Abstract
Background
Increased renin angiotensin aldosterone system (RAAS) activity may contribute to excess cardiovascular disease in people with HIV (PWH). We investigated how RAAS blockade may improve myocardial perfusion, injury, and function among well-treated PWH.
Methods
Forty PWH, on stable ART, without known heart disease were randomized to eplerenone 50 mg PO BID (n = 20) or identical placebo (n = 20) for 12 months. The primary endpoints were (1) myocardial perfusion assessed by coronary flow reserve (CFR) on cardiac PET or stress myocardial blood flow (sMBF) on cardiac MRI or (2) myocardial inflammation by extracellular mass index (ECMi) on cardiac MRI.
Results
Beneficial effects on myocardial perfusion were seen for sMBF by cardiac MRI (mean [SD]: 0.09 [0.56] vs −0.53 [0.68] mL/min/g; P = .03) but not CFR by cardiac PET (0.01 [0.64] vs −0.07 [0.48]; P = .72, eplerenone vs placebo). Eplerenone improved parameters of myocardial function on cardiac MRI including left ventricular end diastolic volume (−13 [28] vs 10 [26] mL; P = .03) and global circumferential strain (GCS; median [interquartile range 25th–75th]: −1.3% [−2.9%–1.0%] vs 2.3% [−0.4%–4.1%]; P = .03), eplerenone versus placebo respectively. On cardiac MRI, improvement in sMBF related to improvement in global circumferential strain (ρ = −0.65, P = .057) among those treated with eplerenone. Selecting for those with impaired myocardial perfusion (CFR <2.5 and/or sMBF <1.8), there was a treatment effect of eplerenone versus placebo to improve CFR (0.28 [0.27] vs −0.05 [0.36]; P = .04). Eplerenone prevented a small increase in troponin (0.00 [−0.13–0.00] vs 0.00 [0.00–0.74] ng/L; P = .03) without effects on ECMi (0.9 [−2.3–4.3] vs −0.7 [−2.2–−0.1] g/m2; P = .38). CD4+ T-cell count (127 [−38–286] vs −6 [−168–53] cells/μL; P = .02) increased in the eplerenone- versus placebo-treated groups.
Conclusions
RAAS blockade with eplerenone benefitted key indices and prevented worsening of myocardial perfusion, injury, and function among PWH with subclinical cardiac disease when compared with placebo.
Clinical Trials Registration
NCT02740179 (https://clinicaltrials.gov/ct2/show/NCT02740179?term=NCT02740179&draw=2&rank=1).
Keywords: HIV, renin-angiotensin-aldosterone system, myocardial dysfunction, myocardial blood flow, eplerenone
The data suggest beneficial effects of eplerenone to prevent worsening of myocardial perfusion, cardiac function, and myocardial injury among people with HIV without known cardiovascular disease.
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality among persons with human immunodeficiency virus (PWH). Large-cohort studies in the Americas and globally have consistently demonstrated a 1.5- to 2.0-fold increased risk for CVD among PWH compared with persons without human immunodeficiency virus (HIV) [1]. Subclinical myocardial dysfunction, including reduced myocardial perfusion, altered cardiac function, and myocardial fibrosis and injury, is increased among well-treated PWH [2]. The underlying mechanism of increased CVD, particularly nonatherosclerotic disease, in HIV remains unknown, and there are no current treatment strategies to prevent or reduce the burden of heart disease.
One potential mediator for subclinical myocardial disease in HIV is microvascular dysfunction. Even in the absence of obstructive coronary disease and/or symptomatic CVD, early impairments in coronary microvascular function may lead to poor perfusion. Impaired flow can be captured by the coronary flow reserve (CFR), an integrative measure of epicardial and microvascular circulations, using cardiac positron emission tomography (PET) or stress myocardial blood flow (sMBF) using cardiac magnetic resonance imaging (MRI). Among the general population, impairment in CFR is associated with an approximate 5-fold increased risk in cardiovascular (CV) mortality and predicts cardiac mortality independent of obstructive coronary artery disease [3, 4]. Recent studies in non-HIV have also reported that sMBF by cardiac MRI is independently associated with major adverse CV events (MACEs) among those without known CVD [5].
Our prior work has shown unique renin-angiotensin-aldosterone system (RAAS) physiology among PWH with increased RAAS activation in association with relevant CVD risk factors [6–8] with effects of eplerenone to improve these indices [9]. In the current study, we hypothesized that excess RAAS activation is a pathological mediator of microvascular dysfunction, inflammation, and fibrosis, and thus may underlie the structural and functional myocardial abnormalities in HIV. In the MIRACLE HIV (MIneralocorticoid Receptor Antagonism for CardiovascuLar hEalth in HIV) study, we conducted a 12-month, double-blinded, randomized, placebo-controlled trial of eplerenone to reduce subclinical myocardial disease among PWH.
METHODS
Participants
This study was conducted between February 2017 and March 2022 at the Massachusetts General Hospital and Brigham and Women's Hospital. Participants were between the ages of 40 and 65 years and were required to be on a continuous antiretroviral therapy regimen for more than 12 months with an HIV viral load less than 100 copies/mL. Participants were selected for increased visceral adiposity using a visceral adipose tissue cutoff of 110 cm2 or greater as measured on abdominal computed tomography (CT), a threshold associated with increased RAAS activation in our prior study [1]. Participants were excluded for known history of CVD (coronary artery disease and heart failure), cerebrovascular disease, uncontrolled diabetes (glycated hemoglobin [HbA1c] ≥7.5% and/or insulin use), liver disease (alanine aminotransferase [ALT] >3× upper limit of normal [ULN]), and kidney disease (creatinine >1.5 mg/dL or estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2). For further details, see the Supplementary Methods. All participants provided informed consent to participate. This study was approved by the Mass General Brigham Human Research Committee.
Randomization and Masking
The randomization was stratified by sex, presence of plaque (yes or no), and statin use (yes or no). A permuted block algorithm with a random block size of either 2 or 4 was utilized, and individuals were allocated 1:1 to eplerenone or matching placebo. Following baseline study procedures, eplerenone dosing was started at 50 mg daily for 1 week, then escalated to 50 mg twice daily for the remaining 12-month study duration. Adherence to study medication was assessed by manual pill count of the returned supply. Study participants and investigators were blinded to the randomization.
Study Outcomes
Participants underwent cardiac PET and cardiac MRI at baseline and 12 months under standardized sodium conditions (see Supplementary Methods).
Safety Monitoring
Safety visits were conducted at 1 week, 2 weeks, 4 weeks, 3 months, 6 months, and 9 months following randomization and commencement of the study drug. Interval medical history and blood pressure (BP) were obtained. Laboratory assessment included serum creatinine and potassium, and urine pregnancy test for participants of childbearing potential. A Data and Safety Monitoring Board (DSMB) convened every 3 months.
Statistical Analysis
Normally distributed variables are reported as mean (SD), non–normally distributed variables as median (interquartile range 25th–75th), and categorical variables are shown as percentages. Baseline and change between baseline and 12-month values were compared between randomization groups using the Student's t test or Wilcoxon rank-sum test. In addition, we controlled for any potential effects of sex in adjusted regression analyses. The primary endpoints were (1) changes in myocardial perfusion as assessed by CFR on cardiac PET or sMBF on cardiac MRI or (2) change in myocardial inflammation assessed by extracellular volume normalized by left ventricular (LV) mass index (ECMi) by cardiac MRI. The study was powered at 85% at a 2-sided .05 α level to detect a between-group difference of 0.93 SD in myocardial perfusion [10] with a planned attrition rate of 15%. We performed 2 sensitivity analyses for the primary endpoints. First, we performed longitudinal mixed-effects modeling to assess a time × treatment interaction (see Supplementary Methods). To account for missing data, we also performed analysis of covariance (ANCOVA) with a conservative imputation with last observation carried forward for those with baseline data. We further performed a supplementary analysis to augment the primary analyses using clinically validated cutoffs and stratifying by CFR (< or ≥2.5) and sMBF (< or ≥1.8), both via cardiac PET, to identify those with impaired total flow capacity, an integrative measure of stress flow and flow reserve at baseline [11]. Correlations were determined using Spearman's correlation coefficient. Statistical significance was determined by a 2-sided P < .05. Analyses were performed using SAS JMP (version 16; SAS Institute). The Consolidated Standards of Reporting Trials (CONSORT) 2010 statement was used to guide the reporting of this study [12].
RESULTS
Participant Flow
One hundred and ten participants who signed consent were assessed for eligibility, and 40 participants were randomized to receive eplerenone (n = 20) or placebo (n = 20). A total of 7 participants did not complete the study (eplerenone arm, n = 6; placebo arm, n = 1). Study withdrawals were not deemed to be related to treatment effects in most cases (Figure 1).
Figure 1.
Flowchart of the participants in the study. One hundred and ten participants were assessed for eligibility. Seventy participants were excluded, as 53 did not meet the inclusion criteria per protocol, 10 declined to participate, and 7 were lost to follow-up. A total of 40 participants were randomized and evenly allocated to the eplerenone and placebo treatment arms. Within the eplerenone arm, 1 participant declined to participate further and 5 other participants discontinued intervention, leaving 14 participants for the primary analysis. Within the placebo arm, 1 participant discontinued intervention, leaving 19 participants for the primary analysis. Abbreviations: CTA, computed tomography angiography; VAT, visceral adipose tissue.
Baseline Assessment
Demographics and Clinical Characteristics
Treatment groups (eplerenone vs placebo) did not differ by age (53 [7] vs 56 [6] y) and had similar proportions of male sex (75% vs 75%) and White race (55% vs 55%). Current hypertension and statin use was seen in 20% of participants in both groups. Ten-year atherosclerotic cardiovascular disease (ASCVD) risk scores were similar and of intermediate risk in the eplerenone versus placebo arms (5.3 [3.4%–8.9%] vs 7.2 [3.6%–11.3%]). HbA1c was normal and similar in both groups at 5.5%. Both groups demonstrated good immunological control based on CD4 count (814 [617–916] vs 740 [545–1122] cells/μL) and had a long duration of HIV (20 [8] vs 21 [7] y) and antiretroviral therapy (ART) use (12 [3] vs 15 [7] y). Eighty percent of participants had undetectable viral loads in the eplerenone and placebo arms. Lipids and glycemic parameters were generally well controlled among randomized groups (Table 1).
Table 1.
Baseline and Between-Group Changes in Demographic and Clinical Characteristics After 12 Months of Treatment
| Baseline | Change Over 12 Months | ||||
|---|---|---|---|---|---|
| Eplerenone (n = 20) |
Placebo (n = 20) |
Eplerenone (n = 14) |
Placebo (n = 19) |
P | |
| Demographic characteristics | |||||
| Age, y | 53 (7) | 56 (6) | n/a | n/a | n/a |
| Male sex, % | 75 | 75 | n/a | n/a | n/a |
| Race, % | … | … | n/a | n/a | n/a |
| White | 55 | 55 | n/a | n/a | n/a |
| Black | 25 | 30 | n/a | n/a | n/a |
| Hispanic ethnicity, % | 25 | 10 | n/a | n/a | n/a |
| Current hypertension, % | 20 | 20 | n/a | n/a | n/a |
| Current statin use, % | 20 | 20 | n/a | n/a | n/a |
| ASCVD 10-year risk, % | 5.3 (3.4–8.9) | 7.2 (3.6–11.3) | n/a | n/a | n/a |
| HbA1c, % | 5.5 (5.3–6.1) | 5.5 (5.2–5.8) | n/a | n/a | n/a |
| HIV parameters | |||||
| CD4+ T-cell count, cells/μL | 814 (617–916) | 740 (545–1122) | 127 (−38–286) | −6 (−168–53) | .02 |
| CD8+ T-cell count, cells/μL | 813 (318) | 772 (251) | 134 (209) | 34 (172) | .16 |
| Log HIV viral load, copies/mL | 1.38 (0.23) | 1.36 (0.18) | −0.07 (0.28) | 0.21 (0.48) | .045 |
| Undetectable viral load, % | 80 | 80 | 93 | 61 | .03 |
| Duration of HIV, y | 20 (8) | 21 (7) | n/a | n/a | n/a |
| Duration of ART use, y | 12 (3) | 15 (7) | n/a | n/a | n/a |
| Current PI use, % | 11 | 10 | n/a | n/a | n/a |
| Current NRTI use, % | 100 | 100 | n/a | n/a | n/a |
| Current NNRTI use, % | 40 | 50 | n/a | n/a | n/a |
| Current integrase inhibitor use, % | 60 | 68 | n/a | n/a | n/a |
| Body composition | |||||
| Waist circumference, cm | 110 (11) | 110 (14) | −1 (5) | 0 (7) | .73 |
| BMI, kg/m2 | 31 (5) | 32 (7) | −0.5 (1.1) | 0.2 (2.3) | .23 |
| VAT area, cm2 | 214 (141–254) | 171 (126–305) | −19 (−46–9) | −6 (−34–21) | .35 |
| SAT area, cm2 | 333 (252–428) | 341 (195–457) | −2 (−28–30) | 9 (−8–49) | .28 |
| Metabolic parameters | |||||
| Total cholesterol, mg/dL | 188 (169–209) | 177 (157–195) | −16 (−43–15) | −7 (−17–6) | .56 |
| Triglycerides, mg/dL | 151 (69) | 166 (94) | 22 (72) | −18 (58) | .10 |
| HDL cholesterol, mg/dL | 48 (43–56) | 46 (39–50) | −4 (−7–2) | 0 (−6–3) | .62 |
| LDL cholesterol, mg/dL | 117 (91–130) | 103 (76–123) | −16 (−36–18) | −3 (−12–14) | .26 |
| Fasting glucose, mg/dL | 101 (87–108) | 95 (88–104) | 2 (−7–16) | −1 (−3–8) | .28 |
| ALT, IU/L | 26 (20–31) | 21 (16–28) | 2 (−3–5) | −1 (−4–7) | .95 |
| Atherosclerotic parameters | |||||
| CAC score | 0 (0–43) | 1 (0–160) | n/a | n/a | n/a |
| CAC >0, % | 42 | 50 | n/a | n/a | n/a |
| Presence of plaque, % | 63 | 56 | n/a | n/a | n/a |
Data are presented as mean (SD), median (interquartile range 25th–75th), or percentage. P values determined by Student's t test for normally distributed variables or Wilcoxon rank-sum test for non–normally distributed variables.
Abbreviations: ALT, alanine transaminase; ART, antiretroviral therapy; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CAC, coronary artery calcium; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; n/a, not applicable; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
RAAS Parameters
Under standardized sodium conditions, eplerenone- and placebo-treated groups had similar plasma renin activity (0.2 [0.1–0.6] vs 0.1 [0.1–0.4] ng/mL/h), serum aldosterone (10 [7–13] vs 12 [7–17] ng/dL), urine sodium [270 [82] vs 240 [89] mmol/24 h), and urinary aldosterone excretion (4 [3–7] vs 4 [3–6] ng/24 h) at baseline. Potassium and creatinine were similar among randomized groups at baseline (Table 2).
Table 2.
Baseline and Absolute Between-Group Changes in Renin-Angiotensin-Aldosterone System Parameters After 12 Months of Treatment.
| Baseline | Change Over 12 Months | |||||
|---|---|---|---|---|---|---|
| Eplerenone (n = 20) |
Placebo (n = 20) |
P | Eplerenone (n = 14) |
Placebo (n = 19) |
P | |
| SBP, mmHg | 131 (125–141) | 127 (123–136) | .28 | −14 (−26–−6) | −6 (−20–13) | .11 |
| DBP, mmHg | 80 (11) | 73 (7) | .03 | −6 (8) | 0 (8) | .09 |
| Heart rate, bpm | 70 (6) | 69 (8) | .65 | −4 (7) | −3 (7) | .57 |
| Urine sodium, mmol/24 h | 270 (82) | 240 (89) | .28 | 2 (100) | −25 (90) | .44 |
| Urine potassium, mmol/24 h | 78 (58–116) | 65 (44–93) | .29 | −3 (−66–9) | −3 (−15–13) | .52 |
| Urine creatinine, g/24 h | 1.69 (0.53) | 1.63 (0.41) | .72 | −0.16 (0.52) | 0.03 (0.22) | .23 |
| Urinary aldosterone excretion, ng/24 h | 4 (3–7) | 4 (3–6) | .97 | 5 (0–10) | 0 (−2–2) | .02 |
| PRA, ng/mL/h | 0.2 (0.1–0.6) | 0.1 (0.1–0.4) | .12 | 0.0 (−0.2–0.4) | 0.0 (0.0–0.1) | .83 |
| Serum aldosterone, ng/dL | 10 (7–13) | 12 (7–17) | .65 | 6 (−2–13) | −1 (−8–2) | .03 |
| Serum potassium, mmol/L | 4.33 (0.38) | 4.34 (0.32) | .93 | 0.20 (0.64) | −0.11 (0.18) | .10 |
| Serum creatinine, mg/dL | 0.97 (0.14) | 0.97 (0.14) | .97 | 0.03 (0.12) | −0.02 (0.13) | .24 |
Data are presented as mean (SD) or median (interquartile range 25th–75th). P values determined by Student's t test for normally distributed variables or Wilcoxon rank-sum test for non–normally distributed variables.
Abbreviations: bpm, beats per minute; DBP, diastolic blood pressure; PRA, plasma renin activity; SBP, systolic blood pressure.
Cardiac Parameters
Eplerenone- and placebo-treated groups had a similar presence of plaque (63% vs 56%) and coronary artery calcium (CAC) scores (0 [0–43] vs 1 [0–160]) on coronary CT angiography at baseline (Table 1). Coronary flow reserve by cardiac PET, sMBF by cardiac MRI, and ECMi by cardiac MRI were similar at baseline in the eplerenone versus placebo arms (Table 3). Measures of LV function were also similar between groups. Both groups demonstrated preserved ejection fraction (EF) (58% [5%] vs 58% [5%], eplerenone vs placebo).
Table 3.
Baseline and Absolute Between-Group Changes in Cardiac Parameters After 12 Months of Treatment
| Baseline | Change Over 12 Months | ||||||
|---|---|---|---|---|---|---|---|
| Eplerenone (n = 20) |
Placebo (n = 20) |
P | Eplerenone (n = 14) |
Placebo (n = 19) |
P | P a | |
| Myocardial perfusion | |||||||
| Global CFR by cardiac PET | 2.83 (0.54) | 2.78 (0.65) | .80 | 0.01 (0.64) | −0.07 (0.48) | .72 | .73 |
| Rest MBF by cardiac MRI, mL/min/g | 0.90 (0.22) | 0.88 (0.20) | .77 | 0.15 (0.25) | −0.02 (0.22) | .08 | .09 |
| Stress MBF by cardiac MRI, mL/min/g | 2.78 (0.81) | 2.98 (0.76) | .46 | 0.09 (0.56) | −0.53 (0.68) | .03 | .04 |
| Myocardial function | |||||||
| LVEDV, mL | 139 (44) | 133 (30) | .64 | −13 (28) | 10 (26) | .03 | .02 |
| LVEDVi, mL/m2 | 67 (16) | 64 (15) | .56 | −5 (14) | 5 (12) | .046 | .02 |
| LV mass, g | 104 (86–122) | 106 (90–112) | .78 | 1 (−25–26) | 9 (−3–17) | .56 | .84 |
| GCS, % | −23 (−24–−21) | −23 (−25–−21) | .88 | −1.3 (−2.9–1.0) | 2.3 (−0.4–4.1) | .03 | .04 |
| GLS, % | −19 (3) | −18 (4) | .23 | −1.4 (4.1) | −0.9 (4.8) | .77 | .80 |
| Myocardial inflammation and fibrosis | |||||||
| ECMi, g/m2 | 13.1 (11.4–16.4) | 14.0 (11.4–16.3) | 0.75 | 0.9 (−2.3–4.3) | −0.7 (−2.2–−0.1) | .38 | .17 |
| T1, ms | 1247 (1157–1267) | 1199 (1132–1299) | 0.90 | 25 (5–74) | 1 (−50–85) | .32 | .31 |
Data are presented as mean (SD) or median (interquartile range 25th–75th). P values determined by Student's t test for normally distributed variables or Wilcoxon rank-sum test for non–normally distributed variables.
Abbreviations: CFR, coronary flow reserve; ECMi, extracellular mass index; GCS, global circumferential strain; GLS, global longitudinal strain; LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVEDVi, left ventricular end diastolic volume index; MBF, myocardial blood flow; MRI, magnetic resonance imaging; PET, positron emission tomography.
P values reported after controlling for natal sex.
Treatment Effects
RAAS Parameters
There was a significant increase in both serum (6 [−2–13] vs −1 [−8–2] ng/dL; P = .03) and urinary aldosterone excretion (5 [0–10] vs 0 [−2–2] ng/24 h; P = .02), while changes in urine sodium were similar (2 [100] vs −25 [90] mmol/24 h; P = .44) among participants randomized to eplerenone versus placebo. This is consistent with eplerenone's known mechanism of action blocking aldosterone at the receptor and may be a surrogate measure to suggest good study drug adherence. Eplerenone tended to reduce supine and resting systolic BP (−14 [−26–−6] vs −6 [−20–13] mmHg; P = .11) and diastolic BP (−6 [8] vs 0 [8] mmHg; P = .09) (Table 2).
Cardiac Effects
Myocardial Perfusion
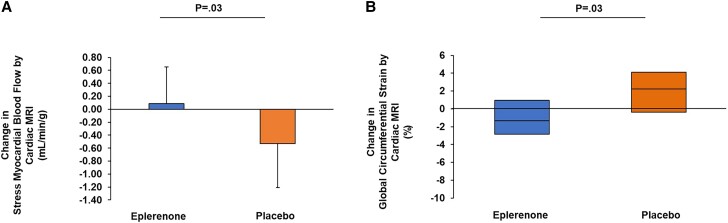
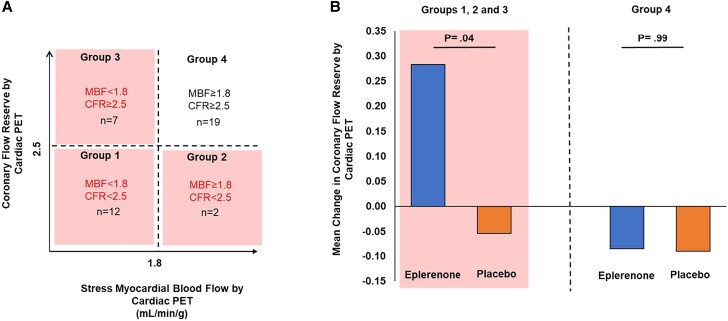
Although significant changes were not seen for CFR by cardiac PET (0.01 [0.64] vs −0.07 [0.48]; P = .72; treatment effect: 0.08; 95% confidence interval [CI]: −0.37, 0.53), a beneficial effect was seen for sMBF by cardiac MRI (0.09 [0.56] vs −0.53 [0.68] mL/min/g; P = .03; treatment effect: 0.62; 95% CI: .05, 1.18 mL/min/g; P = .03) in the eplerenone- versus placebo-treated groups (Table 3, Figure 2A). Changes in sMBF remained significant controlling for sex (Table 3). In supplemental analysis, using clinically validated cutoffs and stratifying by CFR (< or ≥2.5) and sMBF (< or ≥1.8 mL/min/g) [11], both obtained by cardiac PET, we compared treatment effects among those with impaired CFR and/or sMBF (n = 21) and separately among those with normal CFR and sMBF (n = 19) (Figure 3). The majority of participants had impaired CFR and/or sMBF at baseline, and among this group, there was a significant effect of eplerenone to improve CFR versus placebo (0.28 [0.27] vs −0.05 [0.36]; P = .04), while this was not seen in those with normal flow parameters (Figure 3).
Figure 2.
Change in stress myocardial blood flow and global circumferential strain on cardiac MRI over 12 months among treatment groups. A, The bar graph shows the mean change in stress myocardial blood flow measured on cardiac MRI among those treated with eplerenone versus placebo. Error bars represent the standard deviation. B, The box plot shows the median change in global circumferential strain measured on cardiac MRI among those treated with eplerenone versus placebo. The box plot represents the 25th and 75th percentile, and the line within the box represents the median. Abbreviation: MRI, magnetic resonance imaging.
Figure 3.
Change in CFR over 12 months by impaired versus normal total flow capacity on cardiac PET among treatment groups. A, Participants categorized based on integrating CFR and maximal MBF both obtained by cardiac PET to assess total flow capacity identify unique prognostic at-risk phenotypes for CVD. B, The bar graph shows the mean change in CFR stratified by impaired total flow capacity (groups 1–3) versus normal total flow capacity (group 4) in the eplerenone- versus placebo-treated groups. Eplerenone significantly improves mean CFR among those participants with impaired total flow capacity. Abbreviations: CFR, coronary flow reserve; MBF, myocardial blood flow; PET, positron emission tomography.
Myocardial Function
Eplerenone significantly improved multiple additional parameters of myocardial function on cardiac MRI including LV end diastolic volume (LVEDV; −13 [28] vs 10 [26] mL; P = .03) and global circumferential strain (GCS; −1.3% [−2.9%–1.0%] vs 2.3% [−0.4%–4.1%]; P = .03) (Table 3, Figure 2B). Changes in cardiac MRI parameters remained significant controlling for sex (Table 3). Among those who were treated with eplerenone, changes in sMBF on cardiac MRI correlated with CFR on cardiac PET (ρ = 0.69, P = .04) and tended to correlate with GCS (ρ = −0.65, P = .057). The CFR also related to decreases in GCS (ρ = −0.61, P = .047).
Myocardial Inflammation and Injury
No effect was seen on ECMi (0.9 [−2.3–4.3] vs −0.7 [−2.2–−0.1] g/m2; P = .38) (Table 3). At baseline, high-sensitivity cardiac troponin T (hs-cTnT) levels were low and 50% were at or above the level of detection. After 12 months of treatment, eplerenone tended to prevent a small increase in hs-cTnT compared with placebo (0.00 [−0.13–0.00] vs 0.00 [0.00–0.74] ng/L; P = .03) (Table 4).
Table 4.
Baseline and Absolute Between-Group Changes in Inflammatory and Cardiac Biomarkers After 12 Months of Treatment
| Baseline | Change Over 12 Months | |||||
|---|---|---|---|---|---|---|
| Eplerenone (n = 20) |
Placebo (n = 20) |
P a | Eplerenone (n = 14) |
Placebo (n = 19) |
P a | |
| hsIL-6, pg/mL | 3.8 (1.7–5.1) | 2.2 (1.7–3.0) | .17 | −0.8 (−1.8–0.4) | 0.2 (−0.5–2.2) | .09 |
| hsCRP, ng/mL | 3248 (1246–7397) | 1949 (1430–2633) | .05 | 189 (−3841–718) | 591 (−261–2346) | .36 |
| MCP-1, pg/mL | 182 (154–226) | 200 (165–275) | .21 | 285 (49–365) | 292 (241–352) | .88 |
| sCD163, ng/mL | 753 (533–1223) | 832 (490–1113) | .90 | −275 (−384–−11) | −160 (−304–100) | .17 |
| NT-proBNP, ng/L | 34.8 (21.1–63.8) | 37.6 (20.5–85.7) | .99 | 19.4 (6.6–41.0) | 2.8 (−25.6–63.5) | .28 |
| hs-cTnT,b ng/L | 5.99 (5.99–7.69) | 5.99 (5.99–6.91) | .99 | 0.00 (−0.13–0.00) | 0.00 (0.00–0.74) | .03 |
Data are presented as mean (SD) or median (interquartile range 25th–75th).
Abbreviations: hsCRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; hsIL-6, high sensitivity interleukin 6; MCP-1, monocyte chemoattractant protein-1; NT-proBNP, N-terminal pro-hormone brain natriuretic peptide; sCD163, soluble CD163.
P values determined by Student's t test on log-transformed data.
Includes values imputed at 5.99 ng/L in cases where the limit of detection was below assay.
Immune Function and Activation
Those randomized to eplerenone had significantly improved CD4 count (127 [−38–286] vs −6 [−168– 53] cells/μL; P = .02) compared with placebo (Table 1). In addition, small, but positive trends were seen for viral load. Eplerenone also tended to decrease high sensitivity interleukin 6 (hsIL-6; −0.8 [−1.8–0.4] vs 0.2 [−0.5–2.2] pg/mL; P = .09) (Table 4).
Sensitivity Analysis
Baseline demographics, clinical characteristics, and main myocardial endpoints did not differ among those in randomized to eplerenone who completed the study versus those who did not (Supplementary Table 1). The longitudinal mixed-effects models recapitulated the primary results, demonstrating beneficial effects of eplerenone versus placebo on sMBF (P = .03), LVEDV (P = .02), and GCS (P = .0495) on cardiac MRI. Similarly, results were recapitulated accounting for missing data in imputation analyses for these endpoints: sMBF (P = .045), LVEDV (P = .04), and GCS (P = .03) on cardiac MRI for eplerenone versus placebo.
Adverse Events
The medication was generally well tolerated, and no dose reductions were required. There was 1 serious adverse event: a participant was lost to follow-up and later reported to be deceased. The DSMB agreed with the conclusion that this was not related to the study medication. There was no medication-related hyperkalemia or hypotension reported in either group. Renal function was not affected in either group (Supplementary Table 2).
DISCUSSION
In the current study, we demonstrate that mineralocorticoid receptor antagonism with eplerenone may have unique benefits to prevent worsening of subclinical myocardial disease in HIV, particularly among those with worse disease at baseline, when compared with placebo. Assessing a key measure of myocardial perfusion using cardiac MRI, sMBF was preserved in the eplerenone group but decreased in the placebo group over 12 months. In contrast, no overall effects were seen assessing microvascular function by CFR, a ratio of stress to resting blood flow by cardiac PET. However, CFR by PET did improve significantly in supplemental analyses stratified based on impairment of total flow capacity. Thus, we were able to identify a group that benefitted most from RAAS blockade. Moreover, key indices of cardiac function on cardiac MRI, including LVEDV and GCS, a measure of systolic function, also improved, as did measures of cardiac injury—for example, troponin, and immune function.
Eplerenone prevented a reduction in sMBF by cardiac MRI in this study. Knott et al [5] showed that a 1-mL/minute/g decrease in sMBF on cardiac MRI is associated with a hazard ratio of 2.14 and 1.93 with respect to MACEs and death. Stated in another way, a 1.0-SD increase in sMBF is associated with a decreased risk of MACEs and death by 54% and 36%, respectively. To place this in context of our study, we showed a relative difference of 0.62 in sMBF, approximately 1.0 SD, among those treated with eplerenone versus placebo on cardiac MRI, with a primary effect to prevent a deterioration in sMBF over the course of the trial. Assessment of sMBF by cardiac MRI has shown good precision [13] and good correlation with assessment of global CFR by cardiac PET [14]. The effects shown on sMBF using cardiac MRI in this study by a complementary technique to cardiac PET provide additional evidence of a salutary effect of eplerenone in the trial. Compared with other methods in quantifying myocardial blood flow, cardiac MRI has the advantages of high spatial resolution and high temporal resolution [15] and could help explain why it demonstrated a more consistent effect in the current study. While other studies have demonstrated a more moderate correlation between CFR on PET and sMBF on MRI [16], the changes in these indices in our study were in similar directions and correlated significantly among those receiving eplerenone.
Overall, CFRs were relatively low at baseline in our study population, on average less than 3.0, a level that is relatively abnormal. In our primary analysis, we saw no effects of eplerenone on overall CFR. To further address eplerenone's effects on CFR in our study, we performed supplemental analyses, selecting among those with more marked alterations in perfusion at baseline using standard cutoffs for more severe disease, of whom over 50% of participants met the criteria [17, 18]. In the general population, we have shown using cardiac PET that annual CV mortality risk is approximately 3.3% in patients with impairment of both CFR and maximal MBF, 1.7% in patients with impaired CFR with preserved maximal MBF, 0.9% in patients with preserved CFR and impaired maximal MBF, and 0.4% in patients when both CFR and maximal MBF are preserved [11]. Eplerenone appeared to be most effective among those with more severe disease and limitations in myocardial perfusion at baseline, highlighting the importance of identifying this key group. Future studies are now needed to specifically explore this effect in randomized studies of this population with baseline impaired total flow capacity.
Importantly, several parameters of cardiac function improved on MRI, including measures of LVEDV and measures of systolic function, including GCS, following treatment with eplerenone compared with placebo. Recent studies assessing PWH in the absence of CV risk factors showed that GCS was significantly worse in the HIV group compared with the non-HIV group [19]. Interestingly in our study, eplerenone improved GCS and this effect related to changes in sMBF by cardiac MRI. The landmark PARAMOUNT study evaluating those with clinical heart failure with preserved ejection fraction (HFpEF) in the general population demonstrated a relative improvement in GCS with an absolute difference of 4.42% between sacubitril-valsartan and valsartan [20]. This change was similar to the improvement we saw with eplerenone in the current study of PWH with subclinical disease. Given these benefits of eplerenone on LV function and strain as well as microvascular function, eplerenone could have potential therapeutic benefit in the HFpEF phenotype. The MIRAD trial demonstrated that eplerenone reduced N-terminal pro-hormone brain natriuretic peptide (NT-proBNP) by 22% among persons with type 2 diabetes [21]. We did not see a significant effect of eplerenone on NT-proBNP in our study in the HIV population but hypothesize there could be an effect among PWH selected for more disease at baseline. Further evidence in the current study for a benefit in this group of PWH with subclinical myocardial injury was seen in the prevention of increasing hs-cTnT in the eplerenone-treated group relative to placebo. Notably, the troponin was detectable in 52% of participants despite no known CVD. In contrast, we did not see a direct effect of eplerenone on the ECMi or T1 indices.
We also show a signal of eplerenone to improve CD4 count. An effect of eplerenone to increase in CD4 is plausible, although this has not been previously shown among PWH. The mineralocorticoid receptor (MR) can be found on immune cells, including lymphocytes [22]. Spironolactone, another MR antagonist, was shown to block viral TAT-dependent transcription and inhibited HIV infection of permissive T cells [23]. Eplerenone has also been reported to reverse pathologic changes associated with congestive heart failure in rats by working to inhibit the Kv1.3 channel and increasing regulatory T cells (Tregs) [24]. If there are pleotropic effects on the immune system, this would make an MR antagonist an attractive medication to use in combination with ART to reduce CV morbidity and mortality.
This study had a number of strengths and some limitations. In a randomized, placebo-controlled 1-year trial, we performed comprehensive cardiac imaging, which allowed us to evaluate multiple myocardial indices via different imaging modalities in parallel to understand the overall effects of eplerenone. We took great care to standardize sodium conditions, critical to assessing the effects of eplerenone, and did show an increase in urine aldosterone, consistent with an effect of eplerenone on the RAAS. In addition, we saw a trend toward lower BP, consistent with eplerenone's known antihypertensive effects. The study was relatively small, and therefore may have been underpowered; however, the overall dropout rate was consistent with the planned attrition rate. The dropout rate was greater in the eplerenone group, although many of these participants dropped out for reasons unrelated to the use of eplerenone, and clinical characteristics did not differ among those who dropped out versus those who did not. We could not account for unmeasured adverse effects but were able to confirm these findings in analyses accounting for missing data.
In sum, RAAS blockade with eplerenone benefitted key indices of myocardial perfusion, cardiac dysfunction, and cardiac injury and inflammation among PWH. These effects may be larger in those with more severe subclinical disease, and it will be important to now study eplerenone in a broader population of PWH—ie, those with uncontrolled diabetes and hypertension. Moreover, eplerenone may have other novel properties to improve immune indices in HIV, aside from its well-known mechanism of action related to BP regulation. Future studies should assess the effects of RAAS inhibition with eplerenone, targeting the large subset of PWH with subclinical cardiac disease and impaired baseline myocardial perfusion who might derive maximal benefit from this therapeutic strategy. Ultimately, such a strategy may help prevent the development of clinical disease stemming from reduced myocardial perfusion and inflammation seen among PWH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Suman Srinivasa, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Allie R Walpert, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Teressa S Thomas, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Daniel M Huck, Division of Nuclear Medicine and Molecular Imaging, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Michael Jerosch-Herold, Division of Cardiovascular Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Sabeeh Islam, Division of Cardiovascular Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Michael T Lu, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Tricia H Burdo, Department of Neuroscience, Lewis Katz School of Medicine at Temple University, Philadelphia, Pennsylvania, USA.
Christopher R deFilippi, Division of Cardiology, INOVA Heart and Vascular Institute, Falls Church, Virginia, USA.
Carolyn N Dunderdale, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Meghan Feldpausch, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Sanjna Iyengar, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Grace Shen, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Stephen Baak, Department of Neuroscience, Lewis Katz School of Medicine at Temple University, Philadelphia, Pennsylvania, USA.
Martin Torriani, Division of Musculoskeletal Imaging and Intervention, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Gregory K Robbins, Division of Infectious Disease, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Hang Lee, Biostatistics Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Raymond Kwong, Division of Cardiovascular Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Marcelo DiCarli, Division of Nuclear Medicine and Molecular Imaging, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Gail K Adler, Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Steven K Grinspoon, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Notes
Disclaimer. The funding sources had no role in the design of the study, data analysis, or writing of the manuscript.
Financial support. This work was supported by the National Institutes of Health (NIH; grant numbers R01 DK49302 to S. K. G. and G. K. A.; K23 HL136262 and R01 HL151293 to S. S.; K24 HL103845 to G. K. A.; and P30 DK040561 to Nutrition and Obesity Research Center at Harvard).
Data availability
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012; 126:1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011; 124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knott KD, Seraphim A, Augusto JB, et al. The prognostic significance of quantitative myocardial perfusion: an artificial intelligence-based approach using perfusion mapping. Circulation 2020; 141:1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Srinivasa S, Fitch KV, Wong K, et al. RAAS activation is associated with visceral adiposity and insulin resistance among HIV-infected patients. J Clin Endocrinol Metab 2015; 100:2873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Srinivasa S, Burdo TH, Williams KC, et al. Effects of sodium restriction on activation of the renin-angiotensin-aldosterone system and immune indices during HIV infection. J Infect Dis 2016; 214:1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy CA, Fitch KV, Feldpausch M, et al. Excessive adiposity and metabolic dysfunction relate to reduced natriuretic peptide during RAAS activation in HIV. J Clin Endocrinol Metab 2018; 103:1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Srinivasa S, Fitch KV, Wong K, et al. Randomized, placebo-controlled trial to evaluate effects of eplerenone on metabolic and inflammatory indices in HIV. J Clin Endocrinol Metab 2018; 103:2376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg R, Rao AD, Baimas-George M, et al. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015; 64:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017; 136:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010; 152:726–32. [DOI] [PubMed] [Google Scholar]
- 13. Jerosch-Herold M, Kwong RY. Optimal imaging strategies to assess coronary blood flow and risk for patients with coronary artery disease. Curr Opin Cardiol 2008; 23:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engblom H, Xue H, Akil S, et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: a comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J Cardiovasc Magn Reson 2017; 19:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waller AH, Blankstein R, Kwong RY, Di Carli MF. Myocardial blood flow quantification for evaluation of coronary artery disease by positron emission tomography, cardiac magnetic resonance imaging, and computed tomography. Curr Cardiol Rep 2014; 16:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Everaars H, van Diemen PA, Bom MJ, et al. Comparison between quantitative cardiac magnetic resonance perfusion imaging and [(15)O]H(2)O positron emission tomography. Eur J Nucl Med Mol Imaging 2020; 47:1688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serruys PW, di Mario C, Piek J, et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation 1997; 96:3369–77. [DOI] [PubMed] [Google Scholar]
- 18. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015; 8:1445–53. [DOI] [PubMed] [Google Scholar]
- 19. Luetkens JA, Doerner J, Schwarze-Zander C, et al. Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-infected patients. Circ Cardiovasc Imaging 2016; 9:e004091. [DOI] [PubMed] [Google Scholar]
- 20. Biering-Sorensen T, Lassen MCH, Shah A, et al. The effect of sacubitril/valsartan on left ventricular myocardial deformation in heart failure with preserved ejection fraction (PARAMOUNT trial). J Card Fail. 2023. doi: 10.1016/j.cardfail.2023.03.019 [DOI] [PubMed] [Google Scholar]
- 21. Brandt-Jacobsen NH, Lav Madsen P, Johansen ML, et al. Mineralocorticoid receptor antagonist improves cardiac structure in type 2 diabetes: data from the MIRAD trial. JACC Heart Fail 2021; 9:550–8. [DOI] [PubMed] [Google Scholar]
- 22. Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids 2014; 91:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lacombe B, Morel M, Margottin-Goguet F, Ramirez BC. Specific inhibition of HIV infection by the action of spironolactone in T cells. J Virol 2016; 90:10972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shao PP, Liu CJ, Xu Q, et al. Eplerenone reverses cardiac fibrosis via the suppression of Tregs by inhibition of kv1.3 channel. Front Physiol 2018; 9:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.