Abstract
The World Health Organization recommends same-day initiation of antiretroviral therapy (ART) for all persons diagnosed with HIV and ready to start treatment. Evidence, mainly from randomized trials, indicates offering same-day ART increases engagement in care and viral suppression during the first year. In contrast, most observational studies using routine data find same-day ART to be associated with lower engagement in care. We argue that this discrepancy is mainly driven by different time points of enrollment, leading to different denominators. While randomized trials enroll individuals when tested positive, most observational studies start at the time point when ART is initiated. Thus, most observational studies omit those who are lost between diagnosis and treatment, thereby introducing a selection bias in the group with delayed ART. This viewpoint article summarizes the available evidence and argues that the benefits of same-day ART outweigh a potential higher risk of attrition from care after ART initiation.
Keywords: HIV, AIDS, same day, rapid initiation, continuum of care
Same-day versus delayed antiretroviral therapy initiation for people with HIV aims to reduce pretreatment attrition from care. Studies evaluating same-day initiation should start follow-up at the time of HIV testing/linkage to care to avoid selection bias in the delayed group.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/reatment-outcomes-after-offering-same-day-initiation-of-hiv-treatment-how-to-interpret-discrepancies-between-different-studies
During the early years of roll-out of antiretroviral therapy (ART) in low- and middle-income countries, guidelines and clinical practice emphasized the importance of multiple clinic visits to improve treatment literacy, explain the importance of adherence, and assess and ensure readiness for lifelong ART before initiating therapy. At the time, this was driven by the high cost of medication and concerns that drug resistance may emerge in case of suboptimal adherence. A number of studies indicated, however, that during this pre-ART phase a substantial number of patients disengaged from care before ever initiating ART [1–4]. The burden of having to attend multiple preparatory visits over weeks or months prior to ART initiation was frequently cited as one of the reasons why persons newly diagnosed with human immunodeficiency virus (HIV) did not start treatment [5–10]. On the other hand, easily navigable clinics with structures allowing swift ART initiation were found as facilitators to engage persons with HIV in care [6, 9–12]. In light of these findings, the concept of same-day ART initiation emerged, which aimed to reduce requirements to starting ART and reduce pre-ART attrition from care.
Several clinical trials investigated the benefits and harms of rapid or even same-day ART initiation following HIV diagnosis, with all demonstrating feasibility and several concluding that accelerating the initiation process increases overall engagement in care and viral suppression among those eligible for ART [13–20]. In 2017, the World Health Organization (WHO) issued a strong recommendation to initiate ART on the same day as HIV diagnosis for those ready to start ART immediately unless there are clinical reasons, such as symptoms of cryptococcal meningitis or tuberculosis, to delay treatment [21]. The 2021 WHO guidelines noted that meningitis—ie, cryptococcal or tuberculous—was the only medical reason for delaying ART initiation [22].
Since 2017, same-day ART initiation has become the norm in most settings: more than 90% of countries in sub-Saharan Africa report having a policy supporting ART initiation on the same day as diagnosis [23], and a recent study found that 88% of all initiations in Malawi and 91% in Zambia were done on the same day [24]. While the initial WHO recommendation was based primarily on randomized trial results, widespread adoption of same-day initiation has created the opportunity for observational studies to assess the real-world outcomes of this policy. Several of these observational studies have suggested, in contrast to trial results, that same-day initiation may be causally associated with attrition from care.
In this viewpoint article, we consider the differences in findings between studies on same-day ART and discuss why these discrepancies occur and how they might be interpreted.
RANDOMIZED TRIALS ON SAME-DAY ART INITIATION
A literature review to identify studies published up to 31 December 2022 identified 9 randomized trials assessing the effect of offering same-day ART initiation on engagement in care and viral suppression compared with usual care. Table 1 summarizes their key findings, and Supplementary Table 1 provides a more detailed overview on design, trial population, and outcomes. One trial each was conducted in Kenya [15], Lesotho [17], and Uganda [20]; 2 trials in Haiti [16, 19]; and 4 trials were conducted in South Africa [13–15, 18]. Together, these 9 trials analyzed 4393 persons newly diagnosed with HIV, previously diagnosed but not yet taking ART, or re-engaging after defaulting from care. Five trials [14–17, 20] reported significantly higher engagement in care and/or higher rates of viral suppression within the first year among participants offered same-day ART initiation. The other 4 trials found no important differences in terms of engagement in care and viral suppression between arms. An early trial in South Africa observed higher ART initiation with same-day/rapid versus standard ART initiation, but these differences diminished over time [18]. Similarly, the 2 jointly reported implified algorithm for treatment eligibility I trials (SLATE I), which compared an algorithm to assess eligibility for same-day ART initiation with usual care, found that the algorithm increased same-day and rapid ART uptake but did not alter 12-month engagement in care or viral suppression [13]. Finally, 1 trial, available as a preprint, compared same-day tuberculosis workup and same-day initiation of either ART (if tuberculosis excluded) or tuberculosis treatment in adults diagnosed with HIV and presenting with tuberculosis symptoms. This trial found no difference in 12-month engagement in care or viral suppression defined as less than 1000 copies/mL [19].
Table 1.
Key Findings of Randomized Trials Comparing Same-Day Versus Delayed Antiretroviral Therapy Initiation in Low- and Middle-income Countries
| Source | Interpretation | Summarized Point Estimates |
|---|---|---|
| Rosen et al [15] (South Africa; N = 377) |
Offer of same-day ART initiation increases the proportion of patients who initiate ART within 90 d and are engaged in care with viral suppression at 10 mo. | ART initiation within 90 d and engagement in care with HIV VL ≤400 c/mL at 10 mo:
|
| Amanyire et al [20] (Uganda; 437) | Training and infrastructural interventions emphasizing the importance of fast ART initiation led to faster and higher ART initiation rates with higher rates of viral suppression at 12 mo. | In the intervention group, 71% started ART on the same day (vs 18% in control group); 12-month endpoints only assessed in a random subsample of both arms. Viral suppression (threshold not defined) at 12 mo:
|
| Koenig et al [16] (Haiti; N = 703) |
Offer of same-day ART initiation increases the proportion of patients who initiate ART and are engaged in care with viral suppression at 12 mo. | Engagement in care at 12 mo with HIV VL <50 c/mL:
|
| Stevens [18] (South Africa; N = 432) |
Point-of-care diagnostics to facilitate same-day/rapid ART increase uptake of ART, whereas engagement in ART care equalizes within 6 mo. | Engagement in care at 6 mo:
|
| Labhardt et al [17] (Lesotho; N = 274) |
Offer of same-day ART initiation in the context of door-to-door HIV testing increases linkage to and engagement in care, as well as viral suppression at 6 and 12 mo. | Engagement in care with HIV VL <100 c/mL at 12 mo:
|
| Rosen et al [13] (South Africa; N = 600) | The simplified algorithm for treatment eligibility (SLATE I) increases early uptake of ART without significant influence on engagement in care or viral suppression at 8 mo. | ART initiation within 28 d and engagement in care 8 mo:
|
| Rosen et al [13] (Kenya; N = 477) | The simplified algorithm for treatment eligibility (SLATE I) increases early uptake of ART without significant influence on engagement in care or viral suppression at 8 mo. | ART initiation within 28 d and engagement in care 8 mo:
|
| Maskew et al [14] (South Africa; N = 593) |
The simplified algorithm for treatment eligibility II (SLATE II) increases the proportion who initiate ART within 28 d and are engaged in care with viral suppression at 8 mo. | ART initiation within 28 d and engagement in care at 8 mo:
|
| Dorvil et al [19] (Haiti; N = 500) |
In individuals with tuberculosis symptoms at HIV diagnosis, same-day tuberculosis workup and same-day initiation of either ART or tuberculosis treatment does not increase engagement in care or viral suppression at 48 wk. | Engagement in care at 48 wk:
|
Abbreviations: AD, absolute difference; aRR, adjusted relative risk; ART, antiretroviral therapy; c, copies; CI, 95% confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; RD, risk difference; RR, relative risk/risk ratio; VL, viral load.
OBSERVATIONAL STUDIES ON SAME-DAY ART INITIATION
A literature review up to 19 February 2023 found 11 observational studies from low- and middle-income countries comparing engagement in care and/or viral suppression between individuals who received same-day initiation versus usual care (Table 2 summarizes key findings; Supplementary Table 2 shows a more detailed overview on design, patient population and outcomes). Ten studies describe single-country cohorts from South Africa [4], Eswatini, Zambia, Mozambique, Kenya, Rwanda, and Haiti. One study includes data from the International epidemiological Database to Evaluate AIDS (IeDEA), including 11 countries from East, Central, and West Africa. In total, these studies report on 285 958 individuals with sample sizes per study ranging from 410 [25] to 92 609 [26].
Table 2.
Key Findings of Observational Studies Comparing Same-Day Versus Delayed Antiretroviral Therapy and Reporting Engagement in Care and/or Viral Suppression
| Source | Interpretation | Summarized Point Estimates |
|---|---|---|
| Lilian et al [28] (South Africa; N = 32 290) |
Same-day ART was associated with higher loss to follow-up but lower mortality compared with later ART initiation, but individuals who never started ART were excluded from analysis. | Lost to follow-up at 6 mo from HIV diagnosis:
|
| Puttkammer et al [31] (Haiti; N = 51 729) |
Same-day ART was associated with lower engagement in care at 6 mo compared with later ART initiation, but individuals who never started ART were excluded from analysis. | Engagement in care at 6 mo after ART initiation:
|
| Davey et al [26] (South Africa; N = 92 609) |
Same-day ART was associated with higher loss to follow-up compared with later ART initiation, but individuals who never started ART were excluded from analysis. | Lost to follow-up:
|
| Mshweshwe-Pakela et al [33] (South Africa; N = 826) |
No robust differences were observed between same-day and later ART initiation in terms of engagement in care or viral suppression at 6 mo, but individuals who never started ART were excluded from analysis. (The proportion not initiating ART through 12 mo was reported [14%] but excluded from statistical analyses.) | Engagement in care at 6 mo after ART initiation:
|
| Onoya et al [25] (South Africa; N = 410) |
Attrition from care at 12 mo increased after the change in policy to ART initiation on the same day as HIV diagnosis, although only a small minority (16%) of individuals analyzed from the “same-day” policy period actually received same-day ART. | Attrition from care (including pre-ART) at 12 mo from HIV diagnosis:
|
| Kerschberger et al [27] (Eswatini; N = 1328) |
Same-day ART was associated with higher loss to follow-up and lower engagement in care with viral suppression compared with ART initiation 1–14 d after enrolling into HIV care, but individuals who never started ART were excluded from analysis. | Engagement in care with HIV VL <1000 c/mL ≥5 mo after start:
|
| Magro et al [34] (Mozambique; N = 960) |
Same-day ART may be associated with higher loss to follow-up compared with later ART initiation (these findings were not robust to adjustment), but individuals who never started ART were excluded from analysis. (The proportion not initiating ART through 12 mo was reported [16%] but excluded from statistical analysis.) | Loss to follow-up at 6 mo after HIV diagnosis:
|
| Mody et al [35] (Zambia; N = 65 673) |
In a model using regression discontinuity, same-day ART was associated with higher engagement in care. | Engagement 12 mo after nrolment in HIV care and ≥6 mo taking ART: Complier average causal effect (CACE) of 15.8% (CI: 12.1–19.5%, P < .0001) increase in engagement attributable to same-day ART initiation. |
| Kimanga et al [29] (Kenya; N = 8592) |
Same-day ART was associated with higher loss to follow-up compared with later ART initiation, but individuals who never started ART were excluded from analysis. | Non-retention rate at 12 mo after ART initiation per 100 person-months:
|
| Ross et al [32] (11 countries in Africa; N = 29 017) |
Same-day ART was associated with higher loss to follow-up but was not associated with viral suppression rates compared with later ART initiation. Individuals who enrolled in HIV care but never started ART were excluded from the main analysis. A sensitivity analysis including these individuals still showed poorer engagement in care with same-day ART. | Loss to follow-up at 12 mo after ART initiation:
|
| Murenzi et al [30] (Rwanda; N = 2524) |
Same-day ART was associated with higher loss to follow-up but not with viral suppression rates compared with later ART initiation. Individuals who never started ART were excluded from statistical analysis. | Loss to follow-up:
HIV VL <200 c/mL among those with available VL within 455 d after enrollment:
|
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; aRR, adjusted relative risk; ART, antiretroviral therapy; c, copies; CI, 95% confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; ref, reference; VL, viral load.
All of the observational studies are based on routine facility or national program data. Eight studies reported that same-day initiation was associated with lower rates of post-initiation engagement in care and/or viral suppression (Eswatini [27], South Africa [25, 26, 28], Kenya [29], Rwanda [30], Haiti [31], IeDEA [32]). Two smaller studies from South Africa [33] and Mozambique [34] did not find a robust association between the timing of ART initiation and engagement in care or viral suppression. Finally, 1 study from Zambia with 65 673 individuals applying regression discontinuity, a quasi-experimental design, concluded that same-day initiation was associated with higher rates of engagement in care [35].
EXPLAINING THE DISCREPANCY: IT'S THE DENOMINATOR
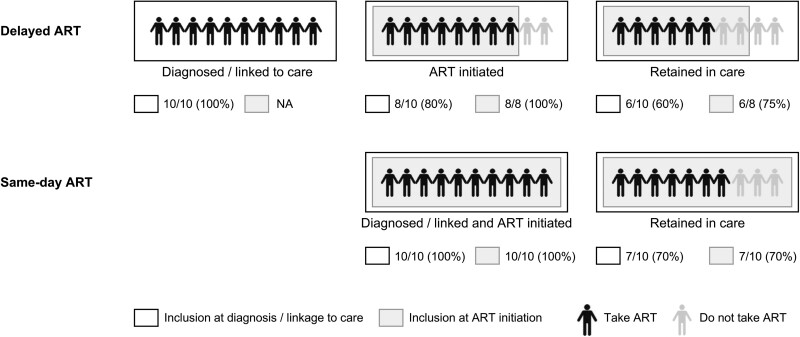
Antiretroviral therapy is not only important for the individual health of persons with HIV but is also a powerful tool to reduce population HIV viral load and thus HIV transmission [36]. At the population level, therefore, the overall proportion of HIV-positive individuals taking ART among all persons with HIV matters more than the proportion engaged on ART among those who started ART. A typical HIV care cascade includes the steps of (re)testing for HIV, (re)linking to HIV care, (re)initiating ART, staying engaged in care, and achieving viral (re)suppression. Studies that start at different steps in the care cascade have, by definition, different denominators. For studies where enrollment starts at the moment of a positive test result, the denominator will be closest to the overall population with HIV and thus comes closest to representing the population-level effect of same-day ART initiation. Studies that start at a later stage in the care cascade, in contrast, focus attention on the effect of same-day ART initiation on a subset of all people with HIV. To capture the full implications of same-day treatment initiation, therefore, studies should ideally start at the point of HIV testing/linkage to care, and not later in the cascade. Finally, studies must avoid using different denominators in the groups they compare (Figure 1).
Figure 1.
Schematic illustration of preselection resulting in different denominators depending on study design. Hypothetical study comparing delayed with same-day ART, with 10 persons diagnosed/linking to care in each group. If in both groups all people diagnosed/linking to care are analyzed (black-bordered box), retention in care is higher in the same-day group (70% same-day vs 60% delayed). If only those initiating ART are included in the analysis (gray box), retention in care is higher in the delayed ART group (75% delayed vs 70% same-day). Abbreviation: ART, antiretroviral therapy.
In the 9 randomized trials on same-day initiation, randomization took place before treatment initiation or pre-ART counselling and before patient readiness to start was confirmed. As such, the modified intention-to-treat population included all those who were tested HIV positive and not taking ART. The lower engagement and viral suppression rates in the control arms observed in 5 of the 9 trials were driven primarily by higher ART initiation rates in the same-day arm—ie higher pre-ART disengagement in the control arms, with relatively similar rates of post-initiation disengagement between study arms over time [15–17].
In considering same-day initiation, it is important to acknowledge that, while a large proportion of those diagnosed with HIV will start ART regardless of timing, a smaller group will not initiate ART even if it is offered on the same day, or will very quickly disengage from care if they accept initiation. In the CASCADE trial comparing the offer of same-day ART versus referral to a clinic among persons who tested HIV positive during home-based HIV testing, for example, 22% of participants who did not return to care and were traced after 2 years never returned to care after they were offered same-day ART and given a first supply of medications at home. Most cited deep skepticism about their HIV diagnosis, the healthcare system as a whole, and concerns about side-effects of ART [17]. Same-day ART as a single intervention does not address the concerns and needs of this subgroup and is thus unlikely to influence their decision on whether to link to and initiate ART or not. They are, however, captured in the denominator of studies that enroll at the point of testing HIV positive or linking to care. Observing higher disengagement from care among those starting the same day compared with those who start later is thus not surprising if only those who ever initiate care are included in the denominator. Indeed, most of the observational studies reporting negative effects of same-day ART on engagement in care, start at the time point when ART is initiated; patients who never start ART are usually excluded (Figure 2).
Figure 2.
Steps in the HIV care cascade covered by identified randomized trials and cohort studies. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
In contrast both to people who will start ART whatever the timing and those who will opt out regardless of how easy initiation is made, same-day ART initiation aims to reduce attrition between HIV testing and initiation for those who would like start but face obstacles or uncertainty. It diminishes the burden that multiple preparatory visits may impose on clients, including long travel distances to the clinic, absence from home or work, and income loss. In the era before the adoption of rapid initiation, the preparation period offered those who were unsure or skeptical about taking ART or faced barriers in attending clinic visits an opportunity to drop out of care before initiating treatment. Under same-day initiation, these same individuals, who are at high risk of disengagement from care, are offered initiation before the obstacles they face have had a chance to get in the way. Same-day ART thus specifically addresses this skeptical or ambivalent subgroup among all persons with HIV.
TIME-ZERO AND SELECTION BIAS
As same-day ART is most likely to have an effect as soon as a person enters the healthcare system after testing HIV positive, studies must ensure that, for both groups, follow-up and comparison start at the same time point, at time zero of the care cascade. However, the majority of published cohort studies report starting from a different time point for those with versus without same-day ART. Five [26–29, 31] of the studies have introduced selection bias by excluding individuals who never started ART in the delayed-initiation group. A further 3 studies [32–34] reported on individuals who had not started ART but included only those who did subsequently start ART as the comparator to same-day ART in the main statistical analyses. In addition to selection bias, the 8 above-mentioned studies are prone to an immortal time bias because those in the delayed ART group have to remain in care for the days or weeks until they initiate ART. Of note, 1 [35] of the observational studies considered all patients at first contact with HIV care using regression discontinuity, a quasi-experimental design aiming to replicate the condition of a randomized trial; this study reports a benefit of same-day ART on engagement in care. Finally, 1 small study [25] comparing time periods for 3 different treatment initiation policies—pre–test-and-treat, test-and-treat, and same-day initiation—enrolled participants from the time of HIV diagnosis but had very high exclusion rates (26%) in the pre–test-and-treat era and only a minority (16%) in the same-day policy period actually received same-day ART. Overall, across cohort studies, many individuals dropped out of care, unobserved, before follow-up began. This was probably exacerbated by the use of routine care datasets, which have the drawback that reliable record-keeping begins when a patient enrolls for HIV care at the clinic (and sometimes only when ART is initiated), rather than at the point of HIV testing, whether tests are conducted in the clinic or in the community. Several observational studies were aware of these limitations and avoided concluding that same-day ART is necessarily unbeneficial, instead rightly arguing for improved mechanisms to support engagement in care in those who start ART.
On the other hand, while published trials may more realistically capture the entire care cascade from testing to viral suppression, even pragmatic trials do not fully reflect real life: they are usually conducted at well-functioning clinic sites, often add human resources to clinics and alter routine clinical management procedures, and may have a Hawthorne effect leading to a general improvement in documentation and quality of care [37]. The way that same-day ART is offered in trial settings may thus be fundamentally different from routine care where same-day initiation may be set as the default option and clinic staff may be too busy to take the time to explore with patients in depth the implications of ART.
WHAT CONCLUSIONS CAN WE DRAW?
Taken together, the available evidence endorses same-day ART initiation as an effective strategy for closing the gap between HIV diagnosis or first clinic contact and treatment provision. The implications of same-day initiation on engagement in care and viral suppression, particularly in the long term, are less clear; there is the potential for improvement in some but not all settings and likely gradual equalization over time, as has also been observed in those studies with longer-term follow-up [38]. Importantly, after accounting for biases, there is no evidence of increased harm.
Both sets of results—clinical trials and observational studies—may each correctly reflect reality for different patient populations. The apparent discrepancy noted above is thus simply an artifact of study design, specifically the time point that participant follow-up starts in the HIV care continuum and the denominator chosen for analysis. As routine data often do not provide reliable documentation from HIV testing to linkage to care and ART initiation, alternative analytic models, such as target trial emulation or marginal structural models [39–41], may be considered and allow more realistic estimates on the effectiveness of same-day ART initiation and engagement in care at the population level.
IMPLICATIONS FOR FUTURE RESEARCH
Perspectives on HIV care are shifting away from a linear model of care cascades and towards a dynamic model of engagement in, disengagement from, and re-engagement in care [42]. Many people with HIV undergo this cycle multiple times over their lifetimes. In consequence, same-day ART initiation should no longer be assessed by its effect on long-term engagement and viral suppression but rather for its benefit in decreasing the time people spend off medication, whether or not there is subsequent disengagement. Same-day initiation lowers the bar for offering medications, contributing to making HIV care more patient-centered. The potential individual risk of higher disengagement post–ART initiation has to be balanced against the population health benefit of same-day ART. The widespread use of integrase-based ART regimens that are less prone to emerging resistance shifts this risk–benefit balance further in favor of same-day ART.
We conclude with the observation that, regardless of the timing of ART initiation and whether individuals drop out of care just before or just after starting ART, all of the research cited here, trials and observational studies alike, point to poor engagement in care in ART programs in the first year of treatment. It is clear that many HIV programs still fail to provide adequate support after initiation, including counselling and treatment literacy, and may place too great a burden of in-person clinic visits on new patients. Work should continue to determine optimal models of care that respond to peoples’ needs. The high rates of disengagement reported in all of the studies thus serve as a reminder of the importance of improving service delivery during the early treatment period.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Niklaus Daniel Labhardt, Division of Clinical Epidemiology, Department of Clinical Research, University Hospital Basel, Basel, Switzerland.
Jennifer Anne Brown, Division of Clinical Epidemiology, Department of Clinical Research, University Hospital Basel, Basel, Switzerland.
Nikita Sass, Division of Clinical Epidemiology, Department of Clinical Research, University Hospital Basel, Basel, Switzerland.
Nathan Ford, Department of HIV, Hepatitis, and Sexually Transmitted Infections, World Health Organization, Geneva, Switzerland; Centre for Infectious Disease Epidemiology and Research, School of Public Health and Family Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Sydney Rosen, Health Economics and Epidemiology Research Office, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; Department of Global Health, Boston University School of Public Health, Boston, Massachusetts, USA.
Notes
Author Contributions . N. D. L. wrote the first draft. N. F., S. R., and J. A. B. contributed significantly to the manuscript text. N. F. designed the literature search. N. S. led the literature screening and data extraction for the supplementary tables, with input from all authors. All authors commented on and approved the final version of the manuscript.
References
- 1. Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS 2014; 28:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larson BA, Brennan A, McNamara L, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health 2010; 15(s1):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNairy ML, Lamb MR, Abrams EJ, et al. Use of a comprehensive HIV care cascade for evaluating HIV program performance: findings from 4 sub-Saharan African countries. J Acquir Immune Defic Syndr 2015; 70:e44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Htun T, Kyaw KWY, Aung TK, et al. Attrition during pre-ART and ART time periods among adolescents enrolled in integrated HIV care programme in Myanmar, 2005–2017. Epidemiol Infect 2019; 147:e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacPherson P, MacPherson EE, Mwale D, et al. Barriers and facilitators to linkage to ART in primary care: a qualitative study of patients and providers in Blantyre, Malawi. J Int AIDS Soc 2012; 15:18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakanwagi S, Matovu JK, Kintu BN, Kaharuza F, Wanyenze RK. Facilitators and barriers to linkage to HIV care among female sex workers receiving HIV testing services at a community-based organization in periurban Uganda: a qualitative study. J Sex Transm Dis 2016; 2016:7673014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tucker JD, Tso LS, Hall B, et al. Enhancing public health HIV interventions: a qualitative meta-synthesis and systematic review of studies to improve linkage to care, adherence, and retention. EBioMedicine 2017; 17:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maughan-Brown B, Kuo C, Galarraga O, et al. Stumbling blocks at the clinic: experiences of seeking HIV treatment and care in South Africa. AIDS Behav 2018; 22:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shamu S, Slabbert J, Guloba G, et al. Linkage to care of HIV positive clients in a community based HIV counselling and testing programme: a success story of non-governmental organisations in a South African district. PLoS One 2019; 14:e0210826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koduah Owusu K, Adu-Gyamfi R, Ahmed Z. Strategies to improve linkage to HIV care in urban areas of sub-Saharan Africa: a systematic review. HIV AIDS (Auckl) 2019; 11:321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hlongwa M, Jama NA, Mehlomakulu V, Pass D, Basera W, Nicol E. Barriers and facilitating factors to HIV treatment among men in a high-HIV-burdened district in KwaZulu-Natal, South Africa: a qualitative study. Am J Mens Health 2022; 16:15579883221120987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vojnov L, Markby J, Boeke C, Harris L, Ford N, Peter T. POC CD4 testing improves linkage to HIV care and timeliness of ART initiation in a public health approach: a systematic review and meta-analysis. PLoS One 2016; 11:e0155256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosen S, Maskew M, Larson BA, et al. Simplified clinical algorithm for identifying patients eligible for same-day HIV treatment initiation (SLATE): results from an individually randomized trial in South Africa and Kenya. PLoS Med 2019; 16:e1002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maskew M, Brennan AT, Fox MP, et al. A clinical algorithm for same-day HIV treatment initiation in settings with high TB symptom prevalence in South Africa: the SLATE II individually randomized clinical trial. PLoS Med 2020; 17:e1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient's first clinic visit: the RapIT randomized controlled trial. PLoS Med 2016; 13:e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koenig SP, Dorvil N, Dévieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med 2017; 14:e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: the CASCADE randomized clinical trial. JAMA 2018; 319:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stevens WS, Gous NM, MacLeod WB, et al. Multidisciplinary point-of-care testing in South African primary health care clinics accelerates HIV ART initiation but does not alter retention in care. J Acquir Immune Defic Syndr 2017; 76:65–73. [DOI] [PubMed] [Google Scholar]
- 19. Dorvil N, Rivera VR, Riviere C, et al. Same-day testing with initiation of antiretroviral therapy or tuberculosis treatment versus standard care for persons presenting with tuberculosis symptoms at HIV diagnosis: a randomized unblinded trial. medRxiv 22283999 [Preprint]. Available from: 10.1101/2022.12.28.22283999. Accessed 21 May 2023. [DOI] [PMC free article] [PubMed]
- 20. Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV 2016; 3:e539–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WHO Guidelines Review Committee . Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva, Switzerland: World Health Organization; 2017. [PubMed] [Google Scholar]
- 22. World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: WHO; 2021. [PubMed] [Google Scholar]
- 23. World Health Organization & Joint United Nations Programme on HIV/AIDS (UNAIDS) . Laws and policies analytics. 2022. Available at: https://lawsandpolicies.unaids.org/topicresult?i=749&lan=en. Accessed 21 May 2023.
- 24. Huber A, Hirasen K, Brennan A, et al. Uptake of same-day initiation of HIV treatment in Malawi, South Africa, and Zambia as reported in routinely collected data: the SPRINT retrospective cohort study. Gates Open Res 2023; 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onoya D, Hendrickson C, Sineke T, et al. Attrition in HIV care following HIV diagnosis: a comparison of the pre-UTT and UTT eras in South Africa. J Int AIDS Soc 2021; 24:e25652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davey DJ, Kehoe K, Serrao C, et al. Same-day antiretroviral therapy is associated with increased loss to follow-up in South African public health facilities: a prospective cohort study of patients diagnosed with HIV. J Int AIDS Soc 2020; 23:e25529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerschberger B, Boulle A, Kuwengwa R, Ciglenecki I, Schomaker M. The impact of same-day antiretroviral therapy initiation under the World Health Organization treat-all policy. Am J Epidemiol 2021; 190:1519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RPH. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: analysis of routine data. PLoS One 2020; 15:e0227572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kimanga DO, Oramisi VA, Hassan AS, et al. Uptake and effect of universal test-and-treat on twelve months retention and initial virologic suppression in routine HIV program in Kenya. PLoS One 2022; 17:e0277675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murenzi G, Kim HY, Shi Q, et al. Association between time to antiretroviral therapy and loss to care among newly diagnosed Rwandan people living with human immunodeficiency virus. AIDS Res Hum Retroviruses 2023; 39:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puttkammer N, Parrish C, Desir Y, et al. Toward universal HIV treatment in Haiti: time trends in art retention after expanded ART eligibility in a national cohort from 2011 to 2017. J Acquir Immune Defic Syndr 2020; 84:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross J, Brazier E, Fatti G, et al. Same-day antiretroviral therapy initiation as a predictor of loss to follow-up and viral suppression among people with human immunodeficiency virus in sub-Saharan Africa. Clin Infect Dis 2023; 76:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mshweshwe-Pakela N, Hansoti B, Mabuto T, et al. Feasibility of implementing same-day antiretroviral therapy initiation during routine care in Ekurhuleni district, South Africa: retention and viral load suppression. South Afr J HIV Med 2020; 21:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magro P, Cerini C, da Gloria A, Tembe S, Castelli F, Tomasoni LR. The cascade of care of HIV after one year of follow-up in a cohort of HIV-positive adult patients in three health settings of Morrumbene in rural Mozambique. Trop Med Int Health 2021; 26:1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mody A, Sikazwe I, Namwase AS, et al. Effects of implementing universal and rapid HIV treatment on initiation of antiretroviral therapy and retention in care in Zambia: a natural experiment using regression discontinuity. Lancet HIV 2021; 8:e755–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Havlir D, Lockman S, Ayles H, et al. What do the universal test and treat trials tell us about the path to HIV epidemic control? J Int AIDS Soc 2020; 23:e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 2014; 67:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amstutz A, Brown JA, Ringera I, et al. Engagement in care, viral suppression, drug resistance, and reasons for nonengagement after home-based same-day antiretroviral therapy initiation in Lesotho: a two-year follow-up of the CASCADE trial. Clin Infect Dis 2020; 71:2608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cain LE, Saag MS, Petersen M, et al. Using observational data to emulate a randomized trial of dynamic treatment-switching strategies: an application to antiretroviral therapy. Int J Epidemiol 2016; 45:2038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petersen ML, Tran L, Geng EH, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS 2014; 28:2097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hernán MA, Wang W, Leaf DE. Target trial emulation: a framework for causal inference from observational data. JAMA 2022; 328:2446–7. [DOI] [PubMed] [Google Scholar]
- 42. Ehrenkranz P, Rosen S, Boulle A, et al. The revolving door of HIV care: revising the service delivery cascade to achieve the UNAIDS 95-95-95 goals. PLoS Med 2021; 18:e1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.