Abstract
Background
Reduced plasma vitamin C (vitC) concentrations in human immunodeficiency virus (HIV) may result from abnormal urinary excretion: a renal leak. VitC renal leak indicates underlying nutritional dysregulation independent of diet. We hypothesized that increased renal leak prevalence in HIV would be associated with deficient vitC concentrations.
Methods
We conducted an outpatient cross-sectional study of 96 women (40 HIV [PWH] and 56 without HIV [PWOH]) at the National Institutes of Health and Georgetown University. Renal leak was defined as abnormal urinary vitC excretion at fasting plasma concentrations <43.2µM, 2 SDs below vitC renal threshold in healthy women. To determine the primary outcome of renal leak prevalence, matched urine and plasma samples were collected the morning after overnight fast. Secondary outcomes assessed group differences in mean plasma vitC concentrations and prevalence of vitC deficiency. Exploratory outcomes assessed clinical parameters associated with renal leak. VitC was measured by high-performance liquid chromatography with coulometric electrochemical detection.
Results
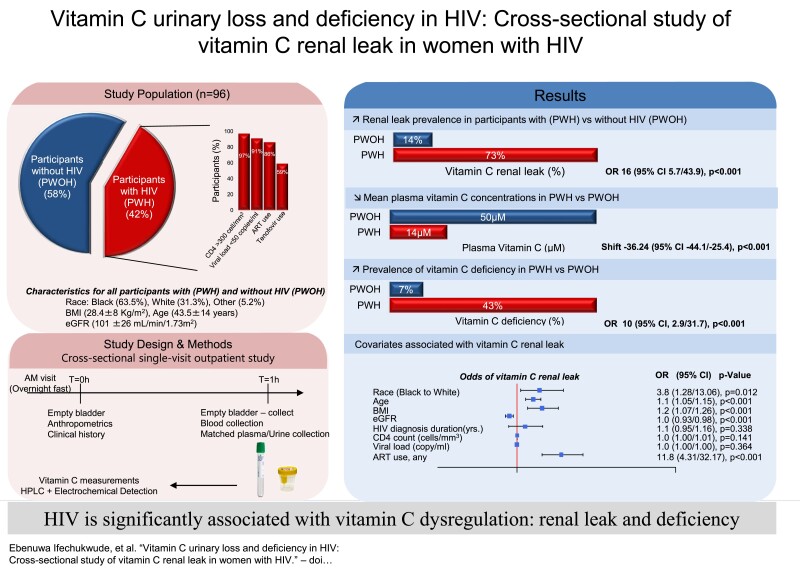
PWH had significantly higher renal leak prevalence (73%vs14%; OR (odds ratio):16; P<.001), lower mean plasma vitC concentrations (14µMvs50µM; P<.001), and higher prevalence of vitC deficiency (43%vs7%; OR:10; P<.001) compared with PWOH, unchanged by adjustments for confounding factors. Significant predictors of renal leak included antiretroviral therapy (ART), Black race, older age, and metabolic comorbidities but not viral load or CD4 count. When compared with other chronic disease cohorts, PWH had the highest prevalence of renal leak and vitC deficiency (P<.001).
Conclusions
High prevalence of vitC renal leak in HIV was associated with vitC deficiency, ART use, and race/ethnicity differences.
Keywords: HIV, antiretroviral therapy, vitamin C, ascorbate, renal leak
High prevalence of abnormal urinary loss of vitamin C or renal leak was associated with deficient vitamin C concentrations in participants with HIV. Findings suggest underlying vitamin C dysregulation in HIV and should prompt measures to monitor and prevent deficiency.
Graphical Abstract
Graphical Abstract.
Vitamin C is an important antioxidant nutrient required for many enzymatic functions in humans [1, 2]. Low vitamin C concentration in chronic diseases, even if nondeficient, may have consequences for disease pathogenesis and wellbeing [3–6]. Cohorts with human immunodeficiency virus (HIV) have been shown to have lower vitamin C concentrations compared with seronegative controls, even with optimal dietary intake [7–11]. Findings were attributed to increased oxidative stress and antioxidant utilization in HIV [7, 9]. Beyond utilization, low vitamin C concentrations may also result from impaired renal reabsorption of vitamin C, resulting in abnormal or increased urinary loss: a renal leak [12–14]. In healthy individuals, at plasma vitamin C concentrations 2 SDs below sex-specific renal thresholds (the minimal elimination threshold [MET]), vitamin C is fully reabsorbed in the proximal renal tubules by sodium-dependent vitamin C transporter 1 (SVCT1), and no vitamin C is excreted into urine. A renal leak occurs when there is inappropriate urinary excretion at low plasma concentrations below the MET [12]. Renal leak indicates underlying nutritional pathophysiology that is independent of dietary intake [12]. For a chronic disease like HIV with accelerated antioxidant utilization, renal leak could increase the risk of vitamin C deficiency.
Renal leak assessment in chronic disease serves 2 key functions. First, renal leak characterizes the scope and contribution of abnormal vitamin C urinary loss to low vitamin C concentrations [12, 15]. Second, because renal leak incorporates both fasting plasma and urine measurements using a specific vitamin C assay, renal leak outcomes are an effective means of investigating disease-specific factors that contribute to vitamin C nutritional pathophysiology and disease pathogenesis [12, 15]. In recent studies, we showed that participants with diabetes had 5-fold higher odds of renal leak compared with nondiabetic controls [12]. There was an even higher, 16-fold, odds of renal leak observed in Fabry disease, a rare chronic disease associated with renal tubular dysfunction, indicating that renal leak may reflect underlying renal tubular dysfunction [15]. Like Fabry disease and diabetes, HIV is a chronic disease with complex multifactorial pathophysiology that includes chronic inflammation, increased antioxidant requirements, renal tubular dysfunction, and low plasma vitamin C concentrations [12, 15–21]. However, in populations with HIV, the relationship between renal leak, low vitamin C concentrations, and vitamin C deficiency is unknown. An understanding of renal leak prevalence and the contributory factors could provide foundational information in uncovering disease-related vitamin C pathophysiology in populations with HIV.
This study had 3 main objectives. The first was to determine the relationship between renal leak prevalence, vitamin C plasma concentrations, and vitamin C deficiency in female participants with HIV (PWH) and without HIV (PWOH). The second was to investigate the demographic and clinical factors associated with vitamin C renal leak. The third was to understand how renal leak outcomes in HIV vary from previously studied cohorts with diabetes and Fabry disease [12, 15]. We hypothesized that PWH would have a higher prevalence of vitamin C renal leak, reduced plasma vitamin C concentrations, and higher prevalence of vitamin C deficiency. We tested our hypotheses using sex-specific renal leak criteria and a sensitive/specific vitamin C assay.
METHODS
Study Design & Setting
This was an outpatient cross-sectional cohort study. There were 2 study locations: the National Institutes of Health Clinical Center's metabolic unit, Bethesda, Maryland (ClinicalTrials.gov NCT00071526), and Georgetown University, Washington, DC (ClinicalTrials.gov NCT00000797). The research protocols were approved by the institutional review boards (IRBs) of both institutions.
Participants
Recruitment
Participants were recruited from the Washington, DC, Women's Interagency HIV Study (WIHS), now combined with the Multicenter AIDS Cohort Study (MACS) (DC MACS/WIHS Combined Cohort Study [DC MWCCS]) and from the Washington, DC, area community. In accordance with the IRB-approved protocols in both institutions, PWH were recruited from MWCCS, while PWOH were recruited from both MWCCS and the community. Recruitment was conducted using recruitment flyers and advertisements displayed in the community, social media, and online registries.
Inclusion/Exclusion Criteria
Among the MWCCS participants, there were no additional inclusion/exclusion criteria beyond the MWCCS requirements [22]. Among participants recruited from the community, inclusion criteria comprised participants aged 18–65 years. Exclusion criteria included acute or chronic illness, alcohol abuse or tobacco use, and use of chronic medications. Exclusion criteria were chosen to avoid confounding the study findings, given the reported association of these conditions with vitamin C dysregulation.
Assessment of Vitamin C Renal Leak
Renal leak was assessed using matched urine and plasma vitamin C measurements, as previously described [12, 15]. Briefly, approximately 2 weeks prior to study sampling, participants were instructed to avoid vitamin C supplements. Participants fasted overnight and arrived at the research center the morning of the study visit. Participants were then instructed to empty their bladder. After a 1-hour interval, urine and blood samples were obtained for vitamin C measurements, chemistry, and related studies. See Supplementary Methods for additional details on sampling approaches, measures to minimize bias, dietary considerations, and assay measurements.
Study Outcomes
The primary outcome was difference in vitamin C renal leak prevalence between PWH and PWOH. Vitamin C renal leak was defined as the presence of urinary fasting plasma concentrations below 43.2 µM, the MET in women [12]. Secondary outcomes were between-group differences in mean plasma vitamin C concentrations and prevalence of vitamin C deficiency, defined as plasma vitamin C concentrations below 11 mol/L [23]. Exploratory outcomes evaluated associations between clinical variables and renal leak outcome in all participants.
Study Size Considerations
As noted in recent renal leak publications, there were no actual, extant data upon which to base a sample size calculation [12, 15]. Sample size calculations of a related single-site study were based on scant anecdotal evidence indicating substantial differences in 24-hour urinary vitamin C excretion between nondiabetic controls and the diabetes cohort. In the combined cohort of participants from the National Institutes of Health and the MWCCS cohort, sample size was effectively determined by logistical constraints and participant willingness, rather than any a priori sample size calculations.
Statistical Methods
In this cross-sectional study, descriptive statistics were calculated for baseline characteristics of study participants. Counts and proportions were used for categorical variables, accompanied by Pearson's chi-square test or Fisher's exact tests, as indicated by expected cell counts, while means and SDs were used for continuous variables, accompanied by Welch's t test or Wilcoxon-Mann-Whitney tests, as indicated by approximate normality. The primary outcome of vitamin C renal leak prevalence in PWH versus PWOH was assessed using group-specific proportions accompanied by a series of unadjusted and covariate-adjusted odds ratios (ORs) estimated via logistic regression [24]. The secondary outcome evaluating group differences (estimated shifts) in mean plasma vitamin C concentrations was assessed using linear regression analyses. Exploratory analyses reported multiplicity-adjusted P values for groups of related variables’ respective associations with the primary endpoint of renal leak via Wald-type tests for nonzero logistic regression coefficients . See Supplementary Methods for additional description of statistical methods.
RESULTS
Baseline Characteristics of Study Cohorts
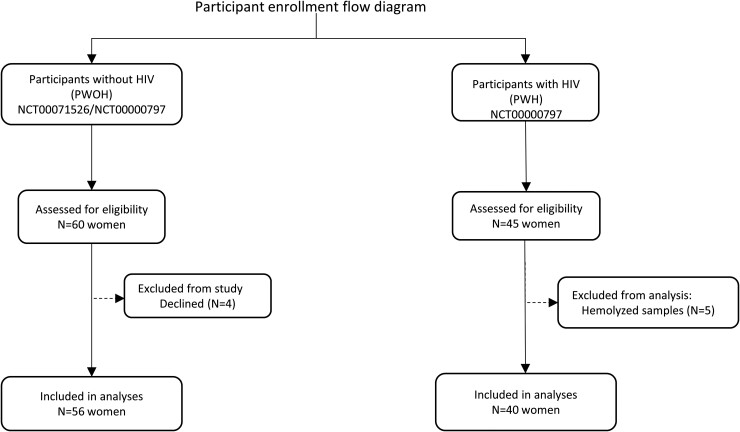
A total of 105 female volunteers were assessed for eligibility in both study groups. Of the 60 PWOH assessed, 4 were unable to participate (Figure 1). Among PWH, all 45 who were assessed were studied; however, data from 5 participants were excluded from the primary analysis due to sample hemolysis (Figure 1). A total of 96 participants were included in the primary analyses, 40 PWH and 56 PWOH (Figure 1). The baseline characteristics of participants in both groups are shown in Table 1. The racial/ethnic composition of both groups was different, with significantly more Black/non-Hispanic PWH versus PWOH (84% vs 48%) (Table 1). The mean age was higher in PWH (53 vs 37 years; P < .05), whereas body mass index (BMI) was not significantly different (P > .05) (Table 1). Among the baseline clinical parameters, compared with PWOH, PWH had lower estimated glomerular filtration rate (eGFR) (84 vs 111 mL/min/1.73 m2; P < .05) and a higher prevalence of hypertension (70% vs 27%; P < .05) and tobacco use (54% vs 4%; P < .05) (Table 1).
Figure 1.
Enrollment and patient flow of study participants with HIV (PWH) and without HIV (PWOH). Abbreviation: HIV, human immunodeficiency virus.
Table 1.
Baseline Characteristics of Female Participants With HIV and Those Without HIV
| PWOH (n = 56) | PWH (n = 40) | |
|---|---|---|
| Demographics & Anthropometrics: mean (SD) or n (%) | ||
| Race/ethnicity,a n (%) | ||
| Black/non-Hispanic | 27 (48) | 34 (84) |
| White/non-Hispanic | 25 (45) | 5 (13) |
| Other | 4 (7) | 1 (3) |
| Age,b y | 37 (14) | 53 (7) |
| BMI, kg/m2 | 29 (8) | 28 (6) |
| Systolic blood pressure, mmHg | 117 (13) | 123 (21) |
| Diastolic blood pressure,b mmHg | 70 (9) | 77 (12) |
| Clinical laboratory: mean (SD) | ||
| Fasting glucose,b mg/dL | 88 (11) | 86 (36) |
| Hemoglobin A1c,b % | 5.3 (0.4) | 6.2 (2) |
| eGFR,c mL/min/1.73 m2 | 111 (21) | 84 (24) |
| Chronic medical conditions: n/total (%) | ||
| Hypertensionb,d | 15/56 (27) | 28/40 (70) |
| Hyperlipidemiae | 9/48 (19) | 1/12 (8) |
| Diabetesf | 7/56 (13) | 5/40 (13) |
| Metabolic syndromeg | 1/54 (2) | 2/25 (8) |
| Obesityh | 20/56 (36) | 15/40 (38) |
| Chronic kidney diseasei | 1/56 (2) | 4/33 (12) |
| Elevated liver enzymesj | 2/56 (2) | 4/33 (12) |
| Tobacco use | 2/56 (4) | 19/35 (54) |
| HIV parameters: mean (SD) or n/total (%) | ||
| Duration of HIV diagnosis, y | … | 17 (7) |
| CD4 count, cells/mm3 | … | 739 (282) |
| CD4 count >300 cells/mm3 | … | 32/37 (97) |
| Viral load, copies/mL | … | 494 (1863) |
| Viral load <50 copies/mL | … | 30/37 (91) |
| ART use, any | … | 32/37 (86) |
| ART use, by class | ||
| Nucleoside RTI | … | 32/32 (100) |
| Tenofovir | … | 18/32 (56) |
| TDF | … | 14/32 (44) |
| TAF | … | 4/32 (13) |
| Non-nucleoside RTI | … | 11/32 (34) |
| Protease inhibitors | … | 13/32 (41) |
| Integrase inhibitor | … | 14/32 (44) |
| Entry inhibitor | … | 1/32 (3) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CD4, cluster of differentiation number 4 estimated blood cell count; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; PWH, participants with HIV; PWOH, participants without HIV; RTI, reverse transcriptase inhibitor; SBP, systolic blood pressure; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Racial categories were based on National Institutes of Health reporting guidelines on racial and ethnic categories (NOT-OD-15-089). “Other” comprises Asian, Hispanic and Latino, and multiethnic subjects.
Variables that are statistically different between groups (P < .05).
The eGFR was calculated using the Modification of Diet in Renal Disease v4 (MDRD4) equation: [186 × (creatinine/88.4) − 1.154 × (age) − 0.203 × (0.742 if female) × (1.210 if Black)].
Any indication of hypertension: SBP ≥130 mmHg or DBP ≥80 mmHg, self-report, or use of antihypertensive medications.
Defined as high-density lipoprotein (HDL) <40 mg/dL or triglycerides >150 mg/dL or low-density lipoprotein (LDL) >130 mg/dL.
Diabetes: any indication of diabetes based on clinical history, medication use, or laboratory values (hemoglobin A1c >6.5% or fasting glucose >126 mg/dL).
Metabolic syndrome: based on American Association of Clinical Endocrinology 2003 criteria.
Obesity: BMI >30 kg/m2.
Chronic kidney disease: eGFR <60 mL/min/1.73 m2.
Elevated liver enzymes: aspartate aminotransferase ≥35 units/L or alanine aminotransferase ≥36 units/L.
Vitamin C Renal Leak Prevalence in Participants With HIV and Participants Without HIV
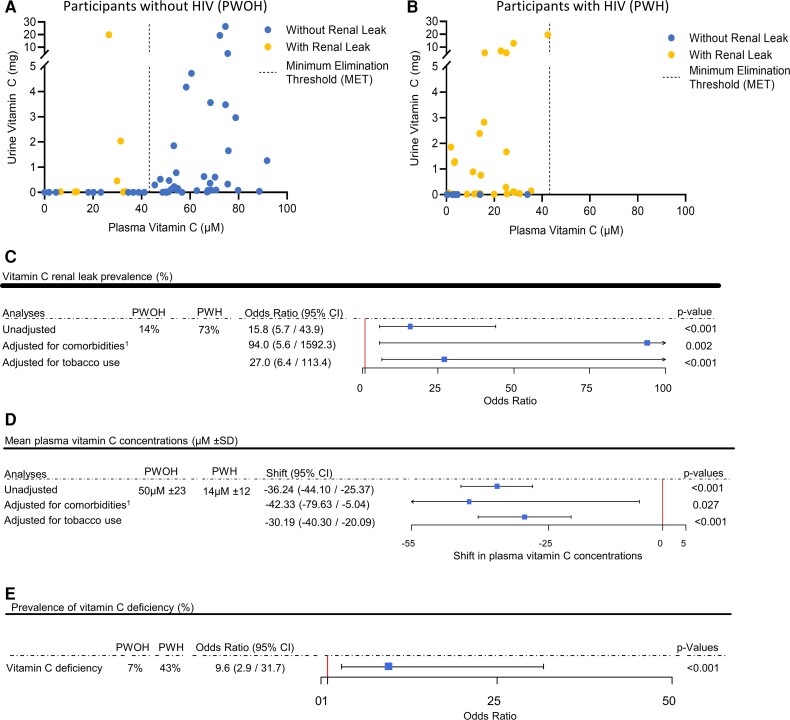
Almost all PWOH had no detectable vitamin C in urine at plasma concentrations below the MET (Figure 2A). In contrast, almost all PWH had detectable vitamin C in urine at plasma vitamin C concentrations below the MET: a renal leak (Figure 2B). Renal leak prevalence was significantly higher in PWH versus PWOH (73% vs 14%; unadjusted OR: 15.8; 95% confidence interval [CI]: 5.7, 43.9; P < .001) (Figure 2C). The odds of renal leak in the PWH versus PWOH cohort remained significant when adjusted for medical comorbidities (adjusted OR: 94; 95% CI: 5.6, 1592.3; P = .002) (Figure 2C, Table 1) and active tobacco use (adjusted OR: 27; 95% CI: 6.4, 113.4; P < .001) (Figure 2C, Table 1). Change-in-effect analyses adjusting for group differences in baseline demographic, anthropometric, and clinical parameters (Table 1) did not change the significant association between HIV-positive status and renal leak (Supplementary Table 1).
Figure 2.
Vitamin C renal leak prevalence, mean plasma concentrations, and prevalence of deficiency in participants with HIV (PWH) and without HIV (PWOH). A, B, Urinary vitamin C excretion as a function of plasma vitamin C concentrations in PWOH (A) and PWH (B). Renal leak symbol (yellow circles), including those that appear to be on the x-axis, indicate vitamin C amounts above 0.01 mg. C, Vitamin C renal leak prevalence with and without adjustments for medical comorbidities and tobacco use. Squares indicate odds ratios, with horizontal lines indicating 95% CIs. D, Mean plasma vitamin C concentrations, with and without adjustment for medical comorbidities and tobacco use. Squares indicate estimated shift in mean plasma vitamin C, with horizontal lines indicating 95% CIs. E, Prevalence of vitamin C deficiency. The square indicates the odds ratio, with the horizontal line indicating 95% CI. P values are shown for estimated shift. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus. 1Comorbidities include chronic medical conditions (Table 1, see Supplementary Methods).
Plasma Vitamin C Concentrations and Prevalence of Vitamin C Deficiency in Participants With and Without HIV
The mean plasma vitamin C concentration was significantly lower in PWH versus PWOH (mean ± SD: 14 ± 12 µM vs 50 ± 23 µM; P < .001) (Figure 2D), even with adjustments for higher prevalence of medical comorbidities and tobacco use in PWH versus PWOH (P = .027 and P < .001, respectively). Change-in-effect analyses adjusting for group differences in baseline demographic, anthropometric, and clinical variables did not change the significant association between HIV-positive status and low plasma vitamin C concentrations (Supplementary Table 2). The prevalence of vitamin C deficiency was significantly higher in PWH versus PWOH (43% vs 7%; OR: 9.6; 95% CI: 2.9, 31.7; P < .001) (Figure 2E).
Variables Associated With Vitamin C Renal Leak in All Participants
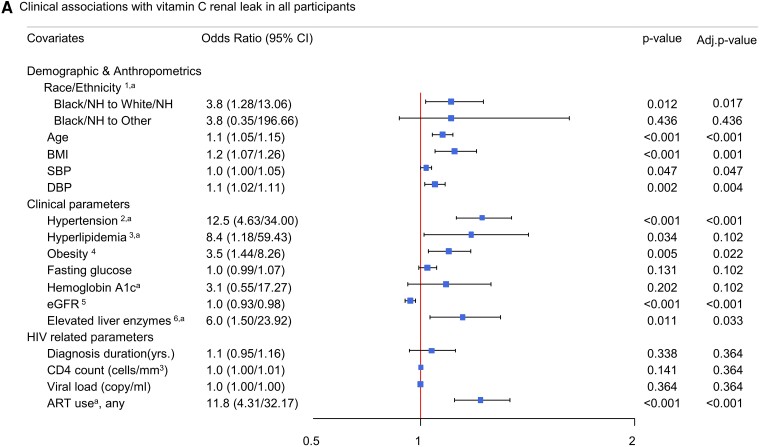
Exploratory analyses were performed to evaluate demographic and clinical variables associated with vitamin C renal leak (Figure 3), with category-based multiplicity adjustments (see Methods). Among the demographic and anthropometric parameters, Black/non-Hispanic participants had significantly higher odds of having a renal leak compared with White/non-Hispanic participants (P = .013) (Figure 3). Older age and higher BMI were also associated with increased renal leak (P < .001 and P = .001, respectively) (Figure 3). Among the clinical variables assessed via single-predictor regression, renal leak was associated with higher systolic blood pressure (P = .047), higher diastolic blood pressure (P = .004), hypertension (P < .001), obesity (P = .022), lower eGFR (P < .001), and elevated liver enzymes (P = .033). Among the HIV-related variables, renal leak was associated with antiretroviral therapy (ART) use (P < .001) but not duration of HIV diagnoses, CD4 count, or viral load (P = .364 for all).
Figure 3.
Demographic and clinical variables and their predictive association with vitamin C renal leak in all participants. Squares indicate odds ratios, with horizontal lines indicating 95% CIs. Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CD4, cluster of differentiation number 4; CI, confidence interval; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; NH, non-Hispanic; RTI, reverse transcriptase inhibitor; SBP, systolic blood pressure. 1Black and White groups were composed of non-Hispanic participants. “Other” comprised Asians, those of Hispanic/Latino ethnicity, and multiracial participants. 2Hypertension was defined as participants with any indication of hypertension: SBP ≥130 mmHg or DBP ≥80 mmHg, self-report, or use of antihypertensive medications. 3Hyperlipidemia was defined as participants with either high-density lipoprotein (HDL) <40 mg/dL or triglycerides >150 mg/dL or low-density lipoprotein (LDL) >130 mg/dL. 4Obesity was defined as participants with a BMI >30 kg/m2. 5The eGFR for all groups was calculated using the Modification of Diet in Renal Disease v4 (MDRD4). 6Elevated liver enzymes were defined as aspartate transaminase (AST) ≥35 units/L or alanine transaminase (ALT) ≥56 units/L. aValues were adjusted (divided by 10) to fit the scale of the forest plot.
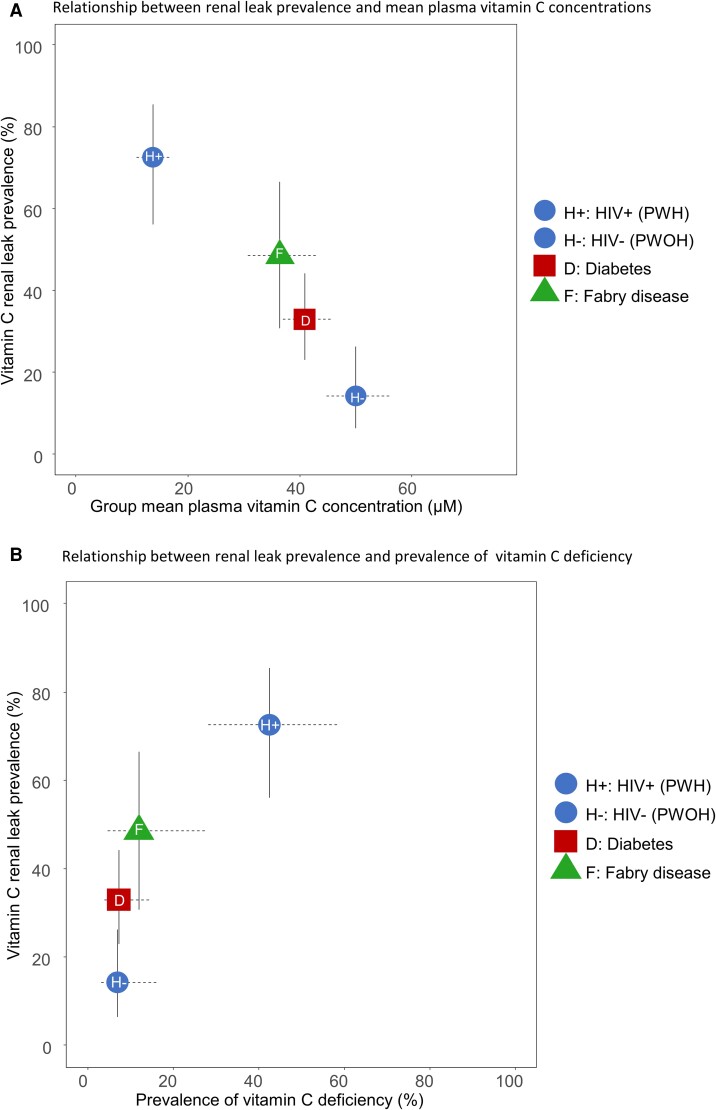
Comparative Renal Leak Outcomes in Participants With and Without HIV and Previously Studied Cohorts With Diabetes and Fabry Disease
Across the 4 groups, a higher renal leak prevalence was associated with lower mean plasma vitamin C concentrations (P < .001) (Figure 4A) and a higher prevalence of vitamin C deficiency (P < .001) (Figure 4B). In this continuum of chronic diseases studied, PWH had the highest renal leak prevalence, lowest plasma vitamin C concentrations, and highest prevalence of vitamin C deficiency (Figure 4).
Figure 4.
Comparative renal leak outcomes in PWH, PWOH, and previously studied cohorts with diabetes and Fabry disease. Relationship between renal leak prevalence and mean plasma vitamin C concentrations (A) and the prevalence of vitamin C deficiency (B) in the 4 groups. Abbreviations: HIV, human immunodeficiency virus; PWH, participants with HIV; PWOH, participants without HIV.
DISCUSSION
We investigated the prevalence and clinical characteristics of vitamin C renal leak in PWH and PWOH, with renal leak indicating the abnormal urinary excretion of vitamin C when plasma concentrations are at least 2 SDs below the renal threshold [12]. Our findings showed a significantly higher prevalence of vitamin C renal leak and vitamin C deficiency in PWH compared with PWOH. The HIV–renal leak association remained significant following statistical adjustments for potentially confounding factors: medical comorbidities, tobacco use, and demographic differences. Renal leak was associated with ART use, medical comorbidities, and demographic parameters, but not viral load, CD4 count, or duration of HIV diagnosis. This study is the first description of vitamin C renal and nutritional dysregulation in HIV and suggests an increased risk of renal leak–mediated vitamin C deficiency in PWH, independent of dietary intake.
To broaden the context of our findings, we analyzed renal leak outcomes in PWH and PWOH, compared with diabetes and Fabry disease cohorts who were previously studied using the same methodology for renal leak assessment and vitamin C measurements (see Methods) [12, 15]. Across all groups, there was a relationship between a higher group prevalence of renal leak, low vitamin C concentrations, and a high prevalence of vitamin C deficiency, with the highest degree of vitamin C dysregulation and deficiency in PWH (P < .001) (Figure 4). Other investigators reported higher vitamin C values than those reported here [7–11]. Prior findings may be explained by nonspecific vitamin C measurements, lack of adjustment for variations in sex and medical comorbidities, and collection of samples from nonfasted subjects. Due to postprandial fluctuations in vitamin C measurements, the use of nonfasting measurements may artifactually inflate plasma vitamin C concentrations and underestimate the true prevalence of vitamin C deficiency [13, 14, 25].
Among the HIV-related parameters, the use of ART was significantly associated with renal leak (Figure 3). Of the 86% of PWH on ART, 100% were on a nucleoside reverse transcriptase inhibitor (NRTI), with smaller proportions on a non-nucleoside reverse transcriptase inhibitor (NNRTI) (34%), protease inhibitor (41%), and integrase inhibitor (44%) and 1 participant on an entry inhibitor (Table 1). We found no association with HIV disease severity biomarkers CD4 count or viral load, suggesting that immune suppression did not contribute to the study findings. However, given that 97% had a CD4 count greater than 300 cells/mm3 and 91% had a suppressed viral load less than 50 copies/mL, findings should be viewed in the context of a generally well-treated cohort.
It is uncertain whether ART use alone accounts for the much higher renal leak prevalence in PWH compared with the other chronic diseases (Figure 4). Nevertheless, there are several potential mechanisms in which ART may directly or indirectly contribute to increased odds of renal leak. The first possibility is that vitamin C renal leak is secondary to ART-induced renal tubular dysfunction and renal impairment [15, 26]. Renal leak describes an aberrant process in which proximal renal tubular reabsorption of vitamin C is dysregulated, resulting in abnormal excretion into urine [12]. Antiretroviral therapies, including NRTIs like tenofovir, are associated with renal tubular dysfunction and renal failure [26, 27]. In our study cohort, 56% of PWH on ART were taking tenofovir (Table 1), but our study was inadequately powered for subgroup analysis evaluating the effects of individual ART drugs and/or classes on renal leak outcomes. In our study of participants with Fabry disease, a rare chronic disease associated with renal tubular dysfunction, we found a significantly high prevalence of vitamin C renal leak [15]. While renal tubular function was not specifically assessed in this HIV study, we found a significant association between reduced renal function (eGFR) and vitamin C renal leak. The second possibility is that ART may have a direct drug effect that is specific to vitamin C transporters in the kidney, although, to date, this has not been tested experimentally. The third possibility is that ART-associated renal leak may be indirectly related to ART-induced metabolic complications in HIV. Antiretroviral therapy has been associated with HIV lipodystrophy, a severe form of metabolic syndrome, with an estimated 40–50% prevalence in individuals with HIV characterized by fat redistribution and cardiometabolic risk [28, 29]. Like other obesity-related conditions, HIV lipodystrophy is associated with increased inflammation and oxidative stress [21, 30, 31], which may potentially contribute to vitamin C dysregulation. The relationship between altered metabolism and vitamin C dysregulation is supported by our study findings that showed significant associations between renal leak and metabolic comorbidities (obesity, hypertension, hyperlipidemia), as well as other studies showing low plasma vitamin C concentrations in obesity and diabetes [12, 32]. Mechanistic and longitudinal clinical studies will be needed to investigate relationships between ART, renal leak, and vitamin C deficiency in PWH, but also PWOH on ART for either pre-exposure prophylaxis in HIV prevention, or treatment of chronic viral infections such as hepatitis. Independent of ART, longitudinal studies are also needed to investigate the clinical implications of renal leak–mediated vitamin C deficiency in PWH with non-scorbutic plasma concentrations, particularly with comorbidities associated with low plasma vitamin C concentrations, including obesity-related conditions such as diabetes and HIV lipodystrophy, neuropsychiatric health outcomes, and low bone density [12, 32–37].
There were several notable demographic associations with renal leak, including older age and Black/non-Hispanic race/ethnicity. While the association with older age is consistent with findings in diabetes, the association with race/ethnicity has not been previously reported and may have several explanations [12]. First, findings may be skewed by the comparatively higher enrollment of Black, non-Hispanic PWH as well as the combined study cohort. Independent of statistical considerations, a second explanation is that HIV- and/or ART-induced vitamin C dysregulation exerts a disproportionately more severe nutritional and metabolic pathophysiology in Black individuals compared with other racial groups. Metabolic phenotypes display race-based differences, with Black individuals having comparatively higher rates of insulin resistance despite lower visceral/hepatic fat and triglyceride levels [38, 39]. These factors may be exacerbated by socioeconomic factors such as food insecurity, low income, and poverty [32]. Comprehensive longitudinal studies will be needed to investigate the disparate metabolic and nutritional outcomes across race/ethnic groups and the confluence of clinical, nutritional, and socioeconomic contributory factors.
There are several strengths of the study presented here. One is the foundational basis of the study design: a sex-specific vitamin C renal leak criteria based on vitamin C depletion-repletion and pharmacokinetics studies coupled with a highly sensitive and specific vitamin C measurement. Second, the study methodology for assessing vitamin C renal leak status was specifically designed to minimize bias. In addition, PWH were recruited from the DC MWCCS. The study of this unique cohort is a strength as it includes many participants from underserved, vulnerable, and high-risk communities. Study of the MWCCS cohort may also be a limitation as findings in this unique cohort may not be generalizable to all PWH, despite adjustments for potential confounding factors. Additionally, our limited data and sample size precluded a more detailed analyses of how race/ethnicity and socioeconomic factors (poverty, income, and food insecurity) potentially contribute to vitamin C renal leak and low plasma vitamin C concentrations. Last, while the cross-sectional study design provided is a necessary first step in understanding prevalence and clinical associations, findings are a snapshot in time and do not include dynamic or longitudinal outcomes, nor is causation determined.
Taken together, the data here indicate that HIV is associated with a significant degree of vitamin C dysregulation and deficiency. While mechanisms and long-term clinical implications can be addressed in future studies, current findings suggest a need for proactive preventative measures to prevent deficiency in all PWH, such as monitoring vitamin C concentrations, optimizing dietary intake, and supplementation when necessary. Our findings emphasize the need for dietary intake guidelines to account for altered vitamin C requirements in HIV and other chronic diseases [40].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Ifechukwude Ebenuwa, Molecular and Clinical Nutrition Section, Digestive Diseases Branch, Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Pierre-Christian Violet, Molecular and Clinical Nutrition Section, Digestive Diseases Branch, Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Kate Michel, Department of Medicine, Division of Infectious Disease, Georgetown University School of Medicine, Washington D.C., USA.
Sebastian J Padayatty, Molecular and Clinical Nutrition Section, Digestive Diseases Branch, Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Yaohui Wang, Molecular and Clinical Nutrition Section, Digestive Diseases Branch, Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Hongbin Tu, Molecular and Clinical Nutrition Section, Digestive Diseases Branch, Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Kenneth J Wilkins, Office of Clinical Research Support, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Seble Kassaye, Department of Medicine, Division of Infectious Disease, Georgetown University School of Medicine, Washington D.C., USA.
Mark Levine, Molecular and Clinical Nutrition Section, Digestive Diseases Branch, Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Notes
Author Contributions. Conceptualization: M. L., I. E., P.-C. V., S. J. P., S. K. Methodology: M. L., I. E., P.-C. V., S. J. P, S. K. Investigation: M. L., I. E., P.-C. V., S. J. P., H. T., Y. W., S. K., K. M. Resources: M. L., S. K. Data Curation: P.-C. V., I. E., K. J. W., and M. L. Writing—original draft: I. E., P.-C. V., K. J. W., M. L. Writing—review and editing: I. E., P.-C. V., K. J. W., K. M., S. J. P., S. K., and M. L. Funding acquisition: M. L., S. K. Supervision: M. L., I. E., S. K. Formal analysis: P.-C. V., I. E., K. J. W., and M. L. All co-authors have read and approved the final version.
Acknowledgments. The authors are grateful to subjects who participated in these studies; to the Intramural Research Programs, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH).
Disclaimer. The funders had no input into study outcomes.
Financial support. This work was supported by Intramural Research Program at the NIDDK at the NIH (Z01-DK053211-15 to M. L.). Data collection in the WIHS, now MWCCS, is funded through extramural NIH funding (National Institute of Allergy and Infectious Diseases [NIAID]; U01AI034994 and National Heart, Lung, and Blood Institute (NHLBI; U01-HL146205). The MWCCS is funded primarily by the NHLBI, with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institute on Aging (NIA), National Institute of Dental & Craniofacial Research (NIDCR), NIAID, National Institute of Neurological Disorders and Stroke (NINDS), National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), National Institute of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), NIDDK, National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the NIH, Office of AIDS Research (OAR). DC MWCCS data collection is also supported by the National Center for Advancing Translational Sciences (NCATS) of the NIH under award number KL2TR001432 and UL1-TR001409.
Data availability
Data described in the manuscript will be made available upon request pending application and approval.
References
- 1. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 1989; 86:6377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frei B, Stocker R, England L, Ames BN. Ascorbate: the most effective antioxidant in human blood plasma. Adv Exp Med Biol 1990; 264:155–63. [DOI] [PubMed] [Google Scholar]
- 3. Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis 2016; 22:463–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA 1999; 281:1415–23. [DOI] [PubMed] [Google Scholar]
- 5. Padayatty SJ, Katz A, Wang Y, et al. . Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 2003; 22:18–35. [DOI] [PubMed] [Google Scholar]
- 6. Tu H, Li H, Wang Y, Niyyati M, Leshin J, Levine M. Low red blood cell vitamin C concentrations induce red blood cell fragility: a link to diabetes via glucose, glucose transporters, and dehydroascorbic acid. EBioMedicine 2015; 2:1735–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allard JP, Aghdassi E, Chau J, Salit I, Walmsley S. Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. Am J Clin Nutr 1998; 67:143–7. [DOI] [PubMed] [Google Scholar]
- 8. Stephensen CB, Marquis GS, Jacob RA, Kruzich LA, Douglas SD, Wilson CM. Vitamins C and E in adolescents and young adults with HIV infection. Am J Clin Nutr 2006; 83:870–9. [DOI] [PubMed] [Google Scholar]
- 9. Ngondi JL, Oben J, Forkah DM, Etame LH, Mbanya D. The effect of different combination therapies on oxidative stress markers in HIV infected patients in Cameroon. AIDS Res Ther 2006; 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwenger KJP, Arendt BM, Smieja M, Ma DWL, Smaill F, Allard JP. Relationships between atherosclerosis and plasma antioxidant micronutrients or red blood cell polyunsaturated fatty acids in people living with HIV. Nutrients 2019; 11:1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trepanier LA, Yoder AR, Bajad S, Beckwith MD, Bellehumeur JL, Graziano FM. Plasma ascorbate deficiency is associated with impaired reduction of sulfamethoxazole-nitroso in HIV infection. J Acquir Immune Defic Syndr 2004; 36:1041–50. [DOI] [PubMed] [Google Scholar]
- 12. Ebenuwa I, Violet PC, Padayatty S, et al. . Abnormal urinary loss of vitamin C in diabetes: prevalence and clinical characteristics of a vitamin C renal leak. Am J Clin Nutr 2022; 116:274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine M, Conry-Cantilena C, Wang Y, et al. . Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 1996; 93:3704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA 2001; 98:9842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebenuwa IV, Voilet PC, Padayatty SJ, Wang Y, et al. . Vitamin C urinary loss in Fabry disease: clinical and genomic characteristics of vitamin C renal leak. J Nutr 2022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore DF, Ye F, Brennan ML, et al. . Ascorbate decreases Fabry cerebral hyperperfusion suggesting a reactive oxygen species abnormality: an arterial spin tagging study. J Magn Reson Imaging 2004; 20:674–83. [DOI] [PubMed] [Google Scholar]
- 17. Sundaram RK, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R, Shanmugasundaram KR. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin Sci (Lond) 1996; 90:255–60. [DOI] [PubMed] [Google Scholar]
- 18. Maxwell SR, Thomason H, Sandler D, et al. . Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1997; 27:484–90. [DOI] [PubMed] [Google Scholar]
- 19. Israel N, Gougerot-Pocidalo MA. Oxidative stress in human immunodeficiency virus infection. Cell Mol Life Sci 1997; 53(11–12):864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Treitinger A, Spada C, Verdi JC, et al. . Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur J Clin Invest 2000; 30:454–9. [DOI] [PubMed] [Google Scholar]
- 21. Morimoto HK, Simao AN, de Almeida ER, et al. . Role of metabolic syndrome and antiretroviral therapy in adiponectin levels and oxidative stress in HIV-1 infected patients. Nutrition 2014; 30(11–12):1324–30. [DOI] [PubMed] [Google Scholar]
- 22. Bacon MC, von Wyl V, Alden C, et al. . The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immun 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crook J, Horgas A, Yoon SJ, Grundmann O, Johnson-Mallard V. Insufficient vitamin C levels among adults in the United States: results from the NHANES surveys, 2003–2006. Nutrients 2021; 13:3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall GH, Round AP. Bayes theorem for the clinician. West Engl Med J 1990; 105:106–9. [PMC free article] [PubMed] [Google Scholar]
- 25. Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR, Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res 1997; 14:1133–9. [DOI] [PubMed] [Google Scholar]
- 26. Gara N, Zhao X, Collins MT, et al. . Renal tubular dysfunction during long-term Adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther 2012; 35:1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS 2008; 22:99–103. [DOI] [PubMed] [Google Scholar]
- 28. Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005; 352:48–62. [DOI] [PubMed] [Google Scholar]
- 29. Hadigan C, Meigs JB, Wilson PW, et al. . Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis 2003; 36:909–16. [DOI] [PubMed] [Google Scholar]
- 30. González-Domenech CM, Plaza-Andrades IJ, Garrido-Sanchez L, Queipo-Ortuño MI. Synergic effect of metabolic syndrome and lipodystrophy on oxidative stress and inflammation process in treated HIV-patients. Enferm Infecc Microbiol Clin (Engl Ed) 2022; 40:310–6. [DOI] [PubMed] [Google Scholar]
- 31. Lagathu C, Eustace B, Prot M, et al. . Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther 2007; 12:489–500. [PubMed] [Google Scholar]
- 32. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 2009; 90:1252–63. [DOI] [PubMed] [Google Scholar]
- 33. McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. . Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012; 78:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plevin D, Galletly C. The neuropsychiatric effects of vitamin C deficiency: a systematic review. BMC Psychiatry 2020; 20:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y, Liu XJ, Robitaille L, Eintracht S, MacNamara E, Hoffer LJ. Effects of vitamin C and vitamin D administration on mood and distress in acutely hospitalized patients. Am J Clin Nutr 2013; 98:705–11. [DOI] [PubMed] [Google Scholar]
- 36. Mangano KM, Noel SE, Dawson-Hughes B, Tucker KL. Sufficient plasma vitamin C is related to greater bone mineral density among postmenopausal women from the Boston Puerto Rican Health Study. J Nutr 2021; 151:3764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol 2001; 154:427–33. [DOI] [PubMed] [Google Scholar]
- 38. Chung ST, Cravalho CKL, Meyers AG, et al. . Triglyceride paradox is related to lipoprotein size, visceral adiposity and stearoyl-CoA desaturase activity in black versus white women. Circ Res 2020; 126:94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sumner AE, Micklesfield LK, Ricks M, et al. . Waist circumference, BMI, and visceral adipose tissue in white women and women of African descent. Obesity (Silver Spring) 2011; 19:671–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Institute of Medicine Panel on Dietary A, Related C . Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press, 2000. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request pending application and approval.