríquez-de-Salamanca A.. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 2016; 7(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fomo KN, Schmelter C, Pfeiffer N, Grus FH.. Tear film-specific biomarkers in glaucoma patients. Klin Monbl Augenheilkd. 2022; 239: 165–168. [DOI] [PubMed] [Google Scholar]
- 6. Acera A, Gomez-Esteban JC, Murueta-Goyena A, et al.. Potential tear biomarkers for the diagnosis of Parkinson's disease-a pilot study. Proteomes. 2022; 10(1): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamm-Alvarez SF, Janga SR, Edman MC, et al.. Levels of oligomeric α-Synuclein in reflex tears distinguish Parkinson's disease patients from healthy controls. Biomark Med. 2019; 13(17): 1447–1457. [DOI] [PubMed] [Google Scholar]
- 8. Hamm-Alvarez SF, Okamoto CT, Janga SR, et al.. Oligomeric α-synuclein is increased in basal tears of Parkinson's patients. Biomark Med. 2019; 13(11): 941–952. [DOI] [PubMed] [Google Scholar]
- 9. Gijs M, Ramakers IHGB, Visser PJ, et al.. Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci Rep. 2021; 11(1): 22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schilde LM, Kösters S, Steinbach S, et al.. Protein variability in cerebrospinal fluid and its possible implications for neurological protein biomarker research. PLoS One. 2018; 13: e0206478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halbgebauer S, Ockl P, Wirth K, Steinacker P, Otto M.. Protein biomarkers in Parkinson's disease: focus on cerebrospinal fluid markers and synaptic proteins. Mov Disord. 2016; 31: 848–860. [DOI] [PubMed] [Google Scholar]
- 12. Lowe B, Wahl I, Rose M, et al.. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. 2010; 122: 86–95. [DOI] [PubMed] [Google Scholar]
- 13. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA.. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994; 17: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 14. Hoffken O, Maier C, Richter H, Tegenthoff M, Schwenkreis P.. A simplified screening protocol predicts pathological electroneurographic results in patients with suspected polyneuropathy. Int J Neurosci. 2010; 120: 28–35. [DOI] [PubMed] [Google Scholar]
- 15. Kroenke K, Spitzer RL, Williams JBW, Löwe B.. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009; 50(6): 613–621. [DOI] [PubMed] [Google Scholar]
- 16. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL.. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000; 118: 615–621. [DOI] [PubMed] [Google Scholar]
- 17. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 108–152. [DOI] [PubMed] [Google Scholar]
- 18. Lemp MA. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes. CLAO J. 1995; 21: 221–232. [PubMed] [Google Scholar]
- 19. Guntermann A, Steinbach S, Serschnitzki B, et al.. Human tear fluid proteome dataset for usage as a spectral library and for protein modeling. Data in Brief. 2019; 23: 103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. May C, Serschnitzki B, Marcus K.. Good old-fashioned protein concentration determination by amino acid analysis. Methods Mol Biol. 2021; 2228: 21–28. [DOI] [PubMed] [Google Scholar]
- 21. Gillespie M, Jassal B, Stephan R, et al.. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022; 50: D687–D692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukaka M. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012; 24: 69–71. [PMC free article] [PubMed] [Google Scholar]
- 23. Guntermann A, Steinbach S, Serschnitzki B, et al.. Human tear fluid proteome dataset for usage as a spectral library and for protein modeling. Data Brief. 2019; 23: 103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arroyo C, Byambajav M, Fernández I, et al.. Diurnal variation on tear stability and correlation with tear cytokine concentration. Cont Lens Anterior Eye. 2022; 45: 101705. [DOI] [PubMed] [Google Scholar]
- 25. Pena-Verdeal H, García-Resúa C, Ramos L, Yebra-Pimentel E, Giráldez MJ.. Diurnal variations in tear film break-up time determined in healthy subjects by software-assisted interpretation of tear film video recordings. Clin Exp Optom. 2016; 99;(2): 142–148. [DOI] [PubMed] [Google Scholar]
- 26. Pena-Verdeal H, Garcia-Resua C, Garcia-Queiruga J, Sabucedo-Villamarin B, Yebra-Pimentel E, Giraldez MJ.. Diurnal variations of tear film osmolarity on the ocular surface. Clin Exp Optom. 2023; 106;(4): 351–361. [DOI] [PubMed] [Google Scholar]
- 27. Benito MJ, González-García MJ, Tesón M, et al.. Intra- and inter-day variation of cytokines and chemokines in tears of healthy subjects. Exp Eye Res. 2014; 120: 43–49. [DOI] [PubMed] [Google Scholar]
- 28. González N, Iloro I, Soria J, et al.. Human tear peptide/protein profiling study of ocular surface diseases by SPE-MALDI-TOF mass spectrometry analyses - ScienceDirect. 2017; 3: 206–215. [Google Scholar]
- 29. Hagan S, Martin E, Enriquez-de-Salamanca A.. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 2016; 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Thun Und Hohenstein-Blaul N, Funke S, Grus FH.. Tears as a source of biomarkers for ocular and systemic diseases. Exp Eye Res. 2013; 117: 126–137. [DOI] [PubMed] [Google Scholar]
- 31. Barmada A, Shippy SA.. Tear analysis as the next routine body fluid test. Eye (Lond). 2020; 34: 1731–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolkoff P, Nøjgaard JK, Troiano P, Piccoli B.. Eye complaints in the office environment: precorneal tear film integrity influenced by eye blinking efficiency. Occup Environ Med. 2005; 62: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jylhä A, Nättinen J, Aapola U, et al.. Comparison of iTRAQ and SWATH in a clinical study with multiple time points. Clin Proteomics. 2018; 15: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. García N, Tesón M, Enríquez-de-Salamanca A, et al.. Basal values, intra-day and inter-day variations in tear film osmolarity and tear fluorescein clearance. Curr Eye Res. 2014; 39: 673–679. [DOI] [PubMed] [Google Scholar]
- 35. Pena-Verdeal H, Garcia-Resua C, Garcia-Queiruga J, Sabucedo-Villamarin B, Yebra-Pimentel E, Giraldez MJ.. Diurnal variations of tear film osmolarity on the ocular surface. Clin Exp Optom. 2023; 106: 351–361. [DOI] [PubMed] [Google Scholar]
- 36. Bachhuber F, Huss A, Senel M, Tumani H.. Diagnostic biomarkers in tear fluid: from sampling to preanalytical processing. Sci Rep. 2021; 11: 10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dammeier S, Martus P, Klose F, et al.. Combined targeted analysis of metabolites and proteins in tear fluid with regard to clinical applications. Transl Vis Sci Technol. 2018; 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shimazaki J, Goto E, Ono M, Shimmura S, Tsubota K.. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology. 1998; 105: 1485–1488. [DOI] [PubMed] [Google Scholar]
- 39. Ramos-Remus C, Suarez-Almazor M, Russell AS.. Low tear production in patients with diabetes mellitus is not due to Sjögren's syndrome. Clin Exp Rheumatol. 1994; 12: 375–380. [PubMed] [Google Scholar]
- 40. Abusharha AA, Pearce EI.. The effect of low humidity on the human tear film. Cornea. 2013; 32: 429–434. [DOI] [PubMed] [Google Scholar]
- 41. Crutchfield CA, Thomas SN, Sokoll LJ, Chan DW.. Advances in mass spectrometry-based clinical biomarker discovery. Clin Proteomics. 2016; 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meijers WC, van der Velde AR, Muller Kobold AC, et al.. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur J Heart Fail. 2017; 19: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med. 2004; 42: 758–764. [DOI] [PubMed] [Google Scholar]
- 44. Szklarczyk D, Gable AL, Nastou KC, et al.. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021; 49: D605–D612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Henderson NC, Sethi T.. The regulation of inflammation by galectin-3. Immunol Rev. 2009; 230: 160–171. [DOI] [PubMed] [Google Scholar]
- 46. Uchino Y, Mauris J, Woodward AM, et al.. Alteration of galectin-3 in tears of patients with dry eye disease. Am J Ophthalmol. 2015; 159: 1027–1035.e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xiao T, Shoeb M, Siddiqui MS, et al.. Molecular cloning and oxidative modification of human lens ALDH1A1: implication in impaired detoxification of lipid aldehydes. J Toxicol Environ Health A. 2009; 72: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jester JV, Moller-Pedersen T, Huang J, et al.. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. 1999; 112(Pt 5): 613–622. [DOI] [PubMed] [Google Scholar]
- 49. Jester JV. Corneal crystallins and the development of cellular transparency. Semin Cell Dev Biol. 2008; 19: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tabuchi N, Toshida H, Koike D, et al.. Effect of retinol palmitate on corneal and conjunctival mucin gene expression in a rat dry eye model after injury. J Ocul Pharmacol Ther. 2017; 33: 24–33. [DOI] [PubMed] [Google Scholar]
- 51. Acera A, Suárez T, Rodríguez-Agirretxe I, Vecino E, Durán JA.. Changes in tear protein profile in patients with conjunctivochalasis. Cornea. 2011; 30: 42–49. [DOI] [PubMed] [Google Scholar]
- 52. Manicam C, Perumal N, Wasielica-Poslednik J, et al.. Proteomics unravels the regulatory mechanisms in human tears following acute renouncement of contact lens use: a comparison between hard and soft lenses. Sci Rep. 2018; 8: 11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alves M, Novaes P, Morraye MA, A Reinach PS, Rocha EM. Is dry eye an environmental disease? Arq Bras Oftalmol. 2014; 77(3): 193–200. [DOI] [PubMed] [Google Scholar]
- 54. Chlasta-Twardzik E, Górecka-Nitoń A, Nowińska A, Wylęgała E.. The influence of work environment factors on the ocular surface in a one-year follow-up prospective clinical study. Diagnostics (Basel, Switzerland). 2021; 11(3): 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schilde LM, Kösters S, Steinbach S, et al.. Protein variability in cerebrospinal fluid and its possible implications for neurological protein biomarker research. PLoS One. 2018; 13: e0206478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.