Abstract
Background
Antimicrobial resistance (AMR) is undermining modern medicine, a problem compounded by bacterial adaptation to antibiotic pressures. Phages are viruses that infect bacteria. Their diversity and evolvability offer the prospect of their use as a therapeutic solution. Reported are outcomes of customized phage therapy for patients with difficult-to-treat antimicrobial resistant infections.
Methods
We retrospectively assessed 12 cases of customized phage therapy from a phage production center. Phages were screened, purified, sequenced, characterized, and Food and Drug Administration–approved via the IND (investigational new drug) compassionate-care route. Outcomes were assessed as favorable or unfavorable by microbiologic and clinical standards. Infections were device-related or systemic. Other experiences such as time to treatment, antibiotic synergy, and immune responses were recorded.
Results
Fifty requests for phage therapy were received. Customized phages were generated for 12 patients. After treatment, 42% (5/12) of cases showed bacterial eradication and 58% (7/12) showed clinical improvement, with two-thirds of all cases (66%) showing favorable responses. No major adverse reactions were observed. Antibiotic-phage synergy in vitro was observed in most cases. Immunological neutralization of phages was reported in 5 cases. Several cases were complicated by secondary infections. Complete characterization of the phages (morphology, genomics, and activity) and their production (methods, sterility, and endotoxin tests) are reported.
Conclusions
Customized phage production and therapy was safe and yielded favorable clinical or microbiological outcomes in two-thirds of cases. A center or pipeline dedicated to tailoring the phages against a patient's specific AMR bacterial infection may be a viable option where standard treatment has failed.
Keywords: antibiotic resistance, phage, phage therapy, microbiology
Antimicrobial resistant infections present clinical challenges. Here, we report the experiences and outcomes of a dedicated phage discovery, manufacturing, and characterization platform for customized phage therapy for a range of heterogenous and complex resistant bacterial infections.
The World Health Organization (WHO) states that antimicrobial resistance (AMR) is a top 10 global health threat [1]. Without significant interventions, AMR deaths are expected to rise and may exceed those caused by cancer [2]. Alternative options have been sought, including phage therapy, the specific use of bacteriophages (phages), viruses that lytically kill bacteria, as a treatment modality. Phages have been shown to be safe for therapeutic use when prepared using standard methods and are generally regarded as safe by the Food and Drug Administration (FDA) [3,4]. Their specificity facilitates targeted killing in a strain-specific manner [5]. An advantage of phages compared with antibiotics is their adaptive and diverse properties [6,7]. Although resistance to phages is common, unlike antibiotics, phages can be trained, or adapted, to counter resistant strains, a feature that highlights the evolutionary arms race that bacteria and phages undergo [8–11]. Phage–antibiotic synergy is also common and offers the possibility of killing resistant strains through distinct molecular mechanisms [12].
Although clinical trial data are still forthcoming, numerous successful case studies have been reported, offering evidence that the approach has therapeutic potential [4,13–17]. Indeed, the personalization of phages tailored to a patient's strain has been suggested to fill a clinical need for challenging bacterial infections. Our objective was to generate a pipeline of technicians, scientists, and clinicians dedicated to streamlining the discovery, characterization, and production of safe, customized phages for a patient's infection [12]. Here, we report clinical and microbiological outcomes of phage therapy, as well as phage synergy, genomic, and immune neutralization responses (where possible) for a diverse range of bacterial infections (12 total cases). Although phage therapy has been shown to be safe [13,18–20], safety monitoring procedures and reactions are reported. Finally, we also provide information associated with ineffective outcomes, and obstacles encountered, since negative results in this area are uncommonly reported. Experiences reported herein will aid the development of effective, personalized therapy for bacterial infections while highlighting challenges to overcome.
METHODS
Human Subjects
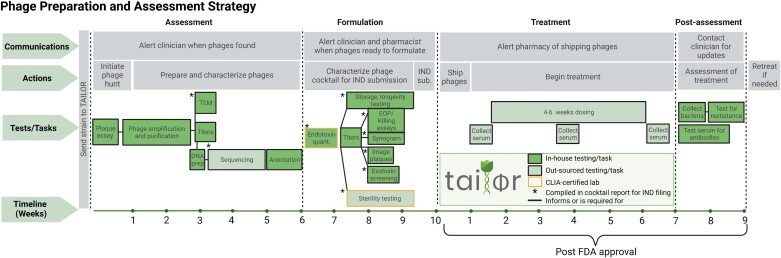
The FDA approved the individual IND (investigational new drug) for each patient on a compassionate-use basis. Each patient case was treated after approval from the local institutional review board (IRB). All patients provided informed consent for clinical use of phages and their data being used for research purposes and publication. The manufacturing institution (Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research [TAILΦR] at Baylor College of Medicine) also received IRB approval. No safety or ethical concerns were noted in either review. Figure 1 shows a Gantt chart that illustrates TAILΦR Lab's end-to-end pipeline. Table 1 lists criteria for inclusion for treatment and Table 2 indicates data provided to the FDA as chemistry, manufacturing and controls (CMC) information.
Figure 1.
TAILΦR plan for phage therapy. Gantt chart showing the TAILΦR process for phage formulation, treatment, and assessment. The timeline (bottom of the chart) is listed in weeks. In-house testing (Baylor College of Medicine) or tasks are shown in dark green boxes. Sequencing, sterility testing, and endotoxin quantification (light green boxes) are outsourced to other laboratories. Starred boxes (*) are test results that are compiled into cocktails reports for filing to the FDA. Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; EOP, Efficienct of Plating; FDA, Food and Drug Administration; IND, investigational new drug; Prep., Preparation; TAILΦR, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research; TEM, Transmission Electron Microscopy; Sub., Submission; Quant., Quantificaiton. The figure was created with BioRender.com.
Table 1.
Inclusion Criteria for Phage Therapy
| Antibiotic treatment has been ineffective |
| Multidrug-resistant organism (or difficult-to-treat infection) |
| Chronically infected patienta |
| Clonal pathogen is the sole or main cause of diseaseb |
Patient is stable during the time frame to manufacture phages.
As determined by genome sequencing.
Table 2.
Cocktail Report Contents
| Name and description of phages |
| Manufacturer |
| Description of manufacturing |
| Cocktail formulation and phage concentration |
| Documentation of lytic activity |
| Stability testing |
| Killing assay results |
| Endotoxin testing results |
| Calculated endotoxin exposure |
| Sterility testing results |
| Toxin screening results |
| Sequencing analysis |
| Antibiotic synergy/antagonism |
| Purification protocols |
| Statement on equipment and facilities |
| Statement on generalized transduction |
| Equipment and materials |
| Certificates of sterility testing or other outside testing |
| References |
Clinical Assessment
There are 2 response classifications for this case series, favorable and unfavorable, and each is classified to either clinical or microbiological criteria. A favorable clinical response is defined as relief of clinical symptoms, regardless of whether the bacteria was detected. An unfavorable clinical response is a lack of or marginal relief of symptoms. These responses were determined by a combination of physician judgment and patient symptom reporting. Cases were also judged by the presence or absence of positive or negative microbiological culture results. No positive culture post-treatment was judged as a favorable microbiological response, whereas a positive culture with the same species was judged as an unfavorable microbiological response.
Phage Isolation
All clinical isolates were grown from individual colonies in lysogeny broth (LB) at 37°C. For plaque (phage) isolation from environmental samples or TAILΦR's library of phages, a double-agar overlay assay was performed using the patient's bacterial isolate as host. Individual plaques were serially passaged by streaking onto a fresh bacterial lawn twice and single plaques used to ensure clonality. Additional methodological details associated with this report can be found in the Supplementary Methods.
RESULTS
TAILΦR Received 50 Phage Therapy Requests
We received 50 case requests from physicians over 30 months (Table 3). Patient infections varied, with a majority (60%) comprising 4 primary infection types, ranging from systemic infection to device-related (left ventricular assist device [LVAD]) (Table 4). Most requests came from the United States (90%), with some from other countries—Canada, Finland, India, and Spain (2% each) (Supplementary Table 1). Thirteen states were represented (Supplementary Table 2), with 67% of requests from California, Texas, and Minnesota. Of the 50 requests, only 12 were treated, with 5 more active cases ongoing (Table 3), thus highlighting a need for a robust pipeline. Some cases improved before treatment (8 cases; 16%). Five (10%) patients expired prior to therapy. Some cases were delayed, spanning from technical related reasons (eg, insufficient phages found) and hospital/pharmacy-related delays (eg, unable to compound on site) to delays associated with meeting regulatory criteria (Supplementary Table 3). For some cases (10%), lytic phages could not be detected, which occurred for the species Escherichia coli, Staphylococcus aureus, Achromobacter xylosoxidans, and Pseudomonas aeruginosa. In other cases (6%), a bacterial agent could not be isolated, although the patient had symptoms of infection. Time to treatment became shorter as we improved efficiency over time (Supplementary Figure 1).
Table 3.
Progress on 50 Phage Requests
| Progress | Cases, n | Cases, % |
|---|---|---|
| Treated | 12 | 24 |
| Improved | 8 | 16 |
| Delayeda | 8 | 16 |
| Expiredb | 5 | 10 |
| Activec | 5 | 10 |
| No phaged | 5 | 10 |
| No isolatee | 4 | 8 |
| Other | 3 | 6 |
| Total | 50 | 100 |
Patient treatment was delayed beyond average (10 wk).
Patient died before treatment started.
Cases that are about to receive phage.
Phage was not able to be isolated for patient.
Bacteria were not able to be isolated for phage isolation.
Table 4.
Clinical Infections Underlying Requests for Phage Therapy
| Indication | Cases, n | Cases, % |
|---|---|---|
| Abdominal aortic graft | 1 | 2 |
| Abdominal infection | 1 | 2 |
| Bacteremia | 7 | 14 |
| CF lunga | 7 | 14 |
| Heart (aortic arch) | 1 | 2 |
| Heart (sternotomy) | 1 | 2 |
| Heart (endocarditis) | 2 | 4 |
| Hip joint | 1 | 2 |
| Hip prosthesis | 1 | 2 |
| Other joint | 3 | 6 |
| Liver | 1 | 2 |
| Lung | 5 | 10 |
| Lung, primary sclerosing cholangitis | 1 | 2 |
| LVAD | 9 | 18 |
| Prostatitis | 2 | 4 |
| UTI | 6 | 12 |
| UTI, abdominal abscess, lung | 1 | 2 |
| Total | 50 | 100 |
Abbreviations: CF, cystic fibrosis; LVAD, left ventricular assist device; UTI, urinary tract infection.
Patients with CF with lung infection.
Ten Phage Cocktails Were Generated Against 6 Different Species
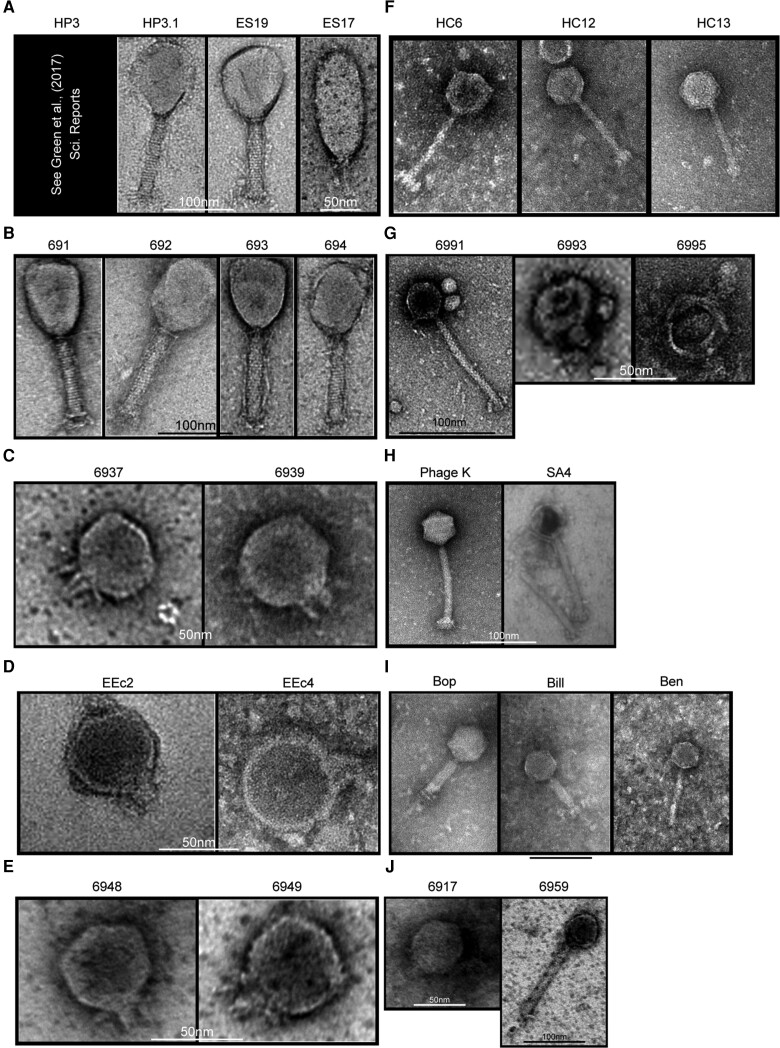
TAILΦR had originally provided phages for 12 patients. For 1 case, we were not provided sufficient clinical information despite treatment being regarded as successful, so we omitted this case. One patient was retreated with a different phage cocktail after developing a new infection (thus, 12 cases) (Tables 5 and 6). The phages used for treatment were characterized. For example, TEM (transmission electron microscopy) images were generated (Figure 2). Table 7 summarizes the source of discovery, sequencing data, genomic analysis, and predicted lifestyle of each phage. Based on a sequence analysis, all phages lacked known bacterial virulence factors (eg, toxins) and antibiotic-resistance genes. Two different methods were used to assess whether the phage existed as temperate (integrated into the genome of the host bacterium) or lytic (lysed the bacteria without integration). Three phages had conflicting results per these 2 methods but were ultimately determined to be lytic by a manual review of their genomes (see Supplementary Methods).
Table 5.
Summary of Case Treatment Details
| Species | Case No. | Phage(s) | Patient Details | Phage Dose, Route, Duration | Safety Outcome | Outcome |
|---|---|---|---|---|---|---|
| Escherichia coli | 1 | HP3, ES17, HP3.1, ES19 |
Patient (IND 19509): Transplant recipient with complex, recurrent prostate and urinary tract infections (UTIs) caused by an extended-spectrum beta-lactamase (ESBL)–producing E. coli (UCS1). Treatment: phage cocktail and ertapenem | 109 PFU/mL every 12 h (q12h), intravenous (IV), 2 wk | No adverse effects | Favorable outcome: There were no further clinical UTI events that required antibiotic therapy. The patient had asymptomatic bacteriuria noted on surveillance urine cultures at 6 and 11 wk following the end of phage therapy that did not require antibiotic treatment. The bacteriuria was caused by a sister strain of E. coli (UCS1.1) that remained susceptible to the original phage cocktail but possessed mutations in virulence genes. See publication by Terwilliger et al., 2021 [4]. |
| 4 | HP3.1, EEc2, EEc4 |
Patient (IND 26982): Recurrent bacteremia for >1 y from ESBL E. coli (USC1) in transplant recipient. Treatment: phage cocktail and ertapenem |
1010 PFU/mL q12h, IV, 6 wk | No adverse effects | Unfavorable outcome: The patient developed bacteremia several months after treatment with an E. coli strain with similar susceptibility profile to original infecting strain. The recurrent bacteremia was successfully treated with ertapenem. The patient has no further episodes of bacteremia. | |
| 5 | 6948, 6949 | Patient (IND 22713): Recurrent bacteremia in transplant recipient, sepsis E. coli (UCS2) infection. Treatment: phage cocktail and ertapenem |
109 PFU/mL q12h, IV, 6 wk | No adverse effects | Unfavorable outcome: The patient developed recurrent symptoms after the end of phage and antibiotic therapy, which resolved with new courses of prolonged ertapenem. Blood cultures, however, remained negative. The patient was re-treated with a new phage cocktail targeting the original pathogen (see row below). Note–cases 5 and 6 are the same patient. | |
| 6 | HC6, HC12, HC13 |
Patient (IND 22713): Recurrent bacteremia, sepsis E. coli (UCS2.1) infection. Treatment: retreatment with new phage cocktail and ertapenem | 109 PFU/mL q12h, IV, 4 wk | No adverse effects | Favorable outcome: No recurrence of symptoms or bacteremia after the end of phage therapy while remaining off antibiotics for 12 mo. However, the patient did develop a carbapenem-resistant E. coli UTI 2 wk afterwards | |
| Enterococcus faecium | 10 | Bop, Bill, Ben |
Patient (IND 22921): Recurrent bacteremia caused by Enterococcus faecium (UMM1). Treatment: phage cocktail, ceftriaxone and daptomycin | 109 PFU/mL q12h, IV, 6 wk | No adverse effects | Unfavorable outcome: The patient developed recurrent enterococcal bacteremia infection 4 wk post–phage therapy treatment that has since been suppressed with antibiotic therapy. |
| Staphylococcus aureus | 8 | Phage K, SA4 | Patient (IND 27384): LVAD-related infection caused by S. aureus (MYC5) with recurrent bacteremia. Treatment: phage cocktail and ceftriaxone | 1010 PFU/mL q12h, IV, 6 wk; 3 × 1010 PFU/mL intraoperative (IO), once | No adverse effects | Favorable outcome: 19 mo after completion of phage therapy, the patient successful underwent heart transplant and all antibiotics for the infection were stopped. |
| 9 | Phage K, SA4 | Patient (IND 27469): LVAD-related infection caused by S. aureus (UCS13). Recurrent bacteremia and ongoing device-associated abscess. Treatment: phage cocktail, cefazolin and ertapenem | 109 PFU/mL q12h, IV, 6 wk | No adverse effects | Unfavorable outcome: Bacteremia recurred after end of phage and antibiotic therapy. He remains on suppressive antibiotics to date. | |
| 12 | Phage K, SA4 | Patient (IND 27952): Bacteremia due to sternal wound caused by S. aureus (UPG1). Persistent infection despite multiple antibiotic treatments and debridement surgeries. Treatment: phage cocktail, daptomycin and ceftaroline | 1010 PFU/mL q12h, IV, 2 wk; 1010 PFU/mL, topical, once | No adverse effects | Favorable outcome: Patient received an abbreviated course of phage IV (2 wk) instead of the usual 4–6-wk dose. The patient did not demonstrate improvement (no bacterial eradication, no clinical improvement). Then, the patient received another 6 wk of the same phage cocktail, dosed IV and topically, together with daptomycin. The wound healed during the 6 wk of topical treatment and remained closed at 2 mo after the 6 wk of treatment. Completed course of phage therapy wand antibiotics on 19 April 2022. | |
| Klebsiella pneumoniae | 7 | 6991, 6993, 6995 | Patient (IND 27614): Recurrent UTI and recurrent bacteremia caused by K. pneumoniae (UMF1) in transplant recipient. Treatment: phage cocktail and ertapenem | 1010 PFU/mL q12h, IV, 6 wk | Mild diarrhea which resolved during treatment | Indeterminate outcome. The K. pneumoniae persisted in the urine, but the bacteremia resolved with no further episodes of sepsis in over 1 y since phage therapy. Clinical symptoms of urinary tract infection resolved except that he experiences intermittent pain consistent with epididymitis every 5–6 wk, which had resolved without antibiotic therapy. However, the patient and clinician both agree that symptoms have improved. |
| Klebsiella aerogenes | 3 | 6937, 6939 | Patient (IND 25016): Prosthetic joint infection (PJI) caused by Klebsiella aerogenes (UCF1). Treatment: phage cocktail, cefepime and ceftazidime-avibactam | 1010 PFU/mL q12h, IV, 6 wk; 1010 PFU/mL intraarticular once | No adverse effects | Indeterminate outcome. Prior drainage and antibiotic therapy had eradicated the K. aerogenes when the joint was entered surgically for repeat drainage, washout, and local installation of phage. The joint instead grew Candida albicans, which likely explained the ongoing infection rather than persistence of the gram-negative infection, and then later vancomycin-resistant enterococci. The joint was eventually removed in a Girdlestone procedure. |
| Pseudomonas aeruginosa | 11 | 6917, 6959 | Patient (IND 27807): Persistent (dissemination) LVAD driveline infection caused by Pseudomonas aeruginosa (MYC4). Treatment: phage cocktail and cefepime |
1010 PFU/mL q12h, IV, 6 wk; 1011 PFU/mL, IO, once | No adverse effects | Favorable outcome: Patient continues to have scant drainage culture positive for P. aeruginosa that is controlled with local wound care. Computed tomography imaging shows no evidence of infection. Currently, the patient is off antibiotic treatment since May 2022 and stable. |
| Enterobacter cloacae | 2 | 691, 692, 693, 694 |
Patient (IND 27401): PJI caused by Enterobacter cloacae (SLC4). Treatment: phage cocktail and cefepime | 1010 PFU/mL q12h, IV, 6 wk | No adverse effects | Favorable outcome: Phage targeted pathogen was eradicated. However, later developed new infection due to Peptoniphilus asaccharolyticus. The patient was treated successfully with oral amoxicillin-clavulanate and will remain on life-long suppression. E. cloacae was not recovered from any of the cultures at the time of recurrence. The treating physician believed the E. cloacae has been eradicated. |
Abbreviations: IND, investigational new drug; LVAD, left ventricular assist device; PFU, plaque-forming units.
Table 6.
Summary of Data on 12 Patients Who Received Phage Therapy Between the Months of March 2019 and September 2022
| Case details | Case details breakdown | Case | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Patient status |
Infection | UTI | PJI | PJI | Bacteremia | Bacteremia, sepsis | Bacteremia, sepsis | UTI, bacteremia | LVAD | LVAD | Bacteremia | LVAD | Sternal wound, bacteremia |
| Bacteria | E. coli | E. cloacae | K. aerogenes | E. coli | E. coli | E. coli | K. pneumonae | S. aureus | S. aureus | E. faecium | P. aeruginosa | S. aureus | |
| Antibiotic | Ertapenem | Cefepime | Cefepime, cetazidime/avibactam | Ertapenem | Ertapenem | Ertapenem | Ertapenem | Ceftriazone | Cefazolin, ertapenem | Ceftriazone, daptomycin | Cefepime | Daptomycin | |
| Immunosuppressed (Y/N) | Y | N | Y | N | Y | Y | Y | N | N | Y | N | Y | |
| Treatment | Phage cocktail | HP3, HP3.1, ES17, ES19 | 691, 692, 693, 694 | 6937, 6939 | HP3.1, EEc2, EEc4 | 6948, 6949 | HC6, HC12, HC13 | 6991, 6993, 6995 | Phage K, SA4 | Phage K, SA4 | Bop, Bill, Ben | 6917, 6959 | Phage K, SA4 |
| Routes | IV | IV | IA, IV | IV | IV | IV | IV | IO, IV | IV | IV | IO, IV | Topical, IV | |
| Dose first route | 109 PFU/mL q12h | 1010 PFU/mL q12h | 1010 PFU/mL once | 1010 PFU/mL q12h | 109 PFU/mL q12h | 109 PFU/mL q12h | 1010 PFU/mL q12h | 3 × 1010 PFU/mL once | 109 PFU/mL q12h | 109 PFU/mL q12h | 1011 PFU/mL once | Once | |
| Dose second route | … | … | 1010 PFU/mL q12h | … | … | … | … | 1010 PFU/mL q12h | … | … | 1010 PFU/mL q12h | 1010 PFU/mL q12h | |
| Duration IV (wk) | 2 | 6 | 6 | 6 | 6 | 4 | 6 | 6 | 6 | 6 | 6 | 8 | |
| Synergy (S/A/AD) | AD | S | S | S | S | S | S | S | S | S | S | AD | |
| Post-treatment | Bacteria cleared (Y/N) | N | Y | Y | Y | N | Y | Y | Y | N | Y | Y | Y |
| Duration bacteria cleared | … | 17 M | 26 M | 3 M | … | 12 M | 6 W | 19 M | … | 4 W | 12 M | 6 M | |
| Symptoms cleared (Y/N) | Y | Y | N | Y | N | Y | Y | Y | N | Y | Y | Y | |
| Duration symptoms cleared | 24 M | 17 M | 6–8 W | 3 M | 12 M | 6 W | 19 M | … | 12 M | 12 M | 6 M | ||
| Secondary infection | E. coli | P. asaccharolyticus | VRE, C. albicans | E. coli | E. coli | E. coli | K. pneumoniae | … | S. aureus | VRE | P. aeruginosa | … | |
| Retreatment (Y/N) | N | N | N | N | Y | N | Y | N | N | N | N | Y | |
| Neutralization (Y/N) | Y | Y | Y | Y | Y | ||||||||
Abbreviations: IA, intra-articular; IO, intra-operative; IV, intravenous; LVAD, left ventricular assist device infection; M, months; N, no; PFU, plaque-forming units; PJI, prosthetic joint infection; S/A/AD, synergy, antagonism, or additive; UTI, urinary tract infection; VRE, vancomycin-resistant Enterococcus; W, weeks; Y, yes.
Figure 2.
A–J, TEM images of phages used in the 12 cases of this report. Scale bars are present in the image, from 50 nm (black) to 100 nm (white).
Table 7.
Phage Characteristics
| Case no. | Phage | Accession No. | Source | Genome Size, bp | G+C, % | ORF | tRNAs | Lifestyle Pred.a | Ab. Res. | Vir. F. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
HP3 | NC_041919.1 | Goose/duck feces | 168 188 | 35.4 | 264 | 11 | V | … | … |
| HP3.1 | OK275722 | Directed evolution | 168 195 | 35.4 | 264 | 11 | V | … | … | |
| ES17 | MN508615.2 | Sewage | 75 134 | 42.12 | 120 | 1 | Vb | … | … | |
| ES19 | MN508616.1 | Sewage | 167 088 | 35.39 | 263 | 11 | V | … | … | |
| 2 |
691 | MN508621 | Sewage | 178 607 | 44.79 | 283 | 2 | V | … | … |
| 692 | MN508622 | Sewage | 176 610 | 44.72 | 275 | 1 | V | … | … | |
| 693 | MN508623 | Sewage | 178 070 | 44.74 | 276 | 2 | V | … | … | |
| 694 | MN508624 | Sewage | 178 230 | 44.74 | 277 | 2 | V | … | … | |
| 3 |
6937 | OL362270 | Sewage | 43 359 | 56.1 | 48 | 0 | V | … | … |
| 6939 | OL362271 | Sewage | 46 039 | 43 | 62 | 0 | V | … | … | |
| 4 |
HP3/1 | OK275722 | Directed evolution | 168 195 | 35.4 | 264 | 11 | V | … | … |
| EEc2 | ON210144 | Sewage | 44 883 | 45 | 57 | 0 | V | … | … | |
| EEc4 | ON210145 | Sewage | 44 590 | 45 | 57 | 0 | V | … | … | |
| 5 |
6948 | OL362272 | Sewage | 44.545 | 45.1 | 53 | 0 | V | … | … |
| 6949 | OL362273 | Sewage | 44 371 | 45 | 54 | 0 | V | … | … | |
| 6 |
HC6 | OL362274 | Sewage | 47 726 | 46.6 | 87 | 0 | Vb | … | … |
| HC12 | OL362275 | Sewage | 39 342 | 50.1 | 50 | 0 | V | … | … | |
| HC13 | OL362276 | Sewage | 39 803 | 50.1 | 52 | 0 | V | … | … | |
| 7 |
6991 | OL362277 | Sewage | 46 373 | 48 | 81 | 0 | Vb | … | … |
| 6993 | OL362278 | Sewage | 44 351 | 53.9 | 53 | 0 | V | … | … | |
| 6995 | OL362279 | Sewage | 42 538 | 54.1 | 53 | 0 | V | … | … | |
| 8, 9, 12 |
Phage K | KF766114 | Unknown | 139 381 | 30.4 | 212 | 4 | V | … | … |
| SA4 | OL362280 | Pig feces | 140 004 | 30.4 | 216 | 4 | V | … | … | |
| 10 |
Bop | ON125307.1 | Sewage | 147 049 | 36.9 | 184 | 14 | V | … | … |
| Bill | OM966901.1 | Sewage | 151 985 | 37.1 | 187 | 0 | V | … | … | |
| Ben | MN027503.1 | Sewage | 153 454 | 37 | 187 | 23 | V | … | … | |
| 11 |
6917 | OL362268 | Sewage | 67 297 | 55.7 | 96 | 0 | V | … | … |
| 6959 | OL362269 | Sewage | 68 080 | 55.6 | 95 | 0 | V | … | … |
Abbreviations: Ab. Res., antibiotic resistance; G+C, guanosine and cytosine content; ORF, open reading frame; tRNA, Transfer RNA; Vir. F, virulence factors
Virulent (V) or temperate (T) as determined by genetic analysis.
Classified as virulent and temperate using 2 different analysis tools, phage.ai and PHACTS.
Clinical Features, Phage Therapy, and Outcomes of the 12 Cases
Clinical Features
The 12 cases consisted of the following infections: LVAD (3), recurrent bacteremia (3 + 1 retreatment), prosthetic joint infection (PJI) (2), urinary tract infection (UTI) (1), bacteremia and UTI (1), and sternal wound infection and bacteremia (1) (Tables 4, 5, and 6). Causative bacteria of those infections are listed as follows: E. coli (3 + 1 retreatment), S. aureus (3), Klebsiella pneumoniae (1), Enterobacter cloacae (1), Klebsiella aerogenes (1), P. aeruginosa (1), and Enterococcus faecium (1). Patient 5 was retreated for the same infection type (bacteremia and sepsis) and the same causative species (E. coli). The reported immune status of the patients was not specifically assessed by our group but was based on a clinical profile provided by the treating physicians. Of the 12 patient cases, more than 50% (7/12 cases) had a secondary immune deficiency.
Mode of Administration
All patients received concomitant antibiotic therapy. All patients were treated with intravenous (IV) phage therapy (Table 5). Four patient cases (33%) received phage therapy through an additional route. Of these, 1 patient (case 3) received phages into the intra-articular space of a prosthetic joint. Two patients (cases 8 and 11) received concomitant intraoperative phages. The fourth patient (case 12) received phages topically. All non-IV doses were single one-time applications. The average duration for IV treatment was 5.6 weeks.
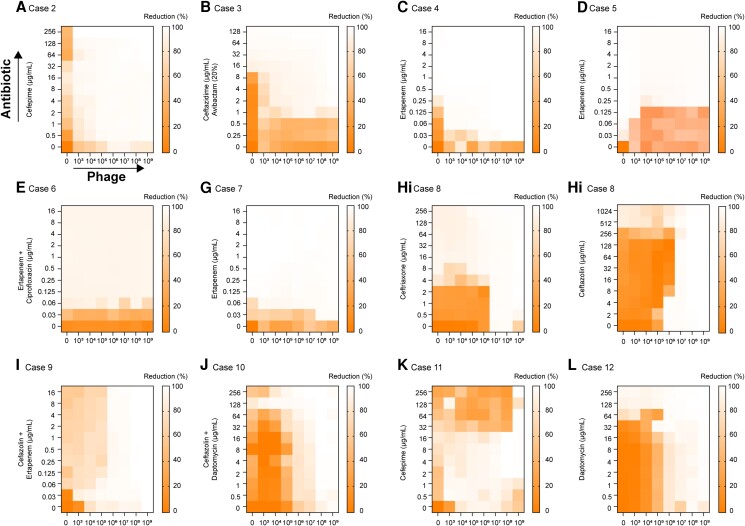
Phage–Antibiotic Combination
Interactions (synergy, antagonism, or additive) between the antibiotic and phage cocktail were determined in vitro using a system developed by TAILΦR Labs termed “synography” (detailed in Gu Liu et al [21] and in Methods [results in Figure 3]). For most cases (10/12), we observed an in vitro synergistic effect; the concentration of antibiotic and phages resulted in greater killing than the same concentration of either alone. A general additive effect was seen in case 1 (not shown, available Terwilliger et al., 2022) and 12 (Figure 3). Of particular note is case 8 in which 2 antibiotics were tested (Figure 1Hi, Hii): ceftriaxone and cefazolin. For the phage cocktail with cefazolin, we observed antagonism. For ceftriaxone, antagonism was also noted, but mostly at low concentrations of antibiotic and was less apparent in the earlier time point. The physician was informed of this and decided to use ceftriaxone.
Figure 3.
The phage–antibiotic combination for each case (A–L) was determined using OD (600-nM) measurements that were taken every 15 minutes. Synograms (t = 24 h) represent the mean reduction percentage of each treatment from 3 biological replicates: Reduction (%) = [(ODgrowth control − ODtreatment)/ODgrowth control] × 100. The “y” axis is the antibiotic concentration and the “x” axis is the phage concentration (PFU/mL). Abbreviations: OD, optical density; PFU, plaque-forming units.
Clinical Outcomes
Although this was not a clinical trial and the phage was not benchmarked against a control or placebo cohort, there were clear outcomes that we classified as either a “favorable response” or “unfavorable response,” which was further divided as either clinical or microbiological. Classifications were determined by the clinical status of the patient, resolution of signs or symptoms, radiographic determination, and finally by microbiological culture data. The duration of follow-up after phage therapy to determine the outcome was based on infection type (eg, UTI, and complicated or recurrent UTI including prostatitis). An unfavorable microbiological response included a recurrence of bacterial infection with an organism whose phenotype matched the original infecting strain, as determined by sequencing.
Favorable Microbiological Responses
Tables 6 and 8 detail each individual case with safety and outcomes. Of the 12 cases, 4 cases (cases 2, 6, 8, and 12) were associated with bacterial eradication (Table 6). The causative organism for these varied by species—E. cloacae, E. coli, and S. aureus. Infection type ranged from recurrent bloodstream infections (1 case), PJI (1 cases), LVAD (1 case), and 1 sternal wound infection and bacteremia. These cases (cases 2, 6, and 12) were also complicated by the development of secondary infections, likely due to comorbidities of the patients. For example, in case 2 (PJI; E. cloacae), E. cloacae was eradicated but 4 weeks later the patient developed an infection caused by Peptoniphilus asaccharolyticus. This patient was treated with an antibiotic and avoided hip disarticulation. For case 8 (LVAD; S. aureus), there were no complications; the patient cleared the initial bacterial infection without developing a secondary infection (favorable microbiological and clinical response).
Table 8.
Outcomes for Each patient
| Favorable Microbiologicala Outcome (Y/N) | Favorable Clinicalb Outcome (Y/N) |
|
|---|---|---|
| Case 1 | N | Y |
| Case 2 | Y | Y |
| Case 3 | Yc | N |
| Case 4 | N | Y/Nd |
| Case 5 | N | N |
| Case 6 | Y | Y |
| Case 7 | N | Y |
| Case 8 | Y | Y |
| Case 9 | N | N |
| Case 10 | N | Y/N |
| Case 11 | N | Y |
| Case 12 | Y | Y |
| Total | 5/12 (42%) | 7/12 (58%) |
Bacterial eradication of initial infection—yes (Y) or no (N).
Clinical improvement of initial symptoms—yes (Y) or no (N).
Although bacteria were eradicated, this likely happened prior to phage therapy.
Y/N indicates that patient did improve but only later after retreatment with antibiotic therapy.
Unfavorable Microbiological With Favorable Clinical Responses
Some patients who did not experience bacterial clearance showed clinical improvement (cases 1, 7, and 11). For case 1, a patient with a multidrug-resistant E. coli UTI, combined antibiotic and phage therapy did not lead to clearance, but the patient became symptom-free with asymptomatic bacteriuria until last follow-up (favorable clinical response).
Unfavorable Clinical and Microbiological Responses
There were 4 cases that we defined as unfavorable responses (clinical and microbiological). It should be noted that 2 of these patients (cases 4 and 10) eventually improved over time with continued antibiotic therapy. Patient 5 had bacteremia and sepsis caused by E. coli and received retreatment with a new phage cocktail (case 6). After the first treatment, neither the patient's bacterial burden nor symptoms were alleviated. However, after the second phage/antibiotic treatment, the case resolved for 12 months. For an LVAD case caused by S. aureus (case 9), phage therapy did not lead to bacterial eradication or clinical improvement. We are not certain why 2 of the 4 unfavorable cases improved with continued antibiotic therapy. Some reasons may include sensitization of the infecting strains to antibiotics (which can occur from phage pressure), duration of treatment, or the spontaneous improvement over time.
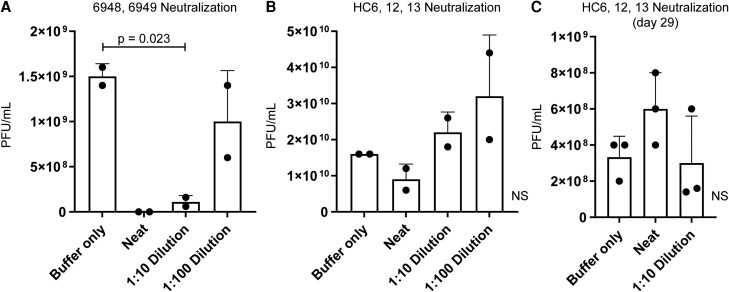
Serum Neutralization
Neutralization assays were performed using serum samples for 5 cases (1, 5, 6, 7, and 9). Neutralization of the phage cocktail was noted for cases 1, 7, and 9 after treatment (Table 6). For patient 5, serum neutralization of phage cocktail was seen at week 4 (Figure 4A). Thus, a new phage cocktail (HC6, HC12, and HC13) was generated for retreatment that was not neutralized by the patient's original serum (case 6) (Figure 4B) or serum attained 4 weeks post-treatment with the new cocktail (day 29) (Figure 4C).
Figure 4.
Serum neutralization of phage. A, Serum neutralization of a cocktail for patient case #5 at 4 weeks post-treatment. B, Serum neutralization of a new patient cocktail for retreatment (case 6) prior to treatment. C, Serum neutralization of a patient cocktail for case 5.1 on day 29 post-treatment with phage therapy. N = 2–3; means ± SDs are shown. Significance was determined with 1-way analysis of variance. Abbreviations: NS, not significant; PFU, plaque-forming units.
Adverse Reactions
Every case was thoroughly reviewed by the physician's IRB, the manufacturing institution's IRB, and the FDA (through the expanded-access pathway). A treatment protocol of the product (phage cocktails) and monitoring of potential adverse effects was submitted. Since each case was different, monitoring was adjusted on a case-by-case basis. Generally, for phage administration in the hospital/clinic, the patient was observed for 3 hours after the first IV dose. Blood was drawn and weekly labs conducted (complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and blood culture). A daily questionnaire was used at the time of administration, vital signs recorded, and symptom diary used to track reactions in the outpatient setting during treatment. The patient was also warned of potential side effects during the consenting process. Only 1 adverse reaction, mild diarrhea, was detected in 1 case (case 7), which resolved during treatment. It should be noted that bacterial sequencing was performed on some strains before and after phage therapy (Supplementary Table 4). In cases 10 and 11, we identified 2 species of bacteria in the sequencing data. Whether both species were simultaneously present at the time of infection is not known.
DISCUSSION
Here, we show that (1) there is demand for phage therapy for difficult-to-treat AMR infections; (2) it is feasible to develop safe, well-characterized, therapeutic phage cocktails that gain FDA compassionate-use approval using an integrated phage discovery and manufacturing pipeline tailored to the patient's bacterial strains; (3) the rate of adverse reactions to phage was low (in fact, in a recent review of 13 clinical trials on phage therapy, no adverse reactions were noted [22]); (4) for 12 infections previously uncontrolled by antibiotics, 42% (5/12) showed favorable microbiological outcomes, with 58% (7/12) showing clinical improvement; and (5) phage–antibiotic synergy (in vitro) was common. Finally, there is a deficit in the literature in the reporting of cases whose outcomes are ineffective. This dearth of knowledge makes it difficult to judge if a personalized approach (difficult to achieve in a clinical trial format) is an option for some patients. As such, we report limitations of this personalized approach, including (1) it was unclear as to why treatment was infective, (2) the time to treatment for some cases was lengthy (>1 y), (3) many cases never proceeded to therapy (for the reasons outlined herein), and (4) anti-phage neutralization was observed in some cases.
There were 2 patient cases (5 and 9) where no clinical improvement after phage and antibiotic treatment was observed. For case 8 (LVAD infection; S. aureus), there was a favorable outcome; however, the same cocktail was used for case 9 (LVAD infection; S. aureus), an ineffective case. For case 8, the patient received a higher dose of phage (>1 log) and was given a second dose via another route. LVAD infections are often driveline infections caused by S. aureus and are difficult to resolve due to biofilms [23]. Topical treatment for drivelines has been shown to be successful [18,24,25], likely due to directly dispersing enzymes capable of degrading the polymetric substances of biofilms [26,27]. The phage dose for the successful case ranged from 1 × 1010 to 3 × 1010 plaque-forming units (PFU)/mL compared with case 9 who received 1 × 109 PFU/mL, IV (Tables 5 and 6). However, in other successful phage therapy cases for LVAD infections, the dose was lower [18,24,25]. One case report of a patient who received 4 × 1010 PFU IV developed fever and wheezing after each dose [16]. The patient from case 8 did not have adverse events from phage therapy. Sequencing of the bacterial strain post-treatment showed mutations that may cause reduced fitness, such as loss of immune evasion factors and virulence regulators (agrC G394A; codY R222C) (Supplementary Table 4). The bacterial strain recovered was still susceptible to the original phage cocktail. Neutralization of the phage cocktail was noted but only tested after treatment, a limitation here.
For the treatment of case 5 (bacteremia, E. coli), the outcome was also unfavorable; however, after retreatment, it became favorable. For both, TAILΦR Labs was able to assess serum neutralization (Figure 4). After the first treatment (week 4r) we saw a reduction in phage titer (1 log decrease). Likely due to varying doses of immunosuppressive drugs, after the second treatment with a different phage cocktail no significant reduction in phage titer was noted, suggesting an absence of anti-phage antibody, perhaps explaining clinical improvement. However, this patient developed a UTI. One well-reported case suggested the reason for failed treatment for a Mycobacterium abscessus infection was due to antibody-mediated phage neutralization [28]. This patient, however, was immunocompetent and received prolonged treatment with phage (6 mo). The patient described here (case 5 and 6) was a transplant recipient who was on immunosuppressants during both treatments. Also, this patient received a new phage cocktail once phage neutralization was noted after the first treatment. A study in Poland assessed the immune response of 20 patients receiving phage therapy and concluded that a weak production of anti-phage antibodies correlated with negative outcomes [29]. For the 12 cases herein, more than 50% of the patients had a form of immunosuppression. It is known that immunosuppression can predispose patients to opportunistic infections [30,31] and are likely to be candidates for phage therapy [4,16]. Finally, in 10 of 12 cases, the phage cocktail showed in vitro synergy with the antibiotic used in treatment. It is uncertain if this synergy improved outcomes. Since phage-antibiotic synergy reduces resistance and increases killing efficiency, clinical trials comparing antibiotics with phages are required to determine the contribution of each.
In conclusion, the data herein provide evidence that a center dedicated to customized phage cocktails can deliver safe and favorable outcomes for AMR infections. There are improvements that would lower cost and increase speed and favorable outcomes (Supplementary Table 5). These include automating phage screening and purification, having regulatory approval of premade “fixed” cocktails, standardized and certified tests associated with biomanufacturing, standard operating procedures (SOPs) for compounding, robust post-treatment characterization, and the development of a discovery pipeline for phages with unique properties (anti-biofilm, etc). Of particular importance is the need to standardize treatment protocols [32]. A global, multidisciplinary effort involving clinicians, hospitals, governments, advocates, the pharmaceutical and biotechnology industry, and basic scientists, all sharing data and agreeing on the parameters of tailored cocktails, will be required for similar centers to make personalized infectious disease therapy a part of mainstream medicine.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Sabrina I Green, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research (TAILΦR), Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA; Laboratory of Gene Technology, KU Leuven, Leuven, Belgium.
Justin R Clark, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research (TAILΦR), Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.
Haroldo H Santos, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research (TAILΦR), Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.
Kyle E Weesner, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research (TAILΦR), Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.
Keiko C Salazar, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research (TAILΦR), Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.
Saima Aslam, Division of Infectious Diseases and Global Public Health, Center for Innovative Phage Applications and Therapeutics, University of California, San Diego, La Jolla, California, USA.
J William Campbell, Division of Infectious Diseases and Infection Prevention, St. Luke's Hospital, Chesterfield, Missouri, USA.
Sarah B Doernberg, Division of Infectious Diseases, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Emily Blodget, Department of Medicine, University of California, Irvine, California, USA.
Michele I Morris, Division of Infectious Diseases, Department of Medicine, University of Miami Miller School of Medicine, Miami, Florida, USA.
Gina A Suh, Division of Infectious Diseases, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Karam Obeid, Division of Infectious Diseases and International Medicine, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA.
Fernanda P Silveira, Division of Infection Diseases, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Andrey A Filippov, Wound Infections Department, Bacterial Diseases Branch, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA.
Katrine L Whiteson, Department of Molecular Biology and Biochemistry, University of California, Irvine, California, USA.
Barbara W Trautner, Department of Medicine, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA; Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
Austen L Terwilliger, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research (TAILΦR), Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.
Anthony Maresso, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research (TAILΦR), Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.
Notes
Author Contributions. S. I. G. wrote and reviewed manuscript edits and performed data analysis and interpretation. K. C. S. reviewed and edited the manuscript. H. H. S. performed experiments and purified phages for treatment. K. E. W. purified phages for treatment. J. R. C. performed data (sequencing) analysis, data interpretation, and edited the manuscript. S. A., J. W. C., S. B. D., E. B., M. I. M., G. A. S., K. O., and F. P. S. contributed to patient treatment and data collection and analysis, reviewed the manuscript, and provided edits. A. A. F. and K. L. W. provided phages for treatment and reviewed and edited the manuscript. A. L. T. organized phage therapy treatments; contributed to data collection, analysis, and interpretation; and reviewed and edited the manuscript. B. W. T. provided necessary funding for the work at TAILOR and reviewed and provided edits for the manuscript. A. M. is the faculty founder and current Center Director of TAILOR, analyzed and interpretated data, and wrote and edited the manuscript.
Acknowledgments. The authors thank Dr. Frank Ramig for originally isolating phages HP3 and ES17. They also thank Micah Forshee for isolating phage 691–694.
Disclaimer. The material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting the true views of the Department of the Army or the Department of Defense.
Financial support. Some of the phage discovery and characterization work was funded by the National Institutes of Health (NIH–National Institute of Allergy and Infectious Diseases [NIAID] 157981), the Mike Hogg Fund, the Kleberg Foundation, and a Veterans Affairs (VA) Merit Award. G. A. S. reports support for this work from Adaptive Phage Therapeutics and Phagelux. K. L. W. reports the following support for this work: R21 AI149354/AI/NIAID (NIH Department of Health and Human Services [HHS]). S. A. reports support for this work in the form of salary support from the University of California San Diego Chancellor’s Innovation Fund.
References
- 1. World Health Organization . Antimicrobial resistance. 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 1 October 2022.
- 2. O’Neill J. Review on antimicrobial resistance antimicrobial resistance: tackling a crisis for the health and wealth of nations. London, 2014; Available at: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf. Accessed 1 October 2022.
- 3. Liu D, Van Belleghem JD, de Vries CR, et al. The safety and toxicity of phage therapy: a review of animal and clinical studies. Viruses 2021; 13:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terwilliger A, Clark J, Karris M, et al. Phage therapy related microbial succession associated with successful clinical outcome for a recurrent urinary tract infection. Viruses 2021; 13:2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young R, Gill JJ. Microbiology. Phage therapy redux—what is to be done? Science 2015; 350:1163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maresso A. Bacterial virulence: a conceptual primer. Berlin, Germany: Springer International Publishing, 2019. [Google Scholar]
- 7. Green S, Ma L, Maresso A. Phage therapy. In: Encyclopedia of microbiology (Schmidt, T, ed.) . 4th ed. Amsterdam, The Netherlands: Elsevier, 2018:485–95. [Google Scholar]
- 8. Rostøl JT, Marraffini L. (Ph)ighting phages: how bacteria resist their parasites. Cell Host Microbe 2019; 25:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woolhouse ME, Webster JP, Domingo E, Charlesworth B, Levin BR. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat Genet 2002; 32:569–77. [DOI] [PubMed] [Google Scholar]
- 10. Samson JE, Magadán AH, Sabri M, Moineau S. Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol 2013; 11:675–87. [DOI] [PubMed] [Google Scholar]
- 11. Salazar KC, Ma L, Green SI, et al. Antiviral resistance and phage counter adaptation to antibiotic-resistant extraintestinal pathogenic E. coli. mBio 2021; 12:e00211-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terwilliger AL, Gu Liu C, Green SI, et al. Tailored antibacterials and innovative laboratories for phage (Φ) research: personalized infectious disease medicine for the most vulnerable at-risk patients. Phage 2020; 1:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cano EJ, Caflisch KM, Bollyky PL, et al. Phage therapy for limb-threatening prosthetic knee Klebsiella pneumoniae infection: case report and in vitro characterization of anti-biofilm activity. Clin Infect Dis 2021; 73:e144–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simner PJ, Cherian J, Suh GA, et al. Combination of phage therapy and cefiderocol to successfully treat. JAC Antimicrob Resist 2022; 4:dlac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paul K, Merabishvili M, Hazan R, et al. Bacteriophage rescue therapy of a vancomycin-resistant Enterococcus faecium infection in a one-year-old child following a third liver transplantation. Viruses 2021; 13:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aslam S, Lampley E, Wooten D, et al. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect Dis 2020; 7:ofaa389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramirez-Sanchez C, Gonzales F, Buckley M, et al. Successful treatment of Staphylococcus aureus prosthetic joint infection with bacteriophage therapy. Viruses 2021; 13:1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tkhilaishvili T, Merabishvili M, Pirnay JP, et al. Successful case of adjunctive intravenous bacteriophage therapy to treat left ventricular assist device infection. J Infect 2021; 83:e1–3. [DOI] [PubMed] [Google Scholar]
- 19. Schooley RT, Biswas B, Gill JJ, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 2017; 61:e00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019; 25:730–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu Liu C, Green SI, Min L, et al. Phage-antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. mBio 2020; 11:e01462-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suh GA, Lodise TP, Tamma PD, et al. Considerations for the use of phage therapy in clinical practice. Antimicrob Agents Chemother 2022; 66:e0207121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamat I, Lamba H, Hines-Munson C, et al. Identifying causative microorganisms in left ventricular assist device infections as a guide for developing bacteriophage therapy. J Surg Res 2021; 271:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mulzer J, Trampuz A, Potapov EV. Treatment of chronic left ventricular assist device infection with local application of bacteriophages. Eur J Cardiothorac Surg 2020; 57:1003–4. [DOI] [PubMed] [Google Scholar]
- 25. Rubalskii E, Ruemke S, Salmoukas C, et al. Bacteriophage therapy for critical infections related to cardiothoracic surgery. Antibiotics (Basel) 2020; 9:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pires DP, Melo LDR, Azeredo J. Understanding the complex phage-host interactions in biofilm communities. Annu Rev Virol 2021; 8:73–94. [DOI] [PubMed] [Google Scholar]
- 27. Pires DP, Oliveira H, Melo LD, Sillankorva S, Azeredo J. Bacteriophage-encoded depolymerases: their diversity and biotechnological applications. Appl Microbiol Biotechnol 2016; 100:2141–51. [DOI] [PubMed] [Google Scholar]
- 28. Dedrick RM, Freeman KG, Nguyen JA, et al. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat Med 2021; 27:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Żaczek M, Łusiak-Szelachowska M, Jończyk-Matysiak E, et al. Antibody production in response to Staphylococcal MS-1 phage cocktail in patients undergoing phage therapy. Front Microbiol 2016; 7:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fishman JA. Opportunistic infections—coming to the limits of immunosuppression? Cold Spring Harb Perspect Med 2013; 3:a015669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Legrand M, Max A, Peigne V, et al. Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med 2012; 40:43–9. [DOI] [PubMed] [Google Scholar]
- 32. Onsea J, Soentjens P, Djebara S, et al. Bacteriophage application for difficult-to-treat musculoskeletal infections: development of a standardized multidisciplinary treatment protocol. Viruses 2019; 11:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.